Abstract
Background
Spinal cord (SC) lesions have been associated with unfavourable clinical outcomes in multiple sclerosis (MS). However, the relation of whole SC lesion number (SCLN) and volume (SCLV) to the future occurrence and type of confirmed disability accumulation (CDA) remains largely unexplored.
Methods
In this monocentric retrospective study, SC lesions were manually delineated. Inclusion criteria were: age between 18 and 60 years, relapsing-remitting MS, disease duration under 2 years and clinical follow-up of 5 years. The first CDA event after baseline, determined by a sustained increase in the Expanded Disability Status Scale over 6 months, was classified as either progression independent of relapse activity (PIRA) or relapse-associated worsening (RAW). SCLN and SCLV were compared between different (sub)groups to assess their prospective value.
Results
204 patients were included, 148 of which had at least one SC lesion and 59 experienced CDA. Patients without any SC lesions experienced significantly less CDA (OR 5.8, 95% CI 2.1 to 19.8). SCLN and SCLV were closely correlated (rs=0.91, p<0.001) and were both significantly associated with CDA on follow-up (p<0.001). Subgroup analyses confirmed this association for patients with PIRA on CDA (34 events, p<0.001 for both SC lesion measures) but not for RAW (25 events, p=0.077 and p=0.22).
Conclusion
Patients without any SC lesions are notably less likely to experience CDA. Both the number and volume of SC lesions on MRI are associated with future accumulation of disability largely independent of relapses.
Keywords: multiple sclerosis, MRI, clinical neurology, image analysis
WHAT IS ALREADY KNOWN ON THIS TOPIC
Spinal cord (SC) lesions contribute considerably to the clinical picture of multiple sclerosis (MS). The vast majority of MRI studies on MS have covered the SC incompletely, if at all. Consequently, the prognostic value of lesions and their volume across the whole SC remain largely unexplored.
WHAT THIS STUDY ADDS
We evaluated the prognostic value of SC lesion number and volume, measured across the whole SC, in regard to confirmed disability accumulation (CDA) on follow-up, either by relapse-associated worsening (RAW) or progression independent of relapses (PIRA).
Our single-centre study comprised 5-year follow-up data from 204 patients with early relapsing-remitting MS.
Patients without SC lesions were found to be notably less likely to experience CDA on follow-up. The number and volume of SC lesions were associated with PIRA (but not RAW).
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
MRI of the whole SC in early MS may allow for better informed treatment decisions in the individual patient.
Introduction
Spinal cord (SC) pathology is a central component of multiple sclerosis (MS), a chronic disorder of the central nervous system comprising both inflammatory and neurodegenerative elements. However, while MRI of the brain is firmly established in both the clinical and research setting, SC imaging is less routinely performed. This can likely be attributed to longer scanning times, the comparatively small cross-sectional area of the SC as well as the susceptibility to imaging artefacts of various origins.1
Atrophy and lesion load are the main two surrogate parameters used to monitor the state of SC pathology via MRI. While the former can only be meaningfully assessed longitudinally or through group comparison, demyelinating lesions are readily detectable on MRI in the individual patient. Although a close association between SC lesions and disability seems anatomically plausible, research findings have been ambivalent in this regard: Some groups have found (mostly moderate) correlations between SC lesion load and disability measured by the Expanded Disability Status Scale (EDSS),2–5 while others have not.6–8 Besides playing an essential role in the diagnosis of MS,9 SC lesions have also been investigated in the context of disease prognosis. They have been identified as a risk factor for conversion to definite MS10 11 with shorter conversion times12 and as a predictor of future disability,13–18 more so than brain MRI measures.19 Here too, other groups have come to contrary conclusions,8 20 especially when looking at asymptomatic SC lesions.21 22 Evidence further suggests that individual lesion extent may be a relevant factor in this context, as larger lesions have been linked to more severe disability23 and worse long-term outcomes.24
Recently, the traditional differentiation between relapsing and progressive disease courses has been challenged by showing that, even in relapsing-remitting MS (RRMS) patients, most confirmed disability accumulation (CDA) happens independently of overt relapses.25–27 This progression independent of relapse activity (PIRA)—as opposed to relapse-associated worsening (RAW)—seems to already occur in the early course of MS27–29 and to be associated with accelerated rates of brain atrophy.25 30
A majority of the studies examining the association between SC lesions and clinical outcome in MS relied on only partial coverage of the SC. Moreover, in many cases, no combined analysis of axial and sagittal slices was performed, which is known to improve detectability of SC lesions.31–33 Finally, only two studies have used volumetric SC lesion data for prognostic purposes, and both of them were restricted to the cervical spine.3 8 In this retrospective study of a large cohort of RRMS patients with full axial and sagittal MRI coverage of the SC, we investigated the prognostic values of two different SC lesion measures, namely SC lesion number (SCLN) and SC lesion volume (SCLV), in respect to the occurrence and the type of disease worsening (PIRA vs RAW) on clinical follow-up.
Methods
Participants
All patients gave written informed consent for the use of their clinical and paraclinical data for research purposes. Inclusion criteria were a diagnosis of RRMS or clinically isolated syndrome (CIS) with conversion on follow-up, an age between 18 and 60 years, a disease duration of less than 2 years, and at least one MRI with sagittal and axial coverage of the whole SC. To achieve a uniform definition, all patients were reclassified according to the 2017 diagnostic criteria.9 Further inclusion criteria were the availability of an EDSS score as well as a standardised quality-checked and processed brain MRI, both within half a year of the SC MRI. If the SC MRI coincided with a relapse, the closest EDSS score without relapse symptoms was chosen as baseline. If no EDSS score existed that conformed to these criteria, the patient was excluded from further analyses. Finally, a clinical follow-up of at least 5 years had to be available in patients who did not experience a CDA event beforehand. The relation of baseline clinical, electrophysiological and SC MRI data of our cohort has been reported recently.5 34
Definition of disability accumulation and subtypes
In accordance with previous work, CDA was defined as an EDSS increase of 1.5 for baseline EDSS scores of 0, an increase of 1 for baseline EDSS scores between 1.0 and 5.0, and an increase of 0.5 for baseline EDSS scores of 5.5 or higher; the increase had to be confirmed on clinical follow-up over at least 6 months.25 29 35 CDA was classified as PIRA if there were no clinical relapses during the 90 days before the EDSS increase and during the period of at least 6 months between the EDSS increase and the confirmation of disability worsening.28–30 CDA events that did not meet these criteria were classified as RAW.
MRI acquisition and processing
SC MRI was performed on three different 3 Tesla scanners (Philips Ingenia, Philips Achieva dStream, Siemens Magnetom Verio). A spine coil was used and optionally an anterior body coil. All scans included two-dimensional T2-weighted (w) turbo spin echo sequences in sagittal and axial orientation. Sagittal scans had a slice thickness of 2 mm with a gap of 0.2 mm, axial scans had a slice thickness of 4 mm with a gap of 1 mm. Typical field of view (FOV) of axial scans was 115 mm with an in-plane spatial resolution of 0.4 mm (ranging from 0.3 mm to 0.5 mm); they were acquired in three consecutive stacks. Sagittal scans had a typical FOV of 250 mm with an in-plane spatial resolution of 0.9 mm (ranging from 0.8 mm to 1 mm).
All scans were converted to NIFTI file format and segmented with the software BrainSeg3D, V.2.2.1. Lesions were segmented manually as described previously.5 34 As acquisitions were planned manually in clinical routine, a few slices were missed in some cases; the regions T6 and T7 (border between middle and caudal stack), and L1 (conus medullaris) were covered by >90% of scans and the remaining regions by >95%.5
Brain MRI was performed on one 3 Tesla scanner (Achieva, Philips Medical Systems, Netherlands). Standardised brain MRI comprised a three-dimensional (3D) spoiled gradient echo T1-weighted (T1w) sequence (voxel size=1 mm isotropic, TR=9 ms, TE=4 ms) and a turbo-spin echo T2-weighted fluid-attenuated inversion recovery (FLAIR) sequence (voxel size=1.0×1.0×1.5 mm, TR=10 000 ms, TE=140 ms, TI=2750 ms). Brain images were processed with the software package SPM12 and its Computational Anatomy Toolbox 12, V.916, as well as the lesion segmentation tool (LST), V. 2.0.15. White matter lesions were segmented from FLAIR and T1-weighted images by the lesion growth algorithm36 as implemented in LST.
Statistical analysis
The Shapiro-Wilk test was performed to determine whether a variable was normally distributed. Since this was the case for neither SCLN nor SCLV, non-parametric tests were chosen for subsequent analyses. Spearman’s rank correlation coefficient (rs) was used for simple correlations. To determine the OR for two dichotomous variables, Fisher’s exact test was used. For group comparisons between patients with and without CDA and patients with PIRA versus RAW, two-sided, independent Wilcoxon-Mann-Whitney tests were performed. To control for potential confounders, logistic regression was performed in both of these cases according to the following general model: clinical outcome parameter=β0+β1 SC lesion measure+ β2 covariate1+β3 covariate 2, etc. To approach normal distribution, the natural logarithm of all lesion measures was entered into the models (adding 1 to both variables beforehand to avoid negative and undefined values). Covariates entered into the models were EDSS at baseline, age, disease duration, sex and disease-modifying therapy (DMT). DMT was defined as present if one disease-modifying drug (DMD) was taken without interruption for at least half the 5-year follow-up or half the time to the first CDA event. DMDs were divided into three levels according to drug efficacy, following the German guideline for diagnosis and treatment of MS (0=no therapy; 1=dimethyl fumarate, glatiramer acetate, interferon, teriflunomide; 2=cladribine, fingolimod, siponimod; 3=alemtuzumab, natalizumab, ocrelizumab, rituximab). All variables were entered into the model initially, followed by a backward elimination process of the variable with the lowest t-value, until p<0.1 was true for all remaining predictors. Statistical comparison of the area under the curve (AUC) for two receiver operating characteristic (ROC) curves was done according to the method described by DeLong et al 37 for paired data. All statistical and graphical analyses were done using the software R (4.1.2) and its packages patchwork, pROC, table1 and tidyverse. P values <0.05 were considered statistically significant.
We used the STROBE (STrengthening the Reporting of OBservational studies in Epidemiology) checklist when writing our report.38
Results
Study participants
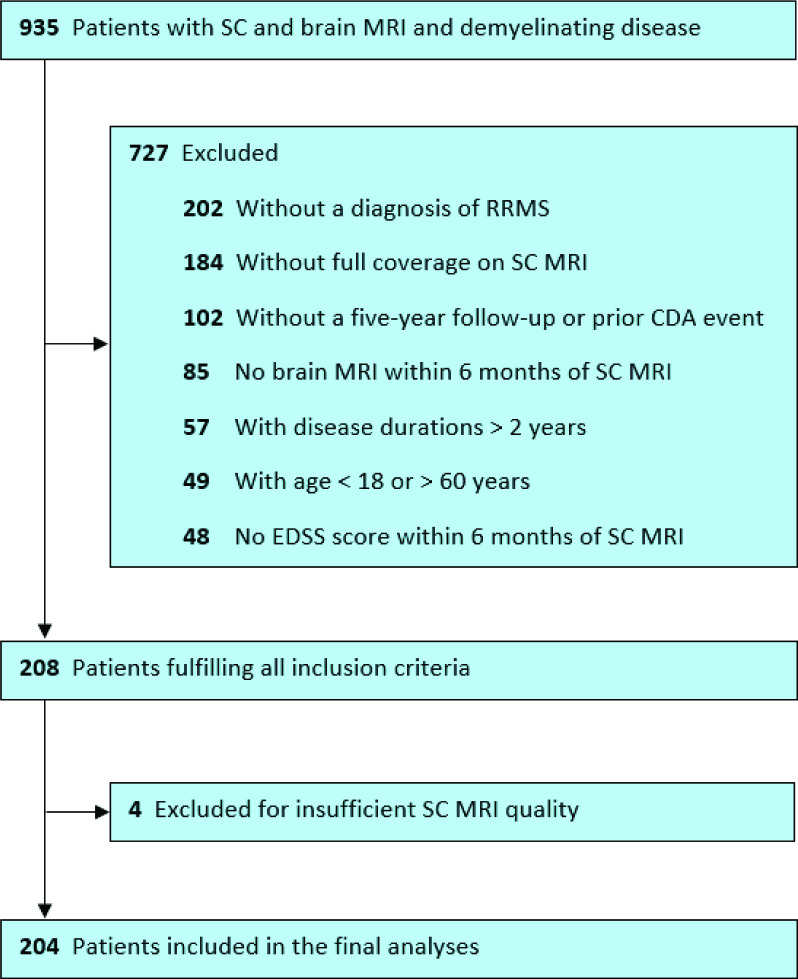
In our database, we identified 935 patients with a demyelinating disease and available brain and SC MRI; 208 patients fulfilled all predefined criteria. Four patients were excluded because of low SC MRI quality, leaving 204 datasets for the final analysis. A patient flowchart is given in figure 1. The median interval between the acquisition of the SC and brain scans was 0.079 years (min: 0, max: 0.48). Key characteristics of this cohort are summarised in table 1.
Figure 1.
Patient flow chart. CDA, confirmed disability accumulation; EDSS, Expanded Disability Status Scale; RRMS, relapsing-remitting multiple sclerosis; SC, spinal cord.
Table 1.
Characteristics of study participants
| Total (n=204) | No CDA (n=145) | CDA (n=59) | PIRA (n=34) | RAW (n=25) | |
| Age at baseline (years) | |||||
| Mean (SD) | 34 (9.2) | 34 (8.9) | 36 (9.6) | 36 (9.7) | 36 (9.5) |
| Median (min, max) | 34 (18, 60) | 34 (18, 55) | 36 (19, 60) | 35 (19, 60) | 37 (21, 58) |
| Sex | |||||
| Female | 137 (67.2%) | 94 (64.8%) | 43 (72.9%) | 25 (73.5%) | 18 (72.0%) |
| Male | 67 (32.8%) | 51 (35.2%) | 16 (27.1%) | 9 (26.5%) | 7 (28.0%) |
| EDSS at baseline | |||||
| Mean (SD) | 1.2 (1.1) | 1.1 (1.1) | 1.3 (1.3) | 1.6 (1.5) | 0.94 (0.92) |
| Median (min, max) | 1.0 (0, 6.5) | 1.0 (0, 5.0) | 1.0 (0, 6.5) | 1.0 (0, 6.5) | 1.0 (0, 3.0) |
| Disease duration at baseline (years) | |||||
| Mean (SD) | 0.18 (0.39) | 0.15 (0.36) | 0.24 (0.46) | 0.35 (0.57) | 0.097 (0.15) |
| Median (min, max) | 0.052 (0.0027, 1.9) | 0.052 (0.0027, 1.9) | 0.055 (0.0027, 1.7) | 0.062 (0.0027, 1.7) | 0.047 (0.0082, 0.58) |
| DMT | |||||
| None | 44 (21.6%) | 35 (24.1%) | 9 (15.3%) | 5 (14.7%) | 4 (16.0%) |
| Level 1 | 120 (58.8%) | 87 (60.0%) | 33 (55.9%) | 20 (58.8%) | 13 (52.0%) |
| Level 2 | 27 (13.2%) | 17 (11.7%) | 10 (16.9%) | 3 (8.8%) | 7 (28.0%) |
| Level 3 | 13 (6.4%) | 6 (4.1%) | 7 (11.9%) | 6 (17.6%) | 1 (4.0%) |
| No of spinal lesions | |||||
| Mean (SD) | 2.6 (3.1) | 2.1 (2.8) | 3.9 (3.4) | 4.6 (3.5) | 3.0 (3.1) |
| Median (min, max) | 2.0 (0, 17) | 1.0 (0, 17) | 3.0 (0, 16) | 3.0 (0, 16) | 2.0 (0, 13) |
| Total lesion volume spine (mL) | |||||
| Mean (SD) | 0.25 (0.44) | 0.17 (0.30) | 0.44 (0.64) | 0.63 (0.77) | 0.19 (0.23) |
| Median (min, max) | 0.079 (0, 3.7) | 0.059 (0, 1.8) | 0.22 (0, 3.7) | 0.38 (0, 3.7) | 0.065 (0, 0.85) |
| No of brain lesions | |||||
| Mean (SD) | 18 (11) | 17 (11) | 20 (10) | 21 (11) | 18 (8.8) |
| Median (min, max) | 16 (0, 59) | 15 (0, 59) | 18 (4.0, 43) | 19 (5.0, 43) | 18 (4.0, 36) |
| Total lesion volume brain (mL) | |||||
| Mean (SD) | 5.3 (9.5) | 4.5 (7.7) | 7.3 (13) | 9.1 (13) | 4.9 (12) |
| Median (min, max) | 2.5 (0, 67) | 2.1 (0, 61) | 2.9 (0.13, 67) | 4.1 (0.51, 67) | 2.4 (0.13, 60) |
CDA, confirmed disability accumulation; DMT, disease-modifying therapy; EDSS, Expanded Disability Status Scale; max, maximum; min, minimum; PIRA, progression independent of relapse activity; RAW, relapse-associated worsening.
Association of baseline lesion measures with disability worsening on follow-up
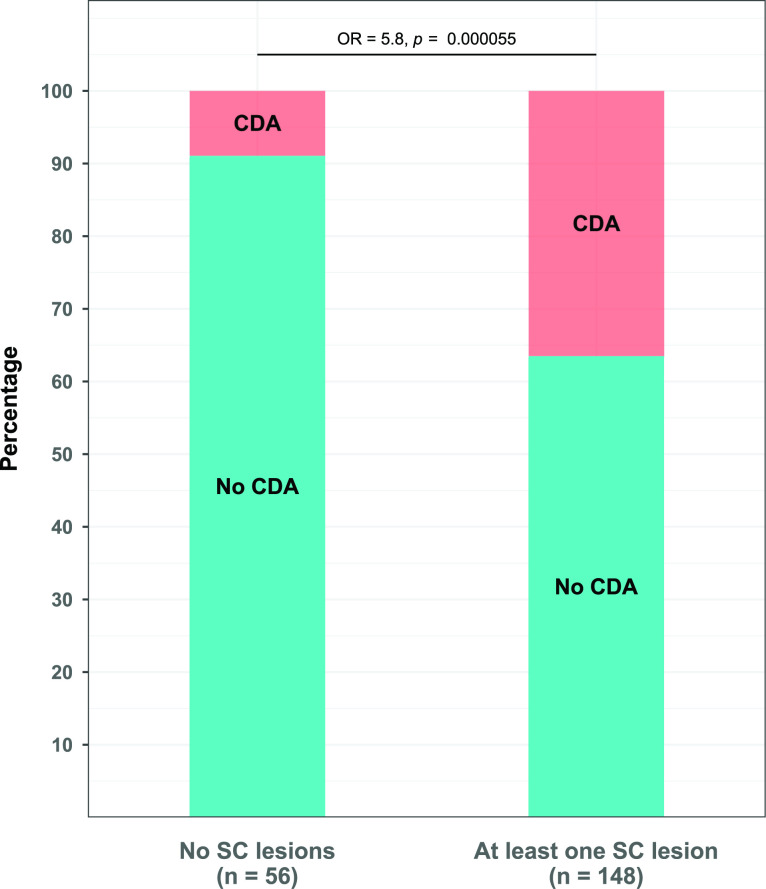
Fifty-nine of the 204 patients from the cohort experienced CDA during follow-up (28.9%) and 148 had at least one SC lesion on MRI (72.5%). Among the 56 patients without SC lesions, 5 experienced CDA during follow-up (8.9%). In comparison, 54 patients with at least one SC lesion had CDA during the 5 years of follow-up (36.5%), corresponding to an OR of 5.8 (95% CI 2.1 to 19.8, p<0.001, figure 2). SCLN and SCLV were strongly associated (rs=0.91, p<0.001). Additionally, there was a positive correlation between both lesion measures and EDSS score at baseline (SCLN: rs=0.22, p=0.0013; SCLV: rs=0.23, p=0.0012). These associations remained similar when only taking into account the 148 patients with at least one SC lesion (SCLN and SCLV: rs=0.77, p<0.001; EDSS and SCLN: rs=0.25, p=0.0023; EDSS and SCLV: rs=0.26, p=0.0018).
Figure 2.
Rate of CDA on follow-up in patients with and without SC lesions. Bar plot showing percentages of disability worsening in both groups. Fisher’s exact test was performed for between-group comparison. CDA, confirmed disability accumulation; SC, spinal cord.
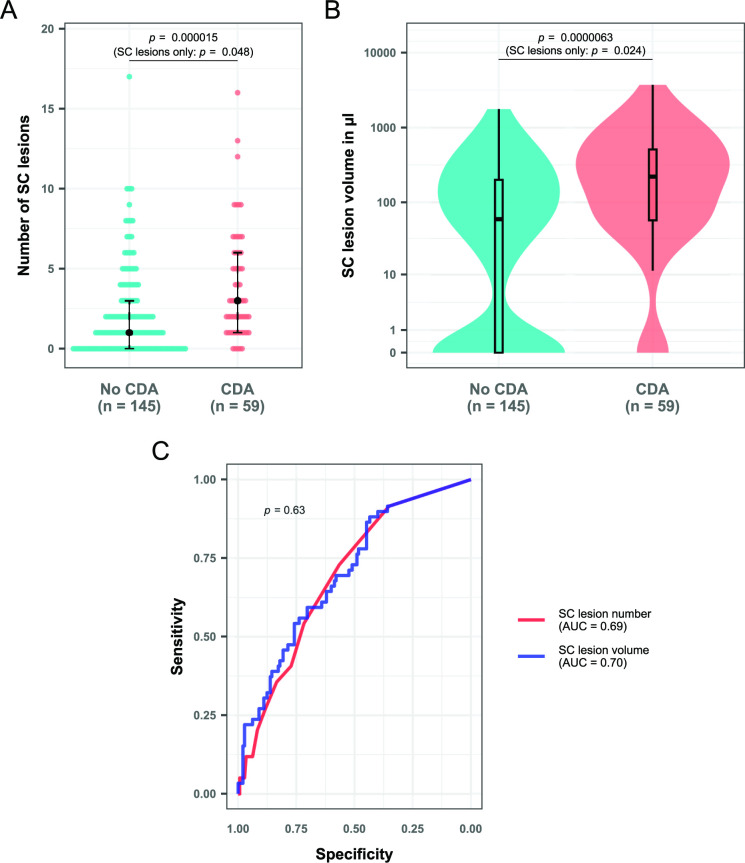
Patients with CDA had significantly more SC lesions (median=3 vs 1, p<0.001, figure 3A) and higher SCLV (median=0.22 vs 0.059 mL, p<0.001, figure 3B) on MRI than patients without clinical worsening. These findings remained significant when controlling for EDSS at baseline, age, sex, disease duration and DMT in a logistic regression model (p<0.001 for both SCLN and SCLV). Similar trends were observed for brain lesion parameters, although without reaching statistical significance (brain lesion number: median=18 vs 15, p=0.058; brain lesion volume: median=2.9 vs 2.1 mL, p=0.069). There was no relevant difference between the prognostic value of SCLN and SCLV for CDA on follow-up assessed through ROC analysis (AUC: 0.69 vs 0.70, p=0.63, figure 3C).
Figure 3.
SC lesion measures in patients with and without CDA on follow-up. (A) Dot plot showing the number of spinal lesions in both groups. The black dots represent the median for the respective group, while the black lines give the IQR. (B) Box/violin plot showing lesion volume in both groups. The y-axis has been log-scaled for better visualisation. P values in parentheses are calculated for the subgroup of patients with at least one spinal lesion. Wilcoxon-Mann Whitney tests were performed in all cases. (C) Receiver operating characteristic curves for both SC lesion measures with CDA as outcome parameter. Between-curve comparison was performed according to the method described by DeLong et al 37 for paired data. AUC, area under the curve; CDA, confirmed disability accumulation; SC, spinal cord.
To account for the relatively high number of zero values among SC lesion measures (27.5%), subgroup analyses of only patients with one or more SC lesions were performed. Here, the association between SC lesion measures and CDA was still present, although statistically weaker (SCLN: median=3 vs 2, p=0.048; SCLV: median=0.26 vs 0.14 mL, p=0.024). We once again controlled for EDSS at baseline, age, sex, disease duration and DMT, after which both parameters remained significantly different between the two groups (SCLN: p=0.046; SCLV: p=0.018). The association between baseline brain lesion parameters and CDA on follow-up did not reach statistical significance in this subgroup analysis (brain lesion number: median=19 vs 15.5, p=0.16; brain lesion volume: median=3.2 vs 2.5 mL, p=0.098).
No correlation was found between the time to CDA and either SC or brain lesion measures (p>0.05 in all cases).
Association of baseline lesion measures with type of disability worsening
Of the 59 CDA events, 34 were classified as PIRA (57.6%) and 25 as RAW (42.4%). Again, there was no association between the time to CDA and lesion parameters for either PIRA or RAW (p>0.05 in all cases). RAW events took place slightly earlier during follow-up than PIRA events (median=2.1 vs 2.8 years, p=0.050).
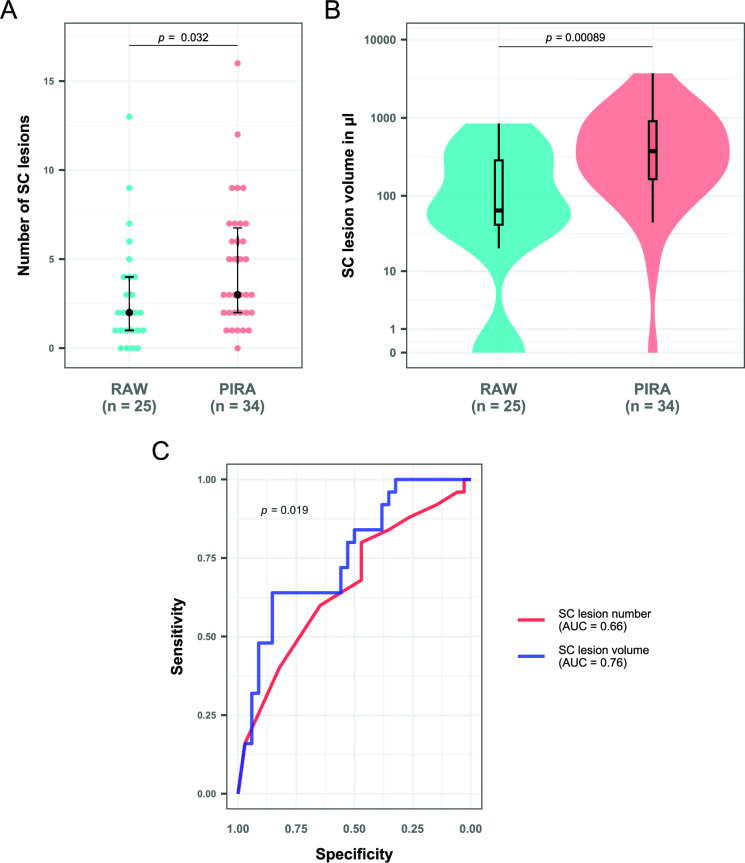
Patients with PIRA on follow-up had significantly higher SCLN and SCLV at baseline than those without CDA (SCLN: median=3 vs 1, p<0.001; SCLV: median=0.38 vs 0.059 mL, p<0.001). This association was still present in a subgroup analysis of only patients with one or more SC lesions (SCLN: median=3 vs 2, p=0.013; SCLV: median=0.40 vs 0.14 mL, p<0.001). There was no difference in SC lesion measures between patients with a RAW event and patients without CDA (SCLN: median=2 vs 1, p=0.077; SCLV: median=0.065 vs 0.059 mL, p=0.22). Furthermore, patients with CDA classified as PIRA had a significantly higher SCLN (median=3 vs 2, p=0.032, figure 4A) and higher SCLV (median=0.38 vs 0.065 mL, p<0.001, figure 4B) on baseline MRI than patients with CDA classified as RAW. The latter association remained significant after controlling for EDSS at baseline, age, sex, disease duration and DMT (SCLN: p=0.032; SCLV: p=0.0062). ROC analysis identified SCLV as a significantly better prognostic parameter than SCLN regarding the occurrence of PIRA versus RAW (AUC: 0.76 vs 0.66, p=0.019, figure 4C)
Figure 4.
SC lesion measures in patients with either RAW or PIRA activity as a confirmed disability accumulation event on follow-up. (A) Dot plot showing the number of spinal lesions in both groups. The black dots represent the median for the respective group, while the black lines give the IQR. (B) Box/violin plot showing lesion volume in both groups. The y-axis has been log-scaled for better visualisation. Wilcoxon-Mann Whitney tests were performed in all cases. (C) Receiver operating characteristic curves for both SC lesion measures with PIRA versus RAW as outcome parameter. Between-curve comparison was performed according to the method described by DeLong et al 37 for paired data. AUC, area under the curve; PIRA, progression independent of relapse activity; RAW, relapse-associated worsening; SC, spinal cord.
While a similar trend regarding PIRA versus RAW events was observed for brain lesion volume on baseline MRI (median=4.1 vs 2.4 mL, p=0.050), no significant associations were found for the number of brain lesions (median=18.5 vs 18, p=0.60). When comparing only patients with PIRA events to patients without CDA, brain lesion volume was significantly higher in patients with PIRA (median=4.1 vs 2.1 mL, p=0.011) whereas the number of brain lesions was not (median=18.5 vs 15, p=0.075). No associations with either brain lesion parameter were observed for patients with RAW events versus patients without CDA (brain lesion number: median=18 vs 15, p=0.29; brain lesion volume: median=2.4 vs 2.1 mL, p=0.99).
Discussion
In this retrospective, longitudinal analysis of 204 people with RRMS, we found whole SC lesion measures to be a significant prognostic factor in determining the occurrence and type of disability accumulation during a 5-year follow-up. This association was largely driven by patients who experienced CDA independently of relapse activity, that is, PIRA, in contrast to RAW. While the number and volume of SC lesions were interchangeable in their association with CDA, SCLV was significantly better in differentiating between PIRA and RAW.
The fraction of patients experiencing CDA during follow-up in our cohort (28.9%) was well within the range reported in recent literature with similar definitions of disability worsening when taking into account the differences in baseline parameters and follow-up time (23.6% in Kappos et al 26; 25.9% in Prosperini et al 35; 37.1% in both Cree et al 25 and Tur et al 29; 45.4% in Portaccio et al 27). One study with a large number of RRMS patients (n=24 469), however, observed much lower disability accumulation rates (7.2% in Lublin et al 28). This discrepancy might be due to the enormous heterogeneity in follow-up time (less than one to more than 15 years) and disease duration (38.6% over 10 years) in that particular study.
The ratio of PIRA to RAW among patients with CDA in our cohort (57.6% vs 42.2%) was among the lower ones encountered in similarly conceived studies (Kappos et al 26: 83.3% vs 17.7%; Lublin et al 28; 63.7% vs 36.3%; Portaccio et al 27; 60.7% vs 39.3%; Prosperini et al 35; 46.6% vs 53.4%). However, patients with PIRA tend to be older and have longer disease courses.27 30 Many of our patients had their baseline MRI when developing MS symptoms for the first time, resulting in a short median disease duration of only 0.05 years. Consequently, we believe that our cohort was unsuitable for detecting a potential association between PIRA and disease duration or age.
In line with earlier observations, we found brain lesion parameters to be notably weaker prognostic factors for disability worsening than SC lesion measures.19 However, we did find a significant difference in baseline brain lesion volume between patients with PIRA and clinically stable patients, which was not seen in another study.30 As was the case for SC lesion measures, this difference was not present between patients with RAW and clinically stable patients. Hence, brain lesion parameters showed similar but weaker associations compared with their spinal counterparts.
Surprisingly, there was no clear advantage to the rather cumbersome and time-consuming task of SC lesion volumetry over simply counting lesions. Both parameters, SCLN and SCLV, were closely correlated and, accordingly, showed very similar associations (with the exception of distinguishing between PIRA and RAW). In other words, volumetry would not have been necessary to obtain the most clinically significant result of this study: an OR of 5.8 for the absence of CDA in patients without any SC lesions. However, considering that larger individual SC lesions have been linked to more severe disability and progressive disease courses in previous studies, both cross-sectionally7 23 and longitudinally,24 the significant association between SCLV and PIRA on follow-up in our data does seem noteworthy. Moreover, the fact that these lesion patterns and clinical courses were observed in our cohort of very early RRMS patients as defined by current standards9 may give further credence to the emerging conception of MS as a continuum rather than a readily subdivisible disease entity.26 Nonetheless, routine SC lesion volumetry currently does not seem advisable from a clinical standpoint. In addition, the clinical and prognostic value of longitudinal SC imaging remains an open question for future studies.
Our study has some limitations. Although we tried to ensure a common temporal framework by restricting disease duration to under 2 years and requiring a follow-up of 5 years in every patient, a prospective design would certainly have been preferable. Furthermore, our decision to classify only the first CDA event during follow-up as either PIRA or RAW without evaluation of potential later events was related to the relatively low overall number of patients with disability worsening (n=59). Since a study with more than double the cohort size only found 14 patients that experienced both PIRA and RAW,30 this number would probably have been even lower in our case. Thus, further subgroup analyses were not undertaken assuming insufficient statistical power. Compared with our sequences, a 3D gradient-recalled echo might have led to better lesion detection, as has been shown for the cervical cord.39 No attempt at differentiating between symptomatic and asymptomatic SC lesions was made.21 22 Finally, the use of three different MRI scanners has likely increased the heterogeneity of our data but may, on the other hand, have enhanced the generalisability of our results.
We conclude that SCLN and SCLV are both of higher prognostic value than their brain counterparts in patients with MS and are more closely associated with PIRA than with RAW on follow-up. The absence of any SC lesions is a significant protective factor and has a stronger bearing on prognosis than the quantification of SC lesion load.
Acknowledgments
We are grateful for the invaluable contributions of the study participants as well as the assistance of the support staff at our clinic.
Footnotes
Contributors: ML designed the study, analysed the data and co-wrote the manuscript. JM contributed to the processing of the data. MB contributed to the acquisition and processing of the data and commented on the manuscript. MEH contributed to the processing of the data. VP contributed to the data acquisition and commented on the manuscript. CE contributed to the data acquisition. AW contributed to the data acquisition. AB contributed to the data acquisition. IR contributed to the data acquisition and commented on the manuscript. JSK contributed to the acquisition, processing, and analysis of the data, and commented on the manuscript. CZ contributed to the acquisition, processing and analysis of the data. BH contributed to the acquisition, processing and analysis of the data, and commented on the manuscript. MM designed the study, analysed the data and co-wrote the manuscript. Guarantor: MM.
Funding: ML received funding through the 'Kommission für klinische Forschung' (KKF) of the School of Medicine, Technical University of Munich (TUM). MM received funding by the Bavarian State Ministry for Science and Art (Collaborative Bilateral Research Program Bavaria—Québec: AI in medicine, grant F.4-V0134.K5.1/86/34), by the German Research Foundation (DFG SPP2177, Radiomics: Next Generation of Biomedical Imaging—project number 428223038), and by the National Institutes of Health (grant 1R01NS112161-01). BH received funding for the study by the MultipleMS EU consortium, the Clinspect-M consortium funded by the Bundesministerium für Bildung und Forschung, and the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany’s Excellence Strategy within the framework of the Munich Cluster for Systems Neurology (EXC 2145 SyNergy—ID 390857198). BH is associated with DIFUTURE (Data Integration for Future Medicine, BMBF 01ZZ1804(A-I)).
Competing interests: VP has received research funding from Novartis (Oppenheim Förderpreis 2017). AB has received consulting and/or speaker fees from Alexion, Biogen, Celgene, Horizon, Novartis, Roche and Sandoz/Hexal. His institution has received compensation for clinical trials from Alexion, Biogen, Merck, Novartis, Roche, and Sanofi Genzyme. JSK has received research funding from the German Research Foundation (Deutsche Forschungsgemeinschaft, DFG; project 432290010), the German Federal Ministry of Education and Research (13GW0469D) and the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (101045128—iBack-epic—ERC-2021-COG). He is Co-Founder of Bonescreen. BH has served on scientific advisory boards for Novartis; he has served as DMSC member for AllergyCare, Sandoz, Polpharma, Biocon and TG therapeutics; his institution received research grants from Roche for multiple sclerosis research. He has received honoraria for counseling (Gerson Lehrmann Group). He holds part of two patents; one for the detection of antibodies against KIR4.1 in a subpopulation of patients with multiple sclerosis and one for genetic determinants of neutralising antibodies to interferon.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available on reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and the study was approved by the internal review board of the Technical University of Munich (reference number: 5848/13). Participants gave informed consent to participate in the study before taking part.
References
- 1. Kearney H, Miller DH, Ciccarelli O. Spinal cord MRI in multiple sclerosis--diagnostic, prognostic and clinical value. Nat Rev Neurol 2015;11:327–38. 10.1038/nrneurol.2015.80 [DOI] [PubMed] [Google Scholar]
- 2. Trop I, Bourgouin PM, Lapierre Y, et al. Multiple sclerosis of the spinal cord: diagnosis and follow-up with contrast-enhanced MR and correlation with clinical activity. AJNR Am J Neuroradiol 1998;19:1025–33. [PMC free article] [PubMed] [Google Scholar]
- 3. Kearney H, Altmann DR, Samson RS, et al. Cervical cord lesion load is associated with disability independently from atrophy in MS. Neurology 2015;84:367–73. 10.1212/WNL.0000000000001186 [DOI] [PubMed] [Google Scholar]
- 4. Andelova M, Uher T, Krasensky J, et al. Additive effect of spinal cord volume, diffuse and focal cord pathology on disability in multiple sclerosis. Front Neurol 2019;10:820. 10.3389/fneur.2019.00820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bussas M, El Husseini M, Harabacz L, et al. Multiple sclerosis lesions and atrophy in the spinal cord: distribution across vertebral levels and correlation with disability. Neuroimage Clin 2022;34:103006. 10.1016/j.nicl.2022.103006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kidd D, Thorpe JW, Thompson AJ, et al. Spinal cord MRI using multi-array coils and fast spin echo II. Findings in multiple sclerosis. Neurology 1993;43:2632–7. 10.1212/wnl.43.12.2632 [DOI] [PubMed] [Google Scholar]
- 7. Nijeholt GJ, van Walderveen MA, Castelijns JA, et al. Brain and spinal cord abnormalities in multiple sclerosis. Correlation between MRI parameters, clinical subtypes and symptoms. Brain 1998;121 (Pt 4):687–97. 10.1093/brain/121.4.687 [DOI] [PubMed] [Google Scholar]
- 8. Weeda MM, Zywicki S, Brouwer I, et al. Upper cervical cord atrophy is independent of cervical cord lesion volume in early multiple sclerosis: a two-year longitudinal study. Mult Scler Relat Disord 2022;60:103713. 10.1016/j.msard.2022.103713 [DOI] [PubMed] [Google Scholar]
- 9. Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol 2018;17:162–73. 10.1016/S1474-4422(17)30470-2 [DOI] [PubMed] [Google Scholar]
- 10. Okuda DT, Mowry EM, Cree BAC, et al. Asymptomatic spinal cord lesions predict disease progression in radiologically isolated syndrome. Neurology 2011;76:686–92. 10.1212/WNL.0b013e31820d8b1d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Patrucco L, Rojas JI, Cristiano E. Assessing the value of spinal cord lesions in predicting development of multiple sclerosis in patients with clinically isolated syndromes. J Neurol 2012;259:1317–20. 10.1007/s00415-011-6345-x [DOI] [PubMed] [Google Scholar]
- 12. Sombekke MH, Wattjes MP, Balk LJ, et al. Spinal cord lesions in patients with clinically isolated syndrome: a powerful tool in diagnosis and prognosis. Neurology 2013;80:69–75. 10.1212/WNL.0b013e31827b1a67 [DOI] [PubMed] [Google Scholar]
- 13. Swanton JK, Fernando KT, Dalton CM, et al. Early MRI in optic neuritis: the risk for disability. Neurology 2009;72:542–50. 10.1212/01.wnl.0000341935.41852.82 [DOI] [PubMed] [Google Scholar]
- 14. D’Amico E, Patti F, Leone C, et al. Negative Prognostic impact of MRI spinal lesions in the early stages of relapsing-remitting multiple sclerosis. Mult Scler J Exp Transl Clin 2016;2:2055217316631565. 10.1177/2055217316631565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Arrambide G, Rovira A, Sastre-Garriga J, et al. Spinal cord lesions: a modest contributor to diagnosis in clinically isolated syndromes but a relevant prognostic factor. Mult Scler 2018;24:301–12. 10.1177/1352458517697830 [DOI] [PubMed] [Google Scholar]
- 16. Brownlee WJ, Altmann DR, Prados F, et al. Early imaging predictors of long-term outcomes in relapse-onset multiple sclerosis. Brain 2019;142:2276–87. 10.1093/brain/awz156 [DOI] [PubMed] [Google Scholar]
- 17. Kerbrat A, Gros C, Badji A, et al. Multiple sclerosis lesions in motor tracts from brain to cervical cord: spatial distribution and correlation with disability. Brain 2020;143:2089–105. 10.1093/brain/awaa162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rocca MA, Valsasina P, Meani A, et al. Spinal cord lesions and brain grey matter atrophy independently predict clinical worsening in definite multiple sclerosis: a 5-year, multicentre study. J Neurol Neurosurg Psychiatry 2023;94:10–8. 10.1136/jnnp-2022-329854 [DOI] [PubMed] [Google Scholar]
- 19. Brownlee WJ, Altmann DR, Alves Da Mota P, et al. Association of asymptomatic spinal cord lesions and atrophy with disability 5 years after a clinically isolated syndrome. Mult Scler 2017;23:665–74. 10.1177/1352458516663034 [DOI] [PubMed] [Google Scholar]
- 20. Cordonnier C, de Seze J, Breteau G, et al. Prospective study of patients presenting with acute partial transverse myelopathy. J Neurol 2003;250:1447–52. 10.1007/s00415-003-0242-x [DOI] [PubMed] [Google Scholar]
- 21. Zecca C, Disanto G, Sormani MP, et al. Relevance of asymptomatic spinal MRI lesions in patients with multiple sclerosis. Mult Scler 2016;22:782–91. 10.1177/1352458515599246 [DOI] [PubMed] [Google Scholar]
- 22. Dekker I, Sombekke MH, Witte BI, et al. Asymptomatic spinal cord lesions do not predict the time to disability in patients with early multiple sclerosis. Mult Scler 2018;24:481–90. 10.1177/1352458517736147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Amezcua L, Lerner A, Ledezma K, et al. Spinal cord lesions and disability in hispanics with multiple sclerosis. J Neurol 2013;260:2770–6. 10.1007/s00415-013-7054-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Coret F, Bosca I, Landete L, et al. Early diffuse demyelinating lesion in the cervical spinal cord predicts a worse prognosis in relapsing-remitting multiple sclerosis. Mult Scler 2010;16:935–41. 10.1177/1352458510371960 [DOI] [PubMed] [Google Scholar]
- 25. University of California, San Francisco MS-EPIC Team, Cree BAC, Hollenbach JA, et al. Silent progression in disease activity-free relapsing multiple sclerosis. Ann Neurol 2019;85:653–66. 10.1002/ana.25463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kappos L, Wolinsky JS, Giovannoni G, et al. Contribution of relapse-independent progression vs relapse-associated worsening to overall confirmed disability accumulation in typical relapsing multiple sclerosis in a pooled analysis of 2 randomized clinical trials. JAMA Neurol 2020;77:1132–40. 10.1001/jamaneurol.2020.1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Portaccio E, Bellinvia A, Fonderico M, et al. Progression is independent of relapse activity in early multiple sclerosis: a real-life cohort study. Brain 2022;145:2796–805. 10.1093/brain/awac111 [DOI] [PubMed] [Google Scholar]
- 28. Lublin FD, Häring DA, Ganjgahi H, et al. How patients with multiple sclerosis acquire disability. Brain 2022;145:3147–61. 10.1093/brain/awac016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tur C, Carbonell-Mirabent P, Cobo-Calvo Á, et al. Association of early progression independent of relapse activity with long-term disability after a first demyelinating event in multiple sclerosis. JAMA Neurol 2023;80:151–60. 10.1001/jamaneurol.2022.4655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cagol A, Schaedelin S, Barakovic M, et al. Association of brain atrophy with disease progression independent of relapse activity in patients with relapsing multiple sclerosis. JAMA Neurol 2022;79:682–92. 10.1001/jamaneurol.2022.1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Weier K, Mazraeh J, Naegelin Y, et al. Biplanar MRI for the assessment of the spinal cord in multiple sclerosis. Mult Scler 2012;18:1560–9. 10.1177/1352458512442754 [DOI] [PubMed] [Google Scholar]
- 32. Galler S, Stellmann J-P, Young KL, et al. Improved lesion detection by using axial T2-weighted MRI with full spinal cord coverage in multiple sclerosis. AJNR Am J Neuroradiol 2016;37:963–9. 10.3174/ajnr.A4638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Breckwoldt MO, Gradl J, Hähnel S, et al. Increasing the sensitivity of MRI for the detection of multiple sclerosis lesions by long axial coverage of the spinal cord: a prospective study in 119 patients. J Neurol 2017;264:341–9. 10.1007/s00415-016-8353-3 [DOI] [PubMed] [Google Scholar]
- 34. Wuschek A, Bussas M, El Husseini M, et al. Somatosensory evoked potentials and magnetic resonance imaging of the central nervous system in early multiple sclerosis. J Neurol 2023;270:824–30. 10.1007/s00415-022-11407-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Prosperini L, Ruggieri S, Haggiag S, et al. Prognostic accuracy of NEDA-3 in long-term outcomes of multiple sclerosis. Neurol Neuroimmunol Neuroinflamm 2021;8:e1059. 10.1212/NXI.0000000000001059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schmidt P, Gaser C, Arsic M, et al. An automated tool for detection of FLAIR-hyperintense white-matter lesions in multiple sclerosis. Neuroimage 2012;59:3774–83. 10.1016/j.neuroimage.2011.11.032 [DOI] [PubMed] [Google Scholar]
- 37. DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a Nonparametric approach. Biometrics 1988;44:837–45. [PubMed] [Google Scholar]
- 38. Elm E von, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ 2007;335:806–8. 10.1136/bmj.39335.541782.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ozturk A, Aygun N, Smith SA, et al. Axial 3D gradient-echo imaging for improved multiple sclerosis lesion detection in the cervical spinal cord at 3T. Neuroradiology 2013;55:431–9. 10.1007/s00234-012-1118-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available on reasonable request.