Abstract
Introduction.
Ketamine is a fast-acting anesthetic with hypnotic properties. Moreover, could potentially improve affective symptoms in patients with refractory depressive disorder.
Objective.
explore the scientific literature available until December 10, 2021, about the efficacy and safety of ketamine in patients with treatment-refractory major depressive disorder.
Material and methods.
Scoping review that included PubMed and Scopus. Records of clinical trials and publications with empirical data in English and Spanish were included.
Results.
31 documents and 12 clinical trial records were included: randomized clinical trials (n = 19), non-randomized clinical trials (n = 11) and retrospective cohort studies (n = 1). The sum of participants in clinical trial registries was 1,318. Some 58.3% (7/12) of the records of clinical trials are not yet recruiting the study population, 25% (3/12) are phase 2 studies and only one study is currently in phase four.
Conclusions.
The evidence supports the use of ketamine for the treatment of refractory depression. Adverse effects are generally mild and self-limited, although more complex adverse effects require monitoring by experienced personnel. Experimental studies are needed to compare the efficacy and safety of ketamine versus electroconvulsive therapy as the first-line treatment for this entity.
Keywords: Depressive disorder, Treatment resistant depression, Ketamine, Electroconvulsive therapy.
KETAMINA PARA LA DEPRESIÓN REFRACTARIA: UNA REVISIÓN EXPLORATORIA
Introducción.
La ketamina es un anestésico de efecto rápido con propiedades hipnóticas. Además, podría mejorar potencialmente los síntomas afectivos en pacientes con trastorno depresivo refractario.
Objetivo.
Explorar la literatura científica disponible hasta el 10 de diciembre de 2021 sobre la eficacia y seguridad de la ketamina en pacientes con trastorno depresivo mayor refractario al tratamiento.
Material y métodos.
Revisión exploratoria que incluyó PubMed y Scopus. Se incluyeron registros de ensayos clínicos y publicaciones con datos empíricos en inglés y español.
Resultados.
Se incluyeron 31 documentos y 12 registros de ensayos clínicos: estudios clínicos aleatorizados (n = 19), estudios clínicos no aleatorizados (n = 11) y estudios de cohortes retrospectivos (n = 1). La suma de participantes en registros de ensayos clínicos fue de 1,318. Un 58,3 % (7/12) de los registros de ensayos clínicos aún no están reclutando la población de estudio, el 25 % (3/12) son estudios de fase 2 y solo un estudio se encuentra actualmente en la fase cuatro.
Conclusiones.
La evidencia apoya el uso de ketamina para el tratamiento de la depresión refractaria. Los efectos adversos son generalmente leves y autolimitados, aunque los efectos adversos más complejos requieren vigilancia por parte de personal experimentado. Son necesarios estudios experimentales que comparen la eficacia y seguridad de la ketamina frente a la terapia electroconvulsiva como tratamiento de primera línea de esta entidad.
Keywords: Trastorno depresivo, Tratamiento refractario, Ketamina, Terapia electroconvulsiva.
INTRODUCTION
Depressive disorders have a high prevalence worldwide and negatively impact functionality and quality of life (1). Major depressive disorder (MDD) is characterized by changes in affective, cognitive and neurovegetative functions that last at least two weeks (2). The etiology of MDD is multifactorial and involves genetic, epigenetic and environmental factors (2). The pathophysiology is associated with biochemical and functional variations in specific areas of the brain such as the dorsolateral prefrontal cortex and the hippocampus (3). Researchers have reported a prevalence of MDD between 16 % to 28.2% in the general population (14).
Treatment of MDD can be pharmacological or nonpharmacological, and typically include changes in lifestyle and psychotherapy (5). Despite the fact that there is currently a wide antidepressant pharmacological arsenal available, control of MDD is not always possible. More than 50% of patients do not respond adequately to treatment regimens and around 30% present refractory or treatment-resistant depression (TRD) (6). Although multiple definitions have been proposed, the current consensus is an insufficient response to two antidepressant drug regimens administered at the appropriate dose, duration and adherence. In addition, bipolar disorder or other nonpsychiatric medical illnesses must be ruled out (7,8)
Ketamine (2-0-chlorophenyl-2-methylamino-cyclo- hexamine) is a drug that acts as a competitive antagonist of phencyclidine through the excitatory receptor of Glutamate N-methyl D'Aspartate (NMDA). It is reported as a hypnotic, analgesic, antidepressant and anti-inflammatory agent (9). The exploration of the antidepressant properties of ketamine dates back to the 1970s. Khor- ramzadeh and Lotfy (10) administered intravenous ketamine 0.2 to 1.0 mg / kg (VI) to 100 hospitalized patients with depressive disorder. The researchers reported a favorable impact by reducing depressive symptoms. Zarate et al. (11) Reported that 17 subjects diagnosed with TRD improved their depressive symptoms after the administration of 0.5 mg / kg of VI ketamine compared to subjects who received placebo within the first two hours after treatment. Veraart et al. (12) conducted a systematic review to explore whether ketamine has an efficacy and safety profile similar to electroconvulsive therapy (ECT). The authors reported that ketamine treatment could address depressive symptoms in a short period of time and with less cognitive impairment. The six studies included in this review, however, had a small sample size, used different therapeutic regimens, and the time of follow-up to the participants made it impossible to study the long-term effects. The objective of this scoping review was to explore the scientific literature available up to 10 December 2021 on the efficacy and safety of ketamine in patients with TRD.
MATERIAL AND METHODS
A scoping review of the literature was carried out following the steps proposed by Arksey and O'Malley (14), and improved by Levac (15): (i) creation of the research question; (ii) identification of relevant studies; (iii) selection of studies; (iv) data extraction; (v) synthesis and reporting of the results. The review sought to answer the question: What is the current state of the scientific literature on the efficacy and safety of ketamine in the management of patients with TRD?
PubMed and Scopus were included using search terms and Boolean operators (Supplementary file 1). The inclusion criteria were: (a) language of the publication is Spanish or English, (b) publications with empirical data (clinical trials or observational studies) with no time limit, (c) documents exploring the efficacy or safety of ketamine, (d) studies include patients with TRD. The documents that did not meet the inclusion criteria were excluded; for example, studies done only in patients with other types of depression, such as bipolar depression. Additionally, the clinical trial records from 18 databases of the WHO (16) International Clinical Trials Registry Platform (Supplemental file 1) were reviewed and included. Inclusion of clinical trial records allow to describe the characteristics of ongoing studies on efficacy and safety of ketamine in TRD.
The free access web application Rayyan was used to select the included studies (17). Two authors (M.E-R and A.H.-P.) independently reviewed the titles and abstracts of the publications found, reaching a consensus on potentially relevant documents. Subsequently, the review of the complete document was carried out for their final selection (based on the eligibility criteria). Two tables were created in Microsoft Word for data extraction. Variables such as authors, type of study, objective, date of publication, journal, country of the authors, occurrence and main findings were extracted. A summary of the characteristics of the documents found in the databases was created. Finally, a narrative synthesis of the results was carried out. The article followed the PRISMA extension for reporting scoping reviews (PRISMA-ScR) (18), which available in supplemental file 2.
RESULTS
31 documents (supplementary file 3) and 12 records of clinical trials (n = 43) were included (Fig. 1, Tables 1 and 2).
Figura 1.
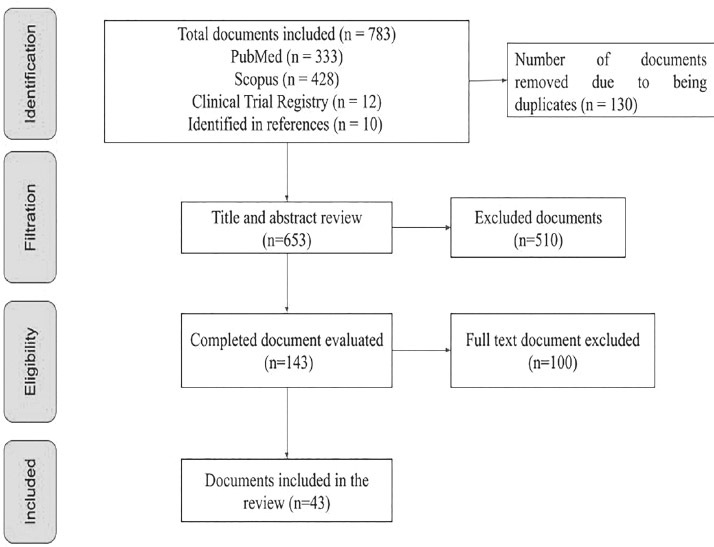
PRISMA diagram of the documents identified in the databases.
Tabla 1.
Characteristics of articles exploring the efficacy and safety of ketamine
| Authors | Type of document | Características de la población | Objective | Fecha de publicación | Journal | País de los autores | Outcome |
|---|---|---|---|---|---|---|---|
| Mu-Hong Chen et al | Randomized clinical trial | 48 males patients between the ages of 20 and 65 | Evaluate the effects of ketamine on frontostriatal connectivity in patients with MDD | 2020 | International Journal of Neuropsychopharmacology | Taiwan | Ketamine at a dose of 0.2 mg / kg IV is effective for a reduction of symptoms of depression associated with frontostriatal disconnect in patients with MDD |
| Orly Lipsitza et al | Clinical trial | 134 males patients and females over 18 years of age | Evaluate early symptomatic improvement after ketamine administration in patients with MDD | 2020 | Neuropsychopharma- cology & Biological Psychiatry | Canada | Ketamine at a dose of 0.5 mg / kg IV is rapid and effective after the administration of 4 doses for symptomatic improvement in patients with MDD |
| Megha M. Va- savada et al | Randomized clinical trial | 44 males patients and females with average age of 38 years | Evaluate the action of ketamine on cortico-limbic connections in the treatment of MDD in patients with a predominance of affective symptoms | 2020 | Biological Psychiatry: CNNI | United States | Ketamine at sub anesthetic doses allows neuroplasticity in all limbic regions allowing improvement in affective symptoms of MDD |
| Liu Weijiana et al | Clinical trial | 60 males patients and females between 18 to 65 years | Evaluate the efficacy and safety of ketamine in the control of MDD in patients with and without anxiety | 2019 | Journal Affective Disorder | China | Ketamine at a dose of 0.5 mg / kg IV is effective and safe in the control of various depressive symptoms in patients with anxiety |
| Mu-Hong Chen et al | Randomized clinical trial | 48 males patients and females between 20 to 65 years | Evaluate the efficacy of ketamine in the control of MDD in patients with suicidal ideation | 2019 | Journal Affective Disorder | Taiwan | Ketamine at a dose of 0.2-0.5 mg / kg IV is effective in the control of various depressive symptoms in patients with suicidal ideation |
| Chuan Jin Zhou et al | Randomized clinical trial | 600 males patients and females between 18 to 65 years | Evaluate the efficacy of ketamine in regulating functional connectivity in patients with MDD | 2019 | Brain and behavior | China | Ketamine at a dose of 0.5 mg / kg IV is effective with just one dose to reduce symptoms and suicidal ideations in patients with MDD |
| Jessica L. Reed et al | Randomized clinical trial | 33 males patients and females with average age of 35 years | Evaluate the effects of ketamine on emotional thinking in magnetic resonance imaging of patients with MDD | 2019 | Biological Psychiatry | United States | Ketamine at a dose of 0.5 mg / kg IV is effective for the normalization of emotional processing in patients with MDD |
| Ashish K. Sahiba et al | Randomized clinical trial | 30 males patients and females between 20 to 64 years | Evaluate neurophysiological changes through MRI that generates the use of Ketamine in patients with MDD | 2019 | European Neuropsy chopharma- cology | United States | Ketamine at sub-anesthetic doses can generate changes in the perfusion of regions such as: hippocampus, cingulate and insular, which allows a significant decrease in the symptoms of MDD |
| Wang C et al | Clinical trial | 97 males patients and females between 18 to 65 years | Evaluate the efficacy of repeated doses of ketamine in the management of MDD and its subtypes | 2019 | Acta Psychia- tr Scand | China | Ketamine at a dose of 0.5 mg / kg IV is a promising and effective treatment for anxiety-type depression in patients with MDD |
| Virginie Ster- penich et al | Clinical trial | 10 males patients and females between 38 to 58 years | Evaluate the impact of Ketamine at the neuronal level and emotional processing of patients with MDD | 2019 | Preoperative medicine | Switzerland | Ketamine at a dose of 0.5 mg / kg IV, with a single bolus, demonstrated changes at the mesolimbic level with an improvement at the thought and emotional level in patients with MDD |
| Ruin Moaddel et al | Randomized clinical trial | 54 males patients and females between 18 to 65 years | Identify the different pathways by which Ketamine can be useful for the treatment of MDD | 2018 | Springer nature | United States | Ketamine at a dose of 0.5mg / kg IV showed an improvement> 50% in the MDRS scale (Montgomery Asberg Depression rating scale) in MDD |
| Rejish K Thomas et al | Retrospective cohort | 50 males patients and females over 50 years of age | Evaluate the rate of remission and maintenance of depressive symptoms in patients with MDD under treatment with ketamine | 2018 | Journal Psychophar macology | Canada | Ketamine at a dose of 0.5 mg / kg IV generates a response rate of 44% and remission of 16% in a patient with MDD |
| Maurizio Fava et al | Clinical trial | 99 males patients and females between 18 to 70 years | Evaluate the efficacy of ketamine infusion management in patients with MDD | 2018 | Springer nature | United States | Ketamine at doses of 0.5 mg / kg and 1.0 mg / kg IV is an effective and rapid treatment for the management of patients with MDD |
| Yoav Domany et al | Randomized clinical trial | 22 males patients and females between 18 to 75 years | Evaluate the efficacy and safety of ketamine in the management of MDD | 2018 | The British Journal of Psychiatry | Israel | Ketamine at a dose of 1 mg / kg OA is fast, effective and safe in the management of MDD; mild and transient adverse effects were reported |
| Chen Mu- Hong et al | Randomized clinical trial | 71 males patients and females with average age of 48 years | Determine the effects of ketamine on pro-inflammatory cytokines and the control of depressive symptoms in patients with MDD | 2018 | Psychiatry Research | China | Ketamine at a dose of 0.5 mg / kg IV reduced TNF-α levels associated with the control of depressive symptoms |
| Kathryn R et al | Randomized clinical trial | 13 males patients and females between 12 to 18 years | Evaluate the efficacy and safety of ketamine in the management of MDD in an adolescent population | 2018 | Journal Child Adolesc. Psychophar- macol | United States | Ketamine at a dose of 0.5 mg / kg IV is effective and safe in the control of various depressive symptoms in the population between 12 and 18 years of age |
| Jennifer L. Phillips et al | Randomized clinical trial | 41 males patients and females between 18 to 65 years | Evaluate the efficacy, duration and safety of ketamine in the control of MDD | 2018 | American Journal Psychiatry | Canada | Ketamine at a dose of 0.5 mg / kg IV is fast, effective and safe in the control of various depressive symptoms; mild and transient adverse effects were reported |
| Duncan George et al | Randomized clinical trial | 16 males patients and females over 60 years of age | Evaluate the efficacy and safety of subcutaneous ketamine in the management of MDD in the geriatric population | 2017 | The American Journal Geriatric Psychiatry | Australia | Ketamine at a dose greater than 0.2 mg / kg VS is effective and safe in the control of various depressive symptoms in a population over 60 years of age |
| B Kadriu et al | Clinical trial | 44 males patients and females between 18 to 65 years | Determine the effects of ketamine on bone marker abnormalities in patients with MDD | 2017 | Molecular psychiatry | United States | Ketamine at a dose of 0.5 mg / kg IV in addition to its antidepressant effects, has anti-inflammatory effects on bone markers in patients with MDD |
| Tung-Ping Su et al | Clinical trial | 71 males patients and females between 30 to 70 years | Determine the dose-effect relationship of Ketamine in patients with MDD | 2017 | Neuropsy chopharma- cology | Taiwan | Ketamine at a dose of 0.5 mg / kg IV is effective compared to the dose of 0.2 mg / kg IV in patients with MDD, predominantly with anxiety |
| Cheng-Ta et al | Randomized clinical trial | 48 males patients and females between 21 to 65 years | Evaluate the effects of ketamine on the prefrontal cortex and amygdala in the management of MDD | 2016 | Human Brain Mapping | China | Ketamine at doses of 0.2 and 0.5 mg / kg IV generates a rapid and effective antidepressant effect at the level of the prefrontal cortex |
| Jaskaran B. Singh et al | Randomized clinical trial | 67 males patients and females between 18 to 64 years | Evaluate the efficacy and safety of ketamine in the management of MDD | 2016 | American Journal Psychiatry | United States | Ketamine at a dose of 0.5 mg / kg VI is fast, effective and safe in the control of various depressive symptoms; mild and transient adverse effects were reported |
| Cristina Cusin et al | Randomized clinical trial | 14 males patients and females between 18 to 65 years | Evaluate the efficacy and duration of the effects of ketamine in the control of depressive symptoms in patients with MDD | 2016 | Australian & New Zealand Journal Psychiatry | United States | Ketamine at doses of 0.75 mg / kg and 0.5 mg / kg proved to be effective for the management of depressive symptoms in patients with MDD |
| JW Murrough et al | Clinical trial | 20 males patients and females over 21 years of age | Evaluate the negative and positive changes in the emotional perception of MDD patients managed with ketamine | 2015 | Translational Psychiatry | United States | Ketamine at a dose of 0.5 mg / kg IV demonstrated positive effects on the emotional perception of patients with MDD |
| Chadi G Abdallah et al | Clinical trial | 13 males patients and females between 44 to 48 years | Evaluate the efficacy of ketamine in patients with MDD in the hippocampal region | 2015 | Journal of Psychopharmacology | United States | Ketamine at a dose of 0.5 mg / kg IV allowed a reduction in symptoms using the Mont- gomery-Asberg Depression Rating Scale in MDD |
Table 2.
Characteristics of clinical trials exploring the effectiveness and safety of ketamine
| ID | Trial design | Country | Sample size | Intervention | Control + | Primary outcome | Start date / registration | Expected end date |
|---|---|---|---|---|---|---|---|---|
| NCT03973268 | Parallel randomized controlled clinical trial aPhase 1 | United States | 70 | Ketamine* | Placebo | Improvement in the initial score of the MADRS scale | January 2020 | February 2022 |
| NCT04101474 | Parallel randomized controlled clinical trial bPhase 1 | United States | 30 | Ketamine 0,5 mg/kg IV | Placebo | Improvement in the initial score of the MADRS scale | December 2019 | No information |
| NCT04352621 | Randomized clinical trialb Phase 4 | United States | 20 | Ketamine 0,5 mg/kg VIn | Placebo | Improvement in depressive symptoms | May 2020 | May 2022 |
| ChiCTR2000041068 | Single arm clinical trial b Phase 2 | China | 200 | Routine antidepressive + esketamine* | Placebo | Improvement in the initial score of the MADRS scale | December 2020 | No information |
| ChiCTR2000040082 | Single arm clinical trial b | China | 50 | Esketami- ne*+ECT | Placebo | Improvement in the initial score of the MADRS scale | November 2020 | No information |
| ChiCTR2000038424 | Multicentric randomized controlled clinical trial a | China | 240 | Arm 1: Routine antidepressive + esketamine 0.25mg/kg VI Arm 2: ECT + esketamine 0.5mg/kg VI | Placebo | Improvement in the initial score of the MADRS and Hamilton scale | October 2020 | No information |
| ChiCTR2000037607 | Parallel randomized clinical trial a | China | 240 | esketamine 0,5 mg/kg IV | Midazolam 0,02 mg/kg | Improvement in depressive symptoms | December 2020 | No information |
| ChiCTR2000036075 | Parallel randomized clinical trial b | China | 200 | Routine antidepressive + esketamine* | Placebo | Improvement in initial score of the Hamilton scale | October 2020 | No information |
| ChiCTR2000032704 | Multicentric randomized controlled clinical trial a | China | 60 | Arm 1: eske- tamine 0.2mg/ kg IV Arm 2: eketa- mine 0.4mg/ kg IV | Placebo | Improvement in the initial score of the MADRS and Hamilton scale | May 2020 | No information |
| CTRI/2020/01/022914 | Parallel randomized controlled clinical trial b Phase 2 | India | 60 | Ketamine 0,5 mg/kg IV | ECT | Improvement in initial score of the Hamilton scale | January 2020 | No information |
| CTRI/2020/08/027340 | Parallel randomized controlled clinical trial b Phase 3 | India | 60 | Ketamine* | ECT | Improvement in the initial score of the QIDS- SR-16 scale | August 2020 | No information |
| EudraCT:2018-001963-22 | Parallel randomized controlled clinical trial a Phase 2 | Poland | 88 | Esketamine* VIn | Placebo | Improvement in the initial score of the MADRS scale | June 2018 | No information |
Notes: a, Initiated; b, No Initiated; + Details of conventional management are not described in the records;*, No information dosis; IV, intravenous; VIn, intranasal administration; ECT, electroconvulsive therapy; MADRS, Montgomery Asberg Depression rating scale;QIDS-SR-16, Quick Inventory of Depressive Symptomatology-Self.
SYNTHESIS OF THE PUBLICATIONS INCLUDED IN THE REVIEW
We found randomized clinical trials (n = 19), non- randomized clinical trials (n = 11), and retrospective cohort studies (n = 1). All documents were written in English, except one in Spanish. The total number of patients recruited in the studies included in the review was 1,946. Some 13 studies included the adolescent population between 12 and 18 years of age. The country of origin of the authors was mostly the United States (n = 15), followed by China (n = 5), Taiwan (n = 3), Canada (n = 3), Australia (n = 2), Israel (n = 1), Japan (n = 1) and Switzerland (n = 1). The main findings of each of these documents are described in Table 1.
In 2019, Liu et al (19) investigated the neurocognitive effects of intravenous ketamine infusion (0.5 mg / kg over 40 minutes) for 12 days in 30 subjects with anxious TRD and 20 participants with non-anxious TRD. The researchers used the Montgomery Asberg Depression rating scale (MADRS) and Hamilton Anxiety Rating Scale (HAM-A) to evaluate the study participants. The patients included in the study met the diagnostic criteria for MDD based on the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5), as well as a score greater than 17 on the Hamilton Depression Rating Scale (HDRS-17). All participants and they had experienced unsuccessful treatments with at least two classes of antidepressants in optimal therapeutic regimens. Both groups of patients presented improvements in MADRS scores on days 13 and 26 of follow-up (p <0.001). In the anxious group, there was a decrease in the HAMA scale scores on days 13 and 26 (p <0.001). Subjects with anxious TRD had significant increases in cognitive processing speed on day 13 and 26 (p <0.001) and memory improved in the non-anxious group on day 13 (p = 0.025). The authors reported neurocognitive improvement and decreased superior anxiety symptoms in subjects with anxious TRD after the ketamine cycle.
George et al (20), evaluated the efficacy and safety of subcutaneous ketamine in 16 subjects older than 60 years with TRD. The participants were evaluated weekly using the MADRS scale. Ascending doses of 0.1 mg / kg to 0.5 mg / kg were administered in sessions one week apart plus 0.01 mg / kg midazolam single dose randomly within the first 3 treatment sessions. Doses up to 0.5 mg / kg did not cause major adverse effects leading to discontinuation of treatment. Some 69% (11/16) of the subjects had an acute response and remission for at least 7 days after treatment. The doses that demonstrated improvements in the MADRS score were 0.2 mg / kg (p <0.001), 0.3 mg / kg (p <0.001) and 0.4 mg / kg (p <0.001). The reported adverse effects included alterations in body perception, time and colors or sounds, but resolved 40 minutes after administration. The authors concluded that ketamine in individually titrated subcutaneous doses is effective in geriatric population with TRD.
Another clinical trial conducted by Cullen et al (21), evaluated the efficacy and safety of intravenous ketamine (0.5 mg / kg) for 2 weeks in 13 subjects between 12 and 18 years of age with TRD. The authors reported an average decrease of 42.5% (p = 0.004) on the CDRS-R (Children's Depression Rating Scale-Revised). The clinical improvement was greater in those patients without a history of other mental disorders. These patients had an average improvement of 60.2% (SD 26.1%) compared with 21.9%, (SD 24.2%) in subjects with a history of other mental disorders (p = 0.002). The adverse effects reported during the study were transient changes in blood pressure, dysphoria, and nausea. All were self-limited and only one subject required medical management with ondansetron. The authors concluded that ketamine may be effective in adolescents with TRD. However, the need to assess long-term safety was highlighted.
In 2013, Murrough et al (22) randomized 73 subjects diagnosed with TRD, who received a single intravenous infusion of ketamine (0.5 mg / kg) or midazolam (0.045 mg / kg) over 40 minutes. The patients were discharged 24 hours after the infusion and received follow-up at 48 hours, 72 hours, and 7 days after the infusion at home. All subjects were evaluated using the MADRS scale. The score was lower in the ketamine group than in the midazolam group by 7.95 points for the MADRS scale (95% CI: 3.20-12.71) and a greater probability of response to treatment in the former 24 hours for the ketamine group (HR: 2.18, 95% CI: 1.214.14, p<0.006). In the intervention group, the most common adverse effects were dizziness, blurred vision, headache, nausea, xerostomia, and restlessness. Severe and persistent depressive symptoms with a single dose of ketamine were quickly and safely controlled.
Okamoto et al (23), compared the antidepressant effect of intravenous ketamine (0.86 mg / kg) and propofol (0.94 mg / kg) as anesthetic during eight ECT sessions in 52 subjects with TRD. To evaluate the improvement of depressive symptoms, the HDRS-17 scale was used as the main outcome. In both groups, a decrease in the HDRS-17 score was observed as the number of ECT sessions performed increased. The subjects who received ketamine presented a marked improvement in depressive symptoms compared to the group that received propofol from the second (p <0.001) and fourth session (p <0.001). The most commonly reported adverse effects in the ketamine group were hypertension during the ECT session (55% vs 20%) (p = 0.049) and feelings of fear with hallucinations upon waking from anesthesia (27% vs 0%, p = 0.014). The authors reported better control of depressive symptoms in subjects who underwent to ECT under ketamine anesthesia during the first four sessions.
RECORDS OF CLINICAL TRIALS EXPLORING THE EFFICACY AND SAFETY OF KETAMINE
We found 12 clinical trials registered in the U.S. National Library of Medicine (n = 3), Chinese Clinical Trial Registry (n = 6), Clinical Trials Registry - India (n = 2) and German Clinical Trials Register (n = 1). The sum of the participants in the trials was 1,318 individuals and 58.3% (7/12) of the studies were not yet recruiting the study population. Some 16.6% (2/12) were studies in phase 1, 25% (3/12) in phase 2, 8.3% (1/12) in phase three, 8.3% (1 / 12) in phase four and five studies did not specify which phase it is in. 58.3 (7/12) of the studies will evaluate esketamine and the remaining 41.6% (5/12) will evaluate ketamine. The characteristics of these clinical trials are described in Table 2.
Some 41.6% (5/12) of the clinical trials will use doses of 0.5 mg / kg intravenously and six studies did not specify the doses used in their intervention. The intravenous route of administration will be used in 41.6% (5/12) of the trials, followed by the intranasal route in 16.6% (2/12). Regarding the control group, 75% (9/12) will use placebo, 16.6% (2/12) ECT and 8.3% (1/12) midazolam.
A single-arm clinical trial will evaluate the efficacy and safety of 0.5 mg / kg ketamine intranasally using an atomization device with a maximum dose of 40 mg for 6 weeks of treatment in 20 subjects diagnosed with TRD. The results of the treatment will be carried out through a self-assessment focused on the improvement of depressive symptoms (NCT04352621). Likewise, the multicenter phase 2 randomized controlled clinical trial (EudraCT: 2018001963-22) will evaluate the efficacy and safety of inhaled esketamine versus placebo in subjects with TRD. The primary outcome will be the MADRS scale score on day 14th after starting the treatment.
A multicenter randomized controlled clinical trial registered in the Chinese Clinical Trial Registry will evaluate the efficacy and safety of anesthesia with esketamine at a dose of 0.25 mg / kg of ketamine intravenously over 40 minutes versus etomidate or propofol in 240 subjects receiving treatment with TEC by DRT. The follow-up of the therapeutic evolution will be carried out using the HDRS-17 scale (ChiCTR2000038424).
DISCUSSION
This scoping review mapped the current medical literature regarding the efficacy and safety of ketamine for DRT. The included studies reported an improvement in depressive symptoms among patients with a diagnosis of DRT who received ketamine compared to placebo and / or midazolam (19,20,22). The most frequently administered dose in the intervention groups was 0.5 mg / kg intravenously (19,21,22,24,25). Some studies reported the efficacy of alternative routes, such as the subcutaneous rout, especially in the geriatric population. Likewise, the anesthetic and antidepressant effects were highlighted in subjects who received complementary ECT (23). Self-limited adverse effects were most reported, although more complex reactions such as hallucinations, restlessness, alterations in body perception, time and colors or sounds were also observed (26,27).
Ketamine has different routes of administration and the most studied for TRD is the intravenous route, the oral, subcutaneous, sublingual and intranasal routes have also been evaluated (28). The oral route has low bioavailability (only 8%) (29), improving up to 24% - 30% with liquid sublingual formulations (30). The bioavailability of subcutaneous ketamine is almost complete, and the intravenous plasma concentration can double the values of the other routes (31). However, oral ketamine has an unpleasant taste, intranasal ketamine produces pain or a sensation of nasal discomfort, and subcutaneous ketamine produces local irritant, effects after administration, which can lead to a decrease in drug adherence (28,32).
Neuroinflammation and oxidative stress play an important role in the neuroprogression of MDD and the response to the treatment. Ketamine has an anti-inflammatory and immunomodulatory effect by reducing plasma levels of interleukin-6 (IL-6), suppressing phosphorylation and inactivating the transcription factor B (33). Immunomodulation of tumor necrosis factor alpha, IL-6 and nitric oxide synthase is similarly proposed, which generates an increase in bone density (34). The drug has an ability to reduce the levels of pro-inflammatory adipokines and resistin (35). Some authors reported that mood disorders were associated with a systemic proinflammatory state and decreased neuroplasticity, although more studies are needed to explore the role of ketamine in the modulation of inflammatory disorders in patients with TRD.
Ketamine has a wider anesthetic safety window and a shorter half-life when compared to other anesthetic drugs (36). It is important to bear in mind, nevertheless, that the dissociative disorders and cardiovascular compromise that could occur after the administration of this drug require strict monitoring by experienced clinicians. Studies still need to be carried out to clarify the pathophysiological processes that explain the dissociative experiences associated with drug administration (37).
The influence of ketamine on the cardiovascular system is the result of the activation of the sympathetic system, as well as the inhibition of the vagus nerve and the reuptake of norepinephrine in the peripheral nerves and myocardium, generating tachycardia, variation in systemic and pulmonary arterial pressure, increase of cardiac output and myocardial oxygen consumption (7,38). This means that the use of the drug in patients with cardiovascular comorbidities and advanced age should be avoided or done under strict medical surveillance (38). In the geriatric population, for example, evidence suggests that ECT treatment is preferable to ketamine due to the safety profile in this age group. However, emerging evidence suggests that ketamine at lower doses (0.2 mg / kg) can be considered a secondary treatment option in this age group (20,39).
Esketamine hydrochloride is the most potent S-enantiomer of ketamine known to date. The Food and Drug Administration (FDA) recently approved the use of intranasal esketamine for the treatment of TRD (40,41). However, it is important to bear in mind that sedative and dissociative adverse effects are more marked than those described by the use of ketamine (40,41). Although esketamine could currently be considered as a therapeutic option over ketamine in patients with TRD, there is insufficient evidence regarding the optimal dose, duration, and frequency for the expected therapeutic effects. Furthermore, esketamine is mostly inaccessibility in low- and middle-income countries.
ECT has been reported as the standard treatment and therapy for patients with TRD (5-8). Despite this, the technique is underused compared to the number of patients suffering from this condition. Due to its restricted access, low availability and incorrect perceptions about its use, only 0.26% of patients with depressive disorder received ECT in 2014 in the United States, (42). Although ECT treatments are the first line of management for TRD, their most feared adverse effect is memory loss. Similarly, long-term exposure to high doses of ketamine could be associated with cognitive impairment, studies are needed to demonstrate its noninferiority in terms of efficacy for the treatment of TDR (42).
LIMITATIONS
Only 2 databases were included, so there may be important studies not included in our review. Additionally, the limited sample size of the included studies limits the results. Finally, we did not conduct a quality assessment of the included studies. The PRISMA extension for scoping reviews, however, does not routinely recommend quality appraisal in scoping reviews (17).
CONCLUSION
The evidence found supports the use of ketamine for the treatment of refractory depression. Its anti-inflammatory and immunomodulatory properties allow a restoration of signaling in the neurocognitive pathways and the neuroplasticity, which positively impacts the control of affective symptoms. There are variations regarding the route of administration, bioavailability and age group. The most common adverse reactions included dissociative symptoms and cardiovascular disorders, which were self-limited. Some of these adverse effects were complex, requiring close monitoring of patients and clinical management by experienced personnel.
GRATITUDE
None
Funding Statement
FINANCING
None
CONFLICTS OF INTEREST
The authors declare that they have no conflict of interest.
ADDITIONAL MATERIAL
Supplementary file 1.
1. Search strategy (last update on February 10, 2021)
Pubmed
(“Depression”[Mesh] OR “Depressive Disorder”[Mesh] OR “Depressive Disorder, Treatment-Resistant”[Mesh] OR “Depressive Disorder, Major”[Mesh]) AND (“Ketamine/therapeutic use”[Mesh] OR “Ketamine"[Mesh]) AND (“Depression”[Title] AND “Ketamine”[Title])
Scopus
(TITLE-ABS-KEY (major AND depressive AND disorder) AND TITLE-ABS-KEY (ketamine) AND ALL (ketamine) AND ALL (depressive AND disorder) AND ALL (major AND depressive AND disorder) AND TITLE-ABS-KEY (treatment-resistant))
2. Number of clinical trials included in each database (last update on February 10, 2021)
| Name | Website | Include |
| U.S. National Library of Medicine | clinicaltrials.gov | 3 |
| Australian New Zealand Clinical Trials Registry (ANZCTR) | anzctr.org.au | 0 |
| Brazilian Clinical Trials Registry (ReBec) | ensaiosclinicos.gov.br | 0 |
| Chinese Clinical Trial Registry (ChiCTR) | chictr.org.cn | 6 |
| Clinical Research Information Service (CRiS), Republic of Korea. | cris.nih.go.kr | 0 |
| Clinical Trials Registry - India (CTRI) | ctri.nic.in/Clinicaltrials/advancesearchmain.php | 2 |
| Cuban Public Registry of Clinical Trials(RPCEC) | registroclinico.sld.cu/en/home | 0 |
| EU Clinical Trials Register (EU-CTR) | clinicaltrialsregister.eu | 0 |
| German Clinical Trials Register (DRKS) | drks.de/drks_web/ | 1 |
| Iranian Registry of Clinical Trials (IRCT) | https://www.irct.ir/ | 0 |
| International Standard Randomised Controlled Trial Number (ISRCTN) | isrctn.com/ | 0 |
| Japan Primary Registries Network (JPRN) | rctportal.niph.go.jp/ | 0 |
| Lebanese Clinical Trials Registry (LBCTR) | http://lbctr.emro.who.int/ | 0 |
| Thai Clinical Trials Registry (TCTR) | http://www.clinicaltrials.in.th/ | 0 |
| The Netherlands National Trial Register (NTR) | trialregister.nl/ | 0 |
| Pan African Clinical Trial Registry (PACTR) | pactr.samrc.ac.za/ | 0 |
| Peruvian Clinical Trial Registry (REPEC) | ensayosclinicos-repec.ins.gob.pe/ | 0 |
| Sri Lanka Clinical Trials Registry (SLCTR) | https://slctr.lk | 0 |
Supplementary file 2.
PRISMA Extension for Scoping reviews (PRISMA-ScR) 2018 Checklist (1)
| Section/topic | PRISMA-ScR Checklist item | Reported on page # | |
|---|---|---|---|
| TITLE | |||
| Title | 1 | Identify the report as a scoping review. | 1 |
| ABSTRACT | |||
| Structured summary | 2 | Provide a structured summary including, as applicable: background; objectives; data sources; study eligibility criteria, participants, and interventions; study synthesis methods; results; limitations; conclusions and implications of key findings. | 2 |
| INTRODUCTION | |||
| Rationale | 3 | Describe the rationale for the review in the context of what is already known. Explain why the review question(s)/objective(s) lend themselves to a scoping review approach. | 4-5 |
| Objectives | 4 | Provide an explicit statement of the question(s) and objective(s) being addressed with reference to their key elements (e.g., population or participants, concepts and context), or other relevant key elements used to conceptualize the review question(s) and/or objective(s)). | 6 |
| METHODS | |||
| Protocol and registration | 5 | Indicate if a review protocol exists, if and where it can be accessed (e.g., Web address), and, if available, provide registration information including registration number. | 6 |
| Eligibility criteria | 6 | Specify the characteristics of the sources of evidence (e.g., years considered, language, publication status) used as criteria for eligibility, and provide a rationale. | 6-7 |
| Information sources | 7 | Describe all information sources (e.g., databases with dates of coverage, contact with study authors to identify additional sources) in the search and date last searched. | 7 |
| Search | 8 | Present full electronic search strategy for at least one database, including any limits used, such that it could be repeated. | Archivo suplementario 1 |
| Selection of sources of evidence | 9 | State the process for selecting studies (i.e., screening, eligibility) included in the scoping review. | 8 |
| Data charting process | 10 | Describe the methods of charting data from the included sources of evidence (e.g. piloted forms; forms that have been tested by the team before their use, whether data charting was done independently, in duplicate) and any processes for obtaining and confirming data from investigators. | 8-9 |
| Data items | 11 | List and define all variables for which data were sought and any assumptions and simplifications made. | 8-9 |
| Critical appraisal of individual sources of evidence | 12 | If done, provide a rationale for conducting a critical appraisal of included sources of evidence; describe the methods used and how this information was used in any data synthesis (if appropriate). | NA |
| Summary measures | 13 | Not applicable for scoping reviews. | NA |
| Synthesis of results | 14 | Describe the methods of handling and summarizing the data that were charted. | 9 |
| Risk of bias across studies | 15 | Not applicable for scoping reviews. | NA |
| Additional analyses | 16 | Not applicable for scoping reviews. | NA |
| RESULTS | |||
| Selection of sources of evidence | 17 | Give numbers of studies screened, assessed for eligibility, and included in the review, with reasons for exclusions at each stage, ideally using a flow diagram. | Figura 1 |
| Characteristics of sources of evidence | 18 | For each source of evidence, present characteristics for which data were charted and provide the citations. | Tablas 1 y 2 |
| Critical appraisal within sources of evidence | 19 | If done, present data on critical appraisal of included sources of evidence (see item 12). | NA |
| Results of individual sources of evidence | 20 | For each included source of evidence, present the relevant data that were charted that relate to the review question(s) and objective(s). | Tablas 1 y 2 |
| Synthesis of results | 21 | Summarize and/or present the charting results as they relate to the review question(s) and objective(s). | 9-14 |
| Risk of bias across studies | 22 | Not applicable for scoping reviews. | NA |
| Additional analysis | 23 | Not applicable for scoping reviews. | NA |
| DISCUSSION | |||
| Summary of evidence | 24 | Summarize the main results (including an overview of concepts, themes, and types of evidence available), explain how they relate to the review question(s) and objectives, and consider the relevance to key groups | 14-15 |
| Limitations | 25 | Discuss the limitations of the scoping review process. | 16 |
| Conclusions | 26 | Provide a general interpretation of the results with respect to the review question(s) and objective(s), as well as potential implications and/or next steps. | 17-18 |
| FUNDING | |||
| Funding | 27 | Describe sources of funding for the included sources of evidence, as well as sources of funding for the scoping review. Describe the role of the funders of the scoping review. | 18 |
1. Tricco AC, Lillie E, Zarin W, et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann Intern Med 2018;1 69(7):467-73.
Supplementary file 3. List of documents included in our study
Murrough JW, Iosifescu DV, Chang LC, Al Jurdi RK, Green CE, Perez AM, Iqbal S, Pillemer S, Foulkes A, Shah A, Charney DS, Mathew SJ. Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial. Am J Psychiatry. 2013 Oct;170(l0):1134-42. doi: 10.ri76/appi.ajp.2013.1 3030392.
Phillips JL, Norris S, Talbot J, Birmingham M, Hatchard T, Ortiz A, Owoeye O, Batten LA, Blier P. Single, Repeated, and Maintenance Ketamine Infusions for Treatment-Resistant Depression: A Randomized Controlled Trial. Am J Psychiatry. 2019 May 1;176(5):401-409. doi: 10.1176/appi.ajp.2018.18070834.
Andrade C. Ketamine for Depression, 4: In What Dose, at What Rate, by What Route, for How Long, and at What Frequency? J Clin Psychiatry. 2017 Jul;78(7):e852-e857. doi: 10.4088/JCP. 17f11738.
Cullen KR, Amatya P, Roback MG, Albott CS, Westlund Schreiner M, Ren Y, Eberly LE, Carstedt P, Samikoglu A, Gunlicks-Stoessel M, Reigstad K, Horek N, Tye S, Lim KO, Klimes-Dougan B. Intravenous Ketamine for Adolescents with Treatment-Resistant Depression: An Open-Label Study. J Child Adolesc Psychopharmacol. 2018 Sep;28(7):437-444. doi: 10.1089/cap.2018.0030.
Singh JB, Fedgchin M, Daly EJ, De Boer P, Cooper K, Lim P, Pinter C, Murrough JW, Sanacora G, Shelton RC, Kurian B, Winokur A, Fava M, Manji H, Drevets WC, Van Nueten L. A Double-Blind, Randomized, Placebo-Controlled, Dose-Frequency Study of Intravenous Ketamine in Patients With Treatment-Resistant Depression. Am J Psychiatry. 2016 Aug 1;173(8):816-26. doi: 10.1176/appi.ajp.2016.16010037.
Murrough JW, Perez AM, Pillemer S, Stern J, Parides MK, aan het Rot M, Collins KA, Mathew SJ, Charney DS, Iosifescu DV. Rapid and longer-term antidepressant effects of repeated ketamine infusions in treatment-resistant major depression. Biol Psychiatry. 2013 Aug 15;74(4):250-6. doi: 10.1016/j.biopsych.2012.06.022.
Domany Y, Bleich-Cohen M, Tarrasch R, Meidan R, Litvak-Lazar O, Stoppleman N, Schreiber S, Bloch M, Hendler T, Sharon H. Repeated oral ketamine for out-patient treatment of resistant depression: randomised, double-blind, placebo-controlled, proof-of-concept study. Br J Psychiatry. 2019 Jan;214(1):20-26. doi: 10.1192/bjp.2018.196.
Price RB, Iosifescu DV, Murrough JW, Chang LC, Al Jurdi RK, Iqbal SZ, Soleimani L, Charney DS, Foulkes AL, Mathew SJ. Effects of ketamine on explicit and implicit suicidal cognition: a randomized controlled trial in treatment-resistant depression. Depress Anxiety. 2014 Apr;31(4):335-43. doi: 10.1002/da.22253.
Chen MH, Lin WC, Tu PC, Li CT, Bai YM, Tsai SJ, Su TP. Antidepressant and antisuicidal effects of ketamine on the functional connectivity of prefrontal cortex-related circuits in treatment-resistant depression: A double-blind, placebo-controlled, randomized, longitudinal resting fMRI study. J Affect Disord. 2019 Dec 1;259:15-20. doi: 10.1016/j.jad.2019.08.022.
Liu W, Zhou Y, Zheng W, Wang C, Zhan Y, Lan X, Zhang B, Li H, Chen L, Ning Y. Repeated intravenous infusions of ketamine: Neurocognition in patients with anxious and nonanxious treatment-resistant depression. J Affect Disord. 2019 Dec 1;259:1-6. doi: 10.1016/j. jad.2019.08.012.
Shiroma PR, Albott CS, Johns B, Thuras P, Wels J, Lim KO. Neurocognitive performance and serial intravenous subanesthetic ketamine in treatment-resistant depression. Int J Neuropsychopharmacol. 2014 Nov;17(11):1805-13. doi: 10.1017/S1461145714001011.
Chen MH, Li CT, Lin WC, Hong CJ, Tu PC, Bai YM, Cheng CM, Su TP. Rapid inflammation modulation and antidepressant efficacy of a low- dose ketamine infusion in treatment-resistant depression: A randomized, double-blind control study. Psychiatry Res. 2018 Nov;269:207- 211. doi: 10.1016/j.psychres.2018.08.078.
Cusin C, Ionescu DF, Pavone KJ, Akeju O, Cassano P, Taylor N, Eikermann M, Durham K, Swee MB, Chang T, Dording C, Soskin D, Kelley J, Mischoulon D, Brown EN, Fava M. Ketamine augmentation for outpatients with treatment-resistant depression: Preliminary evidence for two-step intravenous dose escalation. Aust N Z J Psychiatry. 2017 Jan;51(1):55-64. doi: 10.1177/0004867416631828.
Thomas RK, Baker G, Lind J, Dursun S. Rapid effectiveness of intravenous ketamine for ultraresistant depression in a clinical setting and evidence for baseline anhedonia and bipolarity as clinical predictors of effectiveness. J Psychopharmacol. 2018 Oct;32(10):1110-1117. doi: 10.1177/0269881118793104.
George D, Gálvez V, Martin D, Kumar D, Leyden J, Hadzi-Pavlovic D, Harper S, Brodaty H, Glue P, Taylor R, Mitchell PB, Loo CK. Pilot Randomized Controlled Trial of Titrated Subcutaneous Ketamine in Older Patients with Treatment-Resistant Depression. Am J Geriatr Psychiatry. 2017 Nov;25(11):1199-1209. doi: 10.1016/j.jagp.2017.06.007.
Li CT, Chen MH, Lin WC, Hong CJ, Yang BH, Liu RS, Tu PC, Su TP. The effects of low-dose ketamine on the prefrontal cortex and amygdala in treatment-resistant depression: A randomized controlled study. Hum Brain Mapp. 2016 Mar;37(3):1080-90. doi: 10.1002/hbm.23085.
Okamoto N, Nakai T, Sakamoto K, Nagafusa Y, Higuchi T, Nishikawa T. Rapid antidepressant effect of ketamine anesthesia during electroconvulsive therapy of treatment-resistant depression: comparing ketamine and propofol anesthesia. J ECT. 2010 Sep;26(3):223-7. doi: 10.1097/YCT.0b013e3181c3b0aa.
Lai R, Katalinic N, Glue P, Somogyi AA, Mitchell PB, Leyden J, Harper S, Loo CK. Pilot dose-response trial of i.v. ketamine in treatment-resistant depression. World J Biol Psychiatry. 2014 Sep;15(7):579-84. doi: 10.3109/15622975.2014.922697.
Wang C, Zhou Y, Zheng W, Liu W, Zhan Y, Li H, Chen L, Zhang B, Walter M, Li M, Li MD, Ning Y. Association between depression subtypes and response to repeated-dose intravenous ketamine. Acta Psychiatr Scand. 2019 Nov;140(5):446-457. doi: 10.1111/acps.13096.
Lipsitz O, McIntyre RS, Rodrigues NB, Kaster TS, Cha DS, Brietzke E, Gill H, Nasri F, Lin K, Subramaniapillai M, Kratiuk K, Teopiz K, Lui LMW, Lee Y, Ho R, Shekotikhina M, Mansur RB, Rosenblat JD. Early symptomatic improvements as a predictor of response to repeated-dose intravenous ketamine: Results from the Canadian Rapid Treatment Center of Excellence. Prog Neuropsychopharmacol Biol Psychiatry. 2021 Mar 8;105:110126. doi: 10.1016/j.pnpbp.2020.110126.
Sterpenich V, Vidal S, Hofmeister J, Michalopoulos G, Bancila V, Warrot D, Dayer A, Desseilles M, Aubry JM, Kosel M, Schwartz S, Vutskits L. Increased Reactivity of the Mesolimbic Reward System after Ketamine Injection in Patients with Treatment-resistant Major Depressive Disorder. Anesthesiology. 2019 Jun;130(6):923-935. doi: 10.1097/ALN.0000000000002667.
Chen MH, Chang WC, Lin WC, Tu PC, Li CT, Bai YM, Tsai SJ, Huang WS, Su TP. Functional Dysconnectivity of Frontal Cortex to Striatum Predicts Ketamine Infusion Response in Treatment-Resistant Depression. Int J Neuropsychopharmacol. 2020 Dec 29;23(12):791-798. doi: 10.1093/ijnp/pyaa056.
Fava M, Freeman MP, Flynn M, Judge H, Hoeppner BB, Cusin C, Ionescu DF, Mathew SJ, Chang LC, Iosifescu DV, Murrough J, Debattista C, Schatzberg AF, Trivedi MH, Jha MK, Sanacora G, Wilkinson ST, Papakostas GI. Double-blind, placebo-controlled, dose-ranging trial of intravenous ketamine as adjunctive therapy in treatment-resistant depression (TRD). Mol Psychiatry. 2020 Jul;25(7):1592-1603. doi: 10.1038/s41380-018-0256-5.
Zhuo C, Tian H, Li G, Chen M, Jiang D, Lin X, Xu Y, Wang W. Effects of ketamine on circadian rhythm and synaptic homeostasis in patients with treatment-resistant depression: A protocol for mechanistic studies of its rapid and sustained antidepressant actions in humans. Brain Behav. 2019 Nov;9(11):e01423. doi: 10.1002/brb3.1423.
Moaddel R, Shardell M, Khadeer M, Lovett J, Kadriu B, Ravichandran S, Morris PJ, Yuan P, Thomas CJ, Gould TD, Ferrucci L, Zarate CA. Plasma metabolomic profiling of a ketamine and placebo crossover trial of major depressive disorder and healthy control subjects. Psychopharmacology (Berl). 2018 Oct;235(10):3017-3030. doi: 10.1007/s00213-018-4992-7.
Ashish K. Sahib, Joana R.A. Loureiro, Megha M. Vasavada, Antoni Kubicki, Shantanu H. Joshi, Kai Wang, Roger P. Woods, Eliza Congdon, Danny J.J. Wang, Michael L. Boucher, Randall Espinoza, Katherine L. Narr Single and repeated ketamine treatment induces perfusion changes in sensory and limbic networks in major depressive disorder, European Neuropsychopharmacology, Volume 33, 2020, Pages 89100, ISSN 0924-977X, doi:10.1016j.euroneuro.2020.01.017.
Kadriu B, Gold PW, Luckenbaugh DA, Lener MS, Ballard ED, Niciu MJ, Henter ID, Park LT, De Sousa RT, Yuan P, Machado-Vieira R, Zarate CA. Acute ketamine administration corrects abnormal inflammatory bone markers in major depressive disorder. Mol Psychiatry. 2018 Jul;23(7):1626-1631. doi: 10.1038/mp.2017.109
Reed JL, Nugent AC, Furey ML, Szczepanik JE, Evans JW, Zarate CA Jr. Effects of Ketamine on Brain Activity During Emotional Processing: Differential Findings in Depressed Versus Healthy Control Participants. Biol Psychiatry Cogn Neurosci Neuroimaging. 2019 Jul;4(7):610- 618. doi: 10.1016/j.bpsc.2019.01.005
Murrough JW, Collins KA, Fields J, DeWilde KE, Phillips ML, Mathew SJ, Wong E, Tang CY, Charney DS, Iosifescu DV. Regulation of neural responses to emotion perception by ketamine in individuals with treatment-resistant major depressive disorder. Transl Psychiatry. 2015 Feb 17;5(2):e509. doi: 10.1038/tp.2015.10.
Megha M. Vasavada, Joana Loureiro, Antoni Kubicki, Ashish Sahib, Benjamin Wade, Gerhard Hellemann, Randall T. Espinoza, Eliza Congdon, Katherine L. Narr, Amber M. Leaver, Effects of Serial Ketamine Infusions on Corticolimbic Functional Connectivity in Major Depression, Biological Psychiatry: Cognitive Neuroscience and Neuroimaging,2020, ISSN 2451-9022, doi:10.1016/j.bpsc.2020.06.015
Abdallah CG, Salas R, Jackowski A, Baldwin P, Sato JR, Mathew SJ. Hippocampal volume and the rapid antidepressant effect of ketamine. J Psychopharmacol. 2015 May;29(5):591-5. doi: 10.1177/0269881114544776.
BIBLIOGRAPHIC REFERENCES
- 1.GBD 2017 DALYs and HALE Collaborators. Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018 Nov 10;392(10159):1859–1922. doi: 10.1016/S0140-6736(18)32335-3. 10.1016/S0140-6736(18)32335-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Psychiatric Association. 5th. Washington, DC: American Psychiatric Publishing; 2014. Diagnostic and statistical manual of mental disorders. [Google Scholar]
- 3.Lee S, Jeong J, Kwak Y, Park SK. Depression research: where are we now? Mol Brain. 2010 Mar;10(3):8. doi: 10.1186/1756-6606-3-8. 10.1186/1756-6606-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 5.Dorow M, Löbner M, Pabst A, Stein J, Riedel-Heller SG. Preferences for Depression Treatment Including Internet-Based Interventions: Results From a Large Sample of Primary Care Patients. Front Psychiatry. 2018 May 17;9:181. doi: 10.3389/fpsyt.2018.00181. 10.3389/fpsyt.2018.00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cipriani A, Furukawa TA, Salanti G, Chaimani A, Atkinson LZ, Ogawa Y et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet. 2018 Apr 7;391(10128):1357–1366. doi: 10.1016/S0140-6736(17)32802-7. 10.1016/ S0140-6736(17)32802-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bozymski KM, Crouse EL, Titus-Lay EN, Ott CA, Nofziger JL, Kirkwood CK. Esketamine: A Novel Option for Treatment-Resistant Depression. Ann Pharmacother. 2020 Jun;54(6):567–576. doi: 10.1177/1060028019892644. 10.1177/1060028019892644. [DOI] [PubMed] [Google Scholar]
- 8.Voineskos D, Daskalakis ZJ, Blumberger DM. Management of Treatment-Resistant Depression: Challenges and Strategies. Neuropsychiatr Dis Treat. 2020 Jan 21;16:221–234. doi: 10.2147/NDT.S198774. 10.2147/NDT.S198774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006 Nov;163(11):1905–17. doi: 10.1176/ajp.2006.163.11.1905. 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- 10.Zanos P, Moaddel R, Morris PJ, Riggs LM, Highland JN, Georgiou P et al. Ketamine and Ketamine Metabolite Pharmacology: Insights into Therapeutic Mechanisms. Pharmacol Rev. 2018 Jul;70(3):621–660. doi: 10.1124/pr.117.015198. 10.1124/pr.117.015198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khorramzadeh E, Lotfy AO. The use of ketamine in psychiatry. Psychosomatics. 1973 Nov-Dec;14(6):344–6. doi: 10.1016/S0033-3182(73)71306-2. 10.1016/S0033-3182(73)71306-2. [DOI] [PubMed] [Google Scholar]
- 12.Zarate CA, Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment- resistant major depression. Arch Gen Psychiatry. 2006 Aug;63(8):856–64. doi: 10.1001/archpsyc.63.8.856. 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- 13.Veraart JKE, Smith-Apeldoorn SY, Spaans HP, Kamphuis J, Schoevers RA. Is ketamine an appropriate alternative to ECT for patients with treatment resistant depression? A systematic review. J Affect Disord. 2021 Feb;15(281):82–89. doi: 10.1016/j.jad.2020.11.123. 10.1016/j.jad.2020.11.123. [DOI] [PubMed] [Google Scholar]
- 14.Arksey H, O'Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8:19–32. 10.1080/1364557032000119616. [Google Scholar]
- 15.Levac D, Colquhoun H, O'Brien KK. Scoping studies: advancing the methodology. Implement Sci. 2010;5:69. doi: 10.1186/1748-5908-5-69. 10.1186/1748-5908-5-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.World Health Organization. WHO Registry Network. 2020 [Fecha de consulta: 10 de Febrero de 2021] Disponible en: https://www.who.int/ictrp/network/primary/en/ [Google Scholar]
- 17.Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5(1) doi: 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tricco AC, Lillie E, Zarin W, O'Brien KK, Colquhoun H, Levac D et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. https://doi.org/10.7326/M18-0850. Ann Intern Med. 2018;169:467. doi: 10.7326/M18-0850. [DOI] [PubMed] [Google Scholar]
- 19.Liu W, Zhou Y, Zheng W, Wang C, Zhan Y, Lan X et al. Repeated intravenous infusions of ketamine: Neurocognition in patients with anxious and nonanxious treatment-resistant depression. J Affect Disord. 2019 Dec;1(259):1–6. doi: 10.1016/j.jad.2019.08.012. 10.1016/j.jad.2019.08.012. [DOI] [PubMed] [Google Scholar]
- 20.George D, Galvez V, Martin D, Kumar D, Leyden J, Hadzi- Pavlovic D et al. Pilot Randomized Controlled Trial of Titrated Subcutaneous Ketamine in Older Patients with Treatment-Resistant Depression. Am J Geriatr Psychiatry. 2017 Nov;25(11):1199–1209. doi: 10.1016/j.jagp.2017.06.007. 10.1016/j.jagp.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 21.Cullen KR, Amatya P, Roback MG, Albott CS, Westlund Schreiner M, Ren Y et al. Intravenous Ketamine for Adolescents with Treatment-Resistant Depression: An Open-Label Study. J Child Adolesc Psychopharmacol. 2018 Sep;28(7):437–444. doi: 10.1089/cap.2018.0030. 10.1089/cap.2018.0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murrough JW, Iosifescu DV, Chang LC, Al Jurdi RK, Green CE, Perez AM et al. Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial. Am J Psychiatry. 2013 Oct;170(10):1134–42. doi: 10.1176/appi.ajp.2013.13030392. 10.1176/appi.ajp.2013.13030392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okamoto N, Nakai T, Sakamoto K, Nagafusa Y, Higuchi T, Nishikawa T. Rapid antidepressant effect of ketamine anesthesia during electroconvulsive therapy of treatment-resistant depression: comparing ketamine and propofol anesthesia. J ECT. 2010 Sep;26(3):223–7. doi: 10.1097/YCT.0b013e3181c3b0aa. 10.1097/YCT.0b013e3181c3b0aa. [DOI] [PubMed] [Google Scholar]
- 24.Price RB, Iosifescu DV, Murrough JW, Chang LC, Al Jurdi RK, Iqbal SZ et al. Effects of ketamine on explicit and implicit suicidal cognition: a randomized controlled trial in treatment-resistant depression. Depress Anxiety. 2014 Apr;31(4):335–43. doi: 10.1002/da.22253. 10.1002/da.22253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abdallah CG, Salas R, Jackowski A, Baldwin P, Sato JR, Mathew SJ. Hippocampal volume and the rapid antidepressant effect of ketamine. J Psychopharmacol. 2015 May;29(5):591–5. doi: 10.1177/0269881114544776. 10.1177/0269881114544776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murrough JW, Perez AM, Pillemer S, Stern J, Parides MK, aan het Rot M et al. Rapid and longer-term antidepressant effects of repeated ketamine infusions in treatment-resistant major depression. Biol Psychiatry. 2013 Aug 15;74(4):250–6. doi: 10.1016/j.biopsych.2012.06.022. 10.1016/j. biopsych.2012.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Domany Y, Bleich-Cohen M, Tarrasch R, Meidan R, Litvak-Lazar O, Stoppleman N, Schreiber S, Bloch M, Hendler T, Sharon H. Repeated oral ketamine for outpatient treatment of resistant depression: randomised, double-blind, placebo-controlled, proof-of-concept study. Br J Psychiatry. 2019 Jan;214(1):20–26. doi: 10.1192/bjp.2018.196. 10.1192/bjp.2018.196. [DOI] [PubMed] [Google Scholar]
- 28.Andrade C. Oral Ketamine for Depression, 1: Pharmacologic Considerations and Clinical Evidence. J Clin Psychiatry. 2019 Apr 2;80(2):19f12820. doi: 10.4088/JCP.19f12820. 10.4088/JCP.19f12820. [DOI] [PubMed] [Google Scholar]
- 29.Fanta S, Kinnunen M, Backman JT, Kalso E. Population pharmacokinetics of S-ketamine and norketamine in healthy volunteers after intravenous and oral dosing. Eur J Clin Pharmacol. 2015 Apr;71(4):441–7. doi: 10.1007/s00228-015-1826-y. 10.1007/s00228-015-1826-y. [DOI] [PubMed] [Google Scholar]
- 30.Rolan P, Lim S, Sunderland V, Liu Y, Molnar V. The absolute bioavailability of racemic ketamine from a novel sublingual formulation. Br J Clin Pharmacol. 2014 Jun;77(6):1011–6. doi: 10.1111/bcp.12264. 10.1111/bcp.12264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Andrade C. Ketamine for Depression, 4: In What Dose, at What Rate, by What Route, for How Long, and at What Frequency? J Clin Psychiatry. 2017 Jul;78(7):e852–e857. doi: 10.4088/JCP.17f11738. 10.4088/JCP.17f11738. [DOI] [PubMed] [Google Scholar]
- 32.Bakunina N, Pariante CM, Zunszain PA. Immune mechanisms linked to depression via oxidative stress and neuroprogression. Immunology. 2015 Mar;144(3):365–373. doi: 10.1111/imm.12443. 10.1111/imm.12443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kadriu B, Gold PW, Luckenbaugh DA, Lener MS, Ballard ED, Niciu MJ et al. Acute ketamine administration corrects abnormal inflammatory bone markers in major depressive disorder. Mol Psychiatry. 2018 Jul;23(7):1626–1631. doi: 10.1038/mp.2017.109. 10.1038/mp.2017.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Machado-Vieira R, Gold PW, Luckenbaugh DA, Ballard ED, Richards EM, Henter ID et al. The role of adipokines in the rapid antidepressant effects of ketamine. Mol Psychiatry. 2017 Jan;22(1):127–133. doi: 10.1038/mp.2016.36. 10.1038/mp.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Short B, Fong J, Galvez V, Shelker W, Loo CK. Side- effects associated with ketamine use in depression: a systematic review. Lancet Psychiatry. 2018 Jan;5(1):65–78. doi: 10.1016/S2215-0366(17)30272-9. 10.1016/S2215-0366(17)30272-9. [DOI] [PubMed] [Google Scholar]
- 36.Niesters M, Martini C, Dahan A. Ketamine for chronic pain: risks and benefits. Br J Clin Pharmacol. 2014 Feb;77(2):357–67. doi: 10.1111/bcp.12094. 10.1111/bcp.12094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ballard ED, Zarate CA., Jr The role of dissociation in ketamine's antidepressant effects. Nat Commun. 2020 Dec 22;11(1):6431. doi: 10.1038/s41467-020-20190-4. 10.1038/s41467-020-20190-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sigtermans M, Dahan A, Mooren R, Bauer M, Kest B, Sarton E, Olofsen E. S(+)-ketamine effect on experimental pain and cardiac output: a population pharmacokinetic- pharmacodynamic modeling study in healthy volunteers. Anesthesiology. 2009 Oct;111(4):892–903. doi: 10.1097/ALN.0b013e3181b437b1. 10.1097/ALN.0b013e3181b437b1. [DOI] [PubMed] [Google Scholar]
- 39.Zarate CA., Jr What Should be Done When Elderly Patients with Major Depression Have Failed to Respond to All Treatments? Am J Geriatr Psychiatry. 2017 Nov;25(11):1210–1212. doi: 10.1016/j.jagp.2017.08.009. 10.1016/j.jagp.2017.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Salahudeen MS, Wright CM, Peterson GM. Esketamine: new hope for the treatment of treatment-resistant depression? A narrative review. Ther Adv Drug Saf. 2020 Jul 23;11 doi: 10.1177/2042098620937899. 2042098620937899. 10.1177/2042098620937899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Daly EJ, Trivedi MH, Janik A, Li H, Zhang Y, Li X et al. Efficacy of Esketamine Nasal Spray Plus Oral Antidepressant Treatment for Relapse Prevention in Patients With Treatment-Resistant Depression: A Randomized Clinical Trial. JAMA Psychiatry. 2019 Sep 1;76(9):893–903. doi: 10.1001/jamapsychiatry.2019.1189. 10.1001/jamapsychiatry.2019.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mathew SJ, Wilkinson ST, Altinay M, Asghar-Ali A, Chang LC, Collins KA et al. ELEctroconvulsive therapy (ECT) vs. Ketamine in patients with Treatment- resistant Depression: The ELEKT-D study protocol. Contemp Clin Trials. 2019 Feb;77:19–26. doi: 10.1016/j.cct.2018.12.009. 10.1016/j.cct.2018.12.009. [DOI] [PubMed] [Google Scholar]


