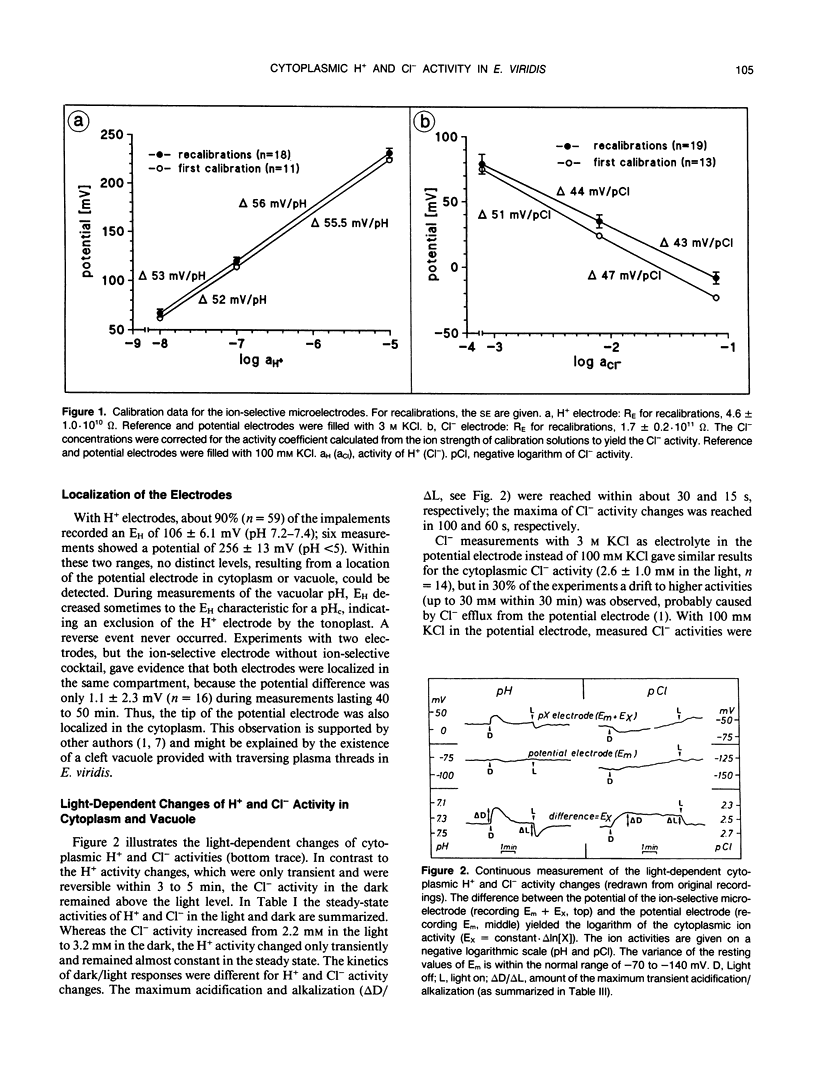
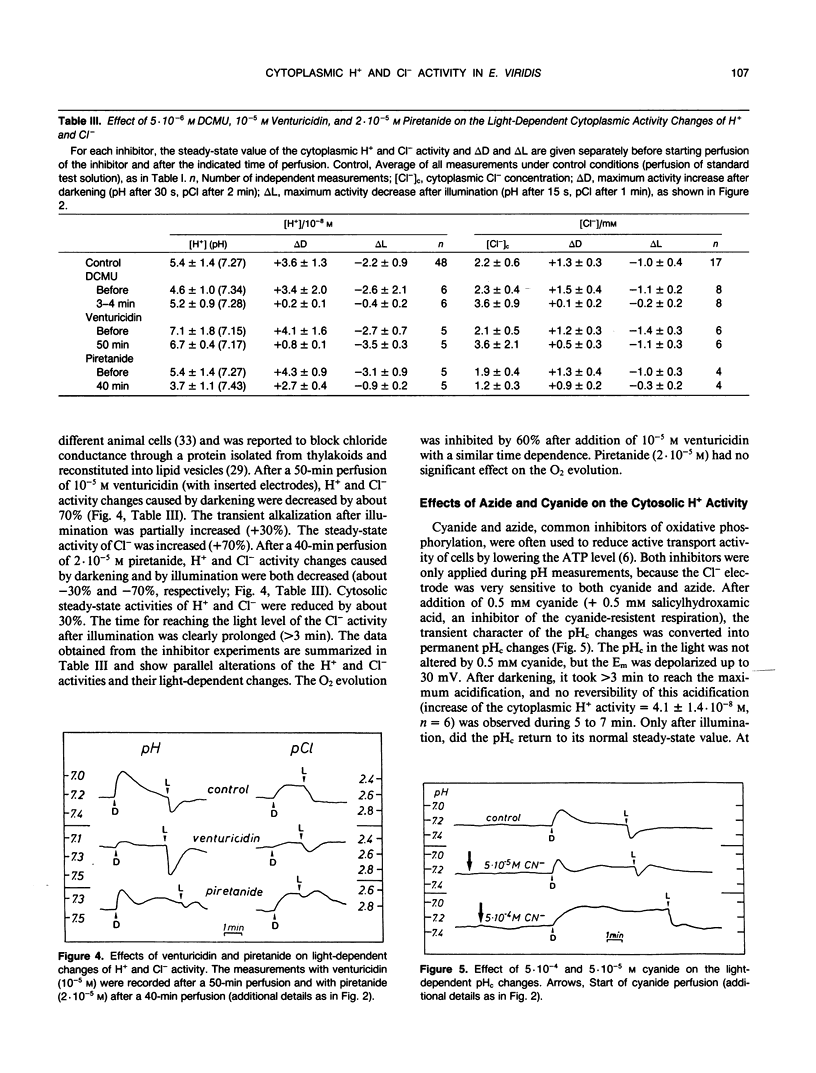
Abstract
Ion-sensitive microelectrodes were used to measure Cl− and H+ activities in the cytoplasm of the unicellular green alga Eremosphaera viridis de Bary. In the light, cytoplasmic Cl− activity was 2.2 millimolar at most and cytoplasmic H+ activity was about 5.4·10−8 molar (pH 7.3). Darkening resulted in a permanent increase of the Cl− activity to 3.2 millimolar and in a transient acidification, which was compensated within 3 to 5 minutes. Switching light on again decreased the Cl− activity to the light level (2.2 millimolar). Simultaneously, a transient alkalization of the cytoplasm was observed. The transient character of the light-dependent pH changes was probably caused by pH-stat mechanisms, whereas the light-dependent Cl− activity changes were compensated to a much smaller degree. Studies with different inhibitors (3-(3,4-dichlorophenyl)-1, 1-dimethylurea, piretanide, venturicidin) indicated a direct relation between the light-driven H+ flow across the thylakoid membrane and the observed light-dependent Cl− and H+ activity changes in the cytoplasm. It is suggested that light-driven H+ flux across the thylakoid membrane was in part electrically compensated by a parallel Cl− flux. The resulting Cl− and H+ activity changes in the stroma were compensated by Cl− and H+ fluxes across the chloroplast envelope giving rise to the observed Cl− and H+ activity changes in the cytoplasm.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chao P., Ammann D., Oesch U., Simon W., Lang F. Extra- and intracellular hydrogen ion-selective microelectrode based on neutral carriers with extended pH response range in acid media. Pflugers Arch. 1988 Feb;411(2):216–219. doi: 10.1007/BF00582318. [DOI] [PubMed] [Google Scholar]
- Hind G., Nakatani H. Y., Izawa S. Light-dependent redistribution of ions in suspensions of chloroplast thylakoid membranes. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1484–1488. doi: 10.1073/pnas.71.4.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo Y., Bührer T., Seiler K., Frömter E., Simon W. A new double-barrelled, ionophore-based microelectrode for chloride ions. Pflugers Arch. 1989 Sep;414(6):663–668. doi: 10.1007/BF00582133. [DOI] [PubMed] [Google Scholar]
- Linnett P. E., Beechey R. B. Inhibitors of the ATP synthethase system. Methods Enzymol. 1979;55:472–518. doi: 10.1016/0076-6879(79)55061-7. [DOI] [PubMed] [Google Scholar]
- Schröppel-Meier G., Kaiser W. M. Ion Homeostasis in Chloroplasts under Salinity and Mineral Deficiency : I. Solute Concentrations in Leaves and Chloroplasts from Spinach Plants under NaCl or NaNO(3) Salinity. Plant Physiol. 1988 Aug;87(4):822–827. doi: 10.1104/pp.87.4.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vambutas V., Beattie D. S., Bittman R. Isolation of protein(s) containing chloride ion transport activity from thylakoid membranes. Arch Biochem Biophys. 1984 Aug 1;232(2):538–548. doi: 10.1016/0003-9861(84)90571-x. [DOI] [PubMed] [Google Scholar]
- Zeuthen T., Ramos M., Ellory J. C. Inhibition of active chloride transport by piretanide. Nature. 1978 Jun 22;273(5664):678–680. doi: 10.1038/273678a0. [DOI] [PubMed] [Google Scholar]


