Abstract
The Haemophilus ducreyi homolog of GroEL, a 58.5-kDa heat shock protein (Hsp), is a dominant protein produced not only in response to heat stress but also under in vitro growth conditions. Extracellular localization of the 58.5-kDa Hsp was investigated by whole-cell enzyme-linked immunosorbent assay (ELISA) and immunoelectron microscopy and in supernatants of washed bacteria by immunoblotting with a Haemophilus ducreyi GroEL-specific mouse monoclonal antibody (BB11). To investigate binding of the Hsp to eukaryotic cells, the 58.5-kDa Hsp was purified by ion-exchange and size exclusion chromatography; incubated with HEp-2 cells, HeLa cells, and human fibroblasts; and then analyzed by immunoblotting. Direct involvement of the 58.5-kDa Hsp in the adherence of H. ducreyi to HEp-2 cells was investigated by using an inhibition assay. An epitope of the 58.5-kDa Hsp was detected by whole-cell ELISA on all of the strains tested, suggesting that it is associated with the cell surface. This was also supported by immunoelectron microscopy results. In supernatants of washed bacteria, the 58.5-kDa Hsp was detected by immunoblotting after 10 h of cultivation. The 58.5-kDa Hsp bound to the eukaryotic cells tested but exerted only limited (about 20%) inhibition of H. ducreyi adherence to HEp-2 cells. These results demonstrate that the 58.5-kDa Hsp of H. ducreyi is associated with the bacterial surface, binds to eukaryotic cells, and partially influences H. ducreyi adherence to HEp-2 cells, indicating possible involvement of the 58.5-kDa Hsp in the attachment of bacteria to host cells and to each other.
Haemophilus ducreyi causes the sexually transmitted disease chancroid (25). This genital ulcerative disease is common in Africa and Asia, where genital ulceration has been related to an increased risk of the spread of the human immunodeficiency virus (25). H. ducreyi has been reported to express a GroEL heat shock protein (Hsp) with a high degree of homology to groEL gene products of other bacterial species (18). Increased expression of the GroEL homolog was induced following heat shock (6), but already at the organism’s optimal cultivation temperature, a higher level of the GroEL Hsp than of the corresponding Escherichia coli protein has been reported (19). Western blot analysis using serum from human chancroid patients and from immunized mice and rabbits has shown the H. ducreyi GroEL to be immunogenic (6). Generally, the GroEL Hsp has been established as an intracellularly located protein and a member of a family of molecular chaperones with ATPase activity that mediate protein folding of newly synthesized polypeptides (30). In several bacterial species, the GroEL homolog has been identified and the heat shock response has been investigated, with Hsp60 acting as an immunodominant antigen inducing both humoral and cellular responses (8, 15, 29). Reports on Helicobacter pylori indicate that Hsp60 is associated with urease and is located at the bacterial surface (10, 14, 28). In Streptococcus suis, surface location of Hsp60 has been suggested, and in Salmonella typhimurium, a role for the GroEL homolog involved in interaction with intestinal mucus has been suggested (4, 9). Decreased adherence of H. ducreyi to human genital cell lines was recently shown by lowering of the level of GroEL (19), and it was suggested that GroEL directly or indirectly affects adherence. H. ducreyi GroEL is a dominant protein at the optimum cultivation temperature. In that context, by using monoclonal antibody (MAb) BB11, we addressed the question of the cellular location and possible binding of the GroEL homolog to eukaryotic cell lines. Direct involvement of the 58.5-kDa Hsp as an inhibitor of H. ducreyi adherence to HEp-2 cells was investigated as well.
Of the six H. ducreyi strains used in this study, two were obtained from the Culture Collection of the University of Gothenburg (CCUG), i.e., CCUG 7470 (CIP 76118) and CCUG 4438 (CIP 542), and one was obtained from the Institute of Tropical Medicine, Antwerp, Belgium, i.e., ITMA 2665. Additionally, three freshly isolated strains (RCU 19, RCU 51, and RCU 188) were obtained from A. W. Sturm in Durban, South Africa. The H. ducreyi strains were cultivated on chocolate-Grand Lux agar plates obtained from the Department of Bacteriology, Sahlgrenska Hospital, Gothenburg, Sweden, in an oxygen-depleted, 6% CO2-enriched, and humid atmosphere for 10 to 48 h at 33°C in an anaerobic jar with anaerocult C (Merck, Darmstadt, Germany) as described previously (21). Cultivation of H. ducreyi in liquid medium was carried out with brain heart infusion broth supplemented with 1% hemin histidine solution (BHI-hemin; Sigma Chemical Company, St. Louis, Mo.), 0.04% l-histidine (Fluka Chemie AG, Buch, Switzerland), 10% fetal calf serum, 1% IsoVitaleX, and 0.03 mg of vancomycin (Department of Bacteriology, Sahlgrenska Hospital) per ml. The bacteria were gently rotated at 75 rpm and 33°C for 18 h in an anaerobic jar as described previously (21) and adjusted to an optical density at 600 nm (OD600) of 0.3.
The accessibility of the GroEL homolog, the 58.5-kDa Hsp, on the surface of six strains of H. ducreyi was investigated by whole-cell enzyme-linked immunosorbent assay (ELISA) with bacteria as the antigen as described previously (1), with some modifications. After cultivation on solid medium for 18 h, bacteria were either not washed or washed three times in phosphate-buffered saline (PBS; pH 7.2) by resuspension, adjusted to an OD600 of 0.3, and used to coat microtitration plates (Greiner, Kebo Lab AB, Stockholm, Sweden). The plates were incubated for 4 h at room temperature, blocked for 1 h with 0.5% bovine serum albumin in PBS, and then washed twice with PBS. Monoclonal antibody (MAb) BB11, which is specific for GroEL of H. ducreyi and was characterized and described previously (6), was used to identify an epitope of the 58.5-kDa Hsp, reflecting the presence of the protein on the bacterial surface. A polyclonal rabbit serum raised against the 58.5-kDa Hsp, cut out and electroeluted from a sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel, was used to detect the Hsp on the bacterial surface as well. An optimum dilution of the BB11 hybridoma cell culture medium was assessed in pilot experiments. MAb BB11 was serially diluted threefold in PBS, starting at 1:300. The plates were incubated overnight at room temperature, and after they had been washed three times with PBS–0.05% Tween, alkaline phosphatase-coupled goat anti-mouse immunoglobulin G (1:2,000; Jackson Immuno Research Laboratories, West Grove, Pa.) in PBS was added and the plates were incubated for 5 h. After being washed, the plates were developed with p-nitrophenylphosphate disodium substrate (Sigma) at 1 mg/ml, and A405 was measured with a Titertek Multiscan photometer (Flow Lab, Irvine, Scotland). MAb II12A4, directed against the 43-kDa major outer membrane protein, was produced from outer membrane antigens extracted from a mixture of strains of H. ducreyi (kindly provided by E. L. Roggen, Institute of Tropical Medicine). Purified MAb II12A4 (6 mg/ml) and PBS buffer were used as negative controls, giving absorbance values of less than 0.3 with all of the strains tested. Absorbance values of wells containing bacteria incubated with PBS served as background values and were subtracted from the values obtained with test samples. In wells without bacteria together with all reagents, no absorbance was observed. The results are expressed as absorbancies and represent the mean ± standard deviation of three experiments done in triplicate.
Absorbance values ranging from 1.54 to 2.85 were obtained with six unwashed strains of H. ducreyi cultivated for 18 h by using MAb BB11 (Table 1). Similar results have been obtained by using 90 strains of H. ducreyi (14a). Absorbance levels similar in magnitude were observed with unwashed bacteria cultivated for 48 h or in liquid medium for 18 h (data not shown). Furthermore, when bacteria washed three times and cultivated for 18 h were used as the antigen in the whole-cell ELISA, absorbance values of the same magnitude as those obtained with unwashed bacteria were recorded (data not shown). The absorbance value was also elevated, compared with that of preimmune serum, when polyclonal rabbit hyperimmune serum raised against the 58.5-kDa Hsp was used in the whole-cell ELISA (data not shown). Altogether, these results suggest that the 58.5-kDa Hsp is associated with the surface of H. ducreyi and that the absorbance levels are not solely the result of lysis of intracellularly located Hsp, although lysis would be expected with bacteria reaching the stationary phase following 48 h of culturing.
TABLE 1.
Surface exposure of the 58.5-kDa Hsp on strains of H. ducreyi
| Strain | Mean A405 ± SDa |
|---|---|
| CCUG 7470 | 2.83 ± 0.10 |
| CCUG 4438 | 1.62 ± 0.15 |
| ITMA 2665 | 2.85 ± 0.03 |
| RCU 19 | 1.58 ± 0.12 |
| RCU 51 | 1.60 ± 0.09 |
| RCU 188 | 1.54 ± 0.06 |
The mean ± the standard deviation of three measurements done in triplicate is shown. The control MAb gave an absorbance of less than 0.3 for all of the strains tested.
Surface localization of the 58.5-kDa Hsp was investigated by analyzing preparations of negatively stained bacterial cells as described previously (11). Bacteria grown on solid medium were applied unwashed to Formvar carbon-coated 400-mesh Ni grids (Ted Pella, Inc.) and incubated for 10 min. Following incubation with MAb BB11, 10-nm gold particle-labelled goat anti-mouse immunoglobulin G (British BioCell, Cardiff, Great Britain) was incubated for 15 min, and after washing, negative staining was performed with 2% ammonium molybdate–2% ammonium acetate in distilled water (pH 7.0). The grids were examined with an electron microscope (JEOL jem-1200 Ex). As a negative control, MAb II12A4 was used with all other reagents. No binding to different strains of H. ducreyi was demonstrated by this MAb in the whole-cell ELISA. As a positive control, we used MAb MAHD7, which is specific against the lipo-oligosaccharide (LOS) of H. ducreyi and was previously characterized (2). With MAb BB11, a negatively stained preparation of strain CCUG 7470 demonstrated the 58.5-kDa Hsp to be localized within the outer filamentous material (Fig. 1A), as well as more uniformly associated with the surface (Fig. 1B), although there was some variation in the amount of labelling of individual bacteria. Other strains of H. ducreyi showed similar localization of the 58.5-kDa Hsp. With MAb II12A4 as the negative control, very few gold-labelled antibodies were detectable (Fig. 1C). The positive control demonstrated uniformly attached anti-LOS antibodies made clearly visible by immunogold staining on the surface of the bacteria (Fig. 1D).
FIG. 1.
Immunoelectron micrographs of H. ducreyi CCUG 7470 negatively immunostained with anti-GroEL MAb BB11 (A and B), with anti-major outer membrane protein MAb II12A4 as a negative control (C), and with anti-LOS MAb MAHD7 as a positive control (D). Bars, 200 nm (A to C) and 100 nm (D).
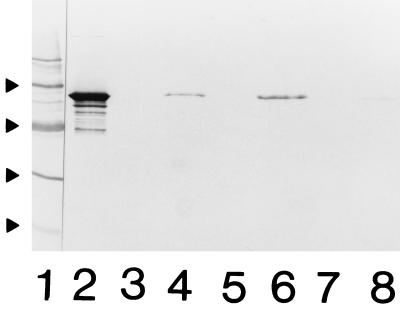
Detection of the 58.5-kDa Hsp in supernatants of washings of bacteria by immunoblot assay was undertaken to estimate the release of the Hsp. Strains CCUG 7470 and RCU 51 were cultivated on solid medium for 10 and 22 h and suspended to an OD600 of 0.8 in identical volumes. The same strains were cultivated in liquid medium for 18 h and treated as bacteria grown on solid medium. After centrifugation, the supernatants of washings were subjected to SDS-PAGE, reduced, heated to 100°C, and separated in 10% acrylamide gels by the method of Laemmli (16). After transfer to a nitrocellulose membrane (immunoblotting) (26), the 58.5-kDa Hsp was identified by using MAb BB11. The 58.5-kDa Hsp was already detected in supernatants of washings from both strains after 10 h of cultivation on solid medium, and an increased amount was detected after 22 h of cultivation (Fig. 2). The results obtained with washings from bacteria cultivated in liquid medium were similar, indicating early production of the GroEL Hsp and a possible capacity for leaking or releasing the material. On the contrary, the fact that only small amounts of Hsp were detected by immunoblot analysis in supernatants of bacteria cultured for up to 48 h in liquid medium (unpublished data) might reflect consumption of the Hsp from the environment and possible binding of this Hsp to adjacent bacteria.
FIG. 2.
Immunoblot analysis of supernatants of washings of bacteria probed with anti-GroEL MAb BB11. Lanes: 2 and 3, strains CCUG 7470 and RCU 51, respectively, after 10 h of cultivation; 4 and 5, strains CCUG 7470 and RCU 51, respectively, after 22 h of cultivation; 1, protein molecular mass markers (from the top, 200, 116.3, 97.4, 66.2, 45, and 31 kDa).
These results, obtained by whole-cell ELISA and immunoelectron microscopy and with supernatants of bacterial washings, taken together, suggest that the 58.5-kDa Hsp is associated with the bacterial cell surface.
The ability of a purified preparation of the 58.5-kDa Hsp from strain CCUG 7470 to bind to eukaryotic cells was determined as described previously by Wang and Stinson (27), with some modification. HEp-2 (ATCC CCL 23) and HeLa (ATCC CCL2) cells were cultivated in Eagle’s minimal essential medium with 5 to 8% fetal calf serum (21). Human fibroblasts (Department of Virology, Gothenburg University) were cultivated in Eagle’s medium with 5% fetal calf serum and 1% l-glutamine. All cells were cultivated at 37°C in 6% CO2. For harvesting of human fibroblasts, 4% trypsin in buffered NaCl (0.54 nM) was used. The cells were seeded in microtitration plates and grown to confluent monolayers.
Purification of the 58.5-kDa Hsp was performed as follows. Bacteria (CCUG 7470) grown on agar plates for 40 h were suspended in 10 mM EDTA in PBS, supplemented with 100 mM phenylmethylsulfonyl fluoride as a protease inhibitor, and incubated at 45°C for 45 min. After centrifugation, the supernatant was dialyzed against PBS.
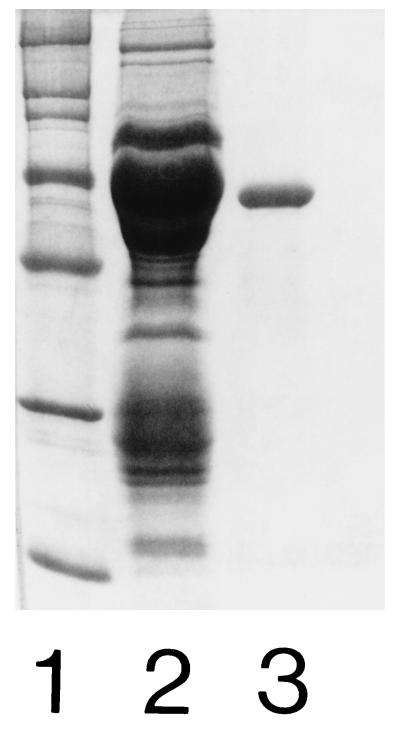
Pooled preparations of EDTA extracts were equilibrated in 0.02 M Bis-Tris buffer (pH 6.5) and applied to an ion-exchange column (Mono Q; Pharmacia, Uppsala, Sweden). The 58.5-kDa protein was eluted with a linear gradient of 0 to 0.6 M NaCl with a flow rate of 1 ml/min. Fractions were pooled and equilibrated in 0.05 M Bis-Tris buffer (pH 6.5) and applied to a Sephacryl S-300 HR 16/60 gel filtration column (Pharmacia) in a fast protein liquid chromatography system (Bio-Rad Laboratories, Richmond, Calif.) with a flow rate of 0.33 ml/min. Fractions were pooled and concentrated, and the amount of protein was determined to be 0.2 mg/ml by the method of Bradford (5). The purity of the Hsp was estimated by SDS-PAGE (Fig. 3) and immunoblotting. With MAb MAHD7, specific for the LOS of H. ducreyi (2), contamination with LOS was detected by immunoblot analysis. The concentration of LOS was determined to be 0.8 μg/mg of protein by a Limulus amoebocyte lysate assay (17). A 10-μg sample of the purified 58.5-kDa Hsp was incubated with confluent monolayers for 5 h. Following washing of the cells five times with PBS, they were detached from the plate and solubilized by SDS in sample buffer, subjected to SDS-PAGE under reducing conditions, and transferred electrophoretically to a nitrocellulose membrane. A rabbit serum raised against HEp-2 cells was used as a positive control. The binding of the purified 58.5-kDa Hsp to HEp-2 cells, HeLa cells, and human fibroblasts was observed by using MAb BB11 (Fig. 4). No bands were detected in controls with only eukaryotic cells (Fig. 4). The results indicate that the 58.5-kDa Hsp is able to bind to the surface of eukaryotic cells.
FIG. 3.
SDS-PAGE analysis of the purified 58.5-kDa Hsp stained with Coomassie blue. Lanes: 1, protein molecular mass markers (from the top, 200, 116.3, 97.4, 66.2, 45, 31, and 21.5 kDa); 2, concentrated crude preparation of 58.5-kDa Hsp; 3, 4 μg of purified protein.
FIG. 4.
Immunoblot analysis of 58.5-kDa Hsp binding to eukaryotic cells probed with anti-GroEL MAb BB11. Lanes: 1, protein molecular mass markers (from the top, 66.2, 45, 31, and 21.5 kDa); 2, purified 58.5-kDa protein; 3 and 4, HEp-2 cells; 5 and 6, HeLa cells; 7 and 8, human fibroblasts; 3, 5, and 7, controls without added Hsp; 4, 6, and 8, detection of bound 58.5-kDa Hsp.
Direct involvement of the 58.5-kDa Hsp was investigated in a competitive inhibition assay using radiolabelled bacteria (unpublished data). In brief, HEp-2 cells were cultivated in 96-well tissue culture plates and seeded with approximately 2 × 105 cells/ml in Eagle’s medium (21). Bacteria cultivated on solid medium for 24 h were used to inoculate liquid medium supplemented with 50 μCi of [35S]methionine (Amersham, Buckinghamshire, United Kingdom) and further cultivated for 18 h as described above. The specific activity incorporated ranged from 0.06 to 0.01 cpm/CFU. No dissociation of radioactivity from the labelled organisms during incubation was found. After washing of the bacteria three times in PBS to remove unbound radioactivity, the OD in Eagle’s medium (pH 7.2) was adjusted to 0.25, corresponding to 7 × 106 CFU/ml. Following preincubation for 10 min of 100 μl of the pure 58.5-kDa Hsp (200 μg/ml) diluted twofold in Eagle’s medium (pH 7.2), a 50-μl bacterial suspension was added for a final volume of 100 μl. Wells containing 0.05 M Bis-Tris buffer (pH 6.5) did not influence adherence, compared with the control. The plates were incubated at 33°C in a 6% CO2-enriched atmosphere for 3 h and then washed five times with buffered saline solution (pH 7.2). Microscopy verified the presence of intact monolayers. Liquid scintillation cocktail (Opti-fluor; Packard, Gröningen, The Netherlands) was added to the 96-well plate, and the radioactivity was measured in counts per minute with a 1450 MicroBeta Trilux counter (Wallac Oy, Turku, Finland). Background counts per minute were subtracted, and inhibition was expressed as relative adherence (mean percentage of control value ± standard deviation) and calculated as 100/C × S, where C is the control counts per minute and S is the sample counts per minute. The results reported are from three experiments done in triplicate.
Coincubation of bacteria with the purified 58.5-kDa Hsp preparation demonstrated a relative adherence of 81% ± 2%, compared with a control adherence of 100%, indicating that this Hsp has the ability to inhibit the adherence of H. ducreyi to HEp-2 cells by 19% ± 2%.
Hsps are considered to be major antigens in a number of bacterial pathogens (12, 23). Although details of their role in pathogenesis are not known, they are clearly of importance. Several organisms have been shown to express immunogenic GroEL homologs (12, 23, 29), giving rise to a well-documented host immune response (15). During infection with a bacterial pathogen, it is estimated that upregulation of bacterial Hsps in response to various stress stimuli is undertaken, suggesting an advantageous way of ensuring the organism’s survival. H. ducreyi has been shown to respond to a thermal shift up by upregulating the GroEL homolog, although from an already high level in vitro (19). Bacterial Hsps have been implicated as virulence factors. For example, Hsps have been suggested to contribute to the intracellular survival of S. typhimurium in macrophages (7), and a 66-kDa Hsp of S. typhimurium has been reported to bind to intestinal mucus (9). Extracellularly located Hsps of H. pylori have been suggested to mediate sulfatide recognition of the gastric mucosa (14), suggesting a direct role for Hsps in the pathogenesis of bacterial infections. Generally, the GroEL homolog is considered to be located intracellularly (22), and our studies of frozen thin sections of H. ducreyi bacteria stained with MAb BB11 confirmed the intracellular localization of the 58.5-kDa Hsp (unpublished data). In this study, by using two methods, whole-cell ELISA and immunoelectron microscopy, we have demonstrated that the GroEL homolog of H. ducreyi is not only intracellularly located but also associated with the cell surface. These results are in agreement with earlier studies of other species showing the presence of Hsps associated with the bacterial surface (4, 28). As abundant amounts of a 60-kDa GroEL homolog have readily been obtained by washing whole-cell suspensions of Legionella species (13) and as the GroEL homolog has been detected in supernatants of washings of H. ducreyi, used in this study, exclusive intracellular localization of this Hsp seems not to be plausible. It has, however, been suggested that autolysis of the H. pylori bacteria was required to have the GroEL homolog surface associated on adjacent bacteria (20), a mechanism that may contribute to the detection of GroEL also on the surface of H. ducreyi. It has been suggested in studies of H. ducreyi that during bacterial adherence to genital cell lines, the level of GroEL homolog was increased and that the adherence and survival of the stressed bacteria were negatively affected when the GroEL level was lowered (19). In this study, we demonstrated the ability of the purified 58.5-kDa Hsp to bind directly to HEp-2 cells, HeLa cells, and human fibroblasts. Bearing in mind the high basal levels of the GroEL homolog of H. ducreyi, it is not unreasonable to speculate that this Hsp has a role in adherence. However, a lower level of inhibition was demonstrated in this study when HEp-2 cells were pretreated with the purified Hsp preparation than when they were pretreated with an EDTA extract containing the 58.5-kDa Hsp (35%) (unpublished data). This may be explained by the fact that either unknown proteins or multiple mechanisms are involved in adherence, since proteoglycans, although not solely, have been shown to play a role in the adherence of H. ducreyi to Chinese hamster ovary (CHO) cells (24). Another explanation for the limited inhibition observed in this study might be that the amount of purified Hsp used was inadequate. LOS has been suggested to be involved in the adherence of H. ducreyi to human fibroblasts (3). As LOS was observed contaminating the purified GroEL preparation, although at a low concentration, and as LOS is surface exposed, one might speculate that a LOS-GroEL complex is involved in adherence. However, LOS was not shown to competitively inhibit the binding of H. ducreyi to HEp-2 cells in a previous study of ours (unpublished data).
In conclusion, in this study, we have demonstrated that the 58.5-kDa Hsp of H. ducreyi is associated with the bacterial surface and has the capacity to bind to eukaryotic cells in vitro, as well as to partially inhibit the adherence of H. ducreyi to HEp-2 cells. This may indicate a possible role of the 58.5-kDa Hsp in the attachment of bacteria to host cells. Further studies are needed to determine if the GroEL homolog is associated with LOS in a complex and responsible for adhesion to human cells, alone or with other potential adhesins.
Acknowledgments
This work was supported by grants from the Swedish International Development Cooperation Agency (Sida).
REFERENCES
- 1.Abdillahi H, Poolman J T. Whole-cell ELISA for typing Neisseria meningitidis with monoclonal antibodies. FEMS Microbiol Lett. 1987;48:367–371. [PubMed] [Google Scholar]
- 2.Ahmed H J, Borrelli S, Jonasson J, Eriksson L, Hanson S, Höjer B, Sunkunyu M, Musaba E, Roggen E L, Lagergård T, Lindberg A A. Monoclonal antibodies against Haemophilus ducreyi lipooligosaccharide and their diagnostic usefulness. Eur J Clin Microbiol Infect Dis. 1995;14:892–898. doi: 10.1007/BF01691496. [DOI] [PubMed] [Google Scholar]
- 3.Alfa M J, Degagne P. Attachment of Haemophilus ducreyi to human foreskin fibroblasts involves LOS and fibronectin. Microb Pathog. 1997;22:39–46. doi: 10.1006/mpat.1996.0089. [DOI] [PubMed] [Google Scholar]
- 4.Benkirane R, Gottschalk M, Jacques M, Dubreuil J D. Abstracts of the 97th General Meeting of the American Society for Microbiology. Washington, D.C: American Society for Microbiology; 1997. Identification of a Streptococcus suis 60-kDa heat-shock protein, abstr. B-491. [Google Scholar]
- 5.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 6.Brown T J, Jardine J, Ison C A. Antibodies directed against Haemophilus ducreyi heat shock proteins. Microb Pathog. 1993;15:131–139. doi: 10.1006/mpat.1993.1063. [DOI] [PubMed] [Google Scholar]
- 7.Buchmeier N A, Heffron F. Induction of Salmonella stress proteins upon infection of macrophages. Science. 1990;248:730–732. doi: 10.1126/science.1970672. [DOI] [PubMed] [Google Scholar]
- 8.Carreiro M M, Laux D C, Nelson D R. Characterization of the heat shock response and identification of heat shock protein antigens of Borrelia burgdorferi. Infect Immun. 1990;58:2186–2191. doi: 10.1128/iai.58.7.2186-2191.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ensgraber M, Loos M. A 66-kilodalton heat shock protein of Salmonella typhimurium is responsible for binding of the bacterium to intestinal mucus. Infect Immun. 1992;60:3072–3078. doi: 10.1128/iai.60.8.3072-3078.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Evans D J, Jr, Evans D G, Engstrand L, Graham D Y. Urease-associated heat shock protein of Helicobacter pylori. Infect Immun. 1992;60:2125–2127. doi: 10.1128/iai.60.5.2125-2127.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frisk A, Roggen E L, Lagergård T. Localisation and immunological properties of a 24-kDa surface protein of Haemophilus ducreyi. J Med Microbiol. 1995;43:192–200. doi: 10.1099/00222615-43-3-192. [DOI] [PubMed] [Google Scholar]
- 12.Hansen K, Bangsborg J M, Fjordvang H, Strandberg Pedersen N, Hindersson P. Immunochemical characterization of and isolation of the gene for a Borrelia burgdorferi immunodominant 60-kilodalton antigen common to a wide range of bacteria. Infect Immun. 1988;56:2047–2053. doi: 10.1128/iai.56.8.2047-2053.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoffman P S, Houston L, Butler C A. Legionella pneumophila htpAB heat shock operon: nucleotide sequence and expression of the 60-kilodalton antigen in L. pneumophila-infected HeLa cells. Infect Immun. 1990;58:3380–3387. doi: 10.1128/iai.58.10.3380-3387.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huesca M, Borgia S, Hoffman P, Lingwood C L. Acidic pH changes receptor binding specificity of Helicobacter pylori: a binary adhesion model in which surface heat shock (stress) proteins mediate sulfatide recognition in gastric colonization. Infect Immun. 1996;64:2643–2648. doi: 10.1128/iai.64.7.2643-2648.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14a.Ison, C. A. Unpublished data.
- 15.Kaufmann S H E. Heat shock proteins and the immune response. Immunol Today. 1990;11:129–136. doi: 10.1016/0167-5699(90)90050-j. [DOI] [PubMed] [Google Scholar]
- 16.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 17.Mattsby-Baltzer I, Lindgren K, Lindholm B, Edebo L. Endotoxin shedding by enterobacteria: free and cell-bound endotoxin differ in Limulus activity. Infect Immun. 1991;59:689–695. doi: 10.1128/iai.59.2.689-695.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parsons L M, Waring A L, Shayegani M. Molecular analysis of the Haemophilus ducreyi groE heat shock operon. Infect Immun. 1992;60:4111–4118. doi: 10.1128/iai.60.10.4111-4118.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parsons L M, Limberger R J, Shayegani M. Alterations in levels of DnaK and GroEL result in diminished survival and adherence of stressed Haemophilus ducreyi. Infect Immun. 1997;65:2413–2419. doi: 10.1128/iai.65.6.2413-2419.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Phadnis S H, Parlow M H, Levy M, Ilver D, Caulkins C M, Connors J B, Dunn B E. Surface localization of Helicobacter pylori urease and a heat shock protein homolog requires bacterial autolysis. Infect Immun. 1996;64:905–912. doi: 10.1128/iai.64.3.905-912.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Purvén M, Lagergård T. Haemophilus ducreyi, a cytotoxin-producing bacterium. Infect Immun. 1992;60:1156–1162. doi: 10.1128/iai.60.3.1156-1162.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scorpio A, Johnson P, Laquerre A, Nelson D R. Subcellular localization and chaperone activities of Borrelia burgdorferi Hsp60 and Hsp70. J Bacteriol. 1994;176:6449–6456. doi: 10.1128/jb.176.21.6449-6456.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shinnick T M, Vodkin M H, Williams J C. The Mycobacterium tuberculosis 65-kilodalton antigen is a heat shock protein which corresponds to common antigen and to the Escherichia coli GroEL protein. Infect Immun. 1988;56:446–451. doi: 10.1128/iai.56.2.446-451.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taylor S N, Figueroa J E, Johnston K H. Program and abstracts of the 12th Meeting of the ISSTDR. Seville, Spain: ISSTDR; 1997. Characterization of the interaction between Haemophilus ducreyi and epithelial cell proteoglycans, abstr. P374; p. 124. [Google Scholar]
- 25.Telzak E E, Chiasson M A, Bevier P J, Stoneburner R L, Castro K G, Jaffe H W. HIV-1 seroconversion in patients with and without genital ulcer disease. Ann Intern Med. 1993;119:1181–1186. doi: 10.7326/0003-4819-119-12-199312150-00005. [DOI] [PubMed] [Google Scholar]
- 26.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang J-R, Stinson M W. M protein mediates Streptococcal adhesion to HEp-2 cells. Infect Immun. 1994;62:442–448. doi: 10.1128/iai.62.2.442-448.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamaguchi H, Osaki T, Taguchi H, Hanawa T, Yamamoto T, Kamiya S. Flow cytometric analysis of the heat shock protein 60 expressed on the cell surface of Helicobacter pylori. J Med Microbiol. 1996;45:270–277. doi: 10.1099/00222615-45-4-270. [DOI] [PubMed] [Google Scholar]
- 29.Young D, Lathigra R, Hendrix R, Sweeter D, Young R A. Stress proteins are immune targets in leprosy and tuberculosis. Proc Natl Acad Sci USA. 1988;85:4267–4270. doi: 10.1073/pnas.85.12.4267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zeilstra-Ryalls J, Fayet O, Georgopoulos C. The universally conserved GroE (Hsp60) chaperonins. Annu Rev Microbiol. 1991;45:301–325. doi: 10.1146/annurev.mi.45.100191.001505. [DOI] [PubMed] [Google Scholar]