Abstract
Aims
Current heart failure (HF) guidelines recommend to prescribe four drug classes in patients with HF with reduced ejection fraction (HFrEF). A clear challenge exists to adequately implement guideline‐directed medical therapy (GDMT) regarding the sequencing of drugs and timely reaching target dose. It is largely unknown how the paradigm shift from a serial and sequential approach for drug therapy to early parallel application of the four drug classes will be executed in daily clinical practice, as well as the reason clinicians may not adhere to new guidelines. We present the design and rationale for the real‐world TITRATE‐HF study, which aims to assess sequencing strategies for GDMT initiation, dose titration patterns (order and speed), intolerance for GDMT, barriers for implementation, and long‐term outcomes in patients with de novo, chronic, and worsening HF.
Methods and results
A total of 4000 patients with HFrEF, HF with mildly reduced ejection fraction, and HF with improved ejection fraction will be enrolled in >40 Dutch centres with a follow‐up of at least 3 years. Data collection will include demographics, physical examination and vital parameters, electrocardiogram, laboratory measurements, echocardiogram, medication, and quality of life. Detailed information on titration steps will be collected for the four GDMT drug classes. Information will include date, primary reason for change, and potential intolerances. The primary clinical endpoints are HF‐related hospitalizations, HF‐related urgent visits with a need for intravenous diuretics, all‐cause mortality, and cardiovascular mortality.
Conclusions
TITRATE‐HF is a real‐world multicentre longitudinal registry that will provide unique information on contemporary GDMT implementation, sequencing strategies (order and speed), and prognosis in de novo, worsening, and chronic HF patients.
Keywords: Design, Registry, Heart failure, Pharmacotherapy, Implementation, Guidelines
Introduction
The cornerstone of guideline‐directed medical therapy (GDMT) in heart failure (HF) with reduced ejection fraction (HFrEF) consists of four drug classes of pharmacotherapy (‘quadruple therapy’). 1 These are angiotensin‐converting enzyme inhibitor (ACE‐I)/angiotensin II receptor blocker (ARB)/angiotensin receptor–neprilysin inhibitor (ARNI), beta‐blocker (BB), mineralocorticoid receptor antagonist (MRA), and sodium–glucose co‐transporter 2 inhibitor (SGLT2i). A recent meta‐analysis has shown an association between the simultaneous use of these therapies and a reduction in mortality rates, emphasizing the need for quadruple therapy in these patients. 2
While the recommendation to include all four drug classes in HFrEF therapy is clearly stated in the guideline, the implementation in clinical practice is not straightforward. In the Netherlands, the CHECK‐HF registry has shown an overall good GDMT prescription rate; however, only a third of the patients with HFrEF used all GDMT in any dose. 3 In the context of persistently high rates of mortality and hospitalization associated with HFrEF, data from CHECK‐HF and other real‐world registries highlight the urgency towards improving GDMT implementation and ensuring that all eligible patients with HFrEF in routine clinical practice receive the recommended four drug classes. 3 , 4 , 5 , 6 , 7
The most recent European Society of Cardiology (ESC) HF 2021 guideline recommends the four life‐saving drug classes to be administered to all eligible patients with HFrEF and that each drug be initiated without significant delay. 1 , 8 Multiple studies have supported this recommendation and show the link between a short implementation time‐frame and reduced mortality, 9 , 10 , 11 of which the results were confirmed in the STRONG‐HF trial. 12 In order to optimize the drug implementation process, several sequencing strategies have been suggested, as summarized in Figure 1 . 13 , 14 , 15 , 16
Figure 1.
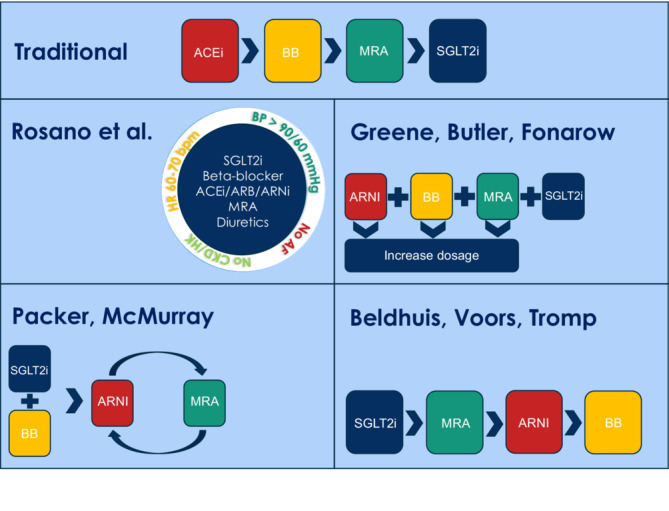
Overview of proposed sequencing strategies. ACE‐I, angiotensin‐converting enzyme inhibitor; AF, atrial fibrillation; ARB, angiotensin II receptor blocker; ARNI, angiotensin receptor–neprilysin inhibitor; BB, beta‐blocker; BP, blood pressure; CKD, chronic kidney disease; HK, hyperkalaemia; HR, heart rate; MRA, mineralocorticoid receptor antagonist; SGLT2i, sodium–glucose co‐transporter 2 inhibitor.
Despite the evidence favouring a high‐intensity and rapid sequencing approach as described in STRONG‐HF, the question of practical feasibility remains. In the first 3 months, patients in the high‐intensity group had an average of five clinic visits compared with one visit in the usual care group. Limited availability of resources and personnel, an increasing and ageing HF population, and substantial health care costs could prove to be limiting factors in the real‐world application of such a strategy.
Identifying barriers for implementation of GDMT and reasons for not initiating recommended drugs and achieving target dose in certain patients are therefore essential to develop new strategies for further improvement and implementation of HF care. These barriers and reasons are an important knowledge gap considering both the scarcity of data on this subject and the available data being incomplete or based on highly selected trial patients. 17 , 18
The TITRATE‐HF registry is designed to evaluate guideline implementation, GDMT sequencing strategies, and quality of care for real‐world HF patients and will provide prospective data on GDMT sequencing strategies and dose titration patterns, as well as its association with HF‐related outcomes. Moreover, barriers for GDMT implementation (including intolerance) will be identified.
Study design
Registry structure and oversight
TITRATE‐HF is a multicentre, longitudinal registry to evaluate the implementation of the current HF guidelines and quality of care for HF patients in daily clinical practice. 1 HF patients should be treated according to the ESC HF 2021 guideline, 1 which is the standard of care for HF patients in the Netherlands. The research protocol has been approved by the ethics committee of the Erasmus University Medical Center Rotterdam under number MEC‐2022‐0252 and complies with the Declaration of Helsinki. HF patients will be asked for oral and written informed consent by their treating physician and will be enrolled consecutively during HF‐related (index) events or outpatient clinic visits in a real‐world all‐comers setting. The informed consent is also related to enable future data linkage and coupling to available external resources for medication use, hospitalization, and death records. HF care in the Netherlands is organized in a structured manner at dedicated HF outpatient clinics with specialized HF nurses and cardiologists. TITRATE‐HF is a national project and all Dutch hospitals can participate, assuming each hospital provides HF care. We aim to include a minimum of 35 participating hospitals. Relevant partners within this national registry consortium are the Working group of Cardiology centres Netherlands (WCN) and the Netherlands Heart Institute (NLHI), representing 60 general and 8 university hospitals in the Netherlands. In addition, TITRATE‐HF is supported by the Dutch Cardiac Society (NVVC), the Dutch Cardiovascular Alliance (DCVA), the National Heart Failure Working Group, and the Netherlands Heart Registration (NHR).
Study participants
Patients (in both inpatient and outpatient settings) with de novo, chronic, or worsening HF are eligible for enrolment. The definitions of each HF type are summarized in Table 1 . HF patients should be treated according to the HF guidelines. Considering the lack of available guideline recommendations for the four drug classes in HF patients with a preserved ejection fraction (HFpEF), only HFrEF, HF with an improved ejection fraction (HFimpEF), and HF with mildly reduced ejection fraction are currently eligible for enrolment. HFpEF patients may become eligible for enrolment in a later stage as add‐on registry project to TITRATE‐HF. The registry will be performed in a real‐world setting, which entails enrolment of eligible participants on an all‐comers and consecutive basis. Accordingly, exclusion criteria are limited and relate only to life expectancy for diseases other than HF, major cardiovascular events interfering with prescription of HF drugs, and advanced or end‐stage HF. The complete list of inclusion and exclusion criteria is summarized in Table 2 . We anticipate to include a minimum of 4000 participants, of which 1600 (40%) with de novo HF and 2400 (60%) with chronic HF. Approximately 600 (25%) of the patients with chronic HF will have worsening HF. Each participating centre is expected to include between 70 and 250 consecutive patients in total, with the above‐mentioned proportions of HF categories.
Table 1.
HF categories and definitions
| Category HF | Definition |
|---|---|
| De novo HF | Confirmed HF diagnosis 3 months or less prior to enrolment. |
| Chronic HF | Confirmed HF diagnosis 6 months or more prior to enrolment. |
| Worsening HF | Confirmed HF diagnosis 6 months or more prior to enrolment and an HF‐related event (hospitalization or urgent visit) 6 months or less prior to enrolment. |
HF, heart failure.
Table 2.
Inclusion and exclusion criteria
| Inclusion criteria | Exclusion criteria |
|---|---|
| Written and oral informed consent. | Life expectancy < 1 year due to comorbidities according to treating physician. |
| Confirmed HF diagnosis (de novo, chronic, or worsening) with type HFrEF (LVEF ≤ 40%), or HFmrEF (LVEF 41–49%), according to EF% measured by echocardiography or MRI. | Major cardiovascular event (e.g. myocardial infarction, open‐heart surgery, or stroke) within 2 months prior to enrolment. |
| Willing and able to comply with follow‐up regimen for usual HF care for at least 1 year. | Advanced or end‐stage HF that are scheduled or likely to undergo HTx or VAD implantation within 6 months after enrolment. |
EF, ejection fraction; HF, heart failure; HFmrEF, heart failure with mildly reduced ejection fraction; HFrEF, heart failure with reduced ejection fraction; HTx, heart transplantation; LVEF, left ventricular ejection fraction; MRI, magnetic resonance imaging; VAD, ventricular assist device.
Data collection and follow‐up
The follow‐up is 3 years, although this may be extended in the future with data linkage from external sources. Data on HF‐related events, patient status, and interventions are investigator reported and will be collected from electronic patient records every 6 months starting from baseline. Additionally, participants will be asked to complete the EQ‐5D‐5L quality of life questionnaire at baseline, 6 months, and 12 months. 19 , 20 Because of the shorter completion time and lower effort required by the patient, the EQ‐5D‐5L was preferred over the longer Kansas City Cardiomyopathy Questionnaire (KCCQ). 21 Additionally, the visual analogue scale of the EQ‐5D‐5L allows for a simple one‐answer quality of life measurement. Detailed information on titration steps of BB, ACE‐I/ARB/ARNI, MRA, and SGLT2i will be collected between baseline (chronic or worsening HF) or diagnosis (de novo HF) and 6 months of follow‐up.
Data will be collected at baseline and every 6 months through electronic case report forms (eCRFs) in the Castor electronic data capture system (Amsterdam, the Netherlands). The data collection and follow‐up frequency are based on the assumption that HF patients in the Netherlands visit their treating physician or nurse at least twice a year, although no schedule for clinical visits is required by the study protocol. All data items collected are part of routine HF care and can be extracted from the electronic health records, except for the very short EQ‐5D‐5L quality of life questionnaire. As the frequency of patient visits may vary between participating centres, wide time windows are included for follow‐up and corresponding data items to provide more overlap between data collection and routine HF care in the centres. The registry does not stipulate a certain treatment scheme in any form as the goal is to learn the current status of quality of care and how guideline implementation takes place. The data collection structure, including the time windows, is summarized in Figure 2 .
Figure 2.
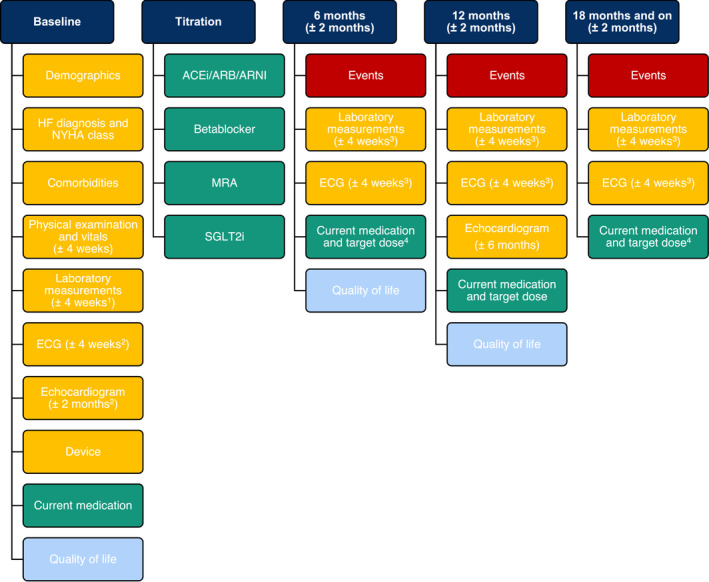
Data collection structure with time windows. ACE‐I, angiotensin‐converting enzyme inhibitor; ARB, angiotensin II receptor blocker; ARNI, angiotensin receptor–neprilysin inhibitor; ECG, electrocardiogram; HF, heart failure; MRA, mineralocorticoid receptor antagonist; NYHA, New York Heart Association; SGLT2i, sodium–glucose co‐transporter 2 inhibitor. 1If unavailable, max 6 months old. 2If unavailable, most recent. 3If unavailable, max 3 months old. 4Target dose only applicable for the four drug classes.
Data items
Data collection at baseline consists of demographics, details on HF diagnosis, New York Heart Association (NYHA) class, comorbidities, physical examination and vital parameters (such as blood pressure and heart rate), electrocardiogram (ECG), laboratory measurements, echocardiogram, details on cardiac implantable electronic devices, current medication use, and quality of life (EQ‐5D‐5L). Data collection at follow‐up consists of clinical endpoints (among which death and HF hospitalizations), NYHA class, ECG, laboratory measurements, echocardiogram (12 months of follow‐up only and if available), current medication use, and quality of life (EQ‐5D‐5L).
The laboratory measurements collected at baseline include creatinine, urea, serum sodium, serum potassium, estimated glomerular filtration rate (eGFR), N‐terminal pro‐brain natriuretic peptide (NT‐proBNP), haemoglobin A1c, total bilirubin, haemoglobin, uric acid, and iron status (including administered intravenous iron treatment). At follow‐up, creatinine, eGFR, serum potassium, NT‐proBNP, and iron status are registered.
The echocardiogram eCRF at baseline and 12 months of follow‐up contains an estimate of the left ventricular ejection fraction (LVEF), a description of all valves and severity of any valvular regurgitation or stenosis, left atrial size, left ventricular (LV) end‐diastolic and end‐systolic dimensions, tricuspid regurgitation gradient, and diameter and collapsibility percentage of the inferior caval vein. The echocardiogram at 12 months of follow‐up focuses only on LVEF, LV dimensions, and mitral valve. In case of moderate or severe mitral regurgitation, the effective regurgitant orifice area, proximal isovelocity surface area, and vena contracta are also registered.
Medication use and four‐drug‐class log
In the eCRFs for current medication, data are collected on the prescription of ACE‐I/ARB/ARNI, BB, MRA, SGLT2i, loop diuretics, anticoagulants, hydralazine, hydrochlorothiazide, digoxin, vericiguat, omecamtiv, ivabradine, amiodarone, sotalol, statins (baseline only), and allopurinol (baseline only). For the four drug classes, type, dose, and whether target dose has been achieved are registered at each visit during follow‐up. If target dose has not been achieved, the reason is registered. For loop diuretics, type, dose, and number of daily administrations are collected.
Between baseline (chronic and worsening HF) or diagnosis (de novo HF) and 6 months of follow‐up, every titration step of the four drug classes (ACE‐I/ARB/ARNI, BB, MRA, and SGLT2i) is recorded in a separate log for each individual drug. At the start of each log, it is noted whether the drug is already prescribed on the start date of the log, and accompanying reason for use or non‐use. After that, all changes are registered. The chosen sequencing and implementation strategy is left to the discretion of the treating physician where the current guideline recommendations on GDMT implementation are leading, including future statements or recommendations. Medication changes during hospitalizations will be simplified by registering a single change corresponding with the difference between the medication when admitted and when discharged, with the exception of index events of de novo HF patients where instead all changes during a hospitalization are registered. The registration logs include date, type of medication change (start, up‐titration, down‐titration, and stop), and the primary reason for change. For the reason of non‐use, the registration options are contra‐indication, intolerance or side effects, use of other drugs, and other. For the reason of down‐titration or stop, the registration options are intolerance or side effects (with options), request by patient, non‐compliance, allergies, change during hospitalization, or switch to other drugs (for ACE‐I/ARB/ARNI). For the reason of start or up‐titration, the registration options are titration or change during hospitalization. In case the registered reason is intolerance or side effects (e.g. hyperkalaemia, hypertension, and bradycardia), 22 additional information is collected on parameter levels when applicable (e.g. serum potassium, systolic blood pressure, and heart rate). The registration options for the four‐drug‐class medication log are listed in Supporting Information, Table S1 ; other options may be added during the study.
Clinical endpoints
At each follow‐up moment, data are collected on clinical endpoints occurring since baseline or the previous follow‐up. The clinical endpoints of interest are mortality (cardiac or non‐cardiac related), hospitalizations (HF related and non‐HF related), urgent HF‐related visits, thoracic surgical procedures, percutaneous valvular interventions, device changes (including implantation, switch, or upgrade of devices), use of telemonitoring, cardiac rehabilitation, and dialysis. The complete list of clinical endpoints and definitions, including the list of relevant non‐HF‐related types of hospitalizations, is shown in Supporting Information, Table S2 .
The primary clinical endpoints are HF‐related hospitalizations, HF‐related urgent visits with a need for intravenous diuretics, all‐cause mortality, and cardiovascular mortality. Endpoints will be studied in relation to percentage of the target dose achieved and speed of drug up‐titration of the four drug classes. Secondary clinical endpoints are freedom from death or first HF‐related hospitalization, quality of life, days alive outside of the hospital, days admitted in the hospital, the number of total hospital presentation visits (outpatient clinic, clinical ward, and emergency department), HF‐related hospitalizations, all‐cause mortality during follow‐up, and freedom from death or first HF‐related hospitalization.
Statistical analysis
Data will be summarized with means and standard deviations or medians and interquartile ranges as appropriate for continuous variables and with counts and percentages for categorical variables. Between‐group comparisons of baseline characteristics will be performed with the two‐sample t‐test or ANOVA for continuous variables with a normal distribution and the Mann–Whitney U test or the Kruskal–Wallis H test for continuous variables with a non‐normal distribution. The χ 2 test will be used for between‐group comparisons of categorical variables.
The survival distributions of clinical events will be calculated and compared between groups with the Kaplan–Meier estimator and the log‐rank test. A Cox proportional hazard regression model will be used for the time to event analysis of clinical endpoints and, for example, the Andersen–Gill extension for the recurrent events analysis. 23 We will include potential confounders in the multivariate models when these have a P‐value < 0.10 in the univariate models, 3 including at least age, sex, body mass index, and NYHA class. Subgroup analyses will be performed for age, gender, ejection fraction, renal function, devices, and relevant comorbidities.
A two‐tailed P‐value ≤ 0.05 will be considered statistically significant in all analyses, and adjustments for repeated significance testing will be considered when appropriate. Multiple imputation will be considered when data are missing conditionally at random or completely at random and when appropriate for the type of analysis. All analyses will be performed with SPSS (Version 28 or later), or R (Version 4.2.1 or later) and RStudio (Version 2022.07.1 or later), depending on the analysis.
Discussion
In the current guidelines, the view on the initiation and titration of HF therapy has changed substantially compared with previous guidelines 1 , 8 and has marked the beginning of a paradigm change of HF care in clinical practice. The sequence of HF drug initiation changes from a serial approach according to the chronology of the landmark randomized controlled trials (RCTs) (titrating one after another to maximum tolerated or target dose) to a parallel approach (combining several drugs at low dose and titrating to maximum tolerated or target dose simultaneously). Although the parallel approach comes with uncertainty regarding the order and precise timing of initiating the drug classes, it is supported by the early event curve separation in the aforementioned major RCTs, 24 , 25 as well as analyses from recent studies. 9 , 10 , 11 Savarese et al. performed a study with 68 172 new users of HF treatment with data from Sweden, the United Kingdom, and the United States and found a high rate of treatment discontinuation within 1 year (24–55%) and poor target dose achievement based on retrospective data (10–30%), showing an urgent need for earlier use of novel GDMTs. 10 In addition, D'Amario et al. reported a lower risk of cardiovascular death or HF hospitalization with the use of two drug classes at 50–99% of the target dose as compared with using one drug at 100% of the target dose. 9 Finally, Shen et al. modelled multiple sequencing approaches and found the conventional serial approach to be less effective as compared with several parallel accelerated approaches. 11
Within the debate on titration strategies, more concrete recommendations have also been given by McMurray and Packer in the form of a three‐step ‘new sequence’ algorithm. 24 This algorithm entails initiating a BB and SGLT2i as Step 1, adding ARNI as Step 2, and adding an MRA as Step 3. 24 The algorithm was based upon five principles: (1) The benefit of each drug class is independent; (2) lower doses are also effective in reducing morbidity and mortality; (3) addition of drug classes provides more benefit than increasing the dose of the ones already in use; (4) proper sequencing improves safety and tolerability; and (5) the algorithm provides additional benefit by achieving therapy of all four drugs within 4 weeks. 24 By comparison, Greene et al. have proposed simultaneous initiation of all four medications at low doses on Day 1 or rapid sequence initiation of all four with 1 week. 15 The rationale is similar to that of McMurray, but with added recognition that there is no evidence that withholding a particular drug in an eligible patient, even if for just 2–4 weeks, accomplishes anything beneficial or improves medication tolerance. Rather, there is evidence that with withholding any of the four drugs for even a couple of weeks, the patient is needlessly exposed to excess clinical risk and the risk that the medication is never implemented at all. Beldhuis et al. advocate initiating an SGLT2i first, then an MRA, followed by ARNI, and lastly a BB. 13 Rosano et al. have proposed the use of patient profiling to provide more specific recommendations according to phenotypic HF profile; however, this initiative is new and validation in clinical practice is not available. 16 , 26
Importantly, the recent STRONG‐HF trial provides direct RCT evidence that simultaneous and rapid sequence initiation and titration of GDMT for HF is safe, well tolerated, and effective for reducing death and HF hospitalization. 12 Aside from benefits on the primary clinical outcome, benefits of high‐intensity care extended across multiple other endpoint domains. Specifically, high‐intensity care improved patient‐reported health status and NYHA class, reduced clinical congestion, lowered NT‐proBNP, and resulted in substantially higher use and dosing of GDMT throughout follow‐up. These benefits were complemented for reassuring safety, with no significant difference in serious adverse events between high‐intensity care and usual care. There were also limitations of STRONG‐HF worth mentioning. These include the design that was altered during the course of the trial (higher sample size and longer follow‐up on primary endpoints), the lack of a protocol for SGLT2i in the implementation strategy, the open label nature of the trial that could have caused potential bias in treatment choices, and finally, the unequal number of visits between the comparison arms. 27 Still, STRONG‐HF is an important first step to address the implementation and up‐titration of GDMT in HF care.
In summary, HF caregivers are confronted with more choices on order and speed of initiation and up‐titration of the recommended drugs. Further guidance of implementation is essential, and large‐scale real‐world registries focusing precisely on this matter can be informative. These registries can also be very insightful on how the implementation of the new ESC HF 2021 guideline is proceeding over time and which choices are made in order and speed of titration and achieving GDMT. Although comparing different protocol‐directed sequencing strategies between subgroups could provide valuable information and certainly have a place for future research, this project is directed to record the chosen implementation strategy (order and speed of GDMT) and dose achieved (intolerance) in daily practice. Capturing the reasons for (non‐)initiation and (non‐)titration of the four drug classes of HF therapy allows for the comparison of different sequencing strategies in relation to outcome, including relevant subgroups.
Moreover, the barriers for reaching the target dose and the reasons for discontinuation need to be prospectively identified in detail to understand which patients do not achieve target dose and why. Most studies on medication use in HF report that there is much room for improvement of guideline implementation but do not report in detail why this is the case. Finally, we can prospectively study in TITRATE‐HF whether maximum tolerated dose of GDMT affects prognosis as compared with those patients at target dose and look in detail to relevant subgroups such as elderly, females, and patients with comorbidities.
It is also relevant to note that TITRATE‐HF includes a separate entity in HF, namely, worsening HF, which has deterioration of chronic HF with the necessity for hospitalization or an urgent visit for intravenous diuretics as globally accepted definition. TITRATE‐HF will study the changes in medication made during and after the worsening HF event and whether this leads to changes in GDMT besides diuretic alterations. HF hospitalizations are recommended to be used as critical opportunity to augment quadruple therapy. 28 , 29 While multiple studies have shown that GDMT optimization occurs only sparsely in worsening HF hospitalizations, 30 , 31 the degree to which medication initiations and dose escalations occur during the hospitalization and early post‐discharge in clinical practice is not well characterized. We will also study the titration steps and prognosis of de novo HF patients in detail, as it is presently unknown what GDMT implementation looks like for patients with a ‘clean slate’. Finally, TITRATE‐HF also includes a recently introduced HFimpEF category, 8 to study the course of this disease entity separately in detail.
Strengths
This study has several strengths to consider. TITRATE‐HF is the first prospective study to assess titration patterns of the four drug classes and to combine this with relevant clinical endpoints for HF. Different titration strategies in terms of the order and speed of titration steps in clinical practice will be prospectively registered, and barriers for not reaching target dose and reasons for discontinuation, such as side effects and intolerance, will be identified. GDMT implementation during HF‐related hospitalizations of worsening HF patients will also be studied, as well as the overall uptake of the latest guideline‐recommended drugs, specifically ARNI and SGLT2i. With a total of 4000 HF patients across different types of HF, TITRATE‐HF will obtain enough relevant prospective data to adequately study different titration strategies and the degree of guideline adherence in relation to HF‐related clinical endpoints and quality of life. The inclusion of patients in this real‐world registry is on an all‐comers, consecutive basis, which is an important strength to avoid or minimize selection bias in HF registries. Finally, in line with the previous CHECK‐HF registry, TITRATE‐HF will explore specific subgroups of HF patients, considering factors such as gender, age, comorbidities, or clinical parameters. 22 , 32 , 33 , 34 , 35 , 36 , 37 This can potentially result in different recommendations among important subgroups or profiles of HF patients and is very relevant in light of earlier mentioned initiatives for tailored medicine, 16 especially as women and elderly are generally underrepresented in RCTs. 17
Limitations
This study also has limitations worth mentioning. First, the structure of HF care in the Netherlands can limit external generalizability of our findings; however, barriers encountered at this level of HF care are most likely exchangeable with other countries. Additionally, clinical endpoints will be investigator reported and not adjudicated due to the nature of this study. However, we use clear definitions of each clinical endpoint of interest to avoid misclassifications. Finally, despite the KCCQ being more HF specific, the TITRATE‐HF registry will use the EQ‐5D‐5L as the faster completion time and lower effort needed from the patient reduce the chance of missing data.
Conclusions
The TITRATE‐HF registry will study the titration process in patients with HF, including the order and speed of drug titration steps, the achieved dose, and the side effects and intolerances that will be analysed in relation to long‐term prospective outcome data. TITRATE‐HF will collect essential information on the ESC HF 2021 guideline implementation, uptake of GDMT and new drugs, and quality of care for HF patients in the Netherlands.
Conflict of interest
P.R.D.C., J.M., and J.S. declare no conflict of interest. S.K. has had speaker engagements with Novartis, Boehringer Ingelheim, and AstraZeneca. M.E. has received speaker fees from AstraZeneca and consulting fees from Vifor. G.C.M.L., G.A.B., and L.H. declare no conflict of interest. C.J.W.B. has had speaker engagements with Novartis, Boehringer Ingelheim, and AstraZeneca. O.C.M. has received advisory fees from Abbott, AstraZeneca, Boehringer Ingelheim, and Novartis. V.E. declares no conflict of interest. S.W. has received grants from Pfizer, Boehringer Ingelheim, and AstraZeneca (HF research, not for this project) and consultancy/speaker fees from Boehringer Ingelheim, AstraZeneca, Novartis, and Roche. M.H. and C.F. declare no conflict of interest. K.D. has received speaker fees from Abbott, Boehringer Ingelheim, and AstraZeneca and has served on advisory board for FIRE1 study. J.R. has received consulting fees from Bayer. R.K. has received consulting fees from Novartis and Bayer and has had speaker engagements with Novartis and Bayer. A.R.T.V., R.A.T., D.V., and F.W.A. declare no conflict of interest. R.A.B. has received research grants and/or fees from AstraZeneca, Abbott, Boehringer Ingelheim, Cardior Pharmaceuticals GmbH, Ionis Pharmaceuticals, Inc., Novo Nordisk, and Roche and has had speaker engagements with Abbott, AstraZeneca, Bayer, Bristol Myers Squibb, Novartis, and Roche. P.M. has received grant support and/or consultancy fees from Novartis, Pharma Nord, Pfizer, Ionis, AstraZeneca, Vifor Pharma, Pharmacosmos, BridgeBio, and Novo Nordisk. S.J.G. has received research support from the Duke University Department of Medicine Chair's Research Award, American Heart Association, National Heart Lung and Blood Institute, Amgen, AstraZeneca, Bristol Myers Squibb, Cytokinetics, Merck & Co., Inc., Novartis, Pfizer, and Sanofi; has served on advisory boards for Amgen, AstraZeneca, Boehringer Ingelheim and Eli Lilly, Bristol Myers Squibb, Cytokinetics, Roche Diagnostics, scPharmaceuticals, and Sanofi; serves as a consultant for Amgen, Bayer, Boehringer Ingelheim and Eli Lilly, Bristol Myers Squibb, Corteria Pharmaceuticals, CSL Vifor, Merck & Co., Inc., PharmaIN, Roche Diagnostics, Sanofi, Tricog Health, and Urovant Pharmaceuticals; and has received speaker fees from Boehringer Ingelheim and Cytokinetics. H.P.B.R. has received unrestricted research grants from Vifor, Novartis, and Roche Diagnostics; declares participation in advisory boards supported by Novartis, Roche Diagnostics, Boehringer Ingelheim, AstraZeneca, Medtronic, and Vifor; and serves as an Exploris Switzerland stock holder. J.J.B. has received unrestricted research grants from Abbott and/or speaker fees from Novartis, Bayer, Vifor, AstraZeneca, Boehringer Ingelheim, and Abbott.
Funding
TITRATE‐HF is made possible in part by funding to ICIN Netherlands Heart Institute (NLHI) as CRO by an independent research grant from Novartis, Boehringer Ingelheim, AstraZeneca, Bayer, Abbott, and Vifor Pharma. This study was initiated by the authors and is designed, conducted, interpreted, and reported independently of the sponsors. The board and steering committee received no funding for this project. All authors have approved the manuscript before submission.
Organization
Project leader: J. J. Brugts. Project management: R. van der Kamp and E. Soeteman (financial management NLHI). Executive board: J. J. Brugts, H. P. Brunner‐La Rocca, and P. van der Meer. Steering committee: R. A. de Boer, F. W. Asselbergs, D. van Veghel, O. Manintveld, J. Schaap, S. Koudstaal, G. C. M. Linssen, M. Emans, A. Mosterd, L. van Heerebeek, C. J. W. Borleffs, V. van Empel, S. van Wijk, M. van den Heuvel, C. da Fonseca, J. van Ramshorst, R. van Kimmenade, R. van der Ven, and R. A. Tio. External/international expert: S. J. Greene.
Supporting information
Table S1. Registration options for the four drug class medication log.
Table S2. Clinical endpoints and definitions.
Acknowledgements
This study is supported by the Working group of Cardiology centres Netherlands (WCN), the Netherlands Heart Institute (NLHI), the Dutch Cardiac Society (NVVC), and the National Heart Failure Working Group as partners of Dutch Cardiovascular Alliance (DCVA). This study collaborates with the Netherlands Heart Registration and the Heart4Data Consortium.
Clephas, P. R. D. , Malgie, J. , Schaap, J. , Koudstaal, S. , Emans, M. , Linssen, G. C. M. , de Boer, G. A. , van Heerebeek, L. , Borleffs, C. J. W. , Manintveld, O. C. , van Empel, V. , van Wijk, S. , van den Heuvel, M. , da Fonseca, C. , Damman, K. , van Ramshorst, J. , van Kimmenade, R. , van de Ven, A. R. T. , Tio, R. A. , van Veghel, D. , Asselbergs, F. W. , de Boer, R. A. , van der Meer, P. , Greene, S. J. , Brunner‐La Rocca, H.‐P. , and Brugts, J. J. (2024) Guideline implementation, drug sequencing, and quality of care in heart failure: design and rationale of TITRATE‐HF. ESC Heart Failure, 11: 550–559. 10.1002/ehf2.14604.
References
- 1. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Bohm M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021;42:3599‐3726. doi: 10.1093/eurheartj/ehab368 [DOI] [PubMed] [Google Scholar]
- 2. Tromp J, Ouwerkerk W, van Veldhuisen DJ, Hillege HL, Richards AM, van der Meer P, et al. A systematic review and network meta‐analysis of pharmacological treatment of heart failure with reduced ejection fraction. JACC Heart Fail 2022;10:73‐84. doi: 10.1016/j.jchf.2021.09.004 [DOI] [PubMed] [Google Scholar]
- 3. Brunner‐La Rocca HP, Linssen GC, Smeele FJ, van Drimmelen AA, Schaafsma HJ, Westendorp PH, et al. Contemporary drug treatment of chronic heart failure with reduced ejection fraction: The CHECK‐HF registry. JACC: Heart Failure 2019;7:13‐21. doi: 10.1016/j.jchf.2018.10.010 [DOI] [PubMed] [Google Scholar]
- 4. Greene SJ, Butler J, Albert NM, DeVore AD, Sharma PP, Duffy CI, et al. Medical therapy for heart failure with reduced ejection fraction: The CHAMP‐HF registry. J Am Coll Cardiol 2018;72:351‐366. doi: 10.1016/j.jacc.2018.04.070 [DOI] [PubMed] [Google Scholar]
- 5. Thorvaldsen T, Benson L, Dahlstrom U, Edner M, Lund LH. Use of evidence‐based therapy and survival in heart failure in Sweden 2003–2012. Eur J Heart Fail 2016;18:503‐511. doi: 10.1002/ejhf.496 [DOI] [PubMed] [Google Scholar]
- 6. Savarese G, Kishi T, Vardeny O, Adamsson Eryd S, Bodegård J, Lund Lars H, et al. Heart failure drug treatment—Inertia, titration and discontinuation: A multinational observational study (EVOLUTION HF). JACC: Heart Failure 2022;11:1‐14. doi: 10.1016/j.jchf.2022.08.009 [DOI] [PubMed] [Google Scholar]
- 7. Odegaard KM, Lirhus SS, Melberg HO, Hallen J, Halvorsen S. Adherence and persistence to pharmacotherapy in patients with heart failure: A nationwide cohort study, 2014–2020. ESC Heart Fail 2022;10:405‐415. doi: 10.1002/ehf2.14206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: A report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol 2022;79:e263‐e421. doi: 10.1161/CIR.0000000000001063 [DOI] [PubMed] [Google Scholar]
- 9. D'Amario D, Rodolico D, Rosano GMC, Dahlstrom U, Crea F, Lund LH, et al. Association between dosing and combination use of medications and outcomes in heart failure with reduced ejection fraction: Data from the Swedish Heart Failure Registry. Eur J Heart Fail 2022;24:871‐884. doi: 10.1002/ejhf.2477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Savarese G, Bodegard J, Norhammar A, Sartipy P, Thuresson M, Cowie MR, et al. Heart failure drug titration, discontinuation, mortality and heart failure hospitalization risk: A multinational observational study (US, UK and Sweden). Eur J Heart Fail 2021;23:1499‐1511. doi: 10.1002/ejhf.2271 [DOI] [PubMed] [Google Scholar]
- 11. Shen L, Jhund PS, Docherty KF, Vaduganathan M, Petrie MC, Desai AS, et al. Accelerated and personalized therapy for heart failure with reduced ejection fraction. Eur Heart J 2022;43:2573‐2587. doi: 10.1093/eurheartj/ehac210 [DOI] [PubMed] [Google Scholar]
- 12. Mebazaa A, Davison B, Chioncel O, Cohen‐Solal A, Diaz R, Filippatos G, et al. Safety, tolerability and efficacy of up‐titration of guideline‐directed medical therapies for acute heart failure (STRONG‐HF): A multinational, open‐label, randomised, trial. Lancet 2022;400:1938‐1952. doi: 10.1016/S0140-6736(22)02076-1 [DOI] [PubMed] [Google Scholar]
- 13. Beldhuis IE, Voors AA, Tromp J. Rapid uptitration: What's the evidence? Eur J Heart Fail 2022;25:223‐225. doi: 10.1002/ejhf.2759 [DOI] [PubMed] [Google Scholar]
- 14. Packer M, McMurray JJV. Rapid evidence‐based sequencing of foundational drugs for heart failure and a reduced ejection fraction. Eur J Heart Fail 2021;23:882‐894. doi: 10.1002/ejhf.2149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Greene SJ, Butler J, Fonarow GC. Simultaneous or rapid sequence initiation of quadruple medical therapy for heart failure‐optimizing therapy with the need for speed. JAMA Cardiol 2021;6:743‐744. doi: 10.1001/jamacardio.2021.0496 [DOI] [PubMed] [Google Scholar]
- 16. Rosano GMC, Moura B, Metra M, Bohm M, Bauersachs J, Ben Gal T, et al. Patient profiling in heart failure for tailoring medical therapy. A consensus document of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2021;23:872‐881. doi: 10.1002/ejhf.2206 [DOI] [PubMed] [Google Scholar]
- 17. Lim YMF, Molnar M, Vaartjes I, Savarese G, Eijkemans MJC, Uijl A, et al. Generalisability of randomised controlled trials in heart failure with reduced ejection fraction. Eur Heart J Qual Care Clin Outcomes 2021;8:761‐769. doi: 10.1093/ehjqcco/qcab070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Malgie J, Clephas PRD, Brunner‐La Rocca HP, de Boer RA, Brugts JJ. Guideline‐directed medical therapy for HFrEF: Sequencing strategies and barriers for life‐saving drug therapy. Heart Fail Rev 2023;28:1221‐1234. doi: 10.1007/s10741-023-10325-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five‐level version of EQ‐5D (EQ‐5D‐5L). Qual Life Res 2011;20:1727‐1736. doi: 10.1007/s11136-011-9903-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jonsson A, Orwelius L, Dahlstrom U, Kristenson M. Evaluation of the usefulness of EQ‐5D as a patient‐reported outcome measure using the Paretian classification of health change among patients with chronic heart failure. J Patient Rep Outcomes 2020;4:50. doi: 10.1186/s41687-020-00216-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Spertus JA, Jones PG, Sandhu AT, Arnold SV. Interpreting the Kansas City Cardiomyopathy Questionnaire in clinical trials and clinical care: JACC state‐of‐the‐art review. J Am Coll Cardiol 2020;76:2379‐2390. doi: 10.1016/j.jacc.2020.09.542 [DOI] [PubMed] [Google Scholar]
- 22. Clephas PRD, Radhoe SP, Linssen GCM, Langerveld J, Plomp J, Smits JPP, et al. Serum potassium level and mineralocorticoid receptor antagonist dose in a large cohort of chronic heart failure patients. ESC Heart Fail 2023;10:1481‐1487. doi: 10.1002/ehf2.14285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Amorim LD, Cai J. Modelling recurrent events: A tutorial for analysis in epidemiology. Int J Epidemiol 2015;44:324‐333. doi: 10.1093/ije/dyu222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McMurray JJV, Packer M. How should we sequence the treatments for heart failure and a reduced ejection fraction?: A redefinition of evidence‐based medicine. Circulation 2021;143:875‐877. doi: 10.1161/CIRCULATIONAHA.120.052926 [DOI] [PubMed] [Google Scholar]
- 25. Tromp J, Voors AA. Heart failure medication: Moving from evidence generation to implementation. Eur Heart J 2022;43:2588‐2590. doi: 10.1093/eurheartj/ehac272 [DOI] [PubMed] [Google Scholar]
- 26. Radhoe SP, Clephas PRD, Linssen GCM, Oortman RM, Smeele FJ, Van Drimmelen AA, et al. Phenotypic patient profiling for improved implementation of guideline‐directed medical therapy: An exploratory analysis in a large real‐world chronic heart failure cohort. Front Pharmacol 2023;14:1081579. doi: 10.3389/fphar.2023.1081579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cox ZL, Lindenfeld J. STRONG start for implementation of guideline‐directed medical therapies. Lancet 2022;400:1901‐1903. doi: 10.1016/S0140-6736(22)02372-8 [DOI] [PubMed] [Google Scholar]
- 28. Greene SJ, Butler J, Fonarow GC. In‐hospital initiation of quadruple medical therapy for heart failure: Making the post‐discharge vulnerable phase far less vulnerable. Eur J Heart Fail 2022;24:227‐229. doi: 10.1002/ejhf.2382 [DOI] [PubMed] [Google Scholar]
- 29. Bhagat AA, Greene SJ, Vaduganathan M, Fonarow GC, Butler J. Initiation, continuation, switching, and withdrawal of heart failure medical therapies during hospitalization. JACC Heart Fail 2019;7:1‐12. doi: 10.1016/j.jchf.2018.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Greene SJ, Ezekowitz JA, Anstrom KJ, Demyanenko V, Givertz MM, Pina IL, et al. Medical therapy during hospitalization for heart failure with reduced ejection fraction: The VICTORIA registry. J Card Fail 2022;28:1063‐1077. doi: 10.1016/j.cardfail.2022.02.011 [DOI] [PubMed] [Google Scholar]
- 31. Carnicelli AP, Lippmann SJ, Greene SJ, Mentz RJ, Greiner MA, Hardy NC, et al. Sacubitril/valsartan initiation and postdischarge adherence among patients hospitalized for heart failure. J Card Fail 2021;27:826‐836. doi: 10.1016/j.cardfail.2021.03.012 [DOI] [PubMed] [Google Scholar]
- 32. Veenis JF, Brunner‐la Rocca HP, Linssen GC, Geerlings PR, van Gent MWF, Aksoy I, et al. Age differences in contemporary treatment of patients with chronic heart failure and reduced ejection fraction. Eur J Prev Cardiol 2019;26:1399‐1407. doi: 10.1177/2047487319835042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Veenis JF, Brunner‐La Rocca HP, Linssen GCM, Van Gent MWF, Hoes AW, Brugts JJ, et al. Treatment differences in chronic heart failure patients with reduced ejection fraction according to blood pressure. Circ Heart Fail 2020;13:e006667. doi: 10.1161/CIRCHEARTFAILURE.119.006667 [DOI] [PubMed] [Google Scholar]
- 34. Veenis JF, Rocca HB, Linssen GCM, Erol‐Yilmaz A, Pronk ACB, Engelen DJM, et al. Impact of sex‐specific target dose in chronic heart failure patients with reduced ejection fraction. Eur J Prev Cardiol 2021;28:957‐965. doi: 10.1177/2047487320923185 [DOI] [PubMed] [Google Scholar]
- 35. Linssen GCM, Veenis JF, Kleberger A, Grosfeld MJW, Viergever EP, van Dalen BM, et al. Medical treatment of octogenarians with chronic heart failure: Data from CHECK‐HF. Clin Res Cardiol 2020;109:1155‐1164. doi: 10.1007/s00392-020-01607-y [DOI] [PubMed] [Google Scholar]
- 36. Veenis JF, Brunner‐la Rocca HP, Linssen GCM, Smeele FJJ, Wouters N, Westendorp PHM, et al. Atrial fibrillation in chronic heart failure patients with reduced ejection fraction: The CHECK‐HF registry. Int J Cardiol 2020;308:60‐66. doi: 10.1016/j.ijcard.2020.03.001 [DOI] [PubMed] [Google Scholar]
- 37. Radhoe SP, Veenis JF, Linssen GCM, van der Lee C, Eurlings LWM, Kragten H, et al. Diabetes and treatment of chronic heart failure in a large real‐world heart failure population. ESC Heart Fail 2022;9:353‐362. doi: 10.1002/ehf2.13743 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Registration options for the four drug class medication log.
Table S2. Clinical endpoints and definitions.


