Abstract
Aims
Myocardial ischemia can affect traditional right ventricular (RV) pacing parameters, but it is unclear whether coronary artery disease (CAD) impact the pacing parameters and electrophysiological characteristics of left bundle branch area pacing (LBBaP) as a physiological pacing representative.
Methods
Patients who underwent coronary angiography (CAG) after/before the LBBaP procedure and underwent percutaneous coronary intervention after LBBaP procedure were divided into CAD group and Non-CAD group according to visual CAG. Pacing parameters and electrophysiological characteristics were recorded at LBBaP implantation. Multivariate logistic regression analysis was implemented to evaluate the association between CAD and higher capture threshold. Sensitivity analyses were conducted to verify result stability.
Results
A total of 176 patients met inclusion criteria (115 Non-CAD patients and 61 CAD patients) with a mean age of 71.1 ± 9.0 years. Compared with the Non-CAD patients, CAD patients had the higher capture threshold (0.67 ± 0.22 V vs. 0.82 ± 0.28 V, P < 0.001) and lower R-wave amplitude (12.5 ± 4.8 mV vs. 10.1 ± 2.7 mV, P = 0.001). Moreover, CAD was independently associated with higher capture threshold (adjusted Odds ratio (OR) 3.418, 95% confidence interval (CI): 1.621–7.206, P = 0.001), which was further validated through sensitivity analyses.
Conclusion
Patients without CAD might have safer pacing parameters in the LBBaP procedure. Besides, CAD might be the risk factor of capture threshold increase during permanent LBBaP implantation.
Keywords: Left bundle branch area pacing (LBBaP), Coronary artery disease (CAD), Pacing parameters, Electrophysiological characteristics, Risk factor, Capture threshold increase
Graphical abstract
Highlights
-
•
This study demonstrated that patients without CAD had relatively lower capture threshold and higher R-wave amplitude during the LBBaP procedure.
-
•
This study used CAG to show CAD as a risk factor for increased capture threshold. QFR further validated the results, demonstrating robustness.
1. Introduction
Prolonged myocardial ischemia has been shown to induce fibrosis in myocardial tissue [[1], [2], [3], [4]], which may affect the pacing parameters leading to changes in the pacemaker's longevity [5,6]. Previous studies have reported that myocardial infarction, diabetes, serum potassium, and antiarrhythmic drugs (AADs) are associated with right ventricular (RV) pacing parameters changes [[7], [8], [9], [10]], with additional research indicating that coronary artery disease (CAD) may influence capture threshold [11,12]. However, with the development of cardiac pacing, an increasing focus has been placed on emerging physiological pacing modalities, including his-bundle pacing (HBP) and left bundle branch area pacing (LBBaP), which have garnered significant attention among researchers. Since the first attempt of HBP to achieve ventricular synchronization, an increasing body of evidence shows the efficacy of HBP [[13], [14], [15], [16]]. Yet, the development of HBP has been hampered by challenges such as suboptimal implant success rates and instability in pacing parameters [17].
LBBaP, initially postulated in 2017 [18], represents a form of physiological pacing characterized by superior R-wave amplitude, relatively lower threshold, and more convenient procedure [19,20]. Considering the existing body of evidence, LBBaP appears to emerge as a viable and efficacious substitute for traditional pacing modalities, demonstrating both safety and effectiveness [[21], [22], [23], [24]]. As LBBaP is increasingly adopted in clinical practice, it is still unknown whether CAD has the same effect on parameters of LBBaP as traditional RV pacing, and early identification of the effect of CAD on LBBaP parameters may help physicians to plan postoperative follow-up for this population in a better way. Therefore, this study aimed to demonstrate whether CAD can affect electrophysiological characteristics and pacing parameters during LBBaP implantation. Furthermore, given the pacing site of LBBaP predominantly receives blood supply from the left anterior descending artery (LAD), the primary objective of this study was also to examine the potential influence exerted by varying locations of diseased vessels and the number of diseased vessels on electrophysiological characteristics and pacing parameters during LBBaP implantation. Finally, this study examined whether CAD is a risk factor for increased capture threshold and further validated the results from a functional perspective using quantitative flow ratio (QFR) as a diagnostic criterion for CAD in sensitivity analyses to ensure robustness of findings [[25], [26], [27]].
2. Methods
2.1. Study population
From April 2018 to February 2021, this retrospective study was carried out at Sir Run Run Shaw Hospital.
Outlined below were the criteria employed for inclusion.
-
(1)
Every patient included was above the age of 18.
-
(2)
Every patient has symptomatic bradycardia indication.
-
(3)
Patients received CAG after/before the LBBaP procedure in our hospital and underwent percutaneous coronary intervention (PCI) after the LBBaP procedure in our hospital.
-
(4)
In the same hospitalization period, both the LBBaP procedure and CAG/PCI were executed at our medical center.
The following criteria were applied for exclusion purposes.
-
(1)
Patients exhibiting either a left ventricular ejection fraction (LVEF) less than 35% or who have undergone cardiac resynchronization therapy (CRT) or implantable cardioverter-defibrillator (ICD) implantation.
-
(2)
Patients who have undergone PCI in the past.
-
(3)
Patients for whom information regarding baseline characteristics, laboratory assessments, or historical medical records is unavailable.
-
(4)
Patients affected by severe valvular heart disease, severe uncontrolled hypertension, metabolic disorders, and a variety of other organic cardiac conditions.
-
(5)
Acute fluctuation in the capture threshold of LBBaP were observed throughout the course of the implantation procedure.
This study enrolled 176 patients and categorized into two cohorts (Non-CAD group and CAD group) by visual CAG. Ethical approval for this study was obtained by submitting an application to the Medical Ethical Review Committee of Sir Run Run Shaw Hospital, referencing 20210420–12.
2.2. Definitions
In accordance with the 2021 ESC guidelines, symptomatic bradycardia encompassed distinct electrocardiographic evidence, including sick sinus syndrome (SSS) captured on an ECG, atrial fibrillation (AF) exhibiting prolonged R–R intervals, as well as high grade atrioventricular block (AVB) [21].
The diagnosis of CAD hinged on the identification of a stenotic lesion, with a degree of at least 70% ascertained via visual angiographic assessment, in either the major arteries or their epicardial branches [[28], [29], [30], [31]]. In sensitivity analyses, QFR ≤0.75 for major arteries or their epicardial branches was used as the diagnostic standard for CAD [32,33].
The quantification of diseased vessels was predicated on the participation of the epicardial segments within the three major arteries [LAD, left circumflex artery (LCX), and right coronary artery (RCA)]. The determination of multivessel lesions was based on the presence of two or more afflicted vessels in patients.
2.3. LBBaP, CAG, and QFR procedure
Tailored to the specific clinical conditions of each patient, a standardized procedural protocol was followed for pacemaker implantation, which incorporated the technique outlined by Huang et al. [34]. A connection was established between the intracardiac electrogram (EGM) and an electrophysiological multichannel recorder (Bard Electrophysiology Lab System, MA, USA), while simultaneously linking a multi-lead surface ECG monitor. Supplementary Material S1 contains comprehensive information regarding the implantation intricacies of the LBBaP procedure. Besides, Supplementary Material S1 also provides the diagnostic criteria utilized in our study to evaluate LBBaP, encompassing two distinct pacing modalities: left bundle branch pacing (LBBP) and either left ventricular septal pacing (LVSP) or deep septal pacing.
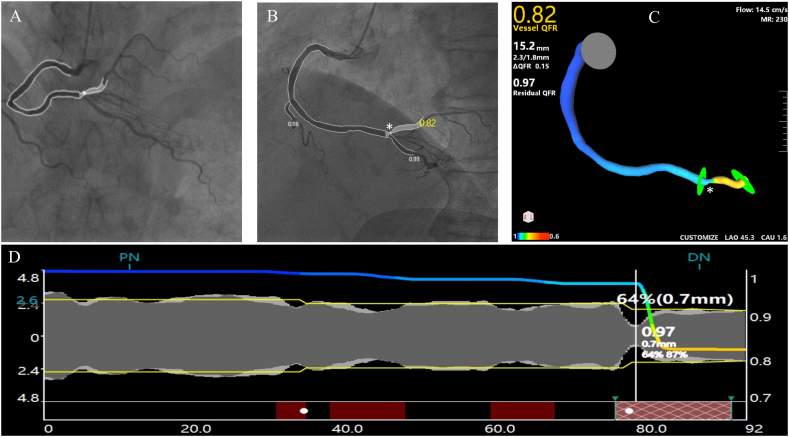
CAG was analyzed by two experienced investigators without their knowledge of other data. A pair of angiographic image runs, obtained at angles greater than or equal to 25°, were transmitted via the local network to the QFR system provided by AngioPlus, a Shanghai-based company specialized in Pulse Medical Imaging Technology. The automated algorithms, which have undergone rigorous validation, were employed to delineate the lumen contour. In instances where the angiographic image quality was suboptimal, a standard operating procedure allowed for manual correction. To compute QFR, the contrast flow model, utilizing a frame count method to derive contrast flow velocity from CAG, was utilized in this investigation (Fig. 1A–D). QFR analysis was carried out by extensively trained technicians, each of whom had conducted QFR assessments on no fewer than 30 patients.
Fig. 1.
Example of quantitative flow ratio (QFR) computation in a right coronary artery (RCA). (A): Coronary angiography (CAG) shows RCA with intermediate stenosis. (B) and (C): QFR was computed as 0.82 at the white asterisk. (D): QFR pull back and calculation analysis.
2.4. Data collection
Demographic features, comorbidities, laboratory data, and echocardiographic parameters were collected. The capture threshold of LBBaP was evaluated using unipolar configuration with a pulse width of 0.4 ms, while simultaneous recordings of intracardiac electrograms and 12-lead surface ECG were conducted throughout the measurements. The R-wave amplitude was measured for the LBBaP lead in the context of unipolar configuration. The R-wave amplitude, when present, was manually assessed using a real-time display on the programmer screen. Subsequently, measurements were printed and repeated three times to calculate the mean value. The final impedance value was determined as the average of three separate tests. Electrophysiology recording systems were employed to measure ECG parameters, including stimulus to left ventricular activation time (Sti-LVAT) and paced QRS duration (QRSd), with a sweep speed of 100 mm/s. These measurements remained consistent across both high and low voltage conditions.
Documentation of periprocedural complications, such as septal perforation and lead revision, pocket hematoma, and other device-related complications. According to the classical Teichholz method, the assessment of LVEF was performed.
2.5. Statistical analysis
The continuous variables were presented through the reporting of both the mean with its corresponding standard deviation (SD), as well as the median accompanied by the interquartile range. In the case of meeting a normal or similar to normal distribution, paired comparisons were conducted utilizing a Student's t-test. Conversely, for non-parametric data, the Mann-Whitney U-tests were employed for paired comparisons. Presentation of the categorical data included the enumeration of cases and corresponding percentages, with differences being analyzed by means of either the χ2 test or Fisher's exact test.
Parameters such as, Sti-LVAT, paced QRSd, capture threshold, R-wave amplitude, and impedance, were analyzed through one-way analysis of variance to assess the effect of CAD and location of diseased vessel on the LBBaP parameters.
Based on previous research and clinical experience [10,11,35,36], this study included 11 potential risk factors associated with increased capture threshold in a univariate logistic regression analysis, using a significance level of P < 0.1 as the criterion. Ultimately, the inclusion of CAD, AADs, Diabetes, and Serum potassium aimed to pinpoint independent risk factors associated with a higher capture threshold in a multivariate logistic regression. The capture threshold was dichotomized by a two-step clustering analysis. Finally, to assess the robustness of the results, sensitivity analyses were conducted using QFR as a diagnostic criterion for CAD to further validate the association between CAD and electrophysiological characteristics as well as pacing parameters.
3. Results
3.1. Patient screening and baseline characteristics
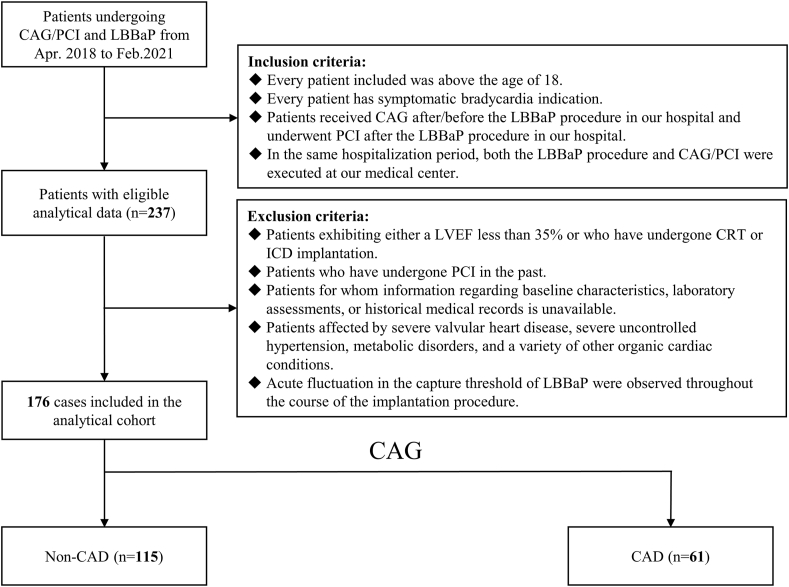
Fig. 2 illustrates the screening of 237 patients who underwent LBBaP procedure and CAG to determine eligibility, with a final enrollment of 176 patients. Subsequent to visual CAG analysis, the patients were categorized into Non-CAD and CAD groups.
Fig. 2.
Flowchart of patient enrollment. CAG: coronary angiography; CAD: coronary artery disease; CRT: cardiac resynchronization therapy; ICD: implantable cardioverter-defibrillator; LBBaP: left bundle branch area pacing; LVEF: left ventricular ejection fraction; PCI: percutaneous coronary intervention.
Table 1 presented the baseline demographic features, comorbidities, laboratory data, and echocardiographic parameters of enrolled patients. The cohort exhibited a mean age of 71.1 ± 9.0 years, with males accounting for 56.8% of the patient population. Among the patients, 52.3% had a history of hypertension, and 28.4% had a history of diabetes. The mean baseline LVEF was 60.5% ± 10.7%, and the mean left ventricular end-diastolic dimension (LVEDD) measured 50.1 ± 7.2 mm. AVB (47.2%) and SSS (35.2%) emerged as the prevailing indications for pacemaker implantation within the study cohort. Cardiac troponin I (cTnI) and N-terminal pro-brain natriuretic peptide (NT-proBNP) were comparable between CAD patients and Non-CAD patients.
Table 1.
Baseline characteristics in two groups.
| Overall (n = 176) | CAG |
|||
|---|---|---|---|---|
| Non-CAD (n = 115) | CAD (n = 61) | P value | ||
| Demographic features | ||||
| Age, yrs | 71.1 ± 9.0 | 70.4 ± 8.5 | 72.5 ± 9.9 | 0.153 |
| Male, n (%) | 100 (56.8) | 63 (54.8) | 37 (60.7) | 0.454 |
| Smoking, n (%) | 95 (54.0) | 60 (52.2) | 35 (57.4) | 0.510 |
| Drinking, n (%) | 48 (27.3) | 30 (26.1) | 18 (29.5) | 0.628 |
| AADs, n (%) | 91 (51.7) | 55 (47.8) | 36 (59.0) | 0.157 |
| Preimplant QRSd, ms | 110.4 ± 22.1 | 109.5 ± 22.7 | 112.1 ± 20.9 | 0.467 |
| Comorbidities | ||||
| Hypertension, n (%) | 92 (52.3) | 57 (49.6) | 35 (57.4) | 0.323 |
| Diabetes, n (%) | 50 (28.4) | 30 (26.1) | 20 (32.8) | 0.348 |
| Hyperlipidemia, n (%) | 52 (29.5) | 32 (27.8) | 20 (32.8) | 0.492 |
| AF, n (%) | 44 (25.0) | 28 (24.3) | 16 (26.2) | 0.784 |
| AVB, n (%) | 83 (47.2) | 53 (46.1) | 30 (49.2) | 0.696 |
| SSS, n (%) | 62 (35.2) | 43 (37.4) | 19 (31.1) | 0.409 |
| LBBB, n (%) | 20 (11.4) | 10 (8.7) | 10 (16.4) | 0.126 |
| RBBB, n (%) | 26 (14.8) | 15 (13.0) | 11 (18.0) | 0.375 |
| Laboratory data | ||||
| NT-proBNP, pg/ml | 351.0 (123.0, 772.8) | 276.0 (122.0, 645.0) | 456.0 (146.5, 884.0) | 0.244 |
| cTnI, ng/ml | 0.009 (0.005, 0.025) | 0.008 (0.004, 0.023) | 0.015 (0.008, 0.032) | 0.110 |
| Serum potassium, mEq/L | 4.02 ± 0.32 | 4.00 ± 0.33 | 4.06 ± 0.29 | 0.192 |
| Echocardiography | ||||
| LVEF (%) | 60.5 ± 10.7 | 61.1 ± 10.6 | 59.4 ± 11.0 | 0.316 |
| LVEDD, mm | 50.1 ± 7.2 | 49.4 ± 7.6 | 51.3 ± 6.4 | 0.108 |
| LVESD, mm | 35.1 ± 8.1 | 34.8 ± 8.1 | 35.6 ± 8.2 | 0.531 |
AADs: antiarrhythmic drugs; AF: atrial fibrillation; AVB: atrioventricular block; CAG: coronary angiography; CAD: coronary artery disease; cTnI: cardiac troponin I; LBBB: left bundle branch block; LVEF: left ventricular ejection fraction; LVEDD: left ventricular end-diastolic dimension; LVESD: left ventricular end-systolic dimension; NT-proBNP: N-Terminal Pro-Brain Natriuretic Peptide; QRSd: QRS duration; RBBB: right bundle branch block; SSS: sick sinus syndrome.
*: P < 0.05.
3.2. Electrophysiological data, pacing parameters, and periprocedural complications
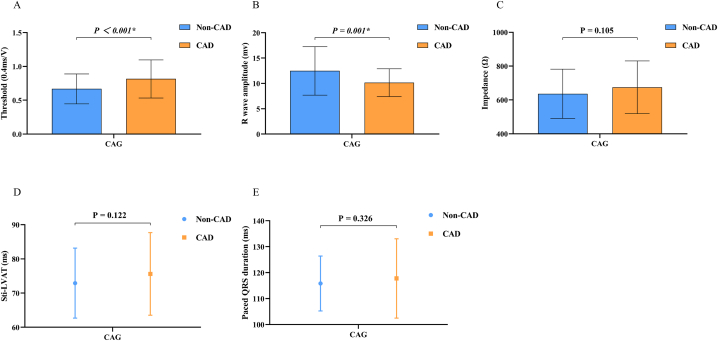
The procedural time and fluoroscopy time demonstrated no significant differences between the two groups, as presented in Table 2. Similarly, between the two groups, there were no significant distinctions observed in the recorded LBB potential, Sti-LVAT, and paced QRSd. After lead fixation, pacing impedance was similar between the two groups, while CAD patients had a higher capture threshold (Non-CAD vs CAD, 0.67 ± 0.22 V vs. 0.82 ± 0.28 V, P < 0.001) and lower R-wave amplitude (Non-CAD vs CAD, 12.5 ± 4.8 mV vs. 10.1 ± 2.7 mV, P = 0.001) than Non-CAD patients (Fig. 3A–E).
Table 2.
Electrophysiological data, pacing parameters, and periprocedural complications were compared between two groups.
| Overall (n = 176) | CAG |
|||
|---|---|---|---|---|
| Non-CAD (n = 115) | CAD (n = 61) | P value | ||
| Electrophysiological Data | ||||
| Procedure time, min | 97.3 ± 32.8 | 95.5 ± 31.0 | 100.8 ± 36.0 | 0.307 |
| Fluoroscopic time, min | 9.9 (7.6, 14.0) | 9.8 (7.5, 13.5) | 10.2 (7.9, 15.7) | 0.480 |
| LBB potential recorded, n (%) | 108 (61.4) | 69 (60.0) | 39 (63.9) | 0.610 |
| Sti-LVAT, ms | 73.9 ± 11.0 | 72.9 ± 10.2 | 75.6 ± 12.1 | 0.122 |
| Paced QRSd, ms | 116.5 ± 12.4 | 115.8 ± 10.6 | 117.8 ± 15.3 | 0.326 |
| Pacing Parameters | ||||
| Threshold, V/0.4 m s | 0.72 ± 0.25 | 0.67 ± 0.22 | 0.82 ± 0.28 | <0.001* |
| R-wave amplitude, mV | 11.7 ± 4.3 | 12.5 ± 4.8 | 10.1 ± 2.7 | 0.001* |
| Impedance, Ω | 649.9 ± 150.1 | 636.5 ± 145.9 | 675.0 ± 155.8 | 0.105 |
| Periprocedural complications | 9 (5.1) | 4 (3.5) | 5 (8.2) | 0.279 |
| Septal perforation, n (%) | 2 (1.1) | 1 (0.9) | 1 (1.6) | 0.647 |
| Lead revision, n (%) | 1 (0.6) | 1 (0.9) | 0 (0.0) | 0.465 |
| Pneumothorax/hemothorax, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1.000 |
| Pocket hematoma, n (%) | 5 (2.8) | 2 (1.7) | 3 (4.9) | 0.343 |
| Pocket infection, n (%) | 1 (0.6) | 0 (0.0) | 1 (1.6) | 0.347 |
CAD: coronary artery disease; CAG: coronary angiography; LBB: left bundle branch; QRSd: QRS duration; Sti-LVAT: time from stimulus to peak ventricular activation.
*: P < 0.05.
Fig. 3.
Electrophysiological data and pacing parameters were compared between Non-CAD and CAD groups by visual CAG. CAG: coronary angiography; CAD: coronary artery disease; Sti-LVAT: time from stimulus to peak ventricular activation. *: P < 0.05.
Regarding complications, the CAD group had one case of pocket infection and three cases of pocket hematoma. Non-CAD group had one case of lead revision and two case of pocket hematoma. In addition, septal perforation was observed in one case each among CAD and Non-CAD patients. Overall, in terms of the incidence of complications, no statistically significant disparities were observed between the two groups (Table 2).
3.3. Comparison within single vessel lesion and comparison between the single vessel lesion and multivessel lesions
Supplementary Table 1 illustrated the electrophysiological data and pacing parameters for the subgroup of patients with single vessel lesions. The capture threshold in the LAD group exhibited a significant elevation compared to both the LCX and RCA groups (LAD vs LCX, 0.87 ± 0.26 V vs. 0.69 ± 0.18 V, P = 0.034; LAD vs RCA, 0.87 ± 0.26 V vs. 0.67 ± 0.16 V, P = 0.023), while concurrently exhibiting a significantly lower R-wave amplitude (LAD vs LCX, 9.4 ± 2.4 mV vs. 11.4 ± 2.7 mV, P = 0.041; LAD vs RCA, 9.4 ± 2.4 mV vs. 12.2 ± 2.5 mV, P = 0.006). In relation to impedance, Sti-LVAT, and paced QRSd, no significant distinctions were observed among the three groups.
Additionally, when comparing the single vessel lesion group with the multivessel lesion group (as shown in Supplementary Table 2), no statistical disparities were observed for capture threshold, impedance, R-wave amplitude, Sti-LVAT, and paced QRSd.
3.4. Regression analysis for the higher capture threshold
Utilizing univariate and multivariate logistic regression analysis, Table 3 illustrated the relationship between the higher capture threshold and different risk factors. The univariate regression analysis indicated that CAD, AADs, Diabetes, and Serum potassium could be included in the multivariate regression analysis based on a significance criterion of P < 0.1. Multivariate regressions showed that CAD, diabetes, and serum potassium were associated with indicated higher odds of the capture threshold increase (CAD (CAG) (OR: 3.418, 95%CI: 1.621–7.206; P = 0.001)); Diabetes (OR: 2.368, 95%CI: 1.091–5.141; P = 0.029); Serum potassium (OR: 3.511, 95%CI: 1.072–11.498; P = 0.038)).
Table 3.
Univariate and multivariate logistic regression analysis for the higher capture threshold.
| Variables | Univariate analysis |
Multivariate analysis |
||||
|---|---|---|---|---|---|---|
| OR | 95%CI | P value | Adjusted OR | 95%CI | P value | |
| CAD (CAG) | 3.742 | 1.828–7.661 | <0.001* | 3.418 | 1.621–7.206 | 0.001* |
| Age, yrs | 1.016 | 0.977–1.056 | 0.417 | |||
| Male | 0.739 | 0.370–1.473 | 0.390 | |||
| AADs | 1.819 | 0.898–3.685 | 0.096 | 1.688 | 0.789–3.609 | 0.177 |
| Hypertension | 1.368 | 0.683–2.741 | 0.376 | |||
| Diabetes | 2.605 | 1.263–5.372 | 0.010* | 2.368 | 1.091–5.141 | 0.029* |
| AF | 1.222 | 0.562–2.655 | 0.613 | |||
| AVB | 1.237 | 0.621–2.461 | 0.546 | |||
| SSS | 1.456 | 0.719–2.949 | 0.296 | |||
| Serum potassium, mEq/L | 4.019 | 1.344–12.016 | 0.013* | 3.511 | 1.072–11.498 | 0.038* |
| Procedure time, min | 1.007 | 0.997–1.017 | 0.178 | |||
AADs: antiarrhythmic drugs; AF: atrial fibrillation; AVB: atrioventricular block; CAD: coronary artery disease; CAG: coronary angiography; CI: confidence interval; OR: Odds ratios; SSS: sick sinus syndrome.
*: P < 0.05.
3.5. Sensitivity analyses
To assess the stability of the research findings, this study utilized QFR as the diagnostic criteria for CAD to evaluate the association between CAD and the electrophysiological characteristics and pacing parameters of LBBaP. According to Supplementary Table 3, no baseline differences were observed between the groups categorized as CAD and Non-CAD under QFR grouping. Supplementary Table 4 demonstrated that compared to the Non-CAD group, the CAD group diagnosed by QFR also exhibited significantly higher capture threshold (Non-CAD vs CAD, 0.68 ± 0.22 V vs. 0.82 ± 0.29 V, P < 0.001) and lower R-wave amplitude (Non-CAD vs CAD, 12.4 ± 4.6 mV vs. 9.9 ± 2.8 mV, P < 0.001). Besides, in both univariate and multivariate logistic regression analyses, CAD (diagnosed by QFR) was also identified as an independent risk factor for higher capture threshold (CAD (QFR) (OR: 4.059, 95%CI: 1.893–8.703; P < 0.001)), consistent with the previously reported main findings (Table 4).
Table 4.
Univariate and multivariate logistic regression analysis for the higher capture threshold in a sensitivity analysis by using QFR as a diagnostic criterion for CAD.
| Variables | Univariate analysis |
Multivariate analysis |
||||
|---|---|---|---|---|---|---|
| OR | 95%CI | P value | Adjusted OR | 95%CI | P value | |
| CAD (QFR) | 4.124 | 1.993–8.532 | <0.001* | 4.059 | 1.893–8.703 | <0.001* |
| Age, yrs | 1.016 | 0.977–1.056 | 0.417 | |||
| Male | 0.739 | 0.370–1.473 | 0.390 | |||
| AADs | 1.819 | 0.898–3.685 | 0.096 | 1.786 | 0.831–3.840 | 0.137 |
| Hypertension | 1.368 | 0.683–2.741 | 0.376 | |||
| Diabetes | 2.605 | 1.263–5.372 | 0.010* | 2.280 | 1.044–4.983 | 0.039* |
| AF | 1.222 | 0.562–2.655 | 0.613 | |||
| AVB | 1.237 | 0.621–2.461 | 0.546 | |||
| SSS | 1.456 | 0.719–2.949 | 0.296 | |||
| Serum potassium, mEq/L | 4.019 | 1.344–12.016 | 0.013* | 4.120 | 1.236–13.729 | 0.021* |
| Procedure time, min | 1.007 | 0.997–1.017 | 0.178 | |||
AADs: antiarrhythmic drugs; AF: atrial fibrillation; AVB: atrioventricular block; CAD: coronary artery disease; CI: confidence interval; OR: Odds ratios; QFR: quantitative flow ratio; SSS: sick sinus syndrome.
*: P < 0.05.
4. Discussion
This retrospective study demonstrated that patients without CAD had relatively lower capture threshold and higher R-wave amplitude during the LBBaP procedure. Besides, this study also found that CAD might be a risk factor for capture threshold increase during permanent LBBaP implantation.
In order to ensure the accuracy of the diagnosis of CAD, this study not only made the diagnosis from the traditional visual CAG but also grouped from the more physiological measurement technology of QFR in sensitivity analyses. Regardless of the diagnostic criteria, this study showed that patients without CAD had safer pacing parameters, which was consistent with previous findings [11,12,37]. Peng et al. and Mittal et al. presented that myocardial infarction may cause an increase in threshold [10,38], however, these studies pay no attention to the R wave amplitude, impedance, and electrophysiological data. Huang et al. documented that CAD might be a standalone risk factor affecting the capture threshold. However, this study lacks sensitivity analyses to evaluate the robustness of the results [11]. Furthermore, all the studies mentioned above only reported the pacing parameters of traditional RV pacing while only few articles have reported the influence of CAD on the pacing parameters of the emerging pacing modality LBBaP. Our study was also the first to add QFR as a diagnostic method to prove the influence of CAD on the LBBaP parameters.
In 2019, CAD was projected to impact 197 million individuals globally and was responsible for 9.1 million fatalities (16.1% of all deaths), making it the leading cause of mortality worldwide [39,40]. CAD patients should be considered as a special population, as they have more severe myocardial ischemia, more co-morbidities such as diabetes and cardio-cerebrovascular disease, and an enhanced risk of bleeding associated with the use of antiplatelet drugs, which contributes to the increased incidence of periprocedural complications. However, in our study, all periprocedural complications demonstrated no differences between the two cohorts, possibly because the low incidence of complications, which hindered the detection of statistical significance. The incidence of septal perforation and lead revision in LBBaP ranged from 0% to 3% in previous studies [41,42], which is relatively low and similar to our findings. Within our study, the recording of LBB potential was observed in 108 individuals, constituting 61.4% of the population. This finding aligns with the reported range of LBB potential, falling within the 50%–80% spectrum as documented in numerous prior studies [[42], [43], [44]].
In concordance with prior investigations, this study revealed that patients with CAD have more unstable pacing parameters during the permanent LBBaP procedure, which might be due to long-term chronic ischemia that cause remolding of myocardial tissue structure and excitability of myocardial cells [45,46]. Furthermore, previous studies have also found that under myocardial ischemia, the production of osmotic active particles through anaerobic metabolism created the osmotic gradients on the cell membrane [47,48], which also affected pacing parameters. Nevertheless, our study found no significant disparity in impedance between CAD cohorts and Non-CAD cohorts, which may be due to multiple factors affecting impedance, especially the depth of wire entry during procedure. Moreover, this study found that when comparing LAD, LCX, and RCA within the single vessel lesion, the capture threshold of LAD was relatively higher and the R-wave amplitude lower. This could be attributed to the fact that the pacing site of LBBaP is mainly supplied by LAD, resulting in greater variation in pacing parameters. However, it must be acknowledged that the sample size employed in this subgroup analysis was comparatively modest, thereby imposing limitations on the reliability of the obtained findings. Future investigations that encompass a more expansive sample size are warranted to allow for more in-depth exploration of the electrophysiological characteristics and disparities in pacing parameters associated with LBBaP within the single vessel lesions.
Previous studies reported that CAD might heighten the risk of the capture threshold increase, which was consistent with our findings [11,37]. Our finding also suggested that diabetes and serum potassium might be associated with capture threshold increase, which was consistent with previous reports by Peng et al. and Dohrmann et al. [10,35]. In individuals with diabetes, the process of oxidative stress-induced aging and subsequent demise of myocardial cells leads to the accumulation of interstitial fibrotic tissues and alterations in myofibrillar proteins, ultimately culminating in myocardial fibrosis and degeneration [[49], [50], [51], [52]]. Therefore, the potential impact of hyperglycemia on cardiomyopathic mechanisms might theoretically affect the myocardial excitability threshold and subsequently alter the capture threshold. In the hyperkalemia, the pacemaker-generated impulse might be inadequate due to an elevated myocardial depolarization threshold. This increase in threshold could potentially diminish the myocardium's response to stimulation in terms of excitability and conductivity. Consequently, while the pacemaker itself may function normally, the capture threshold might elevate, possibly leading to ineffective capture of the myocardium and the induction of depolarization [36,53]. Notably, the effect of AADs on the capture threshold remained controversial due to contradicting reports [7,[54], [55], [56], [57]]. This study also identified no significant association between AADs and capture threshold, which might be because LBBaP beyond the site of the block and AADs had no impact on the conduction beneath the his-bundle lead.
4.1. Study limitations
The limitation of this study pertains to its retrospective nature, as it relied on observational data and encompassed a relatively small patient cohort. To comprehensively examine the influence of varying degrees of stenosis in different vessels on its pacing parameters, further exploration requires a randomized trial with a larger sample size.
5. Conclusion
Patients without CAD might have safer pacing parameters in the LBBaP procedure. Besides, CAD might be the risk factor of capture threshold increase during permanent LBBaP implantation.
Funding
This study was supported by the National Natural Science Foundation of China (81800212 and 82070408).
Ethics approval and consent to participate
Approval was obtained from the Medical Ethical Review Committee of Sir Run Run Shaw Hospital (20210420–12) to conduct this study. Given its retrospective nature, patients who were admitted to the hospital had previously signed a standard informed consent form, thereby authorizing the use of their data and general clinical information. No additional informed consent was required as this study was also non-interventional.
Consent for publication
All authors confirmed and approved for publication.
Data availability statement
Certainly, the corresponding author is willing to furnish the required data upon appropriate inquiries.
CRediT authorship contribution statement
Yu Shan: Writing – review & editing, Writing – original draft, Visualization, Software, Resources, Investigation, Data curation. Maoning Lin: Writing – review & editing, Visualization, Software, Resources, Investigation, Conceptualization. Miao Ye: Visualization, Software, Methodology, Investigation. Xiaohua Shen: Visualization, Validation, Supervision, Project administration, Methodology, Conceptualization. Duanbin Li: Data curation, Conceptualization. Zhezhe Chen: Funding acquisition, Formal analysis. Hangpan Jiang: Methodology, Investigation, Funding acquisition. Guosheng Fu: Resources, Project administration, Methodology. Wenbin Zhang: Validation, Supervision, Funding acquisition. Min Wang: Validation, Supervision, Project administration, Funding acquisition, Data curation, Conceptualization.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:Min Wang reports financial support was provided by National Natural Science Foundation of China. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e24226.
Contributor Information
Wenbin Zhang, Email: 3313011@zju.edu.cn.
Min Wang, Email: wangminsyf30508@zju.edu.cn.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Holmes J.W., Borg T.K., Covell J.W. Structure and mechanics of healing myocardial infarcts. Annu. Rev. Biomed. Eng. 2005;7:223–253. doi: 10.1146/annurev.bioeng.7.060804.100453. [DOI] [PubMed] [Google Scholar]
- 2.Mygind N.D., Michelsen M.M., Pena A., Qayyum A.A., Frestad D., Christensen T.E., et al. Coronary microvascular function and myocardial fibrosis in women with angina pectoris and no obstructive coronary artery disease: the iPOWER study. J. Cardiovasc. Magn. Reson. : Off. J. Soc. Cardiovasc. Magnetic Resonance. 2016;18(1):76. doi: 10.1186/s12968-016-0295-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shah D.J., Kim H.W., James O., Parker M., Wu E., Bonow R.O., et al. Prevalence of regional myocardial thinning and relationship with myocardial scarring in patients with coronary artery disease. JAMA. 2013;309(9):909–918. doi: 10.1001/jama.2013.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sorop O., Heinonen I., van Kranenburg M., van de Wouw J., de Beer V.J., Nguyen I.T.N., et al. Multiple common comorbidities produce left ventricular diastolic dysfunction associated with coronary microvascular dysfunction, oxidative stress, and myocardial stiffening. Cardiovasc. Res. 2018;114(7):954–964. doi: 10.1093/cvr/cvy038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.An Z., Wu J., Li S.H., Chen S., Lu F.L., Xu Z.Y., et al. Injectable conductive hydrogel can reduce pacing threshold and enhance efficacy of cardiac pacemaker. Theranostics. 2021;11(8):3948–3960. doi: 10.7150/thno.54959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vurgun V.K., Baskovski E., Goksuluk H., Ozyuncu N., Tan T.S., Altin A.T., et al. Evaluation of right ventricular pacing parameters in patients with proliferative scar. J. Intervent. Card Electrophysiol. : Int. J. Arrhythmias Pacing. 2018;53(2):249–254. doi: 10.1007/s10840-018-0395-2. [DOI] [PubMed] [Google Scholar]
- 7.Bianconi L., Boccadamo R., Toscano S., Serdoz R., Carpino A., Iesi A.P., et al. Effects of oral propafenone therapy on chronic myocardial pacing threshold. Pacing Clin. Electrophysiol. : PACE (Pacing Clin. Electrophysiol.) 1992;15(2):148–154. doi: 10.1111/j.1540-8159.1992.tb03058.x. [DOI] [PubMed] [Google Scholar]
- 8.Hall R., Jones D.A., Muthumala A., Weerackody R., Sohaib A., Monkhouse C. Transient rise in His-lead threshold due to acute myocardial infarction. Pacing Clin. Electrophysiol. : PACE (Pacing Clin. Electrophysiol.) 2019;42(6):754–757. doi: 10.1111/pace.13612. [DOI] [PubMed] [Google Scholar]
- 9.McVenes R., Hansen N., Lahtinen S.P., Stokes K. The salty dog: serum sodium and potassium effects on modern pacing electrodes. Pacing Clin. Electrophysiol. : PACE (Pacing Clin. Electrophysiol.) 2007;30(1):4–11. doi: 10.1111/j.1540-8159.2007.00573.x. [DOI] [PubMed] [Google Scholar]
- 10.Peng H., Sun Z., Zhang H., Ma W. Long-term performance of right ventricular pacing leads: risk factors associated with permanent right ventricular pacing threshold increase. J. Intervent. Card Electrophysiol. : Int. J. Arrhythmias Pacing. 2019;55(3):349–357. doi: 10.1007/s10840-018-0481-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang G.Y., Peng Z., Zhan Y., Liu D.D., Liu Y.L. Relationship between the change in pacing threshold and the myocardial injury. Eur. Rev. Med. Pharmacol. Sci. 2017;21(24):5774–5780. doi: 10.26355/eurrev_201712_14024. [DOI] [PubMed] [Google Scholar]
- 12.Steendijk P., van Dijk A.D., Mur G., van der Velde E.T., Baan J. Effect of coronary occlusion and reperfusion on local electrical resistivity of myocardium in dogs. Basic Res. Cardiol. 1993;88(2):167–178. doi: 10.1007/BF00798265. [DOI] [PubMed] [Google Scholar]
- 13.Sharma P.S., Naperkowski A., Bauch T.D., Chan J.Y.S., Arnold A.D., Whinnett Z.I., et al. Permanent his bundle pacing for cardiac resynchronization therapy in patients with heart failure and right bundle branch block. Circulation Arrhythmia and Electrophysiol. 2018;11(9) doi: 10.1161/CIRCEP.118.006613. [DOI] [PubMed] [Google Scholar]
- 14.Beer D., Subzposh F.A., Colburn S., Naperkowski A., Vijayaraman P. His bundle pacing capture threshold stability during long-term follow-up and correlation with lead slack. Europace : European pacing, arrhythmias, and cardiac electrophysiology : journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of. Eur. Soc. Cardiol. 2021;23(5):757–766. doi: 10.1093/europace/euaa350. [DOI] [PubMed] [Google Scholar]
- 15.Vijayaraman P., Richter S. His-bundle pacing: promise for the future. Europace : European pacing, arrhythmias, and cardiac electrophysiology : journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of. Eur. Soc. Cardiol. 2019;21(5):686–687. doi: 10.1093/europace/euy318. [DOI] [PubMed] [Google Scholar]
- 16.Sharma P.S., Dandamudi G., Herweg B., Wilson D., Singh R., Naperkowski A., et al. Permanent His-bundle pacing as an alternative to biventricular pacing for cardiac resynchronization therapy: a multicenter experience. Heart Rhythm. 2018;15(3):413–420. doi: 10.1016/j.hrthm.2017.10.014. [DOI] [PubMed] [Google Scholar]
- 17.Teigeler T., Kolominsky J., Vo C., Shepard R.K., Kalahasty G., Kron J., et al. Intermediate-term performance and safety of His-bundle pacing leads: a single-center experience. Heart Rhythm. 2021;18(5):743–749. doi: 10.1016/j.hrthm.2020.12.031. [DOI] [PubMed] [Google Scholar]
- 18.Huang W., Su L., Wu S., Xu L., Xiao F., Zhou X., et al. A novel pacing strategy with low and stable output: pacing the left bundle branch immediately beyond the conduction block. Can. J. Cardiol. 2017;33(12):1736. doi: 10.1016/j.cjca.2017.09.013. e1-.e3. [DOI] [PubMed] [Google Scholar]
- 19.Hua W., Fan X., Li X., Niu H., Gu M., Ning X., et al. Comparison of left bundle branch and his bundle pacing in bradycardia patients. JACC Clin. Electrophysiol. 2020;6(10):1291–1299. doi: 10.1016/j.jacep.2020.05.008. [DOI] [PubMed] [Google Scholar]
- 20.Zhuo W., Zhong X., Liu H., Yu J., Chen Q., Hu J., et al. Pacing characteristics of his bundle pacing vs. Left bundle branch pacing: a systematic review and meta-analysis. Front. Cardiovasc. Med. 2022;9 doi: 10.3389/fcvm.2022.849143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Glikson M., Nielsen J.C., Kronborg M.B., Michowitz Y., Auricchio A., Barbash I.M., et al. ESC Guidelines on cardiac pacing and cardiac resynchronization therapy. Eur. Heart J. 2021;42(35):3427–3520. doi: 10.1093/eurheartj/ehab364. 2021. [DOI] [PubMed] [Google Scholar]
- 22.Chen X., Ye Y., Wang Z., Jin Q., Qiu Z., Wang J., et al. Cardiac resynchronization therapy via left bundle branch pacing vs. optimized biventricular pacing with adaptive algorithm in heart failure with left bundle branch block: a prospective, multi-centre, observational study. Europace : European pacing, arrhythmias, and cardiac electrophysiology : journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of. Eur. Soc. Cardiol. 2022;24(5):807–816. doi: 10.1093/europace/euab249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ji W., Chen X., Shen J., Zhu D., Chen Y., Li F. Left bundle branch pacing improved heart function in a 10-year-old child after a 3-month follow-up. Europace : European pacing, arrhythmias, and cardiac electrophysiology : journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular. Electrophysiol. Eur. Soc. Cardiol. 2020;22(8):1234–1239. doi: 10.1093/europace/euaa090. [DOI] [PubMed] [Google Scholar]
- 24.Liu P., Wang Q., Sun H., Qin X., Zheng Q. Left bundle branch pacing: current knowledge and future prospects. Front. Cardiovasc. Med. 2021;8 doi: 10.3389/fcvm.2021.630399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Song L., Xu B., Tu S., Guan C., Jin Z., Yu B., et al. 2-Year outcomes of angiographic quantitative flow ratio-guided coronary interventions. J. Am. Coll. Cardiol. 2022;80(22):2089–2101. doi: 10.1016/j.jacc.2022.09.007. [DOI] [PubMed] [Google Scholar]
- 26.Xu B., Tu S., Qiao S., Qu X., Chen Y., Yang J., et al. Diagnostic accuracy of angiography-based quantitative flow ratio measurements for online assessment of coronary stenosis. J. Am. Coll. Cardiol. 2017;70(25):3077–3087. doi: 10.1016/j.jacc.2017.10.035. [DOI] [PubMed] [Google Scholar]
- 27.Xu B., Tu S., Song L., Jin Z., Yu B., Fu G., et al. Angiographic quantitative flow ratio-guided coronary intervention (FAVOR III China): a multicentre, randomised, sham-controlled trial. Lancet (London, England) 2021;398(10317):2149–2159. doi: 10.1016/S0140-6736(21)02248-0. [DOI] [PubMed] [Google Scholar]
- 28.Neglia D., Rovai D., Caselli C., Pietila M., Teresinska A., Aguadé-Bruix S., et al. Detection of significant coronary artery disease by noninvasive anatomical and functional imaging. Circulation Cardiovasc. Imag. 2015;8(3) doi: 10.1161/CIRCIMAGING.114.002179. [DOI] [PubMed] [Google Scholar]
- 29.Gutierrez J.A., Bhatt D.L., Banerjee S., Glorioso T.J., Josey K.P., Swaminathan R.V., et al. Risk of obstructive coronary artery disease and major adverse cardiac events in patients with noncoronary atherosclerosis: insights from the Veterans Affairs Clinical Assessment, Reporting, and Tracking (CART) Program. Am. Heart J. 2019;213:47–56. doi: 10.1016/j.ahj.2019.04.004. [DOI] [PubMed] [Google Scholar]
- 30.Vecsey-Nagy M., Szilveszter B., Kolossváry M., Boussoussou M., Vattay B., Gonda X., et al. Association between affective temperaments and severe coronary artery disease. J. Affect. Disord. 2021;295:914–919. doi: 10.1016/j.jad.2021.08.063. [DOI] [PubMed] [Google Scholar]
- 31.Ferencik M., Mayrhofer T., Lu M.T., Bittner D.O., Emami H., Puchner S.B., et al. Coronary atherosclerosis, cardiac troponin, and interleukin-6 in patients with chest pain: the PROMISE trial results. JACC Cardiovasc. Imag. 2022;15(8):1427–1438. doi: 10.1016/j.jcmg.2022.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Milzi A., Dettori R., Marx N., Reith S., Burgmaier M. Quantitative flow ratio (QFR) identifies functional relevance of non-culprit lesions in coronary angiographies of patients with acute myocardial infarction. Clin. Res. Cardiol. : Off. J. German Cardiac Soc. 2021;110(10):1659–1667. doi: 10.1007/s00392-021-01897-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen C., Zhao Y., Li W., Zhang K., Dang H., Liu T., et al. Relation of quantitative flow ratio with transit time coronary artery bypass graft flow measurement. Front. Cardiovasc. Med. 2022;9 doi: 10.3389/fcvm.2022.975759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang W., Chen X., Su L., Wu S., Xia X., Vijayaraman P. A beginner's guide to permanent left bundle branch pacing. Heart Rhythm. 2019;16(12):1791–1796. doi: 10.1016/j.hrthm.2019.06.016. [DOI] [PubMed] [Google Scholar]
- 35.Dohrmann M.L., Goldschlager N.F. Myocardial stimulation threshold in patients with cardiac pacemakers: effect of physiologic variables, pharmacologic agents, and lead electrodes. Cardiol. Clin. 1985;3(4):527–537. [PubMed] [Google Scholar]
- 36.Sanson G., Russo S., Iudicello A., Schiraldi F. Tetraparesis and failure of pacemaker capture induced by severe hyperkalemia: case report and systematic review of available literature. J. Emerg. Med. 2015;48(5):555. doi: 10.1016/j.jemermed.2014.12.048. 61.e3. [DOI] [PubMed] [Google Scholar]
- 37.Heinroth K.M., Unverzagt S., Mahnkopf D., Prondzinsky R. Transcoronary pacing : reliability during myocardial ischemia and after implantation of a coronary stent. Med. Klin. Intensivmed. Notfallmed. 2020;115(2):120–124. doi: 10.1007/s00063-018-0492-0. [DOI] [PubMed] [Google Scholar]
- 38.Mittal S.R., Mahar M.S., Gokhroo R.K. Transvenous pacing in the presence of acute right ventricular infarction. Int. J. Cardiol. 1992;34(1):100–101. doi: 10.1016/0167-5273(92)90088-k. [DOI] [PubMed] [Google Scholar]
- 39.Global age-sex-specific fertility, mortality, healthy life expectancy (HALE), and population estimates in 204 countries and territories, 1950-2019: a comprehensive demographic analysis for the Global Burden of Disease Study 2019. Lancet (London, England) 2020;396(10258):1160–1203. doi: 10.1016/S0140-6736(20)30977-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roth G.A., Mensah G.A., Johnson C.O., Addolorato G., Ammirati E., Baddour L.M., et al. Global burden of cardiovascular diseases and risk factors, 1990-2019: update from the GBD 2019 study. J. Am. Coll. Cardiol. 2020;76(25):2982–3021. doi: 10.1016/j.jacc.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen X., Wei L., Bai J., Wang W., Qin S., Wang J., et al. Procedure-related complications of left bundle branch pacing: a single-center experience. Front. Cardiovasc. Med. 2021;8 doi: 10.3389/fcvm.2021.645947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Su L., Wang S., Wu S., Xu L., Huang Z., Chen X., et al. Long-term safety and feasibility of left bundle branch pacing in a large single-center study. Circulation Arrhythmia and Electrophysiol. 2021;14(2) doi: 10.1161/CIRCEP.120.009261. [DOI] [PubMed] [Google Scholar]
- 43.Jastrzębski M., Burri H., Kiełbasa G., Curila K., Moskal P., Bednarek A., et al. The V6-V1 interpeak interval: a novel criterion for the diagnosis of left bundle branch capture. Europace : Eur. Pacing, Arrhythmias, Cardiac Electrophysiol. : J. Work. Groups on Cardiac Pacing, Arrhythmias, and Cardiac Cellular Electrophysiol. Eur. Soc. Cardiol. 2022;24(1):40–47. doi: 10.1093/europace/euab164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li Y., Chen K., Dai Y., Li C., Sun Q., Chen R., et al. Left bundle branch pacing for symptomatic bradycardia: implant success rate, safety, and pacing characteristics. Heart Rhythm. 2019;16(12):1758–1765. doi: 10.1016/j.hrthm.2019.05.014. [DOI] [PubMed] [Google Scholar]
- 45.Bektik E., Fu J.D. Ameliorating the fibrotic remodeling of the heart through direct cardiac reprogramming. Cells. 2019;8(7) doi: 10.3390/cells8070679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim Y.K., Kim S.J., Kramer C.M., Yatani A., Takagi G., Mankad S., et al. Altered excitation-contraction coupling in myocytes from remodeled myocardium after chronic myocardial infarction. J. Mol. Cell. Cardiol. 2002;34(1):63–73. doi: 10.1006/jmcc.2001.1490. [DOI] [PubMed] [Google Scholar]
- 47.Aggeli I.K., Kapogiannatou A., Paraskevopoulou F., Gaitanaki C. Differential response of cardiac aquaporins to hyperosmotic stress; salutary role of AQP1 against the induced apoptosis. Eur. Rev. Med. Pharmacol. Sci. 2021;25(1):313–325. doi: 10.26355/eurrev_202101_24397. [DOI] [PubMed] [Google Scholar]
- 48.Fiolet J.W., Schumacher C.A., Baartscheer A., Coronel R. Osmotic changes and transsarcolemmal ion transport during total ischaemia of isolated rat ventricular myocytes. Basic Res. Cardiol. 1993;88(5):396–410. doi: 10.1007/BF00795407. [DOI] [PubMed] [Google Scholar]
- 49.Adeghate E., Singh J. Structural changes in the myocardium during diabetes-induced cardiomyopathy. Heart Fail. Rev. 2014;19(1):15–23. doi: 10.1007/s10741-013-9388-5. [DOI] [PubMed] [Google Scholar]
- 50.Epstein A.E., Ellenbogen K.A., Kirk K.A., Kay G.N., Dailey S.M., Plumb V.J. Clinical characteristics and outcome of patients with high defibrillation thresholds. A multicenter study. Circulation. 1992;86(4):1206–1216. doi: 10.1161/01.cir.86.4.1206. [DOI] [PubMed] [Google Scholar]
- 51.Nakamura K., Miyoshi T., Yoshida M., Akagi S., Saito Y., Ejiri K., et al. Pathophysiology and treatment of diabetic cardiomyopathy and heart failure in patients with diabetes mellitus. Int. J. Mol. Sci. 2022;23(7) doi: 10.3390/ijms23073587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ritchie R.H., Abel E.D. Basic mechanisms of diabetic heart disease. Circ. Res. 2020;126(11):1501–1525. doi: 10.1161/CIRCRESAHA.120.315913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.O'Reilly M.V., Murnaghan D.P., Williams M.B. Transvenous pacemaker failure induced by hyperkalemia. JAMA. 1974;228(3):336–337. [PubMed] [Google Scholar]
- 54.Cornacchia D., Fabbri M., Maresta A., Nigro P., Sorrentino F., Puglisi A., et al. Effect of steroid eluting versus conventional electrodes on propafenone induced rise in chronic ventricular pacing threshold. Pacing Clin. Electrophysiol. : PACE (Pacing Clin. Electrophysiol.) 1993;16(12):2279–2284. doi: 10.1111/j.1540-8159.1993.tb02334.x. [DOI] [PubMed] [Google Scholar]
- 55.Grande J.M., Grande A., Molina M., Novo C., Cabestrero F. Atrial selective effect of amiodarone to increase threshold of excitation. Pacing Clin. Electrophysiol. : PACE (Pacing Clin. Electrophysiol.) 2013;36(3):e93–e96. doi: 10.1111/j.1540-8159.2011.03272.x. [DOI] [PubMed] [Google Scholar]
- 56.Stevens S.K., Haffajee C.I., Naccarelli G.V., Schwartz K.M., Luceri R.M., Packer D.L., et al. Effects of oral propafenone on defibrillation and pacing thresholds in patients receiving implantable cardioverter-defibrillators. Propafenone Defibrillation Threshold Investigators. J. Am. Coll. Cardiol. 1996;28(2):418–422. doi: 10.1016/0735-1097(96)00156-8. [DOI] [PubMed] [Google Scholar]
- 57.Su L., Xia X., Liang D., Wu S., Xu L., Xu T., et al. Effects of rhythm and rate-controlling drugs in patients with permanent his-bundle pacing. Front. Cardiovasc. Med. 2020;7 doi: 10.3389/fcvm.2020.585165. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Certainly, the corresponding author is willing to furnish the required data upon appropriate inquiries.