Abstract
Multicellular eukaryotic organisms are hosts to communities of bacteria that reside on or inside their tissues. Often the eukaryotic members of the system contribute to high proportions of metagenomic sequencing reads, making it challenging to achieve sufficient sequencing depth to evaluate bacterial ecology. Stony corals are one such complex community; however, separation of bacterial from eukaryotic (primarily coral and algal symbiont) cells has so far not been successful. Using a combination of hybridization chain reaction fluorescence in situ hybridization and fluorescence activated cell sorting (HCR-FISH + FACS), we sorted two populations of bacteria from five genotypes of the coral Acropora loripes, targeting (i) Endozoicomonas spp, and (ii) all other bacteria. NovaSeq sequencing resulted in 67–91 M reads per sample, 55%–90% of which were identified as bacterial. Most reads were taxonomically assigned to the key coral-associated family, Endozoicomonadaceae, with Vibrionaceae also abundant. Endozoicomonadaceae were 5x more abundant in the ‘Endozoicomonas’ population, highlighting the success of the dual-labelling approach. This method effectively enriched coral samples for bacteria with <1% contamination from host and algal symbionts. The application of this method will allow researchers to decipher the functional potential of coral-associated bacteria. This method can also be adapted to accommodate other host-associated communities.
Keywords: symbiosis, host-associated, metagenomics, coral, FISH, FACS, hybridization chain reaction
Bacteria were separated from coral tissue prior to metagenomics to increase sequencing efficiency.
Introduction
It is now widely accepted that all animals and plants depend on bacteria and other microbes for their health and functioning (McFall-Ngai et al. 2013, Mueller and Sachs 2015, Sessitsch et al. 2023). In eukaryotic hosts, bacteria have been shown to play roles in processes and traits as diverse as immunity, development, digestion of food, adaptation to different environmental conditions, mate choice and other behaviours (McFall-Ngai et al. 2013, McCutcheon 2021). Corals are a notable example, as these marine cnidarians associate with several groups of microbes critical to their health and survival, including bacteria (Blackall et al. 2015, Bourne et al. 2016, Maire et al. 2022, Mohamed et al. 2023). While genomic approaches have provided an in-depth understanding of the composition of bacterial communities associated with reef-building corals (van Oppen and Blackall 2019), the functions of the bacteria within the coral holobiont (i.e. the coral animal and its associated microbiota) are still poorly understood (Sweet and Bulling 2017).
Because not all coral-associated bacteria can be isolated with conventional methods, high-quality assembled genomes derived from metagenomic analyses can be used in their absence to study whole communities. The close symbiotic relationships bacteria have with their coral hosts, in combination with the lack of host reference genomes, make it difficult to eliminate contaminating coral DNA for subsequent analyses. One approach to achieve sufficient bacterial read depth is to physically isolate them from other microorganisms and eukaryotic cells prior to DNA extraction and sequencing (Grieb et al. 2020). Fluorescence in situ hybridization (FISH) can assist with this enrichment process.
FISH was introduced >30 years ago as a valuable molecular tool to detect specific DNA or RNA sequences using complementary DNA- or RNA-probes labelled with fluorescent dyes (DeLong et al. 1989). Host-associated bacterial identification by standard FISH methods (i.e. the use of oligonucleotide probes labelled at either the 5′ or 3′ end with a single fluorophore) targeting ribosomal RNA (rRNA) has been explored in corals (Ainsworth et al. 2006, Apprill et al. 2012, Ainsworth et al. 2015, Damjanovic et al. 2019, Maire et al. 2023), but suffers from several limitations, such as host autofluorescence and non-specific probe binding to certain host cells and structures, that may prevent the successful detection of target organisms (Wada et al. 2016).
Autofluorescence associated with corals is the direct result of high densities of chlorophyll-containing dinoflagellates within the coral tissue and an abundance of host-derived fluorescent proteins, including green, red, cyan, and orange fluorescent protein-like molecules (reviewed in Alieva et al. 2008, Wada et al. 2016). Further, target bacterial cells could be in low abundance in some compartments of the coral animal (Maire et al. 2021) or might not be detected due to low ribosome content (Poulsen et al. 1993), or lack of permeabilization, with additional unsatisfactory signal-to-noise ratio. While some variations of FISH can amplify signal to address these challenges, such as catalysed reporter deposition (CARD)-FISH, they often require reagents that damage DNA (Keller and Pollard 1977) making downstream genomic analyses difficult. Instead, hybridization chain reaction (HCR)-FISH (Choi et al. 2010, Yamaguchi et al. 2015a) can boost the probe signal and transcend specific limitations ranging from low signal detection to the interference of host autofluorescence. In this approach, a specific oligonucleotide probe complementary to the rRNA target, carrying an initiator sequence, is hybridized to the cells. Next, two fluorescently labelled hairpin oligos (X1 and X2) bind subsequently in a chain reaction to the initiator sequence, thus multiplying the fluorescent signal. HCR-FISH has previously been paired with fluorescence activated cell sorting (FACS) to sort bacteria from environmental samples for single cell genomics (Grieb et al. 2020) but has yet to be applied to animals hosting complex communities of microorganisms.
In this study, we developed a combined HCR-FISH + FACS pipeline for the targeted retrieval of bacteria from coral tissues for metagenomic sequencing using the coral Acropora loripes as a model. Coral 16S rRNA gene metabarcoding studies have revealed that while many corals associate with several hundred and sometimes even more than a thousand different bacterial taxa (amplicon sequence variants [ASVs]) (Blackall et al. 2015), adult colonies of A. loripes partner with as few as 20–30 ASVs with only one or a few Endozoicomonas ASVs dominating the communities (Damjanovic et al. 2020). We developed our protocol using mock bacterial communities and A. loripes tissue to select appropriate fluorophores and FACS gates to sort two populations: 1) ‘Endozoicomonas’ and 2) ‘all-bacteria’.
Methods
Coral collections and tissue processing
A detailed protocol for all methods is provided in Supplemental File 1. Three Acropora loripes colonies were collected from Davies Reef (−18.82, 147.64; colonies Al02 and Al05 in June 2020 and Al08 in Feb. 2021) and two colonies were collected from Backnumbers Reef (−18.51, 147.15; Al11 and Al13 in December 2020) (Permit# G12/35236.1). These were transported to the Australian Institute of Marine Science's (AIMS) National Sea Simulator in Townsville, Queensland, where they were housed in outdoor mesocosms with natural light conditions and a flow-through system with seawater volume changed every two hours. The daily average temperature profile followed the average temperature profile recorded at Davies Reef weather station. Corals were maintained on a daily regimen of 0.5 Artemia nauplii ml−1 and 2 000 cells ml−1 of a mixed-species microalgae solution. In July 2021, 2–3 cm fragments were cut from five different locations of each coral genotype without removing the mother colony from the aquarium (to avoid mucus production). To minimise cross-contamination, nitrile gloves were discarded between each sampling location, and all collection equipment (bone cutters and forceps) was sequentially sterilized in 10% sodium hypochlorite, reverse osmosis water, 80% ethanol, with a final wash in 0.22 µm filtered seawater as described in Damjanovic et al. (2020).
Coral tissue was separated from the skeleton with minimal contamination using a divalent cation removal approach for tissue detachment (Domart-Coulon and Ostrander 2015). Coral fragments were rinsed briefly with filter-sterilized (0.22 µm membrane) artificial seawater free of calcium (CafASW, see Supp. File 1 for recipe) (Gates and Muscatine 1992, Frank et al. 1994, Domart-Coulon et al. 2004) to remove loosely associated microbes and transferred to a 50 ml polypropylene tube with ∼10 ml of CafASW. Each sample was incubated for 7 days at 4°C on a rotary mixer in CafASW to induce detachment of cells from the skeleton (Gates and Muscatine 1992, Domart-Coulon et al. 2004, Marshall and Clode 2004, Helman et al. 2008, Reyes-Bermudez et al. 2021). After the CafASW incubation, samples were moved to room temperature (RT) and supplemented with collagenase (Type IV, Sigma, Lecointe et al. 2013) at a final concentration of 0.15% (w/v) and incubated for an additional 6 hrs at 27°C on an orbital incubator (Gates and Muscatine 1992, Gates et al. 1992, Frank et al. 1994, Helman et al. 2008, Khalesi et al. 2008). Each sample was then concentrated by centrifugation (5 250 × g for 30 min at RT) with supernatant discarded but cells and skeleton fragments left behind. To increase permeability of cells, samples were resuspended by vortexing in 10 ml 0.1% Triton in 3x phosphate buffered saline (PBS) and incubated at RT for 5 min. After the incubation, samples were centrifuged at 5 250 × g for 30 min at RT. The supernatant was discarded, and the dissociated coral tissues and skeleton fragments were resuspended in 100% ethanol. At this point, samples were shipped from AIMS to The University of Melbourne (UoM) on ice.
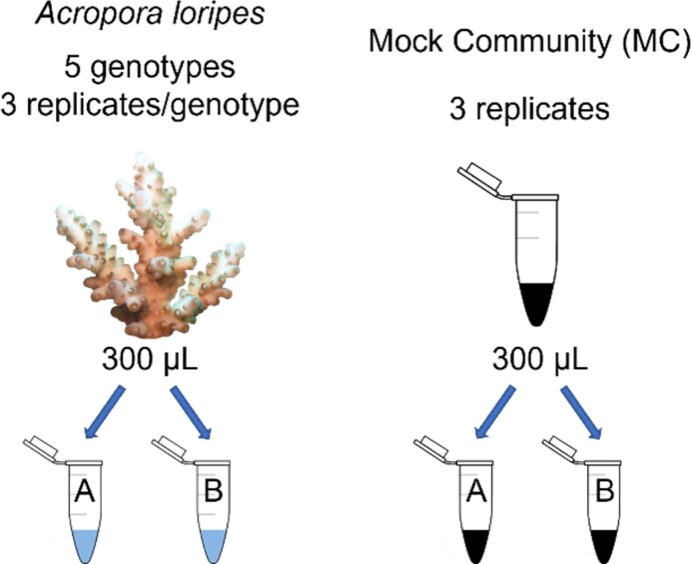
At UoM, samples were concentrated by centrifugation (60 min at 2 885 × g at 4°C), skeleton removed with sterile forceps, and cells were transferred to a 1.5 ml polypropylene tube with 1 ml 100% ethanol. This cell suspension was divided into two fractions: (i) 300 µl to be labelled with EUB-mix probe suite (degenerate primer for bacteria created by merging EUB338, EUB338-II, and EUB338-III, Daims et al. 1999; Table 1) and Endozoicomonas-specific probes (Bayer et al. 2013; Table 1) with HCR-FISH, and (ii) 300 µl as a negative control for HCR-FISH (no probes added). These fractions will hereafter be referred to as labelled and unlabelled, respectively. As a positive control, mock bacterial communities (MC) comprised one Endozoicomonas (ALE010, ALC066, or ALB032; Gotze et al. in prep) and one other cnidarian-associated bacterium (Ruegeria sp. (ALD015), Roseovarius sp. (MMSF01006), or Vibrio sp. (MMSF00650), (Dungan et al. 2021)) and were prepared as labelled and unlabelled fractions (Fig. 1).
Table 1.
Initiator probes for HCR-FISH (Sigma-Aldrich). Underlined portion is the initiator sequence that the amplifier probe binds to along with a five-adenosine linker sequence. The %FA (formamide) column lists the suggested formamide percentage in the hybridization/washing buffer.
| Probe | Sequence (5′-3′) | Target | %FA | Reference |
|---|---|---|---|---|
| Endo663-H | CCGAATACAAAGCATCAACGACTAGAAAAAA GGAAATTCCACACTCCTC | Endozoicomonas spp. | 35 | (Bayer et al. 2013, Yamaguchi et al. 2015a) |
| Endo736-H | CCGAATACAAAGCATCAACGACTAGAAAAAA GTCAGTGTCAGACCAGAG | Endozoicomonas spp. | 35 | (Bayer et al. 2013, Yamaguchi et al. 2015a) |
| EUBMix338-R | TACGCCCTAAGAATCCGAACCCTATGAAAAA GCWGCCWCCCGTAGGWGT | All bacteria | 0–50 | (Daims et al. 1999, Yamaguchi et al. 2015a) |
Figure 1.
Setup for A. loripes (left) and MC (right) samples where A was labelled with EUBMix338 and Endo probes using HCR-FISH, and B was an unlabelled negative control for HCR-FISH. Replicate samples for each coral genotype and MC were processed on three separate days. The MC comprised Endozoicomonas and one other cnidarian-associated bacterium (Ruegeria, Roseovarius, or Vibrio).
HCR-FISH
To remove sampling day as a confounding factor, replicate samples for each A. loripes genotype and the MC were processed on different days. Labelled and unlabelled samples for HCR-FISH were pelleted by centrifuging at 4°C at 10 000 × g for 2 min. The supernatant was discarded, and residual ethanol was removed by drying samples in a heating block set to 40°C for up to 15 min. During this time, 50 ml of hybridization/wash buffer was prepared at a 35% formamide stringency and filtered through a 0.22 µm membrane (see Supp. File 1 for recipe).
Initiator probes were purchased dry from Sigma and resuspended in nuclease-free water to 50 µM. To the labelled fractions, 10 µl of each initiator probe was added with 170 µl hybridization buffer (final probe concentration of 2.5 µM). For the unlabelled fractions, 200 µl of hybridization buffer was added. All samples were resuspended and incubated on a rotary tube mixer at 46°C overnight (12–16 hrs). After hybridization, the samples were centrifuged at 12 000 × g for 5 min to pellet cells and supernatant discarded. Non-specifically bound or unbound probes were removed by resuspending the samples in 500 µl of hybridization (now wash) buffer (warmed to 48°C) and incubating at 48°C for 30 min on a rotary tube mixer. During this incubation, 20 ml of amplification buffer was prepared (Supp. File 1) and filtered through a 0.22 µm membrane. Amplifier probe (Table 2) stocks were kept at 100 µM in milli-Q (MQ) water. For each labelled sample, 1 µl of stock probe was added to 49 µl of amplification buffer for each amplifier probe in the pair (i.e. H1 and H2) for a final concentration of 2 µM. Each amplifier probe was then heated to 95°C for 90 s using a hot block and kept at 25°C for 30 min prior to combining pairs. This resulted in a final concentration of 1 µM for each probe.
Table 2.
Amplifier probe sequences (Choi et al. 2010, Yamaguchi et al. 2015a) ordered from biomers net with fluorophores attached on the 5′ and 3′ end for the amplifiers 1 and 2, respectively. Lowercase letters represent stem structure of amplifier probe. Underlined sequence of H1 and R1 are complementary to the initiator sequences of initiator H and initiator R, respectively. The amplifier H pair was labelled with Atto390. Amplifier R sequences were labelled with Atto550. The emission of the selected fluorophores falls outside of the range of autofluorescence for coral and algal cells.
| Probe | Sequence 5′–3′ |
|---|---|
| H1 | TCTAGTCGTTgatgctttgtattcggCGACAGATAAccgaatacaaagcatc |
| H2 | ccgaatacaaagcatcAACGACTAGAgatgctttgtattcggTTATCTGTCG |
| R1 | CATAGGGTTCggattcttagggcgtaGCAGCATCAAtacgccctaagaatcc |
| R2 | tacgccctaagaatccGAACCCTATGggattcttagggcgtaTTGATGCTGC |
After washing, samples were centrifuged at 15 000 × g for 2 min to pellet cells. The supernatant was discarded, and all samples were resuspended in 200 µl amplification buffer (no probe). After 5 min at RT, cells were pelleted via centrifugation (15 000 × g for 2 min) with supernatant discarded. Labelled samples were resuspended in 200 µl of amplification buffer with the amplifier probe pair (100 µl from each R and H), whereas unlabelled samples were resuspended in 200 µl amplification buffer (no probe). All samples were incubated on a rotary tube mixer at 46°C for 3 h in the dark.
After incubation, cells were centrifuged (15 000 × g for 2 min). After discarding the supernatant, samples were resuspended in 200 µl of amplification buffer (no probe) for 30 min at 4°C to wash away any unbound amplifier probers and prevent probe dissociation. Prior to FACS, samples were centrifuged (15 000 × g for 2 min), supernatant removed, resuspended in 200 µl of MQ water, then filtered through a pluriStrainer Mini 40 µM (pluriSelect Life Science, Germany) as to remove any clumps that might clog the FACS.
FACS
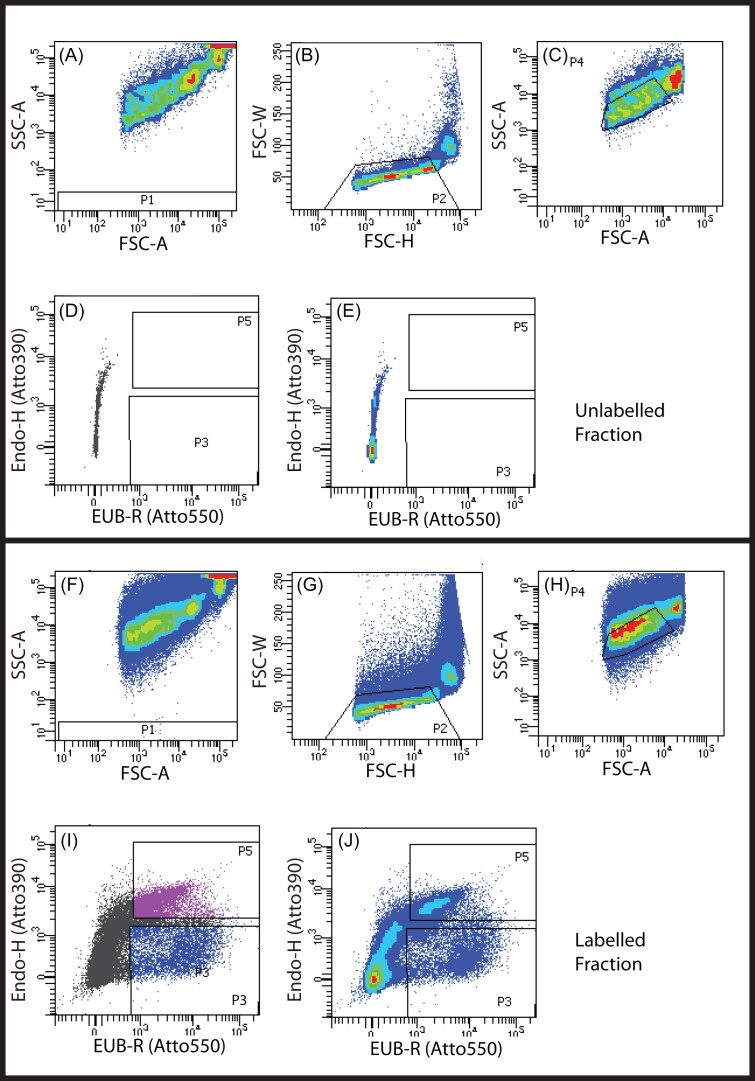
Processed A. loripes samples were sorted on a FACS Aria III (70 µm nozzle/70 psi)/FACS Diva version 8 software (Becton Dickinson (BD), Franklin Lakes, NJ). Bacteria from unlabelled and fluorescent probe-labelled MC samples were used to set and confirm the cytometer scatter and fluorescence parameters and to establish gating strategies for sorting A. loripes-derived bacteria (Fig. S1). Briefly, bacteria were initially resolved from debris and instrument noise (not P1 gate) and single bacterial particles were separated from aggregates using the P2 gate. The P2 gate included a combination of measurements for peak particle size (forward scatter height (FSC-H) and forward scatter width (FSC-W)) or time of flight (side scatter (SSC) and forward scatter (FSC)) (Fig. 2A-C; F-H). These gates were used to select bacteria (exclude large coral or algal cells). FSC measures light scattered by particles and is directly related to the relative size. Relatively large particles, such as coral and algal cells generate higher FSC signals than bacteria. SSC is a measure of relative complexity or granularity—the more complex the internal structure of a particle, the higher this signal. Bacteria are smaller and less complex than eukaryotic cells. Unlabelled bacteria were used to set fluorescence negative boundaries in the EUB-R (Atto550) and Endo-H (Atto390) detectors, with emissions detection ranges of 582+/-7.5 nm and 450+/-20 nm, respectively (Fig. 2D-E). Bacteria from A. loripes labelled with Atto550 only EUB-R+/Endo-H- (‘all-bacteria’, P3) or double positive for Atto550 and Atto390 (EUB-R+/Endo-H+ (‘Endozoicomonas’, P5) were resolved from unlabelled particles and sorted at a rate of under 5 000 events per second into 1.5 ml tubes. Collected fractions containing between 1000 and 300 000 particles were used for subsequent metagenomics (Fig. 2I and J). FlowJo v10 (BD) was used to analyse cytometry data post-acquisition.
Figure 2.
FACS sorting profiles for unlabelled (A-E) and labelled (F-J) A. loripes tissue homogenate (genotype Al08). First, background noise (P1) from the FACS was excluded (A, F). Large coral and Symbiodiniaceae cells were then identified by forward (B, G) and side (C, H) scatter and excluded through sequential gating. Fluorescent channels were used to identify autofluorescence of the remaining events in the unlabelled fraction in the 582 nm (D-E x-axis) and 450 nm (D-E y-axis) emissions. Events in the labelled fraction that surpassed this baseline autofluorescence in both axes were collected as the dual labelled ‘Endozoicomonas’ population (P5) and those that had greater signal on the x-axis only were collected as the single labelled ‘all-bacteria’ population (P3) (I-J).
Sorting validation by microscopic analysis
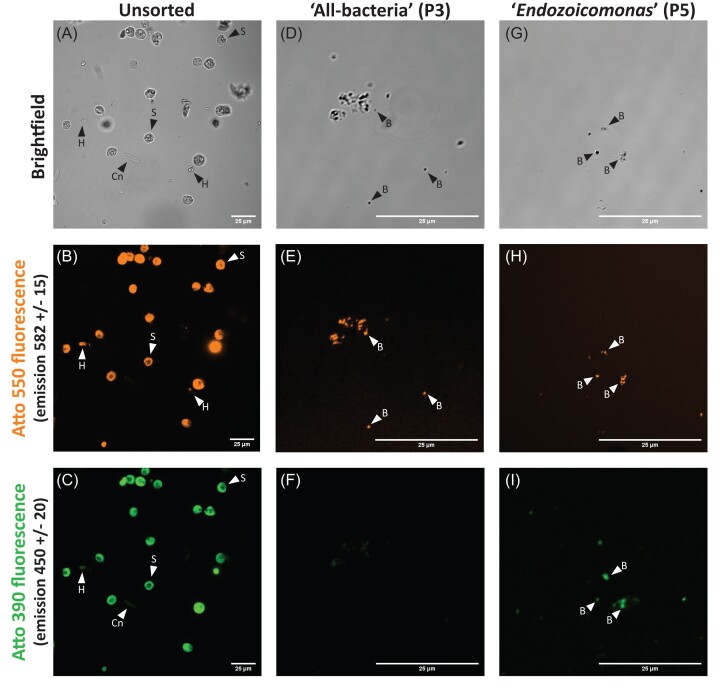
For imaging, sorted cells were concentrated to a final volume of 30 µl by centrifugation at 4°C (5 000 × g; 5 min), removal of supernatant, and resuspension in MQ water. A volume of 10 µl of concentrated cell suspension was placed onto 18 well flat µ-slides coated with poly-L-lysine (Ibidi). Samples (unsorted, ‘all-bacteria’, and ‘Endozoicomonas’ populations) were visualized on a Nikon A1R confocal laser scanning microscope at the Biological Optical Microscopy Platform (UoM) with channels for brightfield, 561 nm excitation, and 405 nm excitation.
Metagenomics preparation and data processing
Cells were pooled by genotype for each of the sorted populations by combining 500 cells from each replicate, resulting in 10 samples. Pooled populations were subjected to multiple displacement amplification (MDA) using a REPLI-g Single Cell Kit (QIAGEN) following manufacturer instructions. Following MDA at 30°C for 8 h, the DNA polymerase was inactivated, and amplified DNA was stored at −20°C. Each sample was quality checked prior to library preparation. Samples were sequenced across two lanes of a NovaSeq 6000 SP 2 × 150 bp flowcell (Illumina, San Diego, CA) at the Ramaciotti Centre for Genomics (UNSW Sydney, Australia).
All data analysis was completed on the Melbourne Research Cloud on a virtual machine with 36vCPUs, 1.5 TB RAM, and 8 TB volume storage, following the approach of Tandon et al. (2023). Raw demultiplexed fastq files were transferred to the virtual machine; data from all samples were merged by read direction and sorted population (‘all-bacteria’ or ‘Endozoicomonas’) following a co-assembly approach. Co-assembling samples together has the advantage of creating one single reliable baseline on which all samples can be easily compared. Paired-end reads were quality checked using FASTQC v0.11.5 (https://www.bioinformatics.babraham.ac.uk/projects/fastqc/). Reads were trimmed with trimmomatic v0.36 (Bolger et al. 2014) with the following parameters: CROP:145, LEADING:30, HEADCROP:10, MINLEN:120. Trimmed reads were mapped to the A. loripes draft genome (Salazar et al. 2022) using bowtie2 v2.4.2 with default settings (Langmead and Salzberg 2012, Langmead et al. 2019). Unmapped paired-end reads were extracted using samtools v1.16.1 (Li et al. 2009). Assembly was performed using MegaHIT v1.2.9 (Li et al. 2015) with kmers 21,33,55,77,99 and a minimum contig length of 2 000. Metaquast v5.0.2 (Mikheenko et al. 2016) was used to extract summary data. Reads were aligned to the contigs with bbwrap (sourceforge.net/projects/bbmap/) to calculate contig coverage using default settings. Contigs were assigned taxonomy using the NCBI non-redundant (NR) database and genome taxonomy database (GTDB) with CAT v5.2.3 (von Meijenfeldt et al. 2019) which uses prodigal v2.6.3 (Hyatt et al. 2010) and DIAMOND v2.0.6 (Buchfink et al. 2015).
Host-filtered microbial reads underwent classification against bacterial genomes using Kraken 2 (Wood et al. 2019, Lu et al. 2022). The classification report was then used by Bracken (Lu et al. 2017) for species abundance estimation, which provides estimated reads per species in the sample. Bracken output files were then passed to KrakenTools (Lu et al. 2022) and Pavian (Breitwieser and Salzberg 2020) for visualization of the data via Krona plots.
Results and Discussion
HCR-FISH + FACS
Traditional in-solution FISH (Hugenholtz et al. 2001) was used in preliminary trials during the development of this method. However, we were unable to sort pure labelled bacteria from some autofluorescent coral cells as evidenced by the presence of mixed populations, observed by confocal laser scanning microscopy post FACS. To combat this issue, we successfully employed HCR-FISH (Yamaguchi et al. 2015a,b, Grieb et al. 2020) to label and sort two populations of bacteria in coral tissue homogenates from A. loripes. This study represents the first application of HCR-FISH in-solution for the enrichment of bacteria from holobiont samples, in this case corals. To date, HCR-FISH has been used to overcome high autofluorescence to visualize bacteria in tissue sections in one coral study (Wada et al. 2022), but it has also been applied to anemones (Goffredi et al. 2021) and the bobtail squid (Nikolakakis et al. 2015, Moriano-Gutierrez et al. 2019). CARD-FISH has been used on histology sections to better resolve bacteria within autofluorescent shallow (Chiu et al. 2012, Neave et al. 2016) and deep-water (Thompson and Gutierrez 2021) coral tissues, but this approach requires reagents that damage DNA (Keller and Pollard 1977). CARD-FISH would therefore not be suitable for post-labelling metagenomic applications.
Previous FACS work in corals have prepared cell suspensions by mechanical disruption after incubation in calcium free media (Rosental et al. 2017, Levy et al. 2021). Here, the dissociation of cells was accomplished using enzymatic tools rather than mechanical approaches to fully dissociate cells and reduce bacterial contamination. Future applications of this method should consider quantifying bacterial load via quantitative or digital PCR to compare the efficiency of a mechanical versus enzymatic approach. This step could be improved further by visualizing cells after dissociation to compare methods. Sorting of FISH-labelled bacterial cells has previously been done using standard FISH on mixed bacterial cultures (Wallner et al. 1997), sludge from a bioreactor (Miyauchi et al. 2007), or marine sediment (Kalyuzhnaya et al. 2006), CARD-FISH on seawater samples (Sekar et al. 2004), or HCR-FISH on marine phytoplankton samples (Grieb et al. 2020). These studies have sequenced PCR products of specific genes like the 16S rRNA gene from sorted cells. Whole genome sequencing has been attempted from FISH labelled and sorted cells, but the recovered genomes suffered from low completeness (Podar et al. 2007, Yilmaz et al. 2010).
Using physical parameter properties FSC and SSC, and fluorescent probes, we established conditions to differentiate bacteria from non-bacterial particles and to distinguish and separate ‘Endozoicomonas’ (dual labelled) and ‘all-bacteria’ (labelled with EUBMix338 only) populations (Fig. 2; Fig. S1). FACS is a highly sensitive technique for detecting and measuring fluorescence signals from individual cells or particles with high precision and resolution. So, while the sorted populations were visually free of host cells and debris (Fig. 3), the sensitivity of confocal microscopy may be limited compared to FACS due to factors such as background noise and detection efficiency. Because coral tissue is known to exhibit non-specific binding of the EUBMix338 probe (Wada et al. 2016), we also trialled HCR-FISH+FACS with a nonsense probe, NONEUB338, which has a nucleotide sequence complementary to the nucleotide sequence of probe EUBMix338 (Christensen et al. 1999). These trials were inconclusive because the NONEUB338 initiator sequence can bind to the 16S rRNA gene in the bacterial DNA and, with HCR-FISH amplification, the signal is sufficient for FACS detection. Future studies should consider ways to address non-specific binding with HCR-FISH+FACS, such with the addition of a blocking reagent (Yamaguchi et al. 2015a).
Figure 3.
A labelled A. loripes sample (colony Al13) prior to sorting with FACS (A–C) or sorted cell populations that were single (‘all-bacteria’, D–F), or dual (‘Endozoicomonas’, G–I) labelled. Each sample was visualized on a Nikon A1R confocal laser scanning microscope with channels for brightfield (A, D, G), 561 nm excitation (B, E, H), and 405 nm excitation (C, F, I). S=Symbiodiniaceae, Cn=host cnidocyst, H=uncharacterised host cell, B=bacteria. All scale bars are 25 µm.
Metagenomics
Merging all data for the ‘Endozoicomonas’ and ‘all-bacteria’ populations resulted in 326 M and 307 M read pairs, with 313 M and 297 M read pairs remaining after quality filter and trimming by trimmomatic, respectively. Of these, only 0.68% of reads in the ‘Endozoicomonas’ and 0.01% in the single-labelled ‘all-bacteria’ population aligned to the reference host A. loripes genome (Salazar et al. 2022). Of the total reads, 169.3 M and 181.5 M from the ‘Endozoicomonas’ and ‘all-bacteria’ populations, respectively, were used to assemble contigs.
A total of 4785 and 4594 contigs were assembled from the ‘all-bacteria’ and ‘Endozoicomonas’ populations, respectively (Table 3). For the ‘all-bacteria’ contigs, 822 were taxonomically identified as eukaryotes (12.0% reads), 2033 contigs were unidentified (18.1% reads), and 1930 contigs were identified as bacteria (69.8% reads). Of those bacterial contigs, the most abundant families were Endozoicomonadaceae (584 contigs, 9.1% reads), Sporolactobacillaceae (160 contigs, 1.1% reads), Peptostreptococcaceae (74 contigs, 1.9% reads), and Vibrionaceae (36 contigs, 0.2% reads). However, the contigs were generally short (Endozoicomonadaceae mean±SD 5 459±3790 bp), with highly variable fold coverage (Endozoicomonadaceae ranges 5–37 744x coverage, mean±SD 1188±3658x). There were only nine symbiodiniacean and four cnidarian contigs, highlighting the success of the sorting in enriching samples for bacteria. For the ‘Endozoicomonas’ contigs, 196 were identified as cnidarian (0.4% reads), three were apicomplexan (0.007% reads), and 18 were Symbiodiniaceae (0.1% reads). Of the remaining contigs, 890 were unidentified (12.0% reads), one contig (0.1% reads) was identified as a virus, and 3 055 contigs were identified as bacteria (75.4% reads; Table 3). Of the bacterial contigs, by far the most abundant family was Endozoicomonadaceae with 1298 contigs (47.8% reads). These contigs averaged 7705±7346 bp (mean±SD), had 75 081–5x fold coverage (mean±SD 2538 ± 5429x) and total length of 10 Mbp.
Table 3.
Stats from metagenomic data analysis for the ‘Endozoicomonas’ and ‘all-bacteria’ populations.
| Sorted population | Raw reads (M) | Reads after trimmomatic (M) | Read pairs after removing A. loripes (M) | Reads used to assemble contigs (M) | Contigs | Total contig length Mbp | Max/Avg contig length (bp) | N50 | Avg. fold coverage | #contigs no hit—read pairs (M) | #contigs euk—read pairs (M) | #contigs bacteria—read pairs (M) | # of Endozoicomonadaceae contigs—read pairs (M) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ‘Endozoicomonas’ (P5) | 326 | 313 | 311 | 169.3 | 4 594 | 27.4 | 165 029/5 972 | 7 643 | 1 434 | 890–20.3 | 648–21.1 (18 Symbiodiniaceae; 196 Cnidarian) | 3 055–127.6 | 1 298–81.0 |
| ‘All-bacteria’ (P3) | 307 | 297 | 297 | 181.5 | 4 785 | 24.9 | 102 783/5 207 | 6 059 | 1 100 | 2 033–32.9 | 822–21.9 (9 Symbiodiniaceae; 4 Cnidarian) | 1 930–126.7 | 584–16.6 |
While Endozoicomonadaceae reads were still present in the ‘all-bacteria’ population, they were ∼5-fold less abundant than in the ‘Endozoicomonas’ population (9.1% reads in ‘all-bacteria’ versus 47.85% reads in ‘Endozoicomonas’; Supp. File 2), highlighting the success of the dual-labelling approach. Because Endozoicomonas spp. are highly abundant in A. loripes (Damjanovic et al. 2020), by concentrating this taxon in one population of sorted cells we hoped to provide greater read depth of rare or novel taxa. This is apparent in the ‘all-bacteria’ population, where 52% of the bacterial reads (Supp. File 2) were unidentified, suggesting that they represent novel environmental microbes not yet present in the GTDB reference database. This is compared to only 22% of bacterial reads assigned as no support in the ’Endozoicomonas’ population (Supp. File 2).
The low level (<1%) of coral and algal symbiont contamination in the metagenomic sequence data for our coral tissue samples is unique. Previous work to enrich prokaryotes from coral samples prior to metagenomic sequencing (Table 4) have applied percoll gradient fractionation (Wegley et al. 2007, Dinsdale et al. 2008, Vega Thurber et al. 2009, Littman et al. 2011), differential centrifugation (Keller-Costa et al. 2021, Keller-Costa et al. 2022), or sequential filtration (Robbins et al. 2019). These approaches however have been unsuccessful in providing researchers with metagenomic data dominated by bacterial reads, in part because percoll fractionation to enrich for bacteria will also capture host mitochondria (Wegley et al. 2007), and residual host DNA can contaminate centrifugation and filtration strategies. When coral tissue has been sequenced more recently without enrichment for prokaryotes, reads from the eukaryotic populations accounted for over 90% of the reads in most cases (Roach et al. 2020, Rosales et al. 2022). Our enrichment method implementing HCR-FISH prior to FACS is the first to average >70% bacterial reads from coral tissue samples.
Table 4.
Details from existing stony coral metagenomics work where the primary sequencing target was coral-associated microbes. *The % of total reads that were classified as bacterial from the authors chosen reference database†. Where studies collected metagenomes from corals and other substrates (i.e. seawater, algae), only the data from the corals is included.
| Coral Species | Geographic location | Target | Enrichment method | Classified bacterial sequences* (% of total reads) | Classification database† | Sequencing Platform | Reference |
|---|---|---|---|---|---|---|---|
| Porites asteroides | Caribbean | All microbes | Percoll fractionation | 1.5% | SEED | 454 Pyrosequencing | (Wegley et al. 2007) |
| Porites compressa | Hawaii | All microbes | Percoll fractionation | 0.6–1.0% | SEED | 454 Pyrosequencing | (Dinsdale et al. 2008, Vega Thurber et al. 2009) |
| Acropora millepora | Magnetic Island, GBR | All microbes | Percoll fractionation | 0.2–0.9% | SEED | 454 Pyrosequencing | (Littman et al. 2011) |
| Platygyra carnosa | Lamma Island and Crescent Bay, South China Sea | Bacteria | Percoll fractionation | 0–40% | NCBI NR | Illumina HiSeq | (Cai et al. 2017) |
| Porites lutea | Orpheus Island, GBR | Bacteria and archaea | Sequential filtration | 0.2–15% | Greengenes/GTDB | Illumina HiSeq2500 (2×150 bp) | (Robbins et al. 2019) |
| Pseudodiploria strigosa Orbicella faveolata | Curaçao, Caribbean | All microbes | NA | 0.93% | SEED | Illumina MiSeq (2×300 bp) | (Roach et al. 2020) |
| Acropora tenuis Goniastrea minuta Pocillopora verrucosa Pocillopora meandrina | Xiane Reef, South China Sea | All microbes | NA | <20% in healthy corals; 33–79% in bleached corals | NCBI NR | Illumina HiSeq (2×150 bp) | (Sun et al. 2020) |
| Pseudodiploria strigosa | Bermuda | Bacteria in coral surface mucus layer (SML) | Modified syringe used to collect SML only | 50.2% average | MG-RAST (Refseq/SEED) | Illumina MiSeq | (Lima et al. 2022) |
| Stephanocoenia intersepta, Diploria labyrinthiformis, Dichocoenia stokesii, and Meandrina meandrites | Looe Key and East Washerwoman Reef, Florida Keys | All microbes in tissue and mucus | NA | 19.2% average (skewed by one sample); ranged 0.9–67.3% | GTDB | Illumina HiSeq 4000 | (Rosales et al. 2022) |
| Porites lutea Goniastrea edwardsi | Abu Shoosha Reef, Red Sea | Skeleton bacteria | Fragmentation to remove tissue | Not provided | GTDB | Illumina HiSeq 4000 (2×151 bp) | (Cardenas et al. 2022) |
| Porites lutea Isopora palifera | Heron Island, GBR | Skeleton bacteria and archaea | Coral tissue was removed from the fragments using a Waterpik and sterile seawater; the remaining skeletons were snap frozen. | Not provided | GTDB | DNBSeq | (Tandon et al. 2023) |
| 270 samples from Pocillopora, Porites, and Millepora | Tara Pacific expedition | All microbes | NA | Not provided | Centrifuge v1.0.3, NCBI NR | Illumina NovaSeq6000 or HiSeq4000 | (Belser et al. 2023, Hochart et al. 2023, Lombard et al. 2023) |
| Acropora loripes | Davies and Backnumbers Reef, GBR | Tissue Bacteria | HCR-FISH + FACS | 72.6% average | GTDB | Illumina NovaSeq6000 SP (2×150 bp) | This study |
When completing metagenomic sequencing on compartments of the coral holobiont that do not include tissue, contamination by host or other eukaryotic reads is less prominent. Tandon et al. (2023: effectively assembled 393 high-quality metagenome assembled genomes (MAGs) from coral skeleton fragments by sequencing samples on individual lanes; in only two samples were there >45% host reads. Cardenas et al. (2022) were able to achieve metagenome sequences that were dominated (∼75%) by bacterial reads when working with skeletal material. However, to compare bacterial communities between skeleton and tissue they had to use 16S rRNA gene metabarcoding. Metagenomics of the surface mucus layer (SML) of a Caribbean coral species contained ∼50% of reads that were identified as bacteria with no additional enrichment required (Lima et al. 2022).
MDA (Dean et al. 2002) was used in this study to obtain sufficient DNA from sorted bacteria. However, it has drawbacks such as amplification bias (Ahsanuddin et al. 2017), poor uniformity, errors and artifacts, low genome coverage, inability to address all variant classes, low accuracy, poor reproducibility, and/or complex protocols that are difficult to automate or scale. Reads generated in this study were heavily skewed toward some regions of bacterial genomes resulting in orders of magnitude differences in coverage and an inability to generate MAGs, which is likely a result of uneven amplification during the 8 hrs of MDA.
Ideally, researchers should aim to collect enough cells so that amplification is not necessary. In these cases, DNA can be extracted with low biomass-input methods (Bramucci et al. 2021). When this is not possible, a potential alternative to MDA is primary template-directed amplification (PTA) (Gonzalez-Pena et al. 2021). PTA is an isothermal whole genome amplification method that reproducibly captures near-complete genomes of single cells while suppressing the formation of experimental artifacts such as chimeric molecules and non-specific priming (Telenius et al. 1992). PTA may be performed directly on DNA from single cells (collected by FACS, microfluidic or other methods), multiple cells, or ultra-low inputs of DNA (>4 pg– 10 ng). Future applications of this method to enrich complex communities for bacteria prior to metagenomics should use caution with MDA and amplify for the shortest duration of time required to get sufficient DNA for sequencing.
Conclusion
Our findings show that HCR-FISH + FACS is a substantially improved method to obtain host-associated bacteria for metagenome sequencing, where standard metagenomic techniques do not work for low-abundant bacteria due to noise from more common species (typically the host species).Our method makes the analysis of uncharacterized microbes simpler and more accessible, and provides researchers with an enhanced platform to address the grand challenge of deciphering the functions of host-associated bacteria in symbiosis. As our method can be implemented in holobionts other than coral, we believe that this innovative approach holds promise for advancing the field of microbial ecology.
Supplementary Material
Acknowledgments
The corals used in this study were originally collected from the sea countries of the Bindal and Wulgurukaba People. We acknowledge their contributions to this research not only by way of the coral samples, but also through Indigenous Knowledge and custodianship of the land and sea country on which we work. We are grateful to A/Prof. Nikki Traylor Knowles for advice on coral cell dissociation and sorting. We would like to thank Gabriela Segal Wasserman from the Biological Optical Microscopy Platform (The University of Melbourne) for assistance with imaging.
Contributor Information
Ashley M Dungan, School of BioSciences, University of Melbourne, Melbourne, VIC 3010, Australia.
Kshitij Tandon, School of BioSciences, University of Melbourne, Melbourne, VIC 3010, Australia.
Vanta Jameson, Melbourne Cytometry Platform, Department of Microbiology and Immunology, University of Melbourne, Melbourne, VIC 3010, Australia.
Cecilie Ravn Gotze, School of BioSciences, University of Melbourne, Melbourne, VIC 3010, Australia; Reef Recovery, Restoration and Adaptation Program, Australian Institute of Marine Science, Townsville, QLD 4810, Australia.
Linda L Blackall, School of BioSciences, University of Melbourne, Melbourne, VIC 3010, Australia.
Madeleine J H van Oppen, School of BioSciences, University of Melbourne, Melbourne, VIC 3010, Australia; Reef Recovery, Restoration and Adaptation Program, Australian Institute of Marine Science, Townsville, QLD 4810, Australia.
Author contributions
Ashley M. Dungan (Conceptualization, Formal analysis, Methodology, Writing – original draft), Kshitij Tandon (Formal analysis, Writing – reviewing and editing), Vanta Jameson (Formal analysis, Resources, Writing – reviewing and editing), Cecilie Ravn Gotze (Methodology, Writing – reviewing and editing), Linda L. Blackall (Conceptualization, Funding acquisition, Supervision, Writing – reviewing and editing), and Madeleine J. H. van Oppen (Conceptualization, Funding acquisition, Supervision, Writing – reviewing and editing)
Conflict of interest
The authors declare no conflicts of interest.
Funding
This research was funded by the Australian Research Council (ARC) (FL180100036 to MJHvO; DP210100630 to MJHvO and LLB). KT is supported by ARC DP200101613 (to Verbruggen, LLB, Medina, and Kuhl). This research was supported by The University of Melbourne's Research Computing Services and the Petascale Campus Initiative. Funding bodies had no influence in the design of the study, the collection, analysis, and interpretation of data, or in writing the manuscript.
Data Availability
The data sets generated and analysed during the current study are available under NCBI BioProject ID PRJNA1036684.
References
- Ahsanuddin S, Afshinnekoo E, Gandara J et al. Assessment of REPLI-g multiple displacement whole genome amplification (WGA) techniques for metagenomic applications. J Biomol Tech. 2017;28:46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ainsworth TD, Fine M, Blackall LL et al. Fluorescence in situ hybridization and spectral imaging of coral-associated bacterial communities. Appl Environ Microb. 2006;72:3016–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ainsworth TD, Krause L, Bridge T et al. The coral core microbiome identifies rare bacterial taxa as ubiquitous endosymbionts. ISME J. 2015;9:2261–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alieva NO, Konzen KA, Field SF et al. Diversity and evolution of coral fluorescent proteins. PLoS One. 2008;3:e2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apprill A, Marlow HQ, Martindale MQ et al. Specificity of associations between bacteria and the coral Pocillopora meandrina during early development. Appl Environ Microb. 2012;78:7467–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer T, Neave MJ, Alsheikh-Hussain A et al. The microbiome of the Red Sea coral Stylophora pistillata is dominated by tissue-associated endozoicomonas bacteria. Appl Environ Microb. 2013;79:4759–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belser C, Poulain J, Labadie K et al. Integrative omics framework for characterization of coral reef ecosystems from the Tara Pacific expedition. Sci Data. 2023;10:326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackall LL, Wilson B, van Oppen MJ. Coral-the world's most diverse symbiotic ecosystem. Mol Ecol. 2015;24:5330–47. [DOI] [PubMed] [Google Scholar]
- Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne DG, Morrow KM, Webster NS. Insights into the coral microbiome: underpinning the health and resilience of reef ecosystems. Annu Rev Microbiol. 2016;70:314–40. [DOI] [PubMed] [Google Scholar]
- Bramucci AR, Focardi A, Rinke C et al. Microvolume DNA extraction methods for microscale amplicon and metagenomic studies. ISME Commu. 2021;1. 10.1038/s43705-021-00079-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitwieser FP, Salzberg SL. Pavian: interactive analysis of metagenomics data for microbiome studies and pathogen identification. Bioinformatics. 2020;36:1303–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchfink B, Xie C, Huson DH. Fast and sensitive protein alignment using DIAMOND. Nat Methods. 2015;12:59–60. [DOI] [PubMed] [Google Scholar]
- Cai L, Zhou G, Tian RM et al. Metagenomic analysis reveals a green sulfur bacterium as a potential coral symbiont. Sci Rep. 2017;7:9320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas A, Raina JB, Pogoreutz C et al. Greater functional diversity and redundancy of coral endolithic microbiomes align with lower coral bleaching susceptibility. ISME J. 2022;16:2406–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu HH, Mette A, Shiu JH et al. Bacterial distribution in the epidermis and mucus of the coral Euphyllia glabrescens by CARD-FISH. Zool Stud. 2012;51:1332–42. [Google Scholar]
- Choi HM, Chang JY, Trinh le A et al. Programmable in situ amplification for multiplexed imaging of mRNA expression. Nat Biotechnol. 2010;28:1208–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen H, Hansen M, Sorensen J. Counting and size classification of active soil bacteria by fluorescence in situ hybridization with an rRNA oligonucleotide probe. Appl Environ Microb. 1999;65:1753–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daims H, Brühl A, Amann R et al. The domain-specific probe EUB338 is insufficient for the detection of all bacteria: development and evaluation of a more comprehensive probe set. Syst Appl Microbiol. 1999;22:434–44. [DOI] [PubMed] [Google Scholar]
- Damjanovic K, Blackall LL, Peplow LM et al. Assessment of bacterial community composition within and among Acropora loripes colonies in the wild and in captivity. Coral Reefs. 2020;39:1245–55. [Google Scholar]
- Damjanovic K, Menéndez P, Blackall LL et al. Early life stages of a common broadcast spawning coral associate with specific bacterial communities despite lack of internalized bacteria. Microb Ecol. 2019;79:1–14. [DOI] [PubMed] [Google Scholar]
- Dean FB, Hosono S, Fang L et al. Comprehensive human genome amplification using multiple displacement amplification. P Natl Acad Sci USA. 2002;99:5261–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLong EF, Wickham GS, Pace NR. Phylogenetic stains: ribosomal RNA-based probes for the identification of single cells. Science. 1989;243:1360–3. [DOI] [PubMed] [Google Scholar]
- Dinsdale EA, Edwards RA, Hall D et al. Functional metagenomic profiling of nine biomes. Nature. 2008;452:629–32. [DOI] [PubMed] [Google Scholar]
- Domart-Coulon I, Ostrander GK. Coral Cell and Tissue Culture methods Diseases of Corals: Wiley Online Library, 2015. https://onlinelibrary.wiley.com/doi/book/10.1002/9781118828502. [Google Scholar]
- Domart-Coulon I, Tambutté S, Tambutté E et al. Short term viability of soft tissue detached from the skeleton of reef-building corals. J Exp Mar Biol Ecol. 2004;309:199–217. [Google Scholar]
- Dungan AM, Bulach D, Lin H et al. Development of a free radical scavenging bacterial consortium to mitigate oxidative stress in cnidarians. Microb Biotechnol. 2021;14:2025–40. 10.1111/1751-7915.13877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank U, Rabinowitz C, Rinkevich B. In vitro establishment of continuous cell cultures and cell lines from ten colonial cnidarians. Mar Biol. 1994;120:491–9. [Google Scholar]
- Gates RD, Baghdasarian G, Muscatine L. Temperature stress causes host cell detachment in symbiotic cnidarians: implications for coral bleaching. Biol Bull. 1992;182:324–32. [DOI] [PubMed] [Google Scholar]
- Gates RD, Muscatine L. Three methods for isolating viable anthozoan endoderm cells with their intracellular symbiotic dinoflagellates. Coral Reefs. 1992;11:143–5. [Google Scholar]
- Goffredi SK, Motooka C, Fike DA et al. Mixotrophic chemosynthesis in a deep-sea anemone from hydrothermal vents in the Pescadero Basin, Gulf of California. BMC Biol. 2021;19:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Pena V, Natarajan S, Xia Y et al. Accurate genomic variant detection in single cells with primary template-directed amplification. P Natl Acad Sci USA. 2021;118:e2024176118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieb A, Bowers RM, Oggerin M et al. A pipeline for targeted metagenomics of environmental bacteria. Microbiome. 2020;8:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helman Y, Natale F, Sherrell RM et al. Extracellular matrix production and calcium carbonate precipitation by coral cells in vitro. P Natl Acad Sci USA. 2008;105:54–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochart C, Paoli L, Ruscheweyh HJ et al. Ecology of endozoicomonadaceae in three coral genera across the Pacific Ocean. Nat Commun. 2023;14:3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugenholtz P, Tyson GW, Blackall LL. Design and Evaluation of 16S rRNA-Targeted Oligonucleotide Probes for Fluorescence in Situ Hybridization. Methods in Molecular Biology. Vol. 176, Totowa, NJ: Humana Press, 2001. [DOI] [PubMed] [Google Scholar]
- Hyatt D, Chen GL, Locascio PF et al. Prodigal: prokaryotic gene recognition and translation initiation site identification. BMC Bioinf. 2010;11:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalyuzhnaya MG, Zabinsky R, Bowerman S et al. Fluorescence in situ hybridization-flow cytometry-cell sorting-based method for separation and enrichment of type I and type II methanotroph populations. Appl Environ Microb. 2006;72:4293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller KM, Pollard EC. Action of hydrogen peroxide on degradation of DNA after irradiation in Escherichia coli. Int J Radiat Biol Relat Stud Phys Chem Med. 1977;31:407–13. [DOI] [PubMed] [Google Scholar]
- Keller-Costa T, Kozma L, Silva SG et al. Metagenomics-resolved genomics provides novel insights into chitin turnover, metabolic specialization, and niche partitioning in the octocoral microbiome. Microbiome. 2022;10:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller-Costa T, Lago-Leston A, Saraiva JP et al. Metagenomic insights into the taxonomy, function, and dysbiosis of prokaryotic communities in octocorals. Microbiome. 2021;9:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalesi MK, Vera-Jimenez NI, Aanen DK et al. Cell cultures from the symbiotic soft coral Sinularia flexibilis. In Vitro CellDevBiol-Animal. 2008;44:330–8. [DOI] [PubMed] [Google Scholar]
- Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods. 2012;9:357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmead B, Wilks C, Antonescu V et al. Scaling read aligners to hundreds of threads on general-purpose processors. Bioinformatics. 2019;35:421–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecointe A, Cohen S, Gèze M et al. Scleractinian coral cell proliferation is reduced in primary culture of suspended multicellular aggregates compared to polyps. Cytotechnology. 2013;65:705–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy S, Elek A, Grau-Bove X et al. A stony coral cell atlas illuminates the molecular and cellular basis of coral symbiosis, calcification, and immunity. Cell. 2021;184:2973–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Liu CM, Luo R et al. MEGAHIT: an ultra-fast single-node solution for large and complex metagenomics assembly via succinct de Bruijn graph. Bioinformatics. 2015;31:1674–6. [DOI] [PubMed] [Google Scholar]
- Li H, Handsaker B, Wysoker A et al. The sequence alignment/map format and SAMtools. Bioinformatics. 2009;25:2078–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima LFO, Alker AT, Papudeshi B et al. Coral and seawater metagenomes reveal key microbial functions to Coral health and ecosystem functioning shaped at reef scale. Microb Ecol. 2022. 10.1007/s00248-022-02094-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littman R, Willis BL, Bourne DG. Metagenomic analysis of the coral holobiont during a natural bleaching event on the Great Barrier Reef. Environ Microbiol Rep. 2011;3:651–60. [DOI] [PubMed] [Google Scholar]
- Lombard F, Bourdin G, Pesant S et al. Open science resources from the Tara Pacific expedition across coral reef and surface ocean ecosystems. Sci Data. 2023;10:324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Breitwieser FP, Thielen P et al. Bracken: estimating species abundance in metagenomics data. PeerJ Comp Sci. 2017:e104:3. [Google Scholar]
- Lu J, Rincon N, Wood DE et al. Metagenome analysis using the Kraken software suite. Nat Protoc. 2022;17:2815–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maire J, Blackall LL, van Oppen MJH. Intracellular bacterial symbionts in corals: challenges and future directions. Microorganisms. 2021;2209:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maire J, Buerger P, Chan WY et al. Effects of ocean warming on the underexplored members of the coral microbiome. Integr Comp Biol. 2022;62:1700–09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maire J, Tandon K, Collingro A et al. Colocalization and potential interactions of endozoicomonas and chlamydiae in microbial aggregates of the coral Pocillopora acuta. Sci Adv. 2023;9:eadg0773. [DOI] [PubMed] [Google Scholar]
- Marshall AT, Clode P. Effects of calcium-free and low-calcium artificial seawater on polyps of a scleractinian coral Galaxea fascicularis. Coral Reefs. 2004;23:277–80. [Google Scholar]
- McCutcheon JP. The genomics and cell biology of host-beneficial intracellular infections. Annu Rev Cell Dev Biol. 2021;37:115–42. 10.1146/annurev-cellbio-120219-024122. [DOI] [PubMed] [Google Scholar]
- McFall-Ngai M, Hadfield MG, Bosch TC et al. Animals in a bacterial world, a new imperative for the life sciences. P Natl Acad Sci USA. 2013;110:3229–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikheenko A, Saveliev V, Gurevich A. MetaQUAST: evaluation of metagenome assemblies. Bioinformatics. 2016;32:1088–90. [DOI] [PubMed] [Google Scholar]
- Miyauchi R, Oki K, Aoi Y et al. Diversity of nitrite reductase genes in “Candidatus Accumulibacter phosphatis”-dominated cultures enriched by flow-cytometric sorting. Appl Environ Microb. 2007;73:5331–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed AR, Ochsenkuhn MA, Kazlak AM et al. The coral microbiome: towards an understanding of the molecular mechanisms of coral-microbiota interactions. FEMS Microbiol Rev. 2023;47:fuad005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriano-Gutierrez S, Koch EJ, Bussan H et al. Critical symbiont signals drive both local and systemic changes in diel and developmental host gene expression. P Natl Acad Sci USA. 2019;116:7990–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller UG, Sachs JL. Engineering microbiomes to improve plant and animal health. Trends Microbiol. 2015;23:606–17. [DOI] [PubMed] [Google Scholar]
- Neave MJ, Apprill A, Ferrier-Pages C et al. Diversity and function of prevalent symbiotic marine bacteria in the genus Endozoicomonas. Appl Microbiol Biotechnol. 2016;100:8315–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolakakis K, Lehnert E, McFall-Ngai MJ et al. Use of hybridization chain reaction-fluorescent In situ hybridization to track gene expression by both partners during initiation of symbiosis. Appl Environ Microb. 2015;81:4728–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podar M, Abulencia CB, Walcher M et al. Targeted access to the genomes of low-abundance organisms in complex microbial communities. Appl Environ Microb. 2007;73:3205–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulsen LK, Ballard G, Stahl DA. Use of rRNA fluorescence in situ hybridization for measuring the activity of single cells in young and established biofilms. Appl Environ Microb. 1993;59:1354–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes-Bermudez A, Hidaka M, Mikheyev A. Transcription profiling of cultured Acropora digitifera adult cells reveals the existence of ancestral genome regulatory modules underlying pluripotency and cell differentiation in Cnidaria. Genome Biol Evol. 2021;13:evab008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roach TNF, Little M, Arts MGI et al. A multiomic analysis of in situ coral-turf algal interactions. P Natl Acad Sci USA. 2020;117:13588–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins SJ, Singleton CM, Chan CX et al. A genomic view of the reef-building coral Porites lutea and its microbial symbionts. Nat Microbiol. 2019;4:2090–100. [DOI] [PubMed] [Google Scholar]
- Rosales SM, Huebner LK, Clark AS et al. Bacterial metabolic potential and micro-eukaryotes enriched in stony coral tissue loss disease lesions. Front Mar Sci. 2022;8:776859. [Google Scholar]
- Rosental B, Kozhekbaeva Z, Fernhoff N et al. Coral cell separation and isolation by fluorescence-activated cell sorting (FACS). BMC Cell Biol. 2017;18:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar OR, NA P, Cui G et al. The coral acropora loripes genome reveals an alternative pathway for cysteine biosynthesis in animals. Sci Adv. 2022;8:eabq0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekar R, Fuchs BM, Amann R et al. Flow sorting of marine bacterioplankton after fluorescence in situ hybridization. Appl Environ Microb. 2004;70:6210–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sessitsch A, Wakelin S, Schloter M et al. Microbiome interconnectedness throughout environments with major consequences for healthy people and a healthy planet. Microbiol Mol Biol Rev. 2023;87:e00212–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun F, Yang H, Wang G et al. Combination analysis of metatranscriptome and metagenome reveal the composition and functional response of coral symbionts to bleaching during an El Niño event. Front Microbiol. 2020;11:448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet MJ, Bulling MT. On the importance of the microbiome and pathobiome in coral health and disease. Front Mar Sci. 2017;4:9. [Google Scholar]
- Tandon K, Ricci F, Costa J et al. Genomic view of the diversity and functional role of archaea and bacteria in the skeleton of the reef-building corals Porites lutea and Isopora palifera. GigaScience. 2023;12:giac127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telenius H, Carter NP, Bebb CE et al. Degenerate oligonucleotide-primed PCR: general amplification of target DNA by a single degenerate primer. Genomics. 1992;13:718–25. [DOI] [PubMed] [Google Scholar]
- Thompson HF, Gutierrez T. Detection of hydrocarbon-degrading bacteria on deepwater corals of the northeast Atlantic using CARD-FISH. J Microbiol Methods. 2021;187:106277. [DOI] [PubMed] [Google Scholar]
- van Oppen MJH, Blackall LL. Coral microbiome dynamics, functions and design in a changing world. Nat Rev Micro. 2019;17:557–67. [DOI] [PubMed] [Google Scholar]
- Vega Thurber R, Willner-Hall D, Rodriguez-Mueller B et al. Metagenomic analysis of stressed coral holobionts. Environ Microbiol. 2009;11:2148–63. [DOI] [PubMed] [Google Scholar]
- von Meijenfeldt FAB, Arkhipova K, Cambuy DD et al. Robust taxonomic classification of uncharted microbial sequences and bins with CAT and BAT. Genome Biol. 2019;20:217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada N, Hsu MT, Tandon K et al. High-resolution spatial and genomic characterization of coral-associated microbial aggregates in the coral Stylophora pistillata. Sci Adv. 2022;8:eabo2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada N, Pollock FJ, Willis BL et al. In situ visualization of bacterial populations in coral tissues: pitfalls and solutions. PeerJ. 2016;4:e2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallner G, Fuchs B, Spring S et al. Flow sorting of microorganisms for molecular analysis. Appl Environ Microb. 1997;63:4223–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegley L, Edwards R, Rodriguez-Brito B et al. Metagenomic analysis of the microbial community associated with the coral Porites astreoides. Environ Microbiol. 2007;9:2707–19. [DOI] [PubMed] [Google Scholar]
- Wood DE, Lu J, Langmead B. Improved metagenomic analysis with Kraken 2. Genome Biol. 2019;20:257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T, Kawakami S, Hatamoto M et al. In situ DNA-hybridization chain reaction (HCR): a facilitated in situ HCR system for the detection of environmental microorganisms. Environ Microbiol. 2015a;17:2532–41. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T, Fuchs BM, Amann R et al. Rapid and sensitive identification of marine bacteria by an improved in situ DNA hybridization chain reaction (quickHCR-FISH). Syst Appl Microbiol. 2015b;38:400–5. [DOI] [PubMed] [Google Scholar]
- Yilmaz S, Haroon MF, Rabkin BA et al. Fixation-free fluorescence in situ hybridization for targeted enrichment of microbial populations. ISME J. 2010;4:1352–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data sets generated and analysed during the current study are available under NCBI BioProject ID PRJNA1036684.