Abstract
Aim
Acyl ghrelin increases cardiac output (CO) in heart failure with reduced ejection fraction (HFrEF). This could impair the right ventricular‐pulmonary arterial coupling (RVPAC), both through an increased venous return and right ventricular afterload. We aim to investigate if acyl ghrelin increases CO with or without worsening the right‐sided haemodynamics in HFrEF assessed by RVPAC.
Methods and results
The Karolinska Acyl ghrelin Trial was a randomized double‐blind placebo‐controlled trial of acyl ghrelin versus placebo (120‐min intravenous infusion) in HFrEF. RVPAC was assessed echocardiographically at baseline and 120 min. ANOVA was used for difference in change between acyl ghrelin versus placebo, adjusted for baseline values. Of the 30 randomized patients, 22 had available RVPAC (acyl ghrelin n = 12, placebo n = 10). Despite a 15% increase in CO in the acyl ghrelin group (from 4.0 (3.5–4.6) to 4.6 (3.9–6.1) L/min, P = 0.003), RVPAC remained unchanged; 5.9 (5.3–7.6) to 6.3 (4.8–7.5) mm·(m/s)−1, P = 0.372, while RVPAC was reduced in the placebo group, 5.2 (4.3–6.4) to 4.8 (4.2–5.8) mm·(m/s)−1, P = 0.035. Comparing change between groups, CO increased in the acyl ghrelin group versus placebo (P = 0.036) while RVPAC and the right ventricular pressure gradient remained unchanged.
Conclusion
Treatment with acyl ghrelin increases CO while preserving or even improving RVPAC in HFrEF, possibly due to increased contractility, reduced PVR and/or reduced left sided filling pressures. These potential effects strengthen the role of acyl ghrelin therapy in HFrEF with right ventricular failure.
Keywords: Acyl ghrelin, Heart failure, Inotrope
Background
Ghrelin is an endogenous appetite‐stimulating peptide hormone with cardiovascular actions, 1 , 2 , 3 , 4 which increases cardiac output (CO) in heart failure with reduced ejection fraction (HFrEF). 5 Apart from inotropic effects, animal data suggest that exogenous ghrelin administration has beneficial effects in the pulmonary vasculature. 6 , 7
Pulmonary hypertension (PH) and right ventricular (RV) failure are common among patients with HFrEF. They are associated with worse prognosis. 8 The RV is afterload sensitive and increasing CO through infusion of acyl ghrelin could possibly worsen the right sided haemodynamics and right ventricular‐pulmonary arterial coupling (RVPAC), both through increased afterload and increased venous return.
Invasive assessment of RVPAC is considered golden standard. Noninvasive echocardiographic assessment of RVPAC using tricuspid annular plane systolic excursion (TAPSE)/tricuspid regurgitation velocity (TRV) has previously been validated. 9 , 10
Aim
We assessed the hypothesis that acyl ghrelin improves CO without worsening the right sided haemodynamics in HFrEF through preserved RVPAC measured echocardiographically as TAPSE/TRV.
Methods
This is a secondary analysis of the Karolinska Acyl Ghrelin Trial (ClinicalTrials.gov NCT05277415), which was a double‐blind placebo‐controlled trial of acyl ghrelin 30 pmol/kg/min versus placebo 0.5 mL/min (NaCl 9 mg/mL) intravenously during 120 min in HFrEF. 5 Patients were ambulatory, had chronic HFrEF in New York Heart Association (NYHA) class III or ambulatory class IV, and a left ventricular ejection fraction (LVEF) ≤ 40%, as well as optimal guideline‐directed medical therapy at time of enrolment. Detailed inclusion and exclusion criteria were presented previously. 5 Echocardiography exams at baseline and 120 min were assessed for RV parameters. Patients were included if both TAPSE and TRV could be assessed at both time‐points. Of 30 patients available, 22 were included (n = 12 acyl ghrelin + 10 placebo).
Non‐invasive resting CO was assessed in duplicate at each measurement time point using a validated inert gas rebreathing method with the Innocor® device (Innovision, Odense, Denmark), as previously described in the main study. 5 LVEF was measured using the Teichholz method. An experienced research echocardiographer blinded to treatment allocation and clinical history reassessed the echocardiographic images for RV parameters: TAPSE, TRV, tricuspid regurgitation (TR) gradient, right ventricular outflow tract (RVOT) proximal diameter, and RV diameter. TAPSE was assessed in the M‐mode (mm), measured between end‐diastole, and peak systole. TRV was assessed using the continuous‐wave Doppler signal of tricuspid regurgitation. All other measurements were according to current guidelines. 11
Continuous variables were reported as median (interquartile range [IQR]). Categorical variables were reported as numbers (n, %). Changes within groups (acyl ghrelin and placebo) at baseline versus 120 min were analysed by Wilcoxon rank sign test. Analysis of variance was used to test for difference in change acyl ghrelin versus placebo, adjusted for baseline values. R 4.0.5 was used for all statistics.
This trial was approved by the ethics committee. For physiological studies of an endogenous peptide, this committee waived approval from medical products agency (MPA) requirement. All patients provided signed informed consent.
Results
Baseline characteristics were similar between acyl ghrelin versus placebo [median (IQR)]: age 71 (65–77) versus 74 (72–77) years, 8 (80%) versus 11 (92%) male. LVEF was 29 (20–35) versus 18 (13–36) %, and N‐terminal pro Brain natriuretic peptide (NT‐proBNP) levels were 2645 (1105–4160) versus 2495 (1078–4555) (Table 1 ).
Table 1.
Baseline characteristics
| Placebo (N = 10) | Ghrelin (N = 12) | P‐value | |
|---|---|---|---|
| Sex (male) | 8 (80) | 11 (92) | 0.571 |
| Age (years) | 74 [72–76] | 71 [66–77] | 0.276 |
| Co‐morbidities | |||
| Ischaemic heart disease | 8 (80) | 9 (75) | 1.000 |
| Atrial fibrillation | 6 (60) | 10 (83) | 0.135 |
| Diabetes mellitus | 4 (40) | 6 (50) | 0.691 |
| Chronic kidney disease | 2 (20) | 3 (25) | 1.000 |
| Hypertension | 6 (60) | 10 (83) | 0.348 |
| Pulmonary disease | 1 (10) | 3 (25) | 0.594 |
| Hyperlipidaemia | 8 (80) | 9 (75) | 1.000 |
| Laboratory | |||
| eGFR (mL/min/1.73 m2) | 59.3 [45.7–64.7] | 60.1 [55.7–69.0] | 0.582 |
| Haemoglobin (g/L) | 136 [125–145] | 129 [125–147] | 0.869 |
| NT‐proBNP (pg/mL) | 2495 [1078–4555] | 2645 [1105–4160] | 0.872 |
| Treatments | |||
| ACEi/ARB | 10 (100) | 12 (100) | 1.000 |
| Beta‐blocker | 10 (100) | 12 (100) | 1.000 |
| MRA | 9 (90) | 10 (83) | 1.000 |
| Loop diuretic | 10 (100) | 11 (92) | 1.000 |
Categorical variables are presented as number (n) and percentage (%) and continuous variables as median and upper and lower quartiles (Q1;Q3). P‐value for Fishers exact test in categorical and Mann–Whitney U in continuous variables. eGFR was calculated by the CKD‐EPI method; chronic kidney disease is defined as eGFR < 60 mL/min/1.73 m2.
ACEi, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; eGFR, estimated glomerular filtration rate; MRA, mineralocorticoid receptor antagonist; NT‐proBNP, N‐terminal pro Brain natriuretic peptide.
At baseline, right‐sided parameters indicated impaired RV function both by a low TAPSE and enlarged RV. In addition, increased TR gradient indicated borderline or manifest pulmonary hypertension. Structural and functional parameters of the RV were similar in both study groups (Table 2 ).
Table 2.
Haemodynamics at baseline and after 120 min infusion of acyl ghrelin/placebo
|
Baseline:acyl ghrelin (N = 12) |
120 min: acyl ghrelin (N = 12) |
P‐value |
Baseline: placebo (N = 10) |
120 min: placebo (N = 10) |
P‐value | |
|---|---|---|---|---|---|---|
| Heart rate (b.p.m.) | 70 [65–79] | 69 [60–72] | 0.264 | 70 [70–70] | 71 [63–81] | 0.325 |
| Saturation (%) | 95 [94–97] | 94 [92–95] | 0.002 | 96 [95–97] | 96 [95–97] | 0.439 |
| Systolic BP (mmHg) | 103 [97–117] | 99 [91–104] | 0.327 | 131[110–144] | 123 [111–128] | 0.109 |
| LVEF (%) | 28.5 [20.0–34.3] | 29 [22.0–41.5] | 0.285 | 18.0 [13.5–32.6] | 17.0 [16.0–31.8] | 0.284 |
| RV basal DM (mm) | 44.5 [40.0–49.5] | 42.0 [40.3–46.5] | 1.000 | 42.0 [38.0–46.0] | 43.0 [36.8–44.0] | 1.000 |
| RVOT prox DM (mm) | 40.5 [34.8–43.3] | 39.0 [32.5–43.0] | 0.472 | 37.0 [34.0–42.0] | 37.0 [35.8–38.0] | 0.671 |
| TR gradient (mmHg) | 29.0 [23.0–40.0] | 32.0 [24.8–38.3] | 0.529 | 29.5 [27.3–34.8] | 29.0 [27.5–35.3] | 0.944 |
| TAPSE (mm) | 18.0 [14.8–20.5] | 17.5 [13.8–19.3] | 0.188 | 14.5 [13.0–17.5] | 13.5 [11.3–15.0] | 0.028 |
Wilcoxon rank sign in statistical analyses.
BP, blood pressure; CO, cardiac output; HR, heart rate; LVEF, left ventricular ejection fraction; RV basal DM, right ventricular basal diameter; RVOT prox DM, right ventricular outflow tract proximal diameter; RVPAC, right ventricular pulmonary arterial coupling; TAPSE, tricuspid annular plane systolic excursion; TRV, tricuspid regurgitation velocity, TR, tricuspid regurgitation gradient.
Figure 1 shows changes during 120 min of acyl ghrelin infusion. CO increased by 15% in the acyl ghrelin group [from 4.0 (3.5–4.6) to 4.6 (3.9–6.1) L/min, P = 0.003]. Despite this, RVPAC remained unchanged: 5.9 (5.3–7.6) to 6.3 (4.8–7.5) mm·(m/s−1), P = 0.372, whereas it was reduced in the placebo group, 5.2 (4.3–6.4) to 4.8 (4.2–5.8) mm·(m/s−1), P = 0.035. Similarly, TAPSE remained unchanged in the acyl ghrelin group 18.0 (14.8–20.5) to 17.5 (13.8–19.3), P = 0.188, while decreased in the placebo group 14.5 (13.0–17.5) to 13.5 (11.3–15.0), P = 0.028 (Table 2 ). In both groups, TRV, RVOT proximal diameter, and RV diameter remained unchanged from baseline to 120 min infusion.
Figure 1.
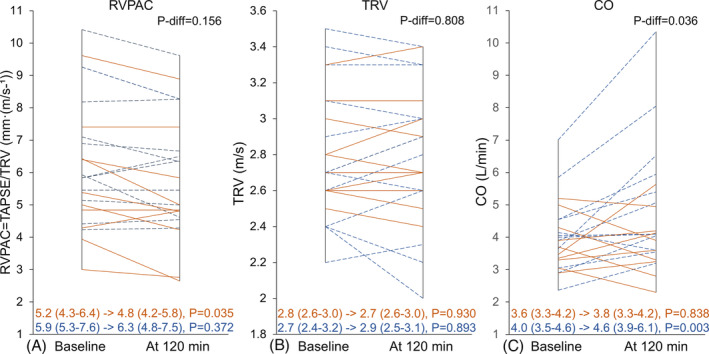
RVPAC, TRV, and CO following 120 min infusion acyl ghrelin/placebo. Ghrelin (BLUE ‘dashed’)/Placebo (RED ‘solid’). Median (IQR), P‐change within group. P‐diff = difference in change between groups. CO, cardiac output; RVPAC, right ventricular pulmonary‐arterial coupling; TAPSE, tricuspid annular plane systolic excursion; TRV, tricuspid regurgitation velocity.
When comparing changes between groups adjusted for baseline, CO increased in the acyl ghrelin group versus placebo (P = 0.036), whereas there were no differences in change in RVPAC, TAPSE, or TRV.
Conclusions
These findings indicate that acyl ghrelin may preserve RVPAC without increasing the pressure gradient. This may be due to improved contractility, reduced PVR and/or reduced left sided filling pressures.
Animal data suggest that exogenous ghrelin administration has beneficial effects in the pulmonary vasculature. Ghrelin improves endothelial cell function, 6 prevents endothelin‐1 mediated vasoconstriction and reduces pulmonary vascular remodelling and RV hypertrophy associated with chronic hypoxia. 7 In our study, patients suffered from impaired RV function and low RVPAC, likely due to left ventricular failure. 12 Increasing CO may worsen RVPAC due to increasing flow and thereby possibly increasing the pressure gradient. However, in the present study, acyl ghrelin did not worsen RVPAC despite increased CO. Possible explanations, apart from increased RV contractility, are a reduced PVR and/or left sided filling pressures. This is based on the physiological principles that increased flow with a preserved pressure gradient requires a reduction in PVR and/or left sided filling pressures.
Notably, RVPAC decreased in the placebo group. This is likely explained by prolonged recumbent state during infusion. Higher venous return in supine position may increase pulmonary arterial pressure. 13 Similarly, TAPSE decreased in the placebo group, possibly explained by recumbent haemodynamics and lower demands at rest. In contrast, RVPAC and TAPSE remained unchanged in the acyl ghrelin group, potentially suggesting that acyl ghrelin counteracts haemodynamic effects of the recumbent state as well as potential increase in the pressure gradient due to higher CO.
This secondary study has several limitations. Data from right heart catheterization was not available and instead RVPAC was estimated echocardiographically by the ratio TAPSE/TRV. Although this method has been validated, 9 interobservability within measurements is a limitation. Lower TAPSE levels in the placebo group could explain the reduction in TAPSE and RVPAC. However, it is unlikely the only explanation given that RVPAC increased, albeit not statistically significant, in the acyl ghrelin group. Sample size was low and as many as 25% of the patients in the original study were excluded due to poor quality of right sided echocardiography images. Nevertheless, these findings strengthen the role of acyl ghrelin therapy in HFrEF 5 by indicating its safety in RV failure in HFrEF. The study is exploratory and the role of acyl ghrelin in RV failure or PH needs to be further investigated.
Funding
Funded by grants to L. H. L. from The Swedish Research Council (grant 523‐2014‐2336), the Swedish Heart‐Lung Foundation (grants 20150557 and 20190310), Karolinska Institutet (grant 2‐70/2014) and Stockholm County Council (grant 20140220). C. H. was supported by the Swedish Research Council (grant 20180899).
Conflict of interest
Lars H Lund: Grants: AstraZeneca, Vifor, Boston Scientific, Boehringer Ingelheim, Novartis. Consulting: Merck, Vifor, AstraZeneca, Bayer, Pharmacosmos, MedScape, Sanofi, Lexicon, Myokardia, Boehringer Ingelheim, Servier. Speaker's honoraria: Abbott, MedScape, Radcliffe, AstraZeneca, Novartis. Stock ownership and founder: AnaCardio (a start‐up company dedicated to developing Acyl ghrelin analogues for the treatment of heart failure). Camilla Hage: consulting fees from Novartis, Roche Diagnostics and AnaCardio, research grants from Bayer and speaker's honoraria from MSD and Novartis. Marcus Ståhlberg: consulting fees, AnaCardio, Impulse dynamics, Swedish agency for health technology assessment and assessment of social services; Speaker's honoraria: Medtronic, Orion Pharma, ALK‐Nordic, Werfen. Ulrika Ljung‐Faxen: consulting fees AnaCardio, lecture fees Orion Pharma. Per Hellström: consulting fees Phamranovia, RenaPharma, Milltons. Tonje Thorvaldsen: lecture fees Orion Pharma, Boehringer Ingelheim, Abbott. Remaining authors Mikael Erhardsson, Ashwin Venkateshvaran, Gianluigi Pironti, Dominic‐Luc Webb, Per M. Hellström, and Daniel C. Andersson report no conflicts of interest.
Erhardsson, M. , Faxén, U. L. , Venkateshvaran, A. , Hage, C. , Pironti, G. , Thorvaldsen, T. , Webb, D.‐L. , Hellström, P. M. , Andersson, D. C. , Ståhlberg, M. , and Lund, L. H. (2024) Acyl ghrelin increases cardiac output while preserving right ventricular‐pulmonary arterial coupling in heart failure. ESC Heart Failure, 11: 601–605. 10.1002/ehf2.14580.
References
- 1. Nagaya N, Kangawa K. Ghrelin improves left ventricular dysfunction and cardiac cachexia in heart failure. Curr Opin Pharmacol 2003;3:146–151. doi: 10.1016/S1471-4892(03)00013-4 [DOI] [PubMed] [Google Scholar]
- 2. Nagaya N, Kangawa K. Therapeutic potential of ghrelin in the treatment of heart failure. Drugs 2006;66:439–448. doi: 10.2165/00003495-200666040-00004 [DOI] [PubMed] [Google Scholar]
- 3. Nagaya N, Kangawa K. Ghrelin, a novel growth hormone‐releasing peptide, in the treatment of chronic heart failure. Regul Pept 2003;114:71–77. doi: 10.1016/s0167-0115(03)00117-4 [DOI] [PubMed] [Google Scholar]
- 4. Nagaya N, Miyatake K, Uematsu M, Oya H, Shimizu W, Hosoda H, et al. Hemodynamic, renal, and hormonal effects of ghrelin infusion in patients with chronic heart failure. J Clin Endocrinol Metab 2001;86:5854–5859. doi: 10.1210/jcem.86.12.8115 [DOI] [PubMed] [Google Scholar]
- 5. Lund LH, Hage C, Pironti G, Thorvaldsen T, Ljung‐Faxen U, Zabarovskaja S, et al. Acyl ghrelin improves cardiac function in heart failure and increases fractional shortening in cardiomyocytes without calcium mobilization. Eur Heart J 2023;44:2009–2025. doi: 10.1093/eurheartj/ehad100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang Y, Narsinh K, Zhao L, Sun D, Wang D, Zhang Z, et al. Effects and mechanisms of ghrelin on cardiac microvascular endothelial cells in rats. Cell Biol Int 2011;35:135–140. doi: 10.1042/CBI20100139 [DOI] [PubMed] [Google Scholar]
- 7. Schwenke DO, Gray EA, Pearson JT, Sonobe T, Ishibashi‐Ueda H, Campillo I, et al. Exogenous ghrelin improves blood flow distribution in pulmonary hypertension‐assessed using synchrotron radiation microangiography. Pflugers Arch 2011;462:397–406. doi: 10.1007/s00424-011-0992-8 [DOI] [PubMed] [Google Scholar]
- 8. Miller WL, Grill DE, Borlaug BA. Clinical features, hemodynamics, and outcomes of pulmonary hypertension due to chronic heart failure with reduced ejection fraction: Pulmonary hypertension and heart failure. JACC Heart failure 2013;1:290–299. doi: 10.1016/j.jchf.2013.05.001 [DOI] [PubMed] [Google Scholar]
- 9. Pestelli G, Fiorencis A, Trevisan F, Luisi GA, Smarrazzo V, Mele D. New measures of right ventricle‐pulmonary artery coupling in heart failure: An all‐cause mortality echocardiographic study. Int J Cardiol 2021;329:234–241. doi: 10.1016/j.ijcard.2020.12.057 [DOI] [PubMed] [Google Scholar]
- 10. Vicenzi M, Caravita S, Rota I, Casella R, Deboeck G, Beretta L, et al. The added value of right ventricular function normalized for afterload to improve risk stratification of patients with pulmonary arterial hypertension. PLoS ONE 2022;17:e0265059. doi: 10.1371/journal.pone.0265059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2015;16:233–270. doi: 10.1093/ehjci/jev014 [DOI] [PubMed] [Google Scholar]
- 12. Reddy YNV, Obokata M, Wiley B, Koepp KE, Jorgenson CC, Egbe A, et al. The haemodynamic basis of lung congestion during exercise in heart failure with preserved ejection fraction. Eur Heart J 2019;40:3721–3730. doi: 10.1093/eurheartj/ehz713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Berlier C, Saxer S, Lichtblau M, Schneider SR, Schwarz EI, Furian M, et al. Influence of upright versus supine position on resting and exercise hemodynamics in patients assessed for pulmonary hypertension. J Am Heart Assoc 2022;11:e023839. doi: 10.1161/JAHA.121.023839 [DOI] [PMC free article] [PubMed] [Google Scholar]


