Abstract
Aims
This systematic review and meta‐analysis aimed to investigate the association between serum uric acid (SUA) levels and the incidence rate and prognosis of heart failure (HF), as well as the impact of uric acid‐lowering treatment on HF patients.
Methods and results
PubMed and Embase were searched for original articles reporting on the association between SUA and HF incidence, adverse outcomes, and the effect of uric acid‐lowering treatment in HF patients. Data were pooled using random effects or fixed effects models. Univariable meta‐regression analysis assessed the influence of study characteristics on research outcomes. Statistical analyses were conducted using RevMan software and STATA software version 15.0. Eleven studies on HF incidence and 24 studies on adverse outcomes in HF patients were included. Higher SUA levels were associated with an increased risk of HF (RR: 1.81, 95% CI: 1.53–2.16), all‐cause mortality (RR: 1.44, 95% CI: 1.25–1.66), cardiac death (RR: 1.56, 95% CI: 1.32–1.84), and HF rehospitalization (RR: 2.07, 95% CI: 1.37–3.13) in HF patients. Uric acid‐lowering treatment was found to increase all‐cause mortality in HF patients (RR: 1.15, 95% CI: 1.05–1.25).
Conclusions
Uric acid is an independent predictor of heart failure occurrence and adverse prognosis. Targeting uric acid lowering as a therapeutic intervention does not improve the prognosis of patients with heart failure. It may not be advisable to use traditional urate‐lowering drugs in young patients with heart failure, and elderly patients should exercise caution when using them.
Keywords: Heart failure, Meta‐analysis, Serum uric acid, Systematic review, Uric acid‐lowering therapy
Introduction
Over the past three decades, heart failure has emerged as a significant public health issue in both developing and developed countries. 1 , 2 , 3 The incidence and prevalence rates of heart failure increase with age. 4 Despite advancements in available therapies, patients with heart failure continue to experience high levels of morbidity and mortality.
Uric acid is the end‐product of purine metabolism in the human body 5 ; it is an antioxidant, 6 and it also has cardiovascular protective effects. There are several possible contributors to increased levels of UA in heart failure. Xanthine oxidoreductase (XO) is a catalytic enzyme in the process of purine metabolism. Under the action of this enzyme, xanthine is metabolized to generate UA, which produces reactive oxygen species (ROS). ROS can lead to various cardiovascular diseases, such as atherosclerosis, cardiac hypertrophy, myocardial fibrosis, left ventricular remodelling, and aggravated heart failure. 7 , 8 Reduced renal excretion and diuretic therapy can also lead to elevated levels of UA. 9 , 10 Numerous recent studies have established a close association between uric acid and the incidence and progression of heart failure. 11 , 12 , 13 This prompts questions regarding whether higher serum uric acid levels indicate a dysregulated pathway in the context of heart failure and whether lowering uric acid through treatment can improve adverse outcomes. The effectiveness of uric acid‐lowering treatment on heart failure outcomes remains controversial, with some studies suggesting its potential to improve adverse outcomes, 14 , 15 , 16 while others indicate no significant improvement in patient survival. 17 , 18 , 19 , 20 , 21
Given the emergence of new studies, a comprehensive systematic review and meta‐analysis are necessary to elucidate the relationship between serum uric acid, the incidence and prognosis of heart failure, and the impact of uric acid‐lowering treatment on heart failure prognosis.
Methods
Data sources and searches
This systematic review and meta‐analysis were performed following the PRISMA statement. 22 The research was registered with PROSPERO (CRD42023400927). To identify the published clinical studies that involved the relation of uric acid and the incidence of heart failure, the effect of uric acid on the prognosis of heart failure, and the impact of uric acid‐lowering therapy on the prognosis of heart failure, we performed a comprehensive online search of the literature through the Medline and Embase databases (to May 2023), without language restriction. The retrieval strategy used relevant keywords and medical subject heading terms including the following: (uric acid) OR (uric acid) OR (urate) OR (serum uric acid) OR (SUA) AND (heart failure) OR (heart failure) OR (cardiac failure) OR (myocardial failure) OR (left ventricular dysfunction) OR (right ventricular dysfunction). The references of the reviewed manuscripts were manually retrieved to avoid missing relevant data.
Inclusion and exclusion criteria
The inclusion criteria for the association between uric acid and incidence rate and prognosis of heart failure were (i) prospective cohort studies, retrospective cohort studies, and case–control studies; (ii) assessing ≥1 of the following outcome measures: all‐cause mortality and cardiovascular mortality, HF hospitalization, and the incidence rates of heart failure; (iii) endpoints were reported as numerical events rather than only hazard ratios, relative risk, or odds rate. 3 The definition of HF was based on physical signs, clinical symptoms, and therapeutic response.
The inclusion criteria for the association between ULT and outcomes of HF were (i) observational, cross‐sectional studies, and randomized control trials; (ii) including patients with heart failure receiving uric acid lowering therapies; (iii) assessing ≥1 of the following outcome measures: all‐cause mortality and cardiovascular mortality, HF hospitalization. 4 endpoints were reported as numerical events rather than only hazard ratios, relative risk, or odds rate. 5 The definition of HF was based on physical signs, clinical symptoms, and therapeutic response.
If related data were not reported in the published articles, we tried to contact the corresponding authors to get relevant information. We excluded multiple studies on the same population. Studies that reported inadequate details were also deleted unless we were able to retrieve the original data.
Study selection process
Two reviewers independently evaluated the records identified from the search for eligibility. The retrieval process consists of four stages. In the first stage, we screened the titles and abstracts to exclude the literature that did not meet the inclusion criteria. In the second stage, the full text of articles identified by two searchers as potentially relevant to the purpose of the study was obtained, and eligible articles were independently reviewed by two researchers. In the third stage, any disagreements regarding inclusion and exclusion were adjudicated by discussion or consultation with a third investigator. In the fourth stage, references of included studies were manually searched to identify any missed articles.
Data extraction and quality assessment
The preparation of data extraction and presentation of this manuscript followed the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta‐Analysis. Two authors conduct the data extraction through using a predefined, standardized protocol, and data collection instrument. The following data were extracted: study region, duration of the trial, publication data, sample size, baseline characteristics, intervention, and control. The Newcastle–Ottawa Quality Assessment Scale for observational trials was used to evaluate the quality of the included studies. 23 The randomized control trials were evaluated through the Cochrane Collaboration tool for assessing the risk of bias.
Date synthesis and analysis
This article is mainly a prognostic study, all outcome variables are dichotomous variables. This meta‐analysis was conducted using the Cochran Mantel–Haenszel test under the random effect model to generate pooled risk ratio (RR) for the endpoints. Statistical heterogeneity within the studies was estimated using the I 2 statistic, and very low, low, moderate, and high levels of heterogeneity were defined as ≤25%, 25% to ≤50%, 50% to ≤75%, and ≥75%, respectively. For the primary outcomes, to inspect the impact of any single study on the pooling summary, sensitivity analysis was conducted by iteratively removing individual studies at each turn. Multiple subgroup analyses (according to region, study design, publication year, sample size, and age of patients) were performed to further test the stability of our meta‐analysis. To examine the source of heterogeneity, we performed a random‐effects meta‐regression analysis. The logarithm of RR for the endpoints was regressed against age, sample size, duration of follow‐up, publication year, left ventricular ejection fraction, diabetes mellitus, and hypertension. It was weighted by the inverse variance of each study. To inspect any publication bias in the primary endpoints, we examined funnel plots for asymmetry in detail and evaluated them further using Egger regression asymmetry tests. The RevMan software package (Review Manager, Version 5.4) and STATA software 15.0 were used to perform all statistical analyses. An alpha criterion of P value <0.05 was considered statistically significant.
Results
Characteristics of included studies
Figure 1 displays the literature selection process. Initially, our search strategy identified a total of 1191 relevant studies, of which 1105 were excluded based on the evaluation of the title and abstract. We further excluded 33 duplicate studies. We conducted a full‐text review of 53 studies to determine the eligible studies, and 17 studies were further removed for the following reasons: unavailable full text, lack of data, conference abstract, and review. Finally, 36 studies were included for further analysis (Figure 1 ). Among them, there were 11 studies on the association between uric acid and the incidence of HF, 10 studies on the association between uric acid and the prognosis of HF, and 14 studies on the association between uric acid lowering treatment and the adverse outcomes of HF.
Figure 1.
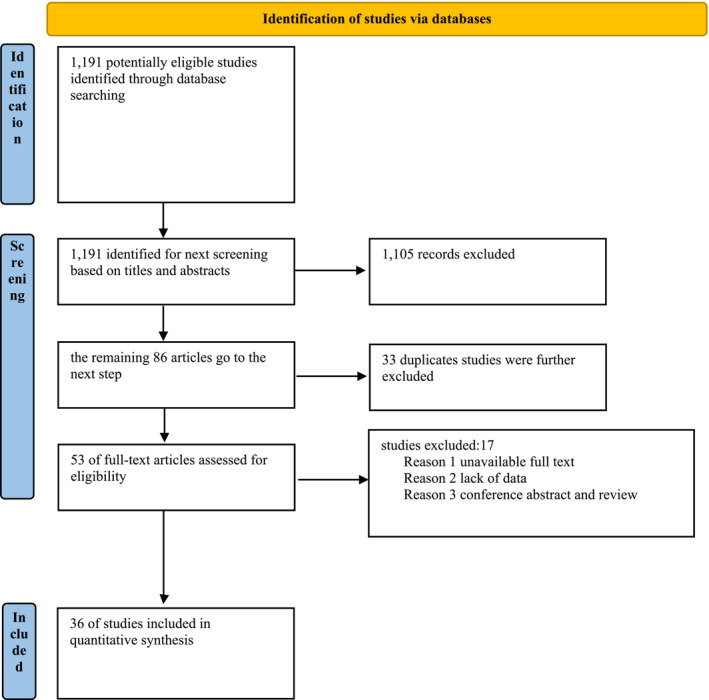
PRISMA 2020 flow diagram for new systematic reviews, including searches of databases and registries.
Table 1 summarized the main patients characteristic of the 11 studies on the relation between uric acid and incident HF. A total of 438 296 patients were identified and analysed, with the median age of the subjects ranged from 36 to 73. The sample size varies from 216 to 353 613. Follow‐up time ranged from 1 to 29 years. These studies were conducted in China, 24 , 25 , 26 Poland, 27 Italy, 28 Japan, 29 USA, 30 , 31 , 32 Turkey, 33 and Korea. 34 Heart failure was diagnosed according to ESC guidelines or based on symptoms, physical signs, and treatment response.
Table 1.
Baseline characteristics of included studies on the association between serum uric acid and incidence of heart failure
| Reference | Year | Study design | Sample size (%male) | Region | Age (year) | Definition of higher UA (mg/dL) | Follow‐up (year) | Quality score |
|---|---|---|---|---|---|---|---|---|
| Wu | 2020 | Cohort | 2749 (55.0%) | China | 70.9 | Male 7.0, women 6.0 | 4 | 5 |
| Liu | 2021 | Cross‐sectional | 216 (20.1%) | China | 64.4 | 6.4 | NR | 7 |
| Wełnicki | 2022 | Cross‐sectional | 829 (55.8%) | Poland | 72.7 | 6.4 | NR | 6 |
| Rebora | 2022 | Cross‐sectional | 1269 (73.7%) | Italy | 68.0 | Male 7.0, women 6.0 | NR | 7 |
| Seki | 2021 | Cohort | 353 613 (57.1%) | Japan | 40.0 | 5.7 | 3.2 | 6 |
| Gu | 2018 | Cohort | 1009 (56.1%) | China | 66.1 | 6.2 | 7.2 | 6 |
| Essex | 2017 | Cohort | 65 329 (69.4%) | USA | 63.7 | 6.0 | 1.0 | 7 |
| Kaya | 2012 | Cohort | 2249 (80.5%) | Turkey | 58.2 | Male 7.0, women 6.0 | 2.0 | 6 |
| Ekundayo | 2010 | Cohort | 5461 (44.0%) | USA | 73.0 | Male 7.0, women 6.0 | 8.1 | 6 |
| Krishnan | 2009 | Cohort | 4912 (47.0%) | USA | 36.0 | 6.2 | 29.0 | 8 |
| Bae | 2007 | Cohort | 660(59.0%) | Korea | 59.2 | 5.7 | 2.3 | 7 |
NR, not reported; UA, uric acid.
Table 2 summarized the main features of included studies on the association between uric acid and heart failure outcomes. A total of 16 742 patients were included, and the sample size vary from 102 to 4795. Follow‐up time ranged from 0.8 to 6.3 years, these studies were conducted in China, 35 , 36 USA, 37 , 38 Turkey, 39 Canada, 38 and Japan. 40 , 41 , 42 , 43 , 44 In addition, there was one international multicentre study. 45 They were all cohort studies. Ten studies focused on the outcomes of all‐cause mortality, eight studies on HF rehospitalization, and five studies were on cardiac death. The baseline proportion of diabetes and hypertension was also presented.
Table 2.
Baseline characteristics of included studies on the association between uric acid and adverse outcomes of heart failure
| References | Year | Age | Sample size (%male) | Study design | LVEF | HTN | DM | Country | Follow up(y) | Definition of higher UA level (mg/dL) | Reported primary outcomes | Quality score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yılmaz | 2022 | 64 | 861 (72.6%) | Cohort | 30 | 53.2 | 38.6 | Turkey | 2.5 | 7 |
All‐cause mortality HF rehospitalization |
6 |
| Wang | 2022 | 61.1 | 102 (69.7%) | Cohort | 31 | 21.9 | 14.1 | China | 2.8 | 7 |
All‐cause mortality HF rehospitalization |
6 |
| Vaduganathan | 2014 | 65.8 | 395 (74.4%) | Cohort | 27.6 | 71 | 38.7 | USA | 0.8 | 8.8 |
All‐cause mortality Cardiac death HF rehospitalization |
7 |
| Filippatos | 2021 | 61 | 2645 (79.0%) | Cohort | 23 | 59 | 35.5 | USA, Canada | 2.1 | Male 8, women 6 |
All‐cause mortality Cardiac death HF rehospitalization |
7 |
| Fujihashi | 2021 | 69.1 | 4652 (65.4%) | Cohort | 55.5 | 87.8 | 39 | Japan | 6.3 | 9.2 |
All‐cause mortality HF rehospitalization |
7 |
| Zhou | 2019 | 66.0 | 535 (63.6%) | Cohort | 48.6 | 25.8 | 28.6 | China | 1.8 | 7 | All‐cause mortality | 8 |
| Hamaguchi | 2011 | 71.1 | 1869 (60.2%) | Cohort | 44.5 | 53.4 | 31.6 | Japan | 2.1 | 7.4 |
All‐cause mortality Cardiac death HF rehospitalization |
6 |
| Selvaraj | 2020 | 72.9 | 4795 (48.4%) | Cohort | 58 | 95.5 | 44.4 | Multiple country | NR | NR |
All‐cause mortality Cardiac death HF rehospitalization |
7 |
| Shimizu | 2015 | 68.1 | 424 (48.6%) | Cohort | 61.1 | 75 | 32.5 | Japan | 2.5 | 7 |
All‐cause mortality Cardiac death |
5 |
| Nishino | 2022 | 82.5 | 464 (49.8%) | Cohort | 60.5 | 88 | 33.4 | Japan | 1.3 | 8.3 |
All‐cause mortality HF rehospitalization |
6 |
| Ambrosio | 2021 | 64.6 | 4938 (72.1%) | Cohort | 37.5 | 7.9 | 34.3 | Italy | 1.5 | 6.6 |
Cardiac death HF rehospitalization |
7 |
| Niizeki | 2006 | 77.5 | 123 (NA) | Cohort | 49.7 | 54.7 | 19.5 | Japan | 1.2 | 6.5 |
Cardiac death HF rehospitalization |
6 |
DM, mellitus diabetes; HF, heart failure; HTN, hypertension; LVEF, left ventricular ejection fractional; NA, not available.
Table 3 summarized the baseline patient characteristic of 10 studies on the association between ULT and the prognosis of heart failure. A total of 19 294 patients were identified and analysed. Ten of the studies used traditional uric acid‐lowering drugs like XOIs and uricosuric drugs, while four studies used novel uric acid‐lowering drugs like sodium‐glucose cotransporter 2 inhibiter (SGLT2i) and angiotensin receptor‐neprilysin inhibitor (ARNi). The median age of the subjects ranged from 51.9 to 75 years, with a sample size ranging from 125 to 6204. Follow‐up time range from 0.5 to 4.8 years. Two studies were from the USA, 17 , 21 two studies were from the Czech Republic, 16 , 46 one study was from Japan, 43 two studies were from the UK, 15 , 47 and the others were from China, 19 Canada, 48 Israel, 14 and Italy, 49 respectively. Additionally, there were three international multicentre studies. 50 , 51 , 52 Four were RCT studies, and the rest were all cohort studies.
Table 3.
Baseline characteristics of the included studies on the association between ULT and the adverse outcomes of heart failure
| References | Year | Country | Study design | Sample size | Age (year) | Follow‐up (year) | Reported primary outcomes | NOS points |
|---|---|---|---|---|---|---|---|---|
| Givertz | 2015 | USA | RCT | 253 | 63 | 0.5 |
All‐cause mortality Cardiac death CV rehospitalization |
RCT |
| Hare | 2008 | Canada | RCT | 405 | 64.5 | 0.5 |
All‐cause mortality Cardiac death CV rehospitalization |
RCT |
| Pavlusova | 2019 | Czech Republic | Cohort | 3160 | 73 | 5 | All‐cause mortality | 7 |
| Málek | 2012 | Czech Republic | Cohort | 1159 | 73.4 | 1 | All‐cause mortality | 6 |
| Xiao | 2016 | China | RCT | 125 | 51.9 | 0.8 |
All‐cause mortality Cardiac death CV rehospitalization |
RCT |
| Gotsman | 2012 | Israel | Cohort | 6204 | 75 | 1.4 | All‐cause mortality | 7 |
| Wu | 2010 | USA | Cohort | 1152 | 64.8 | 1.5 | All‐cause mortality | 7 |
| Wei | 2009 | UK | Cohort | 4785 | 71.9 | 4.8 |
All‐cause mortality Cardiac death CV rehospitalization |
6 |
| Struthers | 2002 | UK | Cohort | 1760 | 66.9 | 4 |
All‐cause mortality Cardiac death CV rehospitalization |
4 |
| Nishino | 2022 | Japan | Cohort | 291 | 81.5 | 1.3 |
All‐cause mortality CV rehospitalization |
6 |
| McDowell | 2022 | Multiple countries | RCT | 253 | 67.3 | 1 |
All‐cause mortality |
RCT |
| Doehner | 2022 | Multiple countries | RCT | 405 | 66.8 | NR |
All‐cause mortality Cardiac death |
RCT |
| Mazza | 2020 | Italy | RCT | 3160 | 78.6 | 1 |
All‐cause mortality CV rehospitalization |
RCT |
| McMurray | 2014 | Multiple countries | RCT | 1159 | 63.8 | 2.3 |
All‐cause mortality Cardiac death CV rehospitalization |
RCT |
NOS, Newcastle–Ottawa Quality Assessment Scale; RCT, randomized controlled trials.
Association between serum uric acid and incidence of heart failure
A total of 11 studies with 434 865 patients reported the incidence of heart failure. Pooled analysis with estimates as categorical variables showed the association between the higher uric acid group and the incidence of heart failure (RR: 1.81, 95% CI: 1.53–2.16) (Figure 2 ). There was a distinct statistical heterogeneity within the studies (I 2 = 90.0%, P < 0.001) (Figure 2 ). We performed meta‐regression including gender, mean age, mean follow‐up time, percentage of hypertension, diabetes mellitus, coronary artery disease, and body mass index as covariables, and the results showed that they all had no statistical significance (P > 0.05) (Table S2); therefore, these covariables were not the primary source of heterogeneity. The pooled analysis of the two studies with the largest sample size showed that the odds of incidence of heart failure in the higher uric acid group increased by 32% and with a slight degree of heterogeneity (I 2 = 32%, P = 0.21) (Figure S2 ). The results of sensitivity analysis showed that there was no significant change in the effect size when we removed any of the studies (Figure S3 ), which suggested that our results were stable. We also conducted subgroup analyses according to publication year, sample size, and ethnicity, and all subsets consistently showed that hyperuricaemia was strongly associated with an increased risk of suffering from HF (Table S3 ). There was obvious publications bias based on funnel plot and Egger's test (Figure S4 ).
Figure 2.
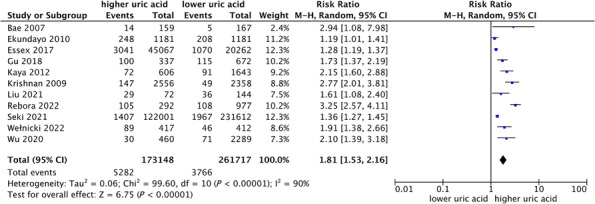
Forest plot of association between uric acid and incidence rate of heart failure.
Relations of serum uric acid and adverse outcomes among patients with heart failure
We included all studies that reported all‐cause mortality by categorical uric acid levels, and as shown in Figure 3 , elevated serum uric acid levels were associated with a significantly increased risk of all‐cause mortality (RR: 1.44, 95% CI: 1.25–1.66) in a random‐effect model, with appreciable heterogeneity (I 2 = 85.0%, P < 0.001) (Figure 3 ). Among these studies, three studies are HF with reduced EF and three studies were HF with preserved EF, with higher UA group led to 41% (RR: 1.41, 95%: 1.02–1.95) and 57% (RR: 1.57, 95%: 1.45–1.69) increases in the risk of all‐cause mortality in heart failure with preserved ejection fraction and heart failure with reduced ejection fraction, respectively (Figure 3 ). Sensitivity analysis indicated that none of the studies significantly influence the overall pooled risk summary (Figure S5 ). Meta‐regression suggested that no significant correlation between the preselected covariables and the all‐cause mortality was observed (Table S4 ). Subgroup analyses showed that in each subset, their effect sizes were significant (Table S5 ). No publication bias was found for the pooled all‐cause mortality of heart failure based on the funnel plot and Egger's test (Figure S6 ).
Figure 3.
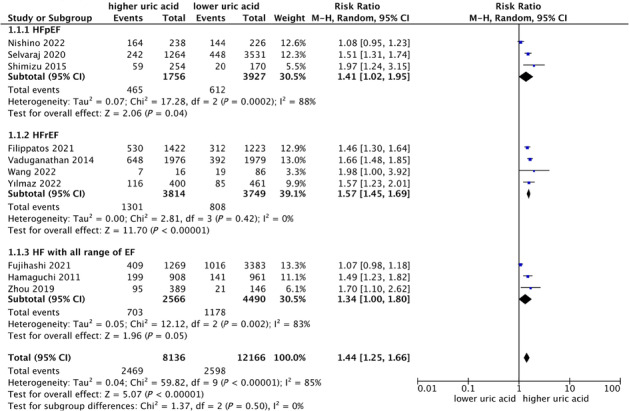
Forest plot of association between uric acid and all‐cause mortality of heart failure.
Of the 10 studies we included, seven studies reported the outcome of cardiovascular death, and seven studies reported the outcome of HF rehospitalization. They also suggested that hyperuricaemia was associated with an increased risk of cardiovascular death and HF hospitalization (cardiovascular death, RR: 1.56, 95% CI: 1.32–1.84; HF rehospitalization, RR: 2.07, 95% CI: 1.37–3.13) (Figures S7 and S8 ).
Effect of lowering uric acid on cardiovascular outcome in patients with heart failure
We included 14 studies involving 32 684 patents on uric acid‐lowering therapy for patients with heart failure, 10 of which were traditional uric acid‐lowering drugs, including XOI and uricosuric drugs, and four were novel uric acid‐lowering drugs, such as SGLT2i and ARNi. A pooled analysis of traditional urate‐lowering drugs showed that lowering uric acid treatment was associated with an increased risk of all‐cause mortality (RR: 1.15, 95% CI: 1.05–1.25) (Figure 4 ), with a moderate heterogeneity (I 2 = 49.0%) (Figure 4 ). In contrast, novel urate‐lowering drugs were associated with reduced all‐cause mortality in heart failure patients (RR: 0.87, 95% CI: 0.81–0.93) (Figure 4 ). To explore the heterogeneity of the studies, a subgroup analysis on all‐cause mortality was performed by ethnicity, study design, publication date, type of heart failure, and sample size. Apart from Asia and RCT did not reach statistical significance, the rest of the subsets showed that in HF patients, elevated UA level was associated with an increased risk of all‐cause mortality (Table S6 ). By univariate meta‐regression analysis (Table S7 ), we found a trend that the risk of all‐cause mortality in patients with heart failure who received urate‐lowering therapy gradually decreased with increasing age (P = 0.07) (Figure 5 ). We divided the age into two subgroups with 70 years old as the cutoff, and we found that among those younger than 70 years old, ULT was not associated with all‐cause mortality in patients with HF (RR: 1.09, 95% CI: 0.97–1.23), whereas in people older than 70 years, ULT is associated with all‐cause mortality in HF patients (RR: 1.26, 95% CI: 1.14–1.41). When we remove any of the studies, the results of sensitivity analysis suggest that there is no significant change in the effect size, indicating that our results have stability (Figure S9 ). No visual publication bias was found based on the funnel plot (Figure S10 ).
Figure 4.
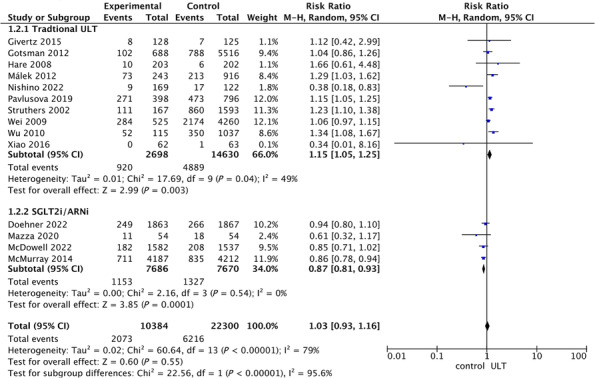
Forest plot of association between uric acid‐lowering therapy and all‐cause mortality of heart failure.
Figure 5.
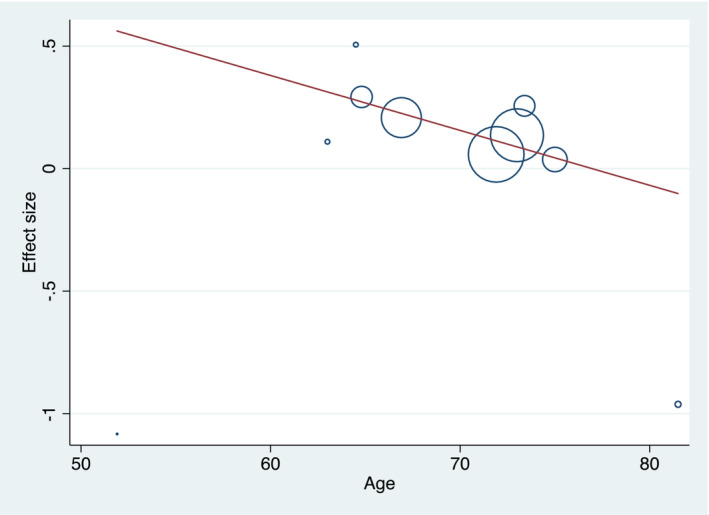
Univariate meta‐regression analysis (age) of association between ULT and all‐cause mortality of heart failure.
Five studies reported cardiovascular mortality, and heart failure patients with lowering uric acid treatment were more susceptible to cardiovascular death (RR: 1.29, 95% CI: 1.04–1.26) with a substantial statistical heterogeneity (I 2 = 56.0%) (Figure S11 ). Five studies showed HF rehospitalization, and the pooled data from the five studies reported that the effect of uric acid‐lowering therapy on cardiovascular hospitalization for heart failure patients did not reach statistical significance (RR: 1.09, 95% CI: 0.75–1.59) (Figure S12 ).
Discussion
Our systematic review and meta‐analysis systematically elucidate the relationship between uric acid and the occurrence and development of heart failure. Our findings indicate that elevated serum uric acid levels are associated with an increased risk of heart failure occurrence and prognosis. Uric acid serves as a predictor of all‐cause mortality, cardiovascular mortality, and HF rehospitalization in heart failure patients. However, it is important to note that reducing uric acid levels in heart failure patients does not improve long‐term prognosis and may even increase all‐cause mortality. Through meta‐regression analysis, we observed that this impact gradually decreases with age.
Several previous meta‐analyses have demonstrated the association between higher serum uric acid and the incidence and prognosis of HF. A meta‐analysis, which included five studies, published by Huang et al., pointed out that hyperuricaemia was associated with an increased risk of suffering from HF (HR: 1.65, 95% CI: 1.41–1.94), for every 1 mg/dL increase in SUA, the odds of development of HF increased by 19% (HR: 1.19, 95% CI: 1.17–1.21). 12 In a meta‐analysis published in 2021 by Miao et al., it was revealed that higher levels of serum uric acid are associated with an increased risk of all‐cause mortality (HR: 2.24, 95% CI: 1.49–3.37), cardiovascular mortality (HR: 1.26, 95% CI: 1.06–1.23), and the composite of death or cardiac events (HR: 1.26, 95% CI: 1.01–1.56) in patients with chronic heart failure. 53 , 54 Additionally, Tamariz et al. found that patients with HF and hyperuricaemia (SUA > 6.5 mg/dL) have an increased risk of death compared with those with normal uric acid levels. Huang et al. demonstrated that higher UA level independently predicts all‐cause mortality and composite of readmission or death in acute heart failure patients. 53 , 54
Although the specific pathological role of uric acid in the occurrence and development of HF is still not very clear, several speculations have been made: Hyperuricaemia has been associated with worse haemodynamic measures. 32 Functional upregulation of XO during purine metabolism can generate ROS and uric acid. ROS may contribute to the pathophysiological process of chronic heart failure, such as ventricular remodelling, myocardial fibrosis, cardiac hypertrophy, and impaired contractility. 55 , 56 , 57 , 58 Radovanovic et al. demonstrated that uric acid is associated with left ventricular remodelling in patients with chronic ischaemic. 59 Uric acid damages vascular endothelial cells and increases blood pressure, leading to a poor prognosis. 60 , 61 , 62 , 63 , 64 Additionally, in patients with heart failure, sympathetic nerve excitation results in the release of catecholamines, which constrict afferent arterioles, decreased glomerular filtration rate, decreased uric acid excretion, and increased blood uric acid concentration. Increased uric acid further activates the RAAS, leading to cardiac remodelling and poor prognosis. 32
Compared with other cardiac‐specific biomarkers, uric acid measurement has several advantages in assessing cardiovascular risk, heart‐related mortality, and heart failure readmissions. These advantages include the routine availability of uric acid measurement as a standard biochemical test that can be easily performed by collecting blood samples. In comparison with other biomarkers, the process of uric acid testing is simpler and more feasible for implementation. 65 Uric acid levels can provide additional information to cardiac‐specific biomarkers like BNP, NT‐proBNP, and troponins. While BNP and NT‐proBNP primarily reflect myocardial stretch and volume overload, and troponins indicate myocardial injury, uric acid reflects other aspects of HF pathophysiology, including vascular dysfunction and metabolic abnormalities. Combining multiple biomarkers, including uric acid, can provide a more comprehensive assessment of cardiovascular risk and prognosis. 66
Therefore, uric acid‐lowering therapy may be one of the potential targets for the treatment of heart failure. However, Kanbay et al. demonstrated that uric acid‐lowering therapy was associated with increased all‐cause mortality in heart failure patients (HR: 1.24, 95% CI: 1.04–1.49). 67 Similarly, in another meta‐analysis of randomized controlled trials conducted by Xu et al., involving 864 elderly patients with heart failure, uric acid‐lowering treatments did not show improvement in brain natriuretic peptide, the 6‐min walk test, left ventricular ejection fraction, cardiovascular death, and all‐cause mortality. Our comprehensive meta‐analysis consolidates previous findings and concludes that solely targeting uric acid‐lowering treatment for heart failure does not improve prognosis, indicating that elevated uric acid levels serve only as a predictive factor for heart failure.
Through meta‐regression analysis, we observed that younger patients were less responsive to traditional uric acid‐lowering drugs, and the effect of uric acid‐lowering therapy on the prognosis of heart failure would gradually decrease with increasing age. This phenomenon may be attributed to XO production of uric acid and generation of ROS, which have oxidative effects and can contribute to various cardiovascular diseases, including atherosclerosis, cardiac hypertrophy, myocardial fibrosis, left ventricular remodelling, and worsened heart failure. XO is upregulated within the heart in both experimental and human heart failure. 68 Thus, patients who are older and have a longer course of disease may have more XO in their hearts. Under such circumstances, the use of XOI may have a certain positive effect on prognosis, but this effect still cannot improve the prognosis of patients. Nevertheless, this is merely our speculation, and more experimental studies are needed to elucidate the specific mechanism. Research by Mazza et al. has shown that ARNi can improve UA levels in patients with heart failure with reduced ejection fraction, and a recent study by Butt et al. found that SGLT2i dapagliflozin had a urate‐lowering effect and reduced the initiation of new treatments for hyperuricaemia and gout. 69 Our study also included two novel urate‐lowering drugs. In contrast to traditional uric acid‐lowering drugs, these medications have been shown to improve the prognosis of heart failure. We further analysed the reasons for this and found that these two medications are not specifically targeted at reducing uric acid. The mechanism by which they lower uric acid is as follows: SGLT2 inhibitors exert their effects by blocking glucose reabsorption, resulting in increased glucosuria and subsequent osmotic diuresis. This diuretic effect may lead to increased uric acid excretion and a subsequent reduction in serum uric acid levels. ARNi reduces the activity of the renin‐angiotensin‐aldosterone system by inhibiting angiotensin‐converting enzyme and blocking the action of angiotensin II (Ang II), thereby reducing pressure in the glomerulus and improving uric acid excretion. These two drugs can improve heart failure, and the improvement of heart failure may also cause the reduction of uric acid. This may also be the reason why these two drugs have the opposite effect on the prognosis of heart failure compared with traditional urate‐lowering drugs. These were just our speculations, and further studies were needed to clarify the specific mechanism.
While uric acid can be used as a biomarker for the occurrence and prognosis of heart failure, it cannot be used as a targeted drug for the treatment of heart failure. In patients with heart failure, there are many factors contributing to increased uric acid levels, such as the use of diuretics, the activation of XO, and the impairment of renal function. Simple uric‐lowering therapy cannot improve the prognosis of patients with heart failure, and even increase the risk of death of patients.
To the best of our knowledge, this study is the first comprehensive meta‐analysis to examine the impact of uric acid on the occurrence and prognosis of heart failure, as well as the effect of uric acid reduction on the prognosis of heart failure patients. Our meta‐analysis revealed that UA is an independent predictor of all‐cause mortality, CV death, or HF rehospitalization. The literature included in our meta‐analysis is relatively comprehensive, with a large sample size, which provides a certain degree of reliability and stability to the results. However, several limitations should be acknowledged. Firstly, our meta‐analysis exhibited significant heterogeneity, which could be attributed to differences in patient characteristics, treatments used, and follow‐up durations among the included studies. Despite conducting subgroup and sensitivity analyses, the exact source of heterogeneity could not be identified. Secondly, individual studies have different thresholds for high or low uric acid, which might contribute to the relatively large heterogeneity observed in our results. Thirdly, the funnel plots for heart failure incidence and all‐cause mortality displayed asymmetrical, suggesting a potential publication bias, possibly due to some small unpublished studies. We did not include conference proceedings, which might mean that we might miss some small, unpublished studies. Fourthly, UA levels in our included literature are all categorical variables, which may lead to biased results. Fifthly, although our findings suggest a decreased effectiveness of uric acid‐lowering therapy on heart failure prognosis with increasing age, the P‐value did not reach statistical significance (P = 0.07). Therefore, future studies involving patients with heart failure across a wider age range are necessary to strengthen this conclusion. Sixthly, for the first question, the results of all the studies are concordant, which tends to reduce the relevance of the meta‐analytic assessment. Finally, due to inadequate data, the relationship between uric acid and different types of heart failure could not be assessed in our meta‐analysis.
Conclusions
Uric acid is an independent predictor of heart failure occurrence and prognosis. Targeting uric acid lowering as a therapeutic intervention does not improve the prognosis of patients with heart failure. It may not be advisable to use traditional urate‐lowering drugs in young patients with heart failure, and elderly patients should exercise caution when using them.
Conflict of interest
The authors have no conflict of interest.
Funding
This work had no relationships with industry and received no financial support.
Supporting information
Appendix S1. The literature search strategies.
Table S1. The quality assessment for literatures of observational studies.
Figure S1. The quality assessment for literatures of randomized controlled trials.
Table S2. meta‐regression for preselected covariates. (Association between higher uric acid and incidence rate of heart failure).
Table S3. subgroup analysis of associations between higher uric acid and incidence rate of heart failure.
Table S4. meta‐regression for preselected covariates. (Association between uric acid and adverse outcomes of heart failure).
Table S5. subgroup analysis of associations between uric acid and adverse outcomes of heart failure.
Table S6. subgroup analysis of associations between uric acid‐lowering therapy and prognosis of heart failure.
Table S7. meta‐regression for preselected covariates. (Association between uric acid‐lowering therapy and prognosis of heart failure patients).
Figure S2. Forest plot of the two studies with the largest sample size on the association between UA and incidence rate of HF.
Figure S3. The sensitivity analysis of the association between uric acid and incidence rate of heart failure.
Figure S4. publication bias of studies on the association between UA and incidence rate of HF:(A) Funnel plot; (B) Egger's test.
Figure S5. The sensitivity analysis of the association between uric acid and all‐cause mortality of heart failure.
Figure S6. publication bias of studies on the association between UA and all‐cause mortality of HF:(A) Funnel plot; (B) Egger's test.
Figure S7. Forest plot of association between uric acid and cardiovascular death of heart failure.
Figure S8. Forest plot of association between uric acid and cardiovascular death of HF hospitalization.
Figure S9. The sensitivity analysis of the association between uric acid‐lowering therapy and all‐cause mortality of heart failure.
Figure S10. publication bias of studies on the association between uric acid‐lowering therapy and all‐cause mortality of HF:(A) Funnel plot; (B) Egger's test.
Figure S11. Forest plot of association between uric acid‐lowering therapy and cardiovascular death of heart failure.
Figure S12. Forest plot of association between uric acid‐lowering therapy and HF rehospitalization of heart failure.
Acknowledgements
We thank all the participants in the study.
Qin, S. , Xiang, M. , Gao, L. , Cheng, X. , and Zhang, D. (2024) Uric acid is a biomarker for heart failure, but not therapeutic target: result from a comprehensive meta‐analysis. ESC Heart Failure, 11: 78–90. 10.1002/ehf2.14535.
Shiwei Qin and Meilin Xiang contributed equally to this work.
Contributor Information
Xiaocheng Cheng, Email: chengxiaocheng@cqmu.edu.cn.
Dongying Zhang, Email: zhangdongying@cqmu.edu.cn.
References
- 1. McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Böhm M, Dickstein K, et al. ESC Committee for Practice GuidelinesESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J 2012;33:1787‐1847. doi: 10.1093/eurheartj/ehs104 [DOI] [PubMed] [Google Scholar]
- 2. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. American Heart Association Statistics Committee and Stroke Statistics SubcommitteeHeart disease and stroke statistics‐‐2015 update: a report from the American Heart Association. Circulation 2015;131:e29‐e322. doi: 10.1161/CIR.0000000000000152 [DOI] [PubMed] [Google Scholar]
- 3. Afsar B, Rossignol P, van Heerebeek L, Paulus WJ, Damman K, Heymans S, et al. Heart failure with preserved ejection fraction: a nephrologist‐directed primer. Heart Fail Rev 2017;22:765‐773. doi: 10.1007/s10741-017-9619-2 [DOI] [PubMed] [Google Scholar]
- 4. Savarese G, Lund LH. Global public health burden of heart failure. Card Fail Rev 2017;3:7‐11. doi: 10.15420/cfr.2016:25:2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Maiuolo J, Oppedisano F, Gratteri S, Muscoli C, Mollace V. Regulation of uric acid metabolism and excretion. Int J Cardiol 2016;213:8‐14. doi: 10.1016/j.ijcard.2015.08.109 [DOI] [PubMed] [Google Scholar]
- 6. Davies KJ, Sevanian A, Muakkassah‐Kelly SF, Hochstein P. Uric acid‐iron ion complexes. A new aspect of the antioxidant functions of uric acid. Biochem J 1986;235:747‐754. doi: 10.1042/bj2350747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Doehner W, Jankowska EA, Springer J, Lainscak M, Anker SD. Uric acid and xanthine oxidase in heart failure ‐ emerging data and therapeutic implications. Int J Cardiol 2016;213:15‐19. doi: 10.1016/j.ijcard.2015.08.089 [DOI] [PubMed] [Google Scholar]
- 8. Kang DH, Ha SK. Uric acid puzzle: dual role as anti‐oxidantand pro‐oxidant. Electrolyte Blood Press 2014;12:1‐6. doi: 10.5049/EBP.2014.12.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bergamini C, Cicoira M, Rossi A, Vassanelli C. Oxidative stress and hyperuricaemia: pathophysiology, clinical relevance, and therapeutic implications in chronic heart failure. Eur J Heart Fail 2009;11:444‐452. doi: 10.1093/eurjhf/hfp042 [DOI] [PubMed] [Google Scholar]
- 10. Reyes AJ. The increase in serum uric acid concentration caused by diuretics might be beneficial in heart failure. Eur J Heart Fail 2005;7:461‐467. doi: 10.1016/j.ejheart.2004.03.020 [DOI] [PubMed] [Google Scholar]
- 11. Abu Sneineh M, Schwartz Y, Nesher G, Freier Dror Y, Breuer GS. Uric acid level as a predictor of long‐term mortality in advanced age population. Am J Med Sci 2020;359:27‐31. doi: 10.1016/j.amjms.2019.10.017 [DOI] [PubMed] [Google Scholar]
- 12. Huang H, Huang B, Li Y, Huang Y, Li J, Yao H, et al. Uric acid and risk of heart failure: a systematic review and meta‐analysis. Eur J Heart Fail 2014;16:15‐24. doi: 10.1093/eurjhf/hft132 [DOI] [PubMed] [Google Scholar]
- 13. Breuer GS, Schwartz Y, Freier‐Dror Y, Nesher G. Uric acid level as predictor of mortality in the acute care setting of advanced age population. Eur J Intern Med 2017;44:74‐76. doi: 10.1016/j.ejim.2017.07.021 [DOI] [PubMed] [Google Scholar]
- 14. Gotsman I, Keren A, Lotan C, Zwas DR. Changes in uric acid levels and allopurinol use in chronic heart failure: association with improved survival. J Card Fail 2012;18:694‐701. doi: 10.1016/j.cardfail.2012.06.528 [DOI] [PubMed] [Google Scholar]
- 15. Wei L, Fahey T, Struthers AD, MacDonald TM. Association between allopurinol and mortality in heart failure patients: a long‐term follow‐up study. Int J Clin Pract 2009;63:1327‐1333. doi: 10.1111/j.1742-1241.2009.02118.x [DOI] [PubMed] [Google Scholar]
- 16. Málek F, Ošťádal P, Pařenica J, Jarkovský J, Vítovec J, Widimský P, et al. Uric acid, allopurinol therapy, and mortality in patients with acute heart failure‐‐results of the acute HEart FAilure database registry. J Crit Care 2012;27:737.e11‐737.e24. doi: 10.1016/j.jcrc.2012.03.011 [DOI] [PubMed] [Google Scholar]
- 17. Givertz MM, Anstrom KJ, Redfield MM, Deswal A, Haddad H, Butler J, et al. Effects of xanthine oxidase inhibition in hyperuricemic heart failure patients: the xanthine oxidase inhibition for hyperuricemic heart failure patients (EXACT‐HF) study. Circulation 2015;131:1763‐1771. doi: 10.1161/CIRCULATIONAHA.114.014536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ansari‐Ramandi MM, Maleki M, Alizadehasl A, Amin A, Taghavi S, Alemzadeh‐Ansari MJ, et al. Safety and effect of high dose allopurinol in patients with severe left ventricular systolic dysfunction. J Cardiovasc Thorac Res 2017;9:102‐107. doi: 10.15171/jcvtr.2017.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Xiao J, Deng SB, She Q, Li J, Kao GY, Wang JS, et al. Allopurinol ameliorates cardiac function in non‐hyperuricaemic patients with chronic heart failure. Eur Rev Med Pharmacol Sci 2016;20:756‐761. [PubMed] [Google Scholar]
- 20. Nasr G, Maurice C. Allopurinol and global left myocardial function in heart failure patients. J Cardiovasc Dis Res 2010;1:191‐195. doi: 10.4103/0975-3583.74262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wu AH, Ghali JK, Neuberg GW, O'Connor CM, Carson PE, Levy WC. Uric acid level and allopurinol use as risk markers of mortality and morbidity in systolic heart failure. Am Heart J 2010;160:928‐933. doi: 10.1016/j.ahj.2010.08.006 [DOI] [PubMed] [Google Scholar]
- 22. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. doi: 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stang A. Critical evaluation of the Newcastle‐Ottawa scale for the assessment of the quality of nonrandomized studies in meta‐analyses. Eur J Epidemiol 2010;25:603‐605. doi: 10.1007/s10654-010-9491-z [DOI] [PubMed] [Google Scholar]
- 24. Wu X, Jian G, Tang Y, Cheng H, Wang N, Wu J. Asymptomatic hyperuricemia and incident congestive heart failure in elderly patients without comorbidities. Nutr Metab Cardiovasc Dis 2020;30:666‐673. doi: 10.1016/j.numecd.2019.12.008 [DOI] [PubMed] [Google Scholar]
- 25. Liu CF, Song KY, Zhou WN, Wei YJ. Association between uric acid and in‐hospital heart failure in patients with acute myocardial infarction undergoing percutaneous coronary intervention. Dis Markers 2021;2021:7883723. doi: 10.1155/2021/7883723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gu J, Fan YQ, Zhang HL, Zhang JF, Wang CQ. Serum uric acid is associated with incidence of heart failure with preserved ejection fraction and cardiovascular events in patients with arterial hypertension. J Clin Hypertens (Greenwich) 2018;20:560‐567. doi: 10.1111/jch.13210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wełnicki M, Gorczyca‐Głowacka I, Lubas A, Wójcik W, Jelonek O, Maciorowska M, et al. Association of Hyperuricemia with impaired left ventricular systolic function in patients with atrial fibrillation and preserved kidney function: analysis of the POL‐AF registry cohort. Int J Environ Res Public Health 2022;19:7288. doi: 10.3390/ijerph19127288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rebora P, Centola M, Morici N, Sacco A, Occhino G, Viola G, et al. Uric acid associated with acute heart failure presentation in acute coronary syndrome patients. Eur J Intern Med 2022;99:30‐37. doi: 10.1016/j.ejim.2022.01.018 [DOI] [PubMed] [Google Scholar]
- 29. Seki H, Kaneko H, Morita H, Itoh H, Morita K, Matsuoka S, et al. Relation of serum uric acid and cardiovascular events in young adults aged 20‐49 years. Am J Cardiol 2021;152:150‐157. doi: 10.1016/j.amjcard.2021.05.007 [DOI] [PubMed] [Google Scholar]
- 30. Essex MN, Hopps M, Bienen EJ, Udall M, Mardekian J, Makinson GT. Evaluation of the relationship between serum uric acid levels and cardiovascular events in patients with gout: a retrospective analysis using electronic medical record data. J Clin Rheumatol 2017;23:160‐166. doi: 10.1097/RHU.0000000000000496 [DOI] [PubMed] [Google Scholar]
- 31. Ekundayo OJ, Dell'Italia LJ, Sanders PW, Arnett D, Aban I, Love TE, et al. Association between hyperuricemia and incident heart failure among older adults: a propensity‐matched study. Int J Cardiol 2010;142:279‐287. doi: 10.1016/j.ijcard.2009.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Krishnan E. Hyperuricemia and incident heart failure. Circ Heart Fail 2009;2:556‐562. doi: 10.1161/CIRCHEARTFAILURE.108.797662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kaya MG, Uyarel H, Akpek M, Kalay N, Ergelen M, Ayhan E, et al. Prognostic value of uric acid in patients with ST‐elevated myocardial infarction undergoing primary coronary intervention. Am J Cardiol 2012;109:486‐491. doi: 10.1016/j.amjcard.2011.09.042 [DOI] [PubMed] [Google Scholar]
- 34. Bae J‐H. Serum uric acid is associated with cardiovascular events in patients with coronary artery disease. 2007.
- 35. Wang X, Fan X, Wu Q, Liu J, Wei L, Yang D, et al. Uric acid predicts recovery of left ventricular function and adverse events in heart failure with reduced ejection fraction: potential mechanistic insight from network analyses. Front Cardiovasc Med 2022;9:853870. doi: 10.3389/fcvm.2022.1107544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhou HB, Xu TY, Liu SR, Bai YJ, Huang XF, Zhan Q, et al. Association of serum uric acid change with mortality, renal function and diuretic dose administered in treatment of acute heart failure. Nutr Metab Cardiovasc Dis 2019;29:351‐359. doi: 10.1016/j.numecd.2019.01.001 [DOI] [PubMed] [Google Scholar]
- 37. Vaduganathan M, Greene SJ, Ambrosy AP, Mentz RJ, Subacius HP, Chioncel O, et al. EVEREST trial investigatorsRelation of serum uric acid levels and outcomes among patients hospitalized for worsening heart failure with reduced ejection fraction (from the efficacy of vasopressin antagonism in heart failure outcome study with tolvaptan trial). Am J Cardiol 2014;114:1713‐1721. doi: 10.1016/j.amjcard.2014.09.008 [DOI] [PubMed] [Google Scholar]
- 38. Filippatos GS, Ahmed MI, Gladden JD, Mujib M, Aban IB, Love TE, et al. Hyperuricaemia, chronic kidney disease, and outcomes in heart failure: potential mechanistic insights from epidemiological data. Eur Heart J 2011;32:712‐720. doi: 10.1093/eurheartj/ehq473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yılmaz Öztekin GM, Genç A, Çağırcı G, Arslan Ş. Prognostic value of the combination of uric acid and NT‐proBNP in patients with chronic heart failure. Hellenic J Cardiol 2022;65:35‐41. doi: 10.1016/j.hjc.2022.03.009 [DOI] [PubMed] [Google Scholar]
- 40. Fujihashi T, Sakata Y, Nochioka K, Miura M, Abe R, Kasahara S, et al. Prognostic impacts of serum uric acid levels in patients with chronic heart failure: insights from the CHART‐2 study. ESC Heart Fail 2021;8:1027‐1038. doi: 10.1002/ehf2.12765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hamaguchi S, Furumoto T, Tsuchihashi‐Makaya M, Goto K, Goto D, Yokota T, et al. Hyperuricemia predicts adverse outcomes in patients with heart failure. Int J Cardiol 2011;151:143‐147. doi: 10.1016/j.ijcard.2010.05.002 [DOI] [PubMed] [Google Scholar]
- 42. Shimizu T, Yoshihisa A, Kanno Y, Takiguchi M, Sato A, Miura S, et al. Relationship of hyperuricemia with mortality in heart failure patients with preserved ejection fraction. Am J Physiol Heart Circ Physiol 2015;309:H1123‐H1129. doi: 10.1152/ajpheart.00533.2015 [DOI] [PubMed] [Google Scholar]
- 43. Nishino M, Egami Y, Kawanami S, Sugae H, Ukita K, Kawamura A, et al. Lowering uric acid may improve prognosis in patients with hyperuricemia and heart failure with preserved ejection fraction. J Am Heart Assoc 2022;11:e026301. doi: 10.1161/JAHA.122.026301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Niizeki T, Takeishi Y, Arimoto T, Okuyama H, Nozaki N, Hirono O, et al. Hyperuricemia associated with high cardiac event rates in the elderly with chronic heart failure. J Cardiol 2006;47:219‐228. [PubMed] [Google Scholar]
- 45. Selvaraj S, Claggett BL, Pfeffer MA, Desai AS, Mc Causland FR, McGrath MM, et al. Serum uric acid, influence of sacubitril‐valsartan, and cardiovascular outcomes in heart failure with preserved ejection fraction: PARAGON‐HF. Eur J Heart Fail 2020;22:2093‐2101. doi: 10.1002/ejhf.1984 [DOI] [PubMed] [Google Scholar]
- 46. Pavlusova M, Jarkovsky J, Benesova K, Vitovec J, Linhart A, Widimsky P, et al. Hyperuricemia treatment in acute heart failure patients does not improve their long‐term prognosis: a propensity score matched analysis from the AHEAD registry. Clin Cardiol 2019;42:720‐727. doi: 10.1002/clc.23197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Struthers AD, Donnan PT, Lindsay P, McNaughton D, Broomhall J, MacDonald TM. Effect of allopurinol on mortality and hospitalisations in chronic heart failure: a retrospective cohort study. Heart 2002;87:229‐234. doi: 10.1136/heart.87.3.229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hare JM, Mangal B, Brown J, Fisher C Jr, Freudenberger R, Colucci WS, et al. Impact of oxypurinol in patients with symptomatic heart failure. Results of the OPT‐CHF study. J Am Coll Cardiol 2008;51:2301‐2309. doi: 10.1016/j.jacc.2008.01.068 [DOI] [PubMed] [Google Scholar]
- 49. Mazza A, Townsend DM, Torin G, Schiavon L, Camerotto A, Rigatelli G, et al. The role of sacubitril/valsartan in the treatment of chronic heart failure with reduced ejection fraction in hypertensive patients with comorbidities: from clinical trials to real‐world settings. Biomed Pharmacother 2020;130:110596. doi: 10.1016/j.biopha.2020.110596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. McDowell K, Welsh P, Docherty KF, Morrow DA, Jhund PS, de Boer RA, et al. Dapagliflozin reduces uric acid concentration, an independent predictor of adverse outcomes in DAPA‐HF. Eur J Heart Fail 2022;24:1066‐1076. doi: 10.1002/ejhf.2433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Doehner W, Anker SD, Butler J, Zannad F, Filippatos G, Ferreira JP, et al. Uric acid and sodium‐glucose cotransporter‐2 inhibition with empagliflozin in heart failure with reduced ejection fraction: the EMPEROR‐reduced trial. Eur Heart J 2022;43:3435‐3446. doi: 10.1093/eurheartj/ehac320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, et al. PARADIGM‐HF Investigators and CommitteesAngiotensin‐neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014;371:993‐1004. doi: 10.1056/NEJMoa1409077 [DOI] [PubMed] [Google Scholar]
- 53. Tamariz L, Harzand A, Palacio A, Verma S, Jones J, Hare J. Uric acid as a predictor of all‐cause mortality in heart failure: a meta‐analysis. Congest Heart Fail 2011;17:25‐30. doi: 10.1111/j.1751-7133.2011.00200.x [DOI] [PubMed] [Google Scholar]
- 54. Huang G, Qin J, Deng X, Luo G, Yu D, Zhang M, et al. Prognostic value of serum uric acid in patients with acute heart failure: a meta‐analysis. Medicine (Baltimore) 2019;98:e14525. doi: 10.1097/MD.0000000000014525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Bendall JK, Cave AC, Heymes C, Gall N, Shah AM. Pivotal role of a gp91(phox)‐containing NADPH oxidase in angiotensin II‐induced cardiac hypertrophy in mice. Circulation 2002;105:293‐296. doi: 10.1161/hc0302.103712 [DOI] [PubMed] [Google Scholar]
- 56. Sun Y. Oxidative stress and cardiac repair/remodeling following infarction. Am J Med Sci 2007;334:197‐205. [DOI] [PubMed] [Google Scholar]
- 57. Prasad K, Kalra J, Chan WP, Chaudhary AK. Effect of oxygen free radicals on cardiovascular function at organ and cellular levels. Am Heart J 1989;117:1196‐1202. doi: 10.1016/0002-8703(89)90396-7 [DOI] [PubMed] [Google Scholar]
- 58. Cicoira M, Zanolla L, Rossi A, Golia G, Franceschini L, Brighetti G, et al. Elevated serum uric acid levels are associated with diastolic dysfunction in patients with dilated cardiomyopathy. Am Heart J 2002;143:1107‐1111. doi: 10.1067/mhj.2002.122122 [DOI] [PubMed] [Google Scholar]
- 59. Radovanovic S, Savic‐Radojevic A, Pekmezovic T, Markovic O, Memon L, Jelic S, et al. Uric acid and gamma‐glutamyl transferase activity are associated with left ventricular remodeling indices in patients with chronic heart failure. Rev Esp Cardiol (Engl Ed) 2014;67:632‐642. doi: 10.1016/j.recesp.2013.11.017 [DOI] [PubMed] [Google Scholar]
- 60. Waring WS, Adwani SH, Breukels O, Webb DJ, Maxwell SR. Hyperuricaemia does not impair cardiovascular function in healthy adults. Heart 2004;90:155‐159. doi: 10.1136/hrt.2003.016121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Farquharson CA, Butler R, Hill A, Belch JJ, Struthers AD. Allopurinol improves endothelial dysfunction in chronic heart failure. Circulation 2002;106:221‐226. doi: 10.1161/01.CIR.0000022140.61460.1D [DOI] [PubMed] [Google Scholar]
- 62. Saito Y, Kitahara H, Nakayama T, Fujimoto Y, Kobayashi Y. Relation of elevated serum uric acid level to endothelial dysfunction in patients with acute coronary syndrome. J Atheroscler Thromb 2019;26:362‐367. doi: 10.5551/jat.45179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Mercuro G, Vitale C, Cerquetani E, Zoncu S, Deidda M, Fini M, et al. Effect of hyperuricemia upon endothelial function in patients at increased cardiovascular risk. Am J Cardiol 2004;94:932‐935. doi: 10.1016/j.amjcard.2004.06.032 [DOI] [PubMed] [Google Scholar]
- 64. Feig DI, Kang DH, Johnson RJ. Uric acid and cardiovascular risk. N Engl J Med 2008;359:1811‐1821. doi: 10.1056/NEJMra0800885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Anker SD, Doehner W, Rauchhaus M, Sharma R, Francis D, Knosalla C, et al. Uric acid and survival in chronic heart failure: validation and application in metabolic, functional, and hemodynamic staging. Circulation 2003;107:1991‐1997. doi: 10.1161/01.CIR.0000065637.10517.A0 [DOI] [PubMed] [Google Scholar]
- 66. Feig DI, Soletsky B, Johnson RJ. Effect of allopurinol on blood pressure of adolescents with newly diagnosed essential hypertension: a randomized trial. JAMA 2008;300:924‐932. doi: 10.1001/jama.300.8.924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kanbay M, Afsar B, Siriopol D, Dincer N, Erden N, Yilmaz O, et al. Effect of uric acid‐lowering agents on cardiovascular outcome in patients with heart failure: a systematic review and meta‐analysis of clinical studies. Angiology 2020;71:315‐323. doi: 10.1177/0003319719897509 [DOI] [PubMed] [Google Scholar]
- 68. Hare JM, Johnson RJ. Uric acid predicts clinical outcomes in heart failure: insights regarding the role of xanthine oxidase and uric acid in disease pathophysiology. Circulation 2003;107:1951‐1953. doi: 10.1161/01.CIR.0000066420.36123.35 [DOI] [PubMed] [Google Scholar]
- 69. Butt JH, Docherty KF, Claggett BL, Desai AS, Petersson M, Langkilde AM, et al. Association of dapagliflozin use with clinical outcomes and the introduction of uric acid‐lowering therapy and colchicine in patients with heart failure with and without gout: a patient‐level pooled meta‐analysis of DAPA‐HF and DELIVER. JAMA Cardiol 2023;8:386‐393. doi: 10.1001/jamacardio.2022.5608 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. The literature search strategies.
Table S1. The quality assessment for literatures of observational studies.
Figure S1. The quality assessment for literatures of randomized controlled trials.
Table S2. meta‐regression for preselected covariates. (Association between higher uric acid and incidence rate of heart failure).
Table S3. subgroup analysis of associations between higher uric acid and incidence rate of heart failure.
Table S4. meta‐regression for preselected covariates. (Association between uric acid and adverse outcomes of heart failure).
Table S5. subgroup analysis of associations between uric acid and adverse outcomes of heart failure.
Table S6. subgroup analysis of associations between uric acid‐lowering therapy and prognosis of heart failure.
Table S7. meta‐regression for preselected covariates. (Association between uric acid‐lowering therapy and prognosis of heart failure patients).
Figure S2. Forest plot of the two studies with the largest sample size on the association between UA and incidence rate of HF.
Figure S3. The sensitivity analysis of the association between uric acid and incidence rate of heart failure.
Figure S4. publication bias of studies on the association between UA and incidence rate of HF:(A) Funnel plot; (B) Egger's test.
Figure S5. The sensitivity analysis of the association between uric acid and all‐cause mortality of heart failure.
Figure S6. publication bias of studies on the association between UA and all‐cause mortality of HF:(A) Funnel plot; (B) Egger's test.
Figure S7. Forest plot of association between uric acid and cardiovascular death of heart failure.
Figure S8. Forest plot of association between uric acid and cardiovascular death of HF hospitalization.
Figure S9. The sensitivity analysis of the association between uric acid‐lowering therapy and all‐cause mortality of heart failure.
Figure S10. publication bias of studies on the association between uric acid‐lowering therapy and all‐cause mortality of HF:(A) Funnel plot; (B) Egger's test.
Figure S11. Forest plot of association between uric acid‐lowering therapy and cardiovascular death of heart failure.
Figure S12. Forest plot of association between uric acid‐lowering therapy and HF rehospitalization of heart failure.


