Abstract
Aims
Cardiac contractility modulation (CCM) is a device therapy for heart failure, based on the delivery of high‐voltage biphasic impulses to the right ventricular septum during the myocardial absolute refractory period. This study evaluated the cost‐effectiveness of CCM therapy plus optimal medical therapy (OMT) vs. OMT alone in patients with heart failure with reduced ejection fraction.
Methods and results
A Markov model with a lifespan time horizon was developed to assess the cost–utility using the FIX trials as main data sources. A deterministic sensitivity analysis and a probabilistic sensitivity analysis were run to analyse the decision uncertainty in the model through cost‐effectiveness acceptability curve (CEAC) and cost‐effectiveness acceptability frontier (CEAF). Value of information analysis was also conducted computing the expected value of perfect information (EVPI) and the expected value of partial perfect information. The base case results showed that the CCM plus OMT option was highly cost‐effective compared with OMT alone with an incremental cost–utility ratio of €7034/quality‐adjusted life year (QALY). The CEAC and CEAF illustrated that for all willingness to pay levels above €5600/QALY, tested up to €50 000/QALY, CCM plus OMT alternative had the highest probability of being cost‐effective. The EVPI per patient was estimated to be €124 412 on a willingness to pay threshold of €30 000/QALY.
Conclusions
For patients with heart failure with reduced ejection fraction, CCM therapy could be cost‐effective when taking a lifetime horizon. Further long‐term, post‐approval clinical studies are needed to verify these results in a real‐world context, particularly concerning the effect of CCM therapy on mortality.
Keywords: Heart failure, Device therapy, Cardiac contractility modulation, Economic evaluation, Costs
Introduction
Heart failure is a clinical syndrome characterized by symptoms and signs that arise from structural and functional disorders of the heart. 1
The prevalence in the general population is 1–2%, and the condition may be undetected in over half of the cases. It is estimated that 64.3 million people are living with heart failure worldwide. 2 , 3
Combined medical treatment with angiotensin receptor–neprilysin inhibitors (ARNIs), beta‐blockers, mineralocorticoid receptor antagonists (MRAs), and sodium–glucose co‐transporter 2 (SGLT2) inhibitors is implemented at the time of diagnosis, as these classes of drugs have been shown to significantly reduce mortality in randomized clinical trials. 1
Device treatment is effective on top of optimal medical therapy (OMT) in selected patient cohorts. Implantable cardiac defibrillators are recommended for prevention of sudden cardiac death in patients with symptomatic heart failure and left ventricular ejection fraction ≤35% despite ≥3 months of optimized medical therapy, particularly in those with ischaemic aetiology.
Cardiac resynchronization therapy improves symptoms and survival in patients with left ventricular ejection fraction ≤35%, QRS duration ≥130 ms, and left bundle branch block. 1
Accounting for these criteria, only a minority of patients are considered suitable for device therapy of heart failure, leaving an unmet need of effective and widely applicable device‐based therapeutic options.
In this regard, cardiac contractility modulation (CCM) is a device therapy based on the delivery of high‐voltage biphasic impulses to the right ventricular septum during the myocardial absolute refractory period. These impulses are defined as non‐excitatory, as they do not initiate cardiac contraction, making CCM possible to use in patients with other implantable devices, such as pacemakers, implantable cardiac defibrillators, or cardiac resynchronization therapy. Consequently, CCM enhances cardiac inotropism with several additive mechanisms, and the full spectrum of effects is yet to be understood. 4 , 5
The known effects mostly rely on reverting the pathological changes in calcium homeostasis that occur in heart failure patients. Acute changes in intracellular calcium levels can be seen right after application of CCM therapy, and administration of ryanodine or verapamil has been shown to blunt inotropy, implying that the effects are mediated by both extra‐cellular calcium and sarcoplasmic reticulum calcium. CCM has also been shown to acutely increase phosphorylation of phospholamban at the site of application, which in turn modulates sarcoplasmic reticulum reuptake of calcium from the cytoplasm. 4 , 6 Interestingly, the marked increase in peak intracellular calcium in cardiac myocytes is not associated with elevation of myocardial oxygen consumption. 7
Chronic changes induced by CCM therapy have been demonstrated at the site of application and in distant locations and involve the reversion of the maladaptive foetal transcriptome induced by heart failure. After 3 months of CCM therapy, the expression of myocardial genes such as A‐ and B‐type natriuretic peptides, α‐myosin heavy chain, sarcoplasmic reticulum calcium adenosine triphosphatase‐2a, and phospholamban is restored to levels similar to those found in healthy controls. 6 , 8
CCM has been studied mainly in patients with heart failure with reduced ejection fraction (<45%) and narrow QRS at 12‐lead electrocardiogram (ECG), not eligible for cardiac resynchronization therapy, with impaired functional capacity according to the New York Heart Association (NYHA) classification (≥2) despite OMT.
Four moderately sized randomized clinical trials and a prospective single‐arm study have shown CCM to significantly improve functional capacity, measured by NYHA class and with the Minnesota Living with Heart Failure Questionnaire (MLHFQ), peak oxygen consumption, and 6 min walking test distance, with a good safety profile. 9 , 10 , 11 , 12 , 13
A recent individual patient data meta‐analysis of these studies, including a total of 861 patients with symptomatic heart failure on OMT, NYHA class ≥2, QRS duration <130 ms, and left ventricular ejection fraction <45%, concluded that the magnitude of the effect of CCM on functional capacity and quality of life is comparable with that achieved with cardiac resynchronization therapy in eligible patients. 14
Detailed cost–utility analyses that quantify the benefits and costs of CCM in terms of patient‐reported outcomes are lacking, even though its cost‐effectiveness has already been shown from an English National Health Service perspective. 15 , 16
This study sought to evaluate the cost–utility of CCM therapy plus OMT vs. OMT alone in patients with heart failure with reduced ejection fraction enrolled in the four FIX studies. 14
Methods
Framing the model
Target population
The present study was based on a cohort of 801 individuals with heart failure, QRS duration ≤130 ms, and left ventricular ejection fraction between 25% and 45%. The chosen population reflects the database population of the following trials: the FIX‐HF‐5 pilot study, the FIX‐CHF‐4 study, the FIX‐HF‐5 study, and the FIX‐HF‐5C study. 14 The FIX‐HF‐5C2 study was excluded for the lack of a control group. 12
Study perspective, setting, and location
The cost‐effectiveness analysis was performed according to the Italian National Health Service's perspective.
Intervention and comparator
We evaluated the cost‐effectiveness of CCM plus OMT compared with OMT alone.
The comparator was OMT, which is a pharmacological‐based approach for the management of heart failure patients. The intervention was based on the CCM therapy, which has been recently developed, plus OMT. The OPTIMIZER™ IVs (OPTIMIZER™ Smart) system is the device commonly used for the application of CCM therapy in the FIX studies.
Time horizon, discount rate, and threshold
The time horizon amounted to 15 years. Considering that, in the Framingham Heart Study, the median survival after the onset of congestive heart failure was 1.7 and 3.2 years in men and women, respectively, and in view of the device battery replacement after ~15 years, we hypothesized that the chosen time horizon was adequate to assess the quality‐adjusted life years (QALYs) of the intervention, being it considered a lifetime horizon. 17 Both costs and effects were discounted by a 3% yearly rate. The eurozone threshold amounting to €30 000–50 000 was chosen.
Model structure
Type of model and health states
A state‐transition Markov model for a hypothetical cohort of 1000 patients in the two intervention arms (i.e. CCM plus OMT and OMT) was built. The model included five health states (i.e. NYHA I, NYHA II, NYHA III, NYHA IV, and death). According to the clinical conditions of included patients and the cardiological indications for device implantation, patients entered the model in NYHA III. Then, the cohort progressed through health states as the disease progressed. Patients could transit to any of the four NYHA states in the next cycle of 6 months. Death could occur from each of the four NYHA states (Figure 1 ).
Figure 1.
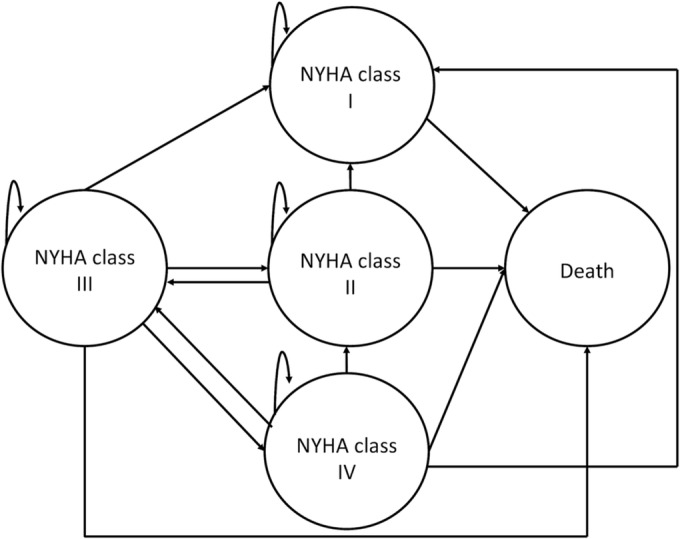
Markov model structure. Schematic representation of the Markov model. Health states are in circles. Arrows represent allowed transitions. NYHA, New York Heart Association.
Model population
Transition probabilities
The transition probabilities across the health states were estimated from the included trials databases, acquired from the manufacturer of the implantable device. Patients missing at 24 weeks were excluded from the model for the two study arms. Probabilities were estimated by dividing the share of patients who changed NYHA class during the follow‐up period (24 weeks) by the total number of patients in the originating class. The transition probabilities were time independent and were measured in a limited time frame of 24 weeks, as reported in the original studies. Table 1 reports the probabilities, for each study arm, used in the base case analysis.
Table 1.
Summary of intervention‐specific clinical parameters adopted in the economic model
| Model parameters | Base estimate | Deterministic sensitivity analysis range | Distribution | Source | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Transition probabilities | |||||
| CCM plus OMT | |||||
| Probability of III to I | 0.170 | — | — | Multinomial | 9 , 10 , 11 , 13 |
| Probability of III to II | 0.445 | — | — | Multinomial | 9 , 10 , 11 , 13 |
| Probability of III to III | 0.335 | — | — | Multinomial | 9 , 10 , 11 , 13 |
| Probability of III to IV | 0.019 | — | — | Multinomial | 9 , 10 , 11 , 13 |
| Probability of III to death | 0.031 | — | — | Multinomial | 9 , 10 , 11 , 13 |
| Probability of I to I | 0.99 | — | — | Multinomial | 9 , 10 , 11 , 13 |
| Probability of I to II | 0 | — | — | Multinomial | 9 , 10 , 11 , 13 |
| Probability of I to III | 0 | — | — | Multinomial | 9 , 10 , 11 , 13 |
| Probability of I to IV | 0 | — | — | Multinomial | 9 , 10 , 11 , 13 |
| Probability of I to death | 0.01 | — | — | Multinomial | 9 , 10 , 11 , 13 |
| Probability of II to I | 0.200 | — | — | Multinomial | 9 , 10 , 11 , 13 |
| Probability of II to II | 0.710 | — | — | Multinomial | 9 , 10 , 11 , 13 |
| Probability of II to III | 0.070 | — | — | Multinomial | 9 , 10 , 11 , 13 |
| Probability of II to IV | 0 | — | — | Multinomial | 9 , 10 , 11 , 13 |
| Probability of II to death | 0.020 | — | — | Multinomial | 9 , 10 , 11 , 13 |
| Probability of IV to I | 0.143 | — | — | Multinomial | 9 , 10 , 11 , 13 |
| Probability of IV to II | 0.286 | — | — | Multinomial | 9 , 10 , 11 , 13 |
| Probability of IV to III | 0.250 | — | — | Multinomial | 9 , 10 , 11 , 13 |
| Probability of IV to IV | 0.286 | — | — | Multinomial | 9 , 10 , 11 , 13 |
| Probability of IV to death | 0.035 | — | — | Multinomial | 9 , 10 , 11 , 13 |
| OMT | |||||
| Probability of III to I | 0.078 | — | — | Multinomial | 9 , 10 , 11 , 13 |
| Probability of III to II | 0.298 | — | — | Multinomial | 9 , 10 , 11 , 13 |
| Probability of III to III | 0.543 | — | — | Multinomial | 9 , 10 , 11 , 13 |
| Probability of III to IV | 0.067 | — | — | Multinomial | 9 , 10 , 11 , 13 |
| Probability of III to death | 0.014 | — | — | Multinomial | 9 , 10 , 11 , 13 |
| Probability of I to I | 0.99 | — | — | Multinomial | 9 , 10 , 11 , 13 |
| Probability of I to II | 0 | — | — | Multinomial | 9 , 10 , 11 , 13 |
| Probability of I to III | 0 | — | — | Multinomial | 9 , 10 , 11 , 13 |
| Probability of I to IV | 0 | — | — | Multinomial | 9 , 10 , 11 , 13 |
| Probability of I to death | 0.01 | — | — | Multinomial | 9 , 10 , 11 , 13 |
| Probability of II to I | 0.182 | — | — | Multinomial | 9 , 10 , 11 , 13 |
| Probability of II to II | 0.707 | — | — | Multinomial | 9 , 10 , 11 , 13 |
| Probability of II to III | 0.091 | — | — | Multinomial | 9 , 10 , 11 , 13 |
| Probability of II to IV | 0 | — | — | Multinomial | 9 , 10 , 11 , 13 |
| Probability of II to death | 0.020 | — | — | Multinomial | 9 , 10 , 11 , 13 |
| Probability of IV to I | 0 | — | — | Multinomial | 9 , 10 , 11 , 13 |
| Probability of IV to II | 0.151 | — | — | Multinomial | 9 , 10 , 11 , 13 |
| Probability of IV to III | 0.455 | — | — | Multinomial | 9 , 10 , 11 , 13 |
| Probability of IV to IV | 0.364 | — | — | Multinomial | 9 , 10 , 11 , 13 |
| Probability of IV to death | 0.030 | — | — | Multinomial | 9 , 10 , 11 , 13 |
| Utility values | |||||
| CCM plus OMT | |||||
| Utility for NYHA I | 0.791 | — | — | Lognormal | 9 , 10 , 11 , 13 |
| Utility for NYHA II | 0.687 | — | — | Lognormal | 9 , 10 , 11 , 13 |
| Utility for NYHA III | 0.573 | — | — | Lognormal | 9 , 10 , 11 , 13 |
| Utility for NYHA IV | 0.531 | 0.398 | 0.664 | Lognormal | 9 , 10 , 11 , 13 |
| OMT | |||||
| Utility for NYHA I | 0.682 | — | — | Lognormal | Algorithm of Kularatna et al. applied to MLHFQ data from several studies 9 , 10 , 11 , 13 |
| Utility for NYHA II | 0.674 | — | — | Lognormal | Algorithm of Kularatna et al. applied to MLHFQ data from several studies 9 , 10 , 11 , 13 |
| Utility for NYHA III | 0.559 | — | — | Lognormal | Algorithm of Kularatna et al. applied to MLHFQ data from several studies 9 , 10 , 11 , 13 |
| Utility for NYHA IV | 0.524 | 0.387 | 0.656 | Lognormal | Algorithm of Kularatna et al. applied to MLHFQ data from several studies 9 , 10 , 11 , 13 |
| Costs | |||||
| State cost for NYHA I | €2121 | — | — | Gamma | 18 |
| State cost for NYHA II | €2121 | €1060 | €3181 | Gamma | 18 |
| State cost for NYHA III (CCM plus OMT) | €3118 | €1559 | €4676 | Gamma | 18 |
| State cost for NYHA III (OMT) | €3335 | €1667 | €5002 | Gamma | 18 |
| State cost for NYHA IV (CCM plus OMT) | €3009 | €1504 | €4513 | Gamma | 18 |
| State cost for NYHA IV (OMT) | €3754 | €1877 | €5631 | Gamma | 18 |
| Cost of device | €20 000 | €16 500 | €23 500 | Gamma | 18 |
CCM, cardiac contractility modulation; MLHFQ, Minnesota Living with Heart Failure Questionnaire; NYHA, New York Heart Association; OMT, optimal medical therapy.
All estimates are computed to be on a 6 month basis. Lognormal distributions are specified by lower and upper limits of the 95% confidence intervals. Gamma distributions are specified by shape and scale parameters.
Costs, currency, and price adjustment
Only direct costs were included in the model. Data on the consumption of healthcare resources for the different NYHA classes were retrieved by Rognoni and Gerzeli, 18 adjusted for the 2021 rates based on the consumer price index. 19 Direct healthcare resources included the hospitalization for heart failure, outpatient services, laboratory exams, imaging, and drugs. Details of the device cost were retrieved directly from the manufacturer. The device cost was assumed to be arm and NYHA class dependent. No currency conversion was needed because all costs were expressed in euros. Table 1 displays the NYHA‐specific costs for the two options.
Utilities
The MLHFQ total scores were used to predict utility values for each NYHA class. MLHFQ scores were obtained from the FIX studies. MLHFQ scores were then converted into utility values adopting the algorithm by Kularatna et al. 20 Calculating the utility values using the above‐cited mapping algorithm is a two‐step process described in the Supporting Information. Table 1 lists the computed utility values for each NYHA class for the two study arms.
Model outcomes
The outcomes of the model are QALYs and expected costs. QALYs are computed as the product between the duration of a patient's stay in a health state and the utility values associated with that state. 21 The expected cost per Markov cycle is estimated by multiplying the cost by the volume of resources needed during the patient's stay in a given health state and study arm. By comparing the two interventions, incremental cost–utility ratio (ICUR) and net monetary benefits can be computed for CCM plus OMT vs. OMT.
Model analyses
Sensitivity analyses
One‐way deterministic sensitivity analysis was performed to investigate the impact of varying one uncertain model parameter, while keeping other parameters equal to their base case values, on study findings. The cost of each NYHA class was varied by 50% above and below the base case values, the cost of device by 17.5%, utilities by 25%, and the discount rate by 50%. Ranges were developed from the literature and expert opinion.
To deal with uncertainty around the estimated mean outcomes, a probabilistic sensitivity analysis was performed. Each parameter was defined by a specific distribution based on the nature of that parameter. For transition probabilities, a multinomial distribution was applied. For the utilities, a lognormal distribution was used. The costs were parameterized by gamma function.
Table 1 depicts the expanded list of model parameters with ranges used in the one‐way deterministic sensitivity analysis and distributions adopted in the probabilistic sensitivity analyses.
For the probabilistic sensitivity analysis, 100 000 Monte Carlo simulations were run. The analysis repeatedly drew sets of parameter values from probability distributions associated with each model parameter, computing incremental costs, incremental QALYs, and ICURs for each randomly generated set. As suggested by the International Society for Pharmacoeconomics and Outcomes Research guideline, 22 probabilistic sensitivity analysis findings were plotted using cost‐effectiveness acceptability curve (CEAC), which illustrates the probability that each intervention would be regarded as the optimal choice at different thresholds, and cost‐effectiveness acceptability frontier (CEAF), which instead displays the net monetary benefit at each willingness to pay level and the decision uncertainty surrounding the optimal choice.
Furthermore, the expected value of perfect information (EVPI) and the expected value of partial perfect information (EVPPI) were calculated to assess the value of collecting further information.
The EVPI is determined, for a specific threshold, by the difference between the expected value with perfect information and the expected value with current information 23 to compute the value of future research to eliminate or decrease uncertainty with respect to the cost‐effectiveness of CCM plus OMT compared with OMT in patients with heart failure.
EVPPI was calculated to compute the value of decreasing uncertainty referred to specific parameters in the model and to identify which parameters were most informative to the estimation of cost‐effectiveness, allowing to steer further research around those parameters requiring additional information.
The study was reported according to the Consolidated Health Economic Evaluation Reporting Standards statement. 24 The analyses were performed using R software (R Development Core Team).
Results
Base case analysis
From the Italian National Health System perspective, the base case results showed that the CCM plus OMT option could be highly cost‐effective compared with the OMT alone alternative. Table 2 summarizes the discounted costs and outcomes as well as the base case findings, respectively, for the simulated cohort.
Table 2.
Base case results over lifetime horizon
| Strategy | Total costs (€) (95% CI) | Incremental costs (€) | QALYs (95% CI) | Incremental QALYs | ICUR | NMB (€) |
|---|---|---|---|---|---|---|
| OMT |
39 723 998 (38 521 793–40 601 424) |
— |
11 237 (10 034–12 399) |
— | Ref. | 297 386 002 |
| CCM plus OMT |
50 230 953 (47 887 392–52 934 904) |
10 506 955 |
12 731 (11 437–13 890) |
1494 | 7034 | 331 699 047 |
CCM, cardiac contractility modulation; CI, confidence interval; ICUR, incremental cost–utility ratio; NMB, net monetary benefit; OMT, optimal medical therapy; QALYs, quality‐adjusted life years; Ref., reference category.
Sensitivity analyses
The deterministic sensitivity analysis resulted in a range of ICURs suggesting cost‐effectiveness of the CCM plus OMT alternative. The ICURs were most sensitive to changes in the NYHA III class cost for the OMT alone option while to changes in the cost of device and in the NYHA III class cost for the CCM plus OMT alternative. The other parameters that, when set at their upper and lower bounds, resulted in a change in the ICUR are depicted in Figure 2 . The variation in the ICURs was lower than 20% in all the parameters except for NYHA III class cost for the OMT alone option. None of the investigated variations in model parameters changed the optimal strategy.
Figure 2.
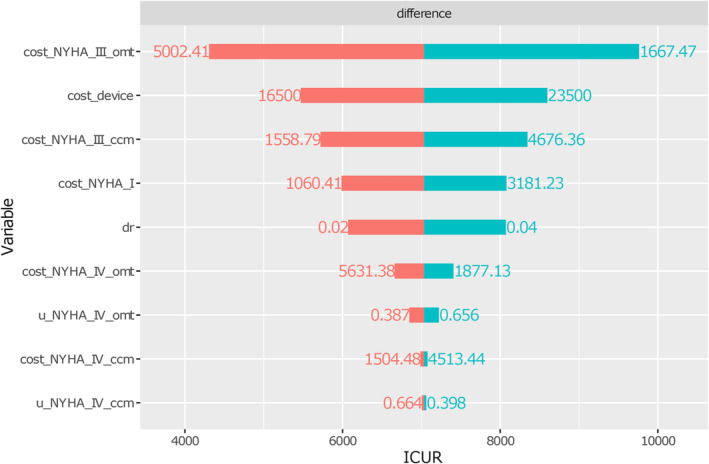
One‐way sensitivity analysis of key model parameters. Tornado diagram for the deterministic sensitivity analysis. Orange bar means that the incremental cost–utility ratio (ICUR) decreases as parameter value decreases; light‐green bar means that ICUR increases as parameter value increases.
As shown in the cost‐effectiveness plane (Figure 3 ), the probabilistic sensitivity analysis confirmed that, compared with OMT alone, the CCM plus OMT alternative could be cost‐effective.
Figure 3.
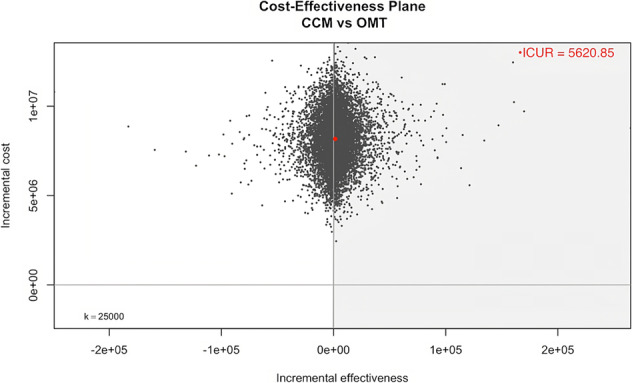
Cost‐effectiveness plane from probabilistic sensitivity analysis. Incremental cost‐effectiveness planes of n = 100 000 bootstrap replicates (grey dots) and point estimate (red dot) for incremental costs and quality‐adjusted life years gained. The grey‐shaded area reflects the sustainability area. CCM, cardiac contractility modulation; ICUR, incremental cost–utility ratio; OMT, optimal medical therapy.
The CEAC and CEAF, given in Supporting Information, Figures S1 and S2 , depicted that the OMT alone option had the highest probability of being cost‐effective for a willingness to pay threshold less than €5600 per QALY gained. For all willingness to pay levels above €5600/QALY, tested up to €50 000/QALY, the CCM plus OMT alternative had the highest probability of being cost‐effective.
Overall, the analysis confirmed the robustness of the conclusion at a threshold of €30 000–50 000/QALY.
Value of information analysis
From the probabilistic sensitivity analysis simulation, the EVPI per patient was estimated to be €124 412 on a willingness to pay threshold of €30 000/QALY (Figure 4 ). The EVPI decreased with lower thresholds, amounting to €44 100 on a threshold of €10 000/QALY. As shown in Supporting Information, Figure S3 , examination of the EVPPI per patient highlighted that the largest value for further research is for perfect information on the cost of NYHA IV class for the OMT alone option followed by the cost and utility of NYHA IV class for the CCM plus OMT alternative.
Figure 4.
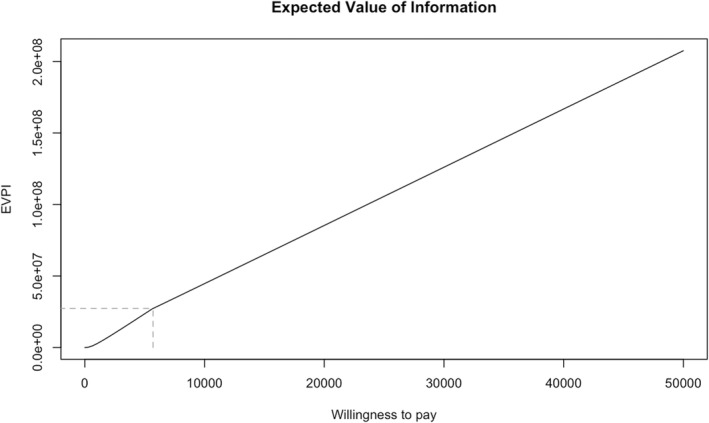
Population expected value of perfect information (EVPI) curve. This graph plots the EVPI in euros (i.e. the y axis) against a willingness to pay per quality‐adjusted life year in euros (i.e. the x axis) for the study results.
Discussion
The present study was conducted to inform policymaking regarding the cost‐effectiveness of CCM therapy in patients with heart failure with reduced ejection fraction.
The analysis demonstrated that implementing CCM therapy plus OMT over a lifetime period would be cost‐effective at a threshold of €30 000 in the Italian National Health System. In sensitivity analysis, the model results were robust to most assumptions and parameter uncertainty.
The study findings are consistent with those provided by a National Institute for Health and Care Excellence guidance document, focused on implantable cardiac defibrillators and cardiac resynchronization therapy 25 for arrhythmias and heart failure, which issued a recommendation for the adoption of implantable cardiac defibrillators and biventricular pacemakers given the cost‐effective ICURs relative to OMT.
Our results support the work of a study performed in the United Kingdom showing the cost‐effectiveness of CCM therapy plus OMT in patients with heart failure with reduced ejection fraction with an ICUR of £16 405. 15
Another study, conducted in England in 2019, assessed whether CCM plus standard of care is a cost‐effective alternative for individuals similar to our population to standard of care alone. 16 Although the authors adopted a different modelling approach, the cost‐effectiveness of CCM strategy (i.e. ICUR of £22 988) was in line with the present results. We evaluated the economic benefit of CCM based on the NYHA class of 801 subjects with heart failure, 14 derived from the results of the four FIX studies. As revised by a large and comprehensive meta‐analysis, CCM significantly improves NYHA class, peak oxygen consumption, and 6 min walk test distance and quality of life (as measured by MLHFQ score) in heart failure patients. The baseline characteristics of all 801 patients were similar: the most common aetiology of heart failure was ischaemic, with left ventricular ejection fraction between 25% and 45% and NYHA III class at baseline. All studies used the OPTIMIZER™ Smart system as the intervention on a background of optimal guideline‐directed medical therapy, and control groups consisted of either sham treatment (FIX‐HF‐5 pilot and FIX‐CHF‐4) or guideline‐directed medical therapy alone (FIX‐HF‐5 and FIX‐HF‐5C).
Cost increased with severity of heart failure from NYHA I class to NYHA IV class. The biggest increment of cost occurred when severity of heart failure increased from NYHA II class to NYHA III class, as reported by Shafie et al. 26 This can be explained by the increase in need for hospitalizations (including intensive care and invasive procedures) expected with increasing disease severity.
Our results show that the use of CCM in heart failure patients and NYHA III class at baseline is likely to be cost saving at the current price, in terms of healthcare costs.
Although our inputs on NYHA class were based on robust data sources such as the four FIX trials, there are also additional data on the effectiveness of CCM by real‐world evidence: the CCM‐REG, 27 a real‐world registry, showed that CCM improved functional status, quality of life, and left ventricular ejection fraction and, compared with patients' prior history, reduced heart failure hospitalization rates; survival at 1 and 3 years was significantly better than that predicted by the MAGGIC risk score.
The findings of this research provide insights for a theoretical implication supporting the idea that CCM therapy allows early and precise disease management, thus reducing the burden of heart failure on both individuals and society. From this perspective, future healthcare costs and negative outcomes, with negative consequences on health systems, could be minimized or even prevented. 28 Given the notable prevalence of people suffering from heart failure worldwide, adopting CCM along with OMT as a first‐tier therapy may have the potential to lower the cost of treating individuals with heart failure with reduced ejection fraction. Nevertheless, even though the technical feasibility of CCM therapy has been proven, 27 more research is needed to reinforce its acceptance among health professionals. 29
Taken together, the findings of the present study suggest another significant implication. A wider implementation and exploitability of CCM should be supported by an analytical assessment of the key issues of each level (i.e. macro‐level, meso‐level, and micro‐level) of the decision‐making process in health systems. Particularly, at macro‐level, policymakers should focus their attention on the assessment of CCM sustainability and its financing mechanisms and on the suggestion of a tailored diagnosis‐related group's tariff for the reimbursement of the inpatient health services related to the application of this treatment strategy. At the meso‐level, healthcare providers need to supervise CCM adoption in clinical practice. Finally, at micro‐level, health professionals should develop the necessary skills to deliver CCM therapy through dedicated education and training programmes.
Notwithstanding, it is essential to review the findings of this study in view of the possible caveats characterizing economic analyses. During the early stages of development and evaluation of a technology, it is not always possible to have long‐term comparative data. Therefore, decisions should be based on extrapolation from intermediate end points. In this case, randomized controlled trials were not designed to collect long‐term data on hospitalization, quality of life, and mortality rates associated with CCM compared with OMT. Moreover, the range of follow‐up from 3 months to 1 year in the four FIX studies was very limited for the heart failure clinical setting. However, the available intermediate end points observed in the trials were used to predict long‐term outcomes. In this regard, the model health states were based on the reported NYHA classes, which are subjective assessments of heart failure severity, leading to possible uncertainty on transitions probabilities. Nonetheless, the NYHA classification is widely endorsed by the European Society of Cardiology in selecting appropriate treatments in heart failure patients. 1
Another limitation is the inclusion of only direct costs, thus excluding potential indirect costs (e.g. productivity loss costs) and out‐of‐pocket costs. Nonetheless, the choice reflects the specific perspective taken (i.e. Italian National Health System) and the assumption that costs associated with productivity loss would be limited, given the high average age of patients with heart failure at baseline. Furthermore, we acknowledge that Markov models have limitations regarding the predetermined number of health states and a specific cycle length, rather than modelling health states and time continuously. Nevertheless, the choice of health states and cycle length was based on clinical information and consistent with other economic analysis models used to evaluate the cost‐effectiveness of diseases with defined health states. An additional limitation was the significant uncertainty on cost and utility parameters associated with the NYHA IV class. However, net of the robustness of the value of information analysis, uncertain parameters may be due to the limited follow‐up time and clinical characteristics of eligible patients in higher NYHA classes.
Currently, modelling was the most suitable method for evaluating cost‐effectiveness given the lack of sufficient long‐term data. Therefore, further long‐term, post‐approval clinical studies, with newest generation CCM technologies, are needed to verify and confirm these results in a real‐world context, particularly concerning the effect of CCM therapy on mortality, not only in heart failure with reduced ejection fraction but also in heart failure with preserved ejection fraction.
Conclusions
This study adds to a growing body of evidence that addresses the important issue of CCM implementation in heart failure management. For patients with heart failure with reduced ejection fraction, the addition of CCM therapy could be cost‐effective when compared with OMT alone, when taking a lifetime horizon. Nonetheless, any decision to implement CCM strategy must be balanced with considerations regarding the feasibility, opportunity cost, and budget impact of its introduction and reimbursement within National Health Systems.
Conflict of interest
M.L.N. worked as a scientific consultant of Value in Health Technology and Academy for Leadership & Innovation (VIHTALI), Spin‐Off of Università Cattolica del Sacro Cuore (Rome, Italy), and received educational grants from Biotronik. M.C.N., N.A., G.E.C., M.M., and G.D. worked as scientific consultants of VIHTALI, Spin‐Off of Università Cattolica del Sacro Cuore (Rome, Italy). All other authors have nothing to disclose. The sponsor had no role in conducting or designing the study, analysing, interpreting the data, and writing the manuscript.
Funding
This paper reports the results of a project of Value in Health Technology and Academy for Leadership & Innovation (VIHTALI), Spin‐Off of Università Cattolica del Sacro Cuore, Rome, Italy. The project was funded by Impulse Dynamics.
Supporting information
Figure S1. Cost‐effectiveness Acceptability Curve.
Figure S2. Cost‐effectiveness Acceptability Frontier Curve.
Figure S3. EVPPI in individual parameters.
Narducci, M. L. , Nurchis, M. C. , Ballacci, F. , Giordano, F. , Calabrò, G. E. , Massetti, M. , Crea, F. , Aspromonte, N. , and Damiani, G. (2024) Cost–utility of cardiac contractility modulation in patients with heart failure with reduced ejection fraction in Italy. ESC Heart Failure, 11: 229–239. 10.1002/ehf2.14538.
References
- 1. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021;42:3599‐3726. doi: 10.1093/eurheartj/ehab368 [DOI] [PubMed] [Google Scholar]
- 2. Groenewegen A, Rutten FH, Mosterd A, Hoes AW. Epidemiology of heart failure. Eur J Heart Fail 2020;22:1342‐1356. doi: 10.1002/ejhf.1858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Conrad N, Judge A, Tran J, Mohseni H, Hedgecott D, Crespillo AP, et al. Temporal trends and patterns in heart failure incidence: A population‐based study of 4 million individuals. Lancet 2018;391:572‐580. doi: 10.1016/S0140-6736(17)32520-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cappannoli L, Scacciavillani R, Rocco E, Perna F, Narducci ML, Vaccarella M, et al. Cardiac contractility modulation for patient with refractory heart failure: An updated evidence‐based review. Heart Fail Rev 2021;26:227‐235. doi: 10.1007/s10741-020-10030-4 [DOI] [PubMed] [Google Scholar]
- 5. Campbell CM, Kahwash R, Abraham WT. Optimizer Smart in the treatment of moderate‐to‐severe chronic heart failure. Future Cardiol 2020;16:13‐25. doi: 10.2217/fca-2019-0044 [DOI] [PubMed] [Google Scholar]
- 6. Borggrefe M, Burkhoff D. Clinical effects of cardiac contractility modulation (CCM) as a treatment for chronic heart failure. Eur J Heart Fail 2012;14:703‐712. doi: 10.1093/eurjhf/hfs078 [DOI] [PubMed] [Google Scholar]
- 7. Butter C, Wellnhofer E, Schlegl M, Winbeck G, Fleck E, Sabbah HN. Enhanced inotropic state of the failing left ventricle by cardiac contractility modulation electrical signals is not associated with increased myocardial oxygen consumption. J Card Fail 2007;13:137‐142. doi: 10.1016/j.cardfail.2006.11.004 [DOI] [PubMed] [Google Scholar]
- 8. Butter C, Rastogi S, Minden H‐H, Meyhöfer J, Burkhoff D, Sabbah HN. Cardiac contractility modulation electrical signals improve myocardial gene expression in patients with heart failure. J Am Coll Cardiol 2008;51:1784‐1789. doi: 10.1016/j.jacc.2008.01.036 [DOI] [PubMed] [Google Scholar]
- 9. Neelagaru SB, Sanchez JE, Lau SK, Greenberg SM, Raval NY, Worley S, et al. Nonexcitatory, cardiac contractility modulation electrical impulses: Feasibility study for advanced heart failure in patients with normal QRS duration. Heart Rhythm 2006;3:1140‐1147. doi: 10.1016/j.hrthm.2006.06.031 [DOI] [PubMed] [Google Scholar]
- 10. Borggrefe MM, Lawo T, Butter C, Schmidinger H, Lunati M, Pieske B, et al. Randomized, double blind study of non‐excitatory, cardiac contractility modulation electrical impulses for symptomatic heart failure. Eur Heart J 2008;29:1019‐1028. doi: 10.1093/eurheartj/ehn020 [DOI] [PubMed] [Google Scholar]
- 11. Abraham WT, Kuck K‐H, Goldsmith RL, Lindenfeld J, Reddy VY, Carson PE, et al. A randomized controlled trial to evaluate the safety and efficacy of cardiac contractility modulation. JACC Heart Fail 2018;6:874‐883. doi: 10.1016/j.jchf.2018.04.010 [DOI] [PubMed] [Google Scholar]
- 12. Wiegn P, Chan R, Jost C, Saville BR, Parise H, Prutchi D, et al. Safety, performance, and efficacy of cardiac contractility modulation delivered by the 2‐lead Optimizer Smart system: The FIX‐HF‐5C2 study. Circ Heart Fail 2020;13:e006512. doi: 10.1161/CIRCHEARTFAILURE.119.006512 [DOI] [PubMed] [Google Scholar]
- 13. Kadish A, Nademanee K, Volosin K, Krueger S, Neelagaru S, Raval N, et al. A randomized controlled trial evaluating the safety and efficacy of cardiac contractility modulation in advanced heart failure. Am Heart J 2011;161:329‐337.e1‐2. doi: 10.1016/j.ahj.2010.10.025 [DOI] [PubMed] [Google Scholar]
- 14. Giallauria F, Cuomo G, Parlato A, Raval NY, Kuschyk J, Stewart Coats AJ. A comprehensive individual patient data meta‐analysis of the effects of cardiac contractility modulation on functional capacity and heart failure‐related quality of life. ESC Heart Fail 2020;7:2922‐2932. doi: 10.1002/ehf2.12902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Maniadakis N, Fragoulakis V, Mylonas C, Sharma R, Stewart CA. Economic evaluation of cardiac contractility modulation (CCM) therapy with the OPTIMIZER IVs in the management of heart failure patients. Int Cardiovasc Forum J 2015;4:43. doi: 10.17987/icfj.v4i0.173 [DOI] [Google Scholar]
- 16. Witte K, Hasenfuss G, Kloppe A, Burkhoff D, Green M, Moss J, et al. Cost‐effectiveness of a cardiac contractility modulation device in heart failure with normal QRS duration. ESC Heart Fail 2019;6:1178‐1187. doi: 10.1002/ehf2.12526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ho KK, Anderson KM, Kannel WB, Grossman W, Levy D. Survival after the onset of congestive heart failure in Framingham Heart Study subjects. Circulation 1993;88:107‐115. doi: 10.1161/01.CIR.88.1.107 [DOI] [PubMed] [Google Scholar]
- 18. Rognoni C, Gerzeli S. Ferric carboxymaltose for patients with heart failure and iron deficiency in Italy: Cost‐effectiveness and budget impact. J Comp Eff Res 2019;8:1099‐1110. doi: 10.2217/cer-2019-0074 [DOI] [PubMed] [Google Scholar]
- 19. Organisation for Economic Co‐operation and Development . Consumer price indices (CPIs) 2023. https://stats.oecd.org/index.aspx?DataSetCode=PRICES_CPI. Accessed 30 December 2022
- 20. Kularatna S, Senanayake S, Chen G, Parsonage W. Mapping the Minnesota Living with Heart Failure Questionnaire (MLHFQ) to EQ‐5D‐5L in patients with heart failure. Health Qual Life Outcomes 2020;18:115. doi: 10.1186/s12955-020-01368-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Whitehead SJ, Ali S. Health outcomes in economic evaluation: The QALY and utilities. Br Med Bull 2010;96:5‐21. doi: 10.1093/bmb/ldq033 [DOI] [PubMed] [Google Scholar]
- 22. Barton GR, Briggs AH, Fenwick EAL. Optimal cost‐effectiveness decisions: The role of the cost‐effectiveness acceptability curve (CEAC), the cost‐effectiveness acceptability frontier (CEAF), and the expected value of perfection information (EVPI). Value Health 2008;11:886‐897. doi: 10.1111/j.1524-4733.2008.00358.x [DOI] [PubMed] [Google Scholar]
- 23. Briggs A, Claxton K, Sculpher M. Decision Modelling for Health Economic Evaluation. Oxford University Press; 2006. [Google Scholar]
- 24. Husereau D, Drummond M, Augustovski F, de Bekker‐Grob E, Briggs AH, Carswell C, et al. Consolidated Health Economic Evaluation Reporting Standards 2022 (CHEERS 2022) statement: Updated reporting guidance for health economic evaluations. BMJ 2022:e067975. doi: 10.1136/bmj-2021-067975 [DOI] [PubMed] [Google Scholar]
- 25. National Institute for Health and Care Excellence . Implantable cardioverter defibrillators and cardiac resynchronisation therapy for arrhythmias and heart failure. 2014.
- 26. Shafie AA, Tan YP, Ng CH. Systematic review of economic burden of heart failure. Heart Fail Rev 2018;23:131‐145. doi: 10.1007/s10741-017-9661-0 [DOI] [PubMed] [Google Scholar]
- 27. Kuschyk J, Falk P, Demming T, Marx O, Morley D, Rao I, et al. Long‐term clinical experience with cardiac contractility modulation therapy delivered by the Optimizer Smart system. Eur J Heart Fail 2021;23:1160‐1169. doi: 10.1002/ejhf.2202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Givertz MM, Yang M, Hess GP, Zhao B, Rai A, Butler J. Resource utilization and costs among patients with heart failure with reduced ejection fraction following a worsening heart failure event. ESC Heart Fail 2021;8:1915‐1923. doi: 10.1002/ehf2.13155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Burkhoff D. Does contractility modulation have a role in the treatment of heart failure? Curr Heart Fail Rep 2011;8:260‐265. doi: 10.1007/s11897-011-0067-3 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Cost‐effectiveness Acceptability Curve.
Figure S2. Cost‐effectiveness Acceptability Frontier Curve.
Figure S3. EVPPI in individual parameters.


