Highlights
-
•
IGEs’ low exercise levels were associated with altered cortical folding and atrophy.
-
•
Increased cerebellar GMV was associated with greater mood disturbance in IGE.
-
•
GMV was positively associated with both exercise levels and cognition in IGE.
-
•
Self-reported exercise levels may highlight neuromorphometric differences in IGE.
Keywords: Hippocampus, Grey matter volume, Exercise levels, Idiopathic generalized epilepsy, Mood, Cognition
Abstract
Regular physical activity may promote beneficial neuroplasticity, e.g., increased hippocampus volume. However, it is unclear whether self-reported physical exercise in leisure (PEL) levels are associated with the brain structure features demonstrated by exercise interventions. This pilot study investigated the relationship between PEL, mood, cognition, and neuromorphometry in patients with idiopathic generalized epilepsy (IGEs) compared to healthy controls (HCs). Seventeen IGEs and 19 age- and sex-matched HCs underwent magnetic resonance imaging (MRI) at 3T. The Baecke Questionnaire of Habitual Physical Activity, Profile of Mood States, and Montreal Cognitive Assessment (MoCA) assessed PEL, mood, and cognition, respectively. Structural MRI data were analyzed by voxel- and surface-based morphometry. IGEs had significantly lower PEL (p < 0.001), poorer mood (p = 0.029), and lower MoCA scores (p = 0.027) than HCs. These group differences were associated with reduced volume, decreased gyrification, and altered surface topology (IGEs < HCs) in frontal, temporal and cerebellar regions involved in executive function, memory retrieval, and emotional regulation, respectively.
These preliminary results support the notion that increased PEL may promote neuroplasticity in IGEs, thus emphasizing the role of physical activity in promoting brain health in people with epilepsy.
Introduction
Epilepsy is a neurological disorder characterized by a predisposition to generate spontaneous, unprovoked seizures, and affects 70–85 million people worldwide [1], [2]. In the United States, up to 50 % of the 3.4 million patients with epilepsy experience significant cognitive impairment [2], [3], [4], [5], [6], [7]. Depression and other mood disorders are common epilepsy-related comorbidities (24.4 % lifetime prevalence), and are bidirectionally related to cognitive declines [8], [9], [10]. Anti-seizure medications (ASMs) and, when indicated, neuromodulation (e.g., vagus nerve stimulation) can help achieve seizure freedom in many patients [11], [12], [13]. Given the limitations of these interventions, researchers have started investigating the role of lifestyle factors, such as increased physical activity and exercise, in improving seizure control and symptoms of epilepsy-related co-morbidities (e.g., cognitive impairment). Although exercise-induced improvements in brain function and structure are well-documented in healthy adults, such research in epilepsy populations is in its infancy [14], [15], [16]. This gap stems from the fact that, historically, epilepsy patients have typically been advised to avoid exercise due to the prevailing misconception that exercise may be unsafe and/or seizure-inducing. Unfortunately, this belief has limited research on exercise in epilepsy [16], [17]. However, in 2016, the International League Against Epilepsy provided guidance on the safety and benefits of exercise for patients with epilepsy [18], and a recent systematic review and meta-analysis provides strong evidence that exercise is not seizure inducing and improves quality of life in epilepsy patients [19].
Intervention studies have demonstrated the safety and benefits of regular exercise for adults and children with epilepsy [20], [21], [22], [23], [24], [25]. However, to date, only five exercise intervention studies in epilepsy patients have specifically examined the effects of exercise on neurocognitive function [20], [21], [22], [23], [24], [25]. All five studies found post-intervention improvements in overall cognitive functioning, verbal learning and memory, attention, verbal fluency, and executive functioning. One cross-sectional study showed low levels of self-reported physical activity in a group of adults with idiopathic generalized epilepsy (IGEs) and healthy controls (HCs) were associated with worse neurocognitive performance [26]. Another cross-sectional study of epilepsy patients with different activity levels showed physical activity levels were inversely associated with depression and anxiety symptoms [27]. Additionally, epilepsy patients who exercised also experienced a significant improvement in quality of life and well-being [23], [24], [28]. Most importantly, these investigations demonstrated that consistent exercise engagement can potentially improve seizure frequency and severity [20], [21], [22], [23], [24], [25].
Few studies have directly explored the link between physical activity and cognition in epilepsy patients, and even fewer have used neuroimaging methods to visualize the neural correlates of exercise-induced improvements [16], [19], [29]. Two of these studies demonstrated the association between post-intervention neurocognitive and functional connectivity changes in the brain [21], [22]. For example, Koirala and colleagues found post-intervention changes in EEG-based functional connectivity corresponded to improvements in neuropsychological functions for 8 epilepsy patients who participated in a 5-week exercise intervention [21]. Similarly, Allendorfer et al. applied resting-state functional magnetic resonance imaging (fMRI) to investigate these relationships, and discovered post-intervention verbal memory improvements were associated with functional connectivity changes in cortical and subcortical brain regions [22]. Overall, the limited but growing body of findings suggests that physical exercise can enhance neurocognitive status, mood, and seizure control in people with epilepsy, and may do so by altering the brain’s function and structure.
Still, neuroimaging studies on exercise-associated changes in epilepsy patients have primarily focused on measuring changes in functional measures before and after an exercise intervention. To date, no previous study has examined whether epilepsy patients’ self-reported recreational activity levels are associated with the neuromorphometric properties linked to higher levels of physical activity in other studies of healthy adults [30], [31], [32], [33]. To address these gaps, this pilot study aimed to investigate the preliminary relationships between neuromorphometric measures (volume- and surface-based), self-reported physical exercise in leisure (PEL), and measures of cognition and mood in IGEs compared to age- and sex-matched HCs. We focus on patients with IGE because they experience a broad range of impairments, regardless of their seizure control status [34], [35], [36], [37], [38], [39], [40]. Additionally, unlike patients with focal epilepsies, patients with IGE do not have seizures originating from a specific brain region (e.g. due to brain injury or abnormality), and therefore typically have structural MRIs without macroscopic damage. These characteristics make the IGE patient population especially well-suited for a study investigating the relationships between neuromorphometry and mood, cognition, and exercise levels in a relatively homogeneous sample of epilepsy patients. We hypothesized that lower PEL levels in IGEs would be associated with decreased grey matter volume (GMV) and altered surface topology, particularly in brain regions that comprise the seizure initiation and propagation network for generalized seizures (i.e. hippocampi, anterior and posterior cingulate, thalamus, bilateral cerebellum, and inferior parietal lobules) [22].
Materials and methods
Study design and participant recruitment
This cross-sectional imaging study recruited participants into two groups: 1) people with IGE, and 2) age- and sex-matched HCs. Unlike focal seizures, which originate in a specific area of the brain, generalized seizures are more diffuse and are related to more widespread structural and functional dysfunction [41], [42]. People with IGE typically have normal brain structure as determined by standard clinical magnetic resonance imaging (MRI), making it difficult to identify the cause of their seizures [42]. Due to the generalized onset of seizures and the corresponding lack of macroscopic MRI abnormalities, IGE participants are an ideal epilepsy patient population for neuromorphometric studies.
Following electronic medical record reviews and physician referrals, adults with clinically-confirmed IGE were contacted by phone or approached in person for potential interest in study participation. If interested, potential participants were screened for study eligibility. HCs were recruited through word of mouth from the general community. For all participants, inclusion criteria were: 1) age of 18 years or older, 2) ability to undergo magnetic resonance imaging (MRI) at 3-Tesla, 3) a negative urine pregnancy test if female of childbearing potential, 4) fluent in the English language, and 5) able to provide written informed consent. Epilepsy patients were included if they had a diagnosis of IGE as determined by a board-certified epilepsy specialist. For all participants, exclusion criteria included: 1) underlying degenerative or metabolic disorders, 2) abnormal general or neurological examination, 3) self-reported alcohol or drug abuse, 4) presence of non-epileptic seizures, 5) documented or self-reported suicidal ideation in the last 3 months, and 6) history of special education or intellectual disability. HCs were excluded if previous medical history included neurological disease or injury, or self-reported previous diagnosis of depression or other psychiatric disorders.
Eligible participants were provided a copy of the informed consent form for review and scheduled for a study session. Participants provided written informed consent prior to study participation. All study procedures were approved by the UAB Institutional Review Board and carried out in accordance with the Declaration of Helsinki ethics principles.
Study visits
A study visit consisted of administering assessments and collecting MRI data at the UAB Civitan International Neuroimaging Laboratory (CINL). Basic demographic data (i.e. sex, age, body mass index (BMI), and years of education) and information regarding clinical characteristics of IGEs (i.e. age of epilepsy onset, seizure frequency and number of current ASMs) were collected. Participants also completed assessments that measured physical activity levels, general cognition, and mood.
Study measures
Physical activity levels
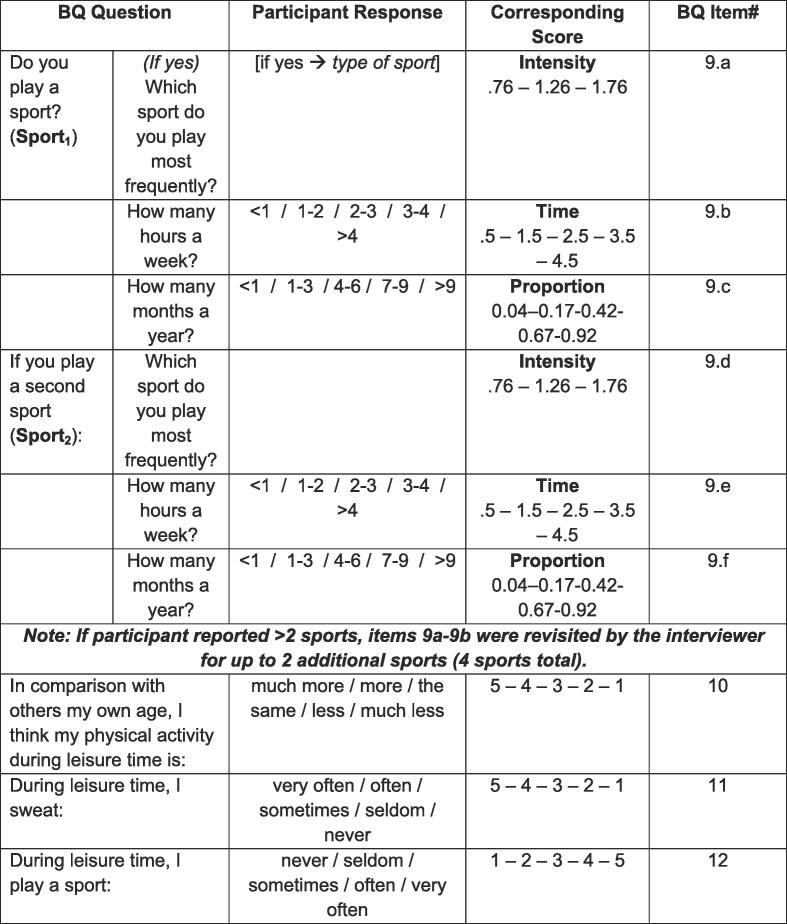
Self-reported physical activity levels were evaluated using the Baecke Questionnaire of Habitual Physical Activity [43]. The Baecke Questionnaire is a validated, reliable, and straight-forward assessment that fragments physical activity across 3 dimensions: work, sports/leisure time exercise, and leisure locomotion. Since the physical exercise in leisure (PEL) score assesses the frequency, intensity, and aerobic quality of sports and high-intensity exercises and has been shown to be correlated with records of physical exercise in the past 12 months, the PEL was the primary outcome of interest (see Fig. 1) [44], [45]. Work and leisure locomotion scores were not calculated due to emphasis on work-related exertion and low-intensity physical activity (e.g., walking), respectively.
Fig. 1.
Physical Exercise in Leisure (PEL) Score Calculation. Participants’ PEL scores were calculated based on responses to the tabulated Baecke Questionnaire of Habitual Physical Activity (BQ) items (Baecke et al 1982). For all responses, lower values (e.g., closer to 0) indicated less frequent or less intense physical activity, while higher values (e.g., closer to 5) indicated more frequent or more intense physical activity.
Cognition and mood
The original version of the Montreal Cognitive Assessment (MoCA, 7.1) was chosen to measure general neurocognitive functioning due to its documented sensitivity for detecting cognitive impairments in PWE who fall within the normal range on tests such as the Mini-Mental State Examination [46], [47]. In our previous behavioral study, we found that adults with IGE had worse MoCA scores compared to HCs [26]. Additionally, an exercise RCT of PWE who did and did not exercise showed that MoCA scores improved in the PWE exercise group as compared to controls with epilepsy [25]. The MoCA incorporates varied tests that visuospatial skills, naming, attention, language, abstraction, and delayed recall; the maximum total score is 30, and scores greater than or equal to 26 are considered within the normal range.
Participants also completed the 65-item Profile of Mood States (POMS), which evaluated mood state over the past week [48]. The primary outcome of interest was the POMS Total Mood Disturbance (TMD) score, which measures overall mood by summing 5 subscale scores (tension-anxiety; depression-dejection; anger-hostility; fatigue-inertia; confusion-bewilderment) and subtracting the subscale score for vigor-activity [48]. Higher TMD scores indicate worse mood problems.
Imaging data acquisition and processing
Participants were scanned on a 3T Siemens Prisma MRI using a 20-channel head coil. A high-resolution T1-weighted anatomical brain scan was acquired with the following parameters: TR/TE 2400/2.22 msec, FOV 25.6 × 24.0 × 19.2 cm, matrix 256 × 256, flip angle 8°, 0.8 mm isotropic voxels. All participants' MRI data were examined for lesions prior to VBM analysis. None of the participants had macroscopic lesions, indicating the suitability of their imaging data for VBM.
Voxel-based morphometry
Participants’ T1-weighted images were processed using the Computational Anatomy Toolbox (CAT12) in Statistical Parametric Mapping (SPM12; https://www.fil.ion.ucl.ac.uk)) running in MatLab 2020a (The MathWorks, Inc., Natick, MA, USA). Data were processed as described in previous publications [49], [50]. Briefly, data processing included removal of non-brain voxels including the skull, bias correction, segmentation of brain tissue with partial volume estimation, denoising, and standardization to Montreal Neurological Institute (MNI) space (1.5 mm voxel size) by the Diffeomorphic Anatomical Registration through the Exponentiated Lie Algebra (DARTEL) approach [51], [52]. Data were modulated and spatially smoothed using an 8-mm full-width-at-half-maximum (FWHM) Gaussian smoothing kernel [50]. Mean GMV estimates for regions of interest (ROIs) were estimated and extracted for external analysis with automated labeling by the Anatomical Automatic Labeling Atlas (AAL) [53], [54], [55], [56]. Given that IGE was the current study’s patient population of interest, ROI analyses were limited to those comprising brain regions in the seizure initiation and propagation network (hippocampi, anterior and posterior cingulate, thalamus, bilateral cerebellum, and inferior parietal lobules) for generalized seizures [22].
Surface-based morphometry
A fully-automated approach integrated with CAT12 tissue segmentation during VBM processing was used to estimate volume-based cortical thickness [57]. Cortical thickness was estimated for the right and left hemispheres using a projection-based approach, which were merged and resampled to allow whole-brain analyses. Projection of local maxima was used to estimate distances between grey matter (GM) and white matter (WM) regions [57]. The central surface was reconstructed on the basis of these neighboring relationships, at the 50 % distance between boundaries of GM/CSF and GM/WM [57]. Topology correction and spherical inflation were applied to the data, with spherical registration by an adapted DARTEL algorithm [57], [58], [59]. Surface data were merged for the left and right hemispheres, then resampled and spatially smoothed using the default 15-mm FWHM Gaussian kernel. In addition to cortical thickness, the following additional surface-based parameters were estimated and processed as described for cortical thickness: fractal dimensionality (FDf), gyrification index, and sulcal depth. Fractal dimensionality and gyrification index measure surface topography features that together characterize cortical folding. Significant surface-based clusters were labeled using the Desikan-Killiany-Tourville atlas [60].
Statistical analysis
Descriptive statistics and group differences were analyzed for sociodemographic, anthropometric, clinical, and assessment variables. For continuous variables, descriptive statistics, outlier testing, correlation analyses (parametric and non-parametric), and independent-samples t-tests of between-group differences were computed using GraphPad Prism version 8.0 for Mac (GraphPad Software, La Jolla, CA, USA, www.graphpad.com). Chi-square tests were performed within IBM SPSS Version 27.0 for Mac (IBM Corp., Armonk, NY, United States) to determine if there were group differences in the proportion of males and females.
The comparison of IGE participants with HCs was important to first establish group differences in behavioral and neuromorphometric measurements. A series of voxel-wise general linear models (GLMs) computed group differences in volume- and surface-based parameters using SPM12. These GLMs additionally modeled the relationship between neuromorphometric data (IGE vs. HC) and PEL, MoCA scores, and TMD, which were measures found to be significantly different between groups. The volume- and surface-based data (GMV, cortical thickness, FDf, gyrification index, and sulcal depth) were the dependent variables (DV) across these GLMs. Scores indicating levels of PEL, cognition (MoCA score), and TMD were the independent variables (IV). For each DV-IV analysis, two different GLMs were constructed in order to investigate 1) if there was an interaction between the DV and the IV (DV*IV), and 2) the impact of modeling the IV as a nuisance covariate that explains variability between-groups.
Voxel-wise GLMs were assessed with permutation-based testing at 5000 iterations to create clusters with the Threshold-Free Cluster Enhancement (TFCE) toolbox running in SPM12. To summarize the methodology, TFCE enhances clusters while minimizing background noise by transforming each voxel’s statistical value based on the height and spatial contiguity of neighboring voxels. This approach removes the need for an arbitrary threshold, ultimately reducing bias and noise to isolate the clusters even when effects are subtle and focal. TFCE-based results were visualized with logarithmically scaled p-values that combined voxel-height and cluster size, with significance considered at p < 0.05, with corrections for multiple comparisons using family-wise error (FWE). For significant clusters, mean data and parameter estimates were extracted and plotted to characterize the magnitude and direction of the effect(s). Cluster-specific masks were created using the Marsbar toolbox, and subsequently used to extract cluster data within SPM12 [61]. The open-source software MRIcroGL (McCausland Center for Brain Imaging, University of South Carolina; https://www.mccauslandcenter.sc.edu/mricrogl/) was used to overlay significant clusters on the MNI template for 3D renderings. For surface data, clusters were visualized using Freesurfer whole-brain vertex-wise templates, and the corresponding figure was created with BioRender. Lastly, once group differences in brain volume and surface topography were established, the within-group correlations in the IGE group were expanded to examine associations between neuromorphometric differences with PEL, mood and cognition.
For VBM data only, total intracranial volume (TIV) was used to partial out the error due to individual head size variation. TIV was calculated during CAT12 tissue segmentation, and extracted within the “Estimate TIV” module. Due to high collinearity between TIV and group parameters of interest, TIV-correction proceeded using a covariate regression method. To summarize, TIV was modeled as a predictor of voxel-wise GMV in multiple regression model, with resultant residuals yielding TIV-free volumes representative of the group effect only. This avoided the need to compute GLMs with TIV as a nuisance covariate, thus conserving the model error for modeling the effects of interest.
In addition to whole-brain voxel- and vertex-wise associations between brain morphometric measures with assessment scores, ROI-based morphometric analyses were also conducted for mean GMV in brain regions comprising the seizure initiation and propagation network for generalized seizures. Mean GMV was estimated and extracted from ROIs delineated by the AAL atlas within CAT12. Independent samples t-tests assessed group differences in mean GMV for atlas-defined brain regions within the seizure initiation and propagation network. To be robust to outliers, Spearman’s rank-order correlation coefficients (rs) were calculated to test the association between mean GMV extracted from these ROIs and study measures found to be significantly different between groups (PEL, MoCA and TMD scores).
Results
Twenty-one IGEs and 19 age-/sex-matched HCs were recruited and completed study procedures. Four epilepsy participants were excluded after a follow-up medical record review showed an updated diagnosis of temporal lobe epilepsy. The final data are presented for 17 IGEs and 19 HCs (total N = 36), ages 18–46 years.
Sociodemographic, anthropometric, and clinical features
Participant characteristics, anthropometric features, and assessment scores are summarized in Table 1. The total sample was 47.2 % female and groups did not significantly differ (p > 0.05) in the distribution of biological sex, or in mean age, body mass index (BMI) and years of education. IGEs reported less physical exercise in leisure than HCs (significantly lower PEL scores, p < 0.001), as well as higher total mood disturbance (significantly higher POMS TMD scores, p = 0.029). Lastly, IGEs scored significantly lower on the MoCA (indicating poorer cognition) than HCs (p = 0.027).
Table 1.
Participant characteristics, assessment scores, and anthropometric features.
| All N = 36 |
Healthy N = 19 |
IGE N = 17 |
Group Difference | ||
|---|---|---|---|---|---|
| Sex, female | 17 (47.2 %) | 10 (52.6 %) | 7 (41.2 %) | χ2(1, N = 36) = 0.47, p = 0.49 | |
| Age at enrollment (yrs) | 27.1 ± 0.3 | 26.9 ± 9.4 | 27.3 ± 5.9 | t(34) = −0.15, p = 0.88 | |
| BMI | 25.9 ± 1.6 | 24.8 ± 3.7 | 27.1 ± 6.7 | t(34) = −1.26, p = 0.22 | |
| Education (years) | 14.6 ± 0.5 | 14.9 ± 1.6 | 14.3 ± 2.6 | t(34) = 0.92, p = 0.37 | |
| General cognition | |||||
| MoCA | 25.4 ± 1.8 | 26.6 ± 2.5 | 24.1 ± 4 | t(34) = 2.31, p = 0.027 * | |
| Physical exercise in leisure levels | |||||
| PEL | 3.0 ± 1.4 | 4.0 ± 1.7 | 2.0 ± 1.0 | t(34) = 4.22, p < 0.001 * | |
| Mood | |||||
| TMD | 23.5 ± 18 | 10.8 ± 26.5 | 36.2 ± 39.6 | t(34) = −2.29, p = 0.029 * | |
| Total Intracranial Volume (TIV) in mm3 |
1460.5 ± 8.3 | 1466.4 ± 158.8 | 1454.6 ± 132.5 | t(34) = 1.02, p = 0.31 | |
Abbreviations: IGE, idiopathic generalized epilepsy; PEL, physical exercise in leisure score of the Baecke Questionnaire of Habitual Physical Activity that represents frequency, intensity, and aerobic quality of sports and high-intensity exercises; TMD, Total Mood Disturbance score for Profile of Mood States; MoCA, Montreal Cognitive Assessment.
IGEs’ mean (SD) age at epilepsy onset was 13.7 (5.4) years, with a mean (SD) illness duration of 13.7 (6.3) years. IGEs’ reported mean (SD) number of seizures over the preceding 6-month period was 0.47 (1.0) seizures. Thirteen participants did not report experiencing any seizures over the preceding 6-months, while the remaining participants reported between 1 and 3 seizures in the past 6 months. On average, IGEs were taking 1.7 (0.88) ASMs, with all patients taking between 1 and 3 ASMs in total. Distribution of the IGE subtypes were 41.2 % juvenile myoclonic epilepsy (JME; n = 7), 23.5 % juvenile absence epilepsy (JAE; n = 4), and 35.3 % IGE with generalized tonic-clonic seizures only (IGE-GTC; n = 6).
Group differences in neuromorphometry and associations with PEL levels and mood
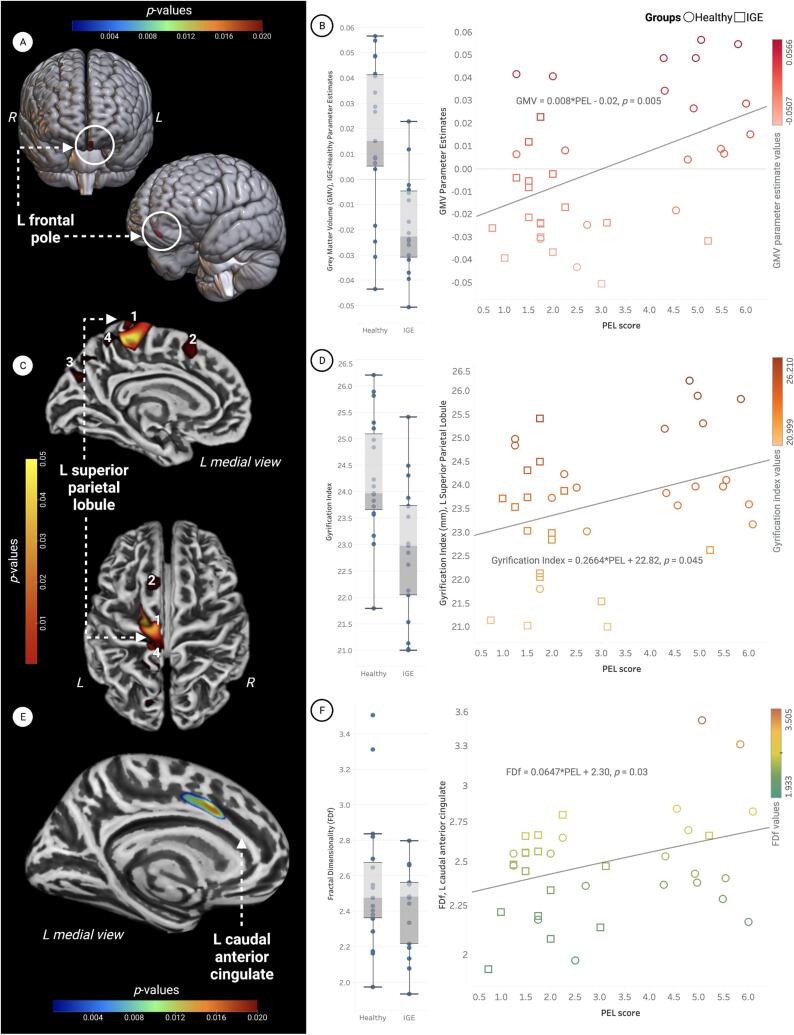
The brain regions where group differences in GMV were most pronounced are visualized in Fig. 2 and summarized in Table 2. IGE participants’ significantly lower PEL levels were associated with atrophy of the left frontal pole (IGE < HC, Fig. 3A–B, Table 2). IGE participants’ significantly elevated TMD scores (indicating worse mood) were associated with increased GMV (IGE > HC) in the right cerebellar lobule IX (Fig. 4, Table 2). Surface-based comparisons are summarized in Table 3 and visualized in Fig. 3C and E. IGE participants’ decreased PEL scores were also associated with decreased gyrification (IGE < HC) in the left superior parietal lobule, superior frontal gyrus, precuneus and cuneus, and a large cluster comprised of the precentral (40 %), paracentral (33 %), and superior frontal (14 %) gyri and precuneus (12 %) (Fig. 3C, Table 3). The association between IGE participants’ decreased PEL and decreased fractal dimensionality (IGE < HC) was localized to the left superior frontal gyrus, caudal anterior cingulate, and posterior cingulate (Fig. 3E, Table 3).
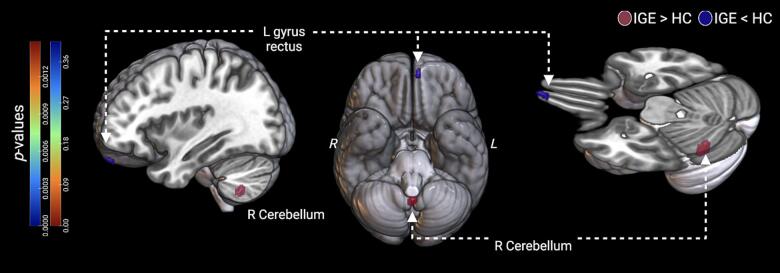
Fig. 2.
Participants with idiopathic generalized epilepsy (IGE) had decreased grey matter volume (GMV) in the left (L) gyrus rectus (IGE < HC), and increased GMV in the right (R) cerebellum (IGE > HC). Significant clusters for voxel-wise analyses are visualized from three perspectives, including a medial cross-section (pFWE < 0.05). The cluster in the right cerebellum extended bilaterally to portions of the left hemisphere, occupying a medial location overall.
Table 2.
Brain regions where group differences in voxelwise grey matter volume (GMV) were most pronounced in patients with idiopathic generalized epilepsy (IGE) and healthy controls (TFCE, pFWE < 0.05). Regions in which group differences in GMV were associated with group differences in physical exercise in leisure (PEL) levels and Total Mood Disturbance (TMD) scores are also summarized.
| Model | Region | Cluster size |
MNI coordinates |
|||
|---|---|---|---|---|---|---|
| (mm3) | X | Y | Z | |||
| GMV differences between groups | ||||||
| IGE > Healthy | R Cerebellum (IX) | 229 | 0 | −56 | −53 | |
| IGE < Healthy | L Gyrus Rectus | 5 | −6 | 60 | 23 | |
| Interaction of GMV*PEL | ||||||
| IGE < Healthy | L Frontal Pole | 140 | −5 | 62 | −24 | |
| Interaction of GMV*TMD | ||||||
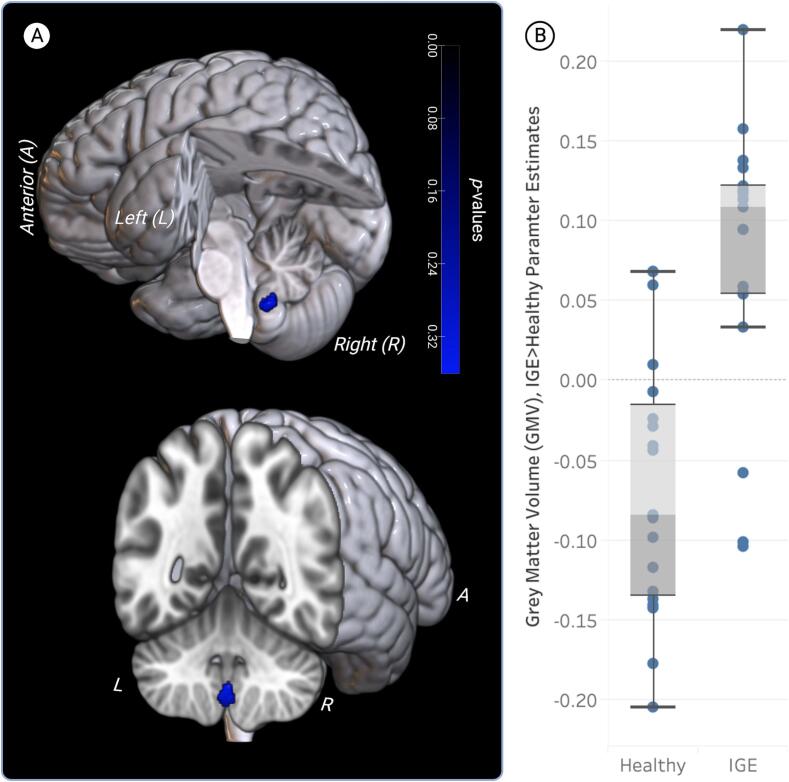
| IGE > Healthy | R Cerebellum (IX) | 180 | 2 | −56 | −51 | |
Abbreviations: GMV, grey matter volume; IGE, idiopathic generalized epilepsy; PEL, physical exercise in leisure score of the Baecke Questionnaire of Habitual Physical Activity that represents frequency, intensity, and aerobic quality of sports and high-intensity exercises; TMD, Total Mood Disturbance score for Profile of Mood States; L, left; R, right; TFCE, threshold-free cluster enhancement.
Fig. 3.
The relationship between self-reported physical exercise in leisure (PEL) and gray matter volume (GMV), gyrification, and cortical folding. All clusters were significant at (TFCE) pFWE < 0.05. (A) PEL scores were significantly associated with GMV in the left frontal pole (red cluster). (B) GMV parameter estimates were extracted from voxels within the left frontal pole (IGE < Healthy) and visualized using box-and-whisker plots. Group means are represented by vertical boxes, with standard deviation indicated by whiskers. The scatterplot shows data from the left frontal pole cluster where the interaction of GMV and PEL was statistically significant. The line of best fit is described by the following regression equation: GMV parameter estimate = 0.008*PEL − 0.02, p = 0.005. (C) PEL scores were significantly associated with cortical gyrification in 4 clusters: 1) superior parietal lobule, 2) superior frontal gyrus, 3) precuneus, and 4) a large cluster consisting of the precentral (40 %), paracentral (33 %), and superior frontal (14 %) gyri and part of the precuneus (12 %). (D) Vertex-wise gyrification indices were extracted and plotted for the superior parietal lobule (IGE < Healthy). The magnitude and direction of group differences are visualized using box-and-whisker plots. The scatterplot shows gyrification indices within the superior parietal lobule where the interaction of gyrification and PEL was statistically significant. The line of best fit for this interaction in the superior parietal lobule is best described by the following regression equation: Gyrification index = 0.2664*PEL + 22.82, p = 0.045. (E) PEL scores were significantly associated with fractional dimensionality (FDf) in a cluster consisting of the left caudal anterior cingulate (42 %), left superior frontal gyrus (40 %) and left posterior cingulate (18 %). (F) Vertex-wise FDf values were extracted from the left caudal anterior cingulate (IGE < Healthy) and visualized using box-and-whisker plots. The scatterplot shows data from this region, where the interaction of FDf and PEL was significant. The line of best fit is described by the following regression equation: FDf in the left caudal anterior cingulate = 0.0647*PEL + 2.30, p = 0.03.
Fig. 4.
The relationship between Total Mood Disturbance (TMD) scores and gray matter volume (GMV) in the right midline cerebellum (lobule IX). Compared to healthy participants, IGE participants had higher GMV in the right cerebellum (blue cluster) associated with higher TMD scores.
Table 3.
Summary of brain regions where decreased gyrification index and fractal dimensionality were associated with lower physical exercise in leisure (PEL) scores (TFCE pFWE < 0.05) in patients with idiopathic generalized epilepsy (IGE) compared to healthy controls.
| Surface Model | Region | Cluster size (mm) | %Contribution |
|---|---|---|---|
| Interaction of Gyrification Index*PEL | |||
| IGE < Healthy | L precentral | 3276 | 40 % |
| L paracentral | 33 % | ||
| L precuneus | 12 % | ||
| L superior frontal | 14 % | ||
| L precuneus | 669 | 77 % | |
| L cuneus | 9 % | ||
| L superior frontal gyrus | 659 | 100 % | |
| L superior parietal lobule | 183 | 100 % | |
| Interaction of Fractal Dimensionality*PEL | |||
| IGE < Healthy | L caudal anterior cingulate | 672 | 42 % |
| L superior frontal | 40 % | ||
| L posterior cingulate | 18 % | ||
Results for ROI-based analyses
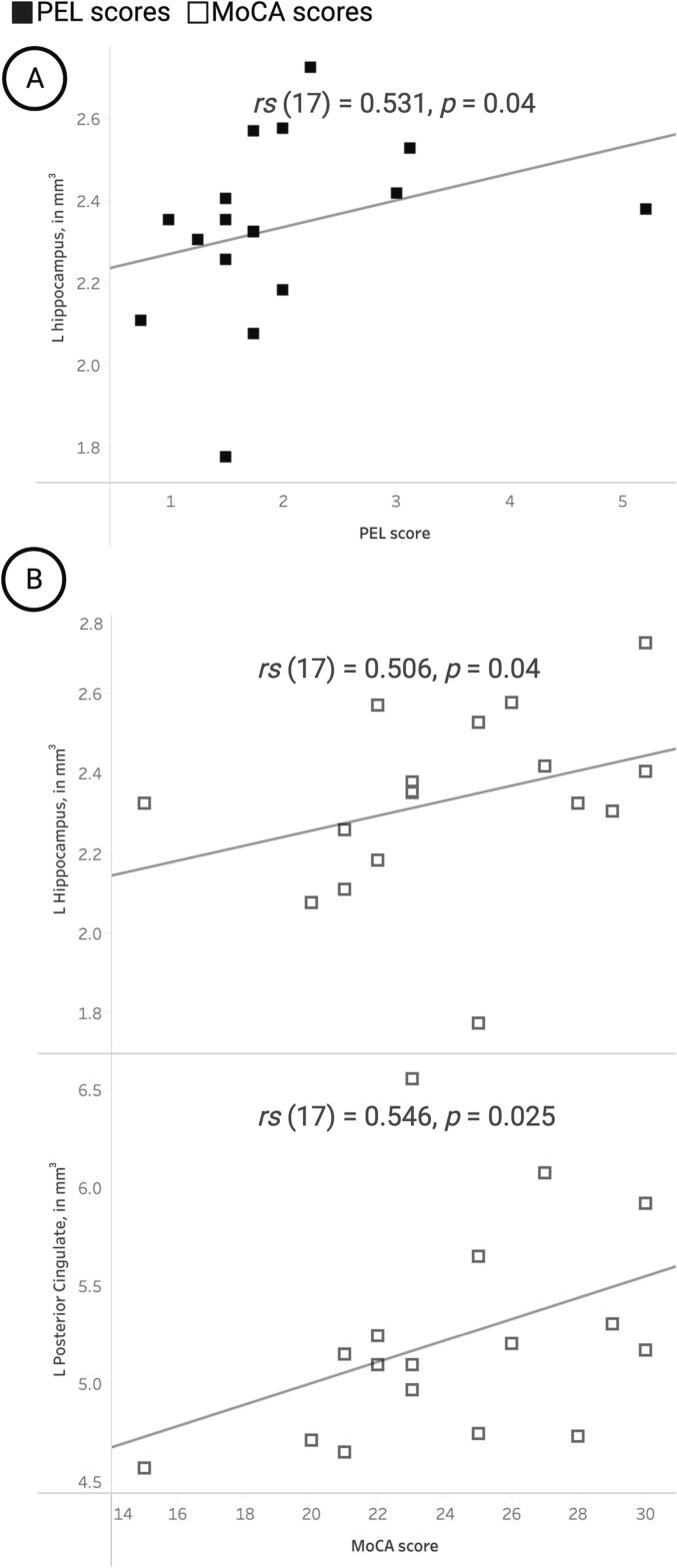
For IGE participants only, PEL scores were positively correlated with mean GMV in the left hippocampus (Fig. 5A). Additionally, IGE participants’ MoCA scores were correlated with mean GMV in the left hippocampus and left posterior cingulate (Fig. 5B).
Fig. 5.
Correlation plots illustrating significant relationships between mean gray matter volume (GMV) in regions of interest and primary study measurements for participants with idiopathic generalized epilepsy (IGE). (A) Physical exercise in leisure (PEL) scores (closed squares) showed significant positive associations with mean GMV in the left (L) hippocampus. (B) Montreal Cognitive Assessment (MoCA) scores (open squares) showed significant positive correlations with mean GMV in the L hippocampus and the L posterior cingulate gyrus.
Discussion
Main findings
The central aim of this pilot study was to investigate preliminary associations between neuromorphometric measures (brain volume- and surface-based) and measures of self-reported physical exercise in leisure (PEL), cognition and mood. As hypothesized, lower PEL and higher TMD scores in IGE showed significant voxel-wise associations with neuromorphometric measures, with several overlapping clusters in regions thought to comprise the seizure initiation and propagation network [22]. More specifically, lower PEL was associated with decreased brain volume in the left frontal pole, decreased gyrification in the left superior parietal lobule, and decreased fractal dimensionality in the left caudal anterior cingulate (IGE < Healthy). Higher TMD scores in IGE (IGE > Healthy) were associated with aberrant increases in GMV in the right midline cerebellum, another region in the seizure initiation and propagation network [22]. There were no voxel-wise group differences associated with MoCA, although ROI analysis showed atrophy of the left hippocampus and posterior cingulate was associated with worse MoCA performance. ROI analysis further showed associations between decreased levels of PEL and atrophy in the left hippocampus. Overall, our findings support the importance of exercise for brain health and wellness in IGE, which we discuss in greater detail below, as well as future studies that incorporate more rigorous study designs and outcome measures (Table 4).
Table 4.
Summary of brain regions in which patients with idiopathic generalized epilepsy had lower mean gray matter volume (GMV) than healthy controls. Analyses were limited to brain regions in the seizure initiation and propagation network for generalized seizures.
| ROIs | Healthy Mean | IGE Mean | Diff | SE of Diff | t-test |
|---|---|---|---|---|---|
| L Cerebellum | 52.02 | 47.47 | 4.551 | 2.124 | t(34) = 2.143, p = 0.039 |
| R Cerebellum | 51.39 | 46.98 | 4.408 | 2.142 | t(34) = 2.058, p = 0.047 |
| R Thalamus | 6.112 | 5.782 | 0.3297 | 0.1561 | t(34) = 2.111, p = 0.042 |
Abbreviations: IGE, idiopathic generalized epilepsy; GMV, gray matter volume; L, left; R, right.
Lower PEL, brain atrophy, and aberrant cortical folding in IGE
IGEs’ lower PEL levels were associated with structural aberrations in brain regions within and adjacent to the seizure initiation and propagation network [22]. Lower levels of physical exercise in leisure time, as indicated by PEL scores, were associated with brain atrophy in the left frontal pole (IGE < HC). These findings are consistent with previous studies indicating that greater levels of physical activity, particularly aerobic exercise, increases GMV in frontal regions. The frontal pole is the most anterior portion of the prefrontal cortex (PFC), a site especially sensitive to the neuroprotective effects of exercise while being particularly vulnerable to age-related cognitive declines that are often amplified in the context of epilepsy [14], [15], [62], [63]. Thus, frontal GMV in IGE participants may be decreased compared to the healthy group, while still occupying the bounds of “normal” GMV. On the other hand, IGEs’ GMV parameter estimates may indicate significant GMV atrophy stemming from underlying disease processes and early onset cognitive aging that is characteristic of epilepsy, both of which may be further exacerbated by the absence of exercise-induced neuroplasticity [14], [15]. IGEs’ lower PEL scores corresponded to significantly decreased vertex-wise fractal dimensionality (FDf) in the superior frontal gyrus, posterior cingulate, and caudal anterior cingulate cortex (ACC), regions implicated in attention, memory, and mood that are typically impacted in epilepsy [64], [65], [66]. Exercise-induced increases in the volume and cortical thickness of the frontal gyri have been previously documented in older adults [14]. The ACC is regulatory hub for cognition and mood, and exercise-induced improvements in mood and emotional regulation may stem from corresponding increases in ACC volume and cortical thickness [65]. Relative to healthy controls, IGE participants’ lower PEL scores were associated with reduced gyrification in left hemisphere brain regions including the precentral, paracentral, and postcentral gyri, the precuneus and cuneus, superior frontal gyrus, and superior parietal lobule. Activation of premotor regions and enhanced functional connectivity of the precuneus and superior parietal lobule have been linked to exercise-induced improvements in executive function and attention [66], [67], [68].
Gray matter volume in the right cerebellum (IX) was increased in IGE, in association with increased mood disturbance (i.e., worse mood) on the POMS. Cerebellar lobule IX is within the vermis, a midline cerebellar structure linked to mood, emotion processing and modulation of affective behavior [69], [70], [71], [72]. Cerebellar GMV loss has been found in people with mood disorders, as well as patients with epilepsy and those with depressive symptoms associated with other neurological disorders [73], [74], [75]. Although both hypertrophy and atrophy can manifest due to abnormal neuroplasticity, the link between cerebellar atrophy and mood aberrations is better documented [73], [74], [75]. While findings of cerebellar hypertrophy are less frequent, they have been reported in at least one study of mood aberrations [76]. Hypertrophy of the cerebellar vermis, as seen in this study, could be a neuroplastic change directly caused by compensatory mechanisms due to epileptic pathophysiology disrupting neural networks [69], [77]. Given the cerebellum’s role in stress-induced behavioral changes, cerebellar hypertrophy could also arise from stress-related changes inherent in the experience of having epilepsy [75], [78]. Based on findings from an investigation of chronic stress-induced behavioral alterations in mice, both depressive symptoms and structural alterations in this context may both derive from cerebellar projections to the ventral tegmental area [78].
ROI-based results and correlations
ROI-based group differences revealed mean GMV decreases in the IGE group, primarily focused in the bilateral cerebellum (all lobules), which also showed atrophy in voxelwise analyses. For IGE participants only, mean GMV in the left hippocampus was positively associated with PEL scores (p = 0.04), as well as MoCA scores (p = 0.04). These findings are consistent with the body of prior work on exercise-induced proliferation of hippocampal cells and corresponding cognitive improvements, which include improved memory retrieval and faster memory processing [63], [79], [80], [81]. The hippocampus also plays a role in fear conditioning via its connections to the amygdala, but these connections may also underlie exercise-induced modulation of anxiety [82]. Given the prevalence of mood disorders in epilepsy patients, and the well-established association between decreased depression and increased physical activity, increased hippocampal volume may underlie some of these positive mood effects [8], [27], [83]. Lastly, MoCA scores were positively associated with mean GMV in the left posterior cingulate ROI. Generalized seizures disproportionally impact frontal brain regions, resulting in executive functioning and attention deficits that are reflected in lower MoCA scores. The posterior cingulate mediates aspects of body consciousness, spatial awareness, and spatial navigation in conjunction with the hippocampus space cells. The posterior cingulate and hippocampus, both regions that comprise the seizure initiation and propagation network, may demonstrate neurodegeneration and/or microstructural abnormalities in the context of mild to severe cognitive impairment [84]. The posterior cingulate cortex is also involved in capacities that decline with cognitive impairment [85], [86].
Study strengths and limitations
The sample size was modest, with 17 participants in the IGE group and 19 healthy controls. In accordance with recommendations that VBM studies enroll 16–32 participants per group, this preliminary study offered sufficient power to detect the primary effects of interest [87], [88]. Power was further increased by recruiting age- and sex-matched controls, eliminating the need to model biological sex or age as nuisance covariates and thus minimizing the overall error variance. Because all epilepsy patients were diagnosed with IGE, statistical analyses did not require adjusting for confounds introduced by variable epilepsy diagnoses and/or ictal onset zones. Overall, the clinical features of the IGE group allowed investigating structural differences between groups on the basis of parameters of interest: level of engagement in leisure time exercise, general neurocognitive function, and mood. While we recognize that differences in IGE subtypes, seizure types, and seizure control may be factors in neuromorphometric and assessment results, the limited sample size in the current study is not conducive to meaningful statistical analyses. Therefore, future studies would benefit from larger sample sizes in order to elucidate the influence of various clinical characteristics in IGE.
The cross-sectional design of present study did not allow for a clear understanding of the relationship between brain structure and PEL levels. Factors such as mood disturbance and exercise motivation could potentially influence this relationship and act as confounding variables. To gain a more comprehensive understanding of the causal nature of these relationships, it is necessary to conduct RCTs of exercise interventions in epilepsy patients. Furthermore, the range of exercise types, degrees of associated exertion, and intensity of aerobic burden are not as accurately quantified in a self-report measure like the PEL score. Longitudinal RCTs are needed to pinpoint the type, frequency, and intensity of exercise necessary for meaningful benefits specific to epilepsy and associated brain changes. The physical activity, cognition, and mood measures used in this study were limited to readily available options due to resource constraints. While the use of more sophisticated measures would have been ideal, it is important to emphasize that this was a pilot imaging study. A future study would be strengthened by including a physiological measure of physical fitness as a comparison to the PEL to examine the congruence of the results and assess whether both measures localize structural abnormalities in the same regions. Despite these limitations, our preliminary findings highlight the importance of future studies investigating exercise-associated changes in brain structure and function in epilepsy, especially those that incorporate a more comprehensive panel of neurocognitive, mood, and physical activity assessments.
Conclusions
This study provides valuable insights into how physical exercise levels measured by a self-report measure can indicate brain health in patients with epilepsy. Self-reported levels of exercise highlighted differences in GMV, cortical complexity, and gyrification between healthy individuals and those with IGE. While early studies are promising, it remains to be determined to what extent and in which brain regions recreational exercise is effective in alleviating impairments in epilepsy.
Ethical statement
We confirm that any aspect of the work covered in this manuscript that has involved human patients or their protected health information has been conducted with the ethical approval of all relevant bodies and that such approvals are acknowledged within the manuscript. The University of Alabama at Birmingham Institutional Review Board approved all study procedures. All participants were screened for MR compatibility. Written informed consent was obtained from all participants before initiating the protocol.
Author contributions
AAS – data analysis, writing and editing final version of the manuscript.
DMT – data analysis, writing original draft, and review and editing of the final version of the manuscript.
JLP – data collection, review and editing of the final version of the manuscript.
JPS – guidance on data collection and analysis, review and editing of the final version of the manuscript.
RCM – guidance on data analysis, review and editing of the final version of the manuscript.
RN – guidance on data analysis, review and editing of the final version of the manuscript.
MK – guidance on data collection and analysis, review and editing of the final version of the manuscript.
GAB – data collection, review and editing of the final version of the manuscript.
MB – development of MRI scan parameters, review and editing of the final version of the manuscript.
JBA – conceptualization, funding, guidance on data collection and analysis, methodology, project administration and supervision, review and editing of the final version of the manuscript.
CRediT authorship contribution statement
Ayushe A. Sharma: . D. Mackensie Terry: . Johanna L. Popp: Investigation, Project administration, Writing – review & editing. Jerzy P. Szaflarski: Investigation, Methodology, Resources, Software, Writing – review & editing. Roy C. Martin: Investigation, Methodology, Resources, Software, Writing – review & editing. Rodolphe Nenert: Investigation, Methodology, Resources, Software, Writing – review & editing. Manmeet Kaur: Investigation, Methodology, Resources, Writing – review & editing. Gabrielle A. Brokamp: Investigation, Resources, Writing – review & editing. Mark Bolding: Methodology, Resources, Validation, Writing – review & editing. Jane B. Allendorfer: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This study was supported by the UAB Faculty Development Grant Program (JBA), the UAB Civitan International Research Center 3T MRI Pilot Research Grants (JBA), funds from the UAB Epilepsy Center to JBA, and the UAB Summer in Biomedical Sciences Program (DMT). During the data processing stages of the study, AAS was supported by an institutional training grant (5T32NS061788-14) through the UAB Department of Neurobiology Pre-doctoral Training Program in Cognition and Cognitive Disorders (C&CD), funded by the National Institute of Neurological Disorders and Stroke (NIH/NINDS). AAS is currently supported by a NIH/NINDS Ruth L. Kirschstein National Research Service Award (1F31NS129288-01) from the NINDS. The authors thank Jasper J. Odell for training in Tableau data visualization, which was instrumental in creating plots for Fig. 2, Fig. 3, Fig. 4. The study was presented, in part, at the 2020 Organization of Human Brain Mapping Meeting.
Data availability statement
The raw data supporting the conclusions of this article will be made available with University of Alabama at Birmingham Institutional Review Board approval and data sharing agreement.
Conflict of interest statement
Dr. Szaflarski is the Editor-in-Chief of Epilepsy & Behavior Reports, associate editor of Journal of Epileptology, editorial board member for Epilepsy & Behavior, Journal of Medical Science, and Folia Medica Copernicana, and receives royalties from Elsevier; receives funding from NIH, NSF, DoD, Shor Foundation for Epilepsy Research, UCB Biosciences, NeuroPace Inc., SAGE Therapeutics Inc., Serina Therapeutics Inc., LivaNova Inc., Greenwich Biosciences Inc., Biogen Inc., Eisai Inc., State of AL; is on consulting /advisory boards for PureTech Health, Biopharmaceutical Research Company, LivaNova Inc., UCB Pharma, AdCel Pharma, iFovea Inc.; has served on the Alabama State Medical Cannabis Study Commission (nominated by Gov. Ivey); serves on the Alabama Medical Cannabis Commission (2021–2025; nominated by Dr. Scott Harris, State Health Officer). Dr. Allendorfer is associate editor of Epilepsy & Behavior Reports; receives funding from NIH, McKnight Brain Institute, DoD, LivaNova Inc.; has served as a consultant for LivaNova, Inc.; has received honoraria from Cleveland Clinic and University of Auckland; serves on a Data Safety Monitoring Board (University of Alabama at Birmingham/University of Colorado Anschutz Medical Campus); serves on the American Epilepsy Society Scientific Program Committee. The remaining authors have no disclosures to declare.
Funding sources
This work was supported by the UAB Faculty Development Grant Program (JBA), the Civitan International Research Center 3T MRI Pilot Research Grants (JBA), and the UAB Epilepsy Center. Additionally, during the data processing and analysis stages of the study, AAS was supported by an institutional training grant (T32-NS061788-13) funded by the National Institute of Health and the National Institute of Neurological Disorders and Stroke (NINDS). AAS was supported by a NIH Ruth L. Kirschstein National Research Service Award (1F31NS129288-01) from the NINDS during the manuscript preparation and submission phase of the study.
Contributor Information
Ayushe A. Sharma, Email: ayushe.sharma@yale.edu.
Jane B. Allendorfer, Email: jallendorfer@uabmc.edu.
References
- 1.Fisher R.S., et al. ILAE Official Report: a practical clinical definition of epilepsy. Epilepsia. 2014;55(4):475–482. doi: 10.1111/epi.12550. [DOI] [PubMed] [Google Scholar]
- 2.Abramovici S., Bagić A. Epidemiol Epilepsy. 2016:159–171. doi: 10.1016/B978-0-12-802973-2.00010-0. [DOI] [PubMed] [Google Scholar]
- 3.Hermann B.P., et al. Cognitive prognosis in chronic temporal lobe epilepsy. Ann Neurol. 2006;60(1):80–87. doi: 10.1002/ana.20872. [DOI] [PubMed] [Google Scholar]
- 4.Helmstaedter C., Hufnagel A., Elger C.E. Seizures during cognitive testing in patients with temporal lobe epilepsy: possibility of seizure induction by cognitive activation. Epilepsia. 1992;33(5):892–897. doi: 10.1111/j.1528-1157.1992.tb02197.x. [DOI] [PubMed] [Google Scholar]
- 5.Elger C.E., Helmstaedter C., Kurthen M. Chronic Epilepsy Cogn. 2004:663–672. doi: 10.1016/S1474-4422(04)00906-8. [DOI] [PubMed] [Google Scholar]
- 6.Holmes GL. Effect of seizures on the developing brain and cognition. [DOI] [PMC free article] [PubMed]
- 7.Motamedi G., Meador K. Epilepsy and cognition. Epilepsy Behav. 2003;4:25–38. doi: 10.1016/j.yebeh.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 8.Salpekar J. Mood Disorders in Epilepsy. 2016;48(1):p. 62-67. doi: 10.1176/appi.focus.20160017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kanner AM. Psychiatric comorbidities in new onset epilepsy: Should they be always investigated? 2017, W.B. Saunders Ltd. p. 79-82. [DOI] [PubMed]
- 10.Kanner A.M. Mood disorder and epilepsy: a neurobiologic perspective of their relationship. Dialogues Clin Neurosci. 2008;10(1):39. doi: 10.31887/DCNS.2008.10.1/amkanner. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benbadis S.R., et al. Putting it all together: Options for intractable epilepsy: An updated algorithm on the use of epilepsy surgery and neurostimulation. Epilepsy Behav. 2018;88:33–38. doi: 10.1016/j.yebeh.2018.05.030. [DOI] [PubMed] [Google Scholar]
- 12.Kwan P, Sperling MR. Refractory seizures: Try additional antiepileptic drugs (after two have failed) or go directly to early surgery evaluation? 2009. pp. 57–62. [DOI] [PubMed]
- 13.Laxer K.D., et al. The consequences of refractory epilepsy and its treatment. Epilepsy Behav. 2014;37:59–70. doi: 10.1016/j.yebeh.2014.05.031. [DOI] [PubMed] [Google Scholar]
- 14.Colcombe S.J., et al. Aerobic exercise training increases brain volume in aging humans. J Gerontol Ser A. 2006;61(11):1166–1170. doi: 10.1093/gerona/61.11.1166. [DOI] [PubMed] [Google Scholar]
- 15.Colcombe S.J., et al. Aerobic fitness reduces brain tissue loss in aging humans. J Gerontol Ser A. 2003;58(2):176–180. doi: 10.1093/gerona/58.2.m176. [DOI] [PubMed] [Google Scholar]
- 16.Johnson EC, Helen Cross J, Reilly C. Physical activity in people with epilepsy: a systematic review. Blackwell Publishing Inc. 2020. pp. 1062–1081. [DOI] [PubMed]
- 17.Shawahna R, Nairat Q. Research productivity in the field of physical exercise and epilepsy: a bibliometric analysis of the scholarly literature with qualitative synthesis (1525-5069 (Electronic)). [DOI] [PubMed]
- 18.Capovilla G., et al. Epilepsy, seizures, physical exercise, and sports: a report from the ILAE Task Force on Sports and Epilepsy. Epilepsia. 2016;57(1):6–12. doi: 10.1111/epi.13261. [DOI] [PubMed] [Google Scholar]
- 19.Duñabeitia I., et al. Effects of physical exercise in people with epilepsy: A systematic review and meta-analysis. Epilepsy Behav. 2022;137(Pt A) doi: 10.1016/j.yebeh.2022.108959. [DOI] [PubMed] [Google Scholar]
- 20.Eom S., et al. The impact of an exercise therapy on psychosocial health of children with benign epilepsy: a pilot study. Epilepsy Behav. 2014;37:151–156. doi: 10.1016/j.yebeh.2014.06.017. [DOI] [PubMed] [Google Scholar]
- 21.Koirala G.R., et al. Altered brain functional connectivity induced by physical exercise may improve neuropsychological functions in patients with benign epilepsy. Epilepsy Behav. 2017 doi: 10.1016/j.yebeh.2017.06.021. [DOI] [PubMed] [Google Scholar]
- 22.Allendorfer J.B., et al. A pilot study of combined endurance and resistance exercise rehabilitation for verbal memory and functional connectivity improvement in epilepsy. Epilepsy Behav. 2019;96:44–56. doi: 10.1016/j.yebeh.2019.04.020. [DOI] [PubMed] [Google Scholar]
- 23.Eriksen H.R., et al. Physical exercise in women with intractable epilepsy. Epilepsia. 1994;35(6):1256–1264. doi: 10.1111/j.1528-1157.1994.tb01797.x. [DOI] [PubMed] [Google Scholar]
- 24.McAuley J.W., et al. A prospective evaluation of the effects of a 12-week outpatient exercise program on clinical and behavioral outcomes in patients with epilepsy. Epilepsy Behav. 2001;2(6):592–600. doi: 10.1006/ebeh.2001.0271. [DOI] [PubMed] [Google Scholar]
- 25.Feter N., et al. Effect of combined physical training on cognitive function in people with epilepsy: results from a randomized controlled trial. Epilepsia. 2020;61(8):1649–1658. doi: 10.1111/epi.16588. [DOI] [PubMed] [Google Scholar]
- 26.Popp J.L., et al. Relationships between cognitive function, seizure control, and self-reported leisure-time exercise in epilepsy. Epilepsy Behav. 2021;118 doi: 10.1016/j.yebeh.2021.107900. [DOI] [PubMed] [Google Scholar]
- 27.Häfele C.A., Freitas M.P., da Silva M.C., Rombaldi A.J. Are physical activity levels associated with better health outcomes in people with epilepsy? Epilepsy Behav. 2017;72:28–34. doi: 10.1016/j.yebeh.2017.04.038. [DOI] [PubMed] [Google Scholar]
- 28.Ko N., et al. Effect of physical training on aerobic capacity, seizure occurrence, and serum level of antiepileptic drugs in adults with epilepsy. Epilepsia. 1990;31(1):88–94. doi: 10.1111/j.1528-1157.1990.tb05365.x. [DOI] [PubMed] [Google Scholar]
- 29.Alexander H.B., Allendorfer J.B. The relationship between physical activity and cognitive function in people with epilepsy: a systematic review. Epilepsy Behav. 2023;142 doi: 10.1016/j.yebeh.2023.109170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koblinsky N.D., Meusel L.C., Greenwood C.E., Anderson N.D. Household physical activity is positively associated with gray matter volume in older adults. BMC Geriatr. 2021;21(1):104. doi: 10.1186/s12877-021-02054-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Erickson K.I., Leckie R.L., Weinstein A.M. Elsevier Inc.; 2014. Physical activity, fitness, and gray matter volume. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Erickson K.I., et al. Aerobic fitness is associated with hippocampal volume in elderly humans. Hippocampus. 2009;19(10):1030–1039. doi: 10.1002/hipo.20547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Erickson KI, et al. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci USA 2011;108(7):3017–3022. [DOI] [PMC free article] [PubMed]
- 34.Thomas R.H., et al. A comprehensive neuropsychological description of cognition in drug-refractory juvenile myoclonic epilepsy. Epilepsy Behav. 2014;36:124–129. doi: 10.1016/j.yebeh.2014.04.027. [DOI] [PubMed] [Google Scholar]
- 35.Valente K.D., et al. Delineating behavioral and cognitive phenotypes in juvenile myoclonic epilepsy: are we missing the forest for the trees? Epilepsy Behav. 2016;54:95–99. doi: 10.1016/j.yebeh.2015.10.022. [DOI] [PubMed] [Google Scholar]
- 36.Cevik N., et al. Evaluation of cognitive functions of juvenile myoclonic epileptic patients by magnetic resonance spectroscopy and neuropsychiatric cognitive tests concurrently. Neurol Sci. 2016;37(4):623–627. doi: 10.1007/s10072-015-2425-5. [DOI] [PubMed] [Google Scholar]
- 37.Bolden L.B., et al. Cortical excitability and seizure control influence attention performance in patients with idiopathic generalized epilepsies (IGEs) Epilepsy Behav. 2018 doi: 10.1016/j.yebeh.2018.10.026. [DOI] [PubMed] [Google Scholar]
- 38.Henkin Y., et al. Cognitive function in idiopathic generalized epilepsy of childhood. Dev Med Child Neurol. 2005 doi: 10.1017/s0012162205000228. [DOI] [PubMed] [Google Scholar]
- 39.Schaffer Y., et al. Auditory verbal memory and psychosocial symptoms are related in children with idiopathic epilepsy. Epilepsy Behav. 2015 doi: 10.1016/j.yebeh.2015.04.069. [DOI] [PubMed] [Google Scholar]
- 40.Bolden L.B., et al. Cortical excitability affects mood state in patients with idiopathic generalized epilepsies (IGEs) Epilepsy Behav. 2019;90:84–89. doi: 10.1016/j.yebeh.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 41.Falco-Walter J.J., Scheffer I.E., Fisher R.S. The new definition and classification of seizures and epilepsy. Epilepsy Res. 2018;139:73–79. doi: 10.1016/j.eplepsyres.2017.11.015. [DOI] [PubMed] [Google Scholar]
- 42.Abela E., et al. Urban und Vogel GmbH; 2014. Neuroimaging of epilepsy: lesions, networks, oscillations; pp. 5–15. [DOI] [PubMed] [Google Scholar]
- 43.Baecke J.A.H., Burema J., Frijters J.E.R. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982;36(5):936–942. doi: 10.1093/ajcn/36.5.936. [DOI] [PubMed] [Google Scholar]
- 44.Ono R., et al. Reliability and validity of the Baecke physical activity questionnaire in adult women with hip disorders. BMC Musculoskelet Disord. 2007;8:61. doi: 10.1186/1471-2474-8-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Florindo A.A., Latorre M.d.R.D.d.O. Validation and reliability of the Baecke questionnaire for the evaluation of habitual physical activity in adult men. Rev Bras Med Esporte. 2003 [Google Scholar]
- 46.Nasreddine Z.S., et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 47.Phabphal K., Kanjanasatien J. Montreal Cognitive Assessment in cryptogenic epilepsy patients with normal Mini-Mental State Examination scores. Epileptic Disord. 2011;13(4):375–381. doi: 10.1684/epd.2011.0469. [DOI] [PubMed] [Google Scholar]
- 48.McNair D, Lorr M, Droppleman LF. Profile of mood states (POMS); 1989.
- 49.Sharma A.A., et al. A preliminary study of the effects of cannabidiol (CBD) on brain structure in patients with epilepsy. Epilepsy Behav Rep. 2019:12. doi: 10.1016/j.ebr.2019.100341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ashburner J., Friston K.J. Voxel-based morphometry—the methods. Neuroimage. 2000;11(6):805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 51.Ashburner J., Friston K.J. Diffeomorphic registration using geodesic shooting and Gauss-Newton optimisation. Neuroimage. 2011;55(3):954–967. doi: 10.1016/j.neuroimage.2010.12.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38(1):95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 53.Tzourio-Mazoyer N., et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 54.Winterburn J.L., et al. A novel in vivo atlas of human hippocampal subfields using high-resolution 3T magnetic resonance imaging. Neuroimage. 2013 doi: 10.1016/j.neuroimage.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 55.Park M.T.M., et al. Derivation of high-resolution MRI atlases of the human cerebellum at 3T and segmentation using multiple automatically generated templates. Neuroimage. 2014;95:217–231. doi: 10.1016/j.neuroimage.2014.03.037. [DOI] [PubMed] [Google Scholar]
- 56.Entis J.J., Doerga P., Barrett L.F., Dickerson B.C. A reliable protocol for the manual segmentation of the human amygdala and its subregions using ultra-high resolution MRI. Neuroimage. 2012 doi: 10.1016/j.neuroimage.2011.12.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dahnke R., Yotter R.A., Gaser C. Cortical thickness and central surface estimation. Neuroimage. 2013;65:336–348. doi: 10.1016/j.neuroimage.2012.09.050. [DOI] [PubMed] [Google Scholar]
- 58.Yotter R.A., Dahnke R., Thompson P.M., Gaser C. Topological correction of brain surface meshes using spherical harmonics. Hum Brain Mapp. 2011;32(7):1109–1124. doi: 10.1002/hbm.21095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yotter R.A., Thompson P.M., Gaser C. Algorithms to improve the reparameterization of spherical mappings of brain surface meshes. J Neuroimaging. 2011;21(2):e134–e147. doi: 10.1111/j.1552-6569.2010.00484.x. [DOI] [PubMed] [Google Scholar]
- 60.Desikan R.S., et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 61.Brett M. MarsBaR documentation. Functional Imaging. 2011 [Google Scholar]
- 62.Baker L.D., et al. Aerobic exercise improves cognition for older adults with glucose intolerance, a risk factor for Alzheimer's disease. J Alzheimers Dis. 2010;22(2):569–579. doi: 10.3233/JAD-2010-100768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Colcombe S.J., et al. Cardiovascular fitness, cortical plasticity, and aging. PNAS. 2004;101(9):3316–3321. doi: 10.1073/pnas.0400266101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex; 2011. pp. 85–93. [DOI] [PMC free article] [PubMed]
- 65.Lin K., et al. Aerobic exercise impacts the anterior cingulate cortex in adolescents with subthreshold mood syndromes: a randomized controlled trial study. Transl Psychiatry. 2020;10(1):1–7. doi: 10.1038/s41398-020-0840-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mehren A., et al. Acute effects of aerobic exercise on executive function and attention in adult patients with ADHD. Front Psychiatry. 2019;10(MAR):132. doi: 10.3389/fpsyt.2019.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yu F., et al. Efficacy and mechanisms of combined aerobic exercise and cognitive training in mild cognitive impairment: Study protocol of the ACT trial. Trials. 2018;19(1):1–13. doi: 10.1186/s13063-018-3054-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chirles T.J., et al. Exercise training and functional connectivity changes in mild cognitive impairment and healthy elders. J Alzheimers Dis. 2017;57(3):845–856. doi: 10.3233/JAD-161151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Klein A.P., et al. Nonmotor functions of the cerebellum: an introduction. Am J Neuroradiol. 2016;37(6):1005–1009. doi: 10.3174/ajnr.A4720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Guell X., Schmahmann J. Cerebellar functional anatomy: a didactic summary based on human fMRI evidence. Cerebellum. 2020;19(1):1–5. doi: 10.1007/s12311-019-01083-9. [DOI] [PubMed] [Google Scholar]
- 71.Saleem A, et al. Functional connectivity of the cerebellar vermis in bipolar disorder and associations with mood. bioRxiv, 2023. [DOI] [PMC free article] [PubMed]
- 72.Pierce J.E., et al. Explicit and implicit emotion processing in the cerebellum: a meta-analysis and systematic review. Cerebellum. 2023;22(5):852–864. doi: 10.1007/s12311-022-01459-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Clausi S., et al. Depression disorder in patients with cerebellar damage: Awareness of the mood state. J Affect Disord. 2019;245:386–393. doi: 10.1016/j.jad.2018.11.029. [DOI] [PubMed] [Google Scholar]
- 74.Ciumas C., Savic I. Structural changes in patients with primary generalized tonic and clonic seizures. Neurology. 2006;67(4):683–686. doi: 10.1212/01.wnl.0000230171.23913.cf. [DOI] [PubMed] [Google Scholar]
- 75.Lazzarotto A., et al. Selective cerebellar atrophy associates with depression and fatigue in the early phases of relapse-onset multiple sclerosis. Cerebellum. 2020;19(2):192–200. doi: 10.1007/s12311-019-01096-4. [DOI] [PubMed] [Google Scholar]
- 76.Li S., et al. Progressive gray matter hypertrophy with severity stages of insomnia disorder and its relevance for mood symptoms. Eur Radiol. 2021;31(8):6312–6322. doi: 10.1007/s00330-021-07701-7. [DOI] [PubMed] [Google Scholar]
- 77.Hellwig S., Gutmann V., Trimble M.R., van Elst L.T. Cerebellar volume is linked to cognitive function in temporal lobe epilepsy: a quantitative MRI study. Epilepsy Behav. 2013;28(2):156–162. doi: 10.1016/j.yebeh.2013.04.020. [DOI] [PubMed] [Google Scholar]
- 78.Baek S.J., et al. VTA-projecting cerebellar neurons mediate stress-dependent depression-like behaviors. Elife. 2022;11 doi: 10.7554/eLife.72981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vaynman S., Ying Z., Gomez-Pinilla F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur J Neurosci. 2004;20(10):2580–2590. doi: 10.1111/j.1460-9568.2004.03720.x. [DOI] [PubMed] [Google Scholar]
- 80.Van Praag H., Shubert T., Zhao C., Gage F.H. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci. 2005;25(38):8680–8685. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Herting M.M., Nagel B.J. Aerobic fitness relates to learning on a virtual Morris Water Task and hippocampal volume in adolescents. Behav Brain Res. 2012;233(2):517–525. doi: 10.1016/j.bbr.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen Y.C., et al. Habitual physical activity mediates the acute exercise-induced modulation of anxiety-related amygdala functional connectivity. Sci Rep. 2019;9(1):19787. doi: 10.1038/s41598-019-56226-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Roth D.L., Goode K.T., Williams V.L., Faught E. Physical exercise, stressful life experience, and depression in adults with epilepsy. Epilepsia. 1994;35(6):1248–1255. doi: 10.1111/j.1528-1157.1994.tb01796.x. [DOI] [PubMed] [Google Scholar]
- 84.Hong Y.J., et al. Microstructural changes in the hippocampus and posterior cingulate in mild cognitive impairment and Alzheimer's disease: a diffusion tensor imaging study. Neurol Sci. 2013;34(7):1215–1221. doi: 10.1007/s10072-012-1225-4. [DOI] [PubMed] [Google Scholar]
- 85.Guterstam A., Björnsdotter M., Gentile G., Ehrsson H.H. Posterior cingulate cortex integrates the senses of self-location and body ownership. Curr Biol. 2015;25(11):1416–1425. doi: 10.1016/j.cub.2015.03.059. [DOI] [PubMed] [Google Scholar]
- 86.Pengas G., Hodges J.R., Watson P., Nestor P.J. Focal posterior cingulate atrophy in incipient Alzheimer's disease. Neurobiol Aging. 2010;31(1):25–33. doi: 10.1016/j.neurobiolaging.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 87.Friston K. Ten ironic rules for non-statistical reviewers. Neuroimage. 2012;61(4):1300–1310. doi: 10.1016/j.neuroimage.2012.04.018. [DOI] [PubMed] [Google Scholar]
- 88.Friston K.J., Holmes A.P., Worsley K.J. How many subjects constitute a study? Neuroimage. 1999;10(1):1–5. doi: 10.1006/nimg.1999.0439. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available with University of Alabama at Birmingham Institutional Review Board approval and data sharing agreement.