Abstract
Aims
We aim to investigate the association between kidney dysfunction and left ventricular diastolic dysfunction parameters and heart failure with preserved ejection fraction (HFpEF) and whether this is sex‐specific.
Methods and results
We included participants from the HELPFul observational study. Outpatient clinical care data, including echocardiography, and an expert panel judgement on HFpEF was collected. Estimated glomerular filtration rate (eGFR) was calculated by creatinine and cystatin C without race. The association between eGFR with E/e′, left ventricular mass index, relative wall thickness, and stage C/D heart failure was tested by multivariable adjusted regression models, stratified by sex, reporting odds ratios and 95% confidence intervals (95% confidence interval). We analysed 880 participants, mean age 62.9 (standard deviation: 9.3) years, 69% female. Four hundred six participants had mild (37.6%) kidney dysfunction (eGFR: 60–89 mL/min/1.73 m2) or moderate (8.5%) kidney dysfunction (eGFR: 30–59 mL/min/1.73 m2). HFpEF was significantly more prevalent in participants with mild and moderate kidney dysfunction (10.3% and 16.0%, respectively) than participants with normal kidney function (3.4%). A lower kidney function was associated with higher E/e′ and higher relative wall thickness values. Participants with moderate kidney dysfunction had a higher likelihood of American College of Cardiology/American Heart Association stage C/D HF (odds ratio: 2.07, 95% confidence interval: 1.23, 3.49) than participants with normal kidney functions.
Conclusions
Both mild and moderate kidney dysfunction are independently associated with left ventricular diastolic dysfunction parameters and HFpEF. This association is independent of sex and strongest for moderate kidney dysfunction. Considering mild‐to‐moderate kidney dysfunction as risk factor for HFpEF may help identify high‐risk groups benefiting most from early intervention.
Keywords: Heart failure with preserved ejection fraction, Kidney dysfunction, Left ventricular diastolic dysfunction, Risk factors
Introduction
Around 50% of the patients with heart failure (HF) have HF with preserved ejection fraction (HFpEF), 1 , 2 and all types of HF, including HFpEF, are associated with an increased mortality risk. 3 , 4 , 5 Kidney dysfunction is seen in 30–60% of the patients with all‐type HF, 6 , 7 whereas, vice versa, in patients with chronic kidney disease (CKD) the prevalence of newly detected HF is estimated to be between 17% and 44%. 8 , 9 Both CKD and HFpEF are more prevalent in females compared with males, 10 , 11 while, on the other hand, more males have heart failure with reduced ejection fraction (HFrEF). The relation between CKD and HFrEF is well established, and the main direction seems to be that HFrEF causes CKD, while in HFpEF, it could well be the other way around; kidney dysfunction increases the risk of HFpEF. 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 Additionally, concurrent CKD is a strong risk factor for increased mortality in established HFpEF. 21 Finally, HFpEF and CKD co‐exist due to common underlying co‐morbidities related to systemic low‐grade inflammation, systemic microvascular dysfunction, neurohormonal activation, oxidative stress, and chronic left ventricular pressure overload. 22 , 23
HFpEF concerns symptoms suggestive of HF plus (echocardiographic) evidence of left ventricular diastolic dysfunction (LVDD). Thus, LVDD may be considered as the underlying pathophysiological process of HFpEF; however, not everybody with LVDD develops HFpEF because LVDD without symptoms of HF may be reversible or develop into LV systolic dysfunction and thus finally HFrEF. 24 , 25 Interestingly, LVDD is equally prevalent in both sexes, while HFpEF is more prevalent in females, and HFrEF is more prevalent in males. 26 , 27 This suggests that there are different ‘preferred pathways’ among sexes from LVDD to heart failure.
Importantly, approximately one third of all patients with CKD also have LVDD. 28 , 29 , 30 Few longitudinal studies found that kidney dysfunction is associated with the progression of asymptomatic LVDD to all‐type HF, also independent of other cardiovascular risk factors. 31 A previous screening study showed that natriuretic peptide‐based screening of high‐risk patients, for example, hypertension, type 2 diabetes mellitus, prior myocardial infarction, in combination with intensified collaborative care in those with marginally increased B‐type natriuretic peptide (BNP) levels (>50 pg/mL), resulted in reduced HF incidence and reduced major adverse cardiac and cerebrovascular events. 32 Importantly, this effect was mainly driven by intensified renin‐angiotensin system (RAS) inhibition treatment. From more recent studies, we know that also sodium‐glucose cotransporter 2 (SGLT2) inhibitors and mineral corticosteroid antagonists may have beneficial potential in patients with HFpEF, with SGLT2 inhibitors also a beneficial effect on kidney function. 33 , 34 , 35
Even though an association between kidney and cardiac dysfunction is apparent, there is a gap in knowledge on whether already mild kidney dysfunction relates with a higher prevalence of LVDD and HFpEF and whether this is sex‐specific. Therefore, we investigated the association of kidney dysfunction with (i) echocardiographic diastolic dysfunction parameters of LVDD and (ii) a panel diagnosis of HFpEF in out‐patient males and females referred for cardiovascular evaluation, with no prior cardiac interventions or congenital heart disease.
Methods
Study participants
For this cross‐sectional study, we included consecutive participants from the HEart failure with Preserved ejection Fraction in patients at risk for cardiovascular disease (HELPFul) study, for which the design has been described in detail elsewhere. 36 A random sample of patients, enriched with participants with an early filling (E) to early diastolic mitral annular velocity (e′) ratio (E/e′ ratio) ≥ 8, measured with echocardiography, were included. All were referred by their general practitioner to an outpatient cardiology clinic (Cardiology Centres of the Netherlands, location Galgenwaard, Utrecht), because of cardiovascular disease suspicion. Participants had to be aged 45 years or older, and without prior cardiac intervention (e.g. percutaneous coronary intervention or coronary artery bypass graft) or congenital heart disease.
Written informed consent was obtained from all participants and this study was approved by the Medical Ethics committee of the UMC Utrecht (number 16‐290/M) and was conducted according to the principles of the Declaration of Helsinki (version 2013) and the Medical Research Involving Human Subjects Act (WMO).
Data collection
Standard care measurements, including blood pressure measurement in sitting position, physical examination, electrocardiography, bicycle exercise testing, echocardiography, and basic laboratory testing (haemoglobin, haematocrit, random glucose, potassium, lipid spectrum, and creatinine levels), were collected from all participants. Additionally, venous blood was collected for storage at the UMC Utrecht biobank. In every participant, BNP and high sensitivity troponin were measured. Creatinine, cystatin C, 25‐hyrdoxy vitamin D, aspartate transaminase, and C‐reactive protein were measured in the first 72% of participants, with the appropriate assay (ARCHITECT i2000 analyser, Abbott Park, Chicago, Illinois, USA). We calculated the estimated glomerular filtration rate (eGFR) with the new CKD‐EPI 2021 equation for creatinine and cystatin C in combination without race. 37
Expert panel diagnosis
An expert panel, consisting of three qualified cardiologists and one general practitioner specialized in heart failure care (R. M., M. C., A. T., and F. R.), was responsible for diagnosing HF and LVDD based on all available diagnostic information, including BNP levels and echocardiography. Classification of the participants was undertaken by the panel that was not aware of the kidney function at the moment of assessment, with a majority of votes or at least after discussion by two panel members. In 10%, diagnoses were re‐evaluated in a blinded fashion. The echocardiographic measurements that were used consisted of left atrial diameter, left atrial volume index (LAVI), interventricular diameter at end‐diastole, left ventricle (LV) dimension at end‐diastole, thickness of the LV posterior wall at end‐diastole, LV dimension at end‐systole, LV ejection fraction, early (E) and late filling (A), blood flow ratio (E/A ratio), E wave deceleration time, peak mitral annual velocity e′, E/e′, LV mass index (LVMI), and relative wall thickness (RWT). 38 The panel diagnosis of LVDD was based on echocardiography parameters, 39 and for the diagnosis of HF, symptoms suggestive of heart failure had to be present. The panel used both the HFA‐PEFF and HF2‐PEF scores 40 , 41 to determine HFpEF diagnosis. Then, all participants were further categorized according to the American College of Cardiology (ACC)/American Heart Association (AHA) staging system in Stage A (no structural cardiac abnormalities), Stage B [structural abnormalities (LVDD), without signs or symptoms of HF], and Stage C/D [signs and symptoms of HF accompanied with structural echocardiographic abnormalities (e.g. HFpEF, HFmrEF, or HFrEF)]. 40 Because we wanted to study the association of kidney function with early HFpEF, participants with possible or probable symptoms of heart failure were classified as ACC/AHA stage C/D.
Data analysis
Normally distributed variables are reported as mean ± standard deviation, non‐normally distributed variables as median and interquartile range, and categorical data as count and percentages. Analyses regarding kidney function were stratified by normal kidney function (GFR ≥ 90 mL/min/1.73 m2), mild kidney dysfunction (eGFR 60–89 mL/min/1.73 m2), and moderate to severe kidney dysfunction (eGFR <60 mL/min/1.73 m2). The association between kidney function and echocardiography results and the diagnosis of ACC/AHA stage C/D was assessed with linear regression models reporting the betas, and logistic regression models reporting the odds ratio [OR, both with the respective 95% confidence interval (95% CI)], respectively. The relationship between continuous variables and outcomes was explored by restricted cubic splines. For the non‐linear variables, log‐transformations were applied. The thresholds for the logistic regression models were based on the third quartile of the distribution, that is, E/e′ > 10, LAVI >30 mL/m2, LVMI for males >90 g/m2 and females >80 g/m2, and RWT > 0.48. The results of multivariable regression analyses were adjusted for cardiovascular risk factors and lifestyle factors based on the literature and previous studies on LV dysfunction, 42 , 43 , 44 , 45 including body mass index, diabetes mellitus, hypertension, hypercholesterolaemia, cardiovascular history, alcohol consumption, smoking status, and education level. Age and sex were not included as confounder given that they are part of the dependent variable (i.e. eGFR). We also reported descriptive statistics and regression analyses sex‐stratified. Missing data were imputed by multiple imputation (iteration = 10) using the ‘mice’ R statistical package and the missing values for all variables are reported in Table S1 . Two‐tailed tests were applied and a P value of <0.05 was considered statistically significant. All statistical analyses were performed using the R v. 3.5.1. software.
Sensitivity analyses
We performed sensitivity analyses excluding all participants with HF (defined as ACC/AHA stage C/D) to see whether the association between kidney dysfunction and echocardiographic parameters was not driven by individuals already affected with HF. Finally, sensitivity analyses have been performed to rule out that our results would depend on cystatin C levels. Therefore, we used creatinine dependent eGFR calculations, that is, the Cockcroft–Gault and the CKD‐EPI equations. 46 , 47 Given that the large majority (>90%) of the included participants was Caucasian, although this was not collected in a standardized manner, for the CKD‐EPI equation, the ethnicity of our sample was assumed to be Caucasian.
Results
Baseline characteristics
In total, 880 participants were included in the main analyses, with a mean age 62.9 [standard deviation (SD): 9.3] years, of whom 69% were female, and the mean body mass index was 27.2 (SD: 4.5) kg/m2 (Table 1 ). The mean eGFR of all included participants was 96.9 (SD: 31.7) mL/min/1.73 m2. Approximately half of the participants (n = 474) had normal kidney function (eGFR of ≥90 mL/min/1.73 m2). In total, 331 (37.6%) had mild kidney dysfunction (eGFR of 60–89 mL/min/1.73 m2), and 75 (8.5%) moderate kidney dysfunction (eGFR <59 mL/min/1.73 m2). Females had a similar eGFR compared with males. Participants with mild kidney dysfunction and moderate kidney dysfunction were on average older, had higher systolic blood pressure, and higher BNP levels compared with participants with normal kidney function.
Table 1.
Baseline characteristics of the included patients stratified by kidney function
| Mean variable (SD) | eGFR (≤59 mL/min/1.73 m2; n = 75) | eGFR (60–89 mL/min/1.73 m2; n = 331) | GFR (≥90 mL/min/1.73 m2; n = 474) | P‐value |
|---|---|---|---|---|
| Age in years | 71.2 (8.8) | 65.2 (8.8) | 59.9 (8.4) | <0.001 |
| Women (%) | 52 (69.3) | 222 (67.1) | 330 (69.6) | 0.738 |
| BMI in kg/m2 | 28.2 (5.4) | 27.5 (4.3) | 26.7 (4.4) | 0.006 |
| Education level: ≥ first year of university | 20 (26.7) | 152 (45.9) | 195 (41.1) | 0.009 |
| Smokers (%) | 0.771 | |||
| Never | 33 (44.0) | 125 (37.8) | 187 (39.5) | |
| Current | 5 (6.7) | 34 (10.3) | 41 (8.6) | |
| Former | 37 (49.3) | 172 (52.0) | 246 (51.9) | |
| Alcohol consumption (%) | 0.898 | |||
| Never | 8 (10.7) | 48 (14.5) | 61 (12.9) | |
| Current | 63 (84.0) | 263 (79.5) | 386 (81.4) | |
| Former | 4 (5.3) | 20 (6.0) | 27 (5.7) | |
| Systolic blood pressure (mmHg) | 151.2 (19.3) | 149.0 (19.7) | 144.0 (19.3) | <0.001 |
| Diastolic blood pressure (mmHg) | 86.4 (11.3) | 87.0 (10.6) | 86.8 (10.7) | 0.84 |
| Hypertension (%) | 55 (77.3) | 200 (60.4) | 249 (52.5) | 0.001 |
| Diabetes (%) | 10 (13.3) | 31 (9.4) | 28 (5.9) | 0.036 |
| Cardiovascular history (%) | 46 (61.3) | 221 (66.8) | 260 (54.9) | 0.003 |
| Haemoglobin (mmol/L) | 9.6 (0.9) | 9.5 (1.1) | 9.4 (1.0) | 0.13 |
| Potassium (mmol/L) | 4.2 (0.4) | 4.1 (0.4) | 4.2 (0.4) | 0.44 |
| Total cholesterol (mmol/L) | 5.3 (1.3) | 5.5 (1.2) | 5.2 (1.1) | <0.001 |
| Dyslipidaemia (%) | 31 (41.3) | 147 (44.4) | 184 (38.8) | 0.284 |
| B‐type natriuretic peptide (pg/mL)* | 45.0 (21.4–80.3) | 29.1 (17.3–49.3) | 21.9 (12.9–42.2) | <0.001 |
| High‐sensitivity Troponin I (pg/mL)* | 3.9 (2.7–6.9) | 2.9 (2.1–4.8) | 2.3 (1.6–3.7) | <0.001 |
| 25‐hydroxy vitamin D (ng/mL) | 28.55 (9.1) | 24.7 (9.8) | 24.7 (10.3) | 0.007 |
| Aspartate transaminase (IU/L)* | 26.0 (20.5–33.7) | 25.0 (21.0–30.0) | 22.0 (18.3–26.0) | <0.001 |
| C‐reactive protein (mg/L)* | 3.5 (1.3–6.6) | 1.7 (0.7–6.2) | 1.2 (0.5–3.3) | <0.001 |
| Cystatin C (mg/L) | 1.4 (0.2) | 1.1 (0.1) | 0.8 (0.1) | <0.001 |
| Albumin (g/L) | 43.1 (3.7) | 43.7 (3.6) | 41.0 (3.9) | <0.001 |
| Creatinine (μmol/L) | 93.7 (16.3) | 73.9 (10.7) | 63.1 (9.3) | <0.001 |
Renal function was estimated using the CKD‐EPI 2021 equation for creatinine and cystatin C in combination without race.
BMI, body mass index; eGFR, estimated glomerular filtration rate; SD, standard deviation.
Median and interquartile range.
Heart failure
HFpEF was more often diagnosed in participants with moderate kidney dysfunction (n = 12, 16%) and mild kidney dysfunction (n = 34, 10%) compared with participants with normal kidney function (n = 16, 3%) (Table 2 ). ACC/AHA stage C/D HF was diagnosed in 41 (55%), 128 (38%), and 153 (32%) participants with moderate kidney dysfunction, mild kidney dysfunction, and normal kidney function, respectively. More females (n = 47, 8% of females) than males (n = 15, 5% of males) were diagnosed with HFpEF, and also more females (n = 252, 42% of females) than males (n = 70, 25% of males) were diagnosed with ACC/AHA stage C/D HF (Table S2A and Table S2B ). Participants with moderate kidney dysfunction were at increased risk, after adjustment for other cardiovascular risk factors, of being diagnosed with ACC/AHA stage C/D HF compared with participants with a normal kidney function (adjusted OR: 2.07, 95% CI: 1.23, 3.49) (Table 3 ). This was not statistically significant for patients with mild kidney dysfunction (adjusted OR: 1.16, 95% CI: 0.85, 1.58). Additionally, only in males, moderate kidney dysfunction was associated with ACC/AHA stage C/D HF (adjusted OR: 3.52, 95% CI: 1.34, 9.26) (Table S3A and Table S3B ).
Table 2.
Echocardiography values and panel diagnoses regarding heart failure and left ventricular diastolic dysfunction stratified by stages of kidney dysfunction
| Mean variable (SD) | eGFR (≤59 mL/min/1.73 m2; n = 75) | eGFR (60–89 mL/min/1.73 m2; n = 331) | GFR (≥90 mL/min/1.73 m2; n = 474) | P‐value |
|---|---|---|---|---|
| Echocardiography values | ||||
| Interventricular diameter at end‐diastole (mm) | 10.2 (1.9) | 9.9 (1.9) | 9.4 (1.7) | <0.001 |
| Left ventricle dimension at end‐diastole (mm) | 43.8 (5.8) | 44.2 (5.4) | 44.9 (5.0) | 0.056 |
| Thickness of the left ventricular posterior wall at end‐diastole | 9.7 (1.5) | 9.7 (1.7) | 9.3 (1.5) | <0.001 |
| Left ventricle dimension at end‐systole (mm) | 28.4 (5.7) | 27.7 (4.4) | 27.9 (4.2) | 0.687 |
| Left ventricular ejection fraction (%) | 66.0 (9.2) | 67.2 (8.1) | 67.6 (7.8) | 0.273 |
| E/A ratio | 0.85 (0.30) | 0.92 (0.40) | 1.00 (0.30) | <0.001 |
| E wave deceleration time (ms) | 204.7 (55.6) | 204.0 (52.4) | 204.3 (49.9) | 0.994 |
| E velocity (cm/s) | 70.6 (19.8) | 68.5 (16.6) | 70.6 (16.2) | 0.222 |
| E/e′ ratio | 10.4 (3.2) | 9.5 (2.9) | 8.9 (2.2) | <0.001 |
| LVMI (g/m2) | ||||
| Men | 87.8 (29.4) | 82.1 (22.7) | 80.7 (20.7) | 0.559 |
| Women | 77.7 (22.0) | 72.7 (17.1) | 70.2 (14.9) | 0.023 |
| Relative wall thickness | 0.45 (0.09) | 0.44 (0.10) | 0.42 (0.08) | <0.001 |
| LA volume index (mL/m2) | 27.7 (17.7) | 24.9 (8.9) | 25.3 (8.6) | 0.071 |
| Panel diagnoses | ||||
| Heart failure | <0.001 | |||
| No HF | 34 (45.3) | 203 (61.3) | 321 (67.7) | |
| ‘Intermediate’ HFpEF | 26 (34.7) | 91 (27.5) | 135 (28.5) | |
| HFpEF | 12 (16.0) | 34 (10.3) | 16 (3.4) | |
| HFrEF/HFmrEF | 3 (4.0) | 3 (0.9) | 2 (0.4) | |
| ACC/AHA HF class | 0.002 | |||
| Stage A | 14 (18.7) | 104 (31.4) | 178 (37.6) | |
| Stage B | 20 (26.7) | 99 (29.9) | 143 (30.2) | |
| Stage C/D | 41 (54.7) | 128 (38.7) | 153 (32.3) | |
Renal function was estimated using the CKD‐EPI 2021 equation for creatinine and cystatin C in combination without race.
ACC/AHA, American College of Cardiology/American Heart Association; eGFR, estimated glomerular filtration rate; HF, heart failure; HFmrEF, heart failure mildly reduced ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; LVMI, left ventricular mass index; SD, standard deviation.
Table 3.
Univariable and multivariable logistic regression for the association between stages of renal dysfunction and echocardiographic measures
| E/e′ > 10 | LAVI > 30 mL/m2 | LVMI > 90 g/m2 (male)LVMI > 80 g/m2 (female) | RWT > 0.48 | ACC/AHA Stage C/D | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |
| Model 1: Crude | ||||||||||
| eGFR categories | ||||||||||
| eGFR ≥90 mL/min/1.73 m2 | Ref. | ‐ | Ref. | ‐ | Ref. | ‐ | Ref. | ‐ | ref. | ‐ |
| eGFR 60–89 mL/min/1.73 m2 | 1.62 (1.20, 2.18) | 0.002 | 0.97 (0.70, 1.33) | 0.84 | 1.19 (0.87, 1.62) | 0.28 | 1.85 (1.33, 2.57) | <0.001 | 1.32 (0.99, 1.77) | 0.06 |
| eGFR <60 mL/min/1.73 m2 | 2.01 (1.22, 3.31) | 0.006 | 1.11 (0.64, 1.88) | 0.70 | 2.10 (1.27, 3.46) | 0.004 | 2.43 (1.43, 4.09) | <0.001 | 2.53 (1.55, 4.16) | <0.001 |
| Model 2: Adjusted | ||||||||||
| eGFR categories | ||||||||||
| eGFR ≥90 mL/min/1.73 m2 | Ref. | ‐ | Ref. | ‐ | Ref. | ‐ | Ref. | ‐ | Ref. | ‐ |
| eGFR 60–89 mL/min/1.73 m2 | 1.55 (1.14, 2.11) | 0.005 | 0.92 (0.67, 1.27) | 0.61 | 1.07 (0.77, 1.58) | 0.69 | 1.75 (1.25, 2.45) | 0.001 | 1.16 (0.86, 1.57) | 0.35 |
| eGFR <60 mL/min/1.73 m2 | 1.80 (1.07, 2.98) | 0.02 | 1.06 (0.61, 1.81) | 0.83 | 1.72 (1.02, 2.88) | 0.04 | 2.09 (1.21, 3.55) | 0.007 | 2.09 (1.25, 3.51) | 0.005 |
| Model 3: Adjusted | ||||||||||
| eGFR categories | ||||||||||
| eGFR ≥90 mL/min/1.73 m2 | Ref. | ‐ | Ref. | ‐ | Ref. | ‐ | Ref. | ‐ | Ref. | ‐ |
| eGFR 60–89 mL/min/1.73 m2 | 1.55 (1.10, 2.08) | 0.005 | 0.92 (0.67, 1.27) | 0.62 | 1.07 (0.77, 1.47) | 0.69 | 1.75 (1.25, 2.45) | 0.001 | 1.16 (0.85, 1.58) | 0.35 |
| eGFR <60 mL/min/1.73 m2 | 1.80 (1.07, 3.02) | 0.03 | 1.03 (0.58, 1.76) | 0.93 | 1.70 (1.00, 2.86) | 0.047 | 2.15 (1.24, 3.68) | 0.005 | 2.07 (1.23, 3.49) | 0.006 |
Model 1: Crude. Model 2: Adjusted for BMI, diabetes mellitus, hypertension, hypercholesterolaemia, and history of cardiovascular disease. Model 3: Adjusted for model 2 + alcohol of >2 units/day, current smoking, and education level. Renal function was estimated using the CKD‐EPI 2021 equation for creatinine and cystatin C in combination without race.
ACC/AHA, American College of Cardiology/American Heart Association; BMI, body mass index; CI, confidence interval; eGFR, estimated glomerular filtration rate; LAVI, left atrial volume index; LVMI, left ventricular mass index; OR, odds ratio; RWT, relative wall thickness.
Echocardiography parameters
The echocardiography parameters stratified by categories of kidney dysfunction are presented in Table 2 . The mean E/e′ ratio and LVMI was higher, while the LV dimension at end‐diastole, LV dimension at end‐systole, and E/A ratio was lower for participants with kidney dysfunction compared with participants with normal kidney function (Table 2 ). There were no important differences in echocardiography findings when stratifying for sex (Table S1 A and Table S1 B), except for LVMI, that was expectedly higher in men. Both participants with mild as well as participants with moderate kidney dysfunction had more often an E/e′ > 10 (adjusted OR: 1.55 [95% CI: 1.10, 2.08] and adjusted OR: 1.80 [95% CI: 1.07, 3.02], respectively) compared with participants with normal kidney function (Table 3 ). Participants with moderate kidney dysfunction had a higher risk of increased LVMI (adjusted OR: 1.70 [95% CI: 1.00, 2.86]) compared with participants with normal kidney function. Also, a higher risk was found for both participants with mild and moderate kidney dysfunction for a RWT > 0.48 (respectively, adjusted OR 1.75 [95% CI: 1.25, 2.45] and adjusted OR 2.15 [1.24–3.68]) compared with participants with normal kidney function. There was no relevant association between kidney dysfunction and LAVI. Sex‐stratified analysis resulted in non‐significant findings for most associations. After excluding participants in HF class C/D, there was still a significant association of moderate and severe kidney function with E/e′ ratio; however, for LVMI and RWT, no significant association was observed (Tables S4 and S5 ). The baseline characteristics of the patients without HF class C/D at baseline are described in Tables S6 and S7 .
For the continuous measures of eGFR, in the adjusted analyses, a statistically significant association with E/e′ [β: −0.01 (95% CI: −0.01, −0.003), P = 0.002] and RWT [β: −0.0003 (95% CI: −0.0005, −0.0001), P = 0.004] was also found, but again, there was no association of eGFR with LAVI (Figure 1 and Table 4 ). The association of eGFR with E/e′ ratio was also present when repeating the analysis sex‐stratified (Table S8 ). For the other associations, except for RWT in females, there were no significant findings. After excluding participants with HF class C/D, eGFR was only significantly associated with RWT (P = 0.02, and not with E/e′, LVMI, or LAVI; Table S4 ). No apparent different findings were identified in the sensitivity analyses based on different kidney function equations (Tables S9 and S10 ).
Figure 1.
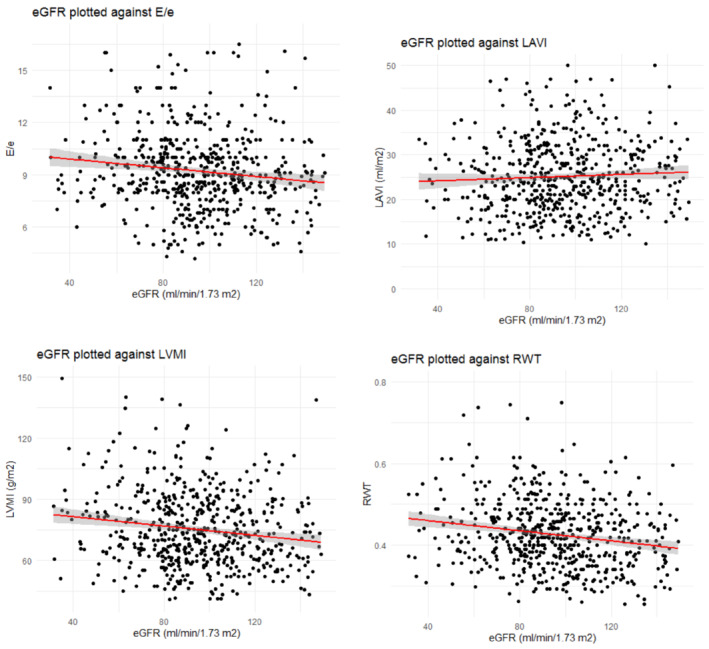
Scatter plot of kidney function against E/e′ (A), against LAVI (B), LVMI (C), and RWT (D).
Table 4.
Univariable and multivariable linear regression for the association between stages of renal dysfunction and echocardiographic measures
| E/e′ | LAVI (mL/m2) | LVMI (g/m2) | RWT | |||||
|---|---|---|---|---|---|---|---|---|
| Beta (95% CI) | P | Beta (95% CI) | P | Beta (95% CI) | P | Beta (95% CI) | P | |
| Model 1: Crude | ||||||||
| eGFR (mL/min/1.73 m2) | −0.01 (−0.02, −0.005) | <0.001 | −0.006 (−0.03, 0.01) | 0.58 | −0.06 (−0.10, −0.02) | 0.005 | −0.0003 (−0.0005, −0.0001) | <0.001 |
| Model 2: Adjusted | ||||||||
| eGFR (mL/min/1.73 m2) | −0.01 (−0.01, −0.003) | 0.001 | −0.003 (−0.02, 0.01) | 0.77 | −0.03 (−0.07, 0.005) | 0.09 | −0.0003 (−0.0005, −0.0001) | 0.004 |
| Model 3: Adjusted | ||||||||
| eGFR (mL/min/1.73 m2) | −0.01 (−0.01, −0.003) | 0.002 | −0.003 (−0.02, 0.02) | 0.77 | −0.03 (−0.07, 0.006) | 0.10 | −0.0003 (−0.0005, −0.0001) | 0.004 |
Model 1: Crude. Model 2: Adjusted for BMI, diabetes mellitus, hypertension, hypercholesterolaemia, and history of cardiovascular disease. Model 3: Adjusted for model 2 + alcohol of >2 units/day, current smoking, and education level. Renal function was estimated using the CKD‐EPI 2021 equation for creatinine and cystatin C in combination without race.
BMI, body mass index; CI, confidence interval; eGFR, estimated glomerular filtration rate; LAVI, left atrial volume index; LVMI, left ventricular mass index; RWT, relative wall thickness.
Discussion
In our cross‐sectional study, we found an association between moderate and mild kidney dysfunction, and diastolic dysfunction and HFpEF, independent of other risk factors (Figure 2 ). This association was already present for mild kidney dysfunction, and stronger for moderate kidney dysfunction. There was a significant association between kidney dysfunction and single echocardiographic parameters of LVDD, notably E/e′ ratio, even after excluding participants with HF. Although the prevalence of HFpEF was higher in females in this study, there was no stronger association of eGFR with worse diastolic function or HFpEF. Hence, our results indicate that the association between reduced kidney function and elevated filling pressures may be evident prior to the development of symptomatic HF and CKD, independent of other cardiovascular risk factors and sex.
Figure 2.
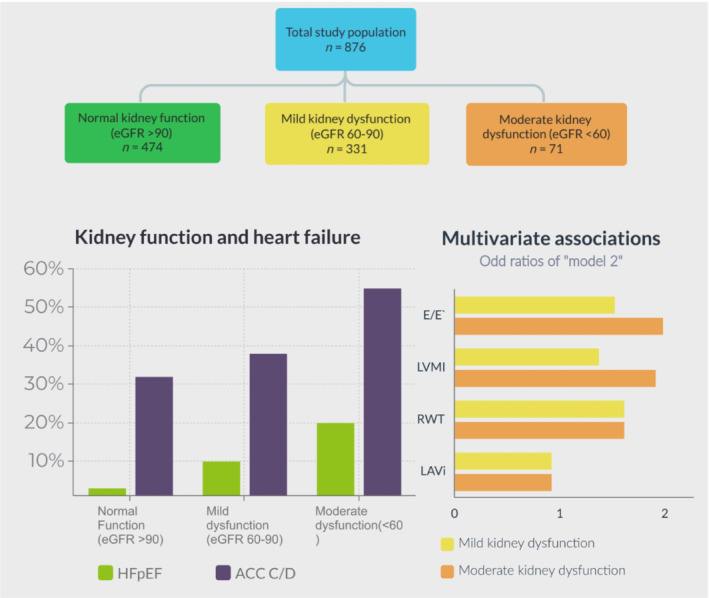
Summary of our results.
Previous studies found differing results between the association of kidney dysfunction and diastolic dysfunction. 23 , 42 , 43 , 45 , 48 , 49 , 50 Comparing previous studies with ours is hampered by heterogeneity in patient characteristics across the studies; the majority of earlier studies evaluated patients with a baseline mean eGFR of approximately 60 mL/min/1.73 m2 or lower. 13 , 23 , 42 , 43 , 44 , 50 Also, other studies were limited to patients with diagnosed CKD at baseline, 47 , 48 established HF at baseline, 21 or were limited to individuals with hypertension or diabetes. 5 , 50 , 51 , 52 Other studies included only outcomes from echocardiography without either the diagnosis of HFpEF or LVDD. 42 , 43 Our study shows a cardiorenal connection in a unique large cohort of patients with largely normal renal function or mild renal dysfunction, that was well‐phenotyped with respect to both kidney function (i.e. creatinine and cystatin C measurement) and cardiac function (i.e. diastolic function and HFpEF diagnosis). Our study adds new information on participants with milder renal function, for example, with a mean eGFR between 60 and 90 mL/min/1.73 m2, and we have phenotyped our patients more extensively on cardiac function, including a panel diagnosis of HF. Additionally, our study provides sex‐specific data on the prevalence of kidney dysfunction, diastolic function abnormalities, and HFpEF. This is important as risk factor associations may differ by sex, especially for HFpEF, but these differences are often not assessed. 53
Our findings show that kidney dysfunction is independently associated with E/e′ ratio, which is considered a parameter of elevated filling pressures. This association remained after we excluded individuals that had already symptoms suggestive of HF (Supporting Information). However, when these individuals were excluded, the association of kidney function with structural abnormalities (LVMI and RWT) disappeared. Although we should be cautious to over‐interpret cross‐sectional data, it could be speculated that elevated filling pressures are the first consequence of kidney dysfunction, while overt changes in left ventricular mass and geometry occur later in the disease trajectory. Another study in individuals with hypertension did not find any association of eGFR with functional or structural parameters relating to LVDD but did observe that individuals with albuminuria had higher RWT and E/e′ ratio. 52
We observed that mild renal dysfunction is linked to elevated filling pressures and structural remodelling and HFpEF. These structural echocardiographic abnormalities, representative of diastolic dysfunction can deteriorate to HFpEF. Our data suggest that high‐risk individuals would benefit from early intervention, targeting, for example, pressure overload, volume overload or systemic inflammation, to prevent deterioration towards HF. 32 Drugs that could prove beneficial and need further investigation are, for example, renin‐angiotensin‐aldosterone system (RAAS) inhibitors, SGLT2 inhibitors, statins, or colchicine. 33 , 34 , 35 , 54 , 55 Further investigations are needed to analyse whether these therapeutic options also would lead to (a better) prevention of HF in a population with mild renal dysfunction.
Adequate measurement of both cardiac function and renal function is important to draw reliable conclusions on their association. In our study, we assessed kidney function using a new equation of cystatin C and creatinine, without race, with diastolic function parameters and HF. 37 Previous studies on the cardiorenal connection mostly used an assessment of renal function based on creatinine, such as the Modification in Renal Disease formula, 5 , 42 , 51 , 56 , 57 and Cockroft–Gault, 50 or cystatin C as marker of kidney function. 42 , 43 , 44 The added value of race in eGFR arguments has recently been under debate, as this offers only modest benefits to precision. 58 , 59 Using the new formula, omitting information on race, has recently been reported to be more accurate and lead to smaller differences between Caucasian participants and non‐Caucasian participants than other equations. 37 Similarly, different strategies to classify diastolic function and heart failure have been reported, including an invasive exercise right heart catheterization or non‐invasive stress echocardiography to measure elevated LV filling pressures and increased pulmonary artery pressure 60 when there is uncertainty on findings during rest. Using an expert panel in our study allowed us to provide the best possible final diagnosis, by adding clinical expertise to all available findings including established HF scores in every participant. 61
A limitation is that cross‐sectional data analysis precludes us from drawing conclusions about causality. Another limitation is that we classified participants as having kidney dysfunction based on a single measurement. Consequently, the strict definition of CKD, including two measurements in 3 months, could not be applied in this population. Also, we were not able to validate our kidney function measurement in our study with other type of kidney damage markers (e.g. urinary samples of albumin or protein). Thus, our approach might have led to incorrect classification of some due to temporary alterations in kidney function. For our main analyses, we have used the most recent eGFR equation without race, which provides the most accurate GFR estimates. 37 Finally, a low number of participants with moderate to severe renal dysfunction (eGFR < 60 mL/min/1.73 m2) were present in our study, limiting the precision to explore the association between moderate CKD and HFpEF in specific subgroups. At the same time, some of our analyses have limited power (e.g. HF diagnosis).
Conclusions
Both mild and moderate kidney dysfunction are independently associated with LVDD parameters and HFpEF. This association is independent of sex and strongest for moderate kidney dysfunction. Considering mild‐to‐moderate kidney dysfunction as risk factor for HFpEF may help identify high‐risk groups benefiting most from early intervention.
Conflict of interest
None declared.
Funding
This investigator‐initiated project received funding from the Dutch Heart Foundation 2013T084 (Queen of Hearts). We acknowledge the support from the Dutch CardioVascular Alliance, an initiative with support of the Dutch Heart Foundation, 2020B008 RECONNEXT.
Supporting information
Table S1. Missing values for baseline characteristics and echocardiography values and panel diagnoses.
Table S2A. Echocardiography values and panel diagnoses regarding heart failure and left ventricular diastolic dysfunction stratified by stages of kidney dysfunction for men.
Table S2B. Echocardiography values and panel diagnoses regarding heart failure and left ventricular diastolic dysfunction stratified by stages of kidney dysfunction for women.
Table S3A. Univariable and multivariable logistic regression for the association between stages of renal dysfunction and echocardiographic measures for men.
Table S3B. Univariable and multivariable logistic regression for the association between stages of renal dysfunction and echocardiographic measures for women.
Table S4. Univariable and multivariable logistic regression for the association between stages of renal dysfunction and echocardiographic measures excluding the patients with heart failure at baseline.
Table S5. Univariable and multivariable linear regression for the association between stages of renal dysfunction and echocardiographic measures excluding the patients with heart failure at baseline.
Table S6. Baseline characteristics of the included patients stratified by renal function excluding the patients with heart failure at baseline.
Table S7. Echocardiography values and panel diagnoses regarding heart failure and left ventricular diastolic dysfunction stratified by stages of kidney dysfunction excluding the patients with heart failure at baseline.
Table S8A. Univariable and multivariable linear regression for the association between stages of renal dysfunction and echocardiographic measures for men.
Table S8B. Univariable and multivariable linear regression for the association between stages of renal dysfunction and echocardiographic measures for women.
Table S9. Univariable and multivariable logistic regression for the association between stages of renal dysfunction and echocardiographic measures.
Table S10. Univariable and multivariable linear regression for the association between stages of renal dysfunction and echocardiographic measures.
Vernooij, R. W. M. , van Ommen, A.‐M. L. N. , Valstar, G. B. , Cramer, M. J. , Teske, A. J. , Menken, R. , Hofstra, L. , Rutten, F. H. , Bots, M. L. , den Ruijter, H. M. , and Verhaar, M. C. (2024) Association of mild kidney dysfunction with diastolic dysfunction and heart failure with preserved ejection fraction. ESC Heart Failure, 11: 315–326. 10.1002/ehf2.14511.
Vernooij RWM and van Ommen AMLN contributed equally.
References
- 1. Groenewegen A, Rutten FH, Mosterd A, Hoes AW. Epidemiology of heart failure. Eur J Heart Fail 2020;22:1342‐1356. doi: 10.1002/ejhf.1858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. de Boer AR, Vaartjes I, Gohar A, Valk MJM, Brugts JJ, Boonman‐de Winter LJM, et al. Heart failure with preserved, mid‐range, and reduced ejection fraction across health care settings: An observational study. ESC Heart Fail 2022;9:363‐372. doi: 10.1002/ehf2.13742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med 2006;355:251‐259. doi: 10.1056/NEJMoa052256 [DOI] [PubMed] [Google Scholar]
- 4. Payne J, Sharma S, de Leon D, Lu JL, Alemu F, Balogun RA, et al. Association of echocardiographic abnormalities with mortality in men with non‐dialysis‐dependent chronic kidney disease. Nephrol Dial Transplant 2012;27:694‐700. doi: 10.1093/ndt/gfr282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen SC, Chang JM, Liu WC, Tsai YC, Tsai JC, Su HM, et al. Stepwise increases in left ventricular mass index and decreases in left ventricular ejection fraction correspond with the stages of chronic kidney disease in diabetes patients. Exp Diabetes Res 2012;2012:789325. doi: 10.1155/2012/789325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wu IW, Hung MJ, Chen YC, Hsu HJ, Cherng WJ, Chang CJ, et al. Ventricular function and all‐cause mortality in chronic kidney disease patients with angiographic coronary artery disease. J Nephrol 2010;23:181‐188. [PubMed] [Google Scholar]
- 7. Heywood JT, Fonarow GC, Costanzo MR, Mathur VS, Wigneswaran JR, Wynne J, et al. High prevalence of renal dysfunction and its impact on outcome in 118,465 patients hospitalized with acute decompensated heart failure: A report from the ADHERE database. J Card Fail 2007;13:422‐430. doi: 10.1016/j.cardfail.2007.03.011 [DOI] [PubMed] [Google Scholar]
- 8. Smith GL, Lichtman JH, Bracken MB, Shlipak MG, Phillips CO, DiCapua P, et al. Renal impairment and outcomes in heart failure: Systematic review and meta‐analysis. J Am Coll Cardiol 2006;47:1987‐1996. doi: 10.1016/j.jacc.2005.11.084 [DOI] [PubMed] [Google Scholar]
- 9. Kottgen A, Russell SD, Loehr LR, Crainiceanu CM, Rosamond WD, Chang PP, et al. Reduced kidney function as a risk factor for incident heart failure: The atherosclerosis risk in communities (ARIC) study. J Am Soc Nephrol 2007;18:1307‐1315. doi: 10.1681/ASN.2006101159 [DOI] [PubMed] [Google Scholar]
- 10. Ricardo AC, Yang W, Sha D, Appel LJ, Chen J, Krousel‐Wood M, et al. Sex‐related disparities in CKD progression. J Am Soc Nephrol 2019;30:137‐146. doi: 10.1681/ASN.2018030296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dunlay SM, Roger VL, Redfield MM. Epidemiology of heart failure with preserved ejection fraction. Nat Rev Cardiol 2017;14:591‐602. doi: 10.1038/nrcardio.2017.65 [DOI] [PubMed] [Google Scholar]
- 12. United States Renal Data System . 2019 USRDS Annual Data Report: Epidemiology of kidney disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2019.
- 13. Vogel MW, Slusser JP, Hodge DO, Chen HH. The natural history of preclinical diastolic dysfunction: A population‐based study. Circ Heart Fail 2012;5:144‐151. doi: 10.1161/CIRCHEARTFAILURE.110.959668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. McCullough PA, Kellum JA, Haase M, Müller C, Damman K, Murray PT, et al. Pathophysiology of the cardiorenal syndromes: Executive summary from the eleventh consensus conference of the acute dialysis quality initiative (ADQI). Contrib Nephrol 2013;182:82‐98. doi: 10.1159/000349966 [DOI] [PubMed] [Google Scholar]
- 15. Haase M, Müller C, Damman K, Murray PT, Kellum JA, Ronco C, et al. Pathogenesis of cardiorenal syndrome type 1 in acute decompensated heart failure: Workgroup statements from the eleventh consensus conference of the acute dialysis quality initiative (ADQI). Contrib Nephrol 2013;182:99‐116. doi: 10.1159/000349969 [DOI] [PubMed] [Google Scholar]
- 16. Cruz DN, Schmidt‐Ott KM, Vescovo G, House AA, Kellum JA, Ronco C, et al. Pathophysiology of cardiorenal syndrome type 2 in stable chronic heart failure: Workgroup statements from the eleventh consensus conference of the acute dialysis quality initiative (ADQI). Contrib Nephrol 2013;182:117‐136. doi: 10.1159/000349968 [DOI] [PubMed] [Google Scholar]
- 17. Bongartz LG, Cramer MJ, Doevendans PA, Joles JA, Braam B. The severe cardiorenal syndrome: ‘Guyton revisited’. Eur Heart J 2005;26:11‐17. doi: 10.1093/eurheartj/ehi020 [DOI] [PubMed] [Google Scholar]
- 18. Bagshaw SM, Hoste EA, Braam B, Briguori C, Kellum JA, McCullough PA, et al. Cardiorenal syndrome type 3: Pathophysiologic and epidemiologic considerations. Contrib Nephrol 2013;182:137‐157. doi: 10.1159/000349971 [DOI] [PubMed] [Google Scholar]
- 19. Tumlin JA, Costanzo MR, Chawla LS, Herzog CA, Kellum JA, McCullough PA, et al. Cardiorenal syndrome type 4: Insights on clinical presentation and pathophysiology from the eleventh consensus conference of the acute dialysis quality initiative (ADQI). Contrib Nephrol 2013;182:158‐173. doi: 10.1159/000349972 [DOI] [PubMed] [Google Scholar]
- 20. Mehta RL, Rabb H, Shaw AD, Singbartl K, Ronco C, McCullough PA, et al. Cardiorenal syndrome type 5: Clinical presentation, pathophysiology and management strategies from the eleventh consensus conference of the acute dialysis quality initiative (ADQI). Contrib Nephrol 2013;182:174‐194. doi: 10.1159/000349970 [DOI] [PubMed] [Google Scholar]
- 21. Damman K, Valente MA, Voors AA, O'Connor CM, van Veldhuisen DJ, Hillege HL. Renal impairment, worsening renal function, and outcome in patients with heart failure: An updated meta‐analysis. Eur Heart J 2014;35:455‐469. doi: 10.1093/eurheartj/eht386 [DOI] [PubMed] [Google Scholar]
- 22. Brouwers FP, de Boer RA, van der Harst P, Voors AA, Gansevoort RT, Bakker SJ, et al. Incidence and epidemiology of new onset heart failure with preserved vs. reduced ejection fraction in a community‐based cohort: 11‐year follow‐up of PREVEND. Eur Heart J 2013;34:1424‐1431. doi: 10.1093/eurheartj/eht066 [DOI] [PubMed] [Google Scholar]
- 23. Taqueti VR, Solomon SD, Shah AM, Desai AS, Groarke JD, Osborne MT, et al. Coronary microvascular dysfunction and future risk of heart failure with preserved ejection fraction. Eur Heart J 2018;39:840‐849. doi: 10.1093/eurheartj/ehx721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bobenko A, Duvinage A, Mende M, Holzendorf V, Nolte K, Herrmann‐Lingen C, et al. Outcome assessment using estimation of left ventricular filling pressure in asymptomatic patients at risk for heart failure with preserved ejection fraction. Int J Cardiol Heart Vasc 2020;28:100525. doi: 10.1016/j.ijcha.2020.100525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pugliese NR, de Biase N, Gargani L, Mazzola M, Conte L, Fabiani I, et al. Predicting the transition to and progression of heart failure with preserved ejection fraction: A weighted risk score using bio‐humoural, cardiopulmonary, and echocardiographic stress testing. Eur. J Prev Cardiol 2020;28:zwaa129. doi: 10.1159/000349970 [DOI] [PubMed] [Google Scholar]
- 26. Caballero L, Saura D, García‐Lara J, Oliva MJ, Pinar E, González‐Carrillo J, et al. Influence of aortic regurgitation after TAVI on left ventricular filling pattern. Eur J Clin Invest 2015;45:18‐26. doi: 10.1111/eci.12374 [DOI] [PubMed] [Google Scholar]
- 27. Redfield MM, Jacobsen SJ, Burnett JC Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: Appreciating the scope of the heart failure epidemic. JAMA 2003;289:194‐202. doi: 10.1001/jama.289.2.194 [DOI] [PubMed] [Google Scholar]
- 28. Park M, Hsu CY, Li Y, Mishra RK, Keane M, Rosas SE, et al. Associations between kidney function and subclinical cardiac abnormalities in CKD. J Am Soc Nephrol 2012;23:1725‐1734. doi: 10.1681/ASN.2012020145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kloch‐Badelek M, Kuznetsova T, Sakiewicz W, Tikhonoff V, Ryabikov A, González A, et al. Prevalence of left ventricular diastolic dysfunction in European populations based on cross‐validated diagnostic thresholds. Cardiovasc Ultrasound 2012;10:10. doi: 10.1186/1476-7120-10-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rasmussen‐Torvik LJ, Colangelo LA, Lima JAC, Jacobs DR, Rodriguez CJ, Gidding SS, et al. Prevalence and predictors of diastolic dysfunction according to different classification criteria: The coronary artery risk development in young in adults study. Am J Epidemiol 2017;185:1221‐1227. doi: 10.1093/aje/kww214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Correa de Sa DD, Hodge DO, Slusser JP, Redfield MM, Simari RD, Burnett JC, et al. Progression of preclinical diastolic dysfunction to the development of symptoms. Heart 2010;96:528‐532. doi: 10.1136/hrt.2009.177980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ledwidge M, Gallagher J, Conlon C, Tallon E, O'Connell E, Dawkins I, et al. Natriuretic peptide‐based screening and collaborative care for heart failure: The STOP‐HF randomized trial. JAMA 2013;310:66‐74. doi: 10.1001/jama.2013.7588 [DOI] [PubMed] [Google Scholar]
- 33. Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Böhm M, et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med 2021;385:1451‐1461. doi: 10.1056/NEJMoa2107038 [DOI] [PubMed] [Google Scholar]
- 34. Capuano A, Scavone C, Vitale C, Sportiello L, Rossi F, Rosano GM, et al. Mineralocorticoid receptor antagonists in heart failure with preserved ejection fraction (HFpEF). Int J Cardiol 2015;200:15‐19. doi: 10.1016/j.ijcard.2015.07.038 [DOI] [PubMed] [Google Scholar]
- 35. Solomon SD, McMurray JJV, Claggett B, de Boer RA, DeMets D, Hernandez AF, et al. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med 2022;387:1089‐1098. doi: 10.1056/NEJMoa2206286 [DOI] [PubMed] [Google Scholar]
- 36. Valstar GB, Bots SH, Groepenhoff F, Gohar A, Rutten FH, Leiner T, et al. Discovery of biomarkers for the presence and progression of left ventricular diastolic dysfunction and HEart faiLure with preserved ejection fraction in patients at risk for cardiovascular disease: Rationale and design of the HELPFul case‐cohort study in a Dutch cardiology outpatient clinic. BMJ Open 2019;9:e028408. doi: 10.1136/bmjopen-2018-028408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Inker LA, Eneanya ND, Coresh J, Tighiouart H, Wang D, Sang Y, et al. New creatinine‐ and cystatin C‐based equations to estimate GFR without race. N Engl J Med 2021;385:1737‐1749. doi: 10.1056/NEJMoa2102953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nagueh SF, Smiseth OA, Appleton CP, Byrd BF 3rd, Dokainish H, Edvardsen T, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2016;29:277‐314. doi: 10.1016/j.echo.2016.01.011 [DOI] [PubMed] [Google Scholar]
- 39. Pieske B, Tschöpe C, de Boer RA, Fraser AG, Anker SD, Donal E, et al. How to diagnose heart failure with preserved ejection fraction: The HFA‐PEFF diagnostic algorithm: A consensus recommendation from the heart failure association (HFA) of the European Society of Cardiology (ESC) Eur Heart J 2019;40:3297‐3317. doi: 10.1093/eurheartj/ehz641 [DOI] [PubMed] [Google Scholar]
- 40. Reddy YNV, Carter RE, Obokata M, Redfield MM, Borlaug BA. A simple, evidence‐based approach to help guide diagnosis of heart failure with preserved ejection fraction. Circulation 2018;138:861‐870. doi: 10.1161/CIRCULATIONAHA.118.034646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the Management of Heart Failure: A report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the Heart Failure Society of America. Circulation 2017;136:e137‐e161. doi: 10.1161/CIR.0000000000000509 [DOI] [PubMed] [Google Scholar]
- 42. Jain A, Scott C, Chen HH. The renal‐cardiac connection in subjects with preserved ejection fraction: A population based study. ESC Heart Fail 2017;4:266‐273. doi: 10.1002/ehf2.12143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nerpin E, Ingelsson E, Risérus U, Sundström J, Andren B, Jobs E, et al. The association between glomerular filtration rate and left ventricular function in two independent community‐based cohorts of elderly. Nephrol Dial Transplant 2014;29:2069‐2074. doi: 10.1093/ndt/gfu199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Agarwal S, Thohan V, Shlipak MG, Lima J, Bluemke DA, Siscovick D, et al. Association between cystatin C and MRI measures of left ventricular structure and function: Multi‐ethnic study of atherosclerosis. Int J Nephrol 2011;2011:153868. doi: 10.4061/2011/153868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ix JH, Shlipak MG, Chertow GM, Ali S, Schiller NB, Whooley MA. Cystatin C, left ventricular hypertrophy, and diastolic dysfunction: Data from the heart and soul study. J Card Fail 2006;12:601‐607. doi: 10.1016/j.cardfail.2006.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron 1976;16:31‐41. doi: 10.1159/000180580 [DOI] [PubMed] [Google Scholar]
- 47. Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 2012;367:20‐29. doi: 10.1056/NEJMoa1114248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Agarwal R, Light RP. Determinants and prognostic significance of electrocardiographic left ventricular hypertrophy criteria in chronic kidney disease. Clin J Am Soc Nephrol 2011;6:528‐536. doi: 10.2215/CJN.07770910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Patel RK, Oliver S, Mark PB, Powell JR, McQuarrie EP, Traynor JP, et al. Determinants of left ventricular mass and hypertrophy in hemodialysis patients assessed by cardiac magnetic resonance imaging. Clin J Am Soc Nephrol 2009;4:1477‐1483. doi: 10.2215/CJN.03350509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Masugata H, Senda S, Goda F, Yamagami A, Okuyama H, Kohno T, et al. Echocardiographic assessment of the cardio‐renal connection: Is left ventricular hypertrophy or diastolic function more closely correlated with estimated glomerular filtration rate in patients with cardiovascular risk factors? Clin Exp Hypertens 2010;32:113‐120. doi: 10.3109/10641960902993145 [DOI] [PubMed] [Google Scholar]
- 51. Nardi E, Palermo A, Mulè G, Cusimano P, Cottone S, Cerasola G. Left ventricular hypertrophy and geometry in hypertensive patients with chronic kidney disease. J Hypertens 2009;27:633‐641. doi: 10.1097/HJH.0b013e3283220ecd [DOI] [PubMed] [Google Scholar]
- 52. Shah AM, Lam CS, Cheng S, Verma A, Desai AS, Rocha RA, et al. The relationship between renal impairment and left ventricular structure, function, and ventricular‐arterial interaction in hypertension. J Hypertens 2011;29:1829‐1836. doi: 10.1097/HJH.0b013e32834a4d38 [DOI] [PubMed] [Google Scholar]
- 53. Eikendal ALM, Gohar A, Rutten FH, Bots ML, Appelman Y, Hofstra L, et al. Sex‐specific relations of cardiovascular risk factors with left ventricular diastolic dysfunction/heart failure with preserved ejection fraction are underreported: A call for action. J Card Fail 2018;24:412‐414. doi: 10.1016/j.cardfail.2018.03.001 [DOI] [PubMed] [Google Scholar]
- 54. Nidorf SM, Fiolet ATL, Mosterd A, Eikelboom JW, Schut A, Opstal TSJ, et al. Colchicine in patients with chronic coronary disease. N Engl J Med 2020;383:1838‐1847. doi: 10.1056/NEJMoa2021372 [DOI] [PubMed] [Google Scholar]
- 55. Fukuta H, Goto T, Wakami K, Ohte N. The effect of statins on mortality in heart failure with preserved ejection fraction: A meta‐analysis of propensity score analyses. Int J Cardiol 2016;214:301‐306. doi: 10.1016/j.ijcard.2016.03.186 [DOI] [PubMed] [Google Scholar]
- 56. Jacobs L, Efremov L, Ferreira JP, Thijs L, Yang WY, Zhang ZY, et al. Risk for incident heart failure: A subject‐level meta‐analysis from the heart “OMics“ in AGEing (HOMAGE) study. J Am Heart Assoc 2017;6:e005231. doi: 10.1161/JAHA.116.005231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Dini FL, Demmer RT, Simioniuc A, Morrone D, Donati F, Guarini G, et al. Right ventricular dysfunction is associated with chronic kidney disease and predicts survival in patients with chronic systolic heart failure. Eur J Heart Fail 2012;14:287‐294. doi: 10.1093/eurjhf/hfr176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Gama RM, Clery A, Griffiths K, Heraghty N, Peters AM, Palmer K, et al. Estimated glomerular filtration rate equations in people of self‐reported black ethnicity in the United Kingdom: Inappropriate adjustment for ethnicity may lead to reduced access to care. PLoS ONE 2021;16:e0255869. doi: 10.1371/journal.pone.0255869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Eneanya ND, Yang W, Reese PP. Reconsidering the consequences of using race to estimate kidney function. JAMA 2019;322:113‐114. doi: 10.1001/jama.2019.5774 [DOI] [PubMed] [Google Scholar]
- 60. Obokata M, Kane GC, Reddy YN, Olson TP, Melenovsky V, Borlaug BA. Role of diastolic stress testing in the evaluation for heart failure with preserved ejection fraction: A simultaneous invasive‐echocardiographic study. Circulation 2017;135:825‐838. doi: 10.1161/CIRCULATIONAHA.116.024822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Faxen UL, Venkateshvaran A, Shah SJ, Lam CSP, Svedlund S, Saraste A, et al. Generalizability of HFA‐PEFF and H2FPEF diagnostic algorithms and associations with heart failure indices and proteomic biomarkers: Insights from PROMIS‐HFpEF. J Card Fail 2021;27:756‐765. doi: 10.1016/j.cardfail.2021.02.005 [DOI] [PubMed] [Google Scholar]
- 62. Bertens LC, Broekhuizen BD, Naaktgeboren CA, Rutten FH, Hoes AW, van Mourik Y, et al. Use of expert panels to define the reference standard in diagnostic research: A systematic review of published methods and reporting. PLoS Med 2013;10:e1001531. doi: 10.1371/journal.pmed.1001531 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Missing values for baseline characteristics and echocardiography values and panel diagnoses.
Table S2A. Echocardiography values and panel diagnoses regarding heart failure and left ventricular diastolic dysfunction stratified by stages of kidney dysfunction for men.
Table S2B. Echocardiography values and panel diagnoses regarding heart failure and left ventricular diastolic dysfunction stratified by stages of kidney dysfunction for women.
Table S3A. Univariable and multivariable logistic regression for the association between stages of renal dysfunction and echocardiographic measures for men.
Table S3B. Univariable and multivariable logistic regression for the association between stages of renal dysfunction and echocardiographic measures for women.
Table S4. Univariable and multivariable logistic regression for the association between stages of renal dysfunction and echocardiographic measures excluding the patients with heart failure at baseline.
Table S5. Univariable and multivariable linear regression for the association between stages of renal dysfunction and echocardiographic measures excluding the patients with heart failure at baseline.
Table S6. Baseline characteristics of the included patients stratified by renal function excluding the patients with heart failure at baseline.
Table S7. Echocardiography values and panel diagnoses regarding heart failure and left ventricular diastolic dysfunction stratified by stages of kidney dysfunction excluding the patients with heart failure at baseline.
Table S8A. Univariable and multivariable linear regression for the association between stages of renal dysfunction and echocardiographic measures for men.
Table S8B. Univariable and multivariable linear regression for the association between stages of renal dysfunction and echocardiographic measures for women.
Table S9. Univariable and multivariable logistic regression for the association between stages of renal dysfunction and echocardiographic measures.
Table S10. Univariable and multivariable linear regression for the association between stages of renal dysfunction and echocardiographic measures.


