Abstract
Addition of a monoclonal antibody which binds the Cryptococcus neoformans capsule to suspensions of human monocytes, T lymphocytes, and cryptococcal cells (i) enhances interleukin-1β (IL-1β), tumor necrosis factor alpha, and IL-2 production; (ii) reduces IL-10 secretion; and (iii) promotes T-cell proliferation. The ability of specific antibody to influence cytokine production and lymphoproliferation suggests a mechanism by which humoral immunity can influence cell-mediated immunity.
Cryptococcus neoformans is a yeast-like fungus which causes incurable life-threatening meningoencephalitis in 5 to 10% of patients with AIDS. C. neoformans is unusual among fungal pathogens in that it has a polysaccharide capsule that is important for virulence (10). Cell-mediated immunity has been extensively implicated as an important defense mechanism against C. neoformans infection (13). In contrast, the role of natural antibody-mediated immunity in protection against C. neoformans is uncertain (7). In animal models of infection, there is convincing evidence that administration of preformed antibody to the polysaccharide capsule can prolong survival and reduce organ tissue fungal burden (12). The efficacy of some antibodies against C. neoformans has led to the development of a highly immunogenic polysaccharide-protein conjugate vaccine for the prevention of cryptococcal infection (2).
Granuloma formation has been temporally associated with control of C. neoformans infection in lung tissue (5). Capsular polysaccharide is released during infection into body tissues (11), and it may produce a variety of deleterious effects on host immunity (3, 9, 14, 15, 19, 20). Specific antibody is effective in clearing serum polysaccharide antigen from animals (6) and humans (7). Antibody-treated mice have earlier and better organized granuloma formation than do control mice after pulmonary infection (4). Administration of specific antibody to the polysaccharide capsule also enhances the formation of monocyte histiocytic rings in murine intraperitoneal infection; these rings may be precursors of granuloma formation (16, 17). The mechanism by which antibody administration enhances the inflammatory response is unknown.
In the present study, we tested the ability of a protective monoclonal antibody (MAb 2H1) to modulate cytokine ex-pression and T-cell response against C. neoformans. The experimental approach was to add MAb 2H1 to suspensions of monocytes, T lymphocytes, and C. neoformans cells and to measure supernatant cytokines and lymphoproliferation.
RPMI 1640 and fetal bovine serum were obtained from Eurobio Laboratories (Paris, France). Human serum was obtained from Biosource International (Camarillo, Calif.). Lipopolysaccharide (LPS) from Escherichia coli 055:135 was obtained from Difco Laboratories (Detroit, Mich.). Antiglucuronoxylomannan (anti-GXM) MAb (MAb 2H1) was isolated from ascites fluid as previously described (12). The RPMI 1640, fetal bovine serum, human serum, C. neoformans cells (approximately 5 × 108), and MAb 2H1 (50 μg/ml) were tested for endotoxin contaminations by L. amebocyte lysate assay (Sigma), which had a sensitivity of approximately 0.05 to 0.1 ng of E. coli LPS per ml. All reagents tested negative.
Two strains of C. neoformans var. neoformans were used: a serotype A thinly encapsulated strain (CBS 6995 = NIH 37; National Institutes of Health, Bethesda, Md.) and an acapsular mutant (CBS 7698 = NIH B-4131). The cultures were maintained by serial passage on Sabouraud agar (BioMerieux, Lyon, France). For our experiments, a single colony was grown and cells were collected as previously described (19). C. neoformans cells were killed by autoclaving. Mononuclear cells were separated by Ficoll-Hypaque density gradient centrifugation as previously described (20). Lymphocyte proliferation assays were done as previously described (18). In selected experiments, the cells were not pulsed with 3H[thymidine], supernatants were harvested after 3 or 7 days, and interleukin-10 (IL-10) or IL-2 levels were determined. Phenotypic analysis of proliferating T lymphocytes was evaluated by flow cytometry analysis as previously described (18).
To test for IL-1β and tumor necrosis factor alpha (TNF-α) production, supernatants were obtained as previously described (20). Cytokine levels in culture supernatants were measured with an enzyme-linked immunosorbent assay kit for human IL-1β, IL-2, and IL-10 (Seromed; Biochrom KG, Berlin, Germany) and a bioassay for TNF-α as previously described (20).
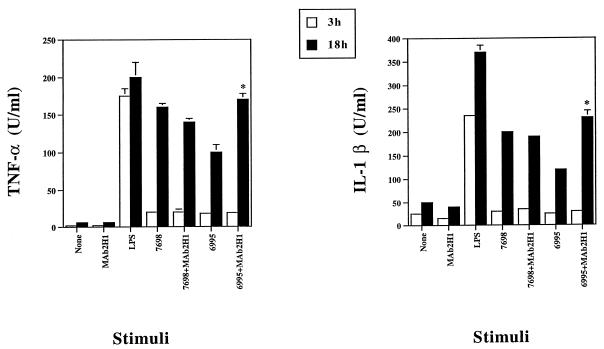
In the absence of MAb 2H1, coincubation of human monocytes with either the acapsular strain 7698 or the encapsulated strain 6995 at an effector-cell-to-target-cell (E-to-T) ratio of 1:1 stimulated TNF-α and IL-1β secretion after 18 h of incubation (Fig. 1). TNF-α and IL-1β secretion were higher for the acapsular strain than for the encapsulated strain, consistent with earlier reports that polysaccharide can down regulate TNF-α production (20). As shown in Fig. 1, addition of MAb 2H1 (10 μg/ml) significantly increased TNF-α and IL-1β production in response to the encapsulated strain but not the acapsular strain. In the presence of MAb 2H1, the levels of proinflammatory cytokine production in response to the encapsulated strain were similar to those observed for the acapsular strain. This result indicates that addition of a capsule-binding antibody can reverse the down-regulatory effect of the capsular polysaccharide.
FIG. 1.
TNF-α and IL-1β production by monocytes treated with LPS (10 μg/ml) or with a C. neoformans encapsulated (6995; E-to-T ratio, 1:1) or acapsular (7698; E-to-T ratio, 1:1) strain in the presence or absence of anti-GXM MAb (MAb 2H1; 10 μg/ml). Results are the means of four separate experiments from four different donors + standard errors of the means (SEMs). ∗, P < 0.01 (MAb 2H1 plus C. neoformans [6995 or 7698]-treated versus C. neoformans [6995 or 7698]-treated cells) according to Student’s t test.
Coincubation of monocytes, lymphocytes, and the acapsular C. neoformans strain resulted in an increase in IL-2 secretion (Table 1). Addition of MAb 2H1 had little or no effect on cytokine secretion in response to the acapsular strain. In contrast, coincubation of monocytes, lymphocytes, and the encapsulated strain significantly enhanced IL-10 and reduced IL-2 levels relative to those measured with the acapsular strain. Addition of MAb 2H1 to the encapsulated strain profoundly reduced IL-10 levels and enhanced IL-2 production.
TABLE 1.
Cytokine levels in supernatants of cocultures of Cryptococcus-laden monocytes plus T lymphocytes in the presence or absence of anti-GXM MAb (MAb 2H1)
| Stimulus(i) | Cytokine production (pg/ml)a
|
|
|---|---|---|
| IL-10 on day 3 | IL-2 on day 7 | |
| None | 2 ± 0 | 4 ± 0 |
| 7698 | 7 ± 1 | 29 ± 2 |
| 7698 + MAb 2H1b | 6 ± 1 | 28 ± 2 |
| 6995 | 42 ± 4 | 17 ± 1 |
| 6995 + MAb 2H1 | 3 ± 2c | 34 ± 2c |
IL-10 and IL-2 were determined in supernatant cocultures of monocytes laden with an encapsulated (6995) or acapsular (7698) strain of C. neoformans plus T lymphocytes. The results are the means of four separate experiments from four different donors ± SEMs.
MAb 2H1 (10 μg/ml) was added together with C. neoformans strains at the time of culture preparation.
P < 0.01 (MAb 2H1 plus C. neoformans [6995]-treated cells versus C. neoformans [6995]-treated cells) according to Student’s t test.
The polysaccharide capsule of C. neoformans is believed to contribute to virulence by interfering with the generation of antigen-specific T-cell responses (18). Since MAb 2H1 is a potent opsonin, we hypothesized that addition of MAb 2H1 would lead to phagocytosis and augment the capacity of peripheral blood monocytes (PBM) to induce T-cell proliferation. Consistent with this premise, coincubation of MAb 2H1-opsonized C. neoformans strains 7698 (acapsular) and 6995 (encapsulated) with T lymphocytes resulted in a significant increase in T-cell proliferation in response to the encapsulated strain but not the acapsular strain (Table 2). The acapsular strain lacks capsular polysaccharide, which is the antigen recognized by MAb 2H1. T-cell proliferation in response to C. neoformans was studied as a function of MAb 2H1 concentration for both the acapsular and encapsulated strains (Table 3). For the acapsular strain, addition of increasing amounts of MAb 2H1 had little or no effect on T-cell proliferation. For the encapsulated strain, the magnitude of T-cell proliferation increased with increasing amounts of MAb 2H1. Phenotypic analysis of proliferating T cells (in the absence or presence of MAb 2H1) to Cryptococcus (6995 or 7698)-laden monocytes was evaluated. Cytofluorometric analysis showed that the cells recovered were >70% CD4 positive.
TABLE 2.
Lymphoproliferative response of T lymphocytes [T(E+)] to monocytes (PBM) laden with an encapsulated (6995) or acapsular (7698) strain of C. neoformans in the presence or absence of anti-GXM MAb (MAb 2H1)
| Cells and treatment | Response (cpm) on day indicateda
|
|||
|---|---|---|---|---|
| 1 | 2 | 3 | 7 | |
| PBMb + T(E+)c | 300 ± 20 | 400 ± 30 | 510 ± 20 | 500 ± 20 |
| (PBM + MAb 2H1) + T(E+) | 310 ± 50 | 400 ± 20 | 500 ± 35 | 530 ± 50 |
| PBM + T(E+) + concanavalin A | 2,000 ± 140 | 28,500 ± 300 | 63,000 ± 500 | 50,700 ± 55 |
| (PBM + 7698) + T(E+) | 780 ± 100 | 1,500 ± 100 | 30,000 ± 980 | 43,000 ± 580 |
| (PBM + 7698 + MAb 2H1) + T(E+) | 780 ± 90 | 1,400 ± 90 | 26,000 ± 250 | 45,000 ± 1,610 |
| (PBM + 6995) + T(E+) | 650 ± 60 | 1,100 ± 120 | 15,000 ± 90 | 25,000 ± 1,100 |
| (PBM + 6995 + MAb 2H1) + T(E+) | 670 ± 60 | 1,300 ± 50 | 17,000 ± 90d | 29,404 ± 1,000d |
Proliferation of T lymphocytes was measured by [3H]thymidine incorporation at the indicated days. The results are the means of four separate experiments from four different donors ± SEMs.
PBM (2 × 104) were incubated with or without a C. neoformans encapsulated (6995) or acapsular (7698) strain (2 × 105) in the presence or absence of MAb 2H1 (10 μg/ml) for 2 h. Then, monocyte monolayers were washed.
Autologous T cells [T(E+), 105] were added subsequently.
P < 0.01 (MAb 2H1 plus C. neoformans [6995]-treated cells versus C. neoformans [6995]-treated cells) according to Student’s t test.
TABLE 3.
Proliferative response of T lymphocytes to monocytes laden with an encapsulated (6995) or acapsular (7698) strain of C. neoformans in the presence or absence of various doses of anti-GXM MAb (MAb 2H1)
| Treatment | Response (cpm) for straina
|
|
|---|---|---|
| 7968 | 6995 | |
| None | 37,000 ± 3,000 | 21,000 ± 1,600 |
| MAb 2H1b | ||
| 0.1 μg/ml | 38,000 ± 1,800 | 23,000 ± 3,000 |
| 0.25 μg/ml | 34,000 ± 1,500 | 21,000 ± 3,600 |
| 5 μg/ml | 39,000 ± 3,000 | 32,000 ± 1,900c |
| 10 μg/ml | 36,000 ± 5,000 | 36,000 ± 2,800c |
Proliferation of T lymphocytes was measured by [3H]thymidine incorporation after 7 days of incubation. The results are the means of four separate experiments from four different donors ± SEMs.
MAb 2H1 was added together with a C. neoformans encapsulated (6995) or acapsular (7698) strain (2 × 105) at the indicated doses to PBM (2 × 104) for 2 h. Then, monocyte monolayers were washed and, subsequently, autologous T cells [T(E+), 105] were added.
P < 0.01 (MAb 2H1 plus C. neoformans [6995]-treated versus C. neoformans [6995]-treated cells) according to Student’s t test.
Our results indicate that antibody to GXM can alter the cytokines produced by human cells in response to C. neoformans in vitro. Our observations are consistent with previous reports that immune complexes can induce IL-1 and TNF (8) production by human monocytes. However, our results are novel in that we observed simultaneous enhancement of TNF-α, IL-1β, and IL-2 production and down regulation of IL-10 after incubation of MAb 2H1, C. neoformans, monocytes, and T cells. Hence, addition of MAb altered the cytokine profile in cell supernatants to favor Th1-associated cytokines. The suppression of IL-10 produced by monocytes in conditions in which MAb 2H1 is added may reflect either neutralization of the IL-10-inducing properties of the C. neoformans polysaccharide or down regulation of IL-10 production by Fc receptor cross-linking or both.
Our results suggest two additional functions for antibody-mediated immunity: the modulation of cytokine synthesis and the enhancement of T-cell responses. The mechanism by which MAb 2H1 modulated cytokine production by monocytes may involve both Fc receptor cross-linking and related signal transduction events and/or neutralization of the down-regulating effects of cryptococcal polysaccharide. Similarly, MAb 2H1 could have enhanced T-cell proliferation by promoting phagocytosis and antigen presentation. Our observations suggest that the protective effects associated with antibody administration in animal models may be a result of enhanced cell-mediated immunity. The finding that specific antibody can affect cytokine secretion and T-cell proliferation provides a link between cellular and humoral immune responses and suggests that the presence of specific antibody may affect the cellular immune response.
Acknowledgments
We are grateful to Eileen Mahoney Zannetti for excellent secretarial and editorial assistance.
This work was supported by the National AIDS Research Program “Opportunistic Infections and Tuberculosis,” contract 50A.0.35, Italy. A. Casadevall is supported by NIH grants AI-13342 and AI-33774 and a Burroughs Wellcome Fund Developmental Therapeutics Award.
REFERENCES
- 1.Casadevall A. Antibody immunity and invasive fungal infections. Infect Immun. 1995;63:1725–1731. doi: 10.1128/iai.63.11.4211-4218.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Casadevall A, Mukherjee J, Devi S J N, Schneerson R, Robbins J B, Scharff M D. Antibodies elicited by a Cryptococcus neoformans glucuronoxylomannan-tetanus toxoid conjugate vaccine have the same specificity as those elicited in infection. J Infect Dis. 1992;65:1086–1093. doi: 10.1093/infdis/165.6.1086. [DOI] [PubMed] [Google Scholar]
- 3.Dong Z M, Murphy J W. Effects of the two varieties of Cryptococcus neoformans cells and culture filtrate antigens on neutrophil locomotion. Infect Immun. 1995;63:2632–2644. doi: 10.1128/iai.63.7.2632-2644.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feldmesser M, Casadevall A. Effect of IgG1 in murine pulmonary Cryptococcus neoformans infection. J Immunol. 1997;158:790–799. [PubMed] [Google Scholar]
- 5.Goldman D L, Lee S C, Casadevall A. Pathogenesis of pulmonary Cryptococcus neoformans infection in the rat. Infect Immun. 1994;62:4755–4761. doi: 10.1128/iai.62.11.4755-4761.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldman D L, Lee S C, Casadevall A. Tissue localization of Cryptococcus neoformans glucoronoxylomannan in the presence and absence of specific antibody. Infect Immun. 1995;63:3448–3453. doi: 10.1128/iai.63.9.3448-3453.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gordon M A, Casadevall A. Serum therapy of cryptococcal meningitis. Clin Infect Dis. 1995;21:1477–1479. doi: 10.1093/clinids/21.6.1477. [DOI] [PubMed] [Google Scholar]
- 8.Hoffman T, Tripathi A K, Lee Y L, Bonvini E, Golding B. Inflammatory mediator release from human monocytes via immobilized Fc receptors. Transplantation. 1992;54:343–346. doi: 10.1097/00007890-199208000-00027. [DOI] [PubMed] [Google Scholar]
- 9.Kozel T R, Gotschlich E. The capsule of Cryptococcus neoformans passively inhibits phagocytosis of the yeast by macrophages. J Immunol. 1982;129:1675–1680. [PubMed] [Google Scholar]
- 10.Kwon-Chung K J, Rhodes J C. Encapsulation and melanin formation as indicators of virulence in Cryptococcus neoformans. Infect Immun. 1986;51:218–223. doi: 10.1128/iai.51.1.218-223.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee S C, Casadevall A, Dickson D W. Immunohistochemical localization of capsular polysaccharide antigen in the central nervous system cells in cryptococcal meningoencephalitis. Am J Pathol. 1996;148:1267–1274. [PMC free article] [PubMed] [Google Scholar]
- 12.Mukherjee S, Lee S, Mukherjee J, Scharff M D, Casadevall A. Monoclonal antibodies to Cryptococcus neoformans capsular polysaccharide modify the course of intravenous infection in mice. Infect Immun. 1994;62:1079–1088. doi: 10.1128/iai.62.3.1079-1088.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murphy J W. Cytokine profiles associated with induction of the anticryptococcal cell-mediated immune response. Infect Immun. 1993;61:4750–4759. doi: 10.1128/iai.61.11.4750-4759.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murphy J W, Cozad G C. Immunological unresponsiveness induced by cryptococcal polysaccharide assayed by the hemolytic plaque technique. Infect Immun. 1972;5:896–901. doi: 10.1128/iai.5.6.896-901.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pettoello-Mantovani M, Casadevall A, Kollman T R, Rubinstein A, Goldstein H. Enhancement of HIV-1 infection by the capsular polysaccharide of Cryptococcus neoformans. Lancet. 1992;339:21–23. doi: 10.1016/0140-6736(92)90142-p. [DOI] [PubMed] [Google Scholar]
- 16.Schneerson-Portat S, Sharar A, Aronson M. Formation of histiocyte rings in response to Cryptococcus neoformans infection. Res J Reticuloendothel Soc. 1965;2:249–255. [PubMed] [Google Scholar]
- 17.Sharar A, Kletter Y, Aronson M. Granuloma formation in cryptococcosis. Isr J Med Sci. 1969;5:1164–1172. [PubMed] [Google Scholar]
- 18.Veccharelli A, Pietrella D, Dottorini M, Monari C, Retini C, Todisco T, Bistoni F. Encapsulation of Cryptococcus neoformans regulates fungicidal activity and the antigen presentation process in human alveolar macrophages. Clin Exp Immunol. 1994;98:217–223. doi: 10.1111/j.1365-2249.1994.tb06128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vecchiarelli A, Retini C, Monari C, Tascini C, Bistoni F, Kozel T R. Purified capsular polysaccharide of Cryptococcus neoformans enhances IL-10 secretion by human monocytes. Infect Immun. 1996;64:2846–2849. doi: 10.1128/iai.64.7.2846-2849.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vecchiarelli A, Retini C, Pietrella D, Monari C, Tascini C, Beccari T, Kozel T R. Downregulation by cryptococcal polysaccharide of tumor necrosis factor alpha and interleukin-1β secretion from human monocytes. Infect Immun. 1995;63:2919–2923. doi: 10.1128/iai.63.8.2919-2923.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]