Abstract
Context:
The genus Mentha is one of the most aromatic and well-known members of the Lamiaceae family. A wide range of bioactive compounds has been reported in mints. Regarding the high economic importance of Mentha plants due to the presence of valuable metabolites, the demand for their products is growing exponentially. Therefore, to supply such demand, new strategies should be adopted to improve the yield and medicinal quality of the products.
Evidence Acquisition:
The current review is written based on scientific literature obtained from online databases, including Google Scholar, PubMed, Scopus, and Web of Science regarding the characteristic features of some species of the genus Mentha, their distribution and cultivation, main uses and benefits, phytochemical composition, biotechnological approaches for the production of secondary metabolites, and strategies for enhanced production of mints secondary metabolites.
Results:
In this article, we offer an overview of the key characteristics, natural compounds, biological properties, and medicinal uses of the genus Mentha. Current research describes biotechnological techniques such as in vitro culture methods for the production of high-value secondary metabolites. This review also highlights the strategies such as elicitation, genetic, and metabolic engineering to improve the secondary compounds production level in mint plants. Overall, it can be concluded that identifying the biosynthetic pathways, leading to the accumulation of pharmaceutically important bioactive compounds, has paved the way for developing highly productive mint plants with improved phytochemical profiles.
Keywords: Mentha, Lamiaceae, Bioactive compound, Secondary metabolite
1. Background
The genus Mentha is one of the well-known genera of the Lamiaceae family, which is named after the legendary Greek character “Minthe” ( 1 , 2 ). Mint with a long history of use (more than 2000 years), is a symbol of hospitality in many cultures. This plant had been used extensively in ancient civilizations, from folk medicinal purposes to non-medicinal uses such as aromatherapy and cooking ( 3 ). In traditional Chinese medicine, mint due to its medicinal properties and health effects has been at the forefront of medical treatments ( 4 , 5 ). Today, the metabolites in this plant are widely used in the preparation of cosmetics ( 6 ), painkillers, fever treatment, headache, colds ( 7 ), and as a flavoring for foods and sweets in food industries ( 6 ).
As mentioned, mints represent a great economic and industrial plant due to the presence of highly commercially valuable metabolites. Moreover, the ease of propagation by seed or vegetative means, and the tolerance of the plants to grow under a broad range of agroclimatic conditions resulted in the cultivation and domestication of mints throughout the world ( 2 ).
Essential oils of the mints are promoted due to their commercial, economic, and health benefits. These sub-stances are composed of a wide range of chemotypes. The essential oil composition varies remarkably de-pending on numerous factors including the genetic background, the growing location, weather conditions, harvest period, and the extraction method ( 8 ).
In species of the genus Mentha aromatic essential oils are produced by glandular trichomes that occur especially on the leaf surface. Essential oils can be extracted in a physical process by crushing the leaves or steam distillation as the most popular method ( 2 ).
Extraction of plant active ingredients from the tissues of intact plants by humans causes many problems such as: rapid genetic erosion, allocation of a large part of agricultural resources and inputs to the cultivation of medicinal plants, time-consuming production of medicinal plants with acceptable content of secondary metabolites, dependence of particular metabolites production on specific plant growth stage, necessity of using agricultural chemicals such as chemical fertilizers, herbicides, insecticides and fungicides in the cultivation process, severe impact of biotic and abiotic environmental stresses on the production, harvesting processes, and the preservation of harvested plant materials. Therefore, application of new alternative biotechnological methods for the production of secondary metabolites seems reasonable. To achieve this goal, it is necessary to understand the molecular mechanisms controlling the production of secondary metabolites and their utilization in metabolic engineering. Moreover, elicitor application, induction of hairy roots, and optimization of culture medium are several approaches to increase the production level of secondary compounds in in vitro cultures. Regarding the high importance of mints in pharmaceutical, food, flavoring, and other industries due to the presence of valuable metabolites with diverse biological activities described in this review, it appears that the only use of traditional approaches for secondary metabolites production in this plant is not sufficient and the implementation of new advanced techniques, can help in more vigorous, purposeful and targeted production of these compounds. The aim of the current review is to appraise scientific literature regarding the characteristic features of some main species of the genus Mentha, their distribution and cultivation, uses and benefits, phytochemical composition, and biotechnological approaches for the production of secondary metabolites.
2. Mentha’s Taxonomic Classification
The Lamiaceae (Labiatae) family is a flowering plant family which was first described and named by the French botanist, Jussieu, in 1789. This family contains more than 7000 species in about 260 genera ( 9 ). According to Li et al. (2016), the Lamiaceae comprises 10 subfamilies: Lamioideae, Nepetoideae (the largest subfamily), Ajugoideae, Symphorematoideae, Prostan-theroideae, Scutellarioideae, Viticoideae, Cymarioideae, Peronematoideae, and Premnoideae ( 10 ). The genus Mentha L. (mint) is one of the most aromatic members of the Nepetoideae subfamily which its taxonomy is highly complex and has been always in a state of flux. On the basis of cytological, morphological, and genetic characteristics, the genus Mentha is defined to include 18-30 species ( 11 ) and about 100 varieties and cultivars ( 9 ), placed in five sections: Mentha, Preslia, Audibertia, Eriodontes, and Pulegium ( 12 ). It has a complicated systematic due to diverse basic chromosome numbers, intra- and interspecific hybridization, and the incidence of polyploidy ( 11 , 13 ).
The Mentha section includes 5 basic species: Mentha spicata L. (Spearmint), Mentha aquatica L. (Water mint), Mentha arvensis L. (Corn mint, Wild mint), Mentha longifolia (L.) Huds (Horse mint), and Mentha suaveolens Ehrh (Apple mint). It has been suggested that the frequent interspecific hybridization between these species has given rise to 11 naturally occurring hybrids ( 14 ). Mentha × piperita L. (peppermint) is a sterile hybrid plant, from the crossing between M. spicata and M. aquatic. This perennial herbaceous herb is native to the Mediterranean region and is cultivated as an important hybrid in Iran ( 15 ).
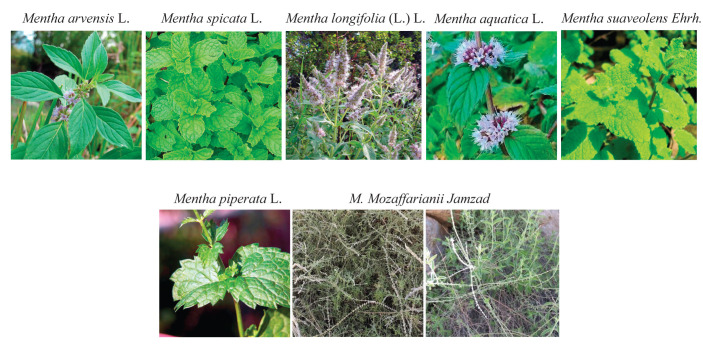
In Iran, various species and subspecies of the genus Mentha such as M. suaveolens, M. spicata, M. longi-folia, M. arvensis, M. Piperita, M. aquatica, and M. Mozaffarianii Jamzad are found (Fig. 1) ( 16 ). Mentha Mozaffarianii Jamzad is an Iranian native species that is rare and growing wild in a limited geographical area in the south and southeast of Iran ( 16 - 18 ).
Figure 1.
The main basic species of the genus Mentha (M. arvensis L. (www.actaplantarum.org); M. spicata L. (https://botanyphoto.botanicalgarden.ubc.ca/); M. longifolia (L.) L. (www.actaplantarum.org); M. aquatica L. (www.marylandbiodiversity.com); M. suaveolens Ehrh. (www.nparks.gov.sg); M. piperita L. (www.actaplantarum.org); M. Mozaffarianii Jamzad ( 21 )).
The scientific classification and hybrids of the genus Mentha are presented in Table 1.
Table 1.
Scientific classification and hybrids of the genus Mentha L. ( 14 )
| Scientific classification of the genus Mentha L. | |
|---|---|
| Kingdom: Plantae – Plants | |
| Subkingdom: Tracheobionta – Vascular plants | |
| Superdivision: Spermatophyta – Seed plants | |
| Division: Magnoliophyta – Flowering plants | |
| Class: Magnoliopsida – Dicotyledons | |
| Subclass: Asteridae | |
| Order: Lamiales | |
| Family: Lamiaceae – Mint family | |
| Genus: Mentha L. – Mint | |
| Hybrid | Parent species |
| Mentha × carinthiaca Host | M. arvensis L. × M. suaveolens Ehrh. |
| Mentha × dalmatica Tausch | M. arvensis L. × M. longifolia (L.) L. |
| Mentha × dumetorum Schultes | M. aquatica L. × M. longifolia (L.) L. |
| Mentha × gracilis Sole | M. arvensis L. × M. spicata L. |
| Mentha × maximilianea F. W. Schults | M. aquatic L. × M. suaveolens Ehrh. |
| Mentha × piperita L. | M. aquatica L. × M. spicata L. |
| Mentha × rotundifolia (L.) Huds. | M. longifolia (L.) L. × M. suaveolens Ehrh. |
| Mentha × smithiana R. Graham | M. aquatic L. × M. arvensis L. × M. spicata |
| Mentha × verticillata L. | M. aquatica L. × M. arvensis L. |
| Mentha × villosa Huds. | M. spicata L. × M. suaveolens Ehrh. |
| Mentha × villosa-nervata Opiz | M. longifolia (L.) L. × M. spicata L. |
3. Characteristic Features of Some Main Mentha Species
Mint species are aromatic, herbaceous perennial plants that are characterized by wide-spreading underground and overground stolons ( 19 ). The branched stems are square-shaped in cross-section and the aromatic leaves are arranged in opposite with serrated or entire margins ( 9 ) and oblong-elliptical to lanceolate in shape ( 20 ). The other characteristic features of the Mentha species include the white or purple zygomorphic bilaterally flowers that are produced in dense clusters known as verticillasters and the small, dry, and woody fruit containing 1 to 4 seeds ( 9 , 20 ). The morphology of the main species of Mentha is given in Table 2.
Table 2.
Characteristic features of some of the main Mentha species (Fig. 1)
| Mentha species | Morphology |
|---|---|
| Mentha arvensis L. | Stem: Erect–ascending branched stem ( 19 , 20 ) |
| Leaves: Arranged in opposite pairs, sparsely hairy, shortly petiole or sessile, oblong-ovate or lanceolate in shape and shiny dark green in color, serrated margins ( 19 , 20 , 32) | |
| Flowers: Mauve in color, occur in whorls and borne on leaf axils ( 20 , 32 ) | |
| Mentha spicata L. | Stem: Square-shaped and may be hairless or hairy ( 33 ) |
| Rhizome: Wide-spreading and creeping underground rhizome | |
| Leaves: Arranged in opposite pairs, ovate to lanceolate in shape and light green in color, often hairless on both sides, serrated margins ( 20 ) | |
| Flowers: Pink or white in color, arranged in a spike inflorescence ( 20 ) | |
| Mentha longifolia (L.) Huds. | Stems: Whitish and branched stems, the stems are erect to creeping 0.5–1 m tall and covered with short and soft hairs |
| Rhizome: Creeping rhizome ( 19 ) | |
| Leaves: The leaves are covered by whitish long hairs, oblong-elliptical to lanceolate in shape with toothed margins and green to greyish-green in color ( 19 , 20 , 33) | |
| Flowers: White to mauve in color, densely arranged into tapering spikes ( 20 , 33 ) | |
| Mentha aquatica L. | Stems: Square-shaped in cross-section, green or purple in color and hairy to almost hairless ( 19 , 33 ) |
| Rhizome: Wide-spreading rhizomes with fibrous roots | |
| Leaves: Opposite, finely toothed, ovate to ovate-lanceolate in shape, green and sometimes purplish in color, hairy to almost hairless | |
| Flowers: The flowers are very small, densely crowded and the inflorescences are near-spherical, pinkish to purple in color ( 19 , 33 ) | |
| Mentha suaveolens Ehrh. | Stems: Erect, square-shaped stems with short internodes, sparsely hairy ( 34 ) |
| Leaves: Opposite, toothed margins, shortly petiole or sessile, ovate-oblong to suborbicular in shape and bright green in color, the leaves are wrinkled with sunken venation and hairy above ( 34 ) | |
| Flowers: Tubular flowers arranged on spike inflorescence and their color is white, pinkish or violet ( 34 ) | |
| Mentha piperita | Stems: Four-angled, purplish, branching towards the top, slightly hairy |
| Leaves: Opposite, petiolate, sharply seriate, pointed, dark green color | |
| Flowers: Purple, small, four-lobed corolla (20-mint/peppermint p.pushpangadan,S.K. Tewari handbook of herbs and spices) | |
| M. Mozaffarianii Jamzad | Stems: Quadrangular, epidermis covered of hairs |
| Leaves: Leaf surface covered of hairs ( 21 , 35 ) |
4. The Genus Mentha Distribution and Cultivation
Mints are widely distributed and commercially cultivated in regions with tropical and temperate climates in different parts of the world ( 22 ). The genus Mentha is believed to be primarily originated in the Mediterranean basin ( 9 , 12 ) and has a cosmopolitan distribution across Asia, Europe, Australia, North America, and North Africa ( 22 , 23 ).
The members of the genus Mentha generally tolerate a broad range of climatic conditions, although most of them prefer moist habitats and thrive in, cool spots with partial shade ( 9 , 14 , 19 , 20 ). Mints as vigorous spreaders and fast-growing herbs, extend a network of underground and overground runners (rhizomes) ( 20 ).
4.1. Agro-Climatic Conditions
Climatic conditions play a significant role in the effective management, successful cultivation, and higher productivity of mints. M. arvensis prefers tropical and subtropical areas ( 24 ) whereas temperate and subtemperate regions are more suitable for M. piperita (Peppermint) and M. spicata (Spearmint) ( 20 ). Peppermint is able to withstand cooler temperatures. The climatic requirements for cultivation of this plant include temperature in the range of 15-25 °C and rainfall between 90 and 105 cm ( 25 ). An investigation by Devi and Sharma (2021) indicated that M. longifolia (L.) L. and M. spicata L. which are growing in subtropical zones, produce more herbage than other climatic zones, which is considered a desirable trait for breeders ( 26 ).
It is reported that the yield and composition of Mentha essential oils are related to climatic conditions ( 27 ). In a study by Heydari et al. (2018), it was suggested that heat stress strongly affects essential oil yield, chemical composition, and antibacterial activities in M. piperita and M. arvensis L ( 28 ). Moreover, Mollaei et al. (2020) recorded the variability of the composition and content of essential oils among 12 M. pulegium L. populations in Iran. Their results indicated the significant effect of environmental factors including average rainfall, temperature, and altitude on antioxidant activity and phytochemical constituents ( 29 ).
4.2. Soil
Mints do best in well-drained soils rich in organic matter with high levels of nutrients ( 20 ). Fertile loam to sandy loam soil with a slightly acidic pH between 6 and 7.5 is favorable for the cultivation of mints ( 12 ). It is reported that the soil composition and bioactive compounds of essential oil are certainly correlated. Therefore, substantial soil parameters such as pH, organic content, and drain capacity should be optimized for mint cultivation ( 27 ). Salehi et al. (2018) reported that the essential oil yield of M. piperita is affected by the pH of the soil ( 12 ).
4.3. Land Preparation Before Propagation
The land must be repeatedly (3-4 times) ploughed and harrowed twice to make a weed-free and fine seedbed before planting the crop ( 12 , 20 ). Farmyard manure (compost) of about 25-30 tons per hectare has to be applied as part of land preparation ( 12 ).
4.4. Propagation
Mints can be propagated either by seed or vegetative means; however, vegetative propagation through stolons and runners (rhizomes) is the main reliable method for raising mints ( 12 ).
Peppermint (Mentha × piperita L.) is one of the most common and popular hybrid mints that rarely produces viable and germinating seeds, therefore vegetative plant parts such as stem cuttings, green shoots, rooty turions, and underground stolons are commonly used for its propagation ( 30 , 31 ).
4.5. Planting
Two different planting methods are developed for mints ( 20 ):
1) Traditional farming: In this method, small pieces of cuttings from the runners or healthy roots with at least one growth bud are placed in shallow furrows (7-10 cm deep). The distance between adjacent rows should be 45-60 cm and after covering the furrow with soil, the plot is irrigated immediately ( 12 ). It has been reported that optimization of cultivation factors such as planting time, methods, and plant density play significant roles in improved productivity and maximum yield of crops like Mentha arvensis and Mentha piperita ( 24 , 36 , 37 ).
2) Micropropagation by using modern plant tissue culture methods: This technique provides an excellent alternative for the mass production of high-quality plantlets in a short period of time. Production of disease-free plants in small spaces, at any time of the year, is the other advantage of this method, in comparison to conventional propagation methods ( 38 , 39 ).
Hydroponics and aquaponics as soilless growth systems have been successfully utilized for the cultivation of several Mentha species like Mentha spicata ( 40 , 41 ), and Mentha piperita var. citrata (Ehrh.) Briq. ( 42 ). Aquaponics system is a relatively new method of mint cultivation that offers many benefits such as environmental compatibility, energy efficiency, minimal maintenance requirement, and increased biomass. Also, it is believed that the flavor and taste of mints might be stronger than those grown in soil ( 40 ).
4.6. Irrigation
As mints are high water-demanding crops, the plants require frequent irrigation (at least three times a week, when the plants are fully developed) ( 12 ) for optimum productivity, growth, maximum yield, high-quality crop, and higher quantities of secondary metabolites ( 43 , 44 ). Water stress can reduce vegetative growth and productivity ( 45 ). Several studies have reported the negative effects of water stress on some growth parameters and biomass production of peppermint and spearmint ( 12 , 45 ). However, the published data regarding the effect of irrigation on essential oil content are conflicting ( 27 ). In a study by Chiappero et al. (2019) drought-stressed M. piperita showed an improvement in total phenolic content ( 46 ). By contrast, a negative impact of drought stress on the flavonoid content, total phenolic content, and the radical scavenging activity of M. piperita extracts was reported by Rahimi et al. (2018) ( 47 ).
4.7. Mint Diseases and Pests
Mints are attacked by a wide variety of diseases and pests, causing serious crop loss and reducing the yield and mint oil quality. Several organisms such as fungi, nematodes, viruses and phytoplasma, bacteria ( 48 , 49 ), and insects ( 12 ) have been reported as causal agents that threaten mint cultivation. The major fungal pathogens that have been reported to cause significant economic loss are Erysiphe cischoracearum (powdery mildew), Puccinia menthae (rust), Alternaria alternata (leaf spot), Rhizoctonia solani (aerial blight), Phoma stasseri (stem rot), Verticillium dahliae (wilt) and Rhizoctonia solani/bataticola (root and stolon rot) ( 12 , 48 ). Mints are susceptible to various viruses, some of them include Cucumber Mosaic Virus (CMV), Mint Vein Banding associated Virus (MVBaV), Peppermint Latent Virus (PeLV), Peppermint Stunt Virus (PmSV), Tobacco Ringspot Virus (TRSV), Tomato Aspermy Virus (TAV), and Tobacco Mosaic Virus (TMV) ( 48 , 50 ). Despite the fast-acting and easy application of chemical control of pests and diseases, the continual application of synthetic pesticides threatens the animals and human health due to environmental pollution and residual toxicity ( 51 ). Therefore, non-chemical disease control strategies such as crop rotation, biological control, use of biopesticides, and producing disease resistance lines through breeding programs, should be developed ( 48 , 49 ).
4.8. Harvesting mint plants
Two important factors that greatly influence forage yield, essential oil quality, and composition are the harvest time and the number of harvests per year ( 52 ). Harvesting the crop too early results in an immature crop and a reduction in essential oil quality ( 12 ), whereas delayed harvesting results in decreased essential oil contents. The proper time for harvesting is when the mint plant is in the full-bloom stage (80%), on dry sunny days ( 20 , 52 ). Based on different species, mints are harvested once or twice a year, and it has been reported that the essential oil quality and content differ between the first and second harvests ( 12 ). Harvesting can be done either manually by hand or mechanically with green-crop loaders and combine harvesters ( 53 ).
4.9. Postharvest Handling
After harvesting, plants may be sold in a fresh or dried form. Due to the rapid senescence of fresh herbs like mints, the postharvest process is considered as a substantial stage to maintain the quality and improve the shelf-life of the fresh plant product. In a study on postharvest quality maintenance of Mentha Piperita by Barbosa, hydroocoling and non-perforated plastic packages were reported as an effective method ( 54 ). Drying as a common method of mint preservation, reduce the moisture content and expand the shelf-life ( 55 ). Different drying approaches like hot air drying, solar drying, open sun drying, freeze drying, and vacuum drying have been used for dehydrating mint leaves. Moreover, desiccant based dehumidified air-drying system has been reported as an effective low-temperature drying option for heat-sensitive leafy vegetables, especially mints ( 56 , 57 ).
Consequently, due to the influence of different growing conditions and cultivation systems on the yield and quality of mints; they should be carefully selected for the cultivation of these valuable plants to achieve a higher concentration of useful phytochemical constituents.
5. Main Uses and Benefits of Mint
Since ancient times, mints as aromatic plants have been used in a wide variety of applications both in Eastern and Western cultures ( 58 ). Members of the genus Mentha are used in the pharmaceutical, cosmetic, and food industries due to their volatile oil compositions ( 23 ).
5.1. Culinary Uses
Mint can be used fresh and dried as a seasoning herb to improve the flavor of foods, confections, and beverages ( 14 , 59 ). Mentha species have traditionally been used as flavoring and aromatic agents in food preparation ( 12 ). The fragrant leaves have a wonderful fresh, sweet, warm flavour with a cool, refreshing aftertaste and are valued as a wonderful appetizer. Species such as M. piperita and M. arvensis are used as popular flavouring in food products such as ice creams, syrups, mint sauces, and drinks ( 20 , 60 ). The most popular mints, peppermint (M. piperita) and spearmint (M. spicata) are commonly used for tea preparation. Mentha essential oil and menthol are often associated with breath fresheners, toothpaste, mint tablets, and chewing gums ( 14 , 19 ).
5.2. Therapeutic Uses
In addition to their culinary uses, Mentha species have been used in traditional medicines since ancient times. Although mint as a calming herb has been used for centuries to cure gastrointestinal ailments, it has a wide spectrum of medical activities ( 9 ). In folkloric remedies, mint is considered a treatment for stomachache, biliary disorders, enteritis ( 9 ), respiratory problems ( 59 ), bronchitis, nausea, coughs ( 61 ), colds, flu, and hemorrhoids ( 54 ). Mint leaves are generally taken as a tea which is a strong diuretic ( 9 ) and aids digestion with its carminative properties ( 58 ). Herbal tea made from this plant has traditionally been used in the treatment of fever, headaches, and different minor health disorders such as indigestion ( 12 ). Different parts of M. longifolia have been used in traditional folk medicine in the treatment of gallstones, jaundice, flatulence, and bladder stone in Iran ( 62 ). Spearmint leaves have been recommended by traditional Iranian medical practitioners to treat digestive disorders ( 63 ). It is reported that peppermint essential oil acts as a smooth muscle relaxant, decreases visceral pain, small bowel contractility, colonic spasm, and modulates psychosocial distress ( 64 ). In terms of biological activities, mint oil act as a low-risk insecticide against several grain pests ( 65 , 66 ). Moreover, antimicrobial ( 64 ), antiviral, antifungal, antioxidant ( 22 , 67 ), and anti-inflammatory ( 14 , 64 ) properties of mints have been frequently reported.
5.2.1. Antioxidant Properties
Mentha species are found to contain high levels of antioxidant compounds such as phenolic acids, flavonoids, ascorbic acid, and carotenoids which act as free radical scavengers. In a study by Park et al. (2019), the extracts of nine Mentha species were investigated for antioxidant and free radical scavenging activities. They reported a range of antioxidant activities among the different Mentha species. According to their results, M. longifolia showed the highest antioxidant activity among the other investigated species ( 68 ). In another study ( 69 ), Nickavar et al. reported that out of five Mentha species (M. longifolia, M. spicata, M. piperita, M. pulegium, and M. rotundifolia) M. piperita showed the highest total phenolic content and the strongest DPPH scavenging activity. As mentioned before, several factors such as cultivation, harvesting, and extraction methods are implicated in these different results.
5.2.2. Antibacterial Properties
Mentha essential oils and extracts are found to demonstrate a wide spectrum of antibacterial activity against bacterial strains. Anwar et al. (2017) reported that the antimicrobial activities of Mentha essential oils are correlated with oxygenated monoterpenoids and monoterpene hydrocarbon contents ( 70 ). It is believed that the presence of the hydroxyl group in phenolic compounds plays a significant role in the antimicrobial properties of essential oils ( 71 ).
5.2.3. Anticancer Properties
Research has developed into an investigation of plant species for their anticancer potential ( 72 ). The cytotoxic effects of some Mentha species have been reported in numerous studies ( 73 - 75 ). Polyphenolic compounds such as flavonoids are considered as anticancer compounds and their cytotoxicity and antioxidant properties have been demonstrated. It has been suggested that polyphenols can induce apoptosis and suppress the proliferation of cancer cells ( 72 ).
Various therapeutic effects reported for Mentha species are summarized in Table 3.
Table 3.
Various therapeutic effects reported for Mentha species
| Therapeutic effects | Mentha species | Preparation used | Reference |
|---|---|---|---|
| Antioxidant | M. spicata | Diethyl ether extract | ( 85 ) |
| Methanolic extract | ( 86 - 88 ) | ||
| Ethanolic extract | ( 88 ) | ||
| M. arvensis | Water extract | ( 89 ) | |
| M. pulegium | Ethanolic extract | ( 90 ) | |
| M. piperita L. | Essential oil | ( 91 ) | |
| Water extract | ( 92 ) | ||
| Water extract | ( 9 ) | ||
| M. longifolia L. | Essential oil | ( 93 ) | |
| Methanolic extract | ( 94 ) | ||
| ( 95 ) | |||
| Antidiabetic | M. piperita (chocolate mint) | Water extract | ( 96 , 97 ) |
| M. spicata | Water extract | ( 98 ) | |
| Ethanolic extract | ( 88 ) | ||
| M. arvensis | Methanolic extract | ( 99 ) | |
| Antifungal | M. spicata | Volatile oil | ( 61 ) |
| M. spicata | Ethanolic extract | ( 88 ) | |
| Methanolic extract | ( 88 ) | ||
| M. piperita | Essential oil | ( 100 , 101 ) | |
| M. longifolia L. | Essential oil | ( 102 ) | |
| M. Mozaffarianii | Essential oil | ( 103 ) | |
| Antibacterial | M. spicata | Volatile oil | ( 61 , 88 ) |
| M. arvensis | Ethanolic extract | ( 90 ) | |
| M. pulegium | Essential oil | ( 91 ) | |
| Essential oil | ( 104 ) | ||
| M. piperita | Essential oil | ( 93 ) | |
| M. aquatica | Essential oil | ( 105 , 106 ) | |
| M. longifolia | Essential oil | ( 106 , 107 ) | |
| M. Mozaffarianii | Essential oil | ( 103 ) | |
| Analgesic | M. arvensis | Ethanolic extract | ( 90 ) |
| M. piperita | Methanolic extract | ( 108 ) | |
| M. suaveolens | Methanolic extract | ( 109 ) | |
| Essential oil | ( 34 ) | ||
| M. spicata | Methanolic extract | ( 88 ) | |
| M. Mozaffarianii | Essential oil | ( 16 ) | |
| Anti-inflammatory | M. piperita | Methanolic extract | ( 108 ) |
| Essential oil | ( 73 , 110 ) | ||
| Ethanolic extract | ( 111 ) | ||
| M. spicata | Water extract | ( 89 ) | |
| Methanolic extract | ( 88 ) | ||
| M. Mozaffarianii | Essential oil | ( 16 ) | |
| Cytotoxic | M. piperita | Essential oil | ( 73 ) |
| Anticancer | M. arvensis | Methanolic extract | ( 74 ) |
| M. longifolia | Methanolic extract | ( 74 , 95 ) | |
| M. spicata | Methanolic extract | ( 74 ) | |
| M. viridis | Methanolic extract | ( 74 ) | |
| M. villosa | Essential oil | ( 75 ) | |
| Antiviral | M. suaveolens | Essential oil | ( 112 ) |
| M. spicata | Essential oil | ( 113 ) | |
| M. piperita | Essential oil | ( 113 ) | |
| Antiallergic | M. arvensis | Water extract | ( 114 ) |
| M. piperita | Water extract | ( 115 ) | |
| M. spicata | Methanolic extract | ( 116 ) |
5.3. The genus Mentha Essential Oils as Biopesticides
Some Mentha species have long been known for their insecticidal and antimicrobial efficacies against plant pathogens (fungi, bacteria) and stored products insects. Biopesticides based on mint essential oils may present efficient alternatives and less harmful crop protectants than commercial pesticides ( 76 ). Essential oil-based botanical insecticides are very effective in controlling the stored product pests and plant pathogens responsible for preharvest and postharvest diseases. Diverse mechanisms of action on insect pests have been reported for essential oils. Many studies have demonstrated the neurotoxic action of essential oils. One of the most reported mechanisms of action in essential oils and constituents is the inhibition of acetylcholinesterase enzyme activity which cause paralysis and final death of the insects occurs ( 77 ). In insects, the octopaminergic system is also the main target site of essential oils activity. It has been reported that Mentha essential oils constituents affect the octopamine receptor which leads to the killing of the larvae and pupae in insects ( 76 ). The insecticidal, antifungal, and antibacterial properties of essential oils from different Mentha species are presented in Table 4.
Table 4.
The insecticidal, antifungal, and antibacterial properties of essential oils from different Mentha species (76, 117, 118)
| Species | Antibacterial activity | Antifungal activity | Insecticidal activity |
|---|---|---|---|
| M. piperita | Pseudomonas syringae pv. syringae | Penicillium digitatum | |
| P. syringae pv. tomato | Aspergillus. flavus | ||
| P. syringae pv. phaseolicola | A. niger | ||
| Xanthomonas campestris pv. campestris | Mucor spp | ||
| X. campestris pv. phaseoli | Fusarium oxysporum | ||
| X. campestris pv.juglandis | A. niger | ||
| Erwinia carotovora subsp. carotovora | Rhizopus solani | Callosobruchusmaculatus | |
| X. vesicatoria | Alternaria alternata | Phthorimaea absoluta | |
| A. flavus | |||
| F. oxysporum f.sp. lycopersici | |||
| Drechslera spicifera | |||
| F. oxysporum f.sp. ciceris | |||
| Macrophomina phaseolina | |||
| Verticillium fungicola | |||
| T. harzianum | |||
| M. spicata | X. campestris pv .juglandis | Rhizopus solani | |
| A. niger | |||
| Alternaria alternata | |||
| Geotrichum citri-aurantii | |||
| P. digitatum | |||
| P. italicum | |||
| A. ochraceus | |||
| A. versicolor | |||
| A. flavus | Rhyzopertha dominica | ||
| A. terreus | Callosobruchus chinensis | ||
| A. alternata | |||
| P. ochrochloron | Ephestia kuehniella | ||
| P. funiculosum | |||
| C. cladosporioides | Plodia interpunctella | ||
| T. viride | |||
| F. tricinctum | Leptinotarsa decemlineata | ||
| Phomopsis helianthi | |||
| Verticillium fungicola | |||
| T. harzianum | |||
| A. terreus | |||
| F. oxysporum | |||
| P. expansum | |||
| V. dahliae | |||
| M. arvensis | Pantoea agglomerans | Aspergillus spp | Tribolium castaneum |
| Erwinia amylovora | Fusarium spp | ||
| Pantoea dispersa | |||
| P. fluorescens | |||
| Ps. syringae pv. syringae | |||
| M. longifolia | A. niger | ||
| A. versicolor | |||
| Cladosporium fulvum | |||
| F. tricinctum | |||
| F. sporotrichioides | |||
| P. funiculosum | |||
| P. ochrochloron | |||
| C. cladosporioides | T. castaneum | ||
| Aspergillus spp | C. maculates | ||
| Fusarium spp | |||
| P. funiculosum | Sitophilus zeamais | ||
| Trichoderma viride | |||
| C. fulvum | |||
| C. cladosporioides | |||
| P. ochrochloron | |||
| Rhizopus solani | |||
| A. niger | |||
| Alternaria alternata |
6. Phytochemical Composition of Mentha Species
The biological properties and medicinal uses of mints are ascribed to essential oils and polyphenolic compounds that have been extensively investigated in literatures.
6.1. Volatile Compounds
Essential oils of mints are highly complex mixtures of volatile compounds characterized by a pleasant and strong odor ( 9 ). They are usually liquid and rarely colored (pale yellow or greenish-yellow) at room temperature. Essential oils are isolated by steam or hydro-distillation ( 9 ) and are freely soluble in organic solvents and lipids ( 12 ). Specialized secreting tissues called glandular trichomes are responsible for the biosynthesis of mint odorous secondary metabolites and are considered important sources of essential oils ( 78 ).
Mint essential oils are largely utilized in perfumery, cosmetics, pharmaceutical industries, and aroma-therapy ( 19 , 79 ). Moreover, thanks to their active constituents, they possess antimicrobial and repellent effects that can be used as safe and potential alternatives for chemical pesticides ( 80 ). The most economically important essential oils belong to Mentha species including, M. piperita L., M. spicata L., M. canadensis L., and M. gracilis sole. In addition, M. citrata and M. pulegium L. oils also have commercial value, but to a lesser extent ( 14 ).
The essential oils of mints are mainly composed of terpenes (monoterpenes and sesquiterpenes) ( 9 ), hydrocarbons, alcohols, ketones, esters, and ethers ( 14 ).
Terpenes are characterized as aromatic compounds which are the major constituents found in essential oils and responsible for the unique fragrance of the mints. It has been reported that terpenes exert antimicrobial activities ( 81 ). In addition, terpenoids are derivatives of terpenes containing oxygen molecules and play an important role in disease resistance ( 82 ).
The terpenoid profile of mint essential oils is highly valued. Menthol as a dominant constituent of the essential oil in Mentha is a cyclic monoterpene alcohol that is used widely in medicinal preparation and cosmetics ( 83 ). The chemical composition of essential oils is reported to be affected by different factors, such as geographical site ( 84 ), environmental conditions, phonological phases of the plant, the part of the plant used, and the essential oil analysis methods ( 12 ).
6.2. Non-Volatile Compounds
A wide range of other bioactive compounds have been reported in mints, including phenolic compounds which comprise the largest group among the different classes of plant secondary metabolites ( 22 ). Phenolics can be classified based on their structures into phenolic acids, tannins, coumarins, flavonoids, stilbenes, and lignans ( 119 ). Mentha plants are potential sources of phenolic compounds, especially flavonoids and phenolic acids as one of the most important groups of mint phenolics ( 120 ).
Table 5 and Table 6 present the major polyphenolic compounds and the main components of some prevalent Mentha species essential oils.
Table 5.
Major chemical constituents of some Mentha species essential oils
| Compounds | Species | Biological activity | Reference |
|---|---|---|---|
| Terpenoid | |||
| Geranyl acetate | M. arvensis | ----- | ( 60 ) |
| M. piperita L. | ( 60 ) | ||
| Pulegone/ Isopulegone | M. arvensis | ----- | ( 60 ) |
| Menthonel lsomenthone | M. arvensis | ----- | ( 60 ) |
| Sabinene | M. piperita L. | ----- | ( 60 ) |
| β- Pinene | M. piperita L. | ----- | ( 60 ) |
| β-Mircene | M. piperita L. | ----- | ( 60 ) |
| α-Terpinene | M. piperita L. | Antioxidant | ( 60 , 134 ) |
| Elixene | M. piperita L. | ----- | ( 60 ) |
| Monoterpene | M. piperita L. | ----- | ( 60 ) |
| Hydrocarbons | |||
| β-caryophyllene | M. piperita L. | Anticancer | ( 84 , 135 ) |
| M. spicata L. | ( 136 , 137 ) | ||
| M. rotundifolia | ( 138 ) | ||
| M. aquatica L. | ( 139 , 140 ) | ||
| Germacrene D | M. piperita L. | ----- | ( 84 ) |
| M. rotundifolia | ( 138 , 141 ) | ||
| M. spicata L. | ( 136 ) | ||
| Limonene | M. spicata L. | Antibacterial | ( 73 , 84 , 142 ) |
| M. gracilis | ( 84 , 135 ) | ||
| M. piperita L. | Antifugal | ( 84 ) | |
| M. pulegium L. | Antiyeast | ( 136 ) | |
| M. rotundifolia | ( 143 ) | ||
| M. aquatica L. | ( 140 , 144 ) | ||
| M. arvensis | ( 60 ) | ||
| Alcohols | |||
| Elemol | M. aquatica L. | ----- | ( 144 ) |
| Geraniol | M. rotundifolia | ----- | ( 143 ) |
| M. suaveolens Ehrh. | ( 12 ) | ||
| M. longifolia | ( 102 ) | ||
| Linalool | M. gentilis L. | ----- | ( 142 ) |
| M. rotundifolia | ----- | ( 143 ) | |
| M. spicata subsp. condensata | ( 12 ) | ||
| M. piperita L. | ( 60 ) | ||
| M. Mozaffarianii | ( 103 ) | ||
| Menthol | M. piperita L. | Antifugal | ( 84 , 135 ) |
| M. rotundifolia | ( 143 ) | ||
| M. spicata L. | Antiyeast | ( 145 ) | |
| M. arvensis L. | Antiviral | ( 135 , 146 , 147 ) | |
| Neomenthol | M. piperita L. | ----- | ( 84 ) |
| M. pulegium L. | ( 141 , 148 ) | ||
| M. rotundifolia | ( 143 ) | ||
| M. arvensis L. | ( 147 ) | ||
| 3-octanol | M. gracilis | ----- | ( 84 ) |
| M. arvensis L. | ( 147 ) | ||
| cis/trans-sabinene hydrate | M. rotundifolia | ----- | ( 12 , 149 ) |
| M. piperita L. | ( 60 ) | ||
| α-terpineol | M. spicata L. | ----- | ( 12 ) |
| M. suaveolens Ehrh. | ( 12 ) | ||
| terpinen-4-ol | M. spicata L. | ----- | ( 12 ) |
| M. longifolia subsp. longifolia | ( 150 ) | ||
| M. piperita L. | ( 60 ) | ||
| viridoflorol | M. aquatica L. | ----- | ( 14 , 151 ) |
| M. piperita L. | ( 60 ) | ||
| Esters | |||
| Decyl acetate | M. verticillata L. | ----- | ( 14 ) |
| Dihydrocarvyl acetate | M. smithiana R. Graham | ----- | ( 14 ) |
| M. villosa Huds. | ( 14 ) | ||
| 1,2-epoxyneomenthyl acetate, | M. suaveolens Ehrh. | ----- | ( 14 ) |
| Menthyl acetate | M. piperita L. | ----- | ( 84 ) |
| M. rotundifolia | ( 143 ) | ||
| M. spicata L. | ( 145 ) | ||
| M. piperita L. | ( 12 ) | ||
| M. arvensis | ( 60 ) | ||
| Neoisomenthyl acetate | M. pulegium L. | ----- | ( 14 ) |
| Neomenthyl acetate | M. diemenica Spreng. | ----- | ( 14 ) |
| M. arvensis L. | ( 146 ) | ||
| 3-octyl acetate | M. arvensis L. | ----- | ( 14 ) |
| α-terpinyl acetate | M. longifolia subsp. longifolia | ----- | ( 150 ) |
| Ketones | |||
| Carvone | M. spicata L. | Antioxidant | ( 73 , 84 , 135 , 152 ) |
| M. gracilis | Antibacterial | ( 9 ) | |
| Antifugal | |||
| Antiyeast | |||
| cis-/trans-dihydrocarvone | M. spicata L. | ----- | ( 12 ) |
| Isomenthone | M. pulegium L. | Antibacterial | ( 135 , 136 ) |
| M. piperita L. | ( 9 ) | ||
| M. rotundifolia | ( 143 ) | ||
| M. arvensis L. | ( 146 , 147 ) | ||
| Menthone | M. piperita L. | Antibacterial | ( 84 , 135 ) |
| M. pulegium L. | ( 136 , 141 ) | ||
| M. rotundifolia | Antifugal | ( 143 ) | |
| M. spicata L. | Antiyeast | ( 145 ) | |
| M. arvensis L. | Anticancer | ( 146 , 147 ) | |
| M. Mozaffarianii | ( 103 ) | ||
| 3-octanone | M. arvensis L. | ----- | ( 14 ) |
| M. verticillata L. | ( 14 ) | ||
| Pulegone | M. pulegium L. | ----- | ( 141 , 149 ) |
| M. spicata L. | ( 142 , 153 ) | ||
| M. rotundifolia | ( 138 ) | ||
| M. longifolia | ( 154 ) | ||
| M. Mozaffarianii | ( 103 ) | ||
| M. piperita L. | ( 60 ) | ||
| Piperitenone | M. pulegium L. | ----- | ( 136 ) |
| M. Mozaffarianii | ( 103 ) | ||
| Piperitone | M. spicata L. | Antifugal | ( 135 , 153 ) |
| M. pulegium L. | ( 136 ) | ||
| M. piperita L. | Antiyeast | ( 60 ) | |
| M. Mozaffarianii | ( 103 ) | ||
| Ethers | |||
| 1,8-cineole, | M. piperita L. | Antioxidant | ( 84 , 135 ) |
| M. spicata L. | ( 73 , 84 , 142 , 155 ) | ||
| M. longifolia | ( 155 ) | ||
| M. rotundifolia | ( 143 ) | ||
| M. aquatica L. | ( 140 ) | ||
| M. arvensis | ( 60 ) | ||
| M. Mozaffarianii | ( 103 ) | ||
| Menthofuran | M. piperita L. | ----- | ( 9 , 84 ) |
| M. rotundifolia | ( 143 ) | ||
| M. spicata L. | ( 145 ) | ||
| M. aquatica L. | ( 140 ) | ||
| Oxides | |||
| Caryophyllene oxide | M. aquatica L. | ( 14 ) | |
| Piperitenone oxide | M. suaveolens | ----- | ( 155 ) |
| M. spicata L. | ( 155 ) | ||
| M. longifolia | ( 154 , 155 ) | ||
| M. rotundifolia | ( 138 , 156 ) | ||
| M. spicata L. | ( 12 ) | ||
| M. Mozaffarianii | ( 103 ) | ||
| Piperitone oxide | M. rotundifolia | ----- | ( 141 ) |
| M. spicata L. | ( 12 ) | ||
| M. longifolia subsp. longifolia | ( 150 ) | ||
Table 6.
Major polyphenolic compounds of some Mentha species
| Species | Essential oil main components | Polyphenolic Compounds | Reference |
|---|---|---|---|
| M. piperita L. | cineol (8.69%) | Rosmarinic acid, caffeic acid, lithospermic acids, salvianolic acid, syringic acid, gallic acid, vanillic acid, p-coumaric acid, ferulic acid, protocatechuic acid glucoside, isosalvianolic acid A, prolithospermic acid, salvianolic acid, danshensu, chlorogenic acid, Luteolin 7-O-rutinoside, isorhoifolin, eriodictyol 7-O-glucoside, hesperidin, eriocitrin, narirutin, diosmin, sorbifolin, thymosin, sideritoflavone, ladanein, xanthomicrol, acacetin, salvigenin, 5-O-demethylnobiletin, pebrellin, hesperidin, Catechin, rutin, quercetin, medioresinol, medioresinol sulfate, trans-resveratrol | ( 3 , 122 , 163 - 168 ) |
| menthol (40.47%) | |||
| menthone (36.58%) | |||
| menthol acetate (4.33%) | |||
| sabinene (1.64%) | |||
| α-pinene (1.11%) | |||
| M. Spicata L. | carvone(42.84%) | Rosmarinic acid, veratric acid, vanillic acid, homovanillic acid, hydroxybenzoic acid, syringic acid, 4-hydroxy cinnamic acid, trans-hydroxy cinnamic , 4-hydroxy cinnamic acid, 2-hydroxy cinnamic ,4-hydroxy cinnamic acid, ferulic acid, gallic acid, protocatechuic acid, chlorogenic, caffeic , 4-hydroxy cinnamic acid, p-coumaric, 4-Hydroxy benzoic acid, diosmin, diosmin-7-glucoside, 6,4′ -trihydroxy-7,3′-dimethoxflavone, desmethoxynobiletin, 5,6-di-hydroxy-7,8,3′,4′-tetramethoxyflavone, thymonin, sideritiflavone, 5-Hydroxy-3′,4′,6,7-tetramethoxyflavone, thymonin, naringenin, luteolin, apigenin, rutin, catechin, chrysoeriol, 5,6-dihydroxy-7, 8,3′,4′-tetramethoxyflavone, nodifloretin, quercetin, luteolin, scopoletin, catechin, epicatechin, myricetin, apigenin, kaempferol, spicatolignan A, spicatolignan B | ( 3 , 122 , 163 , 169 , 170 ) |
| carveol (34.98%) | |||
| menthol (40.47%) | |||
| α-pinene (17.77%) | |||
| M. rotundifolia L. | cineol (2.4%) | Caffeic acid, p-hydroxybenzoic acid, ferulic acid, p-coumaric acid, chlorogenic acid, rosmarinic acid, apigenin, luteolinidin, elargonidin, cyanidin, delphinidin, petunidin, luteolin, thymonin, thymosin, 5,6-dihydroxy-7,8,3′,4′-tetramethoxyflavone, jaceo-sidin, hispidulin, ladanein, sorbifolin, nodifloretin, Esculetin, diosmin, naringenin, kaempferol | ( 9 , 122 , 171 ) |
| limonene (1.8%) | |||
| menthol (40.50%) | |||
| menthone (5%) | |||
| menthofuran (4.2%) | |||
| isomenthone(2.5%) | |||
| linalool (2%) | |||
| linalyl acetate(3.5%) | |||
| piperitone (3.1%) | |||
| menthyl acetate (4.5%) | |||
| M. longifolia L. | limonene (4.3%) | Rosmarinic acid, salvianolic acid, Luteolin-glucuronide, eriodi-ctyol- glucopyranosyl-rhamnopyranoside | ( 167 , 172 , 173 ) |
| piperitenone (43.9%) | |||
| Tripal (14.3%) | |||
| oxathiane (9.3%) | |||
| piperitone oxide (5.9%) | |||
| M. pulgium L. | limonene (1.2%) | Caffeic acid, vanillic acids , ferulic acid, 4-Hydroxy benzoic, p-coumaric acids, chlorogenic acids, rosmarinic acid, Diosmin, Thymonin, jaceosidin, pectolinaringenin, ladanein, sorbifolin, pedalitin, 5,6,4′-trihydroxy-7,3′-dimethoxyflavone; 5,6-dihydroxy-7,3′,4′-trimethoxyflavone; 5-hydroxy-6,7,3′,4′- tetramethoxyflavone, apigenin, luteolin, chrysoeriol, kaemp-ferol, naringenin, catechin | ( 9 , 143 , 170 ) |
| menthol(3.28%) | |||
| menthone (3.09%) | |||
| menthofuran (2.15%) | |||
| pulegone (6.45%) | |||
| isomenthone (1.56%) | |||
| carvone (1.13%) | |||
| piperitenone (21.18%) | |||
| piperitone (35.56%) | |||
| α-terpineol (10.89%) | |||
| M. arvenisis L. | limonene (1.47%) | rosmarinic acid, chlorogenic acid, caffeic acid, neoponcirin, narirutin, biochanin A, apigenin, hesperetin | ( 174 , 175 ) |
| menthol (21.33%) | |||
| menthone (29.41%) | |||
| isomenthone (10.80%) | |||
| eucalyptol (6.91%) ,linalool (2.2%) | |||
| M. suaveolens L. | pulegone (62.3-34.3%) | 4-Hydroxybenzoic acid, vanillic acid, chlorogenic acid, Viola-xanthin, Antheraxanthin, Lutein, Zeaxanthin, 13Z-β Carotene, α-Carotene, E-β-Carotene, 9Z-β-Carotene syringic acid,o-coumaric acid, p-coumaric acid | ( 9 , 176 ) |
| menthone (39.4–10.8%) | |||
| isomenthone (9.3–7.8%) | |||
| M. aquatica L. | menthofuran (66–64%) | Rutin-O-glc, lavandulifolioside, eriodictyol O-rut, quercetin-3-O-soph, verbascoside, caffeic acid, rosmarinic acid | ( 3 , 177 ) |
| cineol (7%) | |||
| limonene (4–9%) | |||
| menthol (0–2%) | |||
| menthone (0–1%) |
6.2.1. Phenolic Acids
Phenolic acids are ubiquitous compounds with a wide range of pharmacological activities, which are present abundantly in plants belonging to the Lamiaceae family, especially Mentha species. They are divided into two subgroups: Hydroxycinnamic acids (HCAs) with a chemical backbone of C6-C3, and hydroxybenzoic acids (HBAs) with a common structure of C6-C1 ( 119 , 121 ). Mentha species are considered a rich source of caffeic acid and its derivatives, rosmarinic acid and chlorogenic acid ( 9 , 14 ). Caffeic acid as a hydroxycinnamic acid has been found in M. spicata L. ( 22 , 23 , 122 , 123 ), M. rotundifolia Huds. ( 122 ), M. australis R. Br. ( 124 ), M. pulegium L. ( 122 ), M. piperita L. ( 125 ), and M. aquatica L. ( 126 ). Hydroxycinnamic acid esters are widely present in plants and are often bound to sugar units in the form of glycosides such as chlorogenic acid that is formed between caffeic acid and quinic acid as a soluble ester and its presence has been reported in several Mentha species such as M. spicata L. ( 22 , 23 , 122 ), M. piperita L. ( 125 ), M. australis R. Br. ( 124 ), and M. longifolia (L.) L. ( 127 ). Rosmarinic acid is a highly valued hydroxycinnamoyl ester that has been abundantly found in a wide variety of Mentha species ( 122 - 124 , 128 ). Moreover, seven salvianolic acids have been detected in mints, such as salvianolic acid B (lithospermic acid B), a dimer of rosmarinic acid ( 22 , 23 , 129 ).
The genus Mentha is a rich source of flavonoids, especially flavones and flavanones ( 9 , 14 ). Luteolin-7-O-glucoside, eriocitrin, and hesperidin have been reported to be the major flavonoids in Mentha species ( 12 ). The content of phenolic compounds within the methanolic extract of M. spicata subsp. spicata is reported to be caffeic acid, rosmarinic acid and luteolin ( 130 ). The main extracted phenolic compounds from M. aquatica L. are rosmarinic acid, eriocitrin, and luteolin-7-O-glucoside ( 6 ), whereas eriocitrin (the dominant flavonoid glycoside), luteolin-7-O-rutinoside, rosmarinic acid, luteolin-7-O-glucoside, and hesperidin are the main polyphenolic components of the peppermint ( 6 , 131 ).
7. Phytochemical Analysis Methods
As mentioned before, secondary metabolites are high-value chemicals that are used as medicines, food supplements, dyes, flavors and fragrances, pigments, and insecticides ( 132 ). Therefore, metabolomic profiling to identify compounds with putative biological activity is highly crucial. In Mentha plants, the gas chromatography-mass spectrometry (GC-MS) method is generally used to identify the chemical compounds in the essential oil, and the spectrophotometry method is performed to measure the amount of metabolites. Identification of compounds present in the extracts of Mentha plants such as phenolic acids, flavonoids, and carotenoids is accomplished using High-Performance Liquid Chromatography (HPLC) and Liquid Chromatography–mass spectrometry (LC-mass) methods ( 12 ).
8. Breeding Efforts of the Genus Mentha
From a commercial point of view, breeding efforts are focused on improving the essential oil quality and verticillium wilt disease resistance of new mint cultivars ( 2 ). Clonal propagation in cultivated commercial mints such as peppermint and spearmint as the main producers of essential oils, leads to unusual ploidy levels ( 2 ). Peppermint and spearmint have complex polyploidy genomes and conventional breeding is not amenable to improving crops due to sterility. The complex polyploidy genome of the cultivated mints, disease sensitivity, and sterility all threaten the sustainability of mint farming ( 133 ).
Therefore, genetic improvement of this economically important crop is needed for its long-term sustainability. Since the advent of genome sequencing technology and the development of molecular markers, marker-assisted selection to improve verticillium wilt disease resistance and desirable essential oil characteristics has increased the importance of breeding programs in mints ( 2 ).
9. Biotechnological Techniques for the Produc-tion of High-Value Secondary Metabolites
Extraction of plant active ingredients from the tissues of intact plants results in biodiversity loss. In addition, production of valuable secondary metabolites by plants is often less than 1% of dry weight and is dependent on the physiological and developmental stage of the plant. To overcome these problems alternative methods such as in vitro culture techniques should be applied for secondary metabolites production ( 157 ).
9.1. Production of Mints Secondary Metabolites by Plant Cell and Tissue Culture Techniques
Plant cell and tissue culture techniques are considered as alternative methods for the production of important secondary metabolites when the supply of these compounds from nature is limited and traditional methods are not practical. Some advantages of the in vitro techniques are including the mass propagation of Mentha plants in an aseptic environment under controlled nutritional and environmental conditions, and the production of bioactive secondary metabolites on a large scale throughout the year without seasonal restrictions. Plant cell and tissue culture is a reliable and novel approach for high-efficient secondary metabolite isolation within a short span of time ( 158 ).
9.1.1. Hairy Root Culture System
Since secondary metabolites are often produced in limited quantities in differentiated tissues of medicinal plants, the extraction, isolation, and purification of these metabolites is generally a major problem. It is advantageous to use an efficient biotechnological method to produce these metabolites in less time and higher yield. Hairy root cultures offer many great features as a persistent source for the production of beneficial secondary metabolites due to their high growth rate, easy preservation, biochemical and genetic stability, and potential to synthesize higher quantities of secondary metabolites relative to natural plant roots. Hairy root disease which is induced by the gram-negative bacterium Agrobacterium rhizogenes, has gained attention as an effective biotechnological method for the production of active plant ingredients ( 23 ). Induction of hairy root and the production of secondary metabolites such as phenolic acids from some species of Mentha plants have been reported ( 22 , 23 , 159 ). In a recent study by Yousefian et al. (2020) the phenolic acids (caffeic acid, rosmarinic acid, chlorogenic acid, lithospermic acid, and cinnamic acid) production was carried out in hairy root cultures of M. spicata using five different A. rhizogenes strains ( 23 ).
10. Strategies for Enhanced Production of Mints Secondary Metabolites
Biotechnology has made it possible to apply several approaches such as metabolic engineering, elicitation, strain improvement, and optimization of culture medium to improve the in vitro secondary compounds production level ( 160 ). A better understanding of the mechanisms stimulated by external signals that can improve the secondary metabolites levels can be valuable for biotechnologists to increase the production of these compounds ( 161 ).
10.1. Optimization of Culture Medium
There are numerous parameters that can be optimized to enhance the secondary metabolite content of Mentha plants, including the medium pH, nutrient composition, nitrate, and phosphate levels, carbon source, and plant growth regulators type and concentration ( 158 ). The effect of phosphate, nitrogen, and pH on the rosmarinic acid content of M. aquatica hairy roots was evaluated by Yousefian et al. (2020). The results indicated that phosphate deficiency, increased phosphate levels, and acidic pH (phosphate 2.5 mM (+P), 0.6 mM (-P), and pH=5.5) significantly increased the rosmarinic acid content of hairy roots in comparison to the control medium (1.25 mM phosphate and pH= 5.8), whereas increasing the amount of nitrogen did not affect its level ( 159 ).
10.2. Elicitation
Stress as an important factor regulates the formation of many secondary metabolites ( 22 ). High-value plant secondary metabolites play a key role in plant responses to biotic and abiotic stresses to protect them against any threats. Stimulating the plant defense response by using substances to improve the production of these secondary metabolites is called elicitation.
The plant tissue cultures can be elicited by biotic or abiotic elicitors (signaling molecules such as phytohormones) ( 23 ). The effect of phytohormones as abiotic elicitors on secondary metabolites production has been widely studied. Methyl jasmonate (MeJA) as a signal molecule has a prominent role in plant signal transduction pathways ( 162 ). It is considered a potent abiotic elicitor which is applied exogenously in plant cell and tissue cultures to induce the production of desirable secondary compounds ( 22 ). Yousefian et al. (2020) investigated the effect of MeJA on phenolic acid biosynthesis in hairy root cultures of M. spicata. The results implied that MeJA has a significant impact on rosmarinic acid, caffeic acid, chlorogenic acid, and cinnamic acid accumulation, which might be the consequence of gene activation from the phenylpropanoid and tyrosine-derived pathways in the elicitor-treated hairy root cultures ( 22 ).
10.3. Genetic and Metabolic Engineering
As mentioned earlier, Mentha plants have been recognized as medicinal herbs in the traditional medicine of many countries ( 178 ). They are known for a range of various bioactive phytochemicals, especially polyphenols (rosmarinic and salvianolic acids) and monoterpenes (menthol and carvone) ( 179 ). Nowadays, due to the economic importance of Mentha plants, the demand for their products is growing exponentially. Therefore, to supply such demand, new strategies should be adopted to improve the yield and medicinal quality of the products. The advanced methods of genetic and metabolic engineering are useful to deal with any obstacles which limit the accumulation of bioactive compounds, and thus, to improve the yield of plants. Up to now, few studies have been carried out regarding the genetic modification of Mentha spp. This could be due to the lack of sufficient information about Mentha genome sequences. For instance, a little information about the number of Coding DNA Sequences (CDS) and Expressed Sequence Tags (EST) of Mentha have been submitted to the National Center for Biotechnology Information (NCBI). Additionally, due to the low number of sequence data, the cloning and sequencing process of regulatory genes are restricted to using the sequence of closely related Mentha species. However, a draft of M. longifolia genome has been recently published that provides a valuable genomic resource for studies aiming to identify novel genes linked to desirable oil characteristics.
In the following paragraphs, we will report and discuss the obtained results about the biosynthetic and regulatory genes that can be used in biotechnological strategies to boost the synthesis of the main class of compounds that have been characterized in Mentha ssp.
10.3.1. Metabolic Engineering of Monoterpene Bio-synthesis
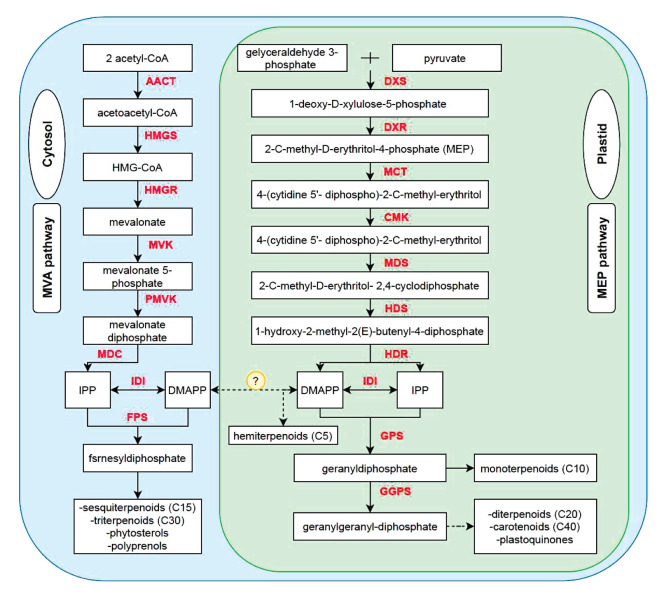
Terpenoids are the main group of pharmacologically active phytochemicals found in Mentha plants ( 180 ). By the number of isoprene units (C5H8), terpenes are classified into different groups such as monoterpenes, sesquiterpenes, diterpenes, triterpenes, tetraterpenes, and polyterpenoids ( 181 ). All these terpenoids arise biosynthetically from the cytoplasmic mevalonate pathway or plastidial 2-C-methyl-D-erythritol 4-phosphate (MEP) pathway (Fig. 2) ( 182 ). Monoterpenes are the main class of mint essential oil, which are generally colorless, lipophilic, and volatile ( 182 ). They are frequently used in pharmaceuticals, cosmetic products, fragrance, and flavor industries ( 183 ). Carvone and menthol are economically valuable monoterpenes found in M.spicata and M. piperita, respectively ( 184 ). During previous attempts, high-efficiency transformation protocols using A. tumefaciens/rhizogenes have been used for transferring genes that are involved in the biosynthesis of monoterpenes in spearmint and peppermint ( 185 , 186 ). The genes were cloned and functionally characterized using metabolic engineering strategies including up-regulation or over-expression of the gene(s)/enzyme(s), down-regulation of the gene(s)/enzyme(s), and engineering of the regulatory gene(s) ( 184 , 187 - 194 ).
Figure 2.
An overview of terpenoids biosynthetic pathway ( 195 )
10.3.1.1. Up-Regulation or Over Expression of Genes
Monoterpenes are mainly derived from the MEP pathway in plastids (Fig. 3). One way to potentially elevate the yield of mint oil is overexpression of the key genes involved in the production of precursors in the monoterpene biosynthesis pathway. For example, Mahmoud and Croteau (2001) reported that the oil yield of Mentha spp. was increased in transgenic plant lines through the overexpression of the 1-deoxy-D-xylulose 5-phosphate reductoisomerase (DXR) gene ( 189 ). They generated transgenic lines of M.piperita with a homologous sense version of DXR cDNA. According to their results, regenerated transgenic samples which remained normal in appearance and development, showed a strong expression of reductoisomerase. The yield of oil was also increased up to 50% without any changes in monoterpene composition. On the other hand, the mRNA or enzyme activity of reductoisomerase was not detected in chlorophyll-deficient transgenic lines. These plants yielded less essential oil than wild-type samples, which indicated the co-suppression of the reductoisomerase gene ( 189 ). In Salvia plants, a close genus to Mentha, a complex metabolic engineering push-pull strategy including simultaneously over-expressing three genes (DXS and GGPS from MEP pathway and HMGR from MVA pathway) was conducted for enhancing monoterpenes biosynthesis by transgenic hairy root cultures. The results showed remarkable achievement of tanshinones production (about 4.74 times higher than the control) ( 196 ). In another study, the co-introduction of DXS and GGPPS yielded monoterpene levels as high as 12.93 mg/g DW, compared to 0.61 mg/g DW in the controls ( 196 ). These findings showed that overexpression of precursor genes involved in monoterpene pathways (alone or in combination) was an appropriate approach to enhance monoterpene production.
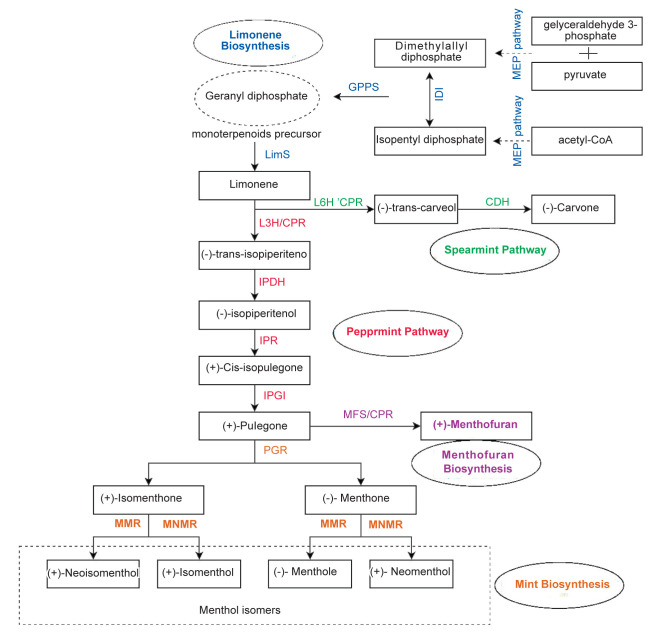
Figure 3.
Monoterpenoids biosynthetic pathway in mint species ( 198 )
Contrary to obtained results from the overexpression of biosynthetic genes, enhancing the expression level of enzymatic genes didn’t have any significant effect on increasing mint oil yield. Cyclization of geranyl pyrophosphate to the olefin (-)-4S-limonene is the first committed enzymatic process in the biosynthesis of monoterpenes which is catalyzed via limonene synthase ((−)-LS) (Fig. 3). This enzyme is considered as a potentially rate-limiting enzyme because of its relatively low catalytic efficiency (187). The previous results demonstrated that elevating the expression level of the gene encoding (−)-LS (LimS) in M. piperita led to only a moderate increase in the oil yield ( 197 ). Mahmoud et al., (2004) transformed cDNA clones of limonene synthase and limonene-3-hydroxylase into M.piperita. Despite the constitutive expression of both genes in transgenic plants, the activity of the corresponding enzyme was not significantly increased. Based on their result, overexpression of this enzyme had no effect on oil yield or its composition in the regenerated plants ( 191 ).
10.3.1.2. Downregulation or Functional Knockout of Genes
Downregulation or functional knockout of the genes is an effective strategy to decrease the production of certain unwanted compounds and boost the concentration and quality of a desired secondary metabolite. RNA interference (RNAi) and antisense RNA technologies approaches have been used for the inhibition or down-regulation of a gene. The principle of antisense RNA technology is that an antisense nucleic acid sequence base pairs with its complementary sense RNA strand and prevents its translation into protein. Whereas, RNAi is a gene silencing process by double-strand RNA (dsRNA) in which only the mRNA associated with dsRNA is specifically degraded ( 181 ).
The gene silencing approaches have been successfully employed to enhance the quality of mint essential oil. For instance, (+)-menthofuran monoterpenoid and its intermediate (+)-pulegone (common components in several Mentha species essential oils) are considered undesirable components in commercial essential oil (Fig. 3) ( 190 ). Their adverse effects, such as increased liver weight, hepatotoxicity, and cerebellar pathology have been proven in multiple studies ( 190 , 200 ). Therefore, metabolic engineering efforts aimed at improving oil composition have focused on reducing these ingredients. For example, antisense RNA technology was used to downregulate the expression of the (+)-menthofuran synthase (MFS) in M. piperita. Interestingly, the transgenic antisense lines, not only had a reduction in (+)-pulegone and (+)-menthofuran levels but also showed an increased oil yield by roughly 35% ( 189 ). This is inconsistent with the study by Mahmoud et al., which reported that transformed peppermint with the antisense version of the menthofuran synthase cDNA produced less than half of this unfavorable monoterpene than wild-type mint ( 190 ). In another research, Lange et al., transformed M. piperita, with various gene constructs to improve essential oil yield and composition simultaneously. They achieved transforming plants expressing an antisense version of (+)-menthofuran synthase with a construct for the overexpression of the DXR gene. The results showed significantly enhanced oil content (up to 61% oil yield increase over wild-type controls). Also, the level of undesirable side-products like (+)-menthofuran and (+)-pulegone was decreased ( 180 ). The above-mentioned descriptions demonstrated that the reduction of both menthofuran and pulegone levels by metabolic engineering approach has commercial significance in improving the quality of mint’s essential oil.
10.3.1.3. Engineering of Regulatory Genes
Although the cloning and functional characterization of genes involved in terpene biosynthesis has been quite successful in Mentha species, knowledge about the regulation of these secretory trichome-specific pathways is extremely limited. Studies on spearmint indicate that essential oil biosynthesis is highly controlled at the transcriptional level ( 194 ). Hence, recognition of transcription factors (TFs) that regulate metabolic pathways will present an attractive strategy for the engineering of terpenoids biosynthesis. Recently, MsMYB (a R2R3-MYB gene) and MsYABBY5 TFs that are preferentially expressed in the PGTs (peltate glandular trichomes) were isolated from transcriptomic data of spearmint. To investigate the role of these TFs in monoterpene production, transgenic lines were generated in which the MsMYB and MsYABBY5 were either overexpressed or silenced. A High level of monoterpenes was detected in TFs-RNAi transgenic lines, whereas TFs-upregulation transgenic lines showed decreased levels of monoterpenes. These results demonstrated that MsMYB and MsYABBY5 are novel inhibitors of monoterpene biosynthesis in Mentha spp. ( 181 , 194 ). Additionally, according to obtained results from the RNA-seq data of spearmint, MYBs, and WRKY transcription factors were significantly more abundant in PGTs ( 183 ). Therefore, they can be good candidates for engineering the biosynthetic pathway of monoterpenes.
10.3.2. Metabolic Engineering of Phenolic Compounds Biosynthesis Pathway
It has been shown that Mentha species especially M. spicata contain considerable amounts of phenolic compounds specially Rosmarinic acid (RA) ( 201 ). Although progressive genetic improvement was focus-ed on increasing of the Mentha’s essential oils, no efforts have yet been made to improve the production of rosmarinic acid or other antioxidant compounds in Mentha spp. Therefore, developing mint varieties with high levels of rosmarinic acid provides an economically valuable source of rosmarinic acid or other phenolic acids.
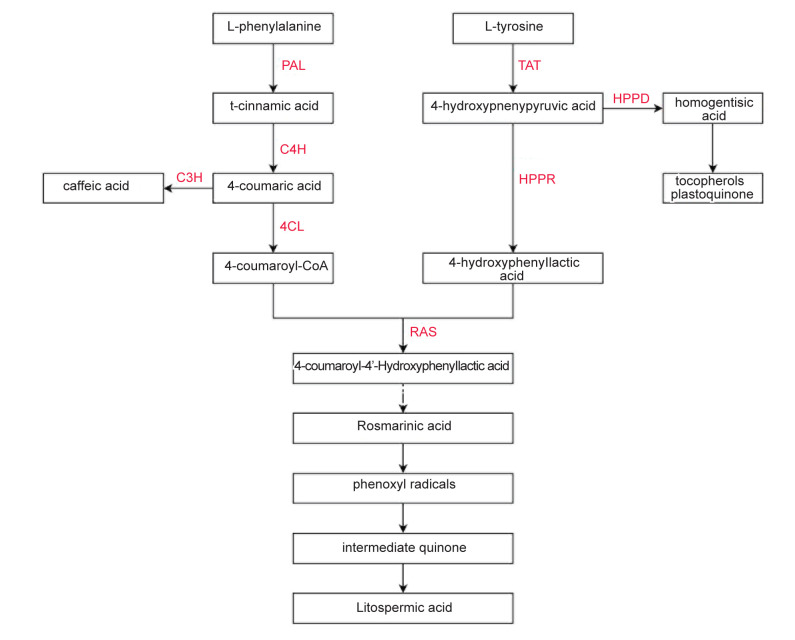
In brief, RA biosynthesis requires the contribution of both the phenylpropanoid branch which leads to the caffeic acid moiety, and the tyrosine-derived branch which drives to the dihydroxyphenyl lactic acid. Conversion of L-phenylalanine to 4-coumaroyl-CoA is done through the phenylpropanoid pathway via multiple reactions catalyzed by phenylalanine ammonia-lyase (PAL), cinnamic acid 4-hydroxylase (C4H), and 4-coumarate CoA ligase (4CL). In the tyrosine-derived pathway, L-tyrosine is converted to 4-hydroxyphenyllactate by two reactions, catalyzed by tyrosine aminotransferase (TAT) and HPPR. Hydroxyphenyllactate is then coupled with 4-coumaroyl CoA by the enzyme rosmarinic acid synthase (RAS) to form rosmarinic acid, and its intermediates (Fig. 4). Since there are no studies focusing on the metabolic engineering of rosmarinic acid and other phenolic compounds of mint plants, this section introduces candidate and engineered biosynthetic genes which are used to increase polyphenol biosynthesis in Salvia species. According to the results of a study by Xiao et al., a strong increment of RA and Salvianolic acid B (SalB) production was observed through overexpression of both TAT and HPPR in s. miltiorrhiza hairy roots ( 202 ). Yousefian et al. reported that upon MJ-elicitation of M. spicata hairy roots, the relative expression levels of phenylpropanoid pathway genes (PAL, C4H, 4CL), and HPPR in the tyrosine-derived pathway were significantly increased compared to untreated controls. Additionally, based on their results, high-performance liquid chromatography analysis indicated that MeJA had a significant impact on RA, CA (caffeic acid), CGA (chlorogenic acid), and CIA (t-cinnamic acid) accumulation ( 22 ). These findings suggested that the overexpression of genes involved in phenylpropanoid and tyrosine-derived pathways can improve the phenolic acid content in Mentha spp. Jin et al. reported the expression of transcripts encoding enzymes for PAL, C4H, and 4CL in the PGT of spearmint ( 183 ).
Figure 4.
Simplified diagram of enzymes and products involved in rosmarinic acid biosynthesis ( 22 )
New reports evidenced that the phenylpropanoid pathway is highly controlled at the transcriptional level ( 203 ). Moreover, several transcription factors have been identified that control the expression of the essential genes (PAL, C4H, and 4CL) in the phenolic acids production process. Different transcriptional factors (TF) and other protein regulators have been characterized and may be used to enhance the precursor flow of the bioactive Salvia phenolic acids ( 196 ). For instance, successful technological results for increasing the amount of both RA and SalB have been obtained by constitutively co-expressed AmDELILA (belonging to the R2R3 MYB family) and AmROSEA1 (belonging to the bHLH family) in S. miltiorrhiza ( 204 ). Genome-wide comparative analysis has identified seven R2R3MYB regulators of Salvia phenolic acids including SmMYB1, SmMYB13, SmMYB16, SmMYB35, SmMYB62, SmMYB63, and SmMYB79. These TFs were considered theoretically as putative regulators which may be proven to be useful, alone or in combination, for the re-direction of metabolic flux towards the desired phenylpropanoid branches ( 205 ). Sequence data and expression values of 4 R2R3-MYBs of spearmint including MYB1, MYB16, MYB35, and MYB79 have been identified by Reddy et al. ( 194 ). Overexpression of heterologous OsMYB4 TF in S. sclarea hairy roots and tobacco plants was also proved to be effective for increasing phenolic acids accumulation. The results of this research suggested that several genes involved in the phenylpropanoid pathway were up-regulated by this MYBTF. These findings indicated that the regulatory role of this TF is evolutionarily conserved in phenylpropanoid biosynthesis ( 196 , 206 ).
11. Conclusion
Identifying the general biosynthetic pathways, leading to the accumulation of pharmaceutically important secondary metabolites, such as terpenoids in Mentha species, has paved the way for developing highly productive plants with improved phytochemical profiles. Nowadays, methods, such as metabolite engineering, and -omics technologies, especially functional genomics and mapping can be useful to improve highly productive, economically feasible platforms for the sustainable production of valuable medicinal phytochemicals of Mentha origin. Elucidation of regulatory mechanisms and evaluation of the potential rate-limiting steps are fundamental biotechnological strategies in many secondary metabolite synthesis pathways. Sequencing and characterization of the M. longifolia genome and the availability of transcriptomic data of M. spicata create opportunities for the identification and characterization of key genes involved in the main secondary metabolites of Mentha species ( 207 ). Such methods could help to a better understanding of the specific biosynthetic and regulatory mechanisms existing in this remarkable genus. On the other hand, an advanced method in metabolic engineering, called genome editing tools, provides a much easier way for engineering secondary metabolites biosynthetic pathways and relevant genes ( 208 ).
Acknowledgments
We thank the Agricultural Biotechnology Department of NIGEB for providing basic facilities to plan and develop this Review Article.
Declaration of Competing Interest
The authors declare that this article has no conflicts of interest.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Elzebroek ATG, Wind K. Guide to Cultivated Plants. Cambridge. CABI; 2008. [Google Scholar]
- 2.Vining KJ, Hummer KE, Bassil NV, Lange BM, Khoury CK, Carver D. Crop wild relatives as germplasm resource for cultivar improvement in mint (Mentha L.) . Front Plant Sci. 2020;11:1217. doi: 10.3389/fpls.2020.01217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anwar F, Abbas A, Mehmood T, Gilani AH, Rehman Nu. Mentha: A genus rich in vital nutra-pharmaceuticals:A review. Phytother Res. 2019;33(10):2548–2570. doi: 10.1002/ptr.6423. [DOI] [PubMed] [Google Scholar]
- 4.Liu J, Feng W, Peng C. A song of ice and fire: cold and hot properties of traditional Chinese medicines. Front Pharmacol. 2021;11:598744. doi: 10.3389/fphar.2020.598744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang J, Lan Y, Li H, Zhang Y, Zhang Q, Cao Y, et al. Percutaneous penetration enhancement effect of essential oil of mint (Mentha haplocalyx Briq.) on Chinese herbal components with different lipophilicity. J Tradit Chin Med. 2014;1(2):109–19. doi: 10.1016/j.jtcms.2014.09.003. [DOI] [Google Scholar]
- 6.Alu’datt MH, Rababah T, Alhamad MN, Gammoh S, Al-Mahasneh MA, Tranchant CC, et al. Pharmaceutical, nutraceutical and therapeutic properties of selected wild medicinal plants: Thyme, spearmint, and rosemary. Elsevier; 2018. pp. 275–90. [DOI] [Google Scholar]
- 7.CHIȘ M-S, MUSTE S, PĂUCEAN A, MAN S, POP A, POP CR, et al. A comprehensive review of medicinal and therapeutic uses of Mentha piperita. 2019; 27:38–49. [Google Scholar]
- 8.Moetamedipoor SA, Saharkhiz MJ, Khosravi AR, Jowkar A. Essential oil chemical diversity of Iranian mints. Ind Crops Prod. 2021;172:114039. doi: 10.1016/j.indcrop.2021.114039. [DOI] [Google Scholar]
- 9.Brahmi F, Khodir M, Mohamed C, Pierre D. Chemical composition and biological activities of Mentha species. IntechOpen; 2017. pp. 47–79. [DOI] [Google Scholar]
- 10.Li B, Cantino PD, Olmstead RG, Bramley GL, Xiang C-L, Ma Z-H, et al. A large-scale chloroplast phylogeny of the Lamiaceae sheds new light on its subfamilial classification. Sci Rep. 2016;6(1):1–18. doi: 10.1038/srep34343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jedrzejczyk I, Rewers M. Genome size and ISSR markers for Mentha L.(Lamiaceae) genetic diversity assessment and species identification. Ind Crops Prod. 2018;120:171–9. doi: 10.1016/j.indcrop.2018.04.062. [DOI] [Google Scholar]
- 12.Salehi B, Stojanović-Radić Z, Matejić J, Sharopov F, Antolak H, Kręgiel D, et al. Plants of genus Mentha: From farm to food factory. Plants. 2018;7(3):70. doi: 10.3390/plants7030070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanafy DM, Prenzler PD, Hill RA, Burrows GE. Leaf micromorphology of 19 Mentha taxa. Aust J Bot. 2019;67 (7):463–72. doi: 10.1071/BT19054. [DOI] [Google Scholar]
- 14.Kapp K. Polyphenolic and essential oil composition of Mentha and their antimicrobial effect. University of Helsinki; 2015. [Google Scholar]
- 15.Shabih S, Hajdari A, Mustafa B, Quave CL. Medicinal plants in the Balkans with antimicrobial properties. Medicinal Plants as Anti-Infectives. 2022: 103–39. doi: 10.1016/B978-0-323-90999-0.00013-6. [DOI] [Google Scholar]
- 16.Daneshbakhsh D, Asgarpanah J, Najafizadeh P, Rastegar T, Mousavi Z. Safety assessment of Mentha mozaffarianii essential oil: acute and repeated toxicity studies. Iran J of Med Sci. 2018;43(5):479. [PMC free article] [PubMed] [Google Scholar]
- 17.Tavakkoli-Khaledi S, Asgarpanah J. Essential oil chemical composition of Mentha mozaffarianii jamzad seeds. J Mex Chem Soc. 2016;60(1):19–22. doi: 10.29356/jmcs.v60i1.66. [DOI] [Google Scholar]
- 18.Jamzad Z. A survey of Lamiaceae in the flora of Iran. Rostaniha. 2013;14(1):59–67. doi: 10.22092/botany.2013.101317. [DOI] [Google Scholar]
- 19.Ahmad RS, Imran A, Arshad MS, Hussain MB, Waheed M, Safdar S, et al. Introductory Chapter: Mentha piperita (a Valuable Herb): Brief Overview. In: Akram M, Ahmad RS, editors. Herbs and Spices. London: IntechOpen; 2020. p. 1. [DOI] [Google Scholar]
- 20.Peter KV. Handbook of herbs and spices Braz. J. Pharm. Sci. 2010; 46(4) doi: 10.1590/S1984-82502010000400030. [DOI] [Google Scholar]
- 21.Roshanibakhsh F, Samsampour D, Askari Seyahooei M, Bagheri A. Strong relationship between molecular and morphological attributes in Iranian mentha populations (Mentha mozaffarianii Jamzad) . Genet Resour Crop Evol. 2023:1–25. doi: 10.1007/s10722-022-01532-1. [DOI] [Google Scholar]
- 22.Yousefian S, Lohrasebi T, Farhadpour M, Haghbeen K. Effect of methyl jasmonate on phenolic acids accumulation and the expression profile of their biosynthesis-related genes in Mentha spicata hairy root cultures. PCTOC. 2020;142(2):285–97. doi: 10.1007/s11240-020-01856-9. [DOI] [Google Scholar]
- 23.Yousefian S, Lohrasebi T, Farhadpour M, Haghbeen K. Production of phenolic acids in hairy root cultures of medicinal plant Mentha spicata L. in response to elicitors. MBRC. 2020;9(1):23. doi: 10.22099/mbrc.2020.36031.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Soltanbeigi A, Özgüven M, Hassanpouraghdam MB. Planting-date and cutting-time affect the growth and essential oil composition of Mentha× piperita and Mentha arvensis. Ind Crops Prod. 2021;170:113790. doi: 10.1016/j.indcrop.2021.113790. [DOI] [Google Scholar]
- 25.Bhattacharya S. Cultivation of essential oils. In: Preedy V, editor. Essential oils in food preservation, flavor and safety. Academic Press; 2016. pp. 19–29. [DOI] [Google Scholar]
- 26.Devi A, Sharma G. Morphological, phenological and cytological comparison of Mentha longifolia and M. spicata from sub-tropical and temperate regions of Jammu province (J&K) . Vegetos. 2022;35(1):179–87. doi: 10.1007/s42535-021-00278-y. [DOI] [Google Scholar]
- 27.Brahmi F, Lounis N, Mebarakou S, Guendouze N, Yalaoui-Guellal D, Madani K, et al. Impact of growth sites on the phenolic contents and antioxidant activities of three algerian mentha species (M. pulegium L., M. rotundifolia (L.) Huds., and M. spicata L.) . Front Pharmacol. 2022; 13: 886337. doi: 10.3389/fphar.2022.886337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heydari M, Zanfardino A, Taleei A, Shahnejat Bushehri AA, Hadian J, Maresca V, et al. Effect of heat stress on yield, monoterpene content and antibacterial activity of essential oils of Mentha x piperita var. Mitcham and Mentha arvensis var. piperascens. Molecules. 2018;23(8):1903. doi: 10.3390/molecules23081903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mollaei S, Ebadi M, Hazrati S, Habibi B, Gholami F, Sourestani MM. Essential oil variation and antioxidant capacity of Mentha pulegium populations and their relation to ecological factors. Biochem Syst Ecol. 2020;91:104084. doi: 10.1016/j.bse.2020.104084. [DOI] [Google Scholar]
- 30.Thuraisingham V, SERAN T. Response of rooting atributes of peppermint (Mentha piperita L.) stem cuttings to natural rooting stimulators. IJCST. 2019;5(1):20–7. [Google Scholar]
- 31.Fontana E, Nicola S, Hoeberechts J. Rooting products and cutting timing for peppermint (Mentha× piperita L.) radication. Acta Hortic. 2006: 297–302. doi: 10.17660/ActaHortic.2006.723.41. [DOI] [Google Scholar]
- 32.Thawkar BS. Phytochemical and pharmacological review of Mentha arvensis. IJGP. 2016;10(2) [Google Scholar]
- 33.Bohloul A, Sayed AV, Hossein AF, Hossein HD. Studying of essential oil variations in leaves of Mentha species. AJPS. 2009;3(10):217–21. doi: 10.5897/AJPS.9000242. [DOI] [Google Scholar]
- 34.Božović M, Pirolli A, Ragno R. Mentha suaveolens Ehrh.(Lamiaceae) essential oil and its main constituent piperitenone oxide: Biological activities and chemistry. Molecules. 2015;20(5):8605–33. doi: 10.3390/molecules20058605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Azizian D. Anatomical studies of Mentha mozaffarianii (Labiatae) and a related species. The Iranian Journal of Botany. 2015;7(1):63–72. doi: 10.22092/IJB.2015.103448. [DOI] [Google Scholar]
- 36.Kumar D, Kumar R, Singh AK, Verma K, Singh KP, Kumar A, et al. A novel and economically viable agro-technique for enhancing productivity and resource use efficiency in menthol mint (Mentha arvensis L.) . Ind Crops Prod. 2021;162:113233. doi: 10.1016/j.indcrop.2020.113233. [DOI] [Google Scholar]
- 37.Yeşil M. The effect of different planting times on yield and quality features in some mint species (Mentha longifolia, Mentha x piperita, Mentha spicata) . EJFA. 2021:671–81. doi: 10.9755/ejfa.2021.v33.i8.2743. [DOI] [Google Scholar]
- 38.Gomes HT, Bartos PMC, Martins AE, Oliveira SOD, Scherwinski-Pereira JE. Assessment of mint (Mentha spp.) species for large-scale production of plantlets by micropropagation. Acta Sci Biol Sci. 2015;37(4):405–10. doi: 10.4025/actascibiolsci.v37i4.26984. [DOI] [Google Scholar]
- 39.Łyczko J, Piotrowski K, Kolasa K, Galek R, Szumny A. Mentha piperita L. micropropagation and the potential influence of plant growth regulators on volatile organic compound composition. Molecules. 2020;25(11):2652. doi: 10.3390/molecules25112652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Knaus U, Zimmermann J, Appelbaum S, Palm HW. Spearmint (Mentha spicata) cultivation in decoupled aquaponics with three hydro-components (grow pipes, raft, gravel) and African catfish (Clarias gariepinus) production in northern Germany. Sustainability. 2022;14(1):305. doi: 10.3390/su14010305. [DOI] [Google Scholar]
- 41.Surendran U, Chandran C, Joseph E. Hydroponic cultivation of Mentha spicata and comparison of biochemical and antioxidant activities with soil-grown plants. Acta Physiol Plant. 2017;39(1):1–14. doi: 10.1007/s11738-016-2320-6. [DOI] [Google Scholar]
- 42.Garlet TMB, Paulus D, Flores R, Garlet TMB. Production and chemical composition of Mentha x piperita var. citrata (Ehrh.) Briq. essential oil regarding to different potassium concentrations in the hydroponic solution. J Biotechnol Biodivers. 2013;4(3):200–6. doi: 10.20873/jbb.uft.cemaf.v4n3.garlet. [DOI] [Google Scholar]
- 43.Behera M, Mahapatra P, Singandhupe R, Kundu D, Kannan K, Mandal K, et al. Effect of drip fertigation on yield, water use efficiency and water productivity of mint (Mentha arvensis L.) . J Agric Phys. 2014;14(1):37–43. [Google Scholar]
- 44.Sommano SR, Kanthawang N, Janpen C, Wongkaew M, Inkham C, Van Doan H, et al. Physiological and Oxidative Responses of Japanese Mint Grown Under Limited Water and Nitrogen Supplies in an Evaporated Greenhouse System. Front Sustain Food Syst. 2022; 5: 533. doi: 10.3389/fsufs.2021.808327. [DOI] [Google Scholar]
- 45.O. Elansary H, Mahmoud EA, El-Ansary DO, Mattar MA. Effects of water stress and modern biostimulants on growth and quality characteristics of mint. Agronomy. 2019;10(1):6. doi: 10.3390/agronomy10010006. [DOI] [Google Scholar]
- 46.Chiappero J, del Rosario Cappellari L, Alderete LGS, Palermo TB, Banchio E. Plant growth promoting rhizobacteria improve the antioxidant status in Mentha piperita grown under drought stress leading to an enhancement of plant growth and total phenolic content. Ind Crops Prod. 2019;139:111553. doi: 10.1016/j.indcrop.2019.111553. [DOI] [Google Scholar]
- 47.Rahimi Y, Taleei A, Ranjbar M. Long-term water deficit modulates antioxidant capacity of peppermint (Mentha piperita L.) . Sci Hortic. 2018;237:36–43. doi: 10.1016/j.scienta.2018.04.004. [DOI] [Google Scholar]
- 48.Pandey R, Singh A, Trivedi S, Suchi S, Smita TP, Shukla A, et al. Diseases of mints and their management. Today and Tomorrow’s Printers and Publishers. 2019: 273–303. [Google Scholar]
- 49.Kalra A, Singh H, Pandey R, Samad A, Patra N, Kumar S. Diseases in mint: causal organisms, distribution, and control measures. J Herbs Spices Med Plants. 2005; 11(1-2): 71–91. doi: 10.1300/J044v11n01_03. [DOI] [Google Scholar]
- 50.Tzanetakis IE, Postman JD, Samad A, Martin RR. Mint viruses: Beauty, stealth, and disease. Plant Dis. 2010;94(1):4–12. doi: 10.1094/PDIS-94-1-0004. [DOI] [PubMed] [Google Scholar]
- 51.Nega A. Review on concepts in biological control of plant pathogens. Journal of Biology, Agriculture and Healthcare. 2014;4(27):33–54. [Google Scholar]
- 52.Aflatuni A, Sari EK JU, Hohtola A. Optimum harvesting time of four Mentha species in Northern Finland. J Essent Oil Res. 2006;18(2):134–8. doi: 10.1080/10412905.2006.9699043. [DOI] [Google Scholar]
- 53.Boor B, Lefebvre N. Harvest and post-harvest handling of herbs. Technical guide. 2021 [Google Scholar]
- 54.Barbosa C, Fonseca M, Silva T, Finger F, Casali V, Cecon P. Effect of hydrocooling, packaging, and cold storage on the post-harvest quality of peppermint (Mentha piperita L.) . Rev bras plantas med. 2016;18:248–55. doi: 10.1590/1983-084X/15_135. [DOI] [Google Scholar]
- 55.Kripanand S, Guruguntla S. Effect of various drying methods on quality and flavor characteristics of mint leaves (Mentha spicata L.) . J Food Pharm Sci. 2015;3(2) doi: 10.14499/jfps. [DOI] [Google Scholar]
- 56.Kannan VS, Arjunan T, Vijayan S. Drying characteristics of mint leaves (Mentha arvensis) dried in a solid desiccant dehumidifier system. J Food Sci Technol. 2021;58(2):777–86. doi: 10.1007/s13197-020-04595-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Venkatachalam SK, Thottipalayam Vellingri A, Selvaraj V. Low-temperature drying characteristics of mint leaves in a continuous-dehumidified air drying system. J Food Process Eng. 2020;43(4):e13384. doi: 10.1111/jfpe.13384. [DOI] [Google Scholar]
- 58.Kee LA, Shori AB, Baba AS. Bioactivity and health effects of Mentha spicata. IFNM. 2017;5(1):1–2. doi: 10.15761/IFNM.1000203. [DOI] [Google Scholar]
- 59.Cirlini M, Mena P, Tassotti M, Herrlinger KA, Nieman KM, Dall’Asta C, et al. Phenolic and volatile composition of a dry spearmint (Mentha spicata L.) extract. Molecules. 2016;21(8):1007. doi: 10.3390/molecules21081007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wei H, Kong S, Jayaraman V, Selvaraj D, Soundararajan P, Manivannan A. Mentha arvensis and Mentha× piperita-Vital Herbs with Myriads of Pharmaceutical Benefits. Horticulturae. 2023;9(2):224. doi: 10.3390/horticulturae9020224. [DOI] [Google Scholar]
- 61.Bayan Y, Küsek M. Chemical composition and antifungal and antibacterial activity of Mentha spicata L. volatile oil. Cienc Investig Agrar. 2018;45(1):64–9. doi: 10.7764/rcia.v45i1.1897. [DOI] [Google Scholar]
- 62.Mikaili P, Mojaverrostami S, Moloudizargari M, Aghajanshakeri S. Pharmacological and therapeutic effects of Mentha Longifolia L. and its main constituent, menthol. Anc Sci Life. 2013;33(2):131. doi: 10.4103/0257-7941.139059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mahendran G, Verma SK, Rahman L-U. The traditional uses, phytochemistry and pharmacology of spearmint (Mentha spicata L.): A review. J Ethnopharmacol. 2021;278:114266. doi: 10.1016/j.jep.2021.114266. [DOI] [PubMed] [Google Scholar]
- 64.Chumpitazi BP, Kearns G, Shulman RJ. the physiological effects and safety of peppermint oil and its efficacy in irritable bowel syndrome and other functional disorders. Aliment Pharmacol Ther. 2018;47(6):738–52. doi: 10.1111/apt.14519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Peeyush K, Sapna M, Anushree M, Santosh S. Insecticidal properties of Mentha species: a review. Ind Crops Prod. 2011;34(1):802–17. doi: 10.1016/j.indcrop.2011.02.019. [DOI] [Google Scholar]
- 66.Saeidi K, Mirfakhraie S. Chemical composition and insecticidal activity Mentha piperita L. essential oil against the cowpea seed beetle Callosobruchus maculatus F.(Coleoptera: Bruchidae) . J Entomol Acarol Res. 2017;49(3) doi: 10.4081/jear.2017.6769. [DOI] [Google Scholar]
- 67.Esmaeili F, Lohrasebi T, Mohammadi-Dehcheshmeh M, Ebrahimie E. Evaluation of the Effectiveness of Herbal Components Based on Their Regulatory Signature on Carcinogenic Cancer Cells. Cells. 2021;10(11):3139. doi: 10.3390/cells10113139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Park YJ, Baek S-A, Choi Y, Kim JK, Park SU. Metabolic profiling of nine Mentha species and prediction of their antioxidant properties using chemometrics. Molecules. 2019;24(2):258. doi: 10.3390/molecules24020258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nikavar B, ALI NA, KAMALNEZHAD M. Evaluation of the antioxidant properties of five Mentha species. 2008; 7(3): 203–209. doi: 10.22037/ijpr.2010.766. [DOI] [Google Scholar]
- 70.Anwar F, Alkharfy KM, Adam EHK. Chemo-geographical variations in the composition of volatiles and the biological attributes of Mentha longifolia (L.) essential oils from Saudi Arabia. Int J Pharmacol. 2017;13(5):408–24. doi: 10.3923/ijp.2017.408.424. [DOI] [Google Scholar]
- 71.Ultee A, Bennik M, Moezelaar R. The phenolic hydroxyl group of carvacrol is essential for action against the food-borne pathogen Bacillus cereus. Appl Environ Microbiol. 2002;68(4):1561–8. doi: 10.1128/AEM.68.4.1561-1568.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Greenwell M, Rahman P. Medicinal plants: their use in anticancer treatment. Int J Pharm Sci Res. 2015;6(10):4103–12. doi: 10.13040/IJPSR.0975-8232.6(10).4103-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chauhan R, Kaul M, Shahi A, Kumar A, Ram G, Tawa A. Chemical composition of essential oils in Mentha spicata L. accession [IIIM (J) 26] from North-West Himalayan region, India. Ind Crops Prod. 2009;29(2-3):654–6. doi: 10.1016/j.indcrop.2008.12.003. [DOI] [Google Scholar]
- 74.Sharma V, Hussain S, Gupta M, Saxena AK. In vitro anticancer activity of extracts of Mentha spp. against human cancer cells. Indian J Biochem Biophys. 2014; 51(5):416–9. [PubMed] [Google Scholar]
- 75.Amaral RG, Fonseca CS, Silva TKM, Andrade LN, França ME, Barbosa-Filho JM, et al. Evaluation of the cytotoxic and antitumour effects of the essential oil from Mentha x villosa and its main compound, rotundifolone. J Pharm Pharmacol. 2015;67(8):1100–6. doi: 10.1111/jphp.12409. [DOI] [PubMed] [Google Scholar]
- 76.Singh P, Pandey AK. Prospective of essential oils of the genus Mentha as biopesticides: A review. Front Plant Sci. 2018;9:1295. doi: 10.3389/fpls.2018.01295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jankowska M, Rogalska J, Wyszkowska J, Stankiewicz M. Molecular targets for components of essential oils in the insect nervous system—A review. Molecules. 2017;23(1):34. doi: 10.3390/molecules23010034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Glas JJ, Schimmel BC, Alba JM, Escobar-Bravo R, Schuurink RC, Kant MR. Plant glandular trichomes as targets for breeding or engineering of resistance to herbivores. J Mol Sci. 2012;13(12):17077–103. doi: 10.3390/ijms131217077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Elshafie HS, Camele I. An overview of the biological effects of some mediterranean essential oils on human health. Biomed Res Int. 2017; 2017: 9268468–9268468. doi: 10.1155/2017/9268468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ebadollahi A, Ziaee M, Palla F. Essential oils extracted from different species of the Lamiaceae plant family as prospective bioagents against several detrimental pests. Molecules. 2020;25(7):1556. doi: 10.3390/molecules25071556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sonmezdag AS, Kelebek H, Selli S. Identification of aroma compounds of Lamiaceae species in Turkey using the purge and trap technique. Foods. 2017;6(2):10. doi: 10.3390/foods6020010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Masyita A, Sari RM, Astuti AD, Yasir B, Rumata NR, Emran TB, et al. Terpenes and terpenoids as main bioactive compounds of essential oils, their roles in human health and potential application as natural food preservatives. Food chemistry. 2022:100217. doi: 10.1016/j.fochx.2022.100217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fuchs LK, Holland AH, Ludlow RA, Coates RJ, Armstrong H, Pickett JA, et al. Genetic manipulation of biosynthetic pathways in mint. Front Plant Sci. 2022; 13: 928178. doi: 10.3389/fpls.2022.928178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wu Z, Tan B, Liu Y, Dunn J, Martorell Guerola P, Tortajada M, et al. Chemical composition and antioxidant properties of essential oils from peppermint, native spearmint and scotch spearmint. Molecules. 2019;24(15):2825. doi: 10.3390/molecules24152825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Choudhury RP, Kumar A, Garg A. Analysis of Indian mint (Mentha spicata) for essential, trace and toxic elements and its antioxidant behaviour. J Pharm Biomed Anal. 2006;41(3):825–32. doi: 10.1016/j.jpba.2006.01.048. [DOI] [PubMed] [Google Scholar]
- 86.Kumar A, Chattopadhyay S. DNA damage protecting activity and antioxidant potential of pudina extract. Food Chem. 2007;100(4):1377–84. doi: 10.1016/j.foodchem.2005.12.015. [DOI] [Google Scholar]
- 87.Scherer R, Lemos MF, Lemos MF, Martinelli GC, Martins JDL, da Silva AG. Antioxidant and antibacterial activities and composition of Brazilian spearmint (Mentha spicata L.) Ind Crops Prod. 2013;50:408–13. doi: 10.1016/J.INDCROP.2013.07.007. [DOI] [Google Scholar]
- 88.El Menyiy N, Mrabti HN, El Omari N, Bakili AE, Bakrim S, Mekkaoui M, et al. Medicinal Uses, Phytochemistry, Pharmacology, and Toxicology of Mentha spicata. Evid Based Complement Alternat Med. 2022 Apr 12; 2022 doi: 10.1155/2022/7990508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gaafar A, Nooman M, brahim E, Ali M, Al-Kashef AS. Prophylactic and Therapeutic Uses of Egyptian Mentha spicata L., Mentha piperita L. and Ocimum basilicum L. Stalks as Agro-industrial Byproducts. J Biol Sci. 2018;18:354–63. doi: 10.3923/jbs.2018.354.363. [DOI] [Google Scholar]
- 90.Biswas NN, Saha S, Ali MK. Antioxidant, antimicrobial, cytotoxic and analgesic activities of ethanolic extract of Mentha arvensis L. Asian Pac J Trop Biomed. 2014;4(10):792–7. doi: 10.12980/APJTB.4.2014C1298. [DOI] [Google Scholar]
- 91.Teixeira B, Marques A, Ramos C, Batista I, Serrano C, Matos O, et al. European pennyroyal (Mentha pulegium) from Portugal: Chemical composition of essential oil and antioxidant and antimicrobial properties of extracts and essential oil. Ind Crops Prod. 2012;36(1):81–7. doi: 10.1016/j.indcrop.2011.08.011. [DOI] [Google Scholar]
- 92.Singh R, Shushni MA, Belkheir A. Antibacterial and antioxidant activities of Mentha piperita L. Arab J Chem. 2015;8(3):322–8. doi: 10.1016/j.arabjc.2011.01.019. [DOI] [Google Scholar]
- 93.Kaur P, Mehta N, Malav OP, Chatli MK, Panwar H. Antimicrobial, Antioxidant and Antibiofilm Potential of Peppermint (Mentha piperita) Essential Oil for Application in Meat Products. J Anim Res. 2020;10(1):33–40. doi: 10.30954/2277-940X.01.2020.4. [DOI] [Google Scholar]
- 94.Farzaei MH, Bahramsoltani R, Ghobadi A, Farzaei F, Najafi F. Pharmacological activity of Mentha longifolia and its phytoconstituents. J Tradit Chin Med. 2017;37(5):710–20. doi: 10.1016/S0254-6272(17)30327-8. [DOI] [PubMed] [Google Scholar]
- 95.Al-Ali KH, El-Beshbishy HA, El-Badry AA, Alkhalaf M. Cytotoxic activity of methanolic extract of Mentha longifolia and Ocimum basilicum against human breast cancer. PJBS. 2013;16(23):1744–50. doi: 10.3923/pjbs.2013.1744.1750. [DOI] [PubMed] [Google Scholar]
- 96.Chandirasegaran G, Elanchezhiyan C, Suhasini S, Babby A. Antihyperglycemic activity of Mentha piperita ethanol leaves extract on streptozotocin induced diabetic rats. Int J Pharm Res Sch. 2014;3:113–7. [Google Scholar]
- 97.Kwon Y-II, Vattem DA, Shetty K. Evaluation of clonal herbs of Lamiaceae species for management of diabetes and hypertension. Asia Pac J Clin Nutr. 2006;15(1):107–118. [PubMed] [Google Scholar]
- 98.Bayani M, Ahmadi-Hamedani M, Javan AJ. Study of hypoglycemic, hypocholesterolemic and antioxidant activities of Iranian Mentha spicata leaves aqueous extract in diabetic rats. IJPR. 2017;16(Suppl):75. doi: 10.22037/ijpr.2017.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Agawane SB, Gupta VS, Kulkarni MJ, Bhattacharya AK, Koratkar SS. Chemo-biological evaluation of antidiabetic activity of Mentha arvensis L. and its role in inhibition of advanced glycation end products. J Ayurveda Integr Med. 2019;10(3):166–70. doi: 10.1016/j.jaim.2017.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Behnam S, Farzaneh M, Ahmadzadeh M, Tehrani AS. Composition and antifungal activity of essential oils of Mentha piperita and Lavendula angustifolia on post-harvest phytopathogens. Commun Agric Appl Biol Sci. 2006;71(3 Pt B):1321–6. [PubMed] [Google Scholar]
- 101.Tullio V, Roana J, Scalas D, Mandras N. Evaluation of the antifungal activity of Mentha x piperita (Lamiaceae) of Pancalieri (Turin, Italy) essential oil and its synergistic interaction with azoles. Molecules. 2019;24(17):3148. doi: 10.3390/molecules24173148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ali HM, Elgat WAA, El-Hefny M, Salem MZ, Taha AS, Al Farraj DA, et al. New approach for using of Mentha longifolia L. and Citrus reticulata L. essential oils as wood-biofungicides: GC-MS, SEM, and MNDO quantum chemical studies. Materials. 2021; 14(6):1361. doi: 10.3390/ma14061361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Teymouri M, Alizadh A. Chemical composition and anti-microbial activity of the essential oil of Mentha mozaffarianii Jamzad growing wild and cultivated in Iran. Nat Prod Res. 2018;32(11):1320–3. doi: 10.1080/14786419.2017.1342082. [DOI] [PubMed] [Google Scholar]
- 104.Ait-Ouazzou A, Lorán S, Arakrak A, Laglaoui A, Rota C, Herrera A, et al. Evaluation of the chemical composition and antimicrobial activity of Mentha pulegium, Juniperus phoenicea, and Cyperus longus essential oils from Morocco. Food Res Int. 2012;45(1):313–9. doi: 10.1016/j.foodres.2011.09.004. [DOI] [Google Scholar]
- 105.Ferhat M, Erol E, Beladjila KA, Çetintaş Y, Duru ME, Öztürk M, et al. Antioxidant, anticholinesterase and antibacterial activities of Stachys guyoniana and Mentha aquatica. Pharm Biol. 2017;55(1):324–9. doi: 10.1080/13880209.2016.1238488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mimica-Dukić N, Božin B, Soković M, Mihajlović B, Matavulj M. Antimicrobial and antioxidant activities of three Mentha species essential oils. Planta Med. 2003;69(05):413–9. doi: 10.1055/s-2003-39704. [DOI] [PubMed] [Google Scholar]
- 107.Mancuso M. The Antibacterial Activity of Mentha. London: IntechOpen; 2020. [DOI] [Google Scholar]
- 108.Belemkar S, Thakre SA, Pata MK. Evaluation of anti-inflammatory and analgesic activities of methanolic extract of Adhatoda vasica Nees and Mentha piperita Linn. Invent rapid : ethnopharmacol. 2013;2:1–6. [Google Scholar]
- 109.Moreno L, Bello R, Primo-Yúfera E, Esplugues J. Pharma-cological properties of the methanol extract from Mentha suaveolens Ehrh. Phytother Res. 2002;16(S1):10–3. doi: 10.1002/ptr.744. [DOI] [PubMed] [Google Scholar]
- 110.Sun Z, Wang H, Wang J, Zhou L, Yang P. Chemical composition and anti-inflammatory, cytotoxic and antioxidant activities of essential oil from leaves of Mentha piperita grown in China. PloS one. 2014;9(12):e114767. doi: 10.1371/journal.pone.0114767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Li Y, Liu Y, Ma A, Bao Y, Wang M, Sun Z. In vitro antiviral, anti-inflammatory, and antioxidant activities of the ethanol extract of Mentha piperita L. Food Sci Biotechnol. 2017;26(6):1675–83. doi: 10.1007/s10068-017-0217-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Civitelli L, Panella S, Marcocci ME, De Petris A, Garzoli S, Pepi F, et al. In vitro inhibition of herpes simplex virus type 1 replication by Mentha suaveolens essential oil and its main component piperitenone oxide. Phytomedicine. 2014;21(6):857–65. doi: 10.1016/j.phymed.2014.01.013. [DOI] [PubMed] [Google Scholar]
- 113.Orhan İE, ÖZÇELİK B, Kartal M, Kan Y. Antimicrobial and antiviral effects of essential oils from selected Umbelliferae and Labiatae plants and individual essential oil components. Turk J Biol. 2012;36(3):239–46. doi: 10.3906/biy-0912-30. [DOI] [Google Scholar]
- 114.Farnaz M, Shahzad H, Alia S, Ghazala P, Amina W, Shazia S, et al. Phyto-chemical analysis, anti-allergic and anti-inflammatory activity of Mentha arvensis in animals. AJPP. 2012;6(9):613–9. doi: 10.5897/AJPP11.702. [DOI] [Google Scholar]
- 115.Hajighasemi F. Suppressive effect of a mint aqueous extract on IL-13 production. 2011; 38:409. [Google Scholar]
- 116.Yamamura S, Ozawa K, Ohtani K, Kasai R, Yamasaki K. Antihistaminic flavones and aliphatic glycosides from Mentha spicata. Phytochemistry. 1998;48(1):131–6. doi: 10.1016/S0031-9422(97)01112-6. [DOI] [Google Scholar]
- 117.Prasannakumar N, Jyothi N, Saroja S, Lokesha A. Insecticidal properties of Ocimum basilicum and Mentha piperita essential oils against South American Tomato moth, Phthorimaea absoluta (Meyrick) (Lepidoptera: Gelichiidae) . PESTIC BIO-CHEM PHYS. 2023;190:105329. doi: 10.1016/j.pestbp.2022.105329. [DOI] [PubMed] [Google Scholar]
- 118.Saeidi K, Mirfakhraie S. Chemical composition and insecticidal activity Mentha piperita L. essential oil against the cowpea seed beetle Callosobruchus maculatus F.(Coleoptera: Bruchidae) . JEAR. 2017;49(3) doi: 10.4081/jear.2017.6769. [DOI] [Google Scholar]
- 119.Soto ML, Falqué E, Domínguez H. Relevance of natural phenolics from grape and derivative products in the formulation of cosmetics. Cosmetics. 2015;2(3):259–76. doi: 10.3390/cosmetics2030259. [DOI] [Google Scholar]
- 120.Ali G, Neda G. Flavonoids and phenolic acids: Role and biochemical activity in plants and human. J Med Plant Res. 2011;5(31):6697–703. doi: 10.5897/JMPR11.1404. [DOI] [Google Scholar]
- 121.Kumar N, Goel N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol Rep (Amst). 2019;24:e00370. doi: 10.1016/j.btre.2019.e00370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Fatiha B, Didier H, Naima G, Khodir M, Martin K, Léocadie K, et al. Phenolic composition, in vitro antioxidant effects and tyrosinase inhibitory activity of three Algerian Mentha species: M. spicata (L.), M. pulegium (L.) and M. rotundifolia (L.) Huds (Lamiaceae) . Ind Crops Prod. 2015;74:722–30. doi: 10.1016/j.indcrop.2015.04.038. [DOI] [Google Scholar]
- 123.Wang H, Provan GJ, Helliwell K. Determination of rosmarinic acid and caffeic acid in aromatic herbs by HPLC. Food Chem. 2004;87(2):307–11. doi: 10.1016/j.foodchem.2003.12.029. [DOI] [Google Scholar]
- 124.Tang KS, Konczak I, Zhao J. Identification and quantification of phenolics in Australian native mint (Mentha australis R. Br.) . Food Chem. 2016;192:698–705. doi: 10.1016/j.foodchem.2015.07.032. [DOI] [PubMed] [Google Scholar]
- 125.Mišan A, Mimica-Dukić N, Mandić A, Sakač M, Milovanović I, Sedej I. Development of a rapid resolution HPLC method for the separation and determination of 17 phenolic compounds in crude plant extracts. Open Chem J. 2011;9(1):133–42. doi: 10.2478/s11532-010-0126-8. [DOI] [Google Scholar]
- 126.Teodor ED, Gatea F, Albu C, Rădulescu CM, Chira A, Radu GL. Polyphenols, radical scavenger activity, short-chain organic acids and heavy metals of several plants extracts from “Bucharest Delta”. Chem Pap. 2015;69(12):1582–90. doi: 10.1515/chempap-2015-0177. [DOI] [Google Scholar]
- 127.Benedec D, Vlase L, Oniga I, Mot AC, Silaghi-Dumitrescu R, Hanganu D, et al. LC-MS analysis and antioxidant activity of phenolic compounds from two indigenous species of Mentha. Note I. Farmacia. 2013;61(2):262–7. doi: 10.3390/molecules18088725. [DOI] [Google Scholar]
- 128.Swamy MK, Sinniah UR, Ghasemzadeh A. Anticancer potential of rosmarinic acid and its improved production through biotechnological interventions and functional genomics. Appl Microbiol Biotechnol. 2018;102(18):7775–93. doi: 10.1007/s00253-018-9223-y. [DOI] [PubMed] [Google Scholar]
- 129.She GM, Xu C, Liu B, Shi RB. Polyphenolic acids from mint (the aerial of Mentha haplocalyx Briq.) with DPPH radical scavenging activity. J Food Sci. 2010;75(4):C359–C62. doi: 10.1111/j.1750-3841.2010.01603.x. [DOI] [PubMed] [Google Scholar]
- 130.Gökbulut A, Şarer E. Simultaneous determination of phenolic compounds in Mentha spicata L. subsp. spicata by RP-HPLC. Turk J Pharm Sci. 2010;7(3):249–54. [Google Scholar]
- 131.Bodalska A, Kowalczyk A, Włodarczyk M, Fecka I. Analysis of polyphenolic composition of a herbal medicinal product—peppermint tincture. Molecules. 2019;25(1):69. doi: 10.3390/molecules25010069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hussain MS, Fareed S, Ansari S, Rahman MA, Ahmad IZ, Saeed M. Current approaches toward production of secondary plant metabolites. J Pharm Bioallied Sci. 2012;4(1):10. doi: 10.4103/0975-7406.92725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kippes N, Tsai H, Lieberman M, Culp D, McCormack B, Wilson RG, et al. Diploid mint (M. longifolia) can produce spearmint type oil with a high yield potential. Sci rep. 2021;11(1):23521. doi: 10.1038/s41598-021-02835-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bardaweel SK, Bakchiche B, ALSalamat HA, Rezzoug M, Gherib A, Flamini G. Chemical composition, antioxidant, antimicrobial and Antiproliferative activities of essential oil of Mentha spicata L.(Lamiaceae) from Algerian Saharan atlas. BMC Complement Altern Med. 2018;18:1–7. doi: 10.1186/s12906-018-2274-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tafrihi M, Imran M, Tufail T, Gondal TA, Caruso G, Sharma S, et al. The wonderful activities of the genus Mentha: Not only antioxidant properties. Molecules. 2021;26(4):1118. doi: 10.3390/molecules26041118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Boukhebti H, Chaker AN, Belhadj H, Sahli F, Ramdhani M, Laouer H, et al. Chemical composition and antibacterial activity of Mentha pulegium L. and Mentha spicata L. essential oils. Der Pharmacia Lettre. 2011;3(4):267–75. doi: 10.26538/tjnpr/v7i4.6. [DOI] [Google Scholar]
- 137.Cao D, Liu J, Zhao Z, Yan X, Wang W, Wei J. Chemical Compounds Emitted from Mentha spicata Repel Aromia bungii Females. Insects. 2022;13(3):244. doi: 10.3390/insects13030244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Riahi L, Elferchichi M, Ghazghazi H, Jebali J, Ziadi S, Aouadhi C, et al. Phytochemistry, antioxidant and antimicrobial activities of the essential oils of Mentha rotundifolia L. in TunisiaL. Ind Crops Proda. 2013;49:883–9. doi: 10.1016/j.indcrop.2013.06.032. [DOI] [Google Scholar]
- 139.Hassanpouraghdam MB, Mohammadzadeh A, Morshedloo MR, Asadi M, Rasouli F, Vojodi Mehrabani L, et al. Mentha aquatica L. Populations from the Hyrcanian Hotspot: Volatile Oil Profiles and Morphological Diversity. Agronomy. 2022;12(10):2277. doi: 10.3390/agronomy12102277. [DOI] [Google Scholar]
- 140.Morteza-Semnani K, Saeedi M, Akbarzadeh M. The essential oil composition of Mentha aquatica L. J Essent Oil-Bear Plants. 2006;9(3):283–6. doi: 10.1080/0972060X.2006.10643505. [DOI] [Google Scholar]
- 141.Brahmi F, Abdenour A, Bruno M, Silvia P, Alessandra P, Danilo F, et al. Chemical composition and in vitro antimicrobial, insecticidal and antioxidant activities of the essential oils of Mentha pulegium L. and Mentha rotundifolia (L.) Huds growing in Algeria. Ind Crops Prod. 2016;88:96–105. doi: 10.1016/j.indcrop.2016.03.002. [DOI] [Google Scholar]
- 142.Gonçalves R, Battistin A, Pauletti G, Rota L, Serafini L. Antioxidant properties of essential oils from Mentha species evidenced by electrochemical methods. Rev bras plantas med. 2009;11:372–82. doi: 10.1590/S1516-05722009000400004. [DOI] [Google Scholar]
- 143.Derwich E, Benziane Z, Boukir A. Antibacterial activity and chemical composition of the leaf essential oil of Mentha rotundifolia from Morocco. Elec J Env Agricult Food Chem. 2010;9(1) [Google Scholar]
- 144.Irina B, Maria-Magdalena Z, Ioan B. Chemical composition of essential oils from Mentha aquatica L. at different moments of the ontogenetic cycle. JMPR. 2013;7(9):470–3. doi: 10.5897/JMPR12.902. [DOI] [Google Scholar]
- 145.Marino S, Ahmad U, Ferreira MI, Alvino A. Evaluation of the effect of irrigation on biometric growth, physiological response, and essential oil of Mentha spicata (L.) . Water. 2019;11(11):2264. doi: 10.3390/w11112264. [DOI] [Google Scholar]
- 146.Makkar MK, Sharma S, Kaur H. Evaluation of Mentha arvensis essential oil and its major constituents for fungitoxicity. J Food Sci Technol. 2018;55(9):3840–4. doi: 10.1007/s13197-018-3291-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Pandey A, Rai M, Acharya D. Chemical composition and antimycotic activity of the essential oils of corn mint (Mentha arvensis) and lemon grass (Cymbopogon flexuosus) against human pathogenic fungi. Pharm Biol. 2003;41(6):421–5. doi: 10.1076/phbi.41.6.421.17825. [DOI] [Google Scholar]
- 148.Abdelli M, Moghrani H, Aboun A, Maachi R. Algerian Mentha pulegium L. leaves essential oil: Chemical composition, antimicrobial, insecticidal and antioxidant activities. Ind Crops Prod. 2016;94:197–205. doi: 10.1016/j.indcrop.2016.08.042. [DOI] [Google Scholar]
- 149.Lorenzo D, Paz D, Dellacassa E, Davies P, Vila R, Cañigueral S. Essential oils of Mentha pulegium and Mentha rotundifolia from Uruguay. Braz Arch Biol Technol. 2002;45:519–24. doi: 10.1590/S1516-89132002000600016. [DOI] [Google Scholar]
- 150.Başer K, Kürkçüoğlu M, Demirci B, Özek T, Tarımcılar G. Essential oils of Mentha species from Marmara region of Turkey. J Essent Oil Res. 2012;24(3):265–72. doi: 10.1080/10412905.2012.676775. [DOI] [Google Scholar]
- 151.Do Ngoc Dai TDT, Emmanuel EE, Oladimeji O, Abdulkabir IAO. Study on essential oil of Mentha aquatica L. from Vietnam+++++ Am J Essent Oil. 2015;2(4):12–6. [Google Scholar]
- 152.Zhao D, Xu YW, Yang GL, Husaini AM, Wu W. Variation of essential oil of Mentha haplocalyx Briq. and Mentha spicata L. from China. Ind Crops Prod. 2013;42:251–60. doi: 10.1016/j.indcrop.2012.06.010. [DOI] [Google Scholar]
- 153.Telci I, Demirtas I, Bayram E, Arabaci O, Kacar O. Environmental variation on aroma components of pulegone/piperitone rich spearmint (Mentha spicata L.) . Ind Crops Prod. 2010;32(3):588–92. doi: 10.1016/j.indcrop.2010.07.009. [DOI] [Google Scholar]
- 154.Gulluce M, Sahin F, Sokmen M, Ozer H, Daferera D, Sokmen A, et al. Antimicrobial and antioxidant properties of the essential oils and methanol extract from Mentha longifolia L. ssp. longifolia. Food Chem. 2007;103(4):1449–56. doi: 10.1016/j.foodchem.2006.10.061. [DOI] [Google Scholar]
- 155.Koliopoulos G, Pitarokili D, Kioulos E, Michaelakis A, Tzakou O. Chemical composition and larvicidal evaluation of Mentha, Salvia, and Melissa essential oils against the West Nile virus mosquito Culex pipiens. Parasitol Res. 2010;107(2):327–35. doi: 10.1007/s00436-010-1865-3. [DOI] [PubMed] [Google Scholar]
- 156.Brada M, Bezzina M, Marlier M, Lognay G. Methyl-jasmonate and salicylic acid as potent elicitors for secondary metabolite production in medicinal plants: a review. J Essent Oil Res. 2006;18(6):663–5. doi: 10.1080/10412905.2006.9699198. [DOI] [Google Scholar]
- 157.Singh A, Dwivedi P. Methyl-jasmonate and salicylic acid as potent elicitors for secondary metabolite production in medicinal plants: a review. J Pharmacogn Phytochem. 2018;7 (1):750–7. [Google Scholar]
- 158.Gonçalves S, Romano A. Production of plant secondary metabolites by using biotechnological tools. In: Vijayakumar R, Raja SS, editors. Secondary metabolites-sources and applications. London: IntechOpen; 2018. pp. 81–99. [DOI] [Google Scholar]
- 159.Yousefian S, Ahmadi Nik F, Lohrasebi T, Mirshahvalad S, Gharanjik S, Esfahani K. Investigation of Nitrogen and Phosphate Effect on Growth and Rosmarinic Acid Production in Transgenic Mentha aquatica Hairy Root Induction. JMPB. 2021;10(2):199. [Google Scholar]
- 160.Naik PM, Al-Khayri JM. Abiotic and biotic elicitors-role in secondary metabolites production through in vitro culture of medicinal plants. In: Shanker AK, Shanker C, editors. Abiotic and biotic stress in plants-recent advances and future perspectives Rijeka: InTech. London: IntechOpen; 2016. pp. 247– 77. [DOI] [Google Scholar]
- 161.Guerriero G, Berni R, Muñoz-Sanchez JA, Apone F, Abdel-Salam EM, Qahtan AA, et al. Production of plant secondary metabolites: Examples, tips and suggestions for biotechnologists. Genes. 2018;9(6):309. doi: 10.3390/genes9060309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Gunjegaonkar Shivshankar M, Joshi Amol A, Wankhede Sagar B, Siraskar Balasaheb D, Merekar Abhijit N, Shinde Sachin D. Potential Defensive Involvement of Methyl Jasmonate in Oxidative Stress and Its Related Molecular Mechanisms. London: IntechOpen; 2022. p. 67. [DOI] [Google Scholar]
- 163.Gharib F, da Silva JT. Composition, total phenolic content and antioxidant activity of the essential oil of four Lamiaceae herbs. Medicinal and aromatic plant science and biotechnology. 2013;7(1):19–27. [Google Scholar]
- 164.Guedon DJ, Pasquier BP. Analysis and distribution of flavonoid glycosides and rosmarinic acid in 40 Mentha x piperita clones. J Agric Food Chem. 1994;42(3):679–84. doi: 10.1021/jf00039a015. [DOI] [Google Scholar]
- 165.Fecka I, Turek S. Determination of water-soluble polyphenolic compounds in commercial herbal teas from Lamiaceae: peppermint, melissa, and sage. J Agric Food Chem. 2007; 55(26):10908–17. doi: 10.1021/jf072284d. [DOI] [PubMed] [Google Scholar]
- 166.Fecka I, Raj D, Krauze-Baranowska M. Quantitative determination of four water-soluble compounds in herbal drugs from Lamiaceae using different chromatographic techniques. Chroma. 2007; 66:87–93. doi: 10.1365/s10337-007-0233-7. [DOI] [Google Scholar]
- 167.Krzyzanowska J, Janda B, Pecio L, Stochmal A, Oleszek W, Czubacka A, et al. Determination of polyphenols in Mentha longifolia and M. piperita field-grown and in vitro plant samples using UPLC-TQ-MS. J AOAC Int. 2011;94(1):43–50. doi: 10.1093/jaoac/94.1.43. [DOI] [PubMed] [Google Scholar]
- 168.Hadjmohammadi M, Karimiyan H, Sharifi V. Hollow fibre-based liquid phase microextraction combined with high-performance liquid chromatography for the analysis of flavonoids in Echinophora platyloba DC. and Mentha piperita. Food Chem. 2013;141(2):731–5. doi: 10.1016/j.foodchem.2013.02.083. [DOI] [PubMed] [Google Scholar]
- 169.Zheng J, Chen G-T, Gao H-Y, Wu B, Wu L-J. Two new lignans from Mentha spicata L. J Asian Nat Prod Res. 2007;9(5):431–5. doi: 10.1080/10286020500384641. [DOI] [PubMed] [Google Scholar]
- 170.Proestos C, Chorianopoulos N, Nychas G-J, Komaitis M. Comparison of pressurised fluid and ultrasonic extraction methods for analysis of plant antioxidants and their antioxidant capacity. J Agric Food Chem. 2005;53(4):1190–5. doi: 10.1021/jf040083t. [DOI] [PubMed] [Google Scholar]
- 171.Dobiáš P, Pavlíková P, Adam M, Eisner A, Beňová B, Ventura K. Comparison of pressurised fluid and ultrasonic extraction methods for analysis of plant antioxidants and their antioxidant capacity. Open Chemistry. 2010;8:87–95. doi: 10.2478/s11532-009-0125-9. [DOI] [Google Scholar]
- 172.Khani A, Asghari J. Insecticide activity of essential oils of Mentha longifolia, Pulicaria gnaphalodes and Achillea wilhelmsii against two stored product pests, the flour beetle, Tribolium castaneum, and the cowpea weevil, Callosobruchus maculatus. J Insect Sci. 2012; 12(1) doi: 10.1673/031.012.7301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Ertaş A, Gören AC, Haşimi N, Tolan V, Kolak U. Evaluation of Antioxidant, Cholinesterase Inhibitory and Antimicrobial Properties of Mentha longifolia subsp. noeana and Its Secondary Metabolites. Rec Nat Prod. 2015;9(1) doi: 10.1055/s-0032-1320810. [DOI] [Google Scholar]
- 174.Aggarwal BB, Kunnumakkara AB. Molecular targets and therapeutic uses of spices: modern uses for ancient medicine. World Scientific. 2009 May 18 doi: 10.1142/7150. [DOI] [Google Scholar]
- 175.Vivek S, Nisha S, Harbans S, Devendra SK, Vijaylata P, Bikram S, et al. Comparative account on GC-MS analysis of Mentha arvensis L.“Cornmint” from three different locations of north India. Int J Drug Dev Res. 2009;1:1–9. doi: 10.1142/7150. [DOI] [Google Scholar]
- 176.Zaidi F, Voirin B, Jay M, Viricel MR. Free flavonoid aglycones from leaves of Mentha pulegium and Mentha suaveolens (Labiatae) . Phytochemistry. 1998;48(6):991–4. doi: 10.1016/S0031-9422(97)01042-X. [DOI] [Google Scholar]
- 177.Andro A-R, Boz I, Zamfirache M-M, Burzo I. Chemical composition of essential oils from Mentha aquatica L. at different moments of the ontogenetic cycle. J Med Plant Res. 2013;7(9):470–3. doi: 10.5897/JMPR12.902. [DOI] [Google Scholar]
- 178.Mimica-Dukic N, Bozin B. species (Lamiaceae) as promising sources of bioactive secondary metabolites. Curr Pharm Des. 2008;14(29):3141–50. doi: 10.2174/138161208786404245. [DOI] [PubMed] [Google Scholar]
- 179.Veronese P, Li X, Niu X, Weller SC, Bressan RA, Hasegawa PM. Bioengineering mint crop improvement. PCTOC. 2001;64(2):133–44. doi: 10.1023/A:1010649207445. [DOI] [Google Scholar]
- 180.Lange BM, Mahmoud SS, Wildung MR, Turner GW, Davis EM, Lange I, et al. Improving peppermint essential oil yield and composition by metabolic engineering. PNAS. 2011;108(41):16944–9. doi: 10.1073/pnas.1111558108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Wang Q, Reddy VA, Panicker D, Mao HZ, Kumar N, Rajan C, et al. Metabolic engineering of terpene biosynthesis in plants using a trichome-specific transcription factor Ms YABBY 5 from spearmint (Mentha spicata) . Plant Biotechnol J. 2016;14(7):1619–32. doi: 10.1111/pbi.12525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Koley S, Grafahrend-Belau E, Raorane ML, Junker BH. The mevalonate pathway contributes to monoterpene production in peppermint. bioRxiv. 2020 doi: 10.1101/2020.05.29.124016. [DOI] [Google Scholar]
- 183.Jin J, Panicker D, Wang Q, Kim MJ, Liu J, Yin J-L, et al. Next generation sequencing unravels the biosynthetic ability of spearmint (Mentha spicata) peltate glandular trichomes through comparative transcriptomics. BMC Plant Biol. 2014;14(1):1–15. doi: 10.1186/s12870-014-0292-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Lange BM, Croteau R. Genetic engineering of essential oil production in mint. Curr Opin Plant Biol. 1999;2(2):139–44. doi: 10.1016/s1369-5266(99)80028-4. [DOI] [PubMed] [Google Scholar]
- 185.Diemer F, Jullien F, Faure O, Moja S, Colson M, Matthys-Rochon E, et al. High efficiency transformation of peppermint (Mentha× piperita L.) with Agrobacterium tumefaciens. Plant Sci. 1998;136(1):101–8. doi: 10.1016/S0168-9452(98)00107-1. [DOI] [Google Scholar]
- 186.Spencer A, Hamill JD, Rhodes MJ. Transformation in Mentha species (mint). InPlant Protoplasts and Genetic Engineering III 1993 (pp. 278-293). Berlin, Heidelberg: Springer Berlin Heidelberg; [Google Scholar]
- 187.Alonso WR, Crock J, Croteau R. Production and characterization of polyclonal antibodies in rabbits to 4S-limonene synthase from spearmint (Mentha spicata) . Arch Biochem Biophys. 1993;301(1):58–63. doi: 10.1006/abbi.1993.1114. [DOI] [PubMed] [Google Scholar]
- 188.Krasnyanski S, May R, Loskutov A, Ball T, Sink K. Transformation of the limonene synthase gene into peppermint (Mentha piperita L.) and preliminary studies on the essential oil profiles of single transgenic plants. Theor Appl Genet. 1999;99(3):676–82. doi: 10.1007/s001220051284. [DOI] [PubMed] [Google Scholar]
- 189.Mahmoud SS, Croteau RB. Metabolic engineering of essential oil yield and composition in mint by altering expression of deoxyxylulose phosphate reductoisomerase and menthofuran synthase. PNAS. 2001;98(15):8915–20. doi: 10.1073/pnas.141237298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Mahmoud SS, Croteau RB. Menthofuran regulates essential oil biosynthesis in peppermint by controlling a downstream monoterpene reductase. PNAS. 2003;100(24):14481–6. doi: 10.1073/pnas.2436325100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Mahmoud SS, Williams M, Croteau R. Cosuppression of limonene-3-hydroxylase in peppermint promotes accumula-tion of limonene in the essential oil. Phytochemistry. 2004; 65(5):547–54. doi: 10.1016/j.phytochem.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 192.Croteau RB, Davis EM, Ringer KL, Wildung MR. (−)-Menthol biosynthesis and molecular genetics. Naturwissenschaften. 2005;92(12):562–77. doi: 10.1007/s00114-005-0055-0. [DOI] [PubMed] [Google Scholar]
- 193.Mahadevappa N, Suvarna DKHPV, Moses V, Chandrashekar S, Cowda S. Study on advanced application of mint oil. J Adv Scient Res. 2014;5(4):1–3.3. [Google Scholar]
- 194.Amarr V, Nori P, Zhu H. Spearmint R2R3-MYB transcription factor MsMYB negatively regulates monoterpene production and suppresses the expression of geranyl diphosphate synthase large subunit (MsGPPS. LSU) . Plant Biotechnol J. 2017; 15(9):1105–1119. doi: 10.1111/pbi.12701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Henry LK, Thomas ST, Widhalm JR, Lynch JH, Davis TC, Kessler SA, et al. Contribution of isopentenyl phosphate to plant terpenoid metabolism. Nature plants. 2018;4(9):721–9. doi: 10.1038/s41477-018-0220-z. [DOI] [PubMed] [Google Scholar]
- 196.Georgiev V, Pavlov A. Salvia biotechnology. Switzerland: Springer; 2017. [DOI] [Google Scholar]
- 197.Diemer F, Caissard J-C, Moja S, Chalchat J-C, Jullien F. Altered monoterpene composition in transgenic mint following the introduction of 4S-limonene synthase. Plant Physiol Biochem. 2001;39(7-8):603–14. doi: 10.1016/S0981-9428(01)01273-6. [DOI] [Google Scholar]
- 198.Toogood HS, Cheallaigh AN, Tait S, Mansell DJ, Jervis A, Lygidakis A, et al. Enzymatic menthol production: one-pot approach using engineered Escherichia coli. ACS Synthetic Biology. 2015;4(10):1112–23. doi: 10.1021/acssynbio.5b00092. [DOI] [PubMed] [Google Scholar]
- 199.Khojasteh SC, Oishi S, Nelson SD. Metabolism and toxicity of menthofuran in rat liver slices and in rats. Chem Res Toxicol. 2010;23(11):1824–32. doi: 10.1021/tx100268g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Malekmohammad K, Rafieian-Kopaei M, Sardari S, Sewell RD. Toxicological effects of Mentha x piperita (peppermint): a review. Toxin Rev. 2021;40(4):445–59. doi: 10.1080/15569543.2019.1647545. [DOI] [Google Scholar]
- 201.Shekarchi M, Hajimehdipoor H, Saeidnia S, Gohari AR, Hamedani MP. Comparative study of rosmarinic acid content in some plants of Labiatae family. Pharmacogn Mag. 2012;8(29):37 doi 104103/0973–1296. doi: 10.4103/0973-1296.93316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Xiao Y, Zhang L, Gao S, Saechao S, Di P, Chen J, et al. The c4h, tat, hppr and hppd genes prompted engineering of rosmarinic acid biosynthetic pathway in Salvia miltiorrhiza hairy root cultures. PLoS One. 2011;6(12):e29713. doi: 10.1371/journal.pone.0029713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Xu F, Ning Y, Zhang W, Liao Y, Li L, Cheng H, et al. An R2R3-MYB transcription factor as a negative regulator of the flavonoid biosynthesis pathway in Ginkgo biloba. Funct Integr Genomics. 2014;14(1):177–89. doi: 10.1007/s10142-013-0352-1. [DOI] [PubMed] [Google Scholar]
- 204.Zhang Y, Yan Y-P, Wu Y-C, Hua W-P, Chen C, Ge Q, et al. Pathway engineering for phenolic acid accumulations in Salvia miltiorrhiza by combinational genetic manipulation. Metab Eng. 2014;21:71–80. doi: 10.1016/j.ymben.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 205.Li C, Lu S. Genome-wide characterization and comparative analysis of R2R3-MYB transcription factors shows the complexity of MYB-associated regulatory networks in Salvia miltiorrhiza. BMC Genet. 2014;15(1):1–12. doi: 10.1186/1471-2164-15-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Park SU, Uddin R, Xu H, Kim YK, Lee SY. Biotechnological applications for rosmarinic acid production in plant. Afr J Biotechnol. 2008;7(25) doi: 10.4314/ajb.v7i25.59707. [DOI] [Google Scholar]
- 207.Vining KJ, Johnson SR, Ahkami A, Lange I, Parrish AN, Trapp SC, et al. Draft genome sequence of Mentha longifolia and development of resources for mint cultivar improvement. Mol Plant. 2017;10(2):323–39. doi: 10.1016/j.molp.2016.10.018. [DOI] [PubMed] [Google Scholar]
- 208.Nishida K, Kondo A. CRISPR-derived genome editing techno-logies for metabolic engineering. Metab Eng. 2021;63:141–7. doi: 10.1016/j.ymben.2020.12.002. [DOI] [PubMed] [Google Scholar]