Abstract
We carried out a retrospective study of acute gastroenteritis (AGE) outbreaks reported between 1 January 2015 and 31 December 2021 in Catalonia (Spain) to compare the incidence from 2015 to 2019 with that observed from 2020 to 2021. We observed a higher incidence rate of outbreaks during the prepandemic period (16.89 outbreaks/1,000,000 person-years) than during the pandemic period (6.96 outbreaks/1,000,000 person-years) (rate ratio (RR) 0.41; 95% confidence interval (CI) 0.34 to 0.51). According to the aetiology of the outbreak, those of viral aetiology decreased from 7.82 to 3.38 outbreaks/1,000,000 person-years (RR 2.31; 95% CI 1.72 to 3.12), and those of bacterial aetiology decreased from 5.01 to 2.78 outbreaks/1,000,000 person-years (RR 1.80; 95% CI 1.29 to 2.52). There was a great reduction in AGE outbreaks in Catalonia. This reduction may have been due to the effect of the nonpharmaceutical measures applied to reduce the transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), but the collapse of the healthcare system and epidemiological surveillance services may also have had a strong influence.
Keywords: acute gastroenteritis, bacterial aetiology, COVID-19 pandemic, epidemiology, outbreaks, viral aetiology
Introduction
On 11 March 2020, the World Health Organization (WHO) declared coronavirus disease 2019 (COVID-19), the disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a pandemic. At the beginning of the pandemic, there was great uncertainty about the transmission mechanisms of the virus. Person-to-person contact, respiratory droplet transmission, and fomite-mediated transmission were identified as the most common routes of the spread of SARS-CoV-2. It was considered that the disease could be transmitted through aerosols generated during the manipulation of patients’ airways [1], although this mode of transmission was considered of little relevance beyond clinical settings [2]. Based on these data, the main WHO recommendation was the implementation of droplet and contact transmission prevention measures, including hand hygiene [2]. Subsequently, outbreaks of COVID-19 were described in restaurants [3] and gyms [4], which led various authors to state the possibility of aerosol transmission [5, 6].
It was expected that measures to prevent COVID-19 transmission would also have an impact on other diseases that share this route of transmission, such as acute gastroenteritis (AGE). Measures to control COVID-19 had already shown a very early impact on the flu season in the Southern Hemisphere (from February to September 2020), with a substantial reduction in the incidence of influenza virus being observed in countries such as Australia, Chile, and South Africa [7].
Huh et al., in the Republic of Korea, studied the consequences of nonpharmaceutical interventions against COVID-19 on the transmission of respiratory infections, comparing the period from January 2016 to January 2020 to the period from February 2020 (when the government decreed the implementation of nonpharmaceutical measures) to July 2020 and observed a reduction of 36.4% in the expected incidence for varicella and 63.4% for mumps during the period in which these measures had been applied compared to the preceding period [8].
The decrease in the reporting of communicable diseases was not only observed for those that shared transmission mechanisms with SARS-CoV-2. In Taiwan (China), between 2019 and 2020, Lai et al. [9] described a 54.8% reduction in the notification of vector-borne diseases, attributing this to less outdoor activity during the pandemic. In contrast, sexually transmitted diseases, especially gonorrhoea, showed an increase of 7.2% during the same period, indicating that the lockdown could have been associated with greater sexual activity or risky sexual practices. In Catalonia, no increase was observed in sexually transmitted diseases; instead, a 19% decrease was observed in 2020 and a return to prepandemic levels in 2021 [10, 11].
Globally, in 2019, diarrhoea was the eighth leading cause of death at all ages [12]. In 2017, there were approximately 1.7 billion cases of AGE among children [13]. In 2018, one in nine deaths among children was due to diarrhoea [14], and in 2019, AGE caused 370,000 deaths among children under 5 years of age, making it the second leading cause of death in this age group [15]. A viral aetiology is the main cause of AGE, and it is estimated that norovirus is responsible for 90% of viral gastroenteritis outbreaks [16].
The main mode of transmission of viral AGE is person-to-person, although transmission by common vehicles (food or water) is also feasible [17]. Petrignani et al. [18], in a meta-analysis carried out in 2014 that included 40 outbreaks and 18 surveillance studies of AGE due to norovirus, observed that the degree of intense contact between workers and nursing home dwellers was associated with the risk of becoming ill and that attack rates were higher among residents with a higher degree of dependence.
All this justifies investigating whether COVID-19 pandemic has influenced the incidence of AGE outbreaks and our objective in this study is to compare the incidence rate of AGE outbreaks in Catalonia from 2015 to 2019 (prepandemic period) with that observed between 2020 and 2021 (pandemic period).
Materials and methods
A retrospective study of AGE outbreaks reported between 1 January 2015 and 31 December 2021 was conducted in Catalonia, an area in northeastern Spain with a population of 7,543,825 inhabitants as of January 2018 [19].
Outbreaks of any aetiology must be reported to the Public Health Agency of Catalonia, which carries out an epidemiological study to determine the causes and establish control measures. In the city of Barcelona, these activities are carried out by the Barcelona Public Health Agency with the same procedure.
Definitions
AGE was defined as a sudden onset of diarrhoea that may also present nausea, vomiting, abdominal pain, or fever. An AGE outbreak was defined as the involvement of two or more epidemiologically related people, either by person-to-person contact or by a common exposure.
Closed and semiclosed institutions are those in which people share the space with other users of the same centre for a considerable part of their time and in which mobility to leave and/or enter the centre has certain restrictions (for example, nursing homes, health institutions, or schools).
Two periods were considered: a first period from 2015 to 2019 (prepandemic period) and a second period from 2020 to 2021 (pandemic period).
Data collection and management
Data were collected by technicians from the Epidemiological Surveillance Services of the Catalan Public Health Agency and the Barcelona Public Health Agency.
For each outbreak, the number and main characteristics of all known exposed people were recorded, and those who had clinical manifestations or microbiological confirmation were classified as cases. The following variables were included: mode of transmission, setting, number of exposed people, number of affected individuals, and aetiology.
Data collection and cleaning were performed using the Access 12.0 database manager of the MS Office 2013 software package (Microsoft, USA), and a statistical analysis was performed using the PASW Statistics 18.0.2 statistical package (IBM Corporation, USA).
Aetiological confirmation
The aetiological confirmation of outbreaks was carried out by collecting stool samples and their subsequent microbiological analysis at the Vall d’Hebron University Hospital Laboratory and Barcelona Public Health Agency Laboratory.
The diagnostic tests performed to confirm the aetiology of the outbreak were determined by the clinical presentation. In outbreaks for which a viral aetiology was suspected, a real-time RT–PCR was performed. If a bacterial aetiology was suspected, culture and/or determination of preformed toxins were performed. To determine the presence of parasites, a direct smear examination of stool samples was performed.
Statistical analysis
The outbreak rate per 1,000,000 person-years for each period was calculated as the quotient of the number of AGE outbreaks divided by the population in the middle of the period studied multiplied by the number of years in the stated period.
For lockdown weeks, this rate was calculated as the ratio of AGE outbreaks during the weeks from 14 March 2020, to 24 June 2020, or the same weeks in prepandemic years and the corresponding number of person-years.
A case was any person who met the criteria of having symptoms compatible with gastroenteritis and who had an epidemiological link with the outbreak. The attack rate was defined as the total number of new cases of AGE divided by the total exposed population. Transmission by common vehicles was considered in outbreaks in which the transmission mechanism was mediated by food or water.
The rate ratio (RR) and its 95% confidence interval (CI) were calculated to compare incidence rates and attack rates in the pandemic period to those in the prepandemic period.
To calculate the statistical significance between the proportions of outbreaks with common vehicle transmission in the two periods (prepandemic and pandemic), a two-tailed chi-squared test with Yates’ continuity correction was performed.
The mean number of people exposed in outbreaks with a viral aetiology and in those with a bacterial aetiology that occurred in the prepandemic period was compared with the mean number of people exposed in the pandemic period by Student’s t-test for the difference between means and its 95% CI.
Institutional review board statement
This study was conducted according to the guidelines of the Declaration of Helsinki, regulations of the Public Health Agency of Catalonia, and ethical protocols established and was approved by the University of Barcelona Bioethics Commission (Institutional Review Board IRB00003099) on 12 April 2016.
Informed consent statement
The authors declare that the Bioethics Committee of the University of Barcelona waived the requirement for informed consent.
All data used in the analysis were collected during routine public health surveillance activities as part of the legislated mandate of the Health Department of Catalonia, which is officially authorized to receive, clean, and temporarily store personal data in the case of infectious diseases. All data were fully anonymized. All study activities formed part of the public health surveillance tasks. The law regulates these activities, and informed consent was not deemed necessary.
Results
Incidence of outbreaks during the study period
During the entire study period, 742 AGE outbreaks were reported: 105 outbreaks in the pandemic period (6.96 outbreaks/1,000,000 person-years) and 637 outbreaks in the prepandemic period (16.88 outbreaks/1,000,000 person-years). RR for the pandemic period versus the prepandemic period was 0.41 (95% CI 0.34 to 0.51). The incidence rate was 3.47 outbreaks/1,000,000 person-years in 2020 and 10.47 outbreaks/1,000,000 person-years in 2021 (Table 1).
Table 1.
Reported outbreaks according to aetiology and year of notification
| Year | Viral aetiology | Bacterial aetiology | Protozoal aetiology | Food poisoning | Unknown | All outbreaks | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of outbreaks | Ratea | Number of outbreaks | Ratea | Number of outbreaks | Ratea | Number of outbreaks | Ratea | Number of outbreaks | Ratea | Number of outbreaks | Ratea | |
| 2015 | 70 | 9.28 | 33 | 4.37 | 0 | 0.00 | 5 | 0.66 | 34 | 4.51 | 142 | 18.82 |
| 2016 | 65 | 8.62 | 39 | 5.17 | 0 | 0.00 | 11 | 1.46 | 26 | 3.45 | 141 | 18.69 |
| 2017 | 57 | 7.56 | 37 | 4.90 | 1 | 0.13 | 15 | 1.99 | 19 | 2.52 | 129 | 17.10 |
| 2018 | 58 | 7.69 | 43 | 5.70 | 2 | 0.27 | 7 | 0.93 | 17 | 2.25 | 127 | 16.83 |
| 2019 | 45 | 5.97 | 37 | 4.90 | 0 | 0.00 | 7 | 0.93 | 9 | 1.19 | 98 | 12.99 |
| 2020 | 8 | 1.06 | 11 | 1.46 | 0 | 0.00 | 3 | 0.40 | 4 | 0.53 | 26 | 3.45 |
| 2021 | 43 | 5.70 | 31 | 4.11 | 0 | 0.00 | 1 | 0.13 | 4 | 0.53 | 79 | 10.47 |
| Total | 346 | 6.55 | 231 | 4.37 | 3 | 0.06 | 49 | 0.93 | 113 | 2.14 | 742 | 14.05 |
| 2020–2021 | 51 | 3.38 | 42 | 2.78 | 0 | 0.00 | 4 | 0.27 | 8 | 0.53 | 105 | 6.96 |
| 2015–2019 (ref) | 295 | 7.82 | 189 | 5.01 | 3 | 0.08 | 45 | 1.19 | 105 | 2.78 | 637 | 16.89 |
| RR (95%CI)b | 0.43 (0.32–0.58) | 0.56 (0.40–0.78) | NC | 0.22 (0.08–0.62) | 0.19 (0.09–0.39) | 0.41 (0.34–0.51) | ||||||
Abbreviation: NC, not calculable.
Number of outbreaks per million person-years.
Rate ratio and 95% confidence interval taking the rate of the period 2015–2019 as reference values.
Lockdown weeks (from 14 March 2020, to 24 June 2020) were compared with the same weeks of the prepandemic period, and the incidence rates were 1.42 outbreaks/1,000,000 person-years and 19.73 outbreaks/1,000,000 person-years, respectively (RR 0.07; 95% CI 0.02 to 0.23) (Table 2).
Table 2.
AGE outbreaks according to the mode of transmission in the prepandemic and pandemic periods and during lockdown weeks
| Common vehicle transmission | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Period of outbreak occurrence | Foodborne | Waterborne | All | Person-to-person transmission | All outbreaks | |||||
| Outbreaks | Ratea | Outbreaks | Ratea | Outbreaks | Ratea | Outbreaks | Ratea | Outbreaks | Ratea | |
| 2015 | 65 | 8.62 | 3 | 0.40 | 68 | 9.01 | 74 | 9.81 | 142 | 18.82 |
| 2016 | 65 | 8.62 | 12 | 1.59 | 77 | 10.21 | 64 | 8.48 | 141 | 18.69 |
| 2017 | 73 | 9.68 | 3 | 0.40 | 76 | 10.07 | 53 | 7.03 | 129 | 17.10 |
| 2018 | 74 | 9.81 | 1 | 0.13 | 75 | 9.94 | 52 | 6.89 | 127 | 16.83 |
| 2019 | 51 | 6.76 | 1 | 0.13 | 52 | 6.89 | 46 | 6.10 | 98 | 12.99 |
| 2020 | 21 | 2.78 | 0 | 0.00 | 21 | 2.78 | 5 | 0.66 | 26 | 3.45 |
| 2021 | 38 | 5.04 | 3 | 0.40 | 41 | 5.43 | 38 | 5.04 | 79 | 10.47 |
| Total | 387 | 7.33 | 23 | 0.44 | 410 | 7.76 | 332 | 6.29 | 742 | 14.05 |
| 2021–2022 | 59 | 3.91 | 3 | 0.20 | 62 | 4.11 | 43 | 2.85 | 105 | 6.96 |
| 2015–2019 | 328 | 8.70 | 20 | 0.53 | 348 | 9.23 | 289 | 7.66 | 637 | 16.89 |
| RR (95%CI)b | 0.45 (0.34 to 0.59) | 3.38 (0.11 to 1.26) | 0.45 (0.34 to 0.58) | 0.37 (0.27 to 0.51) | 0.41 (0.34 to 0.51) | |||||
| From 14 March 2020, to 24 June 2020 | 3 | 9.96 | 0 | 0 | 3 | 1.42 | 0 | NC | 3 | 1.42 |
| From 14 March to 24 June 2015–2019 | 105 | 1.42 | 6 | 0.57 | 109 | 10.34 | 99 | 9.39 | 208 | 19.73 |
| RR (95%CI)b | 0.14 (0.05 to 0.45) | NC | 0.14 (0.04 to 0.43) | NC | 0.07 (0.02 to 0.23) | |||||
Abbreviation: NC, not calculable.
Outbreak rate per 1,000,000 person-years.
Reference: 2015 to 2019 values.
Aetiology of outbreaks
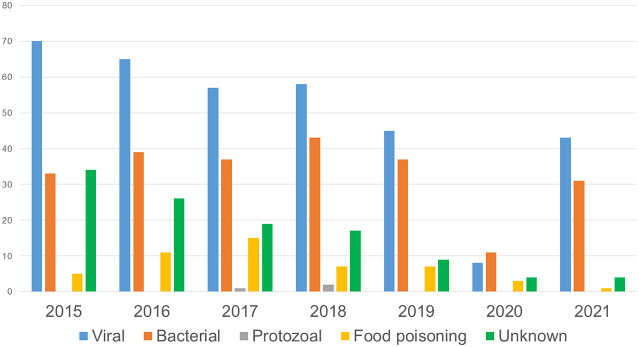
In 346 outbreaks, the aetiology was determined to be viral (97.4% norovirus, 2.02% rotavirus, and 0.58% other viruses); 231 outbreaks had a bacterial aetiology (44.59% Salmonella, 13.85% Shigella, 11.69% Clostridium perfringens, 10% Staphylococcus aureus, 4.32% Bacillus cereus, 4.76% Campylobacter, and 10.82% other bacteria); 52 outbreaks were due to neither bacterial nor viral causes (41 histamine outbreaks, 2 Cryptosporidium, 1 Trichinella spiralis, and 8 due to toxic mushroom ingestion, Jimsonweed poisoning, or beta-agonists); and in 113 outbreaks, the aetiology was not identified (Figure 1).
Figure 1.
Number of reported outbreaks of AGE according to aetiology. Catalonia, 2015 to 2021.
During the entire period studied, norovirus was the most frequently identified aetiological agent, causing 53.58% (337/629) of the outbreaks with a known aetiology.
A decrease in the incidence rate of outbreaks during the pandemic period compared to the prepandemic period was observed for all aetiologies. Among those of viral aetiology, the incidence rate was 3.38 outbreaks/1,000,000 person-years in the pandemic period and 7.82 outbreaks/1,000,000 person-years in the prepandemic period (RR 0.43; 95% CI: 0.32 to 0.58). Among those of bacterial aetiology, the incidence rate was 2.78 outbreaks/1,000,000 person-years in the pandemic period and 5.01 outbreaks/1,000,000 person-years in the prepandemic period (RR 0.56; 95% CI: 0.40 to 0.78).
Transmission mode
During the pandemic period, transmission by common vehicles accounted for 62 outbreaks (59.05%), and during the prepandemic period, this mode of transmission accounted for 348 outbreaks (54.63%). No significant differences were observed between these proportions (p = 0.46).
The incidence rate of outbreaks with common vehicle transmission was 4.11 outbreaks/1,000,000 person-years for the pandemic period versus 9.23 outbreaks/1,000,000 person-years for the prepandemic period (RR 0.45; 95% CI 0.34 to 0.58). For outbreaks with person-to-person transmission, these rates were 2.85/1,000,000 person-years and 7.66/1,000,000 person-years, respectively (RR 0.37; 95% CI 0.27 to 0.51).
Attack rates globally and by transmission mode, setting, and aetiology
In the pandemic and prepandemic periods, the global attack rates were 33.84 and 28.00, respectively (RR 1.21; 95% CI 1.17 to 1.25).
RR in person-to-person transmission outbreaks was 1.58 (95% CI 1.50 to 1.66) and 0.82 (95% CI 0.78 to 0.87) in common vehicle transmission outbreaks.
In closed or semiclosed institutions, the attack rate was higher during the pandemic period than during the prepandemic period for all modes of transmission (RR 1.36; 95% CI: 1.31 to 1.41). In outbreaks in the community setting, the attack rate was lower during the pandemic period than during the prepandemic period in common vehicle transmission outbreaks (RR 0.60; 95% CI 0.54 to 0.66) but not for person-to-person transmission outbreaks (RR 2.68; 95% CI 2.00 to 3.59) (Table 3).
Table 3.
Attack rate and rate ratio in the prepandemic and pandemic periods according to the setting and mode of transmission of the outbreak
| Setting | Transmission mode | Period | Number of outbreaks | Number of cases | All exposed | AR (%) | RRa (95%CI) |
|---|---|---|---|---|---|---|---|
| Closed and semiclosed facilitiesb | Person to person | Pandemic | 37 | 1,220 | 3,447 | 35.39 | 1.50 (1.42 to 1.57) |
| Prepandemic | 171 | 5,122 | 21,640 | 23.67 | 1 | ||
| Common vehicle | Pandemic | 19 | 897 | 2,674 | 33.55 | 1.08 (1.02 to 1.16) | |
| Prepandemic | 54 | 2,165 | 6,993 | 30.96 | 1 | ||
| All | Pandemic | 56 | 2,117 | 6,121 | 34.59 | 1.36 (1.31 to 1.41) | |
| Prepandemic | 225 | 7,287 | 28,633 | 25.45 | 1 | ||
| Community setting | Person to person | Pandemic | 6 | 23 | 46 | 50.00 | 2.68 (2.00 to 3.59) |
| Prepandemic | 118 | 1,172 | 6,279 | 18.67 | 1 | ||
| Common vehicle | Pandemic | 43 | 289 | 1,011 | 28.59 | 0.60 (0.54 to 0.66) | |
| Prepandemic | 294 | 3,181 | 6,690 | 47.55 | 1 | ||
| All | Pandemic | 49 | 312 | 1,057 | 29.52 | 0.88 (0.80 to 0.97) | |
| Prepandemic | 412 | 4,353 | 12,969 | 33.56 | 1 | ||
| All outbreaks | Person to person | Pandemic | 43 | 1,243 | 3,493 | 35.59 | 1.58 (1.50 to 1.66) |
| Prepandemic | 289 | 6,294 | 27,919 | 22.54 | 1 | ||
| Common vehicle | Pandemic | 62 | 1,186 | 3,685 | 32.18 | 0.82 (0.78 to 0.87) | |
| Prepandemic | 348 | 5,346 | 13,683 | 39.07 | 1 | ||
| All | Pandemic | 105 | 2,429 | 7,178 | 33.84 | 1.21 (1.17 to 1.25) | |
| Prepandemic | 637 | 11,640 | 41,602 | 28.00 | 1 |
Abbreviations: AR, attack rate; RR, rate ratio.
Rate ratio taking the prepandemic period (2015–2019) as reference values.
Nursing homes, health institutions, schools, and summer camps.
The attack rate was higher during the pandemic period than during the prepandemic period in both viral outbreaks (RR 1.22; 95% CI 1.16 to 1.27) and bacterial outbreaks (RR 1.16; 95% CI 1.09 to 1.24) (Table 4).
Table 4.
Attack rate (%) and rate ratio according to the mode of transmission in the outbreaks of viral and bacterial aetiology in the prepandemic and pandemic periods
| Viral etiology | Bacterial etiology | |||||
|---|---|---|---|---|---|---|
| Common vehicle | Person to person | All outbreaks | Common vehicle | Person to person | All outbreaks | |
| Period | AR (Cases/exposed) | AR (Cases/exposed) | AR (Cases/exposed) | AR (Cases/exposed) | AR (Cases/exposed) | AR (Cases/exposed) |
| 2020–2021 | 27.05 (267/987) | 35.46 (1,183/3,336) | 33.54 (1,450/4,323) | 33.18 (882/2,658) | 36.51 (23/63) | 33.26 (905/2,721) |
| 2015–2019 | 42.63 (3,192/7,487) | 22.19 (4,460/20,997) | 27.57 (7,852/28,484) | 33.29 (1,448/4,350) | 21.65 (632/2,919) | 28.61 (2,080/7,269) |
| RR (95%CI)a | 0.63 (0.57 to 0.71) | 1.60 (1.52 to 1.68) | 1.22 (1.16 to 1.27) | 1.00 (0.93 to 1.07) | 1.69 (1.21 to 2.35) | 1.16 (1.09 to 1.24) |
Abbreviations: AR, attack rate; RR, rate ratio.
Rate ratio taking the prepandemic period (2015–2019) as reference values.
The median, range, mode, mean, and standard deviation of viral and bacterial outbreaks for exposed individuals and cases in the pandemic and prepandemic periods are shown in Supplementary Table S1. When comparing the pandemic and prepandemic periods, significant differences were observed in the mean numbers of exposed people (p = 0.02) and confirmed cases (p < 0.001).
Discussion
In our study, we observed a decrease in the incidence rate of AGE outbreaks during the COVID-19 pandemic in Catalonia. This decrease was probably due to multiple factors. Aemistead et al. [20] described a 52% decrease in AGE outpatient encounters in 2020 in Colorado (United States) compared to 2017–2019 and stated that the increased use of telemedicine in the pandemic period may have impacted the frequency of stool specimen submission for enteric pathogen testing. Siso et al. [21] described decreases in diagnoses of chronic diseases of up to 50% in Barcelona (Spain) in relation to the prepandemic period. Both authors noted that new consultation models, with a decrease in face-to-face visits and an increase in telemedicine, could have contributed to these phenomena, especially the noncollection of samples for the aetiological diagnosis of AGE. The Catalonia Health Report of 2021 also indicated that there was a significant decrease in the percentage of people who were attended by health services in Catalonia in 2020 and 2021 compared to 2019 [11].Other authors have observed a decrease in the incidence of AGE. Hatun et al. [22] studied the incidence of AGE among children aged 0 to 17 years in Massachusetts from weeks 13 to 18 of 2019 and in the same weeks in 2020 (weeks after the introduction of social distancing measures), observing a decrease in the incidence from 15/100,000 children in 2019 to 1.8/100,000 children in 2020. The authors considered that this decrease could be attributed both to a real decrease in incidence and to a lower frequency of consultations with health services.
Ando et al. [23], in a study of enteric viruses in wastewater carried out between 2018 and 2023 in Sapporo (Japan), observed that after May 2020, the identification of sapovirus and rotavirus A and the concentration of norovirus GII decreased. Lennon et al. [24] compared the months of lockdown in the United States in 2020 with the same months in 2019 and observed a reduction in norovirus outbreaks of 90.57%. Douglas et al. [25] observed a decrease of 85% in the reports of norovirus to the systems that monitor the activity of this virus in England during the pandemic period and pointed out the risk that the paucity of surveillance data on norovirus activity and circulating strains would result in a potential strain replacement event that was not being monitored as effectively as prior to the emergence of COVID-19.
The reduction in the incidence rate of outbreaks in the pandemic period compared to the prepandemic period was greater for outbreaks of viral aetiology than for those of bacterial aetiology. The greater reduction in the incidence of outbreaks of viral aetiology compared to bacterial outbreaks may be related to the fact that nonpharmacological measures had a greater impact on the transmission mechanisms of viral outbreaks. Other authors have also observed a greater decrease in outbreaks of viral aetiology than in bacterial outbreaks. Ondrikova et al. [26] described that the impact of the pandemic was more pronounced in laboratory reports of norovirus than laboratory reporting of Campylobacter in England during 2020 compared to 2015–2019 data, indicating as possible causes the prioritization of the analysis of bacterial AGE samples and that the greater severity of Campylobacter infection entailed a greater frequency of consultation with health services. Wang et al. [27] observed an annual average of 5,521 cases of AGE from 2012 to 2019 in China and only 1,772 cases in 2020, with this decrease occurring for all viral and bacterial AGE cases except those caused by nontyphoid Salmonella and Campylobacter, which increased by 66.53% and 90.48%, respectively. Ahn et al. [28] compared the incidence rates of AGE cases in Korea from March to August 2020 with those of the previous two years and observed a decrease in the rates of viral gastroenteritis and an increase in the rates of Campylobacter and C. perfringens. Mark et al. [29] observed a significant decrease in positive samples among patients with viral AGE but not among those with bacterial AGE in Germany. The decrease in mean samples tested for viruses in the pandemic period compared to the prepandemic period was 40.6%, while for samples tested for bacteria the decrease was 27.1%. One possible explanation is that since viral aetiological symptoms are usually less severe than those of a bacterial aetiology, during the pandemic period, people with viral symptoms either did not consult the health system or were tested less frequently than in the prepandemic period.
We also observed this decrease in outbreak rates during the pandemic period when separately analyzing outbreaks transmitted by a common vehicle or by person-to-person transmission, as well as when comparing lockdown weeks with the same weeks of the prepandemic years.
The decrease in the rate of AGE outbreaks in Catalonia, both in common vehicle transmission outbreaks and in person-to-person transmission outbreaks, seems logical since during the pandemic period there was a substantial decrease in social events and reduced use of collective catering services.
The decrease in outbreak incidence rates detected by us and other authors [30] was not accompanied by a decrease in attack rates in such outbreaks.
The attack rate was higher in the pandemic period than in the prepandemic period for all outbreaks and in outbreaks of person-to-person transmission but not in common vehicle transmission outbreaks. Attack rates in community-based outbreaks were higher in the prepandemic period for outbreaks with person-to-person transmission, but in outbreaks with common vehicle transmission the attack rates were higher in the prepandemic period. Other authors have also observed higher attack rates in AGE outbreaks during the pandemic period. Lu et al. [31] described attack rates of 5.85% in norovirus AGE outbreaks in Guangzhou (China) in 2020 and 4.83% from 2015 to 2019. These authors suggest that efforts dedicated to the control of COVID-19 would have occupied the necessary material and human resources to respond to other diseases, such as diarrhoea. This suggests that, despite the nonpharmacological preventive measures adopted to reduce the transmission of SARS-CoV-2, there were circumstances during the pandemic period that favoured a higher transmission of AGE by direct contact, especially in viral outbreaks, which need a lower infective dose (a mean of 18 viral particles for norovirus [32] versus 106 particles for Salmonella [33]).
It is striking that in closed and semiclosed centres, the attack rates were higher during the pandemic period for both modes of transmission. In community-wide outbreaks, the overall attack rates and transmission by common vehicles were lower in the pandemic period. This could indicate a lower impact of nonpharmacological measures in closed and semiclosed institutions. Another possible explanation could be the lack of human resources in closed and semiclosed centres being significantly affected by COVID-19. Before the pandemic, the average staff/user ratio in Spanish residences was below the recommendations issued by the administration. In some centres, sick leave due to COVID-19 affected 75% of the staff [34]. Additionally, in Canada in 2020, 86% of long-term care facilities (LTCFs) and 71% of nursing homes reported an increase in absenteeism [35].
The mean numbers of exposed and sick people were higher during the pandemic period than during the prepandemic period. Some authors have pointed out that the time elapsed from the notification of the outbreak to the initiation of control measures was longer during the pandemic, and this could have had an impact on a greater number of affected people [31].
Our work has some limitations. We could not rule out that the differences observed in the attack rates were because during the pandemic epidemiological services were overwhelmed and had less capacity to study outbreaks and determine the number of individuals exposed previously.
Thus, Durant et al. [36] studied the impact the pandemic had on the activity of clinical laboratories in the United States and Canada. Comparing the weeks before tests for SARS-CoV-2 available with the weeks after, they observed a decrease in all types of clinical tests requested, with a decrease of 53.9% for microbiological tests and 26.8% for virological tests. Additionally, in Catalonia, the Microbiology Notification System had, in 2020, a decrease of 43.5% (95% CI 42.5 to 44.4%) in the identification and reporting of enteritis-producing agents of all aetiologies compared to 2019 [37]. The decrease in this laboratory activity may result in the emergence of new strains or the replacement of existing strains may go unnoticed and affect the most susceptible groups. This lower intervention capacity of health services was also observed in the control of other diseases, such as tuberculosis contact tracing [38] or cancer diagnoses [11]. Another limitation was the low number of reported outbreaks in 2020, which made it difficult to obtain statistically significant results in any of the analyses.
Conclusion
The COVID-19 pandemic brought a substantial reduction in notifications of AGE outbreaks. This reduction can be attributed to a lower incidence due to the nonpharmaceutical measures implemented to prevent the transmission of SARS-CoV-2 or to a reduction in the diagnosis and notification of outbreaks to epidemiological surveillance services. In addition, changes in the care model, social changes (telemedicine, teleworking), cultural changes, and interpersonal relationships may have played an important role in this reduction. The reduction was greater in 2020, and in 2021, a return to a normal incidence began that affected outbreaks of any aetiology.
It is also possible that affected people did not consult overloaded health services. The lower capacity of surveillance services to investigate reported outbreaks, prioritizing larger outbreaks, may explain the higher attack rate during the pandemic period. Diminished surveillance of the aetiological agents that cause AGE can have a negative impact on the health of the population since agents with a more severe impact on vulnerable groups can go undetected.
The greater reduction in the incidence of outbreaks with a viral aetiology compared to bacterial outbreaks may be related to the fact that nonpharmaceutical measures had a greater impact on the transmission mechanisms of viral outbreaks. Other authors have also observed a greater decrease in outbreaks with a viral aetiology.
Supporting information
Parrón et al. supplementary material
Acknowledgements
The members of the Working Group for the Study of Outbreaks of Acute Gastroenteritis in Catalonia are Miquel Alsedà, Josep Álvarez, Anna de Andrés, Irene Barrabeig, Rosa Bartolomé, Anna Isabel Belver, Javier de Benito, Esteve Camprubí, Neus Camps, Mónica Carol, Thais Cornejo, Lorena Coronas, Montse Cunillé, Àngela Domínguez, Maria Lluïsa Forns, Pere Godoy, Susana Guix, Conchita Izquierdo, Mireia Jané, Ana Martínez, Sofia Minguell, Antonio Moreno-Martínez, Ignacio Parrón, Cristina Pérez, Efrén Razquín, Cristina Rius, Ariadna Rovira, Maria Sabaté, Sara Sabaté, Maria Rosa Sala, Mercé de Simón, Núria Soldevila, Núria Torner, and Rosa Maria Vileu.
Supplementary material
For supplementary material accompanying this paper visit http://doi.org/10.1017/S0950268823001851.
click here to view supplementary material
Data availability statement
Data supporting the findings of this study are available from the corresponding author upon reasonable request.
Author contribution
Conceptualization: A.D., I.P.; Funding acquisition: A.D.; Investigation: A.D., C.I., P.G., M-R.S., S.M., A.M., C.R., I.B., J.F., M.C., N.B., I.P.; Methodology: A.D., I.P.; Project administration: A.D.; Resources: A.D., A.M.; Supervision: A.D.; Writing – original draft: A.D., I.P.; Data curation: C.I., P.G., M-R.S., S.M., A.M., C.R., I.B., J.F., M.C., N.B., I.P.; Writing – review & editing: C.I., P.G., M-R.S., S.M., A.M., C.R., I.B., J.F., M.C., N.B.; Formal analysis: I.P.; Software: I.P.
Financial support
This work was supported by the Instituto de Salud Carlos III through project [PI16/02005] (cofunded by the European Regional Development Fund “Investing in your future”) and the Catalan Agency for the Management of Grants for Universities [AGAUR Grant Number 2017/SGR 1342 and 2021/SGR 00702]. The funders had no role in the design or conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Competing interest
The authors declare none.
References
- [1].World Health Organization. (2020). Modes of transmission of virus causing COVID-19: implications for IPC precaution recommendations. Available at https://www.who.int/news-room/commentaries/detail/modes-of-transmission-of-virus-causing-covid-19-implications-for-ipc-precaution-recommendations (accessed 25 February 2022).
- [2].World Health Organization. (2020). Transmission of SARS-CoV-2: implications for infection prevention precautions: scientific brief. Available at https://www.who.int/news-room/commentaries/detail/transmission-of-sars-cov-2-implications-for-infection-prevention-precautions (accessed 25 February 2022).
- [3].Lu J, et al. (2020). COVID-19 outbreak associated with air conditioning in restaurant, Guangzhou, China, 2020. Emerging Infectious Diseases 26, 1628–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Jang S, Han SH, Rhee JY (2020). Cluster of coronavirus disease associated with fitness dance classes, South Korea. Emerging Infectious Diseases 26, 1917–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Centers for Disease Control and Prevention. (2021). Scientific brief: SARS-CoV-2 transmission. Available at http://www.ncbi.nlm.nih.gov/books/NBK570442/ (accessed 20 December 2021). [PubMed]
- [6].Morawska L, Cao J (2020). Airborne transmission of SARS-CoV-2: the world should face the reality. Environment International 139, 105730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Olsen SJ, et al. (2020). Decreased influenza activity during the COVID-19 pandemic-United States, Australia, Chile, and South Africa, 2020. American Journal of Transplantation 20, 3681–3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Huh K, et al. (2021). Impact of non-pharmaceutical interventions on the incidence of respiratory infections during the coronavirus disease 2019 (COVID-19) outbreak in Korea: a nationwide surveillance study. Clinical Infectious Diseases 72, e184–e191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Lai C-C, et al. (2021). The impact of the coronavirus disease 2019 epidemic on notifiable infectious diseases in Taiwan: a database analysis. Travel Medicine and Infectious Disease 40, 101997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Centre d’Estudis Epidemiològics sobres les Infeccions de Transmissió Sexual i Sida de Catalunya (CEEISCAT). (2022). Vigilància epidemiològica de les infeccions de transmissió sexual (ITS) a Catalunya. Informe anual 2021. Available at https://canalsalut.gencat.cat/web/.content/_A-Z/S/sida/enllasos/anual_ITS.pdf (accessed 5 July 2023).
- [11].Mompart A, Planella A. (2023). Informe de salut de Catalunya 2021. Available at https://scientiasalut.gencat.cat/handle/11351/9286 (accessed 5 July 2023).
- [12].World Health Organization. (2020). The top 10 causes of death (Internet). Available at https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed 25 February 2022).
- [13].World Health Organization. (2017). Diarrhoeal disease. Available at https://www.who.int/news-room/fact-sheets/detail/diarrhoeal-disease (accessed 25 February 2022).
- [14].Centers for Disease Control and Prevention. (2018). Global water, sanitation and hygiene. Available at https://www.cdc.gov/healthywater/global/diarrhea-burden.html (accessed 22 January 2022).
- [15].World Health Organization. (2021). Diarrhoea. Available at https://www.who.int/westernpacific/health-topics/diarrhoea (accessed 22 January 2022).
- [16].Lian Y, et al. (2019). Epidemiology of norovirus outbreaks reported to the public health emergency event surveillance system, China, 2014−2017. Viruses 11, E342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kroneman A, et al. (2008). Analysis of integrated virological and epidemiological reports of norovirus outbreaks collected within the foodborne viruses in Europe network from 1 July 2001 to 30 June 2006. Journal of Clinical Microbiology 46, 2959–2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Petrignani M, et al. (2015). Norovirus introduction routes into nursing homes and risk factors for spread: a systematic review and meta-analysis of observational studies. Journal of Hospital Infection 89, 163–178. [DOI] [PubMed] [Google Scholar]
- [19].Institut d’ Estadistica de Catalunya. (2020). Statistical yearbook of Catalonia. Population on 1 January. Provinces. Available at https://www.idescat.cat/pub/?id=aec&n=245&lang=en&t=2017 (accessed 20 December 2021).
- [20].Armistead I, et al. (2022) Trends in outpatient medical-care seeking for acute gastroenteritis during the COVID-19 pandemic, 2020. Foodborne Pathogens and Disease 19, 290–292. [DOI] [PubMed] [Google Scholar]
- [21].Sisó-Almirall A, et al. (2022). Impact of the COVID-19 pandemic on primary health care disease incidence rates: 2017 to 2020. Annals of Family Medicine 20, 63–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hatoun J, et al. (2020). Social distancing for COVID-19 and diagnoses of other infectious diseases in children. Pediatrics 146, e2020006460. [DOI] [PubMed] [Google Scholar]
- [23].Ando H, et al. (2023). Tracking the effects of the COVID-19 pandemic on viral gastroenteritis through wastewater-based retrospective analyses. The Science of the Total Environment 905, 166557. Available at https://ssrn.com/abstract=4436075 (accessed 7 July 2023). [DOI] [PubMed] [Google Scholar]
- [24].Lennon RP, et al. (2020). Norovirus infections drop 49% in the United States with strict COVID-19 public health interventions. Acta Medica Academica 49, 278–280. [DOI] [PubMed] [Google Scholar]
- [25].Douglas A, et al. (2021). Impact of COVID-19 on national surveillance of norovirus in England and potential risk of increased disease activity in 2021. Journal of Hospital Infection 112, 124–126. [DOI] [PubMed] [Google Scholar]
- [26].Ondrikova N, et al. (2021). Differential impact of the COVID-19 pandemic on laboratory reporting of norovirus and Campylobacter in England: a modelling approach. PLoS One 16, e0256638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Wang LP, et al. (2021). The changing pattern of enteric pathogen infections in China during the COVID-19 pandemic: a nation-wide observational study. The Lancet Regional Health Western Pacific 16, 100268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ahn SY, et al. (2021). Changes in the occurrence of gastrointestinal infections after COVID-19 in Korea. Journal of Korean Medical Science 21, e180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Mack D, et al. (2021). Where have the enteric viruses gone? - Differential effects on frequent causes of infectious diarrhoea by SARS-CoV-2 pandemic lockdown measures. Infection Prevention in Practice 3, 100184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].UK Health Security Agency. (2023). National norovirus and rotavirus report, week 11 report: Data up to week 9 (5 March 2023). Available at https://www.gov.uk/government/statistics/national-norovirus-and-rotavirus-surveillance-reports-2022-to-2023-season/national-norovirus-and-rotavirus-report-week-11-report-data-up-to-week-9-5-march-2023 (accessed 5 July 2023).
- [31].Lu Y, et al. (2021). The rise in norovirus-related acute gastroenteritis during the fight against the COVID-19 pandemic in southern China. Frontiers in Public Health 9, 785373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Dolin R, Treanor JJ (2020). Noroviruses and sapoviruses (Caliciviruses). In Bennett JE, Dolin R, Blaser MJ (eds), Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases, 9th Edn. Philadelphia, PA: Elsevier/Saunders, pp. 2269–2276. [Google Scholar]
- [33].Pegues DA, Miller SI (2020). Salmonella species. In Bennett JE, Dolin R, Blaser MJ (eds), Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases, 9th Edn. Philadelphia, PA: Elsevier/Saunders, pp. 2725–2736. [Google Scholar]
- [34].Codorniu JM. (2021). El impacto de la pandemia en las residencias para personas mayores y las nuevas necesidades de personal en la etapa pos-COVID. Available at https://www.funcas.es/wp-content/uploads/2021/07/Montserrat.pdf (accessed 5 July 2023).
- [35].Clarke J. (2021). Impacts of the COVID-19 pandemic in nursing and residential care facilities in Canada. Available at https://www.researchgate.net/publication/360445618_Impacts_of_the_COVID-19_pandemic_in_nursing_and_residential_care_facilities_in_Canada (accessed 6 July 2023).
- [36].Durant TJS, et al. (2020). Impact of COVID-19 pandemic on laboratory utilization. The Journal of Applied Laboratory Medicine 5, 1194–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Ciruela P, et al. (2022). Informe sobre els microorganismes causants de malalties infeccioses declarats durant l’any 2020. Available at https://scientiasalut.gencat.cat/handle/11351/8970 (accessed 2 July 2023).
- [38].Godoy P, et al. (2022). Impact of the COVID-19 pandemic on contact tracing of patients with pulmonary tuberculosis. European Journal of Public Health 32, 643–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Parrón et al. supplementary material
For supplementary material accompanying this paper visit http://doi.org/10.1017/S0950268823001851.
click here to view supplementary material
Data Availability Statement
Data supporting the findings of this study are available from the corresponding author upon reasonable request.