Abstract
Aims
Red cell distribution width (RDW) is a strong prognostic marker in patients with heart failure (HF) and reduced ejection fraction and other conditions. However, very little is known about its prognostic significance in HF with preserved ejection fraction. We examined the relationship between RDW and outcomes and the effect of sacubitril/valsartan, compared with valsartan, on RDW and clinical outcomes in PARAGON‐HF.
Methods and results
PARAGON‐HF enrolled patients with a left ventricular ejection fraction of ≥45%, structural heart disease, and elevated N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP). The primary endpoint was a composite of total HF hospitalizations and cardiovascular deaths. Median RDW at randomization was 14.1% (interquartile range 13.5–15.0%). Patients with higher RDW levels were more often men and had more comorbidity, a higher heart rate and NT‐proBNP concentration, more advanced New York Heart Association class, and worse Kansas City Cardiomyopathy Questionnaire scores. There was a graded relationship between quartiles of RDW at randomization and the primary endpoint, with a significantly higher risk associated with increasing RDW, even after adjustment for NT‐proBNP and other prognostic variables {Quartile 1, reference; Quartile 2, rate ratio 1.03 [95% confidence interval (CI) 0.83 to 1.28]; Quartile 3, 1.25 [1.01 to 1.54]; Quartile 4, 1.70 [1.39 to 2.08]}. This association was seen for each of the secondary outcomes, including cardiovascular and all‐cause death. Compared with valsartan, sacubitril/valsartan reduced RDW at 48 weeks [mean change −0.09 (95% CI −0.15 to −0.02)]. The effect of sacubitril/valsartan vs. valsartan was not significantly modified by RDW levels at randomization.
Conclusions
RDW, a routinely available and inexpensive biomarker, provides incremental prognostic information when added to established predictors. Compared with valsartan, sacubitril/valsartan led to a small reduction in RDW.
Keywords: Heart failure, Red cell width distribution, Sacubitril–valsartan, Clinical trial, Outcomes
Introduction
Red cell distribution width (RDW) is a measure of the heterogeneity in the size of circulating red cells, and it is routinely assessed as part of a complete blood cell count, but not always reported. 1 , 2 RDW is calculated by dividing the standard deviation of the red blood cell volume by the mean corpuscular volume multiplied by 100. 1 , 2 Higher RDW values reflect greater heterogeneity in red blood cell size (i.e. anisocytosis), and, together with other measures, it has traditionally been used to aid diagnosis of the cause of anaemia. 1 , 2
RDW is an independent predictor of poor outcomes in the general population and individuals with various chronic diseases. 3 , 4 , 5 , 6 , 7 , 8 , 9 This measure is of particular interest in heart failure (HF), because inflammation and neurohormonal activation (especially adrenergic activation), all of which are part of the pathophysiology of HF, 10 , 11 may alter erythropoiesis, red blood cell circulation half‐life, and red blood cell membrane deformability and thereby lead to increased RDW. 12 RDW is associated with incident HF, 13 , 14 , 15 and it is also a predictor of worse outcomes in ambulatory and hospitalized patients with established HF with reduced ejection fraction (HFrEF). 16 , 17 , 18 , 19 , 20 , 21 However, few of these studies were rigorously adjusted for known prognostic variables, including natriuretic peptides. 16 , 17 , 18 , 19 , 20 , 21 In addition, remarkably little is known about its prognostic significance in patients with HF with preserved ejection fraction (HFpEF), especially in the ambulatory setting. 19 , 20 , 21
Consequently, we have examined the relationship between RDW and outcomes in ambulatory patients with HFpEF enrolled in the Prospective Comparison of ARNI With ARB Global Outcomes in HF With Preserved Ejection Fraction trial (PARAGON‐HF). 22 Because sacubitril/valsartan improves overall HF status and directly or indirectly suppresses neurohumoral activation and inflammation and other processes that could lead to anisocytosis, we have also examined the effect of sacubitril/valsartan, compared with valsartan, on RDW levels during follow‐up.
Methods
PARAGON‐HF was a randomized, double‐blind, parallel‐group, active‐controlled trial in patients with HFpEF, evaluating the efficacy and safety of sacubitril/valsartan compared with valsartan. The design and primary results of the trial are published. 22 , 23 The corresponding author had full access to all the trial data and takes responsibility for its integrity and the data analysis. Trial data will be made available by the sponsor, Novartis, in accordance with their data sharing policy.
Study patients and trial procedures
Key inclusion criteria were signs and symptoms of HF [New York Heart Association (NYHA) functional class II–IV], a left ventricular ejection fraction (LVEF) of ≥45% within 6 months of screening, elevated natriuretic peptide level (with different cut‐offs depending on the occurrence of a recent HF hospitalization and the presence of atrial fibrillation/flutter), evidence of structural heart disease (left atrial enlargement or left ventricular hypertrophy), and diuretic therapy. Key exclusion criteria were any previous echocardiographic measurement of LVEF < 40%; recent acute coronary syndrome, cardiac surgery, or percutaneous coronary intervention; acute decompensated HF at the time of screening; intolerance to either study drug (or similar classes) or a history of angioedema; systolic blood pressure of >180 or <110 mmHg; estimated glomerular filtration rate (eGFR) < 30 mL/min/1.73 m2; and serum potassium level > 5.2 mmol/L. A complete list of exclusion criteria is provided in the design paper. 23
During the single‐blind run‐in periods, all participants first received valsartan at half the target dose and then sacubitril/valsartan at half the target dose. Participants who tolerated this dose in both run‐in phases were randomized to treatment with either sacubitril/valsartan (target dose, 97/103 mg twice daily) or valsartan (target dose, 160 mg of valsartan twice daily) in a 1:1 ratio.
Red cell distribution width measurements
Blood samples were collected at baseline, that is, immediately before the single‐blind run‐in period; at the end of the run‐in period, that is, while taking sacubitril/valsartan immediately before randomization; 16 weeks after randomization; and 48 weeks after randomization. All samples were analysed in a central laboratory.
Trial outcomes
The primary outcome in the trial was a composite of total (first and recurrent) HF hospitalizations and cardiovascular death. In the present analysis, we examined the components of the primary outcome; first HF hospitalization or cardiovascular death; first HF hospitalization; total cardiovascular hospitalizations; total all‐cause hospitalizations; all‐cause death; death due to worsening HF (‘pump failure’); sudden cardiac death; and non‐cardiovascular death. We also examined the change in RDW levels from baseline to 16 and 48 weeks.
Statistical analyses
Baseline characteristics were summarized as frequencies with percentages, means with standard deviation, or medians with interquartile ranges. Differences in baseline characteristics were tested using the Cochran–Armitage trend test for binary variables, the Cochran–Mantel–Haenszel test for categorical variables, and the Jonckheere–Terpstra test and linear regression for non‐normal and normally distributed continuous variables, respectively.
Regardless of treatment allocation, total (first and recurrent) events were evaluated with Nelson–Aalen cumulative hazard curves and semiparametric proportional‐rates models, stratified according to region, and adjusted for treatment group assignment. 24 Time‐to‐event data were evaluated with the Kaplan–Meier estimator, the Aalen–Johansen estimator, and Cox proportional‐hazards models, stratified according to region, and adjusted for treatment group assignment. In addition, semiparametric proportional‐rates models and Cox proportional‐hazards models, respectively, stratified according to geographic region and adjusted for treatment assignment, age, sex, systolic blood pressure, heart rate, body mass index, eGFR, log of N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP), haemoglobin, mean corpuscular volume, LVEF, NYHA functional class, HF duration, prior HF hospitalization, history of myocardial infarction, atrial fibrillation/flutter, and diabetes, were performed. The relationship between RDW as a continuous variable and the risk of outcomes was also examined in restricted cubic spline analyses (with the median value as the reference).
In a landmark analysis, the association between the change in RDW levels from baseline to 16 weeks after randomization and the risk of outcomes was examined in restricted cubic spline analyses (with 0, i.e. no change, as the reference). In this analysis, only patients who were alive and had a measurement of RDW at 16 weeks after randomization were included, and patients were followed from 16 weeks after randomization.
To compare the effects of sacubitril/valsartan vs. valsartan on clinical outcomes, total (first and recurrent) events and time‐to‐event data were evaluated with semiparametric proportional‐rates models and Cox proportional‐hazards models, respectively, and models were stratified according to region.
The difference between treatment groups in the change in RDW from baseline to 16 and 48 weeks, respectively, was analysed using analysis of covariance models, adjusted for baseline RDW, and results are presented as least‐squares mean differences with 95% confidence intervals (CIs). The difference between treatment groups in the change in RDW is also presented as ratios of geometric means with 95% CIs.
All analyses were conducted using SAS 9.4 and STATA 17.0. A P‐value of 0.05 was considered statistically significant.
Results
In total, 4742, 4795, 4519, and 4270 patients had RDW measurements available at baseline, randomization, 16 weeks after randomization, and 48 weeks after randomization, respectively. Median RDW at randomization was 14.1% (25th–75th percentile, 13.5–15.0%). The four groups defined by quartiles of RDW at randomization were (i) <13.6%, (ii) 13.6–14.1%, (iii) 14.2–15.0%, and (iv) >15.0%.
Patient characteristics
Baseline characteristics according to quartiles of RDW at randomization are presented in Table 1 and Supporting Information, Table S1 . Compared with patients with a lower RDW, those with a higher RDW were older and more often male and Asian (and less often White). They had a higher heart rate (if atrial fibrillation/flutter was not present on the electrocardiogram) and body mass index, and they were more likely to be current or former smokers and to have a prior HF hospitalization, atrial fibrillation/flutter, peripheral artery disease, diabetes, chronic obstructive pulmonary disease, renal disease, obstructive sleep apnoea, and anaemia. Patients with a higher RDW also had more signs or symptoms of HF (lower Kansas City Cardiomyopathy Questionnaire scores and higher NYHA functional class) than individuals with a lower RDW.
Table 1.
Baseline characteristics of the study population according to quartiles of red cell distribution width at randomization
|
RDW Quartile 1 <13.6% N = 1256 |
RDW Quartile 2 13.6–14.1% N = 1177 |
RDW Quartile 3 14.2–15.0% N = 1247 |
RDW Quartile 4 >15.0% N = 1115 |
P‐value | |
|---|---|---|---|---|---|
| Age (years), mean (SD) | 72.3 ± 8.6 | 72.5 ± 8.3 | 72.7 ± 8.5 | 73.5 ± 8.2 | <0.001 |
| Sex, N (%) | <0.001 | ||||
| Female | 727 (57.9) | 609 (51.7) | 596 (47.8) | 547 (49.1) | |
| Male | 529 (42.1) | 568 (48.3) | 651 (52.2) | 568 (50.9) | |
| Race, N (%) | <0.001 | ||||
| Asian | 156 (12.4) | 98 (8.3) | 142 (11.4) | 210 (18.8) | |
| Black | 18 (1.4) | 19 (1.6) | 28 (2.2) | 37 (3.3) | |
| White | 1034 (82.3) | 1018 (86.5) | 1032 (82.8) | 823 (73.8) | |
| Other | 48 (3.8) | 42 (3.6) | 45 (3.6) | 45 (4.0) | |
| Physiological measures, mean (SD) | |||||
| Systolic blood pressure (mmHg) | 130.4 ± 14.9 | 130.5 ± 15.3 | 131.1 ± 15.8 | 130.2 ± 15.8 | 0.94 |
| Diastolic blood pressure (mmHg) | 74.7 ± 10.4 | 74.9 ± 10.3 | 74.6 ± 10.4 | 73.0 ± 11.0 | <0.001 |
| Heart rate (b.p.m.) | 69.1 ± 11.6 | 70.4 ± 12.4 | 70.6 ± 12.3 | 71.8 ± 12.7 | <0.001 |
| No atrial fibrillation/flutter on ECG | 67.1 ± 10.2 | 68.3 ± 10.9 | 68.4 ± 11.1 | 69.2 ± 11.9 | <0.001 |
| Atrial fibrillation/flutter on ECG | 76.1 ± 13.2 | 75.5 ± 14.1 | 75.2 ± 13.4 | 76.2 ± 12.9 | 0.89 |
| BMI (kg/m2) | 29.7 ± 4.8 | 30.3 ± 5.0 | 30.8 ± 4.9 | 30.1 ± 5.3 | <0.001 |
| Sign/symptoms, N (%) | |||||
| Orthopnoea | 196 (15.6) | 206 (17.5) | 231 (18.6) | 253 (22.7) | <0.001 |
| Oedema | 426 (33.9) | 444 (37.8) | 494 (39.7) | 461 (41.4) | <0.001 |
| Rales | 78 (6.2) | 77 (6.6) | 97 (7.8) | 93 (8.3) | 0.02 |
| Jugular venous distention | 141 (11.4) | 160 (13.7) | 178 (14.4) | 175 (15.8) | 0.002 |
| KCCQ‐OSS, mean (SD) | 73.5 ± 17.7 | 71.5 ± 18.9 | 70.6 ± 19.8 | 69.9 ± 19.3 | <0.001 |
| NYHA class, N (%) | 0.001 | ||||
| I–II | 1035 (82.4) | 955 (81.3) | 988 (79.2) | 864 (77.5) | |
| III–IV | 221 (17.6) | 220 (18.7) | 259 (20.8) | 251 (22.5) | |
| LVEF, mean (SD) | 57.9 ± 8.0 | 57.3 ± 7.9 | 57.2 ± 7.7 | 57.9 ± 7.9 | 0.79 |
| Current or former smoker, N (%) | 456 (36.5) | 474 (40.5) | 492 (39.7) | 490 (44.2) | <0.001 |
| Medical history, N (%) | |||||
| Previous MI | 264 (21.0) | 267 (22.7) | 298 (23.9) | 254 (22.8) | 0.21 |
| Atrial fibrillation/flutter | 539 (42.9) | 619 (52.6) | 690 (55.4) | 713 (63.9) | <0.001 |
| Hypertension | 1189 (94.7) | 1135 (96.4) | 1189 (95.3) | 1070 (96.0) | 0.28 |
| Stroke | 135 (10.8) | 120 (10.2) | 118 (9.5) | 135 (12.2) | 0.45 |
| Diabetes at randomization | 467 (37.2) | 472 (40.1) | 556 (44.6) | 573 (51.4) | <0.001 |
| Hospitalization for HF | 509 (40.5) | 526 (44.7) | 610 (48.9) | 661 (59.3) | <0.001 |
| Chronic obstructive pulmonary disease | 135 (10.7) | 167 (14.2) | 171 (13.8) | 197 (17.7) | <0.001 |
| Cancer | 113 (9.0) | 107 (9.1) | 100 (8.0) | 113 (10.1) | 0.57 |
| Biomarkers, median (25th–75th percentile) | |||||
| NT‐proBNP, pg/mL | 721 (401–1384) | 833 (459–1482) | 957 (488–1657) | 1186 (583–2055) | <0.001 |
| No atrial fibrillation/flutter on ECG | 557 (365–973) | 603 (387–1036) | 663 (393–1177) | 746 (433–1503) | <0.001 |
| Atrial fibrillation/flutter on ECG | 1521 (1126–2120) | 1477 (1060–2065) | 1584 (1184–2261) | 1680 (1204–2668) | <0.001 |
| Creatinine, μmol/L | 86.0 (73.0–105.0) | 90.0 (76.0–109.0) | 95.0 (78.0–114.0) | 98.0 (80.0–121.0) | <0.001 |
| eGFR, mL/min/1.73 m2 | 63.6 (51.0–77.0) | 61.7 (50.6–75.0) | 59.7 (47.5–72.5) | 56.6 (44.6–70.4) | <0.001 |
| eGFR < 60 mL/min/1.73 m2, N (%) | 520 (41.4) | 545 (46.3) | 636 (51.0) | 640 (57.4) | <0.001 |
| Albumin, g/L | 43.0 (41.0–45.0) | 42.0 (41.0–44.0) | 42.0 (40.0–44.0) | 42.0 (40.0–44.0) | <0.001 |
| Blood urea nitrogen, mmol/L | 7.1 (5.7–8.9) | 7.1 (5.8–9.0) | 7.6 (6.0–9.8) | 8.1 (6.1–10.7) | <0.001 |
| Total cholesterol, mmol/L | 4.50 (3.80–5.40) | 4.40 (3.75–5.20) | 4.30 (3.60–5.20) | 4.19 (3.50–5.00) | <0.001 |
| Haemoglobin, g/L | 136 (127–146) | 137 (128–146) | 136 (126–146) | 128 (118–139) | <0.001 |
| Mean corpuscular volume, fL | 97.0 (94.0–101.0) | 96.0 (93.0–100.0) | 95.0 (92.0–99.0) | 94.0 (89.0–99.0) | <0.001 |
| Lymphocyte count, 109/L | 1.70 (1.40–2.10) | 1.60 (1.30–2.00) | 1.60 (1.30–2.00) | 1.50 (1.20–2.00) | <0.001 |
| Neutrophil count, 109/L | 3.90 (3.10–4.90) | 4.00 (3.20–5.00) | 4.20 (3.30–5.10) | 4.20 (3.30–5.20) | <0.001 |
| Neutrophil/lymphocyte ratio | 2.3 (1.8–3.1) | 2.4 (1.9–3.2) | 2.6 (1.9–3.4) | 2.7 (2.0–3.8) | <0.001 |
| Platelet count, 109/L | 213 (179–255) | 210 (172–253) | 202 (168–244) | 199 (165–247) | <0.001 |
| HbA1c, % | 6.0 (5.7–6.7) | 6.1 (5.8–6.8) | 6.2 (5.8–7.0) | 6.4 (5.9–7.2) | <0.001 |
| Alanine aminotransferase, U/L | 17.0 (13.0–22.0) | 17.0 (13.0–22.0) | 17.0 (13.0–22.0) | 15.0 (12.0–20.0) | <0.001 |
| Alkaline phosphatase, U/L | 70.0 (56.0–84.0) | 72.0 (58.0–88.0) | 74.0 (61.0–92.0) | 77.0 (63.0–99.0) | <0.001 |
| Bilirubin, mg/dL | 8.0 (6.0–11.0) | 9.0 (6.0–12.0) | 9.0 (6.0–12.0) | 8.6 (6.0–12.0) | 0.07 |
| High‐sensitivity troponin T, μg/L | 0.015 (0.010–0.022) | 0.015 (0.010–0.020) | 0.017 (0.012–0.025) | 0.021 (0.014–0.033) | <0.001 |
| sST2, ng/mL | 21.3 (17.8–25.9) | 22.2 (17.8–27.2) | 22.8 (18.4–26.8) | 23.3 (18.9–28.3) | 0.002 |
| CITP, μg/L | 5.2 (4.1–6.7) | 5.5 (4.6–7.1) | 6.3 (5.0–8.4) | 7.7 (5.8–11.6) | <0.001 |
| PINP, μg/L | 37.0 (27.0–48.0) | 37.0 (29.0–49.0) | 39.0 (30.0–53.0) | 41.0 (30.0–54.0) | 0.002 |
| PIIINP, μg/L | 4.1 (3.3–5.3) | 4.2 (3.5–5.2) | 4.6 (3.6–5.5) | 4.8 (4.0–6.2) | <0.001 |
| TIMP‐1, ng/mL | 121.3 (101.7–146.8) | 124.2 (106.2–149.5) | 131.8 (112.7–160.3) | 145.4 (116.9–176.4) | <0.001 |
| Treatment, N (%) | |||||
| ACEI/ARB | 1090 (86.8) | 1044 (88.7) | 1093 (87.7) | 912 (81.8) | <0.001 |
| Beta‐blocker | 1020 (81.2) | 921 (78.2) | 1001 (80.3) | 878 (78.7) | 0.30 |
| Mineralocorticoid receptor antagonist | 304 (24.2) | 277 (23.5) | 334 (26.8) | 324 (29.1) | 0.002 |
| Diuretic | 1199 (95.5) | 1122 (95.3) | 1191 (95.5) | 1072 (96.1) | 0.42 |
| Antiplatelet | 156 (12.4) | 143 (12.1) | 166 (13.3) | 169 (15.2) | 0.04 |
| Oral anticoagulant | 300 (23.9) | 381 (32.4) | 421 (33.8) | 449 (40.3) | <0.001 |
ACEI, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; CITP, collagen I telopeptide; ECG, electrocardiogram; eGFR, estimated glomerular filtration rate; HbA1c, glycated haemoglobin; HF, heart failure; KCCQ‐OSS, Kansas City Cardiomyopathy Questionnaire‐Overall Summary Score; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; NYHA, New York Heart Association; PIIINP, N‐terminal propeptide of collagen III; PINP, N‐terminal propeptide of collagen I; RDW, red cell distribution width; SD, standard deviation; sST2, soluble ST2; TIMP‐1, tissue inhibitor of matrix metalloproteinase‐1.
High‐sensitivity troponin T, N = 1260; sST2, N = 1228; N = 1195; CITP, N = 1112; PINP, N = 1116; PIIINP, N = 1108; TIMP‐1, N = 1117. CITP, PINP, PIIINP, and TIMP‐1 were collected at the screening visit.
Regarding biomarkers, patients with greater RDW had higher levels of many biomarkers, including NT‐proBNP, creatinine, blood urea nitrogen, uric acid, erythrocyte count, neutrophil/lymphocyte and neutrophil/leucocyte ratio, markers of collagen turnover, and high‐sensitivity troponin T. Conversely, levels of other biomarkers were lower in individuals with higher RDW levels, including albumin, cholesterol, haemoglobin, platelets, haematocrit, mean corpuscular haemoglobin concentration, mean corpuscular volume, and alanine aminotransferase.
Regarding pharmacological treatment, those with a higher RDW were less frequently treated with a renin–angiotensin system inhibitor but more often with a mineralocorticoid receptor antagonist, digoxin, and oral anticoagulant.
Outcomes according to red cell distribution width at randomization
Compared with the lowest quartile of RDW at randomization, patients in the higher quartiles had higher rates of the primary composite outcome of total HF hospitalizations and cardiovascular death [Quartile 1, reference; Quartile 2, 1.19 (95% CI 0.95 to 1.48); Quartile 3, 1.55 (1.25 to 1.93); Quartile 4, 2.72 (2.23 to 3.30)] and each of the secondary clinical outcomes (Figure 1 and Table 2 ). After adjustment for NT‐proBNP and other prognostic variables, these associations remained statistically significant for all outcomes (Table 2 ). When RDW was examined as a continuous variable, a similar picture was evident (Supporting Information, Figure S1 ).
Figure 1.
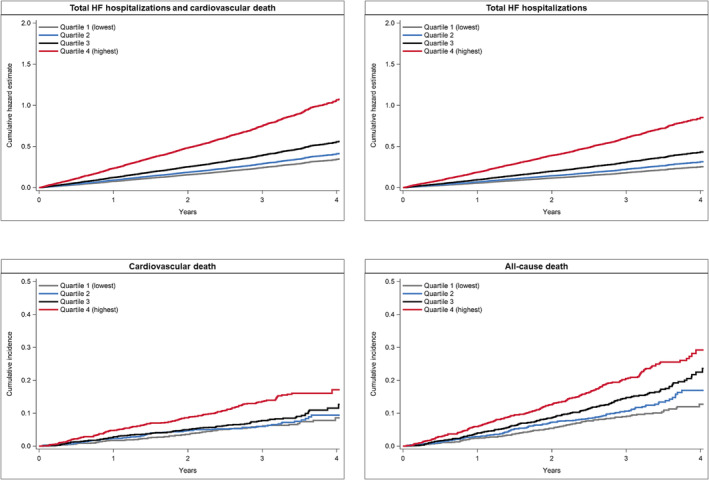
Outcomes according to quartiles of red cell distribution width at randomization. This figure shows the cumulative hazard estimate for total heart failure (HF) hospitalizations and cardiovascular death, and total HF hospitalizations, and the cumulative incidence of cardiovascular death and all‐cause death according to quartiles of red cell distribution width at randomization.
Table 2.
Outcomes according to quartiles of red cell distribution width at randomization
|
RDW Quartile 1 <13.6% N = 1256 |
RDW Quartile 2 13.6–14.1% N = 1177 |
RDW Quartile 3 14.2–15.0% N = 1247 |
RDW Quartile 4 >15.0% N = 1115 |
|
|---|---|---|---|---|
| Primary composite outcome | ||||
| No. of events | 302 | 333 | 481 | 786 |
| Event rate per 100 person‐years (95% CI) | 8.2 (7.3–9.2) | 9.8 (8.8–10.9) | 13.3 (12.1–14.5) | 25.2 (23.5–27.1) |
| RR (95% CI) a | Reference | 1.19 (0.95–1.48) | 1.55 (1.25–1.93) | 2.72 (2.23–3.30) |
| RR (95% CI) b | Reference | 1.03 (0.83–1.28) | 1.25 (1.01–1.54) | 1.70 (1.39–2.08) |
| Total HF hospitalizations | ||||
| No. of events | 224 | 255 | 376 | 631 |
| Event rate per 100 person‐years (95% CI) | 6.1 (5.3–6.9) | 7.5 (6.6–8.4) | 10.4 (9.4–11.5) | 20.3 (18.7–21.9) |
| RR (95% CI) a | Reference | 1.23 (0.95–1.58) | 1.62 (1.27–2.08) | 2.85 (2.29–3.56) |
| RR (95% CI) b | Reference | 1.05 (0.83–1.34) | 1.28 (1.01–1.63) | 1.72 (1.37–2.14) |
| Total cardiovascular hospitalizations | ||||
| No. of events | 665 | 655 | 847 | 1105 |
| Event rate per 100 person‐years (95% CI) | 18.0 (16.7–19.4) | 19.2 (17.8–20.7) | 23.3 (21.8–25.0) | 35.5 (33.5–37.6) |
| RR (95% CI) a | Reference | 1.07 (0.92–1.24) | 1.26 (1.08–1.46) | 1.77 (1.53–2.05) |
| RR (95% CI) b | Reference | 0.99 (0.85–1.15) | 1.10 (0.95–1.27) | 1.36 (1.17–1.58) |
| Total all‐cause hospitalizations | ||||
| No. of events | 1391 | 1324 | 1804 | 2256 |
| Event rate per 100 person‐years (95% CI) | 37.7 (35.8–39.7) | 38.8 (36.7–40.9) | 49.7 (47.5–52.1) | 72.5 (69.5–75.5) |
| RR (95% CI) a | Reference | 1.03 (0.91–1.16) | 1.27 (1.13–1.42) | 1.69 (1.51–1.90) |
| RR (95% CI) b | Reference | 0.97 (0.86–1.09) | 1.13 (1.01–1.26) | 1.34 (1.19–1.51) |
| Cardiovascular death | ||||
| N (%) | 78 (6.2) | 78 (6.6) | 105 (8.4) | 155 (13.9) |
| Event rate per 100 person‐years (95% CI) | 2.1 (1.7–2.6) | 2.3 (1.8–2.8) | 2.9 (2.4–3.5) | 5.0 (4.2–5.8) |
| HR (95% CI) a | Reference | 1.08 (0.79–1.48) | 1.35 (1.01–1.81) | 2.30 (1.74–3.03) |
| HR (95% CI) b | Reference | 0.97 (0.71–1.34) | 1.14 (0.84–1.53) | 1.62 (1.21–2.19) |
| Pump failure death | ||||
| N (%) | 21 (1.7) | 17 (1.4) | 28 (2.2) | 52 (4.7) |
| Event rate per 100 person‐years (95% CI) | 0.6 (0.4–0.9) | 0.5 (0.3–0.8) | 0.8 (0.5–1.1) | 1.7 (1.3–2.2) |
| HR (95% CI) a | Reference | 0.88 (0.47–1.68) | 1.29 (0.73–2.28) | 2.62 (1.57–4.38) |
| HR (95% CI) b | Reference | 0.80 (0.42–1.52) | 0.97 (0.54–1.72) | 1.47 (0.84–2.55) |
| Sudden cardiac death | ||||
| N (%) | 28 (2.2) | 27 (2.3) | 42 (3.4) | 57 (5.1) |
| Event rate per 100 person‐years (95% CI) | 0.8 (0.5–1.1) | 0.8 (0.5–1.2) | 1.2 (0.9–1.6) | 1.8 (1.4–2.4) |
| HR (95% CI) a | Reference | 1.05 (0.62–1.79) | 1.55 (0.96–2.50) | 2.50 (1.58–3.96) |
| HR (95% CI) b | Reference | 0.96 (0.56–1.63) | 1.31 (0.80–2.14) | 2.08 (1.27–3.40) |
| Non‐cardiovascular death | ||||
| N (%) | 41 (3.3) | 57 (4.8) | 90 (7.2) | 86 (7.7) |
| Event rate per 100 person‐years (95% CI) | 1.1 (0.8–1.5) | 1.7 (1.3–2.2) | 2.5 (2.0–3.0) | 2.8 (2.2–3.4) |
| HR (95% CI) a | Reference | 1.48 (0.99–2.21) | 2.21 (1.52–3.20) | 2.56 (1.76–3.73) |
| HR (95% CI) b | Reference | 1.32 (0.88–1.99) | 1.84 (1.26–2.69) | 1.79 (1.20–2.67) |
| All‐cause death | ||||
| N (%) | 119 (9.5) | 135 (11.5) | 195 (15.6) | 241 (21.6) |
| Event rate per 100 person‐years (95% CI) | 3.2 (2.7–3.9) | 4.0 (3.3–4.7) | 5.4 (4.7–6.2) | 7.7 (6.8–8.8) |
| HR (95% CI) a | Reference | 1.22 (0.95–1.56) | 1.65 (1.31–2.07) | 2.39 (1.92–2.99) |
| HR (95% CI) b | Reference | 1.10 (0.85–1.41) | 1.39 (1.10–1.75) | 1.69 (1.33–2.14) |
| First HF hospitalization or cardiovascular death | ||||
| N (%) | 208 (16.6) | 211 (17.9) | 280 (22.5) | 383 (34.3) |
| Event rate per 100 person‐years (95% CI) | 6.0 (5.3–6.9) | 6.6 (5.8–7.6) | 8.4 (7.5–9.5) | 14.3 (13.0–15.9) |
| HR (95% CI) a | Reference | 1.10 (0.91–1.33) | 1.36 (1.13–1.62) | 2.18 (1.84–2.59) |
| HR (95% CI) b | Reference | 0.96 (0.79–1.17) | 1.12 (0.93–1.35) | 1.44 (1.20–1.73) |
| First HF hospitalization | ||||
| N (%) | 156 (12.4) | 156 (13.3) | 220 (17.6) | 305 (27.4) |
| Event rate per 100 person‐years (95% CI) | 4.5 (3.9–5.3) | 4.9 (4.2–5.7) | 6.6 (5.8–7.6) | 11.4 (10.2–12.8) |
| HR (95% CI) a | Reference | 1.08 (0.87–1.35) | 1.41 (1.15–1.73) | 2.26 (1.86–2.75) |
| HR (95% CI) b | Reference | 0.94 (0.75–1.17) | 1.14 (0.92–1.41) | 1.42 (1.15–1.76) |
CI, confidence interval; HF, heart failure; HR, hazard ratio; RDW, red cell distribution width; RR, rate ratio.
Stratified by region and adjusted for treatment assignment.
Stratified by region and adjusted for treatment assignment, age, sex, systolic blood pressure, heart rate, body mass index, estimated glomerular filtration rate, log of N‐terminal pro‐B‐type natriuretic peptide, haemoglobin, mean corpuscular volume, left ventricular ejection fraction, New York Heart Association functional class, HF duration, prior HF hospitalization, history of myocardial infarction, atrial fibrillation/flutter, and diabetes.
We further examined the two principal modes of cardiovascular death, that is, sudden death and death due to worsening HF (‘pump failure’). Compared with those in the lowest quartile of RDW, patients in the highest quartile had a significantly higher adjusted risk of sudden death (Table 2 ).
RDW provided independent incremental prognostic information when added to established prognostic cardiac biomarkers (NT‐proBNP and troponin), eGFR, and haemoglobin (Figure 2 ).
Figure 2.
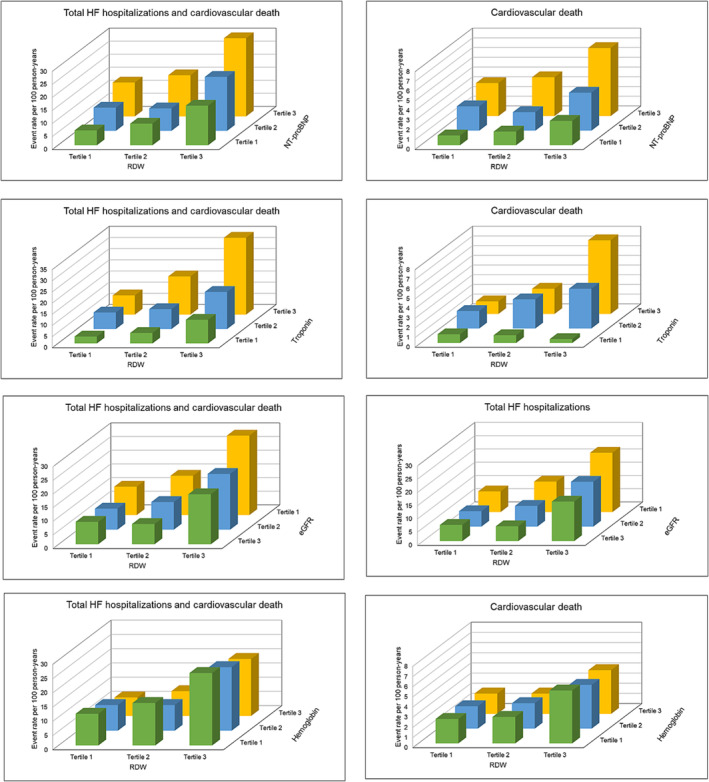
Outcomes according to red cell distribution width (RDW) in conjunction with established prognostic cardiac biomarkers, kidney function, and haemoglobin. This figure shows the event rates per 100 person‐years of total heart failure (HF) hospitalizations or cardiovascular death according to RDW in conjunction with N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP), troponin, estimated glomerular filtration rate (eGFR), and haemoglobin.
Outcomes according to the change in red cell distribution width from baseline to 16 weeks after randomization
In a landmark analysis of patients who were alive at 16 weeks after randomization, a reduction in RDW levels from baseline to 16 weeks after randomization was associated with a lower risk of the primary composite outcome, each of its components, and all‐cause death, compared with no change (Supporting Information, Figure S2 ). An increase in RDW levels from baseline to 16 weeks after randomization was not significantly associated with a higher risk of these outcomes compared with no change (Supporting Information, Figure S2 ).
Effect of sacubitril/valsartan on red cell distribution width
There was no meaningful difference between patients treated with sacubitril/valsartan and valsartan in the change in RDW from baseline to 16 weeks [mean treatment difference 0.02 (95% CI −0.04 to 0.07), P = 0.51; ratio of geometric means 1.00 (1.00 to 1.00), P = 0.55] (Supporting Information, Figure S3 and Tables S2 and S3 ). However, there was a significant attenuation of the increase in RDW from baseline to 48 weeks after randomization in patients treated with sacubitril/valsartan vs. valsartan [mean treatment difference −0.09 (95% CI −0.15 to −0.02), P = 0.009; ratio of geometric means 0.99 (0.99 to 1.00), P = 0.009] (Supporting Information, Figure S3 and Tables S2 and S3 ).
Effect of sacubitril/valsartan on clinical outcomes according to red cell distribution width at randomization
Interaction testing showed no significant modification of the effects of sacubitril/valsartan, compared with valsartan, by RDW at randomization examined as a categorical variable. However, the trend to a favourable effect of sacubitril/valsartan, compared with valsartan, was attenuated among patients in the highest RDW quartile (Figure 3 and Supporting Information, Table S4 ). When RDW at randomization was analysed as a continuous variable, there was a significant interaction with treatment for the primary endpoint and HF hospitalizations, with an absence of benefit in patients at the higher end of the RDW range (Figure 4 ).
Figure 3.
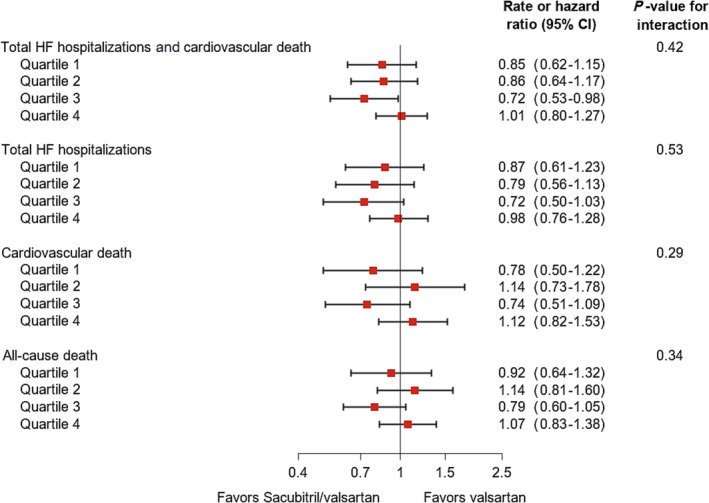
Effects of sacubitril/valsartan, compared with valsartan, on outcomes according to quartiles of red cell distribution width at randomization. A rate ratio or hazard ratio < 1 favours sacubitril/valsartan, and a rate ratio or hazard ratio > 1 favours valsartan. Models were stratified by region. CI, confidence interval; HF, heart failure.
Figure 4.

Effects of sacubitril/valsartan, compared with valsartan, on outcomes according to continuous red cell distribution width (RDW) at randomization. The black line represents the rate ratio or hazard ratio, and the shaded blue area represents the 95% confidence interval. A rate ratio or hazard ratio < 1 favours sacubitril/valsartan, and a rate ratio or hazard ratio > 1 favours valsartan. Models were stratified by region. HF, heart failure.
Discussion
In ambulant patients with HFpEF, higher levels of RDW were associated with worse outcomes, with the greatest risk among patients in the fourth quartile of RDW values at randomization. The lower boundary of this quartile (15%) corresponded with a commonly used upper limit of normal for RDW. RDW remained a predictor of outcomes independently of other established prognostic variables, including established cardiac biomarkers, kidney function, and haemoglobin. Treatment with sacubitril/valsartan, compared with valsartan, led to a small reduction in RDW, and a decrease in RDW over time was associated with better outcomes. Finally, the possible beneficial effect of sacubitril/valsartan on clinical outcomes appeared to be attenuated in patients with the highest levels of RDW.
Association between red cell distribution width and outcomes
RDW was first shown to be predictive of outcomes in chronic HF enrolled in the Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity (CHARM) trials. In CHARM, RDW was among the strongest prognostic markers of morbidity and mortality and remained an independent predictor of worse outcomes in a multivariable model, although that model did not include natriuretic peptides. 25 These findings have subsequently been confirmed in both ambulant and hospitalized patients in several observational reports, some of which have included measurements of natriuretic peptides. 16 , 17 , 18 , 19 , 20 , 21 While elevated levels of RDW have been consistently associated with worse outcomes in patients with HF, most prior studies either did not distinguish between HF phenotype (HFrEF or HFpEF) or included patients with HFrEF only. 16 , 17 , 18 , 19 , 20 , 21 We are not aware of any robust prior analysis of the association between RDW levels and adverse outcomes in ambulant patients with HFpEF. Therefore, the present analysis of approximately 4800 ambulatory patients with HFpEF extends previous findings on the prognostic value of RDW in HF. Specifically, we found that higher levels of RDW were associated with substantially higher rates of the primary composite outcome (total HF hospitalizations and cardiovascular death), each of its components, and all‐cause death. Importantly, these associations persisted after comprehensive adjustment for prognostic variables, including NT‐proBNP, and whether RDW was examined as a categorical or continuous variable.
Although elevated RDW has recently been linked to worse outcomes in other cardiovascular and non‐cardiovascular diseases, the unadjusted risk among patients in the highest RDW quartile in PARAGON‐HF was greatest for HF hospitalization and smallest for all‐cause hospitalization (and intermediate for cardiovascular hospitalization), suggesting some specificity for HF outcomes. However, the risks of cardiovascular and non‐cardiovascular death were similarly elevated in patients with high RDW.
As a biomarker, RDW has caused intrigue because the mechanisms underlying its association with adverse clinical outcomes are not clear, although several explanations have been proposed. First, anisocytosis may be directly harmful by altering the haemorheological properties and functioning of erythrocytes. Large red cells that are less deformable may not traverse the microcirculation and may be less effective in delivering oxygen and modulating nitric oxide levels. Associated membrane fragility and increased cell lysis, with the liberation of iron, lipids, and microparticles, may contribute to several pathophysiological processes including the progression of atherosclerosis and cardiovascular fibrosis. Second, erythropoiesis is influenced by the sympathetic nervous system, and activity of this system increases with the increasing severity of HF. Third, higher RDW may reflect multiple underlying abnormalities themselves associated with worse outcomes including nutritional deficiencies causing impaired erythropoiesis (e.g. iron, folate, and vitamin B12), kidney and liver failure, thyroid dysfunction, inflammation, oxidative stress, bleeding, haemolysis, myelodysplastic syndromes, haemoglobinopathies, and premature senescence. Which, if any of these, might explain the association between RDW levels and adverse outcomes in the present study is uncertain. Our analyses were adjusted for haemoglobin and mean corpuscular volume (as a surrogate for B12 and folate stores). We did not have measurements of iron status, and the greater use of anticoagulants and antiplatelet agents may have led to more blood loss in patients with the highest RDW values at baseline. Kidney function was worse in patients with higher RDW, although RDW still provided independent incremental prognostic information when added to eGFR. Hepatic function was not worse in patients in the upper quartile of RDW values at baseline. The higher NT‐proBNP in patients with higher RDW supports the possibility of greater neurohumoral activation, potentially including adrenergic activation in these patients. The higher heart rate in patients with the highest RDW values is also consistent with greater sympathetic nervous system activity. Interestingly, in the adjusted analyses, patients with the highest RDW values had an elevated risk of sudden death, which is consistent with the finding that greater erythrocyte anisocytosis is associated with cardiac autonomic dysfunction. 26 Unfortunately, we did not have any measure of inflammation such as high‐sensitivity C‐reactive protein. However, perhaps the strength of RDW as a prognostic biomarker is that it serves as an integrator of a broad range of detrimental processes that cumulatively lead to worse outcomes.
Effects of sacubitril/valsartan according to baseline red cell distribution width
Compared with valsartan, sacubitril/valsartan seemed to reduce the risk of the primary endpoint and HF hospitalizations in patients with an RDW < 15%, with no benefit among those in the highest quartile of RDW values. This unexpected finding may reflect the play of chance as the interaction test was only significant when RDW was analysed as a continuous rather than categorical variable (and because sacubitril/valsartan did not reduce the primary outcome significantly in the trial overall). Although reminiscent of the interaction seen with LVEF, there was no association between RDW and LVEF. The other subgroup interaction detected in the primary analysis of PARAGON‐HF was by sex, with greater benefit in women than men. Interestingly, the proportion of women decreased significantly as RDW increased.
Effects of sacubitril/valsartan on red cell distribution width during follow‐up
Compared with valsartan, treatment with sacubitril/valsartan to a small absolute reduction in RDW of approximately 0.1% was not observed until 48 weeks. The significance of such a small change, after such a long period of follow‐up, is uncertain. However, treatment with sacubitril/valsartan has been associated with a reduction in markers of inflammation and fibrosis, 27 , 28 and the reduction in RDW could reflect reduced inflammation.
Limitations
In addition to the limitations mentioned above, the inclusion and exclusion criteria in PARAGON‐HF precluded the enrolment of very high‐risk patients, which may affect the generalizability of our results. Second, given the observational nature of the analyses on the association between RDW and clinical outcomes, the possibility of residual confounding cannot be fully excluded despite adjustment for measured, known confounders. Finally, we did not have data on reticulocyte count.
Conclusions
In ambulant patients with HFpEF, RDW, a routinely available and inexpensive biomarker, provides incremental prognostic information when added to NT‐proBNP and other established predictors. Compared with valsartan, sacubitril/valsartan reduced RDW levels, and its effect on clinical outcomes was not significantly modified by RDW levels at randomization.
Conflict of interest
Dr Butt reports advisory board honoraria from Bayer; consultant honoraria from Novartis; and travel grants from AstraZeneca. Dr Kondo has received speaker fees from Abbott, Ono Pharma, Otsuka Pharma, Novartis, AstraZeneca, Bristol‐Myers Squibb, and Abiomed. Dr Desai has received personal fees from Abbott, Biofourmis, Boston Scientific, Boehringer Ingelheim, Merck, Regeneron, and Relypsa and grants and personal fees from AstraZeneca, Alnylam, and Novartis outside the submitted work. Dr Lefkowitz is an employee of Novartis. Dr Packer has received consulting fees from AbbVie, Akcea, Actavis, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Cardiorentis, Daiichi Sankyo, Gilead, Johnson & Johnson, Novo Nordisk, Pfizer, Relypsa, Sanofi, Synthetic Biologics, and Theravance. Dr Petrie has received research grants or consultancy fees from SQ Innovations, AstraZeneca, Roche, Boehringer Ingelheim, Pharmacosmos, Eli Lilly, Napp Pharmaceuticals, Novartis, and Novo Nordisk and has served on clinical events committees for AbbVie, Alnylam, AstraZeneca, Bayer, Boehringer Ingelheim, GlaxoSmithKline, Resverlogix, and Novo Nordisk. Dr Rouleau has received grants and consulting fees from Novartis and consulting fees from Abbott, AstraZeneca, MyoKardia, and Sanofi. Dr Vaduganathan has received research grant support or served on advisory boards for American Regent, Amgen, AstraZeneca, Bayer AG, Baxter Healthcare, Boehringer Ingelheim, Cytokinetics, Lexicon Pharmaceuticals, and Relypsa; reports speaker engagements with Novartis and Roche Diagnostics; and participates on clinical endpoint committees for studies sponsored by Galmed and Novartis. Dr Zile has received research funding from Novartis and has been a consultant for Novartis, Abbott, Boston Scientific, CVRx, EBR, Endotronics, Ironwood, Merck, Medtronic, and Myokardia V Wave. Dr Jhund's employer, the University of Glasgow, has been remunerated by AstraZeneca for working on the DAPA‐HF and DELIVER trials, has received personal fees from Novartis and Cytokinetics, and has received grants from Boehringer Ingelheim. Dr Køber reports compensation from Novartis for other services; compensation from Novo Nordisk for other services; and compensation from AstraZeneca for other services. Dr Solomon has received research grants from Actelion, Alnylam, Amgen, AstraZeneca, Bellerophon, Bayer, Bristol Myers Squibb, Celladon, Cytokinetics, Eidos, Gilead, GlaxoSmithKline, Ionis, Lilly, Mesoblast, MyoKardia, National Institutes of Health/NHLBI, NeuroTronik, Novartis, Novo Nordisk, Respicardia, Sanofi Pasteur, Theracos, and US2.AI and has consulted for Abbott, Action, Akros, Alnylam, Amgen, Arena, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Cardior, Cardurion, Corvia, Cytokinetics, Daiichi‐Sankyo, GlaxoSmithKline, Lilly, Merck, Myokardia, Novartis, Roche, Theracos, Quantum Genomics, Cardurion, Janssen, Cardiac Dimensions, Tenaya, Sanofi‐Pasteur, Dinaqor, Tremeau, CellPro‐Thera, Moderna, American Regent, and Sarepta. Dr McMurray has received payments through Glasgow University from work on clinical trials, consulting and other activities from Alnylam, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, BMS, Cardurion, Cytokinetics, Dal‐Cor, GSK, Ionis, KBP Biosciences, Novartis, Pfizer, and Theracos, and personal lecture fees from the Corpus, Abbott, Hikma, Sun Pharmaceuticals, Medscape/Heart.Org, Radcliffe Cardiology, Servier Director, and Global Clinical Trial Partners (GCTP). The remaining authors have nothing to disclose.
Funding
The PARAGON‐HF trial was funded by Novartis. Drs McMurray and Jhund are supported by a British Heart Foundation Centre of Research Excellence Grant RE/18/6/34217.
Supporting information
Table S1. Baseline characteristics of the study population according to quartiles of red cell distribution width at randomization.
Table S2. Mean change in red cell distribution width during follow‐up according to treatment assignment.
Table S3. Geometric means of red cell distribution width during follow‐up according to treatment assignment.
Table S4. Effects of sacubitril/valsartan compared with valsartan on outcomes according to quartiles of red cell distribution width at randomization.
Figure S1. Outcomes according to continuous red cell distribution width at randomization.
Figure S2. Outcomes according to change in red cell distribution width from baseline to 16 weeks after randomization.
Figure S3. Change in red cell distribution width during follow‐up according to treatment assignment.
Butt, J. H. , McDowell, K. , Kondo, T. , Desai, A. S. , Lefkowitz, M. P. , Packer, M. , Petrie, M. C. , Pfeffer, M. A. , Rouleau, J. L. , Vaduganathan, M. , Zile, M. R. , Jhund, P. S. , Køber, L. , Solomon, S. , and McMurray, J. J. V. (2024) Heart failure with preserved ejection fraction, red cell distribution width, and sacubitril/valsartan. ESC Heart Failure, 11: 65–77. 10.1002/ehf2.14558.
Clinical trial registration: Unique identifier: NCT01920711.
References
- 1. Salvagno GL, Sanchis‐Gomar F, Picanza A, Lippi G. Red blood cell distribution width: A simple parameter with multiple clinical applications. Crit Rev Clin Lab Sci 2015;52:86‐105. doi: 10.3109/10408363.2014.992064 [DOI] [PubMed] [Google Scholar]
- 2. England JM, Down MC. Red‐cell‐volume distribution curves and the measurement of anisocytosis. Lancet 1974;303:701‐703. doi: 10.1016/S0140-6736(74)92904-3 [DOI] [PubMed] [Google Scholar]
- 3. Perlstein TS, Weuve J, Pfeffer MA, Beckman JA. Red blood cell distribution width and mortality risk in a community‐based prospective cohort. Arch Intern Med 2009;169:588‐594. doi: 10.1001/archinternmed.2009.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Patel KV, Ferrucci L, Ershler WB, Longo DL, Guralnik JM. Red blood cell distribution width and the risk of death in middle‐aged and older adults. Arch Intern Med 2009;169:515‐523. doi: 10.1001/archinternmed.2009.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tonelli M, Wiebe N, James MT, Naugler C, Manns BJ, Klarenbach SW, et al. Red cell distribution width associations with clinical outcomes: A population‐based cohort study. PLoS ONE 2019;14:e0212374. doi: 10.1371/journal.pone.0212374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pan J, Borné Y, Engström G. The relationship between red cell distribution width and all‐cause and cause‐specific mortality in a general population. Sci Rep 2019;9:16208. doi: 10.1038/s41598-019-52708-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ferreira JP, Lamiral Z, Bakris G, Mehta C, White WB, Zannad F. Red cell distribution width in patients with diabetes and myocardial infarction: An analysis from the EXAMINE trial. Diabetes Obes Metab 2021;23:1580‐1587. doi: 10.1111/dom.14371 [DOI] [PubMed] [Google Scholar]
- 8. Seyhan EC, Özgül MA, Tutar N, Ömür I, Uysal A, Altin S. Red blood cell distribution and survival in patients with chronic obstructive pulmonary disease. COPD J Chronic Obstr Pulm Dis 2013;10:416‐424. doi: 10.3109/15412555.2012.758697 [DOI] [PubMed] [Google Scholar]
- 9. Yoo KD, Oh HJ, Park S, Kang MW, Kim YC, Park JY, et al. Red blood cell distribution width as a predictor of mortality among patients regularly visiting the nephrology outpatient clinic. Sci Rep 2021;11:24310. doi: 10.1038/s41598-021-03530-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021;42:3599‐3726. doi: 10.1093/eurheartj/ehab368 [DOI] [PubMed] [Google Scholar]
- 11. Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: A report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022;145:e895‐e1032. doi: 10.1161/CIR.0000000000001063 [DOI] [PubMed] [Google Scholar]
- 12. Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med 2005;352:1011‐1023. doi: 10.1056/NEJMra041809 [DOI] [PubMed] [Google Scholar]
- 13. Borné Y, Smith JG, Melander O, Hedblad B, Engström G. Red cell distribution width and risk for first hospitalization due to heart failure: A population‐based cohort study. Eur J Heart Fail 2011;13:1355‐1361. doi: 10.1093/eurjhf/hfr127 [DOI] [PubMed] [Google Scholar]
- 14. Emans ME, Gaillard CAJM, Pfister R, Tanck MW, Boekholdt SM, Wareham NJ, et al. Red cell distribution width is associated with physical inactivity and heart failure, independent of established risk factors, inflammation or iron metabolism; the EPIC—Norfolk study. Int J Cardiol 2013;168:3550‐3555. doi: 10.1016/j.ijcard.2013.05.002 [DOI] [PubMed] [Google Scholar]
- 15. Tonelli M, Sacks F, Arnold M, Moye L, Davis B, Pfeffer M, et al. Relation between red blood cell distribution width and cardiovascular event rate in people with coronary disease. Circulation 2008;117:163‐168. doi: 10.1161/CIRCULATIONAHA.107.727545 [DOI] [PubMed] [Google Scholar]
- 16. Huang YL, De HZ, Liu SJ, Sun Y, Qin Q, Qin BD, et al. Prognostic value of red blood cell distribution width for patients with heart failure: A systematic review and meta‐analysis of cohort studies. PLoS ONE 2014;9:e104861. doi: 10.1371/journal.pone.0115743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shao Q, Li L, Li G, Liu T. Prognostic value of red blood cell distribution width in heart failure patients: A meta‐analysis. Int J Cardiol 2015;179:495‐499. doi: 10.1016/j.ijcard.2014.11.042 [DOI] [PubMed] [Google Scholar]
- 18. Hou H, Sun T, Li C, Li Y, Guo Z, Wang W, et al. An overall and dose‐response meta‐analysis of red blood cell distribution width and CVD outcomes. Sci Rep 2017;7:43420. doi: 10.1038/srep43420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sotiropoulos K, Yerly P, Monney P, Garnier A, Regamey J, Hugli O, et al. Red cell distribution width and mortality in acute heart failure patients with preserved and reduced ejection fraction. ESC Hear Fail 2016;3:198‐204. doi: 10.1002/ehf2.12091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liang L, Huang L, Zhao X, Zhao L, Tian P, Huang B, et al. Prognostic value of RDW alone and in combination with NT‐proBNP in patients with heart failure. Clin Cardiol 2022;45:802‐813. doi: 10.1002/clc.23850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Imai R, Uemura Y, Okumura T, Takemoto K, Uchikawa T, Koyasu M, et al. Impact of red blood cell distribution width on non‐cardiac mortality in patients with acute decompensated heart failure with preserved ejection fraction. J Cardiol 2017;70:591‐597. doi: 10.1016/j.jjcc.2017.03.010 [DOI] [PubMed] [Google Scholar]
- 22. Solomon SD, McMurray JJV, Anand IS, Ge J, Lam CSP, Maggioni AP, et al. Angiotensin–neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med 2019;381:1609‐1620. doi: 10.1056/NEJMoa1908655 [DOI] [PubMed] [Google Scholar]
- 23. Solomon SD, Rizkala AR, Gong J, Wang W, Anand IS, Ge J, et al. Angiotensin receptor neprilysin inhibition in heart failure with preserved ejection fraction: Rationale and design of the PARAGON‐HF trial. JACC Hear Fail 2017;5:471‐482. doi: 10.1016/j.jchf.2017.04.013 [DOI] [PubMed] [Google Scholar]
- 24. Lin DY, Wei LJ, Yang I, Ying Z. Semiparametric regression for the mean and rate functions of recurrent events. J R Stat Soc Series B Stat Methodology 2000;62:711‐730. doi: 10.1111/1467-9868.00259 [DOI] [Google Scholar]
- 25. Felker GM, Allen LA, Pocock SJ, Shaw LK, McMurray JJV, Pfeffer MA, et al. Red cell distribution width as a novel prognostic marker in heart failure. Data from the CHARM Program and the Duke Databank. J Am Coll Cardiol 2007;50:40‐47. doi: 10.1016/j.jacc.2007.02.067 [DOI] [PubMed] [Google Scholar]
- 26. Yamada S, Yoshihisa A, Kaneshiro T, Amami K, Hijioka N, Oikawa M, et al. The relationship between red cell distribution width and cardiac autonomic function in heart failure. J Arrhythmia 2020;36:1076‐1082. doi: 10.1002/joa3.12442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bolla GB, Fedele A, Faggiano A, Sala C, Santangelo G, Carugo S. Effects of sacubitril/valsartan on biomarkers of fibrosis and inflammation in patients with heart failure with reduced ejection fraction. BMC Cardiovasc Disord 2022;22:217. doi: 10.1186/s12872-022-02647-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bunsawat K, Ratchford SM, Alpenglow JK, Park SH, Jarrett CL, Stehlik J, et al. Sacubitril‐valsartan improves conduit vessel function and functional capacity and reduces inflammation in heart failure with reduced ejection fraction. J Appl Physiol 2021;130:256‐268. doi: 10.1152/japplphysiol.00454.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Baseline characteristics of the study population according to quartiles of red cell distribution width at randomization.
Table S2. Mean change in red cell distribution width during follow‐up according to treatment assignment.
Table S3. Geometric means of red cell distribution width during follow‐up according to treatment assignment.
Table S4. Effects of sacubitril/valsartan compared with valsartan on outcomes according to quartiles of red cell distribution width at randomization.
Figure S1. Outcomes according to continuous red cell distribution width at randomization.
Figure S2. Outcomes according to change in red cell distribution width from baseline to 16 weeks after randomization.
Figure S3. Change in red cell distribution width during follow‐up according to treatment assignment.


