Abstract
Aims
The multi‐systemic effects of heart failure (HF) resemble the spread observed during cancer. We propose a new score, named HLM, analogous to the TNM classification used in oncology, to assess the prognosis of HF. HLM refers to H: heart damage, L: lung involvement, and M: systemic multiorgan involvement. The aim was to compare the HLM score to the conventional New York Heart Association (NYHA) classification, American College of Cardiology/American Heart Association (ACC/AHA) stages, and left ventricular ejection fraction (LVEF), to assess the most accurate prognostic tool for HF patients.
Methods and results
We performed a multicentre, observational, prospective study of consecutive patients admitted for HF. Heart, lung, and other organ function parameters were collected. Each patient was classified according to the HLM score, NYHA classification, ACC/AHA stages, and LVEF assessed by transthoracic echocardiography. The follow‐up period was 12 months. The primary endpoint was a composite of all‐cause death and rehospitalization due to HF. A total of 1720 patients who completed the 12 month follow‐up period have been enrolled in the study. 520 (30.2%) patients experienced the composite endpoint of all‐cause death and rehospitalization due to HF. 540 (31.4%) patients were female. The mean age of the study population was 70.5 ± 12.9. The mean LVEF at admission was 42.5 ± 13%. Regarding the population distribution across the spectrum of HLM score stages, 373 (21.7%) patients were included in the HLM‐1, 507 (29.5%) in the HLM‐2, 587 (34.1%) in the HLM‐3, and 253 (14.7%) in the HLM‐4. HLM was the most accurate score to predict the primary endpoint at 12 months. The area under the receiver operating characteristic curve (AUC) was greater for the HLM score compared with the NYHA classification, ACC/AHA stages, or LVEF, regarding the composite endpoint (HLM = 0.645; NYHA = 0.580; ACC/AHA = 0.589; LVEF = 0.572). The AUC of the HLM score was significantly better compared with the LVEF (P = 0.002), ACC/AHA (P = 0.029), and NYHA (P = 0.009) AUC.
Conclusions
The HLM score has a greater prognostic power compared with the NYHA classification, ACC/AHA stages, and LVEF assessed by transthoracic echocardiography in terms of the composite endpoint of all‐cause death and rehospitalization due to HF at 12 months of follow‐up.
Keywords: Heart failure, HLM score, Prognosis, All‐cause mortality, Rehospitalization
Introduction
Heart failure (HF) is a multifaceted syndrome resulting in high rates of morbidity and mortality that can be considered a non‐infectious pandemic of the third millennium. 1 HF affects more than 37 million people worldwide, involving approximately 1–2% of the general population in developed countries. 1 The 1 year hospitalization rate is 32% for HF outpatients and 44% for patients hospitalized due to HF, with an all‐cause mortality rate ranging between 7% and 17%. 2 , 3
In terms of disease progression, HF could be defined as ‘the cancer of the heart’. As HF progresses, other organs get affected, leading to multi‐systemic organ dysfunction and eventually death. The progressive decline of heart function results in variable involvement of other organs, with the worst prognosis reserved for the most advanced stages. Hence, patients' prognostic stratification has a crucial role for optimal patient care, helping clinicians in defining which patient would likely benefit from invasive and expensive therapeutic procedures, such as left ventricular (LV) assist device implantation and heart transplantation. 1 , 2 , 3 According to the latest guidelines released by the European Society of Cardiology 2 and by the American Heart Association (AHA)/American College of Cardiology (ACC), 4 several new strategies have been proposed to classify and stratify HF patients. 2 , 4 , 5 , 6 , 7 , 8 , 9 , 10 However, all these applications have limits: The parameters on which they are based on are often unavailable or are variable and operator dependent and, therefore, not easily reproducible. Solid data regarding control groups are scarce, and the parameters have poor reliability at the individual level. 5 , 6 , 7 , 8 , 9 , 10 In this context, new and reliable risk models are eagerly awaited.
Previously, we proposed a new staging system for HF, named HLM, 11 , 12 in analogy to the TNM classification used in oncology. In the TNM classification system, the first letter ‘T’ indicates the primary tumour extent, likewise ‘H’, for heart, indicates the severity of the cardiac involvement, while ‘L’ stands for lung involvement, the first organ reached by the spread of heart disease, in analogy with the ‘N’ for lymph nodes in TNM. The third letter ‘M’, which stands for metastasis in the TNM classification, represents systemic multiorgan involvement in the HLM classification (i.e. kidney, liver, brain, and haematopoietic system). The similarities between cancer and HF, in terms of disease evolution, as well as the systemic involvement and deleterious consequences on other organs, led us to hypothesize that a holistic approach regarding this multiorgan syndrome could improve the prognostic stratification and therapeutic achievements. In this study, we developed the HLM score, a prognostic risk model used in a cohort of patients previously hospitalized for HF. We compared its prognostic performance with the main current models in predicting the composite of all‐cause death and rehospitalization due to HF, at 12 months of follow‐up.
Methods
Patients' selection
A multicentre, prospective observational study was conducted on consecutive patients hospitalized due to HF between January 2015 and December 2020 at (i) the Department of Clinical, Internal, Anesthesiology and Cardiovascular Sciences of Sapienza University of Rome; (ii) the Department of Cardiovascular Disease, Le Scotte Hospital, University of Siena; and (iii) the Division of Cardiovascular Medicine, University of Pennsylvania, Philadelphia. Inclusion criteria were (i) patients aged 18 and above; (ii) HF diagnosis or risk for HF, according to current guidelines 2 , 4 who were hospitalized during the case period; and (iii) ability to express and sign written informed consent. The following data were collected: epidemiological, clinical, and echocardiographic parameters including left ventricular ejection fraction (LVEF) at admission; data from previous HF hospitalizations; risk factors that predispose to the development of HF (systemic arterial hypertension, diabetes mellitus, dyslipidaemia, smoking habits, and familiar history of cardiovascular diseases); and New York Heart Association (NYHA) class, HLM classification, and ACC/AHA stage at hospital admission. All variables were collected and screened independently by their univariable association with the composite endpoint. Exclusion criteria were (i) diagnosis of any malignancy reducing short‐term life expectancy; (ii) incomplete NYHA, HLM, ACC/AHA stage, and LVEF values at hospital admission; and (iii) lack of follow‐up data.
Each patient was classified according to NYHA and ACC/AHA classifications, LVEF values, and HLM score.
Primary objective
The outcome of the study was the composite of all‐cause death and rehospitalization due to HF. The study protocol (Prot. No. 701/16; Rif. Ce. 4250) was approved by the ethics committee of each centre involved. All the patients gave written informed consent to participate in the study. The study conforms with the principles outlined in the Declaration of Helsinki.
HLM classification
In analogy to the TNM, 13 the HLM classification relies on the evaluation of three parameters: ‘H’ for heart, ‘L’ for lung, and ‘M’ for systemic multiorgan involvement. Each parameter is divided into four levels of severity (H1–H4, L0–L3, and M0–M3) (Table 1 ).
Table 1.
HLM classification
| Heart (H) | Lungs (L) | Other organs (M) |
|---|---|---|
| H1: Diastolic dysfunction and/or presence of structural cardiac damage a in absence of LV systolic dysfunction (LVEF ≥ 50%) | L0: Absence of any lung involvement | M0: Absence of malfunction of other organs b |
| H2: LV systolic (LVEF < 50%) or diastolic dysfunction with structural damage without LV dilation | L1: Haemodynamic lung involvement, assessed by CXR, and/or sPAP ≥ 35 mmHg at rest, assessed by TTE, with absence of clinical signs of lung congestion | M1: Presence of single systemic organ damage (except heart and lungs) |
| H3: LV dilation, structural cardiac damage with systolic (LVEF < 50%) or diastolic dysfunction, or right ventricular systolic dysfunction (TAPSE < 17 mm) | L2: Clinical signs and symptoms for lung congestion assessed by physical examination (crepitation, raised jugular venous pressure, orthopnoea, dyspnoea, and necessity of supplemental oxygen) and increase of left ventricular filling pressure, assessed by echocardiographic evaluation and, if feasible, by right heart catheterization | M2: Presence of double systemic organ damage (except heart and lungs) |
| H4: Biventricular systolic dysfunction (LVEF < 50% and TAPSE < 17 mm) | L3: ‘Cardiac lung’ defined by arterialization of pulmonary vasculature, with post‐capillary hypertension (type II) and necessity of supplemental oxygen at discharge, despite use of congestion‐relief therapy and absence of congestion | M3: Presence of ≥3 systemic organ damage (except heart and lungs) |
CXR, chest X‐rays; HLM, heart, lungs, and systemic multiorgan involvement; LV, left ventricular; LVEF, left ventricular ejection fraction; sPAP, systolic pulmonary arterial pressure; TAPSE, tricuspid annular plane systolic excursion; TTE, transthoracic echocardiography.
Definition of the stages for each parameter included in the HLM classification for heart failure.
Structural damage is defined by at least one among abnormal wall motion, left ventricular hypertrophy, and moderate to severe left‐sided valvular disease.
Malfunction of other organs are estimated glomerular filtration rate < 60 mL/min for kidney dysfunction; at least one among aspartate aminotransferase/alanine aminotransferase/total bilirubin/gamma‐glutamyl transferase/alkaline phosphatase elevated at least two times more than normal, for liver dysfunction; haemoglobin of <13 g/dL for men and <12 g/dL for women, for anaemia; transferrin saturation < 20% with serum ferritin between 100 and 299 ng/mL or serum ferritin < 100 ng/mL alone, for iron deficiency; more than 5% oedema‐free body weight loss during the previous year or less, for HF‐related cachexia; and the Beck Depression Inventory and Cardiac Depression Scale have been used to assess HF‐related depression and anxiety disorders and computed tomography/magnetic resonance imaging to exclude ischaemic or haemorrhagic stroke, for central nervous system involvement.
The parameter ‘H’, for heart, expresses myocardial involvement in terms of structural and/or functional abnormalities, similar to the degree of local cancer infiltration indicated by the parameter ‘T’ of the TNM classification.
Chest examination, chest X‐ray or computed tomography (CT) scan, and specific tests such as arterial blood gas analysis, respiratory function tests, right heart catheterization, 6 min walking test, and cardiopulmonary test were used to define lung involvement (parameter ‘L’). Due to the close relationships between the heart and lungs, the ‘L’ parameter in HLM classification resembles the lymphatic system involvement (‘N’) of the TNM classification. In this context, both anatomically and functionally lungs can be considered as the ‘lymph nodes of the heart’. Lung involvement in HF includes four levels of severity 14 (Table 1 ).
Systemic multiorgan involvement, such as kidney, liver, brain, and the haematopoietic system, was indicated with letter ‘M’. Kidney dysfunction is defined as an estimated glomerular filtration rate < 60 mL/min, when calculated by the Cockcroft and Gault equation. 15 Liver dysfunction is determined as a minimum two‐fold increase of at least one parameter among aspartate aminotransferase, alanine aminotransferase, total bilirubin, gamma‐glutamyl transferase, and alkaline phosphatase. 16 , 17 , 18 Anaemia is defined as haemoglobin of <13 g/dL for men and <12 g/dL for women, while iron deficiency is defined as transferrin saturation < 20% with a serum ferritin between 100 and 299 ng/mL or serum ferritin < 100 ng/mL alone. 19 HF‐related cachexia is defined as more than 5% oedema‐free body weight loss during the previous year. 20 Central nervous system involvement is characterized by HF‐related depression and anxiety disorders, assessed through the Beck Depression Inventory and Cardiac Depression Scale disorders. 2 CT and/or magnetic resonance imaging were used to exclude ischaemic or haemorrhagic stroke. Systemic multiorgan involvement in HF includes four levels of severity (Table 1 ).
Statistical analysis
Mean (standard deviation) was reported for numerical variables, and counts (percentages) were used for categorical variables. Comparisons between groups were made using t‐test or Wilcoxon's rank‐sum test (non‐parametric) for numerical variables and χ 2 test for categorical variables as appropriate. Composite event‐free (all‐cause death and rehospitalization due to HF) survival time and overall survival were summarized with the Kaplan–Meier methods and evaluated with a stratified log‐rank test. Hazard ratios and 95% confidence intervals were calculated using univariate Cox proportional hazards regression models, to estimate the effects of each prognostic score. To compare the survival models, both Akaike information criterion (AIC) and Bayesian information criterion (BIC) were used. A competing risk analysis was performed regarding the effects of the ‘prognostic scores’ and the risk of developing cardiac death. Logistic regressions were fitted to model the relationship between the different prognostic scores and the composite event at 12 months. The fitted models were evaluated using the area under the receiver operating characteristic curve (AUC); 95% confidence intervals for AUC were also obtained. The Hanley and McNeil test was used to assess the difference between the AUCs. For all tests, a P‐value < 0.05 was considered statistically significant. All analyses were performed using the statistical software R (Version 4.0.4).
The HLM score
The risk score was constructed based on the H–L–M values considered as numerical variables. The linear combination of the coefficients (multiplied by 10 and then rounded to the nearest integer) obtained from the Cox proportional hazards model for the hazard of the composite outcome was used. 21 The resulting score was
The corresponding HLM stages were
HLM score 2–6: HLM‐1;
HLM score 7–11: HLM‐2;
HLM score 12–16: HLM‐3; and
HLM score 17–20: HLM‐4.
The risk stages were defined to have a good performance on stratifying patients according to their risk and to guarantee an adequate number of subjects in each class (at least 10% of the total).
The accuracy of each score was evaluated and compared with the others. After discharge, patients were routinely evaluated for all‐cause death and rehospitalization due to HF at 1, 3, 6, and 12 months in the outpatient clinics of participating institutions or by their referring physicians.
Results
A total of 1720 patients were included in the study and completed the 12 month follow‐up, of which 520 patients experienced the composite endpoint of all‐cause death and rehospitalization due to HF. 540 (31.4%) patients were female. The mean age of the study population was 70.5 (±12.9). The mean LVEF was 42.5 ± 13% and 42 ± 11.9% at admission and discharge, respectively. The baseline characteristics of the population according to each HLM severity stage are represented in Table 2 .
Table 2.
Baseline features of the study population
| Baseline features | HLM‐1 (N = 373) | HLM‐2 (N = 507) | HLM‐3 (N = 587) | HLM‐4 (N = 253) | P‐value |
|---|---|---|---|---|---|
| Age, n (±SD) | 64.3 (±13.6) | 69.1 (±12.6) | 73.2 (±12.3) | 76 (±9.5) | <0.001 |
| Female gender, n (%) | 101 (27.1) | 154 (30.4) | 211 (35.9) | 74 (29.2) | 0.022 |
| BMI (kg/m2) (±SD) | 26.1 (±4.1) | 26.1 (±4.6) | 25.7 (±4.3) | 25 (4) | 0.003 |
| Arterial hypertension, n (%) | 275 (±73.7) | 406 (±80.1) | 486 (±82.8) | 217 (85.8) | 0.001 |
| CV disease family history, n (%) | 137 (36.7) | 176 (34.7) | 231 (39.4) | 100 (39.5) | 0.38 |
| Dyslipidaemia, n (%) | 182 (48.8) | 282 (55.6) | 319 (54.3) | 155 (61.3) | 0.02 |
| Smoking habit, n (%) | 113 (30.3) | 150 (29.6) | 155 (26.4) | 63 (24.9) | 0.314 |
| Diabetes mellitus, n (%) | 29 (7.8) | 54 (10.7) | 107 (18.2) | 65 (25.7) | <0.001 |
| LVEF (%) (±SD) | 51.8 (±7.4) | 45.5 (±11.6) | 38.9 (±12.6) | 31.1 (±12.1) | <0.001 |
| LVEDD, mm (±SD) | 50.3 (±5.8) | 52.8 (±7.3) | 54.1 (±7.8) | 57.1 (±7.9) | <0.001 |
| IVS, mm (±SD) | 11.1 (±1.58) | 11.4 (±1.6) | 11.5 (±1.93) | 11.1 (±1.78) | 0.001 |
| PW, mm (±SD) | 10.3 (±1.33) | 10.6 (±1.37) | 10.6 (±1.62) | 10.3 (±1.57) | <0.001 |
| Haemoglobin, g/dL (±SD) | 13.6 (±1.7) | 12.9 (±1.9) | 12.1 (±2.1) | 11.6 (±1.9) | <0.001 |
| Creatinine, mg/dL (±SD) | 1.02 (±0.72) | 1.13 (±0.61) | 1.32 (±0.83) | 1.78 (±1.49) | <0.001 |
| eGFR, mL/min (±SD) | 78.5 (±26.1) | 67.7 (±24.8) | 62.6 (±22.8) | 58 (±23.7) | <0.001 |
| INR (±SD) | 1.08 (±0.21) | 1.14 (±0.32) | 1.25 (±0.59) | 1.43 (±0.72) | <0.001 |
| AST, U/L (±SD) | 25 (±24) | 39.4 (±84.6) | 37 (±92) | 72.1 (±302) | <0.001 |
| ALT, U/L (±SD) | 21.5 (±17.6) | 26.2 (±32.8) | 29.3 (±66) | 67.7 (±229.1) | <0.001 |
| Weight loss, n (%) | 1 (0.3) | 6 (1.2) | 24 (4.1) | 27 (10.7) | 0.087 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; CV, cardiovascular; eGFR, estimated glomerular filtration rate; HLM, heart, lungs, and systemic multiorgan involvement; INR, international normalized ratio; IVS, interventricular septum; LVEDD, left ventricular end‐diastolic diameter; LVEF, left ventricular ejection fraction; PW, posterior wall; SD, standard deviation.
Baseline features have been listed according to each HLM score severity stage.
Overall, 173 (10%) patients had diastolic dysfunction and/or structural cardiac damage without LV systolic dysfunction; 852 (49.5%) patients showed LV systolic or diastolic dysfunction with structural heart damage without LV dilation; and 444 (25.8%) subjects with systolic or diastolic dysfunction showed structural heart damage with LV dilation or right ventricular systolic dysfunction. In 251 patients (14.6%), a biventricular failure was detected (Table 3 ).
Table 3.
Classification according to the HLM, NYHA, ACC/AHA, and LVEF at 12 months of follow‐up
| Classification type | Total population (N = 1720; 100%) | No composite event (N = 1200; 100%) | Composite event (N = 520; 100%) | P‐value | |
|---|---|---|---|---|---|
| H | (1) | 173 (10.1) | 141 (11.7) | 33 (6.3) | <0.001 |
| (2) | 852 (49.5) | 643 (53.6) | 209 (40.2) | ||
| (3) | 444 (25.8) | 282 (23.5) | 162 (31.2) | ||
| (4) | 251 (14.6) | 135 (11.2) | 116 (22.3) | ||
| L | (0) | 505 (29.4) | 417 (34.8) | 88 (16.9) | <0.001 |
| (1) | 371 (21.6) | 267 (22.2) | 104 (20) | ||
| (2) | 573 (33.3) | 383 (31.9) | 190 (36.5) | ||
| (3) | 271 (15.8) | 133 (11.1) | 138 (26.5) | ||
| M | (0) | 446 (25.9) | 354 (29.5) | 92 (17.7) | <0.001 |
| (1) | 577 (33.5) | 411 (34.2) | 166 (31.9) | ||
| (2) | 481 (28) | 321 (26.8) | 160 (30.8) | ||
| (3) | 216 (12.6) | 114 (9.5) | 102 (19.6) | ||
| HLM | (1) | 373 (21.7) | 311 (25.9) | 62 (11.9) | <0.001 |
| (2) | 507 (29.5) | 392 (32.7) | 115 (22.1) | ||
| (3) | 587 (34.1) | 371 (30.9) | 216 (41.5) | ||
| (4) | 253 (14.7) | 126 (10.5) | 127 (24.4) | ||
| NYHA | (I) | 321 (18.7) | 249 (20.8) | 72 (13.8) | <0.001 |
| (II) | 488 (28.4) | 361 (30.1) | 127 (24.4) | ||
| (III) | 626 (36.4) | 424 (35.3) | 202 (38.8) | ||
| (IV) | 285 (16.6) | 166 (13.8) | 119 (22.9) | ||
| ACC/AHA | (A) | 144 (8.4) | 117 (9.8) | 27 (5.2) | <0.001 |
| (B) | 687 (39.9) | 507 (42.2) | 180 (34.6) | ||
| (C) | 449 (26.1) | 323 (26.9) | 126 (24.2) | ||
| (D) | 440 (25.6) | 253 (21.1) | 187 (36) | ||
| LVEF | pEF | 812 (47.2) | 616 (51.3) | 196 (37.7) | <0.001 |
| mrEF | 297 (17.3) | 201 (16.8) | 96 (18.5) | ||
| rEF | 611 (35.5) | 383 (31.9) | 228 (43.8) | ||
ACC/AHA, American College of Cardiology/American Heart Association; HLM, heart, lungs, and systemic multiorgan involvement; LVEF, left ventricular ejection fraction; mrEF, mildly reduced ejection fraction; NYHA, New York Heart Association; pEF, preserved ejection fraction; rEF, reduced ejection fraction.
Considering the different nosologies included in the study, the total population has been divided in population who manifested the composite event of all‐cause death and rehospitalization due to heart failure and patients who did not manifest the composite event.
Pulmonary involvement (‘L’ parameter) was found in 70% of cases. 371 (21.6%) patients had a haemodynamic lung involvement detected by congestion on chest X‐ray and/or a systolic pulmonary arterial pressure ≥ 35 mmHg by transthoracic echocardiography (TTE); 573 (33.3%) subjects showed signs of clinical congestion assessed by physical examination and increased LV filling pressure, by either TTE or right heart catheterization; and 271 (15.8%) patients had post‐capillary pulmonary hypertension (Table 3 ).
Systemic multiorgan involvement (‘M’ parameter), beyond heart and lungs, was found in 74% of cases. Overall, 577 (33.5%) patients had only one organ involvement, 481 (28%) patients showed two organs involved, and 216 (12.6%) patients had ≥3 organs involved (Table 3 ).
Regarding the population distribution across the spectrum of HLM score stages, 373 (21.7%) patients were included in the HLM‐1, 507 (29.5%) in the HLM‐2, 587 (34.1%) in the HLM‐3, and 253 (14.7%) in the HLM‐4.
HLM, NYHA, ACC/AHA, and LVEF were calculated to stratify patients. Regarding the composite endpoint, the HLM model had better AIC and BIC values compared with other classification systems (Table 4 ). Therefore, the HLM score was identified as the most accurate prognostic score to predict the composite of all‐cause death and rehospitalization due to HF, at 12 months of follow‐up, compared with other staging systems (Table 4 ). Kaplan–Meier curves graphically show the same result (Figure 1 ). The 12 month free survival rate according to each severity stage of each nosology is represented in Table 4 .
Table 4.
Free survival event rate at 12 months, regarding the composite endpoint and according to each stage of severity of each nosology
| 12 months of event‐free survival | HR | 2.5% | 97.5% | P‐value | AIC | BIC | |
|---|---|---|---|---|---|---|---|
| HLM‐1 | 0.85 (95% CI 0.8–0.88) | 1 | — | — | — | 6975.1 | 6987.7 |
| HLM‐2 | 0.78 (95% CI 0.74–0.81) | 1.43 | 1.07 | 1.9 | 0.015 | ||
| HLM‐3 | 0.65 (95% CI 0.61–0.68) | 2.52 | 1.93 | 3.28 | <0.001 | ||
| HLM‐4 | 0.53 (95% CI 0.46–0.59) | 4.04 | 2.98 | 5.49 | <0.001 | ||
| NYHA I | 0.79 (95% CI 0.73–0.83) | 1 | — | — | — | 7048.9 | 7061.4 |
| NYHA II | 0.75 (95% CI 0.71–0.79) | 1.2 | 0.89 | 1.62 | 0.24 | ||
| NYHA III | 0.69 (95% CI 0.65–0.73) | 1.53 | 1.16 | 2.02 | 0.003 | ||
| NYHA IV | 0.6 (95% CI 0.54–0.66) | 2.15 | 1.6 | 2.91 | <0.001 | ||
| ACC/AHA A | 0.82 (95% CI 0.75–0.87) | 1 | — | — | — | 7054.3 | 7066.9 |
| ACC/AHA B | 0.75 (95% CI 0.72–0.78) | 1.5 | 0.99 | 2.29 | 0.05 | ||
| ACC/AHA C | 0.73 (95% CI 0.69–0.77) | 1.61 | 1.05 | 2.48 | 0.031 | ||
| ACC/AHA D | 0.6 (95% CI 0.55–0.64) | 2.72 | 1.79 | 4.14 | <0.001 | ||
| HFpEF | 0.77 (95% CI 0.74–0.8) | 1 | — | — | — | 7051.2 | 7059.6 |
| HFmrEF | 0.68 (95% CI 0.63–0.74) | 1.45 | 1.13 | 1.86 | 0.004 | ||
| HFrEF | 0.65 (95% CI 0.61–0.69) | 1.66 | 1.36 | 2.02 | <0.001 |
ACC/AHA, American College of Cardiology/American Heart Association; AIC, Akaike information criterion; BIC, Bayesian information criterion; CI, confidence interval; HFmrEF, heart failure with mildly reduced ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; HLM, heart, lungs, and systemic multiorgan involvement; HR, hazard ratio; NYHA, New York Heart Association.
HLM score is the best prognostic model compared with NYHA class, ACC/AHA stages, and LVEF‐based classification.
Figure 1.
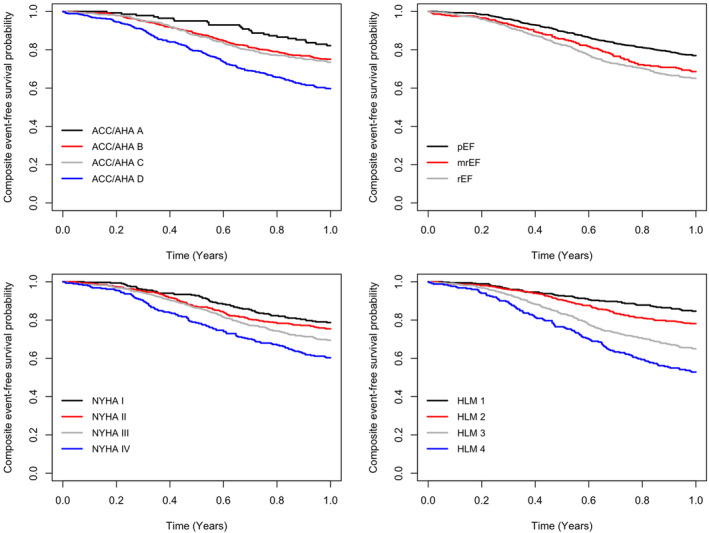
Comparison of Kaplan–Meier curves among the four heart failure (HF) prognostic systems, American College of Cardiology/American Heart Association (ACC/AHA) stages, left ventricular ejection fraction‐based classification, New York Heart Association (NYHA) classification, and HLM (heart, lungs, and systemic multiorgan involvement) score, in terms of the composite of all‐cause death and rehospitalization due to HF prediction, at 12 months of follow‐up. The HLM score shows the best accuracy in terms of the composite endpoint prediction. mrEF, mildly reduced ejection fraction; pEF, preserved ejection fraction; rEF, reduced ejection fraction.
The accuracy of each model was determined using receiver operating characteristic curve analysis and compared with the HLM score. The prognostic performance of each score is depicted in Figure 2 . Considering the composite endpoint, the AUC of the HLM scoring system was 0.645 (0.618–0.673), 0.580 (0.552–0.609) for the NYHA classification, 0.572 (0.544–0.6) for LVEF, and 0.589 (0.560–0.617) for the ACC/AHA stages. The HLM score‐related AUC showed statistically significant differences compared with LVEF (P = 0.002), ACC/AHA (P = 0.029), and NYHA (P = 0.009) AUC.
Figure 2.
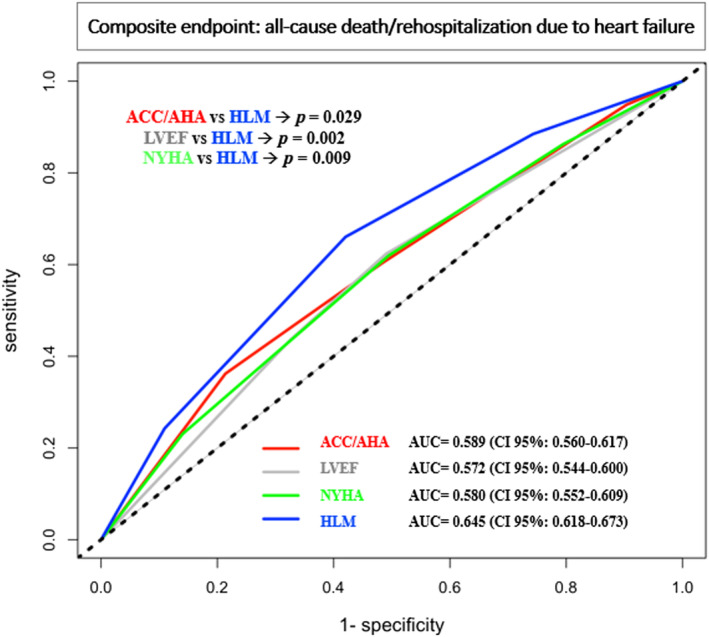
Comparison of receiver operating characteristic (ROC) curves and associated area under the curve (AUC) of the four heart failure (HF) prognostic system, American College of Cardiology/American Heart Association (ACC/AHA) stages, left ventricular ejection fraction (LVEF)‐based classification, New York Heart Association (NYHA) classification, and HLM (heart, lungs, and systemic multiorgan involvement) score, in terms of the composite of all‐cause death and rehospitalization due to HF. HLM shows the best AUC compared with the other nosologies. Differences among HLM and LVEF (P = 0.002), ACC/AHA (P = 0.029), and NYHA (P = 0.009) AUC are statistically significant. CI, confidence interval.
Discussion
This study defines the prognostic performance of a new scoring system, the HLM score, to assess severity and predict prognosis in patients hospitalized for HF and at risk of HF. Most importantly, the HLM score was compared with the currently most used HF staging systems, and it was identified as the best model to predict the composite of all‐cause death and rehospitalization due to HF at 12 months of follow‐up, compared with the other classification systems (Figure 1 ). The HLM score showed an overall good accuracy in stratifying the risk of the composite of all‐cause death and rehospitalization due to HF at 12 months (AUC 0.645), significantly higher when compared with NYHA, ACC/AHA, and LVEF (Figure 2 ).
Prognostic assessment plays a crucial role in the management of HF patients because several medical decisions, such as the use of advanced cardiac therapy and heart transplant, rely on life expectancy and disease severity. 22 , 23 Therefore, during the past years, several prognostic risk scores were proposed and validated to target a therapy for each patient, from the prevention of HF progression to palliative care, and to facilitate shared decisions with patients and their families.
The NYHA scale is the oldest system to classify HF patients and is based on two factors: symptom severity and effort entity related to symptoms. 7 Although the NYHA classification is simple and user‐friendly, without requiring functional and/or structural exams, its main limitation is the large inter‐individual variability in the interpretation of symptom severity and related efforts. This variability leads to a low reproducibility and accuracy of the clinical evaluation and does not account for the patients' classification related to the pathophysiological abnormalities underlying the HF syndrome. 7 Moreover, prognostic discrimination should be driven by objective and detailed measurements and not by subjective symptom assessment. For instance, a patient with a structurally normal heart but with episodes of rapid atrial fibrillation is often more symptomatic than a patient with previous myocardial infarction and a large scar but optimized medical therapy. Clearly, a cardiological evaluation based solely on symptoms and related efforts would lead to a misclassification of patients.
The ACC/AHA classification partially overcomes the NYHA scale by separating patients not only based on symptoms but also based on the underlying structural cardiac damage and risk factors for the development and progression of HF. 4 , 5 Nevertheless, even this classification has some pitfalls, including its cardiocentricity and the unclear definition of advanced HF stages C and D. Indeed, upon the assessment of HF severity together with the underlying structural abnormalities stands the differentiation between advanced HF, characterized by repeated hospitalization and recurrent congestion episodes, and end‐stage HF. 24 , 25 These two stages, although associated with severe cardiac abnormalities, carry a different prognosis and are suitable for different therapeutic strategies: Advanced HF can be treated with ventricular assist device implantation or heart transplantation, whereas palliative care aiming to improve quality of life should be reserved for end‐stage HF patients. 2 , 4 , 23 , 24 In this setting, the right therapy for the right patient has both ethical and economic implications. 24
LVEF is the cornerstone for the evaluation of cardiac mechanical performance, but there are several limitations related to this measurement. Indeed, technical issues affect the reproducibility of two‐dimension echocardiography, possibly leading to incorrect LVEF estimations. 25 Moreover, LVEF does not provide any specific clinical features, pathophysiological distinction, or molecular mechanisms among patients with HF and does not confer any information on diastolic function. 26 At the end of the day, factors beyond LVEF should be used to define prognosis and target therapies in HF patients.
The HLM score system was created given the need to overcome the limitations of the above‐mentioned risk models, with the purpose of developing a holistic approach that can provide a global assessment of HF patients and achieve targeted therapies. Specifically, the HLM score, combining clinical, laboratory, and instrumental parameters, goes beyond the simplistic assessment of HF symptoms and/or LVEF, aiming for a global evaluation of both pathophysiological mechanisms, underlying heart abnormalities and any multi‐systemic involvement. In fact, HLM score had the best overall accuracy among tested risk models in predicting the risk of the composite of all‐cause death and rehospitalization due to HF, at 12 months of follow‐up.
Due to its objective and multiparametric nature, HLM score should overcome common pitfalls of prognostic risk models, such as poor reliability at the individual patient level, because different combinations of HLM staging are possible and each of the HLM parameters is related to different levels of risk. Only a good prognostic discrimination of patients allows for correct estimation of mortality and tailored therapies. 27 , 28 Moreover, the progressive reduction of sudden cardiac death and the increase of non‐cardiac mortality over the past 20 years have shed light on the need to move from a cardiocentric to a global approach. 29 , 30
Of note, the results of our study in terms of prognostic accuracy are like those recently published on a multinational European registry of ambulatory chronic patients. 10 Further research is needed to validate our model in the outpatient settings. The HLM score offers a more comprehensive view of HF, overcoming the cardiocentric approach of other classification systems, while highlighting the negative prognostic impact of HF‐related multiorgan involvement. Moreover, the HLM score utilizes pragmatic cut‐offs, offering cardiologists the possibility to estimate the risk of 12 months of death and rehospitalization according to stage severity. HLM is pragmatic and user‐friendly at the same time, allowing for greater reproducibility in clinical practice. We have also created an interactive application for mobile phones, to make the HLM score calculation easily available for each physician.
Our study has several limitations. First, the HLM score has been validated in a relatively small sample size and, therefore, should be further tested in a larger population. Second, the study population was composed of in‐hospital patients, admitted for HF or for risk factors that predispose to the development of HF, without considering chronic HF patients. Hence, the generalizability and applicability of our model could be somewhat limited and need to be validated in ambulatory HF patients. Additionally, the inclusion of biomarkers, such as natriuretic peptide level, could have increased the prognostic discrimination of our model, but it was not included in the staging system because of substantial missing values. We could not test additional prognostic scores due to the lack of candidate variables.
The complexity of the HF syndrome requires a global, multiparametric, and objective approach to patients, going beyond the cardiocentric view based on LVEF and HF symptoms, addressing the pathophysiological mechanisms underlying heart abnormalities. 25 , 26 , 30 , 31 We propose the HLM score as a new, multivariable, user‐friendly, and pragmatic classification model in the HF population. Although developed in a relatively small study population, HLM demonstrated the best overall accuracy in predicting the composite of all‐cause death and rehospitalization due to HF, at 12 months, compared with currently used prognostic models, representing a promising tool to guide HF staging and management. HLM scores may be valuable in identifying advanced vs. end‐stage HF, because there are therapeutic and ethical considerations that are different in the latter.
Conflict of interest
All the authors have no conflict of interest.
Funding
None.
Acknowledgements
We thank Stefanie Marek‐Iannucci for the critical review of the manuscript and for the English language editing.
Severino, P. , Mancone, M. , D'Amato, A. , Mariani, M. V. , Prosperi, S. , Alunni Fegatelli, D. , Birtolo, L. I. , Angotti, D. , Milanese, A. , Cerrato, E. , Maestrini, V. , Pizzi, C. , Foà, A. , Vestri, A. , Palazzuoli, A. , Vizza, C. D. , Casale, P. N. , Mather, P. J. , and Fedele, F. (2024) Heart failure ‘the cancer of the heart’: the prognostic role of the HLM score. ESC Heart Failure, 11: 390–399. 10.1002/ehf2.14594.
Paolo Severino and Massimo Mancone have contributed equally to this study.
References
- 1. Ponikowski P, Anker SD, AlHabib KF, Cowie MR, Force TL, Hu S, et al. Heart failure: Preventing disease and death worldwide. ESC Heart Fail 2014;1:4‐25. doi: 10.1002/ehf2.12005 [DOI] [PubMed] [Google Scholar]
- 2. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021;42:3599‐3726. doi: 10.1093/eurheartj/ehab368 [DOI] [PubMed] [Google Scholar]
- 3. Committee W, Maddox TM, Januzzi JL Jr, Allen LA, Breathett K, Butler J, et al. 2021 update to the 2017 ACC expert consensus decision pathway for optimization of heart failure treatment: Answers to 10 pivotal issues about heart failure with reduced ejection fraction: A report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol 2021;77:772‐810. doi: 10.1016/j.jacc.2020.11.022 [DOI] [PubMed] [Google Scholar]
- 4. Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: Executive summary: A report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol 2022;79:1757‐1780. doi: 10.1016/j.jacc.2021.12.012 [DOI] [PubMed] [Google Scholar]
- 5. Bozkurt B, Coats AJ, Tsutsui H, Abdelhamid M, Adamopoulos S, Albert N, et al. Universal definition and classification of heart failure: A report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure. J Card Fail 2021;27:387‐413. doi: 10.1002/ejhf.2115 [DOI] [PubMed] [Google Scholar]
- 6. Fedele F, Mancone M, Adamo F, Severino P. Heart failure with preserved, mid‐range, and reduced ejection fraction: The misleading definition of the new guidelines. Cardiol Rev 2017;25:4‐5. doi: 10.1097/CRD.0000000000000131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Raphael C, Briscoe C, Davies J, Ian Whinnett Z, Manisty C, Sutton R, et al. Limitations of the New York Heart Association functional classification system and self‐reported walking distances in chronic heart failure. Heart 2007;93:476‐482. doi: 10.1136/hrt.2006.089656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Codina P, Lupón J, Borrellas A, Spitaleri G, Cediel G, Domingo M, et al. Head‐to‐head comparison of contemporary heart failure risk scores. Eur J Heart Fail 2021;23:2035‐2044. doi: 10.1002/ejhf.2352 [DOI] [PubMed] [Google Scholar]
- 9. Pocock SJ, Ariti CA, McMurray JJ, Maggioni A, Køber L, Squire IB, et al. Predicting survival in heart failure: A risk score based on 39 372 patients from 30 studies. Eur Heart J 2013;34:1404‐1413. doi: 10.1093/eurheartj/ehs337 [DOI] [PubMed] [Google Scholar]
- 10. Canepa M, Fonseca C, Chioncel O, Laroche C, Crespo‐Leiro MG, Coats AJS, et al. Performance of prognostic risk scores in chronic heart failure patients enrolled in the European Society of Cardiology Heart Failure Long‐Term Registry. JACC Heart Fail 2018;6:452‐462. doi: 10.1016/j.jchf.2018.02.001 [DOI] [PubMed] [Google Scholar]
- 11. Fedele F, Gatto MC, D'Ambrosi A, Mancone M. TNM‐like classification: A new proposed method for heart failure staging. Sci World J 2013;2013:175925. doi: 10.1155/2013/175925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fedele F, Severino P, Calcagno S, Mancone M. Heart failure: TNM‐like classification. J Am Coll Cardiol 2014;63:1959‐1960. doi: 10.1016/j.jacc.2014.02.552 [DOI] [PubMed] [Google Scholar]
- 13. Sellers AH. The clinical classification of malignant tumours: The TNM system. Can Med Assoc J 1971;105:836. passim [PMC free article] [PubMed] [Google Scholar]
- 14. Humbert M, Kovacs G, Hoeper MM, Badagliacca R, Berger RMF, Brida M, et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J 2022;43:3618‐3731. doi: 10.1183/13993003.00879-2022 [DOI] [PubMed] [Google Scholar]
- 15. Damman K, Testani JM. The kidney in heart failure: An update. Eur Heart J 2015;36:1437‐1444. doi: 10.1093/eurheartj/ehv010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Horwich TB, Kalantar‐Zadeh K, MacLellan RW, Fonarow GC. Albumin levels predict survival in patients with systolic heart failure. Am Heart J 2008;155:883‐889. doi: 10.1016/j.ahj.2007.11.043 [DOI] [PubMed] [Google Scholar]
- 17. Maeda D, Sakane K, Kanzaki Y, Okuno T, Nomura H, Hourai R, et al. Relation of aspartate aminotransferase to alanine aminotransferase ratio to nutritional status and prognosis in patients with acute heart failure. Am J Cardiol 2021;139:64‐70. doi: 10.1016/j.amjcard.2020.10.036 [DOI] [PubMed] [Google Scholar]
- 18. Batin P, Wickens M, McEntegart D, Fullwood L, Cowley AJ. The importance of abnormalities of liver function tests in predicting mortality in chronic heart failure. Eur Heart J 1995;16:1613‐1618. doi: 10.1093/oxfordjournals.eurheartj.a060785 [DOI] [PubMed] [Google Scholar]
- 19. WHO . Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. In: Vitamin and Mineral Nutrition Information System. Geneva: World Health Organization; 2011. (WHO/NMH/NHD/MNM/11.1); https://www.who.int/vmnis/indicators/haemoglobin.pdf. Accessed 12 December 2022 [Google Scholar]
- 20. Shah P, Abel AAI, Boyalla V, Pellicori P, Kallvikbacka‐Bennett A, Sze S, et al. A comparison of non‐invasive methods of measuring body composition in patients with heart failure: A report from SICA‐HF. ESC Heart Fail 2021;8:3929‐3934. doi: 10.1002/ehf2.13402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jin H, Lu Y. The optimal linear combination of multiple predictors under the generalized linear models. Stat Probab Lett 2009;79:2321‐2327. doi: 10.1016/j.spl.2009.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Howlett JG. Should we perform a heart failure risk score? Circ Heart Fail 2013;6:4‐5. doi: 10.1161/CIRCHEARTFAILURE.112.973172 [DOI] [PubMed] [Google Scholar]
- 23. Crespo‐Leiro MG, Metra M, Lund LH, Milicic D, Costanzo MR, Filippatos G, et al. Advanced heart failure: A position statement of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2018;20:1505‐1535. doi: 10.1002/ejhf.1236 [DOI] [PubMed] [Google Scholar]
- 24. Severino P, Mather PJ, Pucci M, D'Amato A, Mariani MV, Infusino F, et al. Advanced heart failure and end‐stage heart failure: Does a difference exist. Diagnostics (Basel) 2019;9:170. doi: 10.3390/diagnostics9040170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fonarow GC. Refining classification of heart failure based on ejection fraction. JACC Heart Fail 2017;5:808‐809. doi: 10.1016/j.jchf.2017.08.011 [DOI] [PubMed] [Google Scholar]
- 26. Cikes M, Solomon SD. Beyond ejection fraction: An integrative approach for assessment of cardiac structure and function in heart failure. Eur Heart J 2016;37:1642‐1650. doi: 10.1093/eurheartj/ehv510 [DOI] [PubMed] [Google Scholar]
- 27. Straw S, Byrom R, Gierula J, Paton MF, Koshy A, Cubbon R, et al. Predicting one‐year mortality in heart failure using the ‘Surprise Question’: A prospective pilot study. Eur J Heart Fail 2019;21:227‐234. doi: 10.1002/ejhf.1353 [DOI] [PubMed] [Google Scholar]
- 28. Bo X, Zhang Y, Liu Y, Kharbuja N, Chen L. Performance of the heart failure risk scores in predicting 1 year mortality and short‐term readmission of patients. ESC Heart Fail 2023;10:502‐517. doi: 10.1002/ehf2.14208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Harjola VP, Mullens W, Banaszewski M, Bauersachs J, Brunner‐La Rocca HP, Chioncel O, et al. Organ dysfunction, injury and failure in acute heart failure: From pathophysiology to diagnosis and management. A review on behalf of the Acute Heart Failure Committee of the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur J Heart Fail 2017;19:821‐836. doi: 10.1002/ejhf.872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Severino P, D'Amato A, Prosperi S, Dei Cas A, Mattioli AV, Cevese A, et al. Do the current guidelines for heart failure diagnosis and treatment fit with clinical complexity? J Clin Med 2022;11:857. doi: 10.3390/jcm11030857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Severino P, D'Amato A, Prosperi S, Myftari V, Canuti ES, Labbro Francia A, et al. Heart failure pharmacological management: Gaps and current perspectives. J Clin Med 2023;12:1020. doi: 10.3390/jcm12031020 [DOI] [PMC free article] [PubMed] [Google Scholar]


