Abstract
Aims
Valvular heart disease (VHD) is one of the leading causes of heart failure. Clinically significant VHD can induce different patterns of cardiac remodelling, and risk stratification is challenging in patients with various degrees of cardiac dysfunction. The study aimed to investigate the prognostic implications of Meta‐Analysis Global Group in Chronic Heart Failure (MAGGIC) score in patients with VHD.
Methods and results
This study used data from the China Valvular Heart Disease (China‐VHD) registry, which was a multicentre, prospective, observational cohort study for patients with significant (at least moderate) VHD. In total, 10 446 patients with moderate or greater VHD from the China‐VHD study were included in the present analysis. The primary outcome of interest was all‐cause mortality within 2 years. Among 10 446 patients with VHD, the mean age was 61.98 ± 13.47 years, and 5819 (55.7%) were male. During 2 years of follow‐up, 895 (8.6%) patients died. The MAGGIC score was monotonically and independently associated with mortality in both total cohort [adjusted hazard ratio: 1.095, 95% confidence interval (CI): 1.084–1.107, P < 0.001] and most types of VHD (aortic regurgitation, mitral stenosis, mitral regurgitation, tricuspid regurgitation, mixed aortic stenosis and aortic regurgitation, and multiple VHD). The score was also an independent prognostic factor in patients with or without symptoms or preserved left ventricular ejection fraction (LVEF) and exhibited both satisfactory discrimination and calibration properties in predicting mortality. The prognostic value of MAGGIC score was robust in most quartiles of N‐terminal pro‐brain natriuretic peptide level, with no significant interaction observed (P interaction = 0.498). Compared with the EuroSCORE II, the MAGGIC score achieved significantly better predictive performance in overall population [C index: 0.769 vs. 0.727; net reclassification improvement index (95% CI): 0.354 (0.313–0.396), P < 0.001; integrated discrimination improvement index (95% CI): 0.069 (0.052–0.085), P < 0.001] and in subgroups of patients divided by therapeutic strategy, LVEF, symptomatic status, stage of VHD, and aetiology of VHD.
Conclusions
The MAGGIC score is a reliable prognostic factor across the range of cardiac dysfunction in VHD and may assist in risk stratification and guide clinical decision‐making.
Keywords: Meta‐Analysis Global Group in Chronic Heart Failure score, Valvular heart disease, Heart failure, Mortality
Introduction
There is an increasing burden of valvular heart disease (VHD) with the aging population. 1 , 2 In patients with significant VHD, valvular lesions can cause or exacerbate cardiac remodelling and dysfunction, resulting in clinical heart failure (HF) eventually if left untreated. The emergence of HF symptoms and imaging parameters of cardiac geometry and function are crucial components in the mainstream decision‐making framework for the management of significant VHD. 3 , 4 However, VHD can induce diverse patterns of cardiac remodelling, 5 , 6 and VHD patients with various degrees of cardiac dysfunction can be a heterogeneous population with different risk profiles, which challenges clinical decision‐making. The existing risk stratification tools are largely confined to evaluate operative outcomes in patients requiring cardiac intervention, 7 , 8 with few models focusing on discriminating risk among patients with various levels of cardiac dysfunction, which is important to tailor management strategies for patients with VHD.
The Meta‐Analysis Global Group in Chronic Heart Failure (MAGGIC) score, which included 13 routinely available predictors, was previously developed to quantify mortality risk of HF patients within 3 years in a huge database integrating 30 cohort studies. 9 The score was validated in HF patients with reduced or preserved left ventricular ejection fraction (LVEF) 10 , 11 , 12 and was recommended to estimate mortality risk in current guidelines for the management of HF. 13 There is scarce evidence on the implications of MAGGIC score in patients with VHD. Two studies suggested that MAGGIC score might be useful in patients mainly undergoing aortic valve intervention and appeared to outperform existing surgical risk stratification tools. 14 , 15 Nevertheless, the small series of the study cohorts were far from enough to support the generalization of MAGGIC score in VHD. The role of MAGGIC score is not established in most subtypes of VHD, and its prognostic value in patients with VHD and HF is unknown.
The main purposes of the present study were to investigate the prognostic performance of MAGGIC score in a large cohort of VHD and to determine its implications in patients with different degrees of cardiac dysfunction.
Methods
Study population
The present analysis was based on data from the China Valvular Heart Disease (China‐VHD; NCT03484806) study, which was a nationwide, multicentre, prospective, observational study for adult patients (≥18 years) with significant (≥moderate) VHD. 16 Patients with at least moderate VHD, as identified by echocardiography, were enrolled consecutively from inpatient wards and outpatient clinics at 46 medical centres in China. Details of the China‐VHD study have been described elsewhere. 16 The investigation conforms with the principles outlined in the Declaration of Helsinki and was approved by the Institutional Review Board at Fuwai Hospital, National Center for Cardiovascular Diseases of China (Approval No. 2017‐968). Written informed consent was given by all patients before registration.
From April to June 2018, 13 917 patients with various VHDs were included in the China‐VHD study. Patients with moderate or greater tricuspid stenosis (n = 6), pulmonary stenosis (n = 50), or pulmonary regurgitation (n = 154) were excluded due to the limited numbers of patients with these VHDs. We further excluded those with missing or invalid data on any components of MAGGIC score (Supporting Information, Table S1 ; n = 2216), those with previous valvular interventions (n = 848), those with infective endocarditis (n = 186), and patients without any follow‐up information (n = 11). Finally, the present analysis was performed in 10 446 patients with moderate or greater VHD (Figure 1 and Supporting Information, Figure S1 ).
Figure 1.
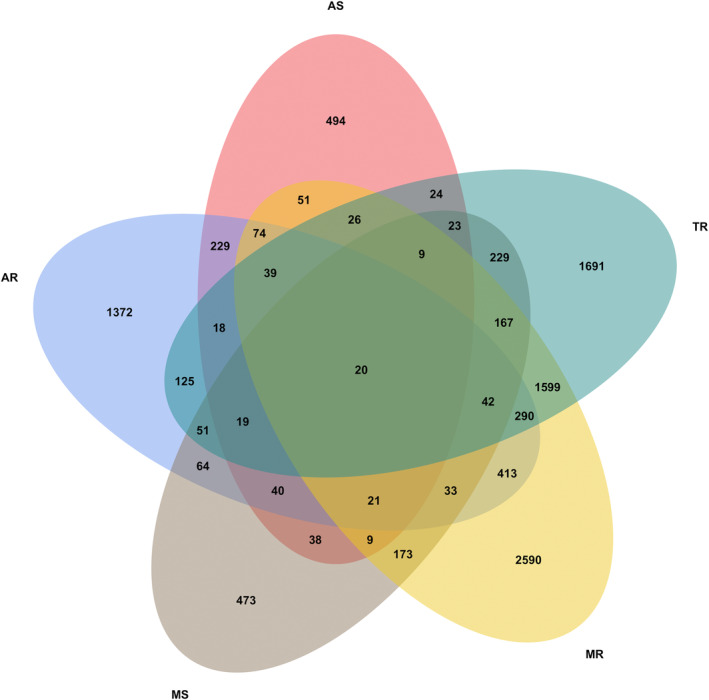
Venn diagram on the distribution of valvular heart disease. AR, aortic regurgitation; AS, aortic stenosis; MR, mitral regurgitation; MS, mitral stenosis; TR, tricuspid regurgitation.
Echocardiography
The echocardiographic measurements and quality control of the China‐VHD study have been described elsewhere. 16 In detail, all patients received comprehensive transthoracic two‐dimensional and Doppler echocardiography by standard ultrasound systems. The quantification of heart chamber was performed according to the recommendations of the American Society of Echocardiography and the European Association of Cardiovascular Imaging. 17 The biplane modified Simpson method was adopted to calculate LVEF. Standard echocardiographic definitions of different subtypes of VHD are detailed in the Supplementary Methods.
Meta‐Analysis Global Group in Chronic Heart Failure score
The MAGGIC score consisted of 13 variables including age, gender, body mass index (BMI), current smoker, diabetes, chronic obstructive pulmonary disease, HF duration (>18 or ≤18 months), systolic blood pressure (SBP), New York Heart Association (NYHA) class, creatinine, LVEF, beta‐blocker, and angiotensin‐converting enzyme inhibitor (ACE‐I)/angiotensin receptor blocker (ARB). 9 Patients were divided into six risk groups according to the scores of 0–16, 17–20, 21–24, 25–28, 29–32, and ≥33. 9 Given that the main purpose of the study was to investigate the prognostic value of MAGGIC score in patients with VHD, the time since first diagnosis of VHD was considered as one predictive component to calculate the MAGGIC score, instead of HF duration.
Follow‐up and clinical outcome
Follow‐up information of the China‐VHD study was collected by medical records, clinical visits, or telephone calls at 6 months, 12 months, 18 months, and 2 years. The outcome of interest in the present study was all‐cause mortality. In the analyses of outcome in patients under conservative treatment, patients were censored at the time of the last follow‐up or valvular intervention if performed.
Statistical analysis
Baseline characteristics were descriptively summarized by numbers (percentages) for categorical variables and using mean ± standard deviation or medians (interquartile ranges) for continuous variables. Differences among groups were compared using the Kruskal–Wallis test or the Mann–Whitney U‐test according to number of groups for continuous variables and using the χ 2 test or Fisher's exact test for categorical variables where appropriate. Spearman's correlation was applied to explore the relationship of MAGGIC score with other parameters.
The MAGGIC score was analysed both continuously and categorically by the MAGGIC‐based risk groups mentioned previously, as well as by the disease‐specific cut‐off points, which were determined by time‐dependent receiver operating characteristic (ROC) curve with the Youden index that maximized the sum of sensitivity and specificity. The cumulative survival rates were estimated using the Kaplan–Meier method and compared by log‐rank test among risk groups. To delineate the shape of the association between MAGGIC score and mortality risk, we used restricted cubic spline (RCS) with three knots at the 25th, 50th, and 75th percentiles. We also adopted unadjusted and multivariable adjusted Cox proportional hazards models to analyse the relationship of MAGGIC score with mortality, which were reported as hazard ratios (HRs) with 95% confidence interval (CI). The multivariable Cox models were adjusted for clinically relevant variables, which were not the components of the MAGGIC score, including hypertension, hyperlipidaemia, coronary artery disease, cardiomyopathy, atrial fibrillation or flutter, chronic kidney disease, haemoglobin, albumin, left atrial end‐diastolic dimension, left ventricular end‐diastolic dimension, pulmonary hypertension, severity of VHD, and valvular intervention. The proportional hazards assumptions were assessed by the Schoenfeld residual plots for continuous variables and log–log survival plots for categorical variables. Relative importance of the predictive components of MAGGIC score was ranked by the proportion of explainable log‐likelihood ratio χ 2 statistics.
We used C index and calibration curves to assess the discrimination and calibration of MAGGIC score separately, as well as to compare its predictive performance with the EuroSCORE II (n = 10 357), which was calculated without poor mobility due to lack of data, a previous risk stratification model for aortic and/or mitral heart valve surgery (n = 8755), 18 and a newly developed prognostic nomogram named the China‐VHD model (Supplementary Methods). The additive value of N‐terminal pro‐brain natriuretic peptide (NT‐proBNP) to MAGGIC score in terms of predicting mortality was also evaluated in patients with available NT‐proBNP value (n = 6600), using C index, integrated discrimination improvement index (IDI), net reclassification improvement index (NRI), likelihood ratio test, and Bayesian information criterion. Subgroup analyses were performed in patients divided by therapeutic option, LVEF, symptom status (NYHA I/II–IV), stage of VHD (B, moderate VHD; C, asymptomatic severe VHD; D, symptomatic severe VHD), aetiology of VHD, and quartiles of NT‐proBNP. Missing data and imputation approaches are provided in Supporting Information, Table S2 . A two‐tailed P < 0.05 was considered as statistical significance. All statistical analyses were conducted using R software (Version 4.2.2, R Foundation for Statistical Computing, Vienna, Austria).
Results
Baseline characteristics
Tables 1 and 2 and Supporting Information, Table S3 summarize the characteristics of the study population. Among 10 446 patients, the mean age was 61.98 ± 13.47 years, and 5819 (55.7%) were male. The majority of patients (69.6%) had NYHA ≥ II, and 3770 (36.1%) underwent valvular intervention. The mean MAGGIC scores were 17.42 ± 7.08, 16.99 ± 6.36, 14.75 ± 6.48, 14.30 ± 5.28, 17.43 ± 6.92, 17.78 ± 7.14, 16.28 ± 6.69, 14.68 ± 5.98, and 19.02 ± 7.27 in total cohort, aortic stenosis (AS), aortic regurgitation (AR), mitral stenosis (MS), mitral regurgitation (MR), tricuspid regurgitation (TR), AS + AR, MS + MR, and multiple VHD (MVHD) (Supporting Information, Figure S2 ). Correlations of MAGGIC score with other variables are shown in Supporting Information, Table S4 .
Table 1.
Baseline characteristics in patients with isolated valvular heart disease
| Variables | AS (n = 494) | AR (n = 1372) | MS (n = 473) | MR (n = 2590) | TR (n = 1691) | P value |
|---|---|---|---|---|---|---|
| Age, years | 63.34 ± 11.53 | 60.28 ± 13.72 | 55.43 ± 10.66 | 61.61 ± 12.73 | 62.95 ± 16.14 | <0.001 |
| Male | 292 (59.1) | 1025 (74.7) | 138 (29.2) | 1561 (60.3) | 806 (47.7) | <0.001 |
| BMI, kg/m2 | 23.90 ± 3.26 | 24.09 ± 3.42 | 23.23 ± 3.22 | 24.02 ± 3.58 | 23.27 ± 4.03 | <0.001 |
| Current smoker | 86 (17.4) | 277 (20.2) | 34 (7.2) | 518 (20.0) | 205 (12.1) | <0.001 |
| Hypertension | 221 (44.7) | 807 (58.8) | 102 (21.6) | 1315 (50.8) | 741 (43.8) | <0.001 |
| Hyperlipidaemia | 103 (20.9) | 215 (15.7) | 55 (11.6) | 441 (17.0) | 191 (11.3) | <0.001 |
| Diabetes | 74 (15.0) | 156 (11.4) | 32 (6.8) | 521 (20.1) | 262 (15.5) | <0.001 |
| Coronary artery disease | 158 (32.0) | 505 (36.8) | 64 (13.5) | 1229 (47.5) | 560 (33.1) | <0.001 |
| Prior MI | 23 (4.7) | 116 (8.5) | 9 (1.9) | 494 (19.1) | 149 (8.8) | <0.001 |
| Prior PCI | 32 (6.5) | 145 (10.6) | 11 (2.3) | 413 (15.9) | 156 (9.2) | <0.001 |
| Prior CABG | 5 (1.0) | 12 (0.9) | 3 (0.6) | 60 (2.3) | 39 (2.3) | 0.001 |
| Cardiomyopathy | 6 (1.2) | 42 (3.1) | 0 (0.0) | 376 (14.5) | 102 (6.0) | <0.001 |
| Atrial fibrillation or flutter | 31 (6.3) | 120 (8.7) | 215 (45.5) | 533 (20.6) | 607 (35.9) | <0.001 |
| Chronic lung disease | 30 (6.1) | 70 (5.1) | 12 (2.5) | 130 (5.0) | 177 (10.5) | <0.001 |
| Chronic kidney disease | 18 (3.6) | 52 (3.8) | 8 (1.7) | 170 (6.6) | 103 (6.1) | <0.001 |
| VHD duration > 18 months | 120 (24.3) | 208 (15.2) | 183 (38.7) | 334 (12.9) | 260 (15.4) | <0.001 |
| Systolic blood pressure, mmHg | 124.09 ± 18.00 | 133.64 ± 20.26 | 119.01 ± 16.26 | 125.61 ± 20.76 | 125.65 ± 21.59 | <0.001 |
| NYHA functional class | <0.001 | |||||
| I | 122 (24.7) | 550 (40.1) | 120 (25.4) | 781 (30.2) | 778 (46.0) | |
| II | 177 (35.8) | 514 (37.5) | 161 (34.0) | 707 (27.3) | 332 (19.6) | |
| III | 172 (34.8) | 243 (17.7) | 173 (36.6) | 787 (30.4) | 403 (23.8) | |
| IV | 23 (4.7) | 65 (4.7) | 19 (4.0) | 315 (12.2) | 178 (10.5) | |
| NT‐proBNP, pg/mL | 851.35 (280.15–2604.75) | 524.70 (140.33–1953.24) | 653.10 (308.93–1256.50) | 1533.00 (510.00–3975.00) | 1409.00 (526.10–3447.00) | <0.001 |
| Haemoglobin, g/L | 134.08 ± 17.96 | 136.34 ± 18.40 | 135.02 ± 16.88 | 132.63 ± 20.04 | 132.55 ± 24.19 | <0.001 |
| Creatinine, μmol/L | 72.29 (64.00–86.50) | 79.00 (67.40–94.00) | 73.80 (63.00–87.00) | 81.00 (68.00–99.00) | 77.00 (64.00–94.95) | <0.001 |
| Albumin, g/L | 41.38 ± 5.03 | 40.55 ± 4.70 | 41.72 ± 4.47 | 39.58 ± 5.11 | 39.00 ± 5.41 | <0.001 |
| LA, mm | 39.08 ± 6.50 | 39.51 ± 6.48 | 50.91 ± 9.41 | 46.06 ± 8.33 | 40.77 ± 8.12 | <0.001 |
| LVEDD, mm | 49.32 ± 7.32 | 58.81 ± 10.50 | 46.87 ± 5.20 | 58.10 ± 10.06 | 46.15 ± 8.54 | <0.001 |
| LVEF, % | 62.00 (56.00–66.00) | 59.00 (52.95–63.11) | 61.00 (56.00–65.00) | 52.00 (37.00–62.00) | 60.00 (53.00–64.00) | <0.001 |
| Pulmonary hypertension | 54 (10.9) | 123 (9.0) | 187 (39.5) | 745 (28.8) | 1042 (61.6) | <0.001 |
| Severe VHD | 371 (75.1) | 506 (36.9) | 263 (55.6) | 876 (33.8) | 524 (31.0) | <0.001 |
| Symptomatic severe VHD | 290 (58.7) | 376 (27.4) | 205 (43.3) | 705 (27.2) | 350 (20.7) | <0.001 |
| Valvular interventions | 347 (70.2) | 649 (47.3) | 376 (79.5) | 684 (26.4) | 170 (10.1) | <0.001 |
| Aetiology | <0.001 | |||||
| Primary | 494 (100.0) | 954 (73.5) | 473 (100.0) | 1035 (41.3) | 544 (36.7) | |
| Rheumatic | 56 (11.3) | 85 (8.9) | 449 (94.9) | 240 (23.2) | 44 (8.1) | |
| Degenerative | 277 (56.1) | 638 (66.9) | 22 (4.7) | 503 (48.6) | 291 (53.5) | |
| Congenital | 138 (27.9) | 191 (20.0) | 1 (0.2) | 101 (9.8) | 193 (35.5) | |
| Others | 23 (4.7) | 40 (4.2) | 1 (0.2) | 191 (18.5) | 16 (2.9) | |
| Secondary | 0 (0.0) | 344 (26.5) | 0 (0.0) | 1474 (58.7) | 939 (63.3) | |
| Ischaemic | 0 (0.0) | 0 (0.0) | 0 (0.0) | 560 (38.0) | 0 (0.0) | |
| Functional | 0 (0.0) | 344 (100.0) | 0 (0.0) | 914 (62.0) | 939 (100.0) | |
| Medications | ||||||
| Diuretics | 389 (78.7) | 958 (69.8) | 406 (85.8) | 1954 (75.4) | 1031 (61.0) | <0.001 |
| Beta‐blockers | 307 (62.1) | 853 (62.2) | 220 (46.5) | 1737 (67.1) | 856 (50.6) | <0.001 |
| ACE‐I/ARB | 147 (29.8) | 669 (48.8) | 101 (21.4) | 1367 (52.8) | 657 (38.9) | <0.001 |
| ARNI | 0 (0.0) | 9 (0.7) | 1 (0.2) | 53 (2.0) | 18 (1.1) | <0.001 |
| Warfarin | 307 (62.1) | 665 (48.5) | 387 (81.8) | 749 (28.9) | 373 (22.1) | <0.001 |
| Aspirin | 146 (29.6) | 514 (37.5) | 67 (14.2) | 1240 (47.9) | 600 (35.5) | <0.001 |
| P2Y12 inhibitors | 103 (20.9) | 353 (25.7) | 30 (6.3) | 909 (35.1) | 457 (27.0) | <0.001 |
| EuroSCORE II, % | 1.42 (0.92–2.46) | 1.15 (0.75–2.04) | 1.61 (1.07–2.52) | 1.61 (1.00–2.69) | 1.55 (1.01–2.71) | <0.001 |
| The risk score for heart valve surgery | 4.12 ± 2.29 | 3.78 ± 2.39 | 5.64 ± 2.35 | 5.96 ± 2.57 | — | <0.001 |
| MAGGIC score | 16.99 ± 6.36 | 14.75 ± 6.48 | 14.30 ± 5.28 | 17.43 ± 6.92 | 17.78 ± 7.14 | <0.001 |
ACE‐I, angiotensin‐converting enzyme inhibitor; AR, aortic regurgitation; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor‐neprilysin inhibitor; AS, aortic stenosis; BMI, body mass index; CABG, coronary artery bypass graft; LA, left atrial end‐diastolic dimension; LVEDD, left ventricular end‐diastolic dimension; LVEF, left ventricular ejection fraction; MAGGIC, Meta‐Analysis Global Group in Chronic Heart Failure; MI, myocardial infarction; MR, mitral regurgitation; MS, mitral stenosis; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; TR, tricuspid regurgitation; VHD, valvular heart disease.
Data are presented as mean ± standard deviation, median (interquartile range), or number (percentage). Baseline characteristics are shown without imputation of missing values.
Table 2.
Baseline characteristics in patients with mixed or multiple valvular heart disease
| Variables | AS + AR (n = 229) | MS + MR (n = 173) | MVHD (n = 3424) | P value |
|---|---|---|---|---|
| Age, years | 61.32 ± 12.53 | 56.01 ± 10.25 | 63.52 ± 12.82 | <0.001 |
| Male | 145 (63.3) | 59 (34.1) | 1793 (52.4) | <0.001 |
| BMI, kg/m2 | 23.60 ± 3.51 | 23.21 ± 4.06 | 23.26 ± 3.71 | 0.208 |
| Current smoker | 33 (14.4) | 10 (5.8) | 424 (12.4) | 0.020 |
| Hypertension | 87 (38.0) | 38 (22.0) | 1463 (42.7) | <0.001 |
| Hyperlipidaemia | 35 (15.3) | 20 (11.6) | 334 (9.8) | 0.023 |
| Diabetes | 15 (6.6) | 16 (9.2) | 503 (14.7) | 0.001 |
| Coronary artery disease | 45 (19.7) | 26 (15.0) | 1100 (32.1) | <0.001 |
| Prior MI | 7 (3.1) | 3 (1.7) | 289 (8.4) | <0.001 |
| Prior PCI | 11 (4.8) | 4 (2.3) | 284 (8.3) | 0.004 |
| Prior CABG | 0 (0.0) | 0 (0.0) | 39 (1.1) | 0.104 |
| Cardiomyopathy | 2 (0.9) | 0 (0.0) | 322 (9.4) | <0.001 |
| Atrial fibrillation or flutter | 16 (7.0) | 88 (50.9) | 1429 (41.7) | <0.001 |
| Chronic lung disease | 11 (4.8) | 3 (1.7) | 229 (6.7) | 0.020 |
| Chronic kidney disease | 3 (1.3) | 3 (1.7) | 225 (6.6) | <0.001 |
| VHD duration > 18 months | 54 (23.6) | 58 (33.5) | 786 (23.0) | 0.006 |
| Systolic blood pressure, mmHg | 127.56 ± 19.11 | 117.61 ± 16.40 | 123.70 ± 20.16 | <0.001 |
| NYHA functional class | <0.001 | |||
| I | 58 (25.3) | 31 (17.9) | 734 (21.4) | |
| II | 89 (38.9) | 68 (39.3) | 917 (26.8) | |
| III | 68 (29.7) | 61 (35.3) | 1252 (36.6) | |
| IV | 14 (6.1) | 13 (7.5) | 521 (15.2) | |
| NT‐proBNP, pg/mL | 1096.00 (261.75–4019.50) | 1011.50 (439.00–2003.80) | 2470.00 (1043.00–5728.25) | <0.001 |
| Haemoglobin, g/L | 133.99 ± 17.40 | 134.86 ± 16.75 | 129.95 ± 20.00 | <0.001 |
| Creatinine, μmol/L | 78.00 (65.50–87.78) | 75.00 (63.00–88.00) | 81.00 (67.00–100.00) | <0.001 |
| Albumin, g/L | 40.55 ± 4.28 | 41.02 ± 4.18 | 38.95 ± 5.13 | <0.001 |
| LA, mm | 41.45 ± 6.55 | 54.31 ± 10.61 | 50.48 ± 10.30 | <0.001 |
| LVEDD, mm | 57.47 ± 8.81 | 48.56 ± 6.76 | 55.81 ± 10.91 | <0.001 |
| LVEF, % | 59.00 (50.00–65.00) | 60.00 (54.00–65.00) | 55.00 (41.00–62.00) | <0.001 |
| Pulmonary hypertension | 40 (17.5) | 75 (43.4) | 2139 (62.5) | <0.001 |
| Severe VHD | 166 (72.5) | 94 (54.3) | 2168 (63.3) | 0.001 |
| Symptomatic severe VHD | 128 (55.9) | 81 (46.8) | 1796 (52.5) | 0.194 |
| Valvular interventions | 180 (78.6) | 131 (75.7) | 1233 (36.0) | <0.001 |
| Aetiology | <0.001 | |||
| Primary | 229 (100.0) | 173 (100.0) | 2044 (60.9) | |
| Rheumatic | 43 (18.8) | 158 (91.3) | 1001 (49.0) | |
| Degenerative | 98 (42.8) | 4 (2.3) | 753 (36.8) | |
| Congenital | 47 (20.5) | 1 (0.6) | 152 (7.4) | |
| Others | 41 (17.9) | 10 (5.8) | 138 (6.8) | |
| Secondary | 0 (0.0) | 0 (0.0) | 1311 (39.1) | |
| Ischaemic | 0 (0.0) | 0 (0.0) | 284 (21.7) | |
| Functional | 0 (0.0) | 0 (0.0) | 1027 (78.3) | |
| Medications | ||||
| Diuretics | 201 (87.8) | 159 (91.9) | 2904 (84.8) | 0.020 |
| Beta‐blockers | 124 (54.1) | 94 (54.3) | 2066 (60.3) | 0.061 |
| ACE‐I/ARB | 75 (32.8) | 45 (26.0) | 1569 (45.8) | <0.001 |
| ARNI | 0 (0.0) | 0 (0.0) | 59 (1.7) | 0.018 |
| Warfarin | 172 (75.1) | 145 (83.8) | 1597 (46.6) | <0.001 |
| Aspirin | 52 (22.7) | 29 (16.8) | 976 (28.5) | 0.001 |
| P2Y12 inhibitors | 26 (11.4) | 15 (8.7) | 689 (20.1) | <0.001 |
| EuroSCORE II, % | 1.34 (0.93–2.17) | 1.73 (1.01–2.63) | 3.27 (2.06–5.69) | <0.001 |
| The score for heart valve surgery | 3.94 ± 2.44 | 5.89 ± 2.34 | 8.85 ± 2.73 | <0.001 |
| MAGGIC score | 16.28 ± 6.69 | 14.68 ± 5.98 | 19.02 ± 7.27 | <0.001 |
ACE‐I, angiotensin‐converting enzyme inhibitor; AR, aortic regurgitation; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor‐neprilysin inhibitor; AS, aortic stenosis; BMI, body mass index; CABG, coronary artery bypass graft; LA, left atrial end‐diastolic dimension; LVEDD, left ventricular end‐diastolic dimension; LVEF, left ventricular ejection fraction; MAGGIC, Meta‐Analysis Global Group in Chronic Heart Failure; MI, myocardial infarction; MR, mitral regurgitation; MS, mitral stenosis; MVHD, multiple valvular heart disease; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; VHD, valvular heart disease.
Data are presented as mean ± standard deviation, median (interquartile range), or number (percentage). Baseline characteristics are shown without imputation of missing values.
Prognostic value of Meta‐Analysis Global Group in Chronic Heart Failure score in patients with valvular heart disease
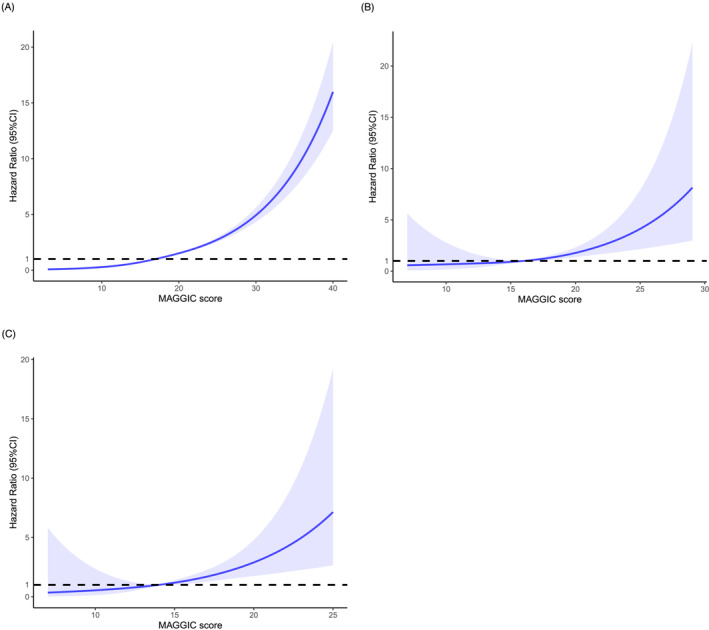
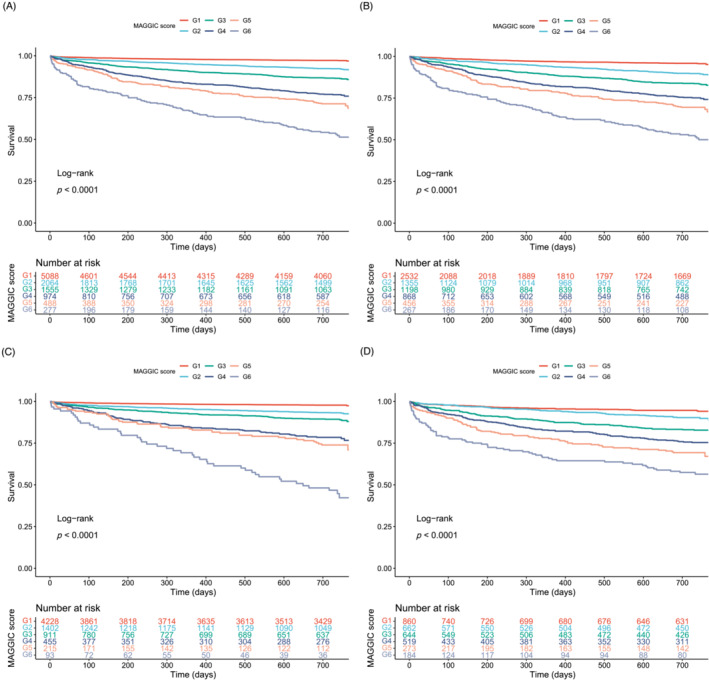
The median follow‐up of the study population was 732 days, with 895 (8.6%) deaths recorded. The RCS analysis revealed a monotonically positive association between MAGGIC score and mortality (Figures 2 and 3 ). The score showed satisfactory discrimination and calibration in predicting mortality in total cohort and various types of VHD (Supporting Information, Table S5 and Figures S3 and S4 ). After multivariable adjustment, the MAGGIC score, as a continuous variable, was an independent predictor of mortality in total cohort [adjusted HR (95% CI): 1.095 (1.084–1.107), P < 0.001], as well as in patients with AR [adjusted HR (95% CI): 1.074 (1.030–1.120), P < 0.001], MS [adjusted HR (95% CI): 1.156 (1.025–1.303), P = 0.018], MR [adjusted HR (95% CI): 1.088 (1.065–1.112), P < 0.001], TR [adjusted HR (95% CI): 1.113 (1.086–1.141), P < 0.001], AS + AR [adjusted HR (95% CI): 1.217 (1.069–1.387), P = 0.003], and MVHD [adjusted HR (95% CI): 1.097 (1.080–1.114), P < 0.001]. When categorized by the disease‐specific thresholds determined by ROC curve, the MAGGIC score remained a powerful prognostic factor in AR, MS, MR, TR, AS + AR, and MVHD (Table 3 and Supporting Information, Figures S5 and S6 ), with a higher score indicating more than 2.3‐fold, 5.3‐fold, 2.6‐fold, 4.2‐fold, 9.7‐fold, and 2.8‐fold mortality risk, respectively. The score was also independently associated with mortality after further adjustment for diuretics, angiotensin receptor‐neprilysin inhibitor (ARNI), warfarin, aspirin, and P2Y12 inhibitor [adjusted HR (95% CI): 1.090 (1.078–1.101), P < 0.001]. The six‐group assignment according to MAGGIC score discriminated risk clearly in patients with VHD (Figure 4 ). The relative importance of MAGGIC components is described in Figure 5 and Supporting Information, Figure S7 , which showed that age, NYHA functional class, creatinine, and LVEF were the four most important predictors in the study population.
Figure 2.
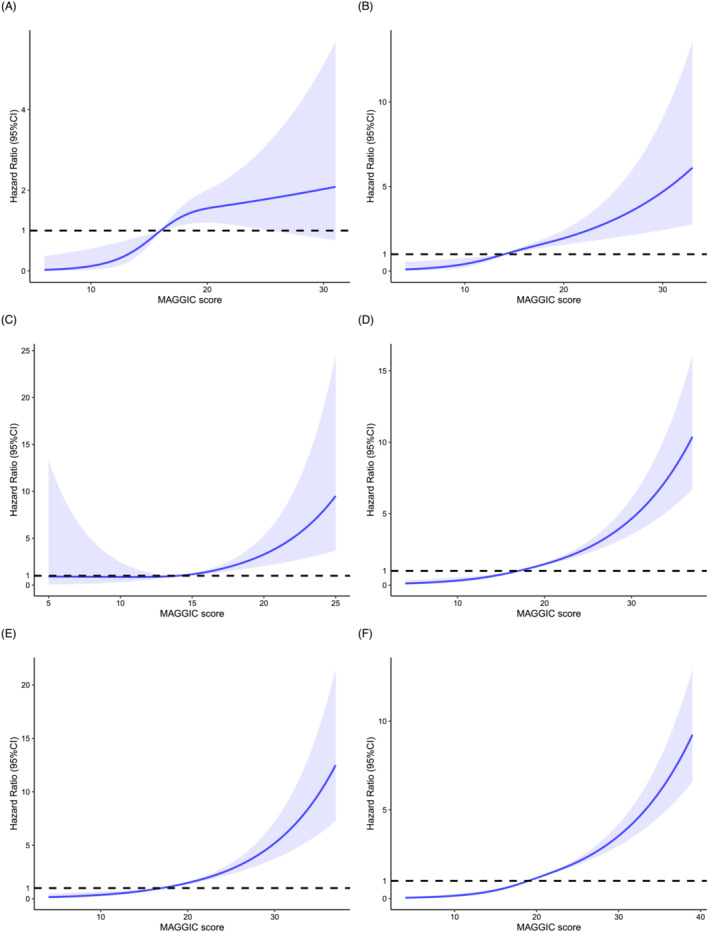
Restricted cubic splines for the association of Meta‐Analysis Global Group in Chronic Heart Failure (MAGGIC) score with mortality in isolated or multiple valvular heart disease. (A) Restricted cubic spline for the association of MAGGIC score with mortality in aortic stenosis. (B) Restricted cubic spline for the association of MAGGIC with mortality in aortic regurgitation. (C) Restricted cubic spline for the association of MAGGIC with mortality in mitral stenosis. (D) Restricted cubic spline for the association of MAGGIC score with mortality in mitral regurgitation. (E) Restricted cubic spline for the association of MAGGIC score with mortality in tricuspid regurgitation. (F) Restricted cubic spline for the association of MAGGIC score with mortality in multiple valvular heart disease. The mortality risks corresponding to median values of MAGGIC score were selected as references. CI, confidence interval.
Figure 3.
Restricted cubic splines for the association of Meta‐Analysis Global Group in Chronic Heart Failure (MAGGIC) score with mortality in total cohort and mixed valvular heart disease. (A) Restricted cubic spline for the association of MAGGIC score with mortality in total cohort. (B) Restricted cubic spline for the association of MAGGIC with mortality in mixed aortic stenosis and aortic regurgitation. (C) Restricted cubic spline for the association of MAGGIC with mortality in mixed mitral stenosis and mitral regurgitation. CI, confidence interval.
Table 3.
Association of Meta‐Analysis Global Group in Chronic Heart Failure score with mortality according to types of valvular heart disease
| Univariable analysis | Multivariable analysis a | |||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Total cohort (n = 10 446) | ||||
| MAGGIC score (per 1 point increase) | 1.139 (1.130–1.149) | <0.001 | 1.095 (1.084–1.107) | <0.001 |
| AS (n = 494) b | ||||
| MAGGIC score (per 1 point increase) | 1.101 (1.051–1.154) | <0.001 | 1.053 (0.992–1.117) | 0.087 |
| MAGGIC score ≥ 17 (vs. <17) | 3.193 (1.551–6.575) | 0.002 | 1.970 (0.909–4.268) | 0.086 |
| AR (n = 1372) | ||||
| MAGGIC score (per 1 point increase) | 1.119 (1.083–1.157) | <0.001 | 1.074 (1.030–1.120) | <0.001 |
| MAGGIC score ≥ 19 (vs. <19) | 4.280 (2.545–7.198) | <0.001 | 2.351 (1.312–4.212) | 0.004 |
| MS (n = 473) c | ||||
| MAGGIC score (per 1 point increase) | 1.202 (1.107–1.306) | <0.001 | 1.156 (1.025–1.303) | 0.018 |
| MAGGIC score ≥ 19 (vs. <19) | 8.742 (2.692–28.388) | <0.001 | 5.306 (1.392–20.224) | 0.015 |
| MR (n = 2590) | ||||
| MAGGIC score (per 1 point increase) | 1.132 (1.112–1.152) | <0.001 | 1.088 (1.065–1.112) | <0.001 |
| MAGGIC score ≥ 20 (vs. <20) | 4.864 (3.612–6.552) | <0.001 | 2.677 (1.939–3.695) | <0.001 |
| TR (n = 1691) | ||||
| MAGGIC score (per 1 point increase) | 1.138 (1.113–1.163) | <0.001 | 1.113 (1.086–1.141) | <0.001 |
| MAGGIC score ≥ 18 (vs. <18) | 6.086 (3.992–9.276) | <0.001 | 4.272 (2.733–6.676) | <0.001 |
| AS + AR (n = 229) b | ||||
| MAGGIC score (per 1 point increase) | 1.161 (1.081–1.246) | <0.001 | 1.217 (1.069–1.387) | 0.003 |
| MAGGIC score ≥ 19 (vs. <19) | 6.854 (1.886–24.907) | 0.003 | 9.740 (1.827–51.935) | 0.008 |
| MS + MR (n = 173) d | ||||
| MAGGIC score (per 1 point increase) | 1.193 (1.096–1.299) | <0.001 | 1.127 (0.981–1.295) | 0.092 |
| MAGGIC score ≥ 16 (vs. <16) | 6.571 (1.418–30.436) | 0.016 | 3.506 (0.629–19.549) | 0.152 |
| MVHD (n = 3424) | ||||
| MAGGIC score (per 1 point increase) | 1.135 (1.120–1.150) | <0.001 | 1.097 (1.080–1.114) | <0.001 |
| MAGGIC score ≥ 22 (vs. <22) | 5.073 (4.083–6.303) | <0.001 | 2.837 (2.238–3.596) | <0.001 |
AR, aortic regurgitation; AS, aortic stenosis; CI, confidence interval; HR, hazard ratio; MAGGIC, Meta‐Analysis Global Group in Chronic Heart Failure; MR, mitral regurgitation; MS, mitral stenosis; MVHD, multiple valvular heart disease; TR, tricuspid regurgitation.
Adjusted for hypertension, hyperlipidaemia, coronary artery disease, cardiomyopathy, atrial fibrillation or flutter, chronic kidney disease, haemoglobin, albumin, left atrial end‐diastolic dimension, left ventricular end‐diastolic dimension, pulmonary hypertension, severity of valvular heart disease, and valvular intervention.
In patients with isolated AS or mixed AS and AR, cardiomyopathy was not adjusted because no death occurred in patients with cardiomyopathy.
In patients with MS, cardiomyopathy was not adjusted because no patient had cardiomyopathy.
In patients with mixed MS and MR, hyperlipidaemia and cardiomyopathy were not adjusted because no death occurred in patients with hyperlipidaemia and no patient had cardiomyopathy.
Figure 4.
Kaplan–Meier curves according to six risk groups of Meta‐Analysis Global Group in Chronic Heart Failure (MAGGIC) score. (A) Kaplan–Meier curve in total cohort. (B) Kaplan–Meier curve in patients under medical treatment. (C) Kaplan–Meier curve in patients with left ventricular ejection fraction ≥ 50%. (D) Kaplan–Meier curve in patients with left ventricular ejection fraction < 50%.
Figure 5.
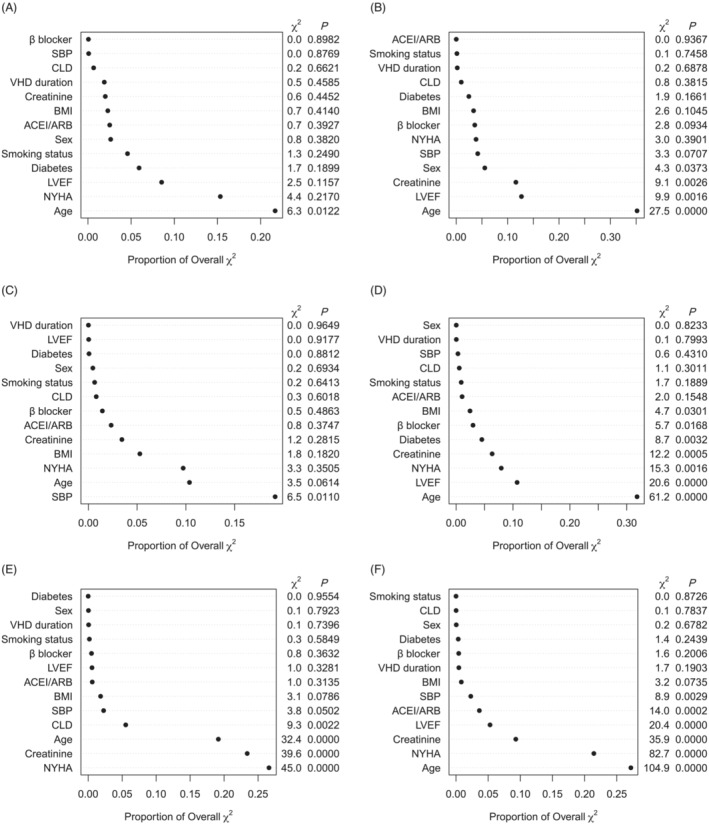
Relative importance of predictors by the proportion of explainable log‐likelihood ratio χ 2 statistics in patients with isolated valvular heart disease (VHD) or multiple VHD. (A) Relative importance of predictors in aortic stenosis. (B) Relative importance of predictors in aortic regurgitation. (C) Relative importance of predictors in mitral stenosis. (D) Relative importance of predictors in mitral regurgitation. (E) Relative importance of predictors in tricuspid regurgitation. (F) Relative importance of predictors in multiple VHD. The relative importance of predictors was ranked and evaluated by the proportion of explainable log‐likelihood ratio χ 2 statistics. ACE‐I, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; CLD, chronic lung disease; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; SBP, systolic blood pressure.
Meta‐Analysis Global Group in Chronic Heart Failure score in subgroups of patients
In patients under conservative treatment, the median follow‐up was 731 days, with 789 (11.8%) deaths recorded. The MAGGIC score achieved satisfactory discrimination and calibration in patients under conservative care [C index: 0.729 (0.712–0.746); Supporting Information, Table S6 and Figure S8 ], which were also observed in those under valvular intervention [0.725 (0.675–0.774); Supporting Information, Figure S8 ], or those divided by LVEF [LVEF ≥ 50%: 0.774 (0.751–0.796); LVEF < 50%: 0.698 (0.675–0.722); Supporting Information, Figure S9 ], symptom status [NYHA I: 0.719 (0.679–0.759); NYHA II–IV: 0.770 (0.754–0.786); Supporting Information, Figure S9 ], stage of VHD [moderate VHD: 0.759 (0.737–0.782); asymptomatic severe VHD: 0.732 (0.675–0.790); symptomatic severe VHD: 0.782 (0.761–0.804); Supporting Information, Figure S10 ], and aetiology of VHD [primary VHD: 0.783 (0.762–0.804); secondary VHD: 0.740 (0.716–0.763)]. Kaplan–Meier survival curves showed that the six‐group assignment according to MAGGIC score properly stratified risk in these subgroups of patients (Figure 4 and Supporting Information, Figures S11 and S12 ).
In multivariable analyses, increased MAGGIC score was independently related to higher mortality risk in patients under medical treatment [Supporting Information, Table S7 ; adjusted HR (95% CI): 1.094 (1.082–1.106), P < 0.001]. Similar results were found in patients under valvular intervention [adjusted HR (95% CI): 1.126 (1.089–1.164), P < 0.001] or those divided by LVEF [LVEF ≥ 50%: adjusted HR (95% CI): 1.104 (1.088–1.121), P < 0.001; LVEF < 50%: adjusted HR (95% CI): 1.079 (1.063–1.095), P < 0.001], symptom status [NYHA I: adjusted HR (95% CI): 1.095 (1.063–1.128), P < 0.001; NYHA II–IV: adjusted HR (95% CI): 1.093 (1.080–1.107), P < 0.001], stage of VHD [moderate VHD: adjusted HR (95% CI): 1.101 (1.086–1.117), P < 0.001; asymptomatic severe VHD: adjusted HR (95% CI): 1.081 (1.030–1.134), P = 0.002; symptomatic severe VHD: adjusted HR (95% CI): 1.090 (1.071–1.109), P < 0.001], and aetiology of VHD [primary VHD: adjusted HR (95% CI): 1.100 (1.084–1.116), P < 0.001; secondary VHD: adjusted HR (95% CI): 1.094 (1.077–1.111), P < 0.001]. Notably, the protective effect of valvular intervention vs. conservative care seemed to be consistent as the MAGGIC score changed (Supporting Information, Figure S13 ). Relative prognostic contribution of MAGGIC components in subgroups of patients is described in Supporting Information, Figures S14 – S16 .
Incremental value of N‐terminal pro‐brain natriuretic peptide to Meta‐Analysis Global Group in Chronic Heart Failure score
Among 10 446 patients, 6600 (63.2%) had baseline NT‐proBNP measurement, with a median value of 1482.50 pg/mL (492.60–3867.07 pg/mL). In the multivariable analyses, the association of MAGGIC score with mortality was found to be statistically significant in patients in the second, third, and fourth quartiles of NT‐proBNP level, with no significant interaction (Supporting Information, Table S8 ; P interaction = 0.498). The addition of NT‐proBNP to MAGGIC score provided a moderate improvement of risk prediction in total cohort, AS, AR, MR, TR, and MVHD (Supporting Information, Table S9 ). The incremental prognostic value of NT‐proBNP beyond MAGGIC score was also observed in patients divided by LVEF, symptom status, and aetiology of VHD, as well as those under conservative care (Supporting Information, Table S10 ).
Comparisons of Meta‐Analysis Global Group in Chronic Heart Failure score with other risk models
The median EuroSCORE II was 1.94 (1.13–3.43) in the study population (Supporting Information, Table S3 ). The MAGGIC score was positively correlated with EuroSCORE II (r = 0.67, P < 0.001). Compared with EuroSCORE II, the MAGGIC score had significantly better discrimination and reclassification properties in predicting mortality within two years in both total cohort [C index: 0.769 vs. 0.727; NRI (95% CI): 0.354 (0.313–0.396), P < 0.001; IDI (95% CI): 0.069 (0.052–0.085), P < 0.001] and different types of VHD (Supporting Information, Table S11 ). The calibration curves also demonstrated excellent calibration of MAGGIC score, while a poor agreement was observed between actual and EuroSCORE‐predicted survival probabilities (Supporting Information, Figure S17 ). Similar results were found in major subgroups of patients (Supporting Information, Table S12 and Figure S17 ). When comparing MAGGIC risk score with the risk model for heart valve surgery by Ambler et al., 18 the score achieved significantly better predictive performance in terms of discrimination and reclassification [C index: 0.768 vs. 0.708; NRI (95% CI): 0.212 (0.158–0.271), P < 0.001; IDI (95% CI): 0.048 (0.033–0.063), P < 0.001] and had a comparable calibration property (Supporting Information, Figure S18 ).
A prognostic nomogram, known as the China‐VHD model, was established using 13 predictors selected by the least absolute shrinkage and selection operator method (Supplementary Methods and Supporting Information, Figures S19 and S20 ), including age, BMI, chronic kidney disease, SBP, NYHA functional class, haemoglobin, creatinine, albumin, LVEF, pulmonary hypertension, valvular intervention, diuretics, and ACE‐I/ARB/ARNI (Supporting Information, Table S13 ). Compared with the China‐VHD model, the MAGGIC score had a relatively lower predictive capability (C index in the derivation cohort: 0.764 vs. 0.797; C index in the validation cohort: 0.771 vs. 0.793; Supporting Information, Table S14 ), yet still exhibited good discrimination (C index > 0.75) and calibration (Supporting Information, Figure S21 ) in both the derivation and validation cohorts of the nomogram. The comparisons of China‐VHD model with EuroSCORE II and the risk score by Ambler et al. are shown in Supporting Information, Table S14 .
Discussion
In this large, contemporary, multicentre cohort study of >10 000 patients with VHD, the following results were found: (i) the MAGGIC score was a significant independent predictor of 2 years of mortality in patients with VHD; (ii) the prognostic value of MAGGIC score was robust and consistent regardless of left ventricular systolic function, symptom status, and therapeutic strategy; (iii) the increase of MAGGIC score indicated poor survival across most degrees of NT‐proBNP, with a moderate improvement observed after the inclusion of this cardiac biomarker to the score; and (iv) the MAGGIC score, which included readily accessible and fewer prognostic components, achieved both substantially better discrimination and calibration compared with EuroSCORE II in patients with VHD.
The MAGGIC risk score was initially created with 30 cohorts of patients with HF to estimate the risk of all‐cause mortality. 9 It achieved satisfactory predictive performance regardless of ejection fraction 9 , 10 , 12 , 19 and was found to outperform other prognostic models of HF. 19 The intimate relationship between valvular disease and HF, and the user‐friendly properties of MAGGIC score make it an attractive choice to assess prognosis in VHD patients with cardiac dysfunction. To the best of our knowledge, the present study for the first time demonstrated that MAGGIC score was a strong and independent predictor of 2 years of mortality in a large series of patients with various types of VHD. The prognostic value of MAGGIC score appeared to be more pronounced in patients with AR, MS, MR, TR, AS + AR, and MVHD, compared with the score in those with isolated AS. A previous study that enrolled 259 patients mainly undergoing surgical aortic valve replacement examined the predictive performance of MAGGIC score in comparison of the existing risk models and found moderate discrimination in assessing both 1 year and 30 days of mortality risk. 15 Data from the Optimized transCathEter vAlvular interveNtion‐Transcatheter Aortic Valve Intervention (OCEAN‐TAVI) registry (n = 1383) also suggested that MAGGIC score provided useful information in evaluating 2 years of mortality risk of patients undergoing TAVI. 14 This study, with more than 7‐fold and 40‐fold sample size of previous reports, represents the crucial step to confirm the prognostic role of MAGGIC score in VHD population.
One of the most important findings of the present study was that the MAGGIC score possessed both satisfactory discrimination and calibration properties for predicting mortality in VHD patients with or without preserved ejection fraction. Additional analyses that quantified cardiac function with the levels of NT‐proBNP yielded similar results. Moreover, the increase of MAGGIC score was associated with higher mortality risk in patients regardless of symptom status, stage of VHD, aetiology of VHD, and therapeutic strategy. All these results were first obtained and should be interpreted with the consideration of the clinical uniqueness of VHD with HF. Patients with VHD can exhibit different patterns of cardiac remodelling and various degrees of cardiac dysfunction, 5 , 6 , 20 which challenge risk stratification and significantly influence the efficacy of valvular intervention. 21 On the other hand, risk profiles can also be different between patients with VHD‐related HF and those with HF due to other aetiologies. From the perspective of comprehensive disease management, the decision‐making of VHD with HF is more complicated and relies more on the multidisciplinary heart team that includes both cardiologists and surgeons to achieve reverse cardiac remodelling, alleviated symptoms, and prolonged life expectancy. 3 , 4 , 13 However, data on risk assessment in patients with VHD and HF are scarce, which may hamper therapeutic decision‐making for this specific population. Indeed, according to the 2022 American Heart Association/American College of Cardiology/Heart Failure Society of America guideline for HF, 13 all patients with significant VHD should be classified as at least stage B of HF (pre‐HF) and require appropriate management to prevent the development or worsening of clinical HF. Our findings imply that the MAGGIC score is a valuable instrument for risk stratification, with the potential to assist in tailoring individualized management strategies of VHD with cardiac dysfunction.
In the present analysis, we found that age, NYHA functional class, creatinine, and LVEF were the four most contributive prognostic factors in overall study population. Interestingly, although these robust predictors were considered in EuroSCORE II, it exhibited significantly poorer discrimination and calibration compared with MAGGIC score. The MAGGIC also outperformed a previous risk score for aortic and/or mitral valve surgery. 18 These results should be mainly attributed to the fact that the traditional surgical risk prediction tools were designed to estimate operative risk of patients requiring cardiac interventions, instead of assessing risk of VHD population beyond short term. 7 Besides, we observed that the use of ACE‐I/ARB, a component only for MAGGIC score, was a highly ranked contributive predictor in patients with LVEF < 50%, NYHA ≥ II, or secondary VHD, which might also partly explain such differences.
In this study, we established a novel risk prediction instrument, known as the China‐VHD model, and compared its prognostic capability with MAGGIC score. Although the newly developed risk model was found to have a better discrimination property than MAGGIC score, it should be noted that the China‐VHD model was only internally tested in the China‐VHD cohort, and its predictive performance might be overestimated. The MAGGIC score was developed and evaluated in different ethnicities and, therefore, possessed more potential to generalize. Regarding the China‐VHD model, more studies are needed to further examine its predictive performance, as well as to compare this risk model with both MAGGIC score and traditional surgical risk assessment tools.
Limitations
There were several limitations in this study. First, the present analysis was based on an observational cohort study. Despite using multivariable statistical approaches, there may exist residual unmeasured confounders and biases that affect the analyses of the prognostic value of the MAGGIC score. However, it is essential to validate the prognostic role of MAGGIC score in real‐world cohorts of VHD before conducting randomized controlled trials to confirm the efficacy of MAGGIC‐guided therapeutic decision‐making, and this study represents the largest effort to address this issue. Second, in the China‐VHD study, the evaluation of severity of valvular lesions was performed by echocardiography, which might be subjective sometimes. The inclusion of other approaches, such as the cardiac magnetic resonance, may further enhance the assessment of valvular dysfunction. However, echocardiography remains to be the first choice of imaging method to evaluate valvular lesions in routine clinical practice and is the widely used approach in large‐scale cohort studies of VHD. Third, compared with those with isolated or multiple VHD, patients with mixed VHD accounted for a small proportion of the study population, which might reduce the generalizability of our findings in this subset. Nevertheless, data on patients with mixed VHD are generally limited in existing literature, and to our best knowledge, this is the first study to examine the value of a prognostic score in mixed VHD. Fourth, although the MAGGIC score has included the use of beta‐blocker and ACE‐I/ARB as predictive components, the inclusion of other anti‐HF drugs, such as sodium‐glucose cotransporter‐2 inhibitors (SGLT2i), might further improve its prognostic capability. The China‐VHD study did not have data on SGLT2i, as well as the titration of medications, which should be investigated in future studies. Fifth, the 2 year follow‐up period in this study may be not enough to reveal the prognostic effects of all predictors in some subsets of patients, and our findings on the relative importance of MAGGIC components should be interpreted with consideration of the follow‐up duration. Sixth, some variables in the Society of Thoracic Surgeons (STS) score were not collected in the China‐VHD study, and therefore, the comparative analyses were not performed between MAGGIC score and STS score. Finally, the present study did not investigate prognostic implications of other risk stratification tools in HF due to the lack of some model components. However, the MAGGIC score well balances the user‐friendly property and predictive performance and has been found to possess the best accuracy among major prognostic models of HF. 19 Further studies are needed to analyse the clinical implications of other models in patients with VHD.
Conclusions
The MAGGIC risk score exhibited excellent discrimination and calibration in assessing mortality risk in VHD. The prognostic value of the score was consistent regardless of symptom status, therapeutic option, and the degree of cardiac dysfunction. The MAGGIC score can serve as a pragmatic tool to enhance risk prediction of VHD.
Conflict of interest
None declared.
Funding
This work was supported by the Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (grant number 2017‐12M‐3‐002).
Supporting information
Table S1. Excluded patients due to missing or invalid value of components of MAGGIC score. (P9).
Table S2. Number of missing values and corresponding dispositions. (P10).
Table S3. Baseline characteristics. (P11–12).
Table S4. Correlations between MAGGIC score and other variables. (P13).
Table S5. Predictive performance of the MAGGIC score. (P14).
Table S6. Predictive performance of the MAGGIC score in subgroups of patients. (P15).
Table S7. Associations of MAGGIC score with mortality in subsets of patients. (P16).
Table S8. Association of MAGGIC score with mortality according to quartiles of NT‐proBNP (n = 6600). (P17).
Table S9. Incremental value of NT‐proBNP over MAGGIC score according to types of VHD. (P18–19).
Table S10. Incremental value of NT‐proBNP over MAGGIC score in subgroups of patients. (P20–22).
Table S11. Comparisons between EuroSCORE II and MAGGIC score in patients with VHD. (P23–24).
Table S12. Comparisons between EuroSCORE II and MAGGIC score in subgroups of patients. (P25–26).
Table S13. Multivariable analyses for variables selected by LASSO method. (P27).
Table S14. Comparisons of the China‐VHD model with MAGGIC score, EuroSCORE II, and the risk score by Ambler et al. (P28).
Figure S1. Flowchart of the study cohort. VHD, valvular heart disease; TS, tricuspid stenosis; AS, aortic stenosis; AR, aortic regurgitation; MS, mitral stenosis; MR, mitral regurgitation; TR, tricuspid regurgitation; MVHD, multiple valvular heart disease; and MAGGIC, Meta‐Analysis Global Group in Chronic Heart Failure. (P29).
Figure S2. The MAGGIC score in different types of VHD. MAGGIC, Meta‐Analysis Global Group in Chronic Heart Failure; VHD, valvular heart disease; AS, aortic stenosis; AR, aortic regurgitation; MS, mitral stenosis; MR, mitral regurgitation; TR, tricuspid regurgitation; and MVHD, multiple valvular heart disease. (P30).
Figure S3. Calibration curves of MAGGIC score in patients with isolated VHD or MVHD. The calibration curves show the relationship between actual and predicted survival probabilities by MAGGIC score. (A) Calibration curve in patients with AS. (B) Calibration curve in patients with AR. (C) Calibration curve in patients with MS. (D) Calibration curve in patients with MR. (E) Calibration curve in patients with TR. (F) Calibration curve in patients with MVHD. MAGGIC, Meta‐Analysis Global Group in Chronic Heart Failure; VHD, valvular heart disease; AS, aortic stenosis; AR, aortic regurgitation; MS, mitral stenosis; MR, mitral regurgitation; TR, tricuspid regurgitation; and MVHD, multiple valvular heart disease. (P31).
Figure S4. Calibration curves of MAGGIC score in total cohort and mixed VHD. The calibration curves show the relationship between actual and predicted survival probabilities by MAGGIC score. (A) Calibration curve in total cohort. (B) Calibration curve in patients with mixed AS and AR. (C) Calibration curve in patients with mixed MS and MR. MAGGIC, Meta‐Analysis Global Group in Chronic Heart Failure; VHD, valvular heart disease; AS, aortic stenosis; AR, aortic regurgitation; MS, mitral stenosis; and MR, mitral regurgitation. (P32).
Figure S5. Kaplan–Meier curves according to disease‐specific thresholds of MAGGIC score in patients with isolated VHD or MVHD. (A) Kaplan–Meier curve in patients with AS. (B) Kaplan–Meier curve in patients with AR. (C) Kaplan–Meier curve in patients with MS. (D) Kaplan–Meier curve in patients with MR. (E) Kaplan–Meier curve in patients with TR. (F) Kaplan–Meier curve in patients with MVHD. MAGGIC, Meta‐Analysis Global Group in Chronic Heart Failure; VHD, valvular heart disease; AS, aortic stenosis; AR, aortic regurgitation; MS, mitral stenosis; MR, mitral regurgitation; TR, tricuspid regurgitation; and MVHD, multiple valvular heart disease. (P33).
Figure S6. Kaplan–Meier curves according to disease‐specific thresholds of MAGGIC score in patients with mixed VHD. (A) Kaplan–Meier curve in patients with mixed AS and AR. (B) Kaplan–Meier curve in patients with mixed MS and MR. MAGGIC, Meta‐Analysis Global Group in Chronic Heart Failure; VHD, valvular heart disease; AS, aortic stenosis; AR, aortic regurgitation; MS, mitral stenosis; and MR, mitral regurgitation. (P34).
Figure S7. Relative importance of predictors by the proportion of explainable log‐likelihood ratio χ 2 statistics in total cohort and mixed VHD. The relative importance of predictors was ranked and evaluated by the proportion of explainable log‐likelihood ratio χ2 statistics. (A) Relative importance of predictors in total cohort. (B) Relative importance of predictors in patients with mixed AS and AR. (C) Relative importance of predictors in patients with mixed MS and MR. VHD, valvular heart disease; CLD, chronic lung disease; SBP, systolic blood pressure; ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; AS, aortic stenosis; AR, aortic regurgitation; MS, mitral stenosis; and MR, mitral regurgitation. (P35).
Figure S8. Calibration curves of MAGGIC score according to therapeutic options. The calibration curves show the relationship between actual and predicted survival probabilities by MAGGIC score. (A) Calibration curve in patients under medical treatment. (B) Calibration curve in patients undergoing valvular intervention. MAGGIC, Meta‐Analysis Global Group in Chronic Heart Failure. (P36).
Figure S9. Calibration curves of MAGGIC score in subgroups of patients. The calibration curves show the relationship between actual and predicted survival probabilities by MAGGIC score. (A) Calibration curve in patients with LVEF≥50%. (B) Calibration curve in patients with LVEF<50%. (C) Calibration curve in patients with NYHA I. (D) Calibration curve in patients with NYHA II‐IV. (E) Calibration curve in patients with primary VHD. (F) Calibration curve in patients with secondary VHD. MAGGIC, Meta‐Analysis Global Group in Chronic Heart Failure; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; and VHD, valvular heart disease. (P37).
Figure S10. Calibration curves of MAGGIC score in patients with different stages of VHD. The calibration curves show the relationship between actual and predicted survival probabilities by MAGGIC score. (A) Calibration curve in patients with stage B VHD. (B) Calibration curve in patients with stage C VHD. (C) Calibration curve in patients with stage D VHD. MAGGIC, Meta‐Analysis Global Group in Chronic Heart Failure; and VHD, valvular heart disease. (P38).
Figure S11. Kaplan–Meier curves according to six risk groups of MAGGIC score in subgroups of patients. (A) Kaplan–Meier curve in patients with NYHA I. (B) Kaplan–Meier curve in patients with NYHA II‐IV. (C) Kaplan–Meier curve in patients with primary VHD. (D) Kaplan–Meier curve in patients with secondary VHD. (E) Kaplan–Meier curve in patients undergoing valvular intervention. MAGGIC, Meta‐Analysis Global Group in Chronic Heart Failure; NYHA, New York Heart Association; and VHD, valvular heart disease. (P39).
Figure S12. Kaplan–Meier curves according to six risk groups of MAGGIC score in patients with different stages of VHD. (A) Kaplan–Meier curve in patients with stage B VHD. (B) Kaplan–Meier curve in patients with stage C VHD. (C) Kaplan–Meier curve in patients with stage D VHD. MAGGIC, Meta‐Analysis Global Group in Chronic Heart Failure; and VHD, valvular heart disease. (P40).
Figure S13. Survival benefit of VI beyond MT across the range of MAGGIC score. VI, valvular intervention; MT, medical treatment; MAGGIC, Meta‐Analysis Global Group in Chronic Heart Failure; and CI, confidence interval. (P41).
Figure S14. Relative importance of predictors by the proportion of explainable log‐likelihood ratio χ 2 statistics according to therapeutic options. The relative importance of predictors was ranked and evaluated by the proportion of explainable log‐likelihood ratio χ2 statistics. (A) Relative importance of predictors in patients under medical treatment. (B) Relative importance of predictors in patients undergoing valvular intervention. CLD, chronic lung disease; VHD, valvular heart disease; SBP, systolic blood pressure; LVEF, left ventricular ejection fraction; BMI, body mass index; ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; and NYHA, New York Heart Association. (P42).
Figure S15. Relative importance of predictors by the proportion of explainable log‐likelihood ratio χ 2 statistics in subgroups of patients. The relative importance of predictors was ranked and evaluated by the proportion of explainable log‐likelihood ratio χ2 statistics. (A) Relative importance of predictors in patients with LVEF≥50%. (B) Relative importance of predictors in patients with LVEF<50%. (C) Relative importance of predictors in patients with NYHA I. (D) Relative importance of predictors in patients with NYHA II‐IV. (E) Relative importance of predictors in patients with primary VHD. (F) Relative importance of predictors in patients with secondary VHD. LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; VHD, valvular heart disease; SBP, systolic blood pressure; ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; CLD, chronic lung disease; and BMI, body mass index. (P43).
Figure S16. Relative importance of predictors by the proportion of explainable log‐likelihood ratio χ 2 statistics in patients with different stages of VHD. The relative importance of predictors was ranked and evaluated by the proportion of explainable log‐likelihood ratio χ2 statistics. (A) Relative importance of predictors in patients with stage B VHD. (B) Relative importance of predictors in patients with stage C VHD. (C) Relative importance of predictors in patients with stage D VHD. VHD, valvular heart disease; SBP, systolic blood pressure; CLD, chronic lung disease; BMI, body mass index; ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; LVEF, left ventricular ejection fraction; and NYHA, New York Heart Association. (P44).
Figure S17. Comparisons of calibration between MAGGIC score and EuroSCORE II. The calibration curves show the relationship between actual and predicted survival probabilities by MAGGIC score or EuroSCORE II. (A) Calibration curve of MAGGIC score in total cohort. (B) Calibration curve of EuroSCORE II in total cohort. (C) Calibration curve of MAGGIC score in patients with LVEF≥50%. (D) Calibration curve of EuroSCORE II in patients with LVEF≥50%. (E) Calibration curve of MAGGIC score in patients with LVEF<50%. (F) Calibration curve of EuroSCORE II in patients with LVEF<50%. MAGGIC, Meta‐Analysis Global Group in Chronic Heart Failure; and LVEF, left ventricular ejection fraction. (P45).
Figure S18. Comparisons of calibration between MAGGIC score and the score by Ambler et al. The calibration curves show the relationship between actual and predicted survival probabilities by MAGGIC score or the score by Ambler et al. (A) Calibration curve of MAGGIC score in total cohort. (B) Calibration curve of the score by Ambler et al. in total cohort. MAGGIC, Meta‐Analysis Global Group in Chronic Heart Failure. (P46).
Figure S19. Variable selection process by LASSO method. (A) The plot showing the partial likelihood deviance values versus log(λ). In the LASSO model, the tuning parameter λ selection used 10‐fold cross‐validation. The lambda with 1 standard error of minimum deviance was adopted for variable selection. (B) The coefficient profile plot. When λ corresponded to 1 standard error of minimum deviance, 13 variables were selected. LASSO, least absolute shrinkage and selection operator. (P47).
Figure S20. The nomogram for predicting two‐year mortality risk in patients with VHD. The value of each model component was assigned a point by drawing a vertical line to the “Points” scale. The sum of points for all components was plotted on the “Total points” scale, and the total point corresponded to the two‐year mortality risk at the bottom line. VHD, valvular heart disease; BMI, body mass index; NYHA, New York Heart Association; LVEF, left ventricular ejection fraction; ACEI, angiotensin converting enzyme inhibitors; ARB, angiotensin receptor blocker; and ARNI, angiotensin receptor‐neprilysin inhibitor. (P48).
Figure S21. Comparisons of calibration between MAGGIC score and China‐VHD model. The calibration curves show the relationship between actual and predicted survival probabilities by MAGGIC score or the China‐VHD model. (A) Calibration curve of MAGGIC score in the derivation cohort of the China‐VHD model. (B) Calibration curve of China‐VHD model in the derivation cohort. (C) Calibration curve of MAGGIC score in the validation cohort of the China‐VHD model. (D) Calibration curve of China‐VHD model in the validation cohort. MAGGIC, Meta‐Analysis Global Group in Chronic Heart Failure; and China‐VHD, China Valvular Heart Disease. (P49).
Acknowledgements
We are grateful for all staff members for data collection, data entry, and monitoring as part of the China‐VHD study.
Lv, J. , Xu, H. , Ye, Y. , Li, Z. , Wang, W. , Zhang, B. , Zhao, Q. , Zhang, H. , Zhao, Z. , Liu, Q. , Wang, B. , Duan, Z. , Yu, Z. , Guo, S. , Zhao, Y. , Gao, R. , Ge, J. , Wu, Y. , and CHINA‐VHD collaborators (2024) Meta‐Analysis Global Group in Chronic Heart Failure score for the prediction of mortality in valvular heart disease. ESC Heart Failure, 11: 349–365. 10.1002/ehf2.14586.
Yongjian Wu and Junbo Ge contributed equally to this work as joint corresponding authors.
Contributor Information
Junbo Ge, Email: ge.junbo2@zs-hospital.sh.cn.
Yongjian Wu, Email: wuyongjian@fuwaihospital.org.
References
- 1. Messika‐Zeitoun D, Baumgartner H, Burwash IG, Vahanian A, Bax J, Pibarot P, et al. Unmet needs in valvular heart disease. Eur Heart J 2023;44:1862‐1873. doi: 10.1093/eurheartj/ehad121 [DOI] [PubMed] [Google Scholar]
- 2. Shu S, Yang Y, Sun B, Su Z, Fu M, Xiong C, et al. Alerting trends in epidemiology for calcific aortic valve disease, 1990–2019: An age‐period‐cohort analysis for the Global Burden of Disease Study 2019. Eur Heart J Qual Care Clin Outcomes 2023;9:qcad018. doi: 10.1093/ehjqcco/qcad018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP 3rd, Gentile F, et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: A report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021;143:e72‐e227. doi: 10.1161/CIR.0000000000000923 [DOI] [PubMed] [Google Scholar]
- 4. Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J, et al. ESC/EACTS Scientific Document Group2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J 2022;43:561‐632. doi: 10.1093/eurheartj/ehab395 [DOI] [PubMed] [Google Scholar]
- 5. Malahfji M, Kitkungvan D, Senapati A, Nguyen DT, El‐Tallawi C, Tayal B, et al. Differences in myocardial remodeling and tissue characteristics in chronic isolated aortic and mitral regurgitation. Circ Cardiovasc Imaging 2023;16:e014684. doi: 10.1161/CIRCIMAGING.122.014684 [DOI] [PubMed] [Google Scholar]
- 6. Lamb HJ, Beyerbacht HP, de Roos A, van der Laarse A, Vliegen HW, Leujes F, et al. Left ventricular remodeling early after aortic valve replacement: Differential effects on diastolic function in aortic valve stenosis and aortic regurgitation. J Am Coll Cardiol 2002;40:2182‐2188. doi: 10.1016/s0735-1097(02)02604-9 [DOI] [PubMed] [Google Scholar]
- 7. Nashef SA, Roques F, Sharples LD, Nilsson J, Smith C, Goldstone AR, et al. EuroSCORE II. Eur J Cardiothorac Surg 2012;41:734‐745. doi: 10.1093/ejcts/ezs043 [DOI] [PubMed] [Google Scholar]
- 8. Dreyfus J, Audureau E, Bohbot Y, Coisne A, Lavie‐Badie Y, Bouchery M, et al. TRI‐SCORE: A new risk score for in‐hospital mortality prediction after isolated tricuspid valve surgery. Eur Heart J 2022;43:654‐662. doi: 10.1093/eurheartj/ehab679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pocock SJ, Ariti CA, McMurray JJ, Maggioni A, Køber L, Squire IB, et al. Meta‐Analysis Global Group in Chronic Heart FailurePredicting survival in heart failure: A risk score based on 39 372 patients from 30 studies. Eur Heart J 2013;34:1404‐1413. doi: 10.1093/eurheartj/ehs337 [DOI] [PubMed] [Google Scholar]
- 10. Sartipy U, Dahlström U, Edner M, Lund LH. Predicting survival in heart failure: Validation of the MAGGIC heart failure risk score in 51 043 patients from the Swedish Heart Failure Registry. Eur J Heart Fail 2014;16:173‐179. doi: 10.1111/ejhf.32 [DOI] [PubMed] [Google Scholar]
- 11. Allen LA, Matlock DD, Shetterly SM, Xu S, Levy WC, Portalupi LB, et al. Use of risk models to predict death in the next year among individual ambulatory patients with heart failure. JAMA Cardiol 2017;2:435‐441. doi: 10.1001/jamacardio.2016.5036 [DOI] [PubMed] [Google Scholar]
- 12. Rich JD, Burns J, Freed BH, Maurer MS, Burkhoff D, Shah SJ. Meta‐Analysis Global Group in Chronic (MAGGIC) Heart Failure risk score: Validation of a simple tool for the prediction of morbidity and mortality in heart failure with preserved ejection fraction. J Am Heart Assoc 2018;7:e009594. doi: 10.1161/JAHA.118.009594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: A report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022;145:e895‐e1032. doi: 10.1161/CIR.0000000000001063 [DOI] [PubMed] [Google Scholar]
- 14. Hioki H, Watanabe Y, Kozuma K, Kawashima H, Nagura F, Nakashima M, et al. The MAGGIC risk score predicts mortality in patients undergoing transcatheter aortic valve replacement: Sub‐analysis of the OCEAN‐TAVI registry. Heart Vessels 2019;34:1976‐1983. doi: 10.1007/s00380-019-01443-9 [DOI] [PubMed] [Google Scholar]
- 15. Zhuo DX, Bilchick KC, Shah KP, Mehta NK, Mwansa H, Nkanza‐Kabaso K, et al. MAGGIC, STS, and EuroSCORE II risk score comparison after aortic and mitral valve surgery. J Cardiothorac Vasc Anesth 2021;35:1806‐1812. doi: 10.1053/j.jvca.2020.11.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lv J, Ye Y, Li Z, Zhang B, Liu Q, Zhao Q, et al. CHINA‐VHD Collaborators Prognostic value of modified model for end‐stage liver disease scores in patients with significant tricuspid regurgitation. Eur Heart J Qual Care Clin Outcomes 2023;9:227‐239. doi: 10.1093/ehjqcco/qcac027 [DOI] [PubMed] [Google Scholar]
- 17. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015;28:1‐39.e14. doi: 10.1016/j.echo.2014.10.003 [DOI] [PubMed] [Google Scholar]
- 18. Ambler G, Omar RZ, Royston P, Kinsman R, Keogh BE, Taylor KM. Generic, simple risk stratification model for heart valve surgery. Circulation 2005;112:224‐231. doi: 10.1161/CIRCULATIONAHA.104.515049 [DOI] [PubMed] [Google Scholar]
- 19. Canepa M, Fonseca C, Chioncel O, Laroche C, Crespo‐Leiro MG, Coats AJS, et al. ESC HF Long Term Registry InvestigatorsPerformance of prognostic risk scores in chronic heart failure patients enrolled in the European Society of Cardiology Heart Failure Long‐Term Registry. JACC Heart Fail 2018;6:452‐462. doi: 10.1016/j.jchf.2018.02.001 [DOI] [PubMed] [Google Scholar]
- 20. Zhang B, Xu H, Zhang H, Liu Q, Ye Y, Hao J, et al. CHINA‐DVD Collaborators Prognostic value of N‐terminal pro‐B‐type natriuretic peptide in elderly patients with valvular heart disease. J Am Coll Cardiol 2020;75:1659‐1672. doi: 10.1016/j.jacc.2020.02.031 [DOI] [PubMed] [Google Scholar]
- 21. Grayburn PA, Sannino A, Packer M. Proportionate and disproportionate functional mitral regurgitation: A new conceptual framework that reconciles the results of the MITRA‐FR and COAPT trials. JACC Cardiovasc Imaging 2019;12:353‐362. doi: 10.1016/j.jcmg.2018.11.006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Excluded patients due to missing or invalid value of components of MAGGIC score. (P9).
Table S2. Number of missing values and corresponding dispositions. (P10).
Table S3. Baseline characteristics. (P11–12).
Table S4. Correlations between MAGGIC score and other variables. (P13).
Table S5. Predictive performance of the MAGGIC score. (P14).
Table S6. Predictive performance of the MAGGIC score in subgroups of patients. (P15).
Table S7. Associations of MAGGIC score with mortality in subsets of patients. (P16).
Table S8. Association of MAGGIC score with mortality according to quartiles of NT‐proBNP (n = 6600). (P17).
Table S9. Incremental value of NT‐proBNP over MAGGIC score according to types of VHD. (P18–19).
Table S10. Incremental value of NT‐proBNP over MAGGIC score in subgroups of patients. (P20–22).
Table S11. Comparisons between EuroSCORE II and MAGGIC score in patients with VHD. (P23–24).
Table S12. Comparisons between EuroSCORE II and MAGGIC score in subgroups of patients. (P25–26).
Table S13. Multivariable analyses for variables selected by LASSO method. (P27).
Table S14. Comparisons of the China‐VHD model with MAGGIC score, EuroSCORE II, and the risk score by Ambler et al. (P28).
Figure S1. Flowchart of the study cohort. VHD, valvular heart disease; TS, tricuspid stenosis; AS, aortic stenosis; AR, aortic regurgitation; MS, mitral stenosis; MR, mitral regurgitation; TR, tricuspid regurgitation; MVHD, multiple valvular heart disease; and MAGGIC, Meta‐Analysis Global Group in Chronic Heart Failure. (P29).
Figure S2. The MAGGIC score in different types of VHD. MAGGIC, Meta‐Analysis Global Group in Chronic Heart Failure; VHD, valvular heart disease; AS, aortic stenosis; AR, aortic regurgitation; MS, mitral stenosis; MR, mitral regurgitation; TR, tricuspid regurgitation; and MVHD, multiple valvular heart disease. (P30).
Figure S3. Calibration curves of MAGGIC score in patients with isolated VHD or MVHD. The calibration curves show the relationship between actual and predicted survival probabilities by MAGGIC score. (A) Calibration curve in patients with AS. (B) Calibration curve in patients with AR. (C) Calibration curve in patients with MS. (D) Calibration curve in patients with MR. (E) Calibration curve in patients with TR. (F) Calibration curve in patients with MVHD. MAGGIC, Meta‐Analysis Global Group in Chronic Heart Failure; VHD, valvular heart disease; AS, aortic stenosis; AR, aortic regurgitation; MS, mitral stenosis; MR, mitral regurgitation; TR, tricuspid regurgitation; and MVHD, multiple valvular heart disease. (P31).
Figure S4. Calibration curves of MAGGIC score in total cohort and mixed VHD. The calibration curves show the relationship between actual and predicted survival probabilities by MAGGIC score. (A) Calibration curve in total cohort. (B) Calibration curve in patients with mixed AS and AR. (C) Calibration curve in patients with mixed MS and MR. MAGGIC, Meta‐Analysis Global Group in Chronic Heart Failure; VHD, valvular heart disease; AS, aortic stenosis; AR, aortic regurgitation; MS, mitral stenosis; and MR, mitral regurgitation. (P32).
Figure S5. Kaplan–Meier curves according to disease‐specific thresholds of MAGGIC score in patients with isolated VHD or MVHD. (A) Kaplan–Meier curve in patients with AS. (B) Kaplan–Meier curve in patients with AR. (C) Kaplan–Meier curve in patients with MS. (D) Kaplan–Meier curve in patients with MR. (E) Kaplan–Meier curve in patients with TR. (F) Kaplan–Meier curve in patients with MVHD. MAGGIC, Meta‐Analysis Global Group in Chronic Heart Failure; VHD, valvular heart disease; AS, aortic stenosis; AR, aortic regurgitation; MS, mitral stenosis; MR, mitral regurgitation; TR, tricuspid regurgitation; and MVHD, multiple valvular heart disease. (P33).
Figure S6. Kaplan–Meier curves according to disease‐specific thresholds of MAGGIC score in patients with mixed VHD. (A) Kaplan–Meier curve in patients with mixed AS and AR. (B) Kaplan–Meier curve in patients with mixed MS and MR. MAGGIC, Meta‐Analysis Global Group in Chronic Heart Failure; VHD, valvular heart disease; AS, aortic stenosis; AR, aortic regurgitation; MS, mitral stenosis; and MR, mitral regurgitation. (P34).
Figure S7. Relative importance of predictors by the proportion of explainable log‐likelihood ratio χ 2 statistics in total cohort and mixed VHD. The relative importance of predictors was ranked and evaluated by the proportion of explainable log‐likelihood ratio χ2 statistics. (A) Relative importance of predictors in total cohort. (B) Relative importance of predictors in patients with mixed AS and AR. (C) Relative importance of predictors in patients with mixed MS and MR. VHD, valvular heart disease; CLD, chronic lung disease; SBP, systolic blood pressure; ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; AS, aortic stenosis; AR, aortic regurgitation; MS, mitral stenosis; and MR, mitral regurgitation. (P35).
Figure S8. Calibration curves of MAGGIC score according to therapeutic options. The calibration curves show the relationship between actual and predicted survival probabilities by MAGGIC score. (A) Calibration curve in patients under medical treatment. (B) Calibration curve in patients undergoing valvular intervention. MAGGIC, Meta‐Analysis Global Group in Chronic Heart Failure. (P36).
Figure S9. Calibration curves of MAGGIC score in subgroups of patients. The calibration curves show the relationship between actual and predicted survival probabilities by MAGGIC score. (A) Calibration curve in patients with LVEF≥50%. (B) Calibration curve in patients with LVEF<50%. (C) Calibration curve in patients with NYHA I. (D) Calibration curve in patients with NYHA II‐IV. (E) Calibration curve in patients with primary VHD. (F) Calibration curve in patients with secondary VHD. MAGGIC, Meta‐Analysis Global Group in Chronic Heart Failure; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; and VHD, valvular heart disease. (P37).
Figure S10. Calibration curves of MAGGIC score in patients with different stages of VHD. The calibration curves show the relationship between actual and predicted survival probabilities by MAGGIC score. (A) Calibration curve in patients with stage B VHD. (B) Calibration curve in patients with stage C VHD. (C) Calibration curve in patients with stage D VHD. MAGGIC, Meta‐Analysis Global Group in Chronic Heart Failure; and VHD, valvular heart disease. (P38).
Figure S11. Kaplan–Meier curves according to six risk groups of MAGGIC score in subgroups of patients. (A) Kaplan–Meier curve in patients with NYHA I. (B) Kaplan–Meier curve in patients with NYHA II‐IV. (C) Kaplan–Meier curve in patients with primary VHD. (D) Kaplan–Meier curve in patients with secondary VHD. (E) Kaplan–Meier curve in patients undergoing valvular intervention. MAGGIC, Meta‐Analysis Global Group in Chronic Heart Failure; NYHA, New York Heart Association; and VHD, valvular heart disease. (P39).
Figure S12. Kaplan–Meier curves according to six risk groups of MAGGIC score in patients with different stages of VHD. (A) Kaplan–Meier curve in patients with stage B VHD. (B) Kaplan–Meier curve in patients with stage C VHD. (C) Kaplan–Meier curve in patients with stage D VHD. MAGGIC, Meta‐Analysis Global Group in Chronic Heart Failure; and VHD, valvular heart disease. (P40).
Figure S13. Survival benefit of VI beyond MT across the range of MAGGIC score. VI, valvular intervention; MT, medical treatment; MAGGIC, Meta‐Analysis Global Group in Chronic Heart Failure; and CI, confidence interval. (P41).
Figure S14. Relative importance of predictors by the proportion of explainable log‐likelihood ratio χ 2 statistics according to therapeutic options. The relative importance of predictors was ranked and evaluated by the proportion of explainable log‐likelihood ratio χ2 statistics. (A) Relative importance of predictors in patients under medical treatment. (B) Relative importance of predictors in patients undergoing valvular intervention. CLD, chronic lung disease; VHD, valvular heart disease; SBP, systolic blood pressure; LVEF, left ventricular ejection fraction; BMI, body mass index; ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; and NYHA, New York Heart Association. (P42).
Figure S15. Relative importance of predictors by the proportion of explainable log‐likelihood ratio χ 2 statistics in subgroups of patients. The relative importance of predictors was ranked and evaluated by the proportion of explainable log‐likelihood ratio χ2 statistics. (A) Relative importance of predictors in patients with LVEF≥50%. (B) Relative importance of predictors in patients with LVEF<50%. (C) Relative importance of predictors in patients with NYHA I. (D) Relative importance of predictors in patients with NYHA II‐IV. (E) Relative importance of predictors in patients with primary VHD. (F) Relative importance of predictors in patients with secondary VHD. LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; VHD, valvular heart disease; SBP, systolic blood pressure; ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; CLD, chronic lung disease; and BMI, body mass index. (P43).
Figure S16. Relative importance of predictors by the proportion of explainable log‐likelihood ratio χ 2 statistics in patients with different stages of VHD. The relative importance of predictors was ranked and evaluated by the proportion of explainable log‐likelihood ratio χ2 statistics. (A) Relative importance of predictors in patients with stage B VHD. (B) Relative importance of predictors in patients with stage C VHD. (C) Relative importance of predictors in patients with stage D VHD. VHD, valvular heart disease; SBP, systolic blood pressure; CLD, chronic lung disease; BMI, body mass index; ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; LVEF, left ventricular ejection fraction; and NYHA, New York Heart Association. (P44).
Figure S17. Comparisons of calibration between MAGGIC score and EuroSCORE II. The calibration curves show the relationship between actual and predicted survival probabilities by MAGGIC score or EuroSCORE II. (A) Calibration curve of MAGGIC score in total cohort. (B) Calibration curve of EuroSCORE II in total cohort. (C) Calibration curve of MAGGIC score in patients with LVEF≥50%. (D) Calibration curve of EuroSCORE II in patients with LVEF≥50%. (E) Calibration curve of MAGGIC score in patients with LVEF<50%. (F) Calibration curve of EuroSCORE II in patients with LVEF<50%. MAGGIC, Meta‐Analysis Global Group in Chronic Heart Failure; and LVEF, left ventricular ejection fraction. (P45).
Figure S18. Comparisons of calibration between MAGGIC score and the score by Ambler et al. The calibration curves show the relationship between actual and predicted survival probabilities by MAGGIC score or the score by Ambler et al. (A) Calibration curve of MAGGIC score in total cohort. (B) Calibration curve of the score by Ambler et al. in total cohort. MAGGIC, Meta‐Analysis Global Group in Chronic Heart Failure. (P46).
Figure S19. Variable selection process by LASSO method. (A) The plot showing the partial likelihood deviance values versus log(λ). In the LASSO model, the tuning parameter λ selection used 10‐fold cross‐validation. The lambda with 1 standard error of minimum deviance was adopted for variable selection. (B) The coefficient profile plot. When λ corresponded to 1 standard error of minimum deviance, 13 variables were selected. LASSO, least absolute shrinkage and selection operator. (P47).
Figure S20. The nomogram for predicting two‐year mortality risk in patients with VHD. The value of each model component was assigned a point by drawing a vertical line to the “Points” scale. The sum of points for all components was plotted on the “Total points” scale, and the total point corresponded to the two‐year mortality risk at the bottom line. VHD, valvular heart disease; BMI, body mass index; NYHA, New York Heart Association; LVEF, left ventricular ejection fraction; ACEI, angiotensin converting enzyme inhibitors; ARB, angiotensin receptor blocker; and ARNI, angiotensin receptor‐neprilysin inhibitor. (P48).
Figure S21. Comparisons of calibration between MAGGIC score and China‐VHD model. The calibration curves show the relationship between actual and predicted survival probabilities by MAGGIC score or the China‐VHD model. (A) Calibration curve of MAGGIC score in the derivation cohort of the China‐VHD model. (B) Calibration curve of China‐VHD model in the derivation cohort. (C) Calibration curve of MAGGIC score in the validation cohort of the China‐VHD model. (D) Calibration curve of China‐VHD model in the validation cohort. MAGGIC, Meta‐Analysis Global Group in Chronic Heart Failure; and China‐VHD, China Valvular Heart Disease. (P49).


