Abstract
Hamsters infected with Leishmania donovani develop a disease similar to human kala-azar. They present hypergammaglobulinemia, and their T cells do not respond to parasite antigens. This unresponsiveness has been primarily ascribed to defects in antigen-presenting cells (APCs), because these cells are unable to stimulate proliferation of parasite-specific T cells from immunized animals. In this study, we show that APCs (adherent spleen cells) from L. donovani-infected hamsters produce high levels of the inhibitory cytokine transforming growth factor β (TGF-β). Immunohistochemical studies with an anti-TGF-β monoclonal antibody (MAb) showed that this cytokine is abundantly produced in vivo by the spleen cells of infected animals. In addition, high levels of TGF-β are produced in vitro by infected hamster cells, either spontaneously or after stimulation with parasite antigen or lipopolysaccharide. Furthermore, in vivo-infected adherent cells obtained from spleens of L. donovani-infected hamsters caused profound inhibition of the in vitro antigen-induced proliferative response of lymph node cells from hamsters immunized with leishmanial antigens. Moreover, this inhibition was totally abrogated by the anti-TGF-β MAb. These results suggest that the immunosuppression observed in visceral leishmaniasis is, at least in part, due to the abundant production of TGF-β during the course of the infection.
The protozoa of the genus Leishmania are parasites responsible for diseases of major relevance in tropical and subtropical areas of the world (10, 14). In mammals, these parasites are strictly intracellular and infect mononuclear phagocytic and dendritic cells. The involvement of the immune system in human visceral leishmaniasis is characterized by increased serum immunoglobulin levels with high titers of antileishmania antibodies (6, 16, 17), hepatomegaly, splenomegaly, adenomegaly, negative skin test for leishmania antigens, and the absence of a proliferative response of peripheral blood mononuclear cells to Leishmania donovani antigens (20). The cause of this impaired specific immune response may be ascribed to any of the cell types involved in the immune response.
Hamsters have been used as a good model for the study of visceral leishmaniasis (4, 5, 7, 18, 22). After intracardiac or intraperitoneal infection, they present a progressive disease similar to human kala-azar, developing hypergammaglobulinemia due to B-cell polyclonal activation and the absence of a T-cell proliferative response to parasite antigens (4). The latter dysfunction has been attributed to the inability of the infected antigen-presenting cells (APCs) to stimulate the specific T cells (9, 13, 15, 21).
Transforming growth factor β (TGF-β) is a pleiotropic cytokine involved in many functions of resident tissue cells, but it largely inhibits some activity of immune cells. TGF-β can mediate immunosuppression by inhibiting interleukin 2-dependent T- and B-cell proliferation and interleukin 2-dependent immunoglobulin production by B cells (11, 12) and macrophage activation (8, 23). In addition, TGF-β has been implicated as a cytokine that promotes the in vitro replication and survival of leishmania within the macrophages and is an important factor for determining in vivo susceptibility to experimental infection of mice with species of leishmania that cause cutaneous and mucocutaneous infections in humans (1–3).
Most of the alterations induced by TGF-β on the cellular immune response resemble those of our previous observation in the hamster infected with L. donovani (21). These studies indicated that the impairment of the immune response was caused by a defect in the antigen presentation by the APCs of L. donovani-infected animals.
In the present work, we extend these studies, aiming to investigate the mechanism involved in this defect. We focused on the immunoregulatory effect of TGF-β on the APCs (adherent spleen cells) from L. donovani-infected hamsters. To achieve this proposal, we initially had to validate a standard human TGF-β biological assay (19) for the hamster system. This validation was important because of the unavailability of an anti-hamster TGF-β antibody. Because of the evolutionary conservation of TGF-β, we tested the standard proliferation inhibition assay of CCL64 cells (mink lung epithelial cells) in the absence and in the presence of the mouse anti-human TGF-β monoclonal antibody (MAb) 1D11.16 (Celtrix Pharmaceuticals, Inc., Santa Clara, Calif.). Normal hamster spleen cells were stimulated with lipopolysaccharide (1 μg/ml), and the presence of TGF-β in the culture supernatants was analyzed 24 and 48 h later. Regardless of the incubation time, the culture supernatants caused, in a dose response manner, inhibition of the proliferation of the CCL64 cells ranging from 100% to no inhibition (not shown). More importantly, this activity was totally abrogated by the 1D11.16 MAb, therefore validating the use of this assay for the hamster system.
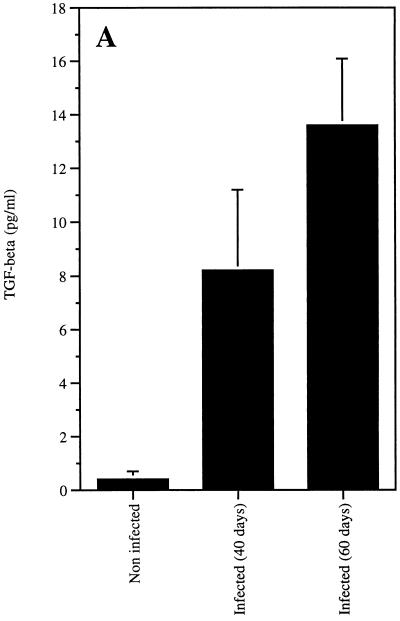
To evaluate the production of TGF-β in L. donovani-infected hamsters, spleen cells were obtained from noninfected inbred CB hamsters (Charles River, Mass.) and from CB hamsters at 40 and 60 days after infection with 10 × 106 amastigote forms of L. donovani (amastigotes were freshly isolated from spleens of infected hamsters). The spontaneous production of TGF-β was measured in the culture (Dulbecco’s modified Eagle’s medium [DMEM] plus 2% fetal calf serum [FCS]) supernatants after 48 h of incubation at 37°C. It is clear from the results shown in Fig. 1 that the spleen cells from hamsters infected for 40 or 60 days produced as much as 20 and 34 times more TGF-β, respectively, than was produced by the cells from age- and sex-matched syngeneic normal hamsters. In addition, in vitro stimulation of these cells with 10 μg of a soluble L. donovani lysate antigen (SLdA) per ml (21) augmented two to three times the production of TGF-β by the spleen cells from either noninfected or infected hamsters. These results are consistent with previous observations that showed that TGF-β is produced by murine macrophages exposed to Leishmania braziliensis (1, 2). However, stimulation of the hamster cells with 1 μg of lipopolysaccharide (Pierce Chemical Company, Rockford, Ill.) augmented more than 10 times the production of TGF-β by the cells from noninfected animals and less than 3 times the production for the cells of hamsters infected for 40 or 60 days (not shown), thus suggesting an exacerbated in vivo prestimulation of the hamster spleen cells by the infectious process.
FIG. 1.
Spontaneous production of TGF-β by spleen cells of noninfected or L. donovani-infected hamsters. Spleen mononuclear cells from hamsters infected for 40 and 60 days and from noninfected animals were suspended at 5 × 106 cells/ml in RPMI medium supplemented with 2% FCS plus antibiotics and cultured at 37°C for 48 h. Supernatants were analyzed for native TGF-β (nonacidified) activity (A) with the proliferation inhibition assay of CCL64 cells (mink lung epithelial cells). CCL64 cells were cultured for 30 h in DMEM supplemented with 2% FCS in the presence of serial dilutions of supernatants. Proliferation was measured by incorporation of [3H]thymidine added during the last 6 h of incubation. An anti-TGF-β-neutralizing MAb was used to confirm the specificity of the assay. Quantification of TGF-β was performed with a standard curve obtained with recombinant human TGF-β (B). Bars represent the arithmetic mean of the results from at least three hamsters per group, and lines give the standard errors.
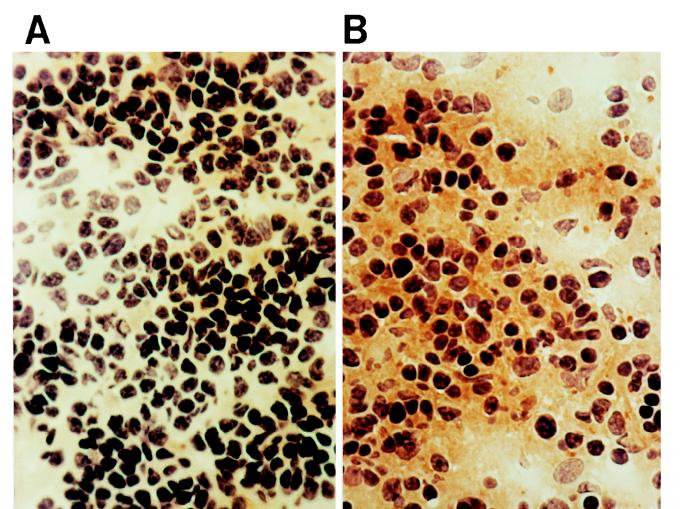
To demonstrate the in vivo production of TGF-β, we performed an immunohistochemical assay with the 1D11.16 MAb. Spleen sections (4 μm thick) from L. donovani-infected and noninfected animals were fixed with acetone and incubated with the 1D11.16 MAb for 4 h at room temperature. The sections were washed four times with phosphate-buffered saline (PBS) containing 0.05% Tween 20 and incubated with a rabbit anti-mouse immunoglobulin-peroxidase conjugate (Pierce Chemical Company) for 1 h at room temperature. The slides were washed four times and incubated with 1 mg of diaminobenzidine (Pierce Chemical Company) per ml and 0.01% hydrogen peroxide in 100 mM Tris-HCl (pH 7.2). The results (Fig. 2) show massive production of TGF-β in the spleens from the infected animals, largely present both in the cytoplasm of mononuclear cells and in the intercellular space. In contrast, the presence of this cytokine was barely noticeable in the spleens from age- and sex-matched syngeneic noninfected controls.
FIG. 2.
Detection of TGF-β production in vivo in spleens of L. donovani-infected hamsters. Spleen sections (4 μm thick) from noninfected hamsters (A) and from hamsters infected with L. donovani for 60 days (B) were fixed with acetone and incubated with anti-TGF-β MAb 1D11.16, washed four times with PBS plus 0.05% Tween 20, and incubated with a rabbit anti-mouse immunoglobulin-peroxidase conjugate for 1 h at room temperature. The slides were washed and incubated with 1 mg of diaminobenzidine per ml and 0.01% hydrogen peroxide in Tris-HCl (0.1 M [pH 7.2]). Brown granules indicate the presence of TGF-β in the spleens of infected animals. Magnification, ×500.
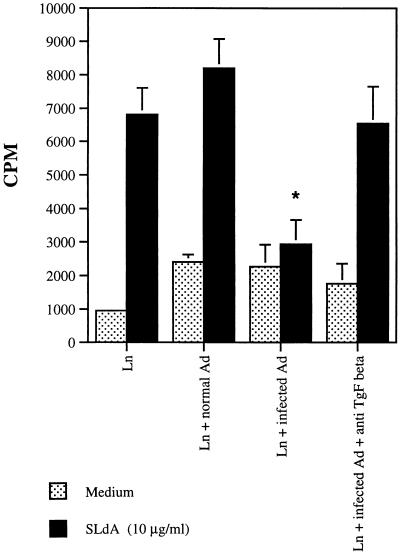
We and others have previously shown that the antigen presentation by L. donovani-infected cells is greatly impaired (9, 13, 15, 21). Because the spleen cells from L. donovani-infected hamsters produced large quantities of TGF-β, we tested the possibility that the production of this antiproliferative cytokine by infected cells was responsible for the observed inability of these cells to perform antigen presentation. To investigate this possibility, noninfected CB hamsters were initially immunized in the footpad with SLdA in complete Freund’s adjuvent, and the draining lymph nodes were harvested 10 days later. Lymph node cells (2 × 105/well in 96-well flat-bottom microtiter culture plates) were obtained and stimulated to proliferate with SLdA in the absence and in the presence of adherent spleen cells obtained from either noninfected or L. donovani-infected hamsters. Adherent spleen cells were obtained by incubating mononuclear spleen cells (5 × 105) for 2 h in the wells of 96-well flat-bottom microtiter culture plates. Nonadherent cells were then removed by three consecutive washes with RPMI medium plus 10% FCS. In addition, some cultures received the 1D11.16 MAb or an isotype-matched immunoglobulin control. Figure 3 shows that adherent cells from the infected hamsters inhibited practically 100% of the specific proliferative response of the lymph node cells from L. donovani-immunized animals. More importantly, the anti-TGF-β MAb 1D11.16 totally restored the responsiveness of the lymph node cells to the leishmanial antigens, thus pointing to a strong participation of TGF-β as an inhibitory cytokine produced by adherent spleen cells of L. donovani-infected hamsters. No inhibition of the proliferative response was observed when adherent spleen cells from noninfected hamsters were used. In addition, no restoration of the proliferation was achieved when an isotype-matched control of the 1D11.16 MAb was added to the cell cultures (not shown). These results expand those related to the L. donovani complex, with previous observations pointing to a pathological role of TGF-β in the course of infection of mice infected with dermotropic Leishmania (1–3). However, in these studies, the inhibitory effect of TGF-β appeared relatively early (a few days) after infection, suggesting that this cytokine is involved in the regulation and development of a pervasive immune response at the initiation of the infection. In contrast, because in the present studies the involvement of TGF-β was detected only late in the infection, it is more likely that the role of this cytokine in visceral leishmaniasis is to maintain or to exacerbate the characteristic immunosuppression observed in this disease.
FIG. 3.
Inhibition of antigen-induced proliferative response of lymph node cells from immunized hamsters by adherent spleen cells from L. donovani-infected hamsters. CB hamsters were immunized in the footpad with soluble L. donovani lysate antigen in complete Freund’s adjuvant, and the draining lymph nodes were harvested 10 days later. Lymph node cells were obtained and stimulated to proliferate with L. donovani antigens in the absence of additional adherent spleen cells (Ln), in the presence of adherent spleen cells obtained from noninfected hamsters (Ln + normal Ad) or from L. donovani-infected hamsters (Ln + infected Ad), or in the presence of adherent spleen cells obtained from L. donovani-infected hamsters plus the anti-TGF-β MAb 1D11.16 (Ln + infected Ad + anti-TGF-β). Cultures were either nonstimulated (medium) or stimulated with 10 μg of SLdA, and proliferation was measured by incorporation of [3H]thymidine added during the last 6 h of incubation in a 3-day assay. Bars represent the arithmetic mean of triplicate cultures, and lines give the standard errors. The results shown are representative of two independent experiments. One-way analysis of variance was used to analyze the results. Statistical significance was set up at P < 0.01. *, P < 0.01 versus Ln + normal Ad or Ln + infected Ad + anti-TGF-β.
Acknowledgments
We thank Fundação de Ensino e Pesquisa de Uberaba for financial support.
REFERENCES
- 1.Barral A, Barral-Netto M, Yong E C, Brownell C E, Twardzik D R, Reed S G. Transforming growth factor β as a virulence mechanism for Leishmania braziliensis. Proc Natl Acad Sci USA. 1993;90:3442–3446. doi: 10.1073/pnas.90.8.3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barral, A., M. Teixeira, P. Reis, V. Vinhas, J. Costa, H. Lessa, A. L. Bittencourt, S. Reed, E. M. Carvalho, and M. Barral-Netto. Transforming growth factor β in human cutaneous leishmaniasis. Am. J. Pathol. 147:947–954. [PMC free article] [PubMed]
- 3.Barral-Netto M, Barral A, Brownell C E, Skeiky Y A, Ellingsworth L R, Twardzik D R, Reed S G. Transforming growth factor β in leishmania infection: a parasite escape mechanism. Science. 1992;257:545–548. doi: 10.1126/science.1636092. [DOI] [PubMed] [Google Scholar]
- 4.Bunn-Moreno M, Madeira E D, Miller K, Menezes J A, Campos-Neto A. Hypergammaglobulinemia in Leishmania donovani infected hamsters: possible association with a polyclonal activator of B cells and with suppression of T cell function. Clin Exp Immunol. 1985;59:427–434. [PMC free article] [PubMed] [Google Scholar]
- 5.Campos-Neto A, Bunn-Moreno M M. Polyclonal B cell activation in hamsters infected with parasites of the genus Leishmania. Infect Immun. 1982;38:871–876. doi: 10.1128/iai.38.3.871-876.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chaves J, Ferri R G. Immunoglobulins in visceral leishmaniasis. Rev Inst Med Trop São Paulo. 1966;8:225–227. [PubMed] [Google Scholar]
- 7.Clinton B A, Stauber L A, Palczuk N C. Leishmania donovani. I. Antibody response to chicken ovalbumin by infected golden hamsters. Exp Parasitol. 1969;25:171–180. doi: 10.1016/0014-4894(69)90063-0. [DOI] [PubMed] [Google Scholar]
- 8.Ding A, Nathan C F, Graycar J, Derynck R, Stuehr D J, Srimal S. Macrophage deactivating factor and transforming growth factors β1, β2, and β3 inhibit induction of macrophage nitrogen oxide synthesis by IFN-γ. J Immunol. 1990;145:940–944. [PubMed] [Google Scholar]
- 9.Fruth U, Solioz N, Louis J A. Leishmania major interferes with antigen presentation by infected macrophages. J Immunol. 1993;150:1857–1864. [PubMed] [Google Scholar]
- 10.Grimaldi G, Jr, Tesh R B. Leishmaniasis of the New World: current concepts and implications for future research. Clin Microbiol Rev. 1993;6:230–250. doi: 10.1128/cmr.6.3.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kehrl J H, Roberts A B, Wakefield L M, Jakowlew S, Sporn M B, Fauci A S. Transforming growth factor β is an important immunomodulatory protein for human B lymphocytes. J Immunol. 1986;137:3855–3860. [PubMed] [Google Scholar]
- 12.Kehrl J H, Wakefield L M, Roberts A B, Jakowlew S, Alvarez-Mon M, Derynck R, Sporin M B, Fauci A S. Production of transforming growth factor β by human T lymphocytes and its potential role in the regulation of T cell growth. J Exp Med. 1986;163:1037–1050. doi: 10.1084/jem.163.5.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kima P E, Soong L, Chicarro C, Ruddle N H, McMahon-Pratt D. Leishmania-infected macrophages sequester endogenously synthesized parasite antigens from presentation to CD4+ T cells. Eur J Immunol. 1996;26:3163–3169. doi: 10.1002/eji.1830261249. [DOI] [PubMed] [Google Scholar]
- 14.Laison R, Shaw J J. Epidemiology and ecology of leishmaniasis in Latin America. Nature. 1978;273:595–600. doi: 10.1038/273595a0. [DOI] [PubMed] [Google Scholar]
- 15.López J A, LeBowitz J H, Beverly S M, Rammensee H-G, Overath P. Leishmania mexicana promastigotes induce cytotoxic T lymphocytes in vivo that do not recognize infected macrophages. Eur J Immunol. 1992;23:217–223. doi: 10.1002/eji.1830230134. [DOI] [PubMed] [Google Scholar]
- 16.Manson-Bahr P E C. Immunity in kala-azar. Trans R Soc Trop Med Hyg. 1961;55:550–555. doi: 10.1016/0035-9203(61)90078-5. [DOI] [PubMed] [Google Scholar]
- 17.Mayrink W, Araujo F G, Magalhães P A. Fluorescent antibody test in visceral leishmaniasis. Rev Inst Med Trop São Paulo. 1967;9:172–174. [PubMed] [Google Scholar]
- 18.Nickol A D, Bonventre P F. Immunosuppression associated with visceral leishmaniasis of hamsters. Parasite Immunol. 1985;7:439–449. doi: 10.1111/j.1365-3024.1985.tb00089.x. [DOI] [PubMed] [Google Scholar]
- 19.Ranchalis J E, Gentry L, Seyedin S M, McPherson J, Purchio A, Twardzik D R. Bone-derived and recombinant transforming growth factor β are potent inhibitors of tumor cell growth. Biochem Biophys Res Commun. 1987;148:783–789. doi: 10.1016/0006-291x(87)90944-2. [DOI] [PubMed] [Google Scholar]
- 20.Rezai H R, Ardehsli S M, Armirhakimi G, Kharazmi A. Immunological features of kala-azar. Am J Trop Med Hyg. 1978;27:1079–1083. doi: 10.4269/ajtmh.1978.27.1079. [DOI] [PubMed] [Google Scholar]
- 21.Rodrigues V, Jr, Santana J S, Campos-Neto A. Selective inability of spleen antigen presenting cells from Leishmania donovani infected hamsters to mediate specific T cell proliferation to parasite antigens. Parasite Immunol. 1992;14:49–58. doi: 10.1111/j.1365-3024.1992.tb00005.x. [DOI] [PubMed] [Google Scholar]
- 22.Sartori A, Oliveira A V, Roque-Barreira M C, Rossi M A, Campos-Neto A. Immune complex glomerulonephritis in experimental kala-azar. Parasite Immunol. 1987;9:93–103. doi: 10.1111/j.1365-3024.1987.tb00491.x. [DOI] [PubMed] [Google Scholar]
- 23.Tsunawaki S, Sporn M, Ding A, Nathan C F. Deactivation of macrophages by transforming growth factor β. Nature. 1988;334:260–262. doi: 10.1038/334260a0. [DOI] [PubMed] [Google Scholar]