Abstract
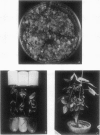
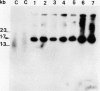
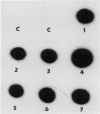
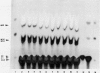
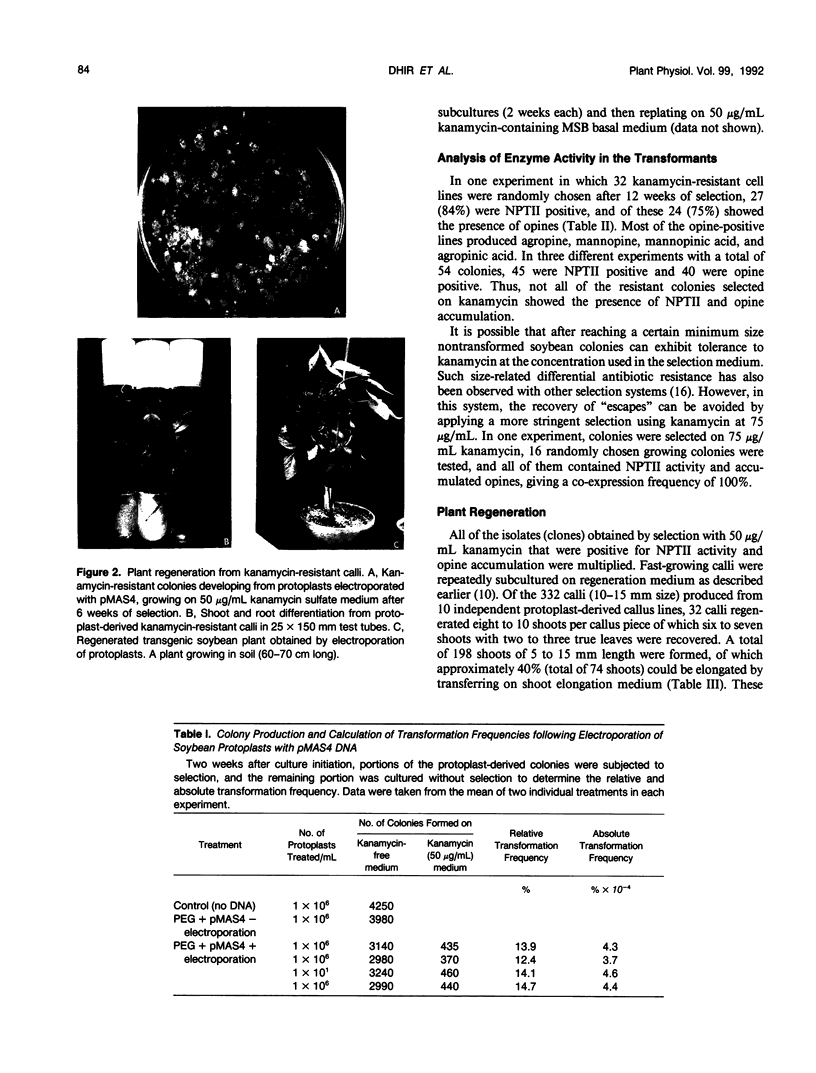
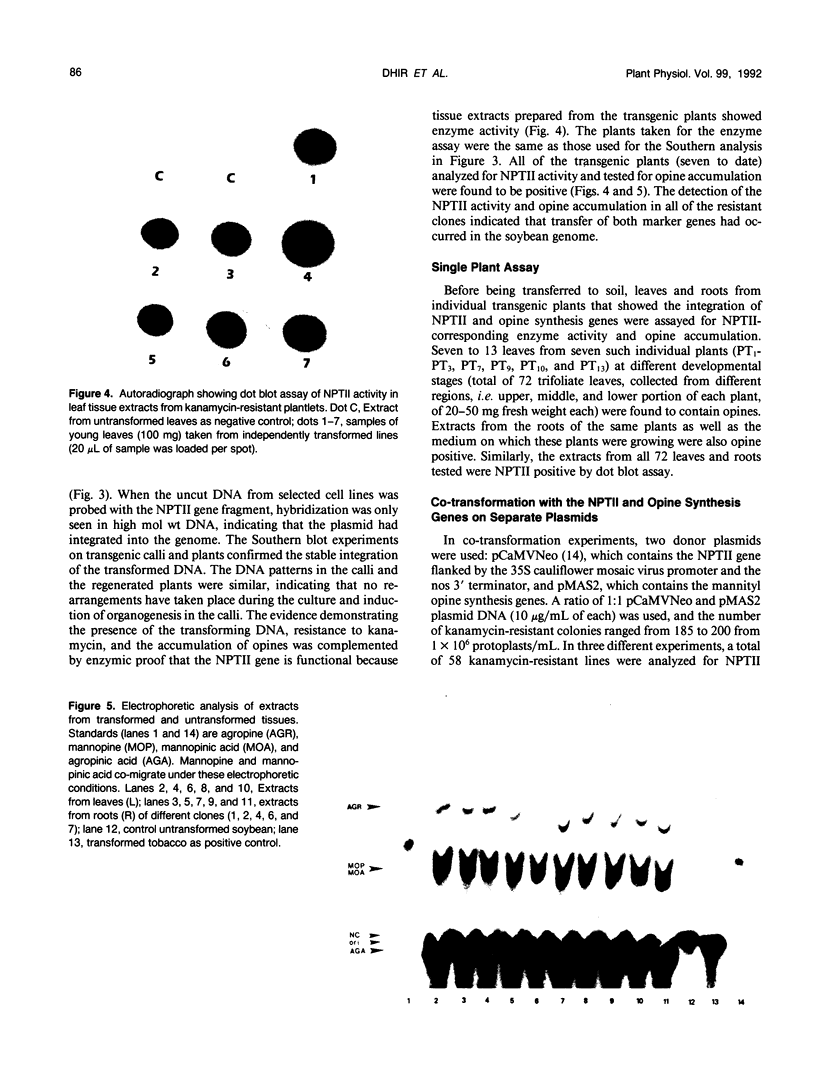
Transgenic soybean (Glycine max [L.] Merr.) plants were regenerated from calli derived from protoplasts electroporated with plasmid DNA-carrying genes for a selectable marker, neomycin phosphotransferase (NPTII), under the control of the cauliflower mosaic virus 35-Svedberg unit promoter, linked with a nonselectable mannityl opine synthesis marker. Following electroporation and culture, the protoplast-derived colonies were subjected to kanamycin selection (50 micrograms per milliliter) beginning on day 15 for 6 weeks. Approximately, 370 to 460 resistant colonies were recovered from 1 × 106 electroporated protoplasts, giving an absolute transformation frequency of 3.7 to 4.6 × 10−4. More than 80% of the kanamycin-resistant colonies showed NPTII activity, and about 90% of these also synthesized opines. This indicates that the linked marker genes were co-introduced and co-expressed at a very high frequency. Plants were regenerated from the transformed cell lines. Southern blot analysis of the transformed callus and leaf DNA demonstrated the integration of both genes. Single-plant assays performed with different plant parts showed that both shoot and root tissues express NPTII activity and accumulate opines. Experiments with NPTII and mannityl opine synthesis marker genes on separate plasmids resulted in a co-expression rate of 66%. These results indicate that electroporation can be used to introduce both linked and unlinked genes into the soybean to produce transformed plants.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chee P. P., Fober K. A., Slightom J. L. Transformation of Soybean (Glycine max) by Infecting Germinating Seeds with Agrobacterium tumefaciens. Plant Physiol. 1989 Nov;91(3):1212–1218. doi: 10.1104/pp.91.3.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christou P., Murphy J. E., Swain W. F. Stable transformation of soybean by electroporation and root formation from transformed callus. Proc Natl Acad Sci U S A. 1987 Jun;84(12):3962–3966. doi: 10.1073/pnas.84.12.3962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damm B., Schmidt R., Willmitzer L. Efficient transformation of Arabidopsis thaliana using direct gene transfer to protoplasts. Mol Gen Genet. 1989 May;217(1):6–12. doi: 10.1007/BF00330935. [DOI] [PubMed] [Google Scholar]
- Fromm M. E., Taylor L. P., Walbot V. Stable transformation of maize after gene transfer by electroporation. 1986 Feb 27-Mar 5Nature. 319(6056):791–793. doi: 10.1038/319791a0. [DOI] [PubMed] [Google Scholar]
- Gallie D. R., Novak S., Kado C. I. Novel high- and low-copy stable cosmids for use in Agrobacterium and Rhizobium. Plasmid. 1985 Sep;14(2):171–175. doi: 10.1016/0147-619x(85)90078-2. [DOI] [PubMed] [Google Scholar]
- Hauptmann R. M., Vasil V., Ozias-Akins P., Tabaeizadeh Z., Rogers S. G., Fraley R. T., Horsch R. B., Vasil I. K. Evaluation of selectable markers for obtaining stable transformants in the gramineae. Plant Physiol. 1988 Feb;86(2):602–606. doi: 10.1104/pp.86.2.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W., Odell J. T., Schreiner R. M. Soybean protoplast culture and direct gene uptake and expression by cultured soybean protoplasts. Plant Physiol. 1987 Jul;84(3):856–861. doi: 10.1104/pp.84.3.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyznik L. A., Ryan R. D., Ritchie S. W., Hodges T. K. Stable co-transformation of maize protoplasts with gusA and neo genes. Plant Mol Biol. 1989 Aug;13(2):151–161. doi: 10.1007/BF00016134. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Widholm J. M. The use of fluorescein diacetate and phenosafranine for determining viability of cultured plant cells. Stain Technol. 1972 Jul;47(4):189–194. doi: 10.3109/10520297209116483. [DOI] [PubMed] [Google Scholar]