Abstract
Quantitative analysis of Yersinia pseudotuberculosis infection of murine gut loops revealed that significantly more wild-type bacteria associated with Peyer’s patch M cells than with dome enterocytes or goblet cells. An invasin-deficient mutant was significantly attenuated for M-cell invasion, while β1 integrin expression was demonstrated in the apical membranes of M cells but not enterocytes. M-cell targeting by Yersinia pseudotuberculosis in vivo may, therefore, be mediated primarily by the interaction of invasin with cell surface β1 integrins.
The genus Yersinia contains three species that are pathogenic to humans: Y. pestis is the cause of plague, while the enteropathogenic species Y. pseudotuberculosis and Y. enterocolitica cause diseases ranging from enteritis and acute mesenteric lymphadenitis to, more rarely, septicemia (18, 30). Infection by the enteropathogenic species is initiated by ingestion of contaminated food or water. The bacteria initially infect the Peyer’s patches, lymphoid follicle colonization resulting in Peyer’s patch destruction and abscess formation (1, 6, 15, 17, 26). From this site, bacteria migrate to the mesenteric lymph nodes and may subsequently be found at more distant sites, including the liver and spleen.
A number of studies have demonstrated that the follicle-associated epithelium (FAE), which overlies the Peyer’s patch lymphoid follicles, is the primary site of intestinal epithelial invasion by the enteropathogenic Yersinia species (1, 11, 14, 17, 25). The FAE includes the specialized antigen-sampling M cells, which are exploited by a number of pathogens, including Salmonella typhimurium (8, 9, 24), as a portal for invasion (reviewed in references 10, 12, and 29), and it may therefore be hypothesized that M cells are also the primary site of Yersinia invasion. While previous studies have demonstrated invasion of mouse and rabbit M cells by the enteropathogenic Yersinia species (1, 11, 14, 27), no attempt has been made to quantify the relative contribution of FAE cell types to Yersinia invasion, primarily because most previous studies (1, 11, 14) have been based on transmission electron microscopy (TEM), which is impracticable for examination of sufficiently large areas of epithelium. The mechanisms by which the enteropathogenic Yersinia species invade the intestinal epithelium are similarly unclear. In vitro studies have demonstrated that the most efficient pathway by which these Yersinia species invade cultured cells is mediated by the bacterial protein invasin encoded by the chromosomal gene inv (21). This protein appears to play a similar role in vivo, since it has recently been found that invasin-defective mutants are attenuated for translocation across mouse FAE (27). Invasin mediates bacterial invasion of cultured cell lines by interaction with multiple β1 chain integrins on the target cell surface (19 [reviewed in references 4 and 40]). The in vivo significance of this interaction is unclear, since, paradoxically, β1 integrin expression is polarized to the basolateral membranes of villous enterocytes (2). This observation raises the possibility that M cells might express β1 integrins on their apical surface, which would permit invasin-mediated M-cell targeting by the enteropathogenic Yersinia species.
The aim of the present study was to characterize the interaction of Y. pseudotuberculosis with intestinal M cells during the early stages of infection by using a mouse gut loop model. Our initial aim was to determine, on a quantitative basis, the relative contribution of M cells to intestinal invasion by Y. pseudotuberculosis. We subsequently investigated the mechanisms by which Y. pseudotuberculosis invades the intestinal epithelium by examining, again on a quantitative basis, the interaction of an invasin-deficient mutant with intestinal M cells. Finally, we analyzed the FAE surface for β1 integrin expression. Our quantitative studies were made possible by the recent identification of M-cell-selective lectins (reviewed in reference 23) which allow us to analyze the interaction of microorganisms with extensive areas of FAE by confocal laser scanning microscopy (CLSM).
To determine the role of M cells during infection with the wild-type Y. pseudotuberculosis strain YPIII/pIB1, bacterial inocula were prepared by infecting Luria-Bertani broth with 4 × 107 bacteria/ml and incubating them for 24 h at 26°C, a temperature which promotes invasin (22) and suppresses YadA expression (reviewed in reference 39). The bacteria were then pelleted and resuspended in phosphate-buffered saline (PBS) to a final total bacterial count of 1010 bacteria/ml. These bacteria were used to infect Peyer’s patch-containing ligated jejunal or ileal gut loops which had been created in anesthetized adult female BALB/c mice as described previously (8). The gut loops were harvested after 15, 30, 60, or 120 min of incubation, and the mice were culled by cervical dislocation. Harvested tissues were rinsed in PBS and either fixed in methanol (−20°C) for at least 60 min and processed as described below or fixed in 2% glutaraldehyde (in 100 mM phosphate buffer [pH 7.4]) at 4°C for at least 120 min. Glutaraldehyde-fixed tissues were prepared for scanning electron microscopy (SEM) as described previously (7) and examined with a Cambridge S240 SEM.
Methanol-fixed tissues were processed for immunocytochemical localization of bacteria by staining with rabbit anti-Y. pseudotuberculosis antibodies (33) at 1:200 for 60 min, followed by fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit immunoglobulin G (IgG) (Sigma, Poole, United Kingdom) at 1:100 for 60 min. M cells were localized with the mouse Peyer’s patch M-cell marker Ulex europaeus agglutinin 1 (UEA1) (7), by immersion in 50 μg of tetramethylrhodamine isothiocyanate (TRITC)-conjugated UEA1 (Vector Laboratories, Peterborough, United Kingdom) per ml for 60 min. Brush cells (also known as tuft or caveolated cells [41]) are also stained by UEA1 (7) but are much smaller than other FAE cell types, while goblet cells, which are occasionally stained by UEA1 (23), are readily distinguished from M cells by their rounded shape and the variable presence of cell surface mucus. Dual-stained tissues were mounted in Vectashield mounting medium (Vector Laboratories) and examined with a Bio-Rad MRC-600 CLSM equipped with a krypton-argon mixed-gas laser.
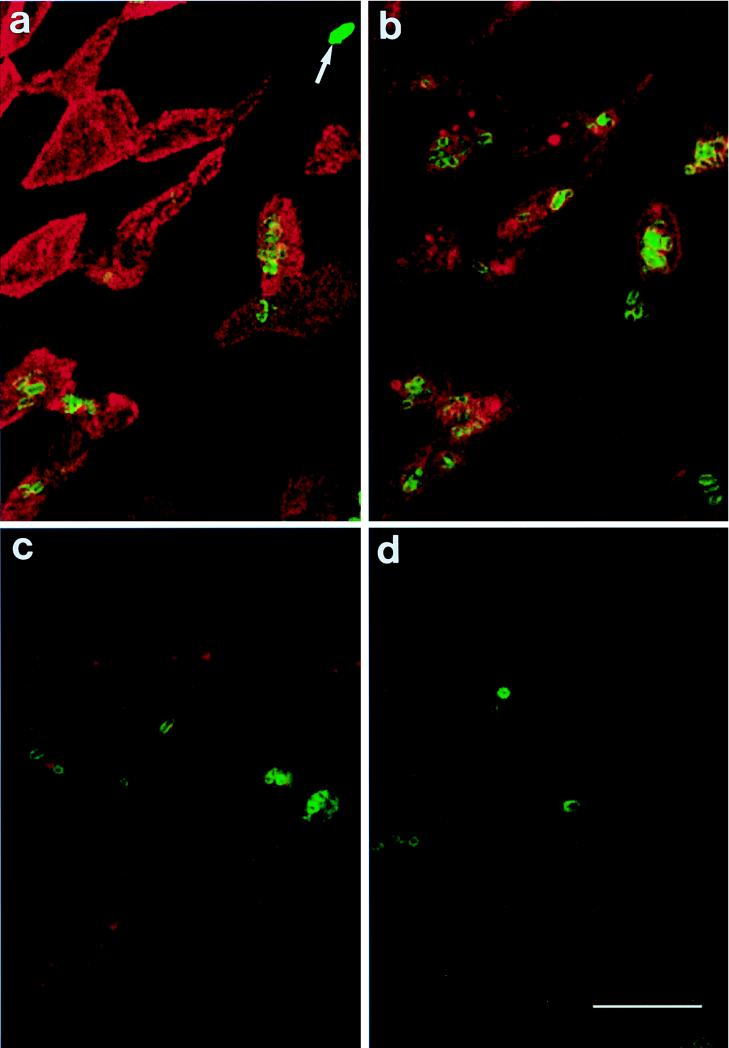
Large numbers of Y. pseudotuberculosis bacteria were observed in mucus which overlay regions of both FAE and the villi (not shown). Bacteria were rarely observed in association with villous epithelium, and they were never observed invading villous enterocytes. In contrast, large numbers of bacteria were associated with the FAE, and of these, the majority were associated with M cells (Fig. 1 and 2). M-cell-associated bacteria were located both on the M-cell surface and within the cells (Fig. 1 and 2), whereas enterocyte-associated bacteria were invariably located on the cell surface (Fig. 1). To quantify Y. pseudotuberculosis interaction with FAE cell types, Z-series of images from depths of 0 (surface) to 10 μm were collected at randomly selected areas of FAE from four discrete predetermined peripheral regions of infected domes. Areas where the FAE was obscured (e.g., by mucus) were excluded from analysis, as were bacteria located at depths greater than 10 μm below the surface, because it was impossible to determine the cell type initially invaded by these bacteria. The surface area occupied by M cells or enterocytes and goblet cells was determined by analysis of the imaged regions of UEA1-stained tissues with Bio-Rad Comos software, and statistical analyses were performed with Mann-Whitney U tests, with P values of <0.05 being considered statistically significant. Analysis of images collected from 31 domes located in six Peyer’s patches obtained from five mice revealed that after 60 min of infection, significantly more bacteria were associated per unit area of M cell (6.85 ± 2.94 bacteria/1,000 μm2 [mean ± standard error]) than per unit area of enterocyte or goblet cell (0.08 ± 0.03 bacteria/1,000 μm2). These data demonstrate that at least during the early stages of infection, M cells are the primary route by which Y. pseudotuberculosis invades the murine intestine. While we failed to observe invasion of either villous or dome epithelial enterocytes, the possibility that enterocytes may represent an alternative route of Yersinia invasion cannot be excluded, particularly during the later stages of infection.
FIG. 1.
CLSM images of mouse Peyer’s patch FAE incubated for 120 min with Y. pseudotuberculosis YPIII/pIB1 and dual stained for M cells (red) and bacteria (green). (a) Surface view. Several bacteria are adherent to M cells, and a single bacterium (arrow) is adherent to an enterocyte. At depths of 2 μm (b) and 6 μm (c), large numbers of bacteria are located within the M cells. (d) Invaded bacteria are present 16 μm below the FAE surface, at which depth the cell type initially entered cannot be determined. Bar, 10 μm.
FIG. 2.
CLSM images of mouse Peyer’s patch FAE incubated for 60 min with Y. pseudotuberculosis YPIII/pIB1 and dual stained for M cells (a and c) and bacteria (b and d). (a and b) Surface views. (c and d) Images at 1 μm in depth. Bacteria are absent from the surface of this region of FAE (a and b). Bacterial invasion (d [arrows]) is accompanied by a redistribution of UEA1 binding sites in the subapical region of the cells, observed on a confocal optical section as rings of UEA1 staining around the invading bacteria (c [arrows]). Bar, 10 μm.
While it was clear that Y. pseudotuberculosis targets M cells within the FAE, the FAE-associated bacteria were unevenly distributed between different domes and between different regions of FAE on single domes. Frequently, bacteria were concentrated on small groups of M cells scattered among regions of FAE containing M cells lacking any associated bacteria. Within the infected M cells, bacteria occurred both singly and (more frequently) as groups, some M cells containing many invaded bacteria (Fig. 1 and 2). The uneven distribution of FAE-associated Y. pseudotuberculosis resembled that observed following incubation of other bacteria, including S. typhimurium, in mouse Peyer’s patch gut loops (8, 12). At present, it is unclear whether this distribution is a consequence of variation in the accessibility of the FAE cell surfaces (bacterial migration to the FAE surface being impeded, for example, by luminal digesta or mucus secretion) or whether the distribution of FAE-associated bacteria may reflect variations in the surface properties of M cells. The frequent occurrence of M-cell invasion by multiple bacteria may indicate the existence of a cooperative binding mechanism, whereby initial bacterial adhesion promotes adhesion of further bacteria (12).
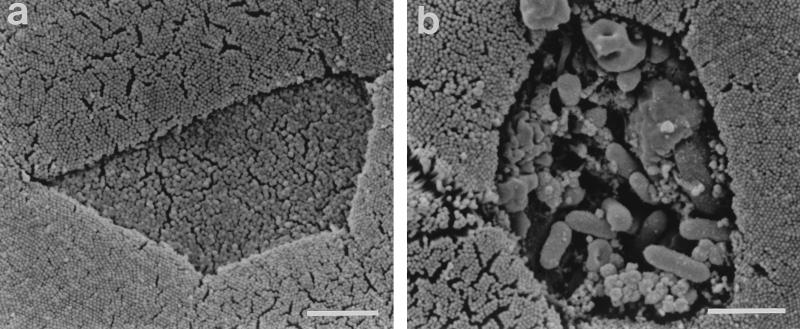
In the present study, we observed Yersinia invasion of M cells after only 15 min of incubation (not shown), although invasion was more frequent at later time points. After 120 min, M-cell invasion was extensive, and groups of bacteria were located within the lymphoid follicle tissues as deep as 16 μm below the FAE surface (Fig. 1). SEM examination revealed that the interaction of multiple Y. pseudotuberculosis bacteria with M cells was occasionally accompanied by severe disruption of the M-cell surface (Fig. 3), suggesting that heavy infection may result in M-cell death. This hypothesis is consistent with previous observations that FAE cell destruction is common at later stages of Yersinia infection (1, 15, 17). We observed evidence of M-cell destruction after only 60 min of infection, and it is therefore possible that a proportion of the more deeply invaded bacteria which we observed at this and later time points and whose precise location, whether intracellular or extracellular, was unclear may have invaded via the paracellular route. However, since M-cell destruction was relatively infrequent, invasion by the paracellular route is likely to have represented only a minor route of bacterial invasion in the current investigation.
FIG. 3.
SEM images of mouse Peyer’s patch FAE incubated for 60 min with Y. pseudotuberculosis YPIII/pIB1. Both images depict a central M cell surrounded by enterocytes (and part of a brush cell in panel b). The M cell in panel a lacks adherent bacteria and exhibits a normal surface morphology. In contrast, the association of large numbers of bacteria with the M cell in panel b is accompanied by disruption of the M-cell surface. Bars, 2 μm.
CLSM examination revealed that the interaction of Y. pseudotuberculosis with mouse M cells was accompanied by a redistribution of UEA1 binding sites around invaded bacteria located in the subapical region of the cells (Fig. 1 and 2). This UEA1 redistribution was most frequently observed in M cells invaded by multiple bacteria and may represent the membrane-bound vacuoles observed around invading bacteria by TEM (1, 11). We have previously reported redistribution of UEA1 staining around M-cell-associated S. typhimurium (8), which is also accompanied by actin redistribution (8), loss of cell surface microvilli, and formation of prominent membrane protrusions, termed membrane ruffles (8, 9, 24). The UEA1 rearrangement and actin redistribution induced by S. typhimurium are thought to correspond to membrane ruffle formation. SEM examination of tissues infected with Y. pseudotuberculosis for up to 120 min demonstrated that this bacterium failed to induce M-cell membrane ruffle formation, which was mirrored by its failure, in contrast to S. typhimurium (8), to induce prominent changes in actin distribution. Together, these observations suggest that the UEA1 rearrangement induced by Y. pseudotuberculosis is distinct from that induced by S. typhimurium and that M-cell invasion by these enteropathogens proceeds by different mechanisms.
Comparison of M cells with other cell types suggests that M cells are unusual in their ability to support invasion by large numbers of Y. pseudotuberculosis bacteria, the Yersinia proteins YopE and YopH mediating resistance to phagocytosis by professional phagocytes (33, 35) and cultured epithelial cells (5). The widespread bacterial invasion of M cells observed in the present study and our failure to consistently identify Yersinia-induced M-cell death or actin redistribution suggest either that the cytotoxic activities of these proteins (35) and the actin depolymerizing activity of YopE (36) are expressed only at very low levels under the conditions employed in the current investigation, as might be predicted at our bacterial culture temperature of 26°C (39), or that M cells are resistant to the effects of these proteins.
The bacterial gene-encoded protein invasin mediates the most efficient route by which the enteropathogenic Yersinia species invade cultured cells (reviewed in references 4 and 40), and it has recently been demonstrated that this protein also plays a role in bacterial translocation across mouse Peyer’s patch FAE (27). To investigate further the in vivo role of invasin and to determine its contribution to M-cell-selective uptake of Y. pseudotuberculosis, we tested an invasin-deficient mutant (inv) SF104 (16) in our mouse model of infection. Parental wild-type YPIII/pIB1 and inv mutant SF104 bacteria were grown under conditions which promote invasin expression (22) as described above, with the addition of 20 μg of kanamycin per ml to the SF104 culture and inoculum media. Mouse gut loops were infected with the bacterial preparations (as above), incubated for 60 min, and then harvested, fixed in methanol, and stained for bacteria and M cells. Z-series of images were collected at four randomly selected, predetermined regions of FAE overlying each infected dome, and bacterial interaction was quantified. Examination of SF104-infected tissues revealed that in common with the wild-type strain YPIII/pIB1, the inv mutant selectively associated with intestinal M cells; extremely few SF104 bacteria were observed in association with enterocytes. Analysis of seven Peyer’s patches from five mice infected with wild-type YPIII/pIB1 and six mice infected with inv mutant SF104 revealed that after 60 min of incubation, significantly fewer SF104 bacteria were associated with the intestinal M cells than the parental wild-type strain YPIII/pIB1 (Table 1). Since the lectin UEA1 accurately localizes on M-cell surfaces, CLSM examination of infected FAE permitted the M-cell-associated bacteria to be classified as either adherent to the cell surface or internalized, the latter bacteria being defined as those at least 2 μm below the UEA1-stained cell surface. These analyses suggested that the inv mutant is attenuated for both adhesion to and invasion of mouse intestinal M cells. After a 60-min infection period, significantly fewer SF104 bacteria were adherent to the M cells than those of the parental wild-type strain, and significantly fewer SF104 bacteria had invaded the M cells compared with the parental wild-type bacteria (Table 1). It is clear that the reduction in bacterial adhesion exhibited by the inv mutant could account only in part for the observed attenuation in invasion, since the attenuation in invasion (approximately 30-fold) exceeded the attenuation in adhesion (approximately 5-fold). Consequently, a lower proportion of M-cell-associated inv mutant bacteria had invaded the M cells than was the case for the wild-type strain (25% compared to 68%), emphasizing that the decrease in bacterial invasion is not solely due to a decrease in adhesion and that invasin mediates both adhesion to and invasion of intestinal M cells. This proposal is consistent with the ability of invasin to mediate both adhesion to and invasion of cultured epithelial cells (21). In addition, using a mutant expressing an uptake-defective invasin protein which retains considerable receptor binding activity, Marra and Isberg (27) have similarly demonstrated that invasin promotes bacterial translocation across mouse FAE.
TABLE 1.
Association of Y. pseudotuberculosis wild-type and inv mutant strains with Peyer’s patch M cells after 60 min of incubation in mouse gut loops
| Strain | Phenotype | No. of M-cell-associated bacteria/1,000 μm2a
|
||
|---|---|---|---|---|
| Total | Adherent | Invaded | ||
| YPIII/pIB1 | Wild type | 8.06 ± 4.72 | 2.58 ± 0.96 | 5.48 ± 3.80 |
| SF104 | Invasin-defective mutant | 0.72 ± 0.17*b | 0.54 ± 0.16* | 0.18 ± 0.06* |
All values are expressed as means ± standard errors (n = 7 Peyer’s patches).
*, Significantly different (P < 0.05; Mann-Whitney U test) from wild-type values.
We have demonstrated that at least during the early stages of infection, intestinal invasion by Y. pseudotuberculosis is primarily mediated by invasin-dependent invasion of intestinal M cells. This observation supports and extends the findings of the recent study performed by Marra and Isberg (27) with nonquantitative microscopic techniques, which demonstrated that wild-type Y. pseudotuberculosis bacteria colocalize with Peyer’s patch M cells and that inv mutants are defective for translocation across mouse Peyer’s patch FAE. Our observations are also consistent with previous studies demonstrating that the inv mutation delays the intestinal translocation of orally administered enteropathogenic Yersinia species (31, 32, 34) and that inv insertion into an attenuated aroA mutant of S. typhimurium results in increased bacterial translocation from the gut lumen to the mesenteric lymph nodes, while oral administration with this recombinant Salmonella strain inhibits intestinal translocation of Y. pseudotuberculosis (38).
In the present study, we have also demonstrated that the inv mutant retained the capacity, albeit at a very reduced frequency, to invade intestinal M cells. It is thus clear that invasin-independent routes of invasion also operate in vivo. This is consistent with the observations that orally administered inv mutants readily kill mice (31, 32, 34) and that an inv yadA mutant efficiently translocates across mouse intestinal FAE (27). Interestingly, Marra and Isberg (27) demonstrated that an inv yadA double mutant was more invasive in vivo than the single inv mutant and proposed that invasion by the single inv mutant was restricted by YadA-mediated adhesion to intestinal mucus. This proposal is not inconsistent with our own observation that large numbers of both wild-type and inv mutant bacteria were entrapped within mucus. In contrast to the present study, Marra and Isberg (27) failed to detect translocation of inv mutant bacteria across mouse FAE, despite demonstrating low levels of Peyer’s patch colonization following oral administration of these bacteria. We suggest these apparent discrepancies may have resulted from the use of tissue sections to screen for invading bacteria (27), a technique which is likely to be less sensitive for detecting low numbers of invading bacteria than our own whole-tissue technique, although it is also possible that the bacteria may invade at alternative sites in the gastrointestinal tract (27, 31). Invasin-independent routes of intestinal invasion may be mediated by the alternative proteins which mediate the interaction of Y. pseudotuberculosis with cultured cells, namely the protein YadA encoded by a plasmid-carried gene or possibly the pH 6 antigen encoded by the chromosomal gene psaA (reviewed in references 4 and 40). Since both inv yadA and psa mutants readily invade the FAE (27), M-cell invasion by Y. pseudotuberculosis may also proceed by mechanisms independent of those previously defined in cultured cells. Future studies may reveal that different proteins encoded by the enteropathogenic Yersinia species mediate invasion of different intestinal cell types or are active at different stages in the infective process.
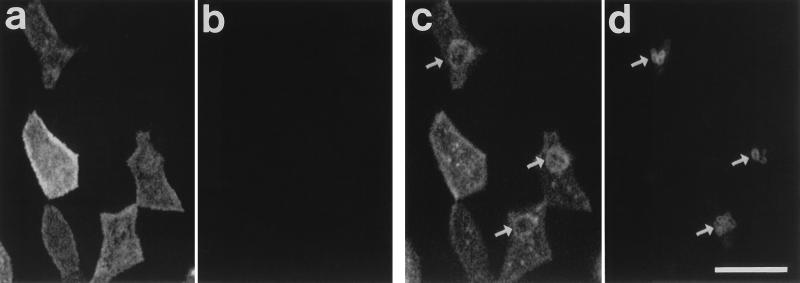
Since in vitro studies have demonstrated that multiple β1 chain cell surface integrins are responsible for invasin-mediated interaction of enteropathogenic Yersinia species with cultured epithelial cells (19), we investigated β1 integrin expression by mouse FAE. Mouse Peyer’s patches were obtained from adult female BALB/c mice and fixed in methanol. After fixation, the tissues were incubated in PBS supplemented with 3% normal rabbit serum (NRS) for 30 min at room temperature, followed by incubation in 20 μg of rat IgG2a anti-mouse β1 integrin monoclonal antibodies (Novus Molecular, Inc., San Diego, Calif.) per ml in PBS–3% NRS for 120 min at 4°C. After being rinsed in PBS, the tissues were then incubated for 60 min at room temperature in 5 μg of biotin-conjugated mouse-adsorbed rabbit anti-rat IgG (Vector Laboratories) per ml in 2% normal mouse serum, rinsed again in PBS, and incubated for a further 60 min at room temperature in 10 μg of FITC-conjugated goat anti-biotin (Vector Laboratories) per ml in 3% normal goat serum. M cells were subsequently localized by a 60-min immersion in 50 μg of TRITC-UEA1 per ml, and the stained tissues were mounted on slides and examined by CLSM as described above.
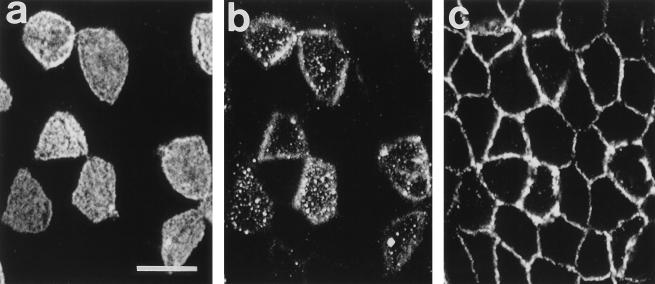
Examination of stained villi revealed that, consistent with previous observations (2), β1 integrins are strongly expressed in the lateral membranes of enterocytes but are undetectable at the apical surfaces. Enterocytes within the FAE exhibited a similar pattern of β1 integrin expression, whereas M cells expressed β1 integrins in both their apical and lateral membranes (Fig. 4). Cell surface expression of β1 integrins clearly distinguished M cells from enterocytes, with the majority of M cells exhibiting a granular pattern of apical membrane staining (Fig. 4b). Surface staining of β1 integrins on the majority of M cells was of moderate intensity, although occasional M cells exhibited significantly brighter levels of staining (not shown). Control tissues in which the anti-mouse β1 integrin antibodies were omitted from the primary staining solution or replaced by 20 μg of rat IgG2a anti-mouse β2 integrin monoclonal antibodies (Chemicon International, Inc., Temecula, Calif.) per ml failed to exhibit similar patterns of FITC staining.
FIG. 4.
CLSM images of mouse Peyer’s patch FAE dual stained for M cells (a) and β1 integrins (b and c). (a and b) Surface views. UEA1-stained M cells express β1 integrins in their apical membranes (stained cells in panels a and b), whereas β1 integrin expression is absent from the apical membranes of enterocytes (unstained cells in panels a and b). (c) At a depth of 2 μm, both M cells and enterocytes express β1 integrins in their lateral membranes. Bar, 10 μm.
We have demonstrated that M cells may be differentiated from enterocytes by their surface expression of β1 integrins and that inv mutants are attenuated for M-cell invasion. Together with previous studies which have demonstrated that invasin mediates Y. pseudotuberculosis association with cultured cells by interaction with cell surface β1 integrins (reviewed in references 4 and 40), our findings suggest that targeting of Y. pseudotuberculosis to mouse intestinal M cells is mediated by the specific interaction of invasin with M-cell surface β1 integrins. Rigorous testing of this hypothesis is outside the scope of the current investigation, but must clearly be addressed in future studies.
Interestingly YadA, which mediates invasion of cultured cells by inv mutants (5, 42), may also interact (possibly indirectly) with cell surface β1 integrins (5). It is, therefore, possible that M-cell surface β1 integrin expression may be responsible for both putative invasin- and YadA-mediated interactions of enteropathogenic Yersinia species with intestinal M cells. While it is probable that cell surface β1 integrins are responsible for the targeting of Y. pseudotuberculosis to intestinal M cells, the β1 integrins expressed in the basolateral membranes of enterocytes may also play a role in infection by the enteropathogenic Yersinia species. Basolateral membrane β1 integrins might permit lateral cell-to-cell spread of bacteria which have initially invaded through the M-cell route, or they may become exposed to luminal bacteria following M-cell destruction or neutrophil transmigration (28). Such mechanisms may contribute to the extensive FAE destruction induced by the enteropathogenic Yersinia species during the later stages of infection (1, 15, 17).
Our finding that M cells are the primary cell type invaded by Y. pseudotuberculosis supports the hypothesis that M cells are a major site of invasion by a diverse range of pathogens (reviewed in references 10, 12, and 29). Indeed, while the antigen-sampling function of M cells is a vital component of the mucosal immune system, it is becoming increasingly clear that M cells also represent a weak point of the intestinal epithelial barrier, a wide range of pathogens selectively invading these cells. The mechanisms by which these microorganisms selectively target and invade M cells remain obscure, although recent studies analyzing the surface properties of M cells have brought us nearer to identifying them. Previous studies have demonstrated that M cells may be distinguished from enterocytes by their differential expression of cell surface carbohydrates (reviewed in reference 23), and in the present study, we have demonstrated that mouse Peyer’s patch M cells may also be identified by their surface expression of β1 integrins. Microorganisms frequently use cell surface carbohydrates as receptors (reviewed in reference 37), and a range of microorganisms in addition to the enteropathogenic Yersinia species interact with cell surface integrins (reviewed in references 3 and 20). Both cell surface glycoconjugates and β1 integrins are, therefore, potential candidates as M-cell receptors for microorganisms. Species- and site-related variations in M-cell surface glycoconjugate expression (reviewed in reference 23) may contribute to the selectivity of microorganism infectivity. In addition, the existence of distinct M-cell subsets, as demonstrated by heterogeneity in their surface glycoconjugate expression (13), may account for the uneven distribution of FAE-associated bacteria observed in this and previous studies. While in the present study we observed some evidence of variation in the intensity of M-cell surface β1 integrin staining, it will be interesting to determine if M-cell surface β1 integrin expression also exhibits site- and species-related variations.
Identification of the mechanisms by which microorganisms target M cells must now be considered a priority area for future M-cell research. Such studies may ultimately permit the development of effective disease control strategies based on the prevention of M-cell invasion. In addition, by targeting vaccines and drugs to the M-cell surfaces, it may be possible to exploit the vast transcytotic capacity of these specialized cells for the effective delivery of orally administered vaccines and drugs.
Acknowledgments
This work was supported by a Wellcome Trust Veterinary Research Fellowship (041573/Z/94/Z) awarded to M.A.C. and a Medical Research Council grant (G9405434) awarded to B.H.H.
We are grateful to H. Wolf-Watz, M. Fällman, and R. Rosqvist, Umeå University, Sweden, for supplying Y. pseudotuberculosis bacteria and anti-Y. pseudotuberculosis antibodies; and V. L. Miller, Washington University, St. Louis, Mo., for allowing us access to Y. pseudotuberculosis SF104. We also thank T. A. Booth, Biomedical Electron Microscopy Unit, University of Newcastle upon Tyne, for assistance with electron microscopy.
REFERENCES
- 1.Autenrieth I B, Firsching R. Penetration of M cells and destruction of Peyer’s patches by Yersinia enterocolitica: an ultrastructural and histological study. J Med Microbiol. 1996;44:285–294. doi: 10.1099/00222615-44-4-285. [DOI] [PubMed] [Google Scholar]
- 2.Beaulieu J-F. Differential expression of the VLA family of integrins along the crypt-villus axis in the human small intestine. J Cell Sci. 1992;102:427–436. doi: 10.1242/jcs.102.3.427. [DOI] [PubMed] [Google Scholar]
- 3.Bergelson J M, Finberg R W. Integrins as receptors for virus attachment and cell entry. Trends Microbiol. 1993;1:287–288. doi: 10.1016/0966-842x(93)90003-a. [DOI] [PubMed] [Google Scholar]
- 4.Bliska J B, Falkow S. Interplay between determinants of cellular entry and cellular disruption in the enteropathogenic Yersinia. Curr Opin Infect Dis. 1994;7:323–328. [Google Scholar]
- 5.Bliska J B, Copass M C, Falkow S. The Yersinia pseudotuberculosis adhesin YadA mediates intimate bacterial attachment to and entry into HEp-2 cells. Infect Immun. 1993;61:3914–3921. doi: 10.1128/iai.61.9.3914-3921.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carter P B. Pathogenicity of Yersinia enterocolitica for mice. Infect Immun. 1975;11:164–170. doi: 10.1128/iai.11.1.164-170.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clark M A, Jepson M A, Simmons N L, Booth T A, Hirst B H. Differential expression of lectin-binding sites defines mouse intestinal M cells. J Histochem Cytochem. 1993;41:1679–1687. doi: 10.1177/41.11.7691933. [DOI] [PubMed] [Google Scholar]
- 8.Clark M A, Jepson M A, Simmons N L, Hirst B H. Preferential interaction of Salmonella typhimurium with mouse Peyer’s patch M cells. Res Microbiol. 1994;145:543–553. doi: 10.1016/0923-2508(94)90031-0. [DOI] [PubMed] [Google Scholar]
- 9.Clark M A, Reed K A, Lodge J, Stephen J, Hirst B H, Jepson M A. Invasion of murine intestinal M cells by Salmonella typhimurium inv mutants severely deficient for invasion of cultured cells. Infect Immun. 1996;64:4363–4368. doi: 10.1128/iai.64.10.4363-4368.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fujimura Y, Owen R L. M cells as portals of infection: clinical and pathophysiological aspects. Infect Agents Dis. 1996;5:144–156. [PubMed] [Google Scholar]
- 11.Fujimura Y, Kihara T, Mine H. Membranous cells as a portal of Yersinia pseudotuberculosis entry into rabbit ileum. J Clin Electron Microsc. 1992;25:35–45. [Google Scholar]
- 12.Giannasca P J, Neutra M R. Interactions of microorganisms with intestinal M cells: mucosal invasion and induction of secretory immunity. Infect Agents Dis. 1993;2:242–248. [PubMed] [Google Scholar]
- 13.Giannasca P J, Giannasca K T, Falk P, Gordon J I, Neutra M R. Regional differences in glycoconjugates of intestinal M cells in mice: potential targets for mucosal vaccines. Am J Physiol. 1994;267:G1108–G1121. doi: 10.1152/ajpgi.1994.267.6.G1108. [DOI] [PubMed] [Google Scholar]
- 14.Grützkau A, Hanski C, Hahn H, Riecken E O. Involvement of M cells in the bacterial invasion of Peyer’s patches: a common mechanism shared by Yersinia enterocolitica and other enteroinvasive bacteria. Gut. 1990;31:1011–1015. doi: 10.1136/gut.31.9.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grützkau A, Hanski C, Neumann M. Comparative study of histopathological alterations during intestinal infection of mice with pathogenic and nonpathogenic strains of Yersinia enterocolitica serotype O:8. Virchows Arch A. 1993;423:97–103. doi: 10.1007/BF01606583. [DOI] [PubMed] [Google Scholar]
- 16.Han Y W, Miller V L. Reevaluation of the virulence phenotype of the inv yadA double mutants of Yersinia pseudotuberculosis. Infect Immun. 1997;65:327–330. doi: 10.1128/iai.65.1.327-330.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanski C, Kutschka U, Schmoranzer H P, Naumann M, Stallmach A, Hahn H, Menge H, Riecken E O. Immunohistochemical and electron microscopic study of interaction of Yersinia enterocolitica serotype O8 with intestinal mucosa during experimental enteritis. Infect Immun. 1989;57:673–678. doi: 10.1128/iai.57.3.673-678.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hubbert W T, Petenyi C W, Glasgow L A, Uyeda C T, Creighton S A. Yersinia pseudotuberculosis infection in the United States. Septicaemia, appendicitis, and mesenteric lymphadenitis. Am J Trop Med Hyg. 1971;20:679–684. doi: 10.4269/ajtmh.1971.20.679. [DOI] [PubMed] [Google Scholar]
- 19.Isberg R R, Leong J M. Multiple β1 chain integrins are receptors for invasin, a protein that promotes bacterial penetration into mammalian cells. Cell. 1990;60:861–871. doi: 10.1016/0092-8674(90)90099-z. [DOI] [PubMed] [Google Scholar]
- 20.Isberg R R, Tran Van Nhieu G. Binding and internalization of microorganisms by integrin receptors. Trends Microbiol. 1994;2:10–14. doi: 10.1016/0966-842x(94)90338-7. . (Review.) [DOI] [PubMed] [Google Scholar]
- 21.Isberg R R, Voorhis D L, Falkow S. Identification of invasin: a protein that allows enteric bacteria to penetrate cultured mammalian cells. Cell. 1987;50:769–778. doi: 10.1016/0092-8674(87)90335-7. [DOI] [PubMed] [Google Scholar]
- 22.Isberg R R, Swain A, Falkow S. Analysis of expression and thermoregulation of the Yersinia pseudotuberculosis inv gene with hybrid proteins. Infect Immun. 1988;56:2133–2138. doi: 10.1128/iai.56.8.2133-2138.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jepson M A, Clark M A, Foster N, Mason C M, Bennett M K, Simmons N L, Hirst B H. Targeting to intestinal M cells. J Anat. 1996;189:507–516. [PMC free article] [PubMed] [Google Scholar]
- 24.Jones B D, Ghori N, Falkow S. Salmonella typhimurium initiates murine infection by penetrating and destroying the specialised epithelial M cells of the Peyer’s patches. J Exp Med. 1994;180:15–23. doi: 10.1084/jem.180.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaneko K, Uehara K, Ogawa M. Uptake of killed Yersinia enterocolitica cells by epithelial cells in the Peyer’s patches of mice. Contrib Microbiol Immunol. 1991;12:156–158. [PubMed] [Google Scholar]
- 26.Lian C-J, Hwang W S, Kelly J K, Pai C H. Invasiveness of Yersinia enterocolitica lacking the virulence plasmid: an in-vivo study. J Med Microbiol. 1987;24:219–226. doi: 10.1099/00222615-24-3-219. [DOI] [PubMed] [Google Scholar]
- 27.Marra A, Isberg R R. Invasin-dependent and invasin-independent pathways for translocation of Yersinia pseudotuberculosis across the Peyer’s patch intestinal epithelium. Infect Immun. 1997;65:3412–3421. doi: 10.1128/iai.65.8.3412-3421.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCormick B A, Nusrat A, Parkos C A, D’Andrea L, Hofman P M, Carnes D, Liang T W, Madara J L. Unmasking of intestinal epithelial lateral membrane β1 integrin consequent to transepithelial neutrophil migration in vitro facilitates inv-mediated invasion by Yersinia pseudotuberculosis. Infect Immun. 1997;65:1414–1421. doi: 10.1128/iai.65.4.1414-1421.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neutra M R, Pringault E, Kraehenbuhl J-P. Antigen sampling across epithelial barriers and induction of mucosal immune responses. Annu Rev Immunol. 1996;14:275–300. doi: 10.1146/annurev.immunol.14.1.275. [DOI] [PubMed] [Google Scholar]
- 30.Paff J R, Triplett D A, Saari T N. Clinical and laboratory aspects of Yersinia pseudotuberculosis infections, with a report of two cases. Am J Clin Pathol. 1976;66:101–110. doi: 10.1093/ajcp/66.1.101. [DOI] [PubMed] [Google Scholar]
- 31.Pepe J C, Miller V L. Yersinia enterocolitica invasin: a primary role in the initiation of infection. Proc Natl Acad Sci USA. 1993;90:6473–6477. doi: 10.1073/pnas.90.14.6473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pepe J C, Wachtel M R, Wagar E, Miller V L. Pathogenesis of defined invasion mutants of Yersinia enterocolitica in a BALB/c mouse model of infection. Infect Immun. 1995;63:4837–4848. doi: 10.1128/iai.63.12.4837-4848.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosqvist R, Bölin I, Wolf-Watz H. Inhibition of phagocytosis in Yersinia pseudotuberculosis: a virulence plasmid-encoded ability involving the Yop2b protein. Infect Immun. 1988;56:2139–2143. doi: 10.1128/iai.56.8.2139-2143.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosqvist R, Skurnik M, Wolf-Watz H. Increased virulence of Yersinia pseudotuberculosis by two independent mutations. Nature. 1988;334:522–525. doi: 10.1038/334522a0. [DOI] [PubMed] [Google Scholar]
- 35.Rosqvist R, Forsberg Å, Rimpiläinen M, Bergman T, Wolf-Watz H. The cytotoxic protein YopE of Yersinia obstructs the primary host defence. Mol Microbiol. 1990;4:657–667. doi: 10.1111/j.1365-2958.1990.tb00635.x. [DOI] [PubMed] [Google Scholar]
- 36.Rosqvist R, Forsberg Å, Wolf-Watz H. Intracellular targeting of the Yersinia YopE cytotoxin in mammalian cells induces actin microfilament disruption. Infect Immun. 1991;59:4562–4569. doi: 10.1128/iai.59.12.4562-4569.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sharon N, Lis H. Lectins as cell recognition molecules. Science. 1989;246:227–234. doi: 10.1126/science.2552581. [DOI] [PubMed] [Google Scholar]
- 38.Simonet M, Fortineau N, Beretti J L, Berche P. Immunization with live aroA recombinant Salmonella typhimurium producing invasin inhibits intestinal translocation of Yersinia pseudotuberculosis. Infect Immun. 1994;62:863–867. doi: 10.1128/iai.62.3.863-867.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Straley S C, Perry R D. Environmental modulation of gene expression and pathogenesis in Yersinia. Trends Microbiol. 1995;3:310–317. doi: 10.1016/s0966-842x(00)88960-x. [DOI] [PubMed] [Google Scholar]
- 40.Straley S C, Skrzypek E, Plano G V, Bliska J B. Yops of Yersinia spp. pathogenic for humans. Infect Immun. 1993;61:3105–3110. doi: 10.1128/iai.61.8.3105-3110.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Trier J S, Allan C H, Marcial M A, Madara J L. Structural features of the apical and tubulovesicular membranes of rodent small intestinal tuft cells. Anat Rec. 1987;219:69–77. doi: 10.1002/ar.1092190112. [DOI] [PubMed] [Google Scholar]
- 42.Yang Y, Isberg R R. Cellular internalization in the absence of invasin expression is promoted by the Yersinia pseudotuberculosis yadA product. Infect Immun. 1993;61:3907–3913. doi: 10.1128/iai.61.9.3907-3913.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]