Key Points
Question
Is concurrent tissue-based and circulating tumor DNA (ctDNA)–based genomic profiling associated with increased discovery of targetable National Comprehensive Cancer Network guideline–based variants vs tissue-based testing alone?
Findings
In this cohort study of 3209 patients undergoing concurrent testing across 4 cancer types who received both tissue-based and ctDNA genomic profiling results, 45.1% had a guideline-based variant detected. Of these patients, 9.3% had a clinically actionable variant detected by ctDNA profiling that was not detected by solid-tissue testing, and 24.2% had a variant detected by solid-tissue testing but not by ctDNA profiling; for patients with breast cancer with actionable variants, 20.2% had a unique, guideline-based variant detected by ctDNA profiling; most (55.0%) of these unique ctDNA variants were in the ESR1 gene.
Meaning
This study suggests that concurrent ctDNA–based and tissue-based genomic profiling identified more patients with targetable, guideline-based variants than would have been discovered by tissue profiling alone, with a higher detection rate among patients with breast cancer.
Abstract
Importance
Tissue-based next-generation sequencing (NGS) of solid tumors is the criterion standard for identifying somatic mutations that can be treated with National Comprehensive Cancer Network guideline–recommended targeted therapies. Sequencing of circulating tumor DNA (ctDNA) can also identify tumor-derived mutations, and there is increasing clinical evidence supporting ctDNA testing as a diagnostic tool. The clinical value of concurrent tissue and ctDNA profiling has not been formally assessed in a large, multicancer cohort from heterogeneous clinical settings.
Objective
To evaluate whether patients concurrently tested with both tissue and ctDNA NGS testing have a higher rate of detection of guideline-based targeted mutations compared with tissue testing alone.
Design, Setting, and Participants
This cohort study comprised 3209 patients who underwent sequencing between May 2020, and December 2022, within the deidentified, Tempus multimodal database, consisting of linked molecular and clinical data. Included patients had stage IV disease (non–small cell lung cancer, breast cancer, prostate cancer, or colorectal cancer) with sufficient tissue and blood sample quantities for analysis.
Exposures
Received results from tissue and plasma ctDNA genomic profiling, with biopsies and blood draws occurring within 30 days of one another.
Main Outcomes and Measures
Detection rates of guideline-based variants found uniquely by ctDNA and tissue profiling.
Results
The cohort of 3209 patients (median age at diagnosis of stage IV disease, 65.3 years [2.5%-97.5% range, 43.3-83.3 years]) who underwent concurrent tissue and ctDNA testing included 1693 women (52.8%). Overall, 1448 patients (45.1%) had a guideline-based variant detected. Of these patients, 9.3% (135 of 1448) had variants uniquely detected by ctDNA profiling, and 24.2% (351 of 1448) had variants uniquely detected by solid-tissue testing. Although largely concordant with one another, differences in the identification of actionable variants by either assay varied according to cancer type, gene, variant, and ctDNA burden. Of 352 patients with breast cancer, 20.2% (71 of 352) with actionable variants had unique findings in ctDNA profiling results. Most of these unique, actionable variants (55.0% [55 of 100]) were found in ESR1, resulting in a 24.7% increase (23 of 93) in the identification of patients harboring an ESR1 mutation relative to tissue testing alone.
Conclusions and Relevance
This study suggests that unique actionable biomarkers are detected by both concurrent tissue and ctDNA testing, with higher ctDNA identification among patients with breast cancer. Integration of concurrent NGS testing into the routine management of advanced solid cancers may expand the delivery of molecularly guided therapy and improve patient outcomes.
This cohort study evaluates whether patients concurrently tested with both tissue and circulating tumor DNA next-generation sequencing testing have a higher rate of detection of guideline-based targeted mutations compared with tissue testing alone.
Introduction
Comprehensive genomic profiling via next-generation sequencing (NGS) of tumor tissue is recommended in clinical guidelines from the National Comprehensive Cancer Network (NCCN) and others.1,2,3,4,5,6,7 The detection of actionable gene variants from tumor profiling has the potential to identify targeted treatment options that improve clinical outcomes compared with nontargeted therapies.8,9,10,11 Genomic profiling of circulating tumor DNA (ctDNA) via a liquid (most commonly, plasma) biopsy may also be used to identify tumor-derived actionable mutations,12,13,14,15 but guidelines typically recommend this option in limited cases.3 However, the clinical use of ctDNA sequencing is evolving, with recognition being given to its ease of use, rapid turnaround time, low failure rate,16,17 ability to detect resistance mutations,18,19,20 and its potential use in monitoring treatment response to immunotherapy.16,21,22,23,24
Although tissue-based NGS testing remains the standard of care for most cancer indications, there are potential limitations to performing tissue-only genomic profiling. First, tissue-based sequencing requires an invasive procedure for metastatic lesions, and some patients are not medically fit for required biopsies.25 Second, a substantial fraction of tissue-based testing orders do not provide results due to insufficient or low-quality material.26,27 Third, tissue-based sequencing may not capture molecular heterogeneity that is present within tumors or across multiple lesions; this heterogeneity is particularly important with regard to the detection of subclonal resistance mutations.24,28,29
Used alone, ctDNA-based testing may produce false-negative results for low-shedding tumors as well as false-positive results from clonal hematopoiesis of indeterminate potential (CHIP).14,30,31,32 Due to the potential for false-negative results, NCCN lung cancer guidelines recommend reflex tissue-based sequencing if actionable variants are not identified in ctDNA results.2 Moreover, published guidelines elaborating standards for analytical performance characteristics of ctDNA have not been well established, in contrast to tissue-based testing. Despite these limitations, ctDNA testing is rapid, noninvasive, and suitable for detecting variants arising from molecular heterogeneity and clonal evolution of resistance mutations.14,33
Concurrent genomic sequencing of both ctDNA and tissue—hereafter, concurrent testing—may provide advantages in timely clinical treatment decision-making for patients with advanced cancer compared with the use of single-modality testing.15,25,27,34,35,36,37,38 At present, there is evolving evidence that concurrent testing may provide benefit to patients as acknowledged by NCCN non–small cell lung cancer (NSCLC) guidelines that support complementary (concurrent) testing.2 In addition, the importance of monitoring ESR1 (OMIM 133430) resistance variants in response to aromatase inhibitor therapy among patients with breast cancer has been recently established and highlights the possible benefits of ctDNA testing for this population.21 However, there is a paucity of large clinical validation studies on the possible benefit to patients of concurrent testing—due to increased detection of clinically actionable variants with targetable therapies—across multiple solid-tumor cancer types.15,25 We conducted a clinical validation study of a multicancer cohort across heterogeneous clinical settings to assess whether patients who undergo concurrent testing have a higher rate of detection of clinically actionable variants based on NCCN guidelines compared with those receiving single-modality testing.
Methods
Data Ethics Statement
Tempus Labs Inc was granted an exemption by the Advarra Institutional Review Board permitting the use of deidentified clinical, molecular, and multimodal data to derive or capture results, insights, or discoveries (eMethods in Supplement 1). The Advarra Institutional Review Board deemed the study exempt from approval and the need for informed consent owing to the use of deidentified data. This study followed the Strengthening the Reporting Studies of Observational Studies in Epidemiology (STROBE) reporting guideline.
Cohort Description
The complete cohort consisted of 3209 deidentified patient records with primary diagnoses of NSCLC (n = 1302), breast cancer (n = 660), prostate cancer (n = 324), or colorectal cancer (CRC; n = 923), and metastatic disease. Patients underwent sequencing between May 2020 and December 2022, and had liquid (Tempus xF) and solid-tumor (Tempus xT, including matched normal) NGS testing, with biopsies collected within 30 days of each other.33,39,40 eTable 1 in Supplement 1 shows demographic and clinical characteristics. The subcohort consisting solely of patients who had at least 1 actionable variant identified by 1 or more tests (n = 1448) is referred to as the actionable cohort. Results are presented for either cohort at the aggregate level or split according to cancer type as indicated. Clinically actionable variants were identified using indication-matched recommendations from NCCN guidelines for all 4 cancer types included in the study (eTable 2 in Supplement 1). See eMethods in Supplement 1 for additional details on study inclusion and exclusion criteria and NGS testing.
Race and ethnicity designations were determined via abstraction from order forms and/or clinical records; these designations may originate from patient self-reports or be filled in by a health care professional (eg, nurse, physician, pathologist). We assessed race and ethnicity in our study to permit comparisons between present and future studies, as well as to better characterize the overall diversity of our cohort.
Circulating Tumor DNA Burden
Tumor shedding—hereafter referred to as ctDNA burden—was calculated as the maximum somatic variant allele frequency (VAF) after removal of potential germline variants based on the patient’s paired normal sample from peripheral blood mononuclear cells, considering all assay variants detected above the assay limit of detection. Samples with no eligible variants were defined as having a ctDNA burden of 0.
Statistical Analysis
Group differences were assessed using the Mann-Whitney test, the Kruskal-Wallis test, and the t test, as noted in Results. Differences in count distributions were assessed using the χ2 test and the Fisher exact test. Statistical analyses were performed using Python, version 3.7.10 (Python Software Foundation), with the Python package SciPy, version 1.6.2, and R, version 4.3.1 (R Project for Statistical Computing). All reported statistical tests were 2-sided and results were deemed statistically significant at P < .05. In the event of multiple hypothesis testing, Bonferroni correction was used to control type I error.
Results
Concordance of Clinically Actionable Variants Between Solid and ctDNA NGS Testing
The cohort of 3209 patients (median age at diagnosis of stage IV disease, 65.3 years [2.5%-97.5% range, 43.3-83.3 years]) who underwent concurrent tissue and ctDNA testing included 1693 women (52.8%) and 95 Asian patients (3.0%), 290 Black or African American patients (9.0%), 179 Hispanic or Latino patients (5.6%), 1539 White patients (48.0%), 97 patients of other race (any self-identified race; 3.0%), and 1009 patients of unknown race (31.4%) (eTable 1 in Supplement 1). The actionable cohort comprised 1448 patients (45.1%) who had at least 1 clinically actionable variant detected by 1 or both tests. The percentage of patients with detected actionable variants varied considerably by cancer subtype, ranging from 12.7% of patients with prostate cancer (41 of 324) to 61.2% of patients with CRC (565 of 923) (Figure 1A).
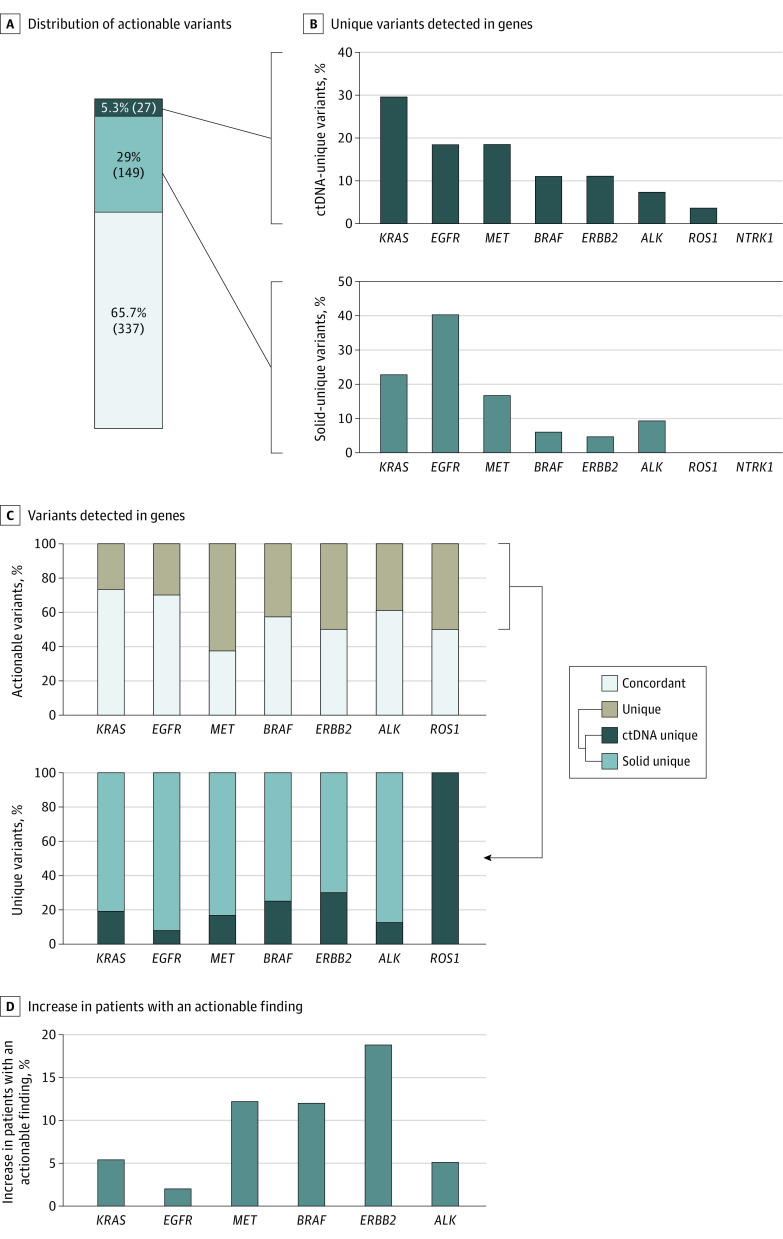
Figure 1. Percentage of Patients With Actionable Variants.
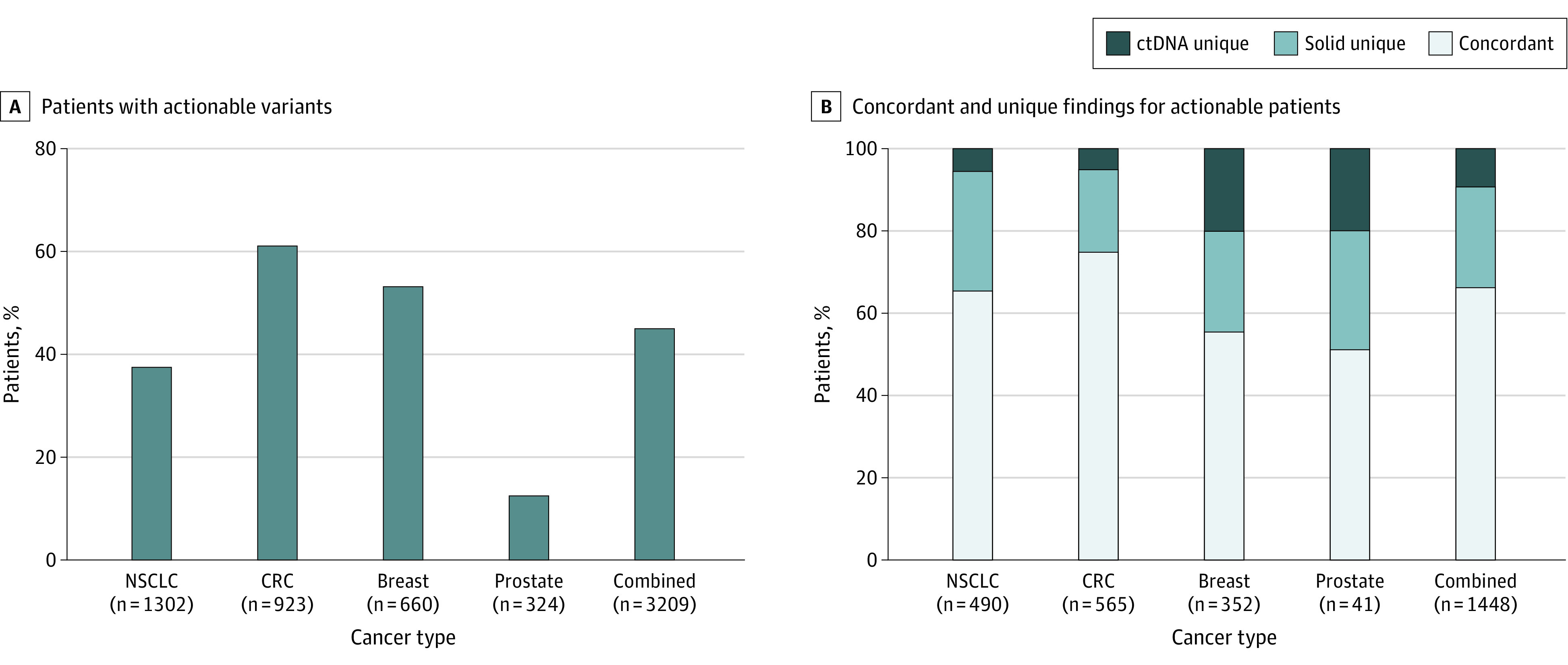
A, Percentage of patients with at least 1 actionable variant detected by 1 or both assays across individual cancer types as well as in the combined cohort. B, Patient-level findings by type: concordant refers to patients with identical findings in both assays, solid unique references patients who were not perfectly concordant across assays but where solid-tissue testing detected all unique variants, and ctDNA unique refers to all patients who had a unique actionable finding detected via ctDNA testing. CRC indicates colorectal cancer; ctDNA, circulating tumor DNA; and NSCLC, non–small cell lung cancer.
In the actionable cohort, the concordance rate between ctDNA and tissue NGS assays was 66.4% (962 of 1448). The concordance rate differed across cancer subtypes, with the highest concordance observed in CRC (75.0% [424 of 565]) and the lowest in prostate cancer (51.2% [21 of 41]) (Figure 1B). Examining ctDNA-specific variants, 9.3% of patients (135 of 1448) had at least 1 actionable variant detected uniquely by ctDNA profiling, with 5.1% of variants (29 of 565) in CRC, 5.5% of variants (27 of 490) in NSCLC, 19.5% of variants (8 of 41) in prostate cancer, and 20.2% of variants (71 of 352) in breast cancer (Figure 1B). Similarly, 24.2% of patients (351 of 1448) had at least 1 actionable variant detected uniquely by tissue profiling (with no additional variants uniquely detected in ctDNA), with 19.8% of variants (112 of 565) in CRC, 29.0% of variants (142 of 490) in NSCLC, 29.3% of variants (12 of 41) in prostate cancer, and 24.1% of variants (85 of 352) in breast cancer (Figure 1B).
Association Between Tumor Burden and Purity on Assay Variant Detection
We hypothesized that ctDNA burden may be associated with the concordance rate of detected variants between ctDNA and tissue NGS testing. The positive percentage agreement (PPA) of solid tissue–detected variants detected in ctDNA increased as a function of ctDNA burden (Figure 2A; see Methods). The PPA was 95.1% when the ctDNA burden was 10% or higher and decreased to 74.3% when all ctDNA burden levels were included. This association between PPA and tumor burden was observed for all individual cancer subtypes (eFigure 1 in Supplement 1).
Figure 2. Positive Percentage Agreement (PPA) for Thresholds of Circulating Tumor DNA (ctDNA) Burden.
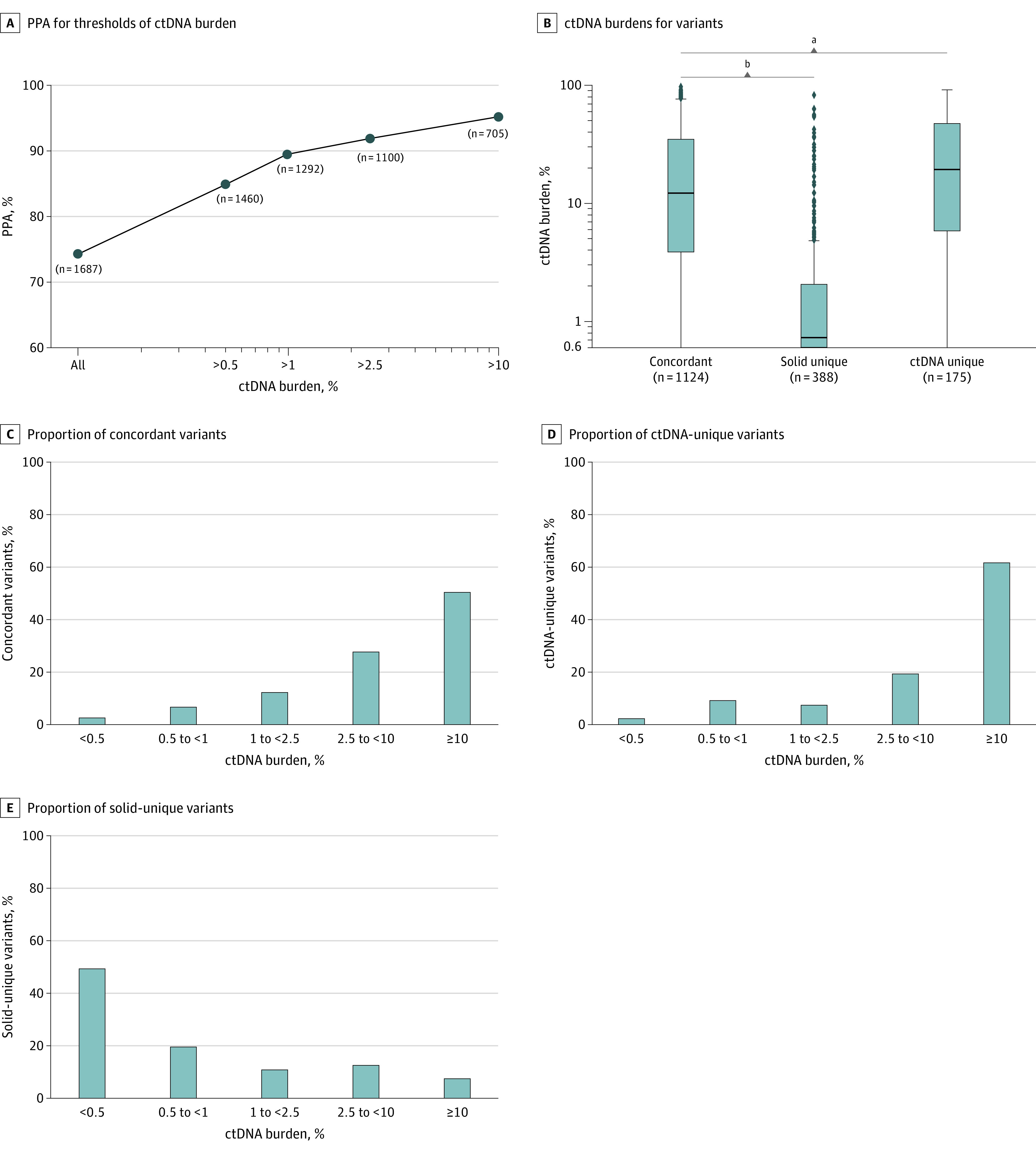
A, Using solid-tissue results as the comparator, PPA is shown for different thresholds of ctDNA burden, where “n” is the number of variants in the cohort greater than or above the sample’s ctDNA burden threshold (ctDNA burden is maximum somatic variant allele frequency). B, Distributions of ctDNA burdens for concordant, ctDNA unique, and solid unique variants. The horizontal lines represent medians, while the boxes represent IQRs; whiskers represent 1.5 × IQRs. The dots represent outliers outside of these distributions. C, Proportion of concordant variants (n = 1124) at varying ctDNA burden thresholds. D, Proportion of ctDNA unique variants (n = 175) at varying ctDNA burden thresholds. E, Proportion of solid-unique variants (n = 388) at varying ctDNA burden thresholds.
aP < .01.
bP < .001.
Detected variants in the actionable cohort were grouped according to whether they were concordant (n = 1124), uniquely detected in ctDNA (ctDNA-unique; n = 175), or uniquely detected in tissue (solid-unique; n = 388). We observed that solid-unique variants detected were significantly associated with lower ctDNA burden compared with concordant variants (U statistic, 53124.5; median solid unique [0.5%] vs median concordant [10.3%]; P = 5.6 × 10−110, Mann-Whitney test) (Figure 2B). Conversely, ctDNA-unique variants were associated with higher ctDNA burden compared with concordant variants (U statistic, 86384.0; median ctDNA unique [16.8%] vs median concordant [10.3%]; P = 4.8 × 10−3, Mann-Whitney test) (Figure 2B).
For both the concordant and ctDNA-unique variant groups, we observed an increase in the proportion of total variants detected with increasing ctDNA burden (Figure 2C and D), with more than 61.7% of ctDNA-unique variants (108 of 175) detected among patients with the highest ctDNA burden (>10%; Figure 2D). Conversely, the proportion of variants uniquely detected in tissue decreased as a function of increasing ctDNA burden, with 49.5% of all solid-unique variants (192 of 388) detected among patients with the lowest ctDNA burden (≤0.5%; Figure 2E). The analyses were repeated for each individual cancer type, resulting in distinct patterns (eFigure 1 in Supplement 1).
We observed no significant association between tumor purity and concordance rate in our evaluable cohort nor within each of the cancer subtypes (eFigure 2A-E in Supplement 1), with the exception of NSCLC, where variants uniquely detected in ctDNA were associated with lower tumor purity compared with concordant and solid-unique variants (H statistic, 23.07; median, 20%, 50%, and 50% for ctDNA unique, concordant, and solid unique variants, respectively; P = 3.9 × 10−5, Bonferroni-corrected Kruskal-Wallis test; eFigure 2C in Supplement 1). To evaluate the possible association of subclonality with variant detection, we compared the VAFs of concordant and uniquely detected variants in the combined cohort and in each cancer type (eFigure 3 in Supplement 1). The VAFs of assay-unique variants were consistently lower than the VAFs of concordant variants across all cancer subtypes and both the solid-tissue (U statistic, 121831.0; median VAF solid unique [24.1%] vs median VAF concordant [32.4%]; P = 1.13 × 10−12, Mann-Whitney test, combined) and ctDNA (U statistic, 34298.0; median VAF ctDNA unique [0.9%] vs median VAF concordant [8.0%]; P = 1.15 × 10−30, Mann-Whitney test, combined) testing modalities, suggesting that assay-unique variants are more likely to be subclonal.
Analysis of Concurrent Testing in Metastatic NSCLC
Most patients with NSCLC with actionable findings had 1 actionable variant, with 4.7% (23 of 490) having more than 1 actionable variant (eTable 3 in Supplement 1). In the NSCLC actionable cohort, 65.7% of detected variants (337 of 513) were concordant, 29.0% (149 of 513) were detected uniquely in solid tissue, and 5.3% (27 of 513) were detected uniquely in ctDNA (Figure 3A).
Figure 3. Actionable Variants Detected in Non–Small Cell Lung Cancer (NSCLC) Cohort.
A, Distribution of actionable variants in NSCLC by assay concordance: concordant (gray), circulating tumor DNA (ctDNA) unique (dark blue), and solid unique (light blue). B, Proportion of all ctDNA unique variants detected in the indicated genes (top) and proportion of all solid unique variants detected in the indicated genes (bottom). C, At the individual gene level, variants detected in the NSCLC cohort are classified as either concordant or assay-unique (top), with concordance varying across genes. Assay-unique variants are separately categorized according to whether they were uniquely detected by ctDNA or tissue testing (bottom). D, Per gene, the percentage increase in the number of patients with an actionable finding identified by concurrent testing compared with solid-tissue testing alone.
KRAS (OMIM 190070) variants were the most common ctDNA-unique variants at 29.6% (8 of 27), while among solid-unique variants, EGFR (OMIM 131550) variants were the most common at 40.3% (60 of 149) (Figure 3B). There was no difference in the gene proportions uniquely detected by ctDNA vs those uniquely detected by tissue testing (unique-ctDNA [KRAS, 29.6%; EGFR, 18.5%; MET, 18.5%; BRAF, 11.1%; ERBB2, 11.1%; ALK,7.4%; ROS1, 3.7%] vs unique-solid [KRAS, 22.8%; EGFR, 40.2%; MET, 16.8%; BRAF, 6.0%; ERBB2, 4.7%; ALK, 9.3%; ROS1, 0%]; P > .05, Fisher exact test; Figure 3B), although significant differences in gene proportions were observed between ctDNA-unique and concordant variants (concordant [KRAS, 34.2%; EGFR, 44.9%; MET, 5.4%; BRAF, 4.7%; ERBB2, 2.9%; ALK, 7.4%; ROS1, 0.2%]; P = .002, Fisher exact test) and between solid-unique and concordant variants (χ2 = 22.21; P < .001; Figure 3B; eFigure 4B in Supplement 1).
We next examined the relative proportion of variants per gene that were concordant, solid-unique, or ctDNA-unique (Figure 3C). Overall, concordance rates varied significantly across genes (P = 8.52 × 10−5, χ2 test). MET (OMIM 164860) had the smallest percentage (37.5% [18 of 48]) of concordant variants (ie, most MET variants were detected uniquely in one or the other assay). However, except for ROS (OMIM 165020; n = 1), most assay-unique variants (>70%) across all genes were detected by tissue testing (Figure 3C).
Polyclonal variants (ie, multiple pathogenic variants within a patient’s gene) were observed in 3 genes, most frequently in ERBB2 (OMIM 164870; 5.3% [1 of 19]; eTable 4 in Supplement 1). To account for this, we examined the proportional increase of patients with an actionable gene-level alteration due to concurrent testing compared with tissue results. For KRAS, there was a 5.4% (8 of 149) increase in the number of patients found to harbor an actionable KRAS variant due to concurrent vs tissue-only testing (Figure 3D), with EGFR exhibiting the smallest increase of patients (2.0% [4 of 202]) vs ERBB2 with the largest increase (18.8% [3 of 16]).
Analysis of Concurrent Testing in Metastatic Breast Cancer
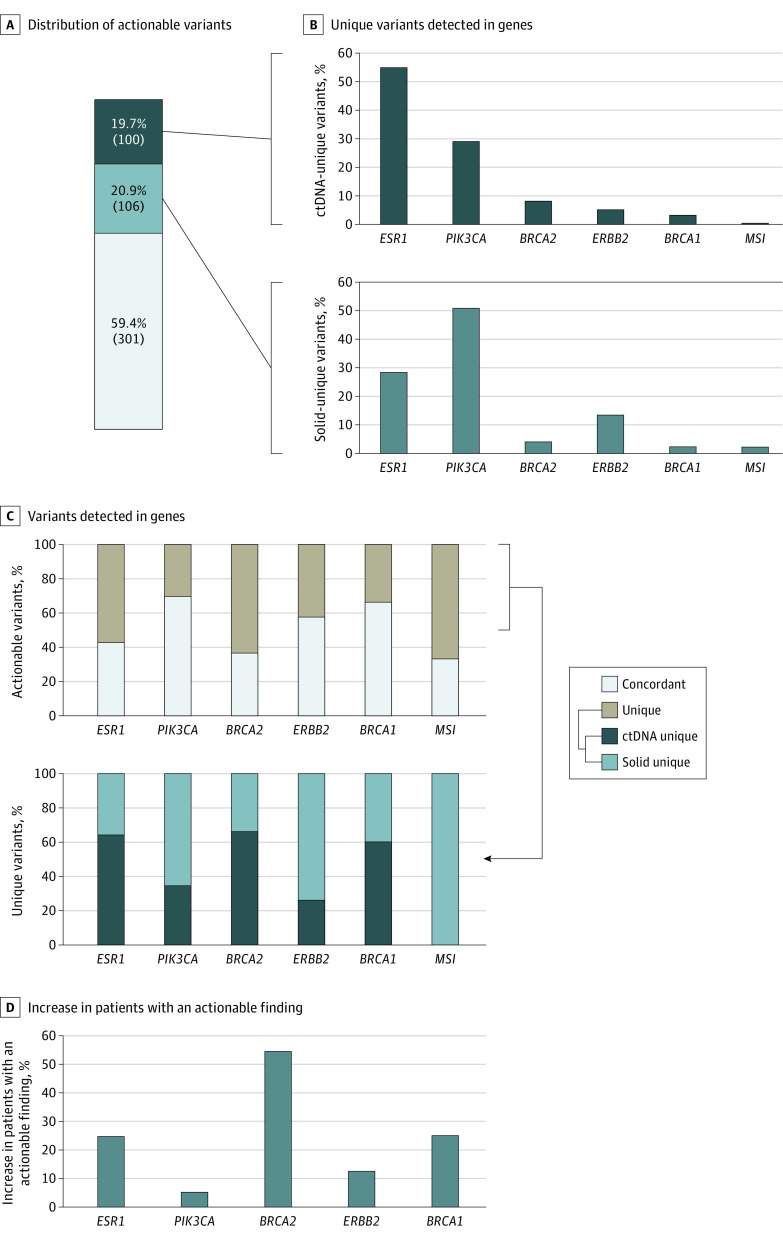
In the actionable cohort with metastatic breast cancer (n = 352), the concordance rate of variant detection by both assays was 59.4% (301 of 507); 19.7% of the variants (100 of 507) were detected uniquely by ctDNA, and the remaining 20.9% (106 of 507) were detected uniquely by tissue testing (Figure 4A). ESR1 variants were the most prevalent actionable findings detected uniquely via ctDNA (55.0% [55 of 100]), while PIK3CA (OMIM 171834) variants accounted for 29.0% (29 of 100) (Figure 4B). Overall, the difference in the proportion of genes with actionable variants detected uniquely by ctDNA compared with that uniquely detected by tissue testing, and the difference uniquely detected by ctDNA compared with that of concordant variants, was statistically significant at P = 3 × 10−4 and P = 1 × 10−10, respectively (Fisher exact test) while the difference between concordant variants and that of unique detected variants in solid tissue was not significant (unique-ctDNA [ESR1, 55%; PIK3CA, 29%; BRCA2, 8%; ERBB2, 5%; BRCA1, 3%; MSI, 0%]; unique-solid [ESR1, 28.3%; PIK3CA, 50.9%; BRCA2, 3.8%; ERBB2, 13.2%; BRCA1, 1.9%; MSI, 1.9%]; concordant [ESR1, 21.3%; PIK3CA, 64.1%; BRCA2, 2.3%; ERBB2, 8.6%; BRCA1, 3.3%; MSI, 0%]) (Figure 4B; eFigure 5B in Supplement 1).
Figure 4. Actionable Variants Detected in Breast Cancer Cohort.
A, Distribution of actionable variants in breast cancer by assay concordance: concordant (gray), circulating tumor DNA (ctDNA) unique (dark blue), and solid unique (light blue). B, Proportion of all ctDNA unique variants detected in the indicated genes (top) and proportion of all solid unique variants detected in the indicated genes (bottom). C, At the individual gene level, variants detected in the breast cancer cohort are classified as either concordant or assay-unique (top), with concordance varying across genes. Assay-unique variants are separately categorized according to whether they were uniquely detected by ctDNA or solid-tissue testing (bottom). D, Per gene, the percentage increase in the number of patients with an actionable finding identified by concurrent testing compared with solid-tissue testing alone.
Next, we examined intragene concordance rates, which varied significantly across genes (χ2 = 34.62; P = 1.8 × 10−6), with PIK3CA showing the highest concordance (69.9% [193 of 276]) (Figure 4C). Focusing on the unique variants detected at a gene level for each assay, we observed variability in detection rates across genes, with 64.7% of all ESR1 variants (55 of 85) that were uniquely detected by either assay modality associated with ctDNA testing (Figure 4C). By contrast, 73.6% of assay-unique ERBB2 variants (14 of 19) were detected by tissue testing (Figure 4C).
Overall, there was a much larger association between polyclonality and patient-level variant detection in advanced breast cancer than NSCLC, especially in the ESR1 and PIK3CA genes (eTables 3 and 4 in Supplement 1). For ESR1, there was a 24.7% increase (23 of 93) in the number of patients found to harbor an actionable variant due to concurrent testing, with PIK3CA exhibiting the smallest increase of patients (5.2% [12 of 233]) and BRCA2 (OMIM 600185) the largest increase (54.5% [6 of 11]) (Figure 4D).
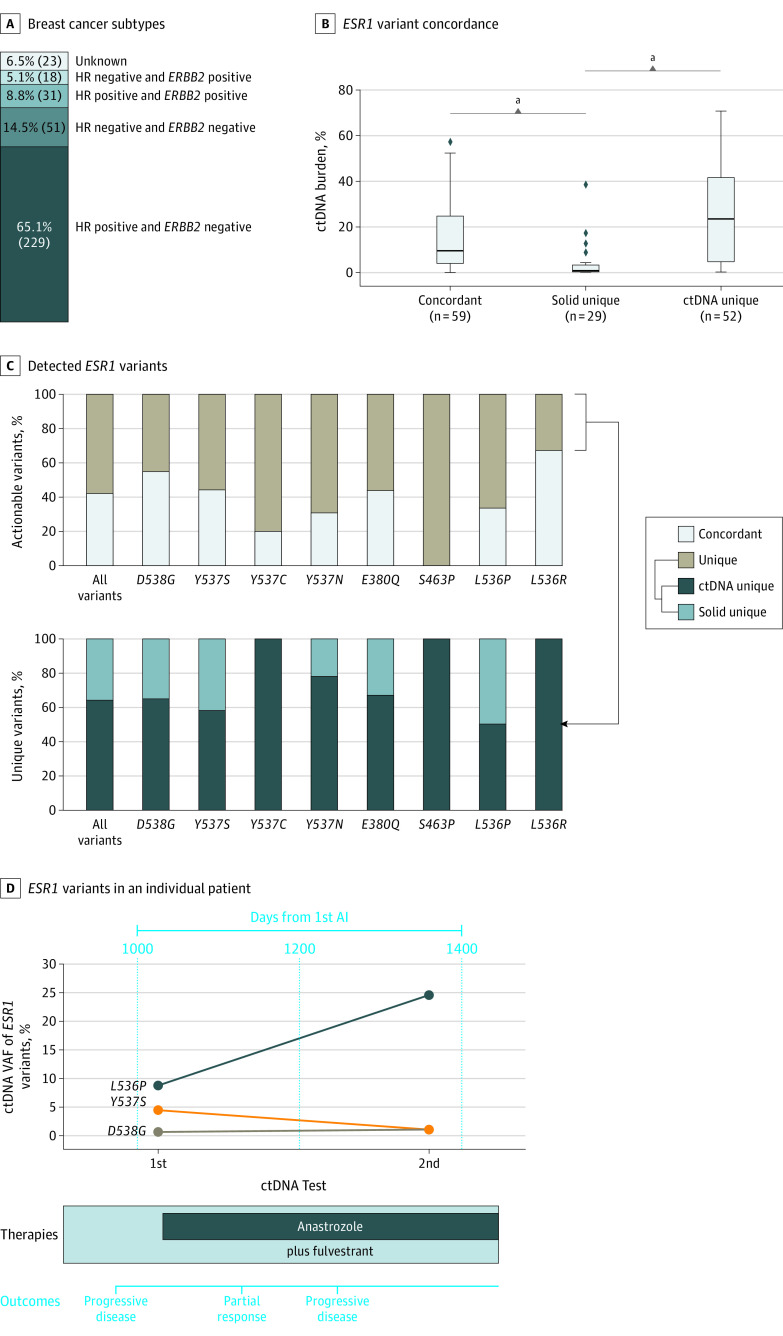
Within the actionable cohort of patients with metastatic breast cancer, 65.1% (229 of 352) were hormone receptor (HR) positive and ERBB2 negative, while 73.8% (260 of 352) were HR positive irrespective of ERBB2 status (Figure 5A). Among the patients with HR-positive breast cancer, the population in which ESR1 variants have been shown to be an actionable biomarker, patients with high ctDNA burden were more likely to harbor ctDNA-unique (U statistic, 1331.5; median ctDNA unique [26.9%] vs median solid unique [1.0%]; P = 6.6 × 10−8, Mann-Whitney test) and concordant (U statistic, 1465.5; median concurrent [11.2%] vs median solid unique [1.0%]; P = 2.5 × 10−7, Mann-Whitney test) ESR1 variants, relative to solid-tissue unique ESR1 variants (Figure 5B). Most of the ESR1 variants had less than 50% assay concordance, and the proportion of assay-unique variants identified in ctDNA ranged from 100% for Y537C (8 of 8) to 50% for L536P (2 of 4) when considering variants that occurred 3 or more times in the actionable breast cancer cohort (Figure 5C).
Figure 5. Breast Cancer Variant Subtypes.
A, The distribution of breast cancer subtypes among patients in the actionable cohort. B, Distribution of circulating tumor DNA (ctDNA) burden values by ESR1 variant assay concordance among patients with hormone receptor–positive breast cancer. The horizontal lines represent medians, while the boxes represent IQRs; whiskers represent 1.5 × IQRs. The dots represent outliers outside of these distributions. C, At the individual ESR1 variant level, variants detected in the breast cancer cohort are classified as either concordant or assay-unique (top), with concordance varying across genes. Assay-unique variants are separately categorized according to whether they were uniquely detected by ctDNA or solid-tissue testing (bottom). D, Sequential ctDNA monitoring of ESR1 variants in an individual patient with metastatic breast cancer. The variant allele frequencies (VAFs) of the 3 ESR1 variants plotted show distinct trajectories over time, with indicated therapies and outcomes shown below. AI indicates aromatase inhibitor.
aP < .001.
We examined a single patient with HR-positive and ERBB2-negative metastatic breast cancer with prior antiestrogen treatment with 2 ctDNA tests separated by 325 days (Figure 5D). At the time of the first test, 3 ESR1 variants (L536P, Y537S, and D538G) were detected by ctDNA profiling—most likely resistant variants selected from prior aromatase and CDK4/6 inhibitor therapy. In the interval between the 2 ctDNA tests, the patient was placed on an antiestrogen regimen of anastrozole and fulvestrant and clinical notes indicated initial partial response to therapy followed by progressive disease. At the time of the second ctDNA test, there was a large increase in the ctDNA VAF of the L536P variant and a modest decrease in VAF of the Y537S variant, reflecting clonal selection arising from treatment pressure.
Concurrent Testing in Metastatic CRC and Prostate Cancer
The analyses were repeated among the CRC and prostate cancer cohorts (eFigures 6-9 in Supplement 1). Among the 4 indications, CRC and prostate cancer had the highest and lowest proportion of patients with actionable variants, respectively (Figure 1B). Among actionable gene-level variants detected in CRC, KRAS represented the largest proportion (75.7% [439 of 580 total variants]). We observed a significant difference in the proportion of genes with variants uniquely detected by ctDNA vs those uniquely detected by tissue testing between ctDNA-unique and concordant variants, but not between solid-unique and concordant variants (eFigure 6B and eFigure 7B in Supplement 1). In prostate cancer, given the low number of patients with actionable variants in this cohort (n = 41), and the fact that many guideline-based variants for prostate cancer may be associated with CHIP (eg, ATM [OMIM 607585]), patterns of unique variant detection by assay were difficult to interpret and require additional follow-up analysis to assess clinical relevance (eFigures 8 and 9 in Supplement 1).
Discussion
To our knowledge, this is the largest advanced multicancer, solid tumor study to date that examines concurrent ctDNA and tissue NGS testing results across heterogeneous clinical settings, comprising a geographically and racially and ethnically diverse patient population, with 20.6% of the patient cohort with stated race and ethnicity as other than White. In addition, 9.3% and 24.2% of patients with an actionable finding had at least 1 NCCN recommended actionable variant that was uniquely detected by ctDNA and solid-tissue testing, respectively.
Although variant detection by both testing methods was largely concordant, concordance rates varied considerably by cancer subtype. Spatial heterogeneity is likely a dominant biological factor influencing the detection of ctDNA-unique and solid-tissue unique variants, either through intratumor heterogeneity, or intertumor heterogeneity across metastatic lesions. Other potential biological confounders, including germline variants and CHIP, were minimized in our study by restricting the patient cohort to those with matched tumor and normal profiling.
Among patients with advanced breast cancer with actionable findings, 20.2% had an actionable variant uniquely detected by ctDNA testing. More than half of these unique genomic abnormalities (55.0%) were somatic mutations in the ESR1 gene, which are a common cause of acquired resistance to aromatase inhibitor endocrine therapy. Detection of ESR1 mutation is critical information that will enable oncologists to optimize treatment decisions, with recent clinical evidence supporting the use of ESR1 mutations as actionable biomarkers for treatment change, as well as the US Food and Drug Administration granting approval for ESR1 mutations as a companion diagnostic to elacestrant therapy for patients with pretreated ESR1-mutated, HR-positive, advanced breast cancer.41 Moreover, the clinical evidence is rapidly evolving on serial ctDNA monitoring of ESR1 mutation detection to capture quantitative changes in its allele frequency among patients with frontline advanced HR-positive breast cancer.21,42,43 Patients undergoing concurrent testing specifically in this setting had a higher detection rate of this actionable variant, supporting baseline ctDNA detection to enable serial ctDNA monitoring of molecular evolution in patients with advanced HR-positive breast cancer.
In advanced NSCLC, while NCCN guidelines acknowledge the value of complementary concurrent ctDNA testing in circumstances when (1) the patient is not medically fit for invasive tissue sampling, (2) there is insufficient tissue for analysis, or (3) the timing of tissue acquisition is uncertain, our study provides clinical evidence on the value of concurrent testing when successful tissue and ctDNA results are available. In our study, 5.5% of patients with actionable variants in NSCLC had a variant identified uniquely via ctDNA despite successful tissue profiling. Published work by Aggarwal et al25 that supports our observation found that 6.3% of patients with NSCLC overall had a targetable mutation detected uniquely by plasma ctDNA-based profiling.
Limitations
Our study has some limitations, including its retrospective nature and lack of data on adherence to matched targeted therapy based on actionable findings along with associated patient outcomes. Nonetheless, our study was based on a large and diverse patient cohort reflective of the evolving changes in the pattern of care observed in heterogeneous clinical settings, where concurrent testing (≤30 days between biopsies) has proven to be feasible, practical, and clinically applicable. Although each assay modality has technical and biological limitations, these are largely mutually exclusive and may provide complementary evidence for therapeutic actionability when used together. Our study attempted to control for CHIP by excluding samples that lacked matched normal tissue sequencing from the same patient, but we cannot rule out that some ctDNA unique variants may still be confounded with CHIP. Finally, our study focused on a limited set of cancer subtypes based on the higher prevalence seen in our data set, reflecting patterns of clinical care. We expect that future studies will include additional cancer subtypes to evaluate clinical benefit for patients with advanced pancancer solid tumors.
Conclusions
Findings from our study—using a large, clinically heterogeneous cohort—support the claim that concurrent testing is feasible and clinically beneficial for detecting more actionable variants among patients with advanced cancer. Although the benefits associated with concurrent testing varied in magnitude by cancer type, higher detection persisted across all 4 cancer types and was most pronounced in breast cancer and NSCLC, reaffirming NCCN guideline support for ctDNA testing in these populations. Future studies will examine how concurrent testing results in more optimal treatment selection and improved outcomes for patients by expanding the number of matched therapies.
eMethods.
eReferences.
eTable 1. Overview of Cohort Demographics and Clinical Features for Individual Cancer Types and the Combined Cohort
eTable 2. List of Indication-Matched, Guidelines-Based Variants Used to Assess Actionability
eTable 3. Overview of Actionable Variant Counts per Patient for Each Cancer Type and in the Combined Cohort
eTable 4. Overview of Actionable Variant Counts Within an Individual Gene at the Patient-Level for Each Cancer Type, Highlighting That Many Patients Have More Than One Actionable Variant Identified Within the Same Gene
eFigure 1. (A) Positive Percent Agreement (PPA) for Different Thresholds of ctDNA Burden. (B) Distributions of ctDNA Burden Tumor Shedding Values for Concordant, ctDNA-Unique, and Solid-Unique Variants. Proportion of all Concordant (C), ctDNA-Unique (D), and Solid-Unique (E) Variants at Varying ctDNA Burden Thresholds
eFigure 2. Distributions of Solid-Tumor Purity Values for Concordant, Solid-Unique, and ctDNA-Unique Variants
eFigure 3. Comparison of VAFs by Assay Concordance
eFigure 4. (A) Gene-Specific Actionable Variant Prevalence in the Actionable NSCLC Cohort. (B) Concordance Values Between ctDNA and Solid-Tissue at the Individual Gene Level in the NSCLC Cohort. ctDNA Burden Values for Individual Variant Types Stratified According to Gene: (C) KRAS, (D) EGFR, (E) MET, (F) BRAF, (G) ERBB2, and (H) ALK
eFigure 5. (A) Gene-Specific Actionable Variant Prevalence in the Actionable Breast Cancer Cohort. (B) Concordance Values Between ctDNA and Solid-Tissue at the Individual Gene Level in the Breast Cancer Cohort. ctDNA Burden Values for Individual Variant Types Stratified According to Gene/Biomarker: (C) PIK3CA, (D) HER2, (E) BRCA1, (F) BRCA2 and (G) MSI
eFigure 6. (A) Actionable Variants in CRC by Type. (B) Proportion of All ctDNA-Unique and Solid-Unique Variants Detected in the Indicated Genes. (C) Variants Detected in the CRC Cohort Are Classified as Either Concordant or Assay-Unique. (D) Percent Increase in the Number of Patients With an Actionable Finding Identified by Concurrent Testing Compared to Solid-Tissue Testing Alone
eFigure 7. (A) Gene-Specific Actionable Variant Prevalence in the Actionable CRC Cohort. (B) Concordance Values Between ctDNA and Solid-Tissue at the Individual Gene Level in the CRC Cohort. ctDNA Burden Values for Individual Variant Types Stratified According to Gene/Biomarker: (C) KRAS, (D) NRAS, (E) ERBB2, (F) BRAF, (G) NTRK1 and (H) MSI
eFigure 8. (A) Actionable Variants in Prostate Cancer by Type. (B) Proportion of all ctDNA-Unique and Solid-Unique Variants Detected in the Indicated Genes. (C) Variants Detected in the Prostate Cancer Cohort Are Classified as Either Concordant or Assay-Unique. (D) Percent Increase in the Number of Patients With an Actionable Finding Identified by Concurrent Testing Compared to Solid-Tissue Testing Alone
eFigure 9. (A) Gene-Specific Actionable Variant Prevalence in the Actionable Prostate Cancer Cohort. (B) Concordance Values Between ctDNA and Solid-Tissue at the Individual Gene Level in the Prostate Cancer Cohort. ctDNA Burden Values for Individual Variant Types Stratified According to Gene/Biomarker: (C) ATM, (D) BRCA1, (E) BRCA2, (F) PALB2, (G) RAD51C and (H) MSI
Data Sharing Statement
References
- 1.National Comprehensive Cancer Network . Breast cancer (version 3.2023). Accessed March 22, 2023. https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf
- 2.National Comprehensive Cancer Network . Non-small cell lung cancer (version 2.2023). Accessed March 22, 2023. https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf
- 3.National Comprehensive Cancer Network . Prostate cancer (version 1.2023). Accessed March 22, 2023. https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf
- 4.National Comprehensive Cancer Network . Colon cancer (version 1.2023). Accessed April 13, 2023. https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf
- 5.National Comprehensive Cancer Network . Rectal cancer (version 1.2023). Accessed April 13, 2023. https://www.nccn.org/professionals/physician_gls/pdf/rectal.pdf
- 6.ESMO . Guidelines by topic. Accessed April 13, 2023. https://www.esmo.org/guidelines/guidelines-by-topic
- 7.Chakravarty D, Johnson A, Sklar J, et al. Somatic genomic testing in patients with metastatic or advanced cancer: ASCO provisional clinical opinion. J Clin Oncol. 2022;40(11):1231-1258. doi: 10.1200/JCO.21.02767 [DOI] [PubMed] [Google Scholar]
- 8.Sicklick JK, Kato S, Okamura R, et al. Molecular profiling of cancer patients enables personalized combination therapy: the I-PREDICT study. Nat Med. 2019;25(5):744-750. doi: 10.1038/s41591-019-0407-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pishvaian MJ, Blais EM, Brody JR, et al. Overall survival in patients with pancreatic cancer receiving matched therapies following molecular profiling: a retrospective analysis of the Know Your Tumor Registry trial. Lancet Oncol. 2020;21(4):508-518. doi: 10.1016/S1470-2045(20)30074-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsimberidou AM, Hong DS, Ye Y, et al. Initiative for Molecular Profiling and Advanced Cancer Therapy (IMPACT): an MD Anderson precision medicine study. JCO Precis Oncol. 2017;2017:PO.17.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yuan M, Huang LL, Chen JH, Wu J, Xu Q. The emerging treatment landscape of targeted therapy in non–small-cell lung cancer. Signal Transduct Target Ther. 2019;4:61. doi: 10.1038/s41392-019-0099-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cescon DW, Bratman SV, Chan SM, Siu LL. Circulating tumor DNA and liquid biopsy in oncology. Nat Cancer. 2020;1(3):276-290. doi: 10.1038/s43018-020-0043-5 [DOI] [PubMed] [Google Scholar]
- 13.Siravegna G, Mussolin B, Venesio T, et al. How liquid biopsies can change clinical practice in oncology. Ann Oncol. 2019;30(10):1580-1590. doi: 10.1093/annonc/mdz227 [DOI] [PubMed] [Google Scholar]
- 14.Ignatiadis M, Sledge GW, Jeffrey SS. Liquid biopsy enters the clinic—implementation issues and future challenges. Nat Rev Clin Oncol. 2021;18(5):297-312. doi: 10.1038/s41571-020-00457-x [DOI] [PubMed] [Google Scholar]
- 15.Leighl NB, Page RD, Raymond VM, et al. Clinical utility of comprehensive cell-free DNA analysis to identify genomic biomarkers in patients with newly diagnosed metastatic non-small cell lung cancer. Clin Cancer Res. 2019;25(15):4691-4700. doi: 10.1158/1078-0432.CCR-19-0624 [DOI] [PubMed] [Google Scholar]
- 16.Rolfo C, Mack P, Scagliotti GV, et al. Liquid biopsy for advanced NSCLC: a consensus statement from the International Association for the Study of Lung Cancer. J Thorac Oncol. 2021;16(10):1647-1662. doi: 10.1016/j.jtho.2021.06.017 [DOI] [PubMed] [Google Scholar]
- 17.Pascual J, Attard G, Bidard FC, et al. ESMO recommendations on the use of circulating tumour DNA assays for patients with cancer: a report from the ESMO Precision Medicine Working Group. Ann Oncol. 2022;33(8):750-768. doi: 10.1016/j.annonc.2022.05.520 [DOI] [PubMed] [Google Scholar]
- 18.Papadimitrakopoulou VA, Wu YL, Han JY, et al. Analysis of resistance mechanisms to osimertinib in patients with EGFR T790M advanced NSCLC from the AURA3 study. Ann Oncol. 2018;29(suppl_8):viii741. doi: 10.1093/annonc/mdy424.064 [DOI] [Google Scholar]
- 19.McCoach CE, Blakely CM, Banks KC, et al. Clinical utility of cell-free DNA for the detection of ALK fusions and genomic mechanisms of ALK inhibitor resistance in non–small cell lung cancer. Clin Cancer Res. 2018;24(12):2758-2770. doi: 10.1158/1078-0432.CCR-17-2588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li X, Lu J, Zhang L, Luo Y, Zhao Z, Li M. Clinical implications of monitoring ESR1 mutations by circulating tumor DNA in estrogen receptor positive metastatic breast cancer: a pilot study. Transl Oncol. 2020;13(2):321-328. doi: 10.1016/j.tranon.2019.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bidard FC, Hardy-Bessard AC, Dalenc F, et al. ; PADA-1 investigators . Switch to fulvestrant and palbociclib versus no switch in advanced breast cancer with rising ESR1 mutation during aromatase inhibitor and palbociclib therapy (PADA-1): a randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 2022;23(11):1367-1377. doi: 10.1016/S1470-2045(22)00555-1 [DOI] [PubMed] [Google Scholar]
- 22.Passaro A, Jänne PA, Mok T, Peters S. Overcoming therapy resistance in EGFR-mutant lung cancer. Nat Cancer. 2021;2(4):377-391. doi: 10.1038/s43018-021-00195-8 [DOI] [PubMed] [Google Scholar]
- 23.Zheng D, Ye X, Zhang MZ, et al. Plasma EGFR T790M ctDNA status is associated with clinical outcome in advanced NSCLC patients with acquired EGFR-TKI resistance. Sci Rep. 2016;6:20913. doi: 10.1038/srep20913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tukachinsky H, Madison RW, Chung JH, et al. Genomic analysis of circulating tumor DNA in 3,334 patients with advanced prostate cancer identifies targetable BRCA alterations and AR resistance mechanisms. Clin Cancer Res. 2021;27(11):3094-3105. doi: 10.1158/1078-0432.CCR-20-4805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aggarwal C, Thompson JC, Black TA, et al. Clinical implications of plasma-based genotyping with the delivery of personalized therapy in metastatic non–small cell lung cancer. JAMA Oncol. 2019;5(2):173-180. doi: 10.1001/jamaoncol.2018.4305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palmero R, Taus A, Viteri S, et al. Biomarker discovery and outcomes for comprehensive cell-free circulating tumor DNA versus standard-of-care tissue testing in advanced non–small-cell lung cancer. JCO Precis Oncol. 2021;5:93-102. doi: 10.1200/PO.20.00241 [DOI] [PubMed] [Google Scholar]
- 27.Bayle A, Peyraud F, Belcaid L, et al. Liquid versus tissue biopsy for detecting actionable alterations according to the ESMO Scale for Clinical Actionability of Molecular Targets in patients with advanced cancer: a study from the French National Center for Precision Medicine (PRISM). Ann Oncol. 2022;33(12):1328-1331. doi: 10.1016/j.annonc.2022.08.089 [DOI] [PubMed] [Google Scholar]
- 28.Nong J, Gong Y, Guan Y, et al. Circulating tumor DNA analysis depicts subclonal architecture and genomic evolution of small cell lung cancer. Nat Commun. 2018;9(1):3114. doi: 10.1038/s41467-018-05327-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vendramin R, Litchfield K, Swanton C. Cancer evolution: Darwin and beyond. EMBO J. 2021;40(18):e108389. doi: 10.15252/embj.2021108389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Passiglia F, Rizzo S, Di Maio M, et al. The diagnostic accuracy of circulating tumor DNA for the detection of EGFR-T790M mutation in NSCLC: a systematic review and meta-analysis. Sci Rep. 2018;8(1):13379. doi: 10.1038/s41598-018-30780-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stover DG, Parsons HA, Ha G, et al. Association of cell-free DNA tumor fraction and somatic copy number alterations with survival in metastatic triple-negative breast cancer. J Clin Oncol. 2018;36(6):543-553. doi: 10.1200/JCO.2017.76.0033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jensen K, Konnick EQ, Schweizer MT, et al. Association of clonal hematopoiesis in DNA repair genes with prostate cancer plasma cell-free DNA testing interference. JAMA Oncol. 2021;7(1):107-110. doi: 10.1001/jamaoncol.2020.5161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Finkle JD, Boulos H, Driessen TM, et al. Validation of a liquid biopsy assay with molecular and clinical profiling of circulating tumor DNA. NPJ Precis Oncol. 2021;5(1):63. doi: 10.1038/s41698-021-00202-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jee J, Lebow ES, Yeh R, et al. Overall survival with circulating tumor DNA-guided therapy in advanced non–small-cell lung cancer. Nat Med. 2022;28(11):2353-2363. doi: 10.1038/s41591-022-02047-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Choudhury Y, Tan MH, Shi JL, et al. Complementing tissue testing with plasma mutation profiling improves therapeutic decision-making for patients with lung cancer. Front Med (Lausanne). 2022;9:758464. doi: 10.3389/fmed.2022.758464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sacher AG, Paweletz C, Dahlberg SE, et al. Prospective validation of rapid plasma genotyping for the detection of EGFR and KRAS mutations in advanced lung cancer. JAMA Oncol. 2016;2(8):1014-1022. doi: 10.1001/jamaoncol.2016.0173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sivakumar S, Jin DX, Tukachinsky H, et al. Tissue and liquid biopsy profiling reveal convergent tumor evolution and therapy evasion in breast cancer. Nat Commun. 2022;13(1):7495. doi: 10.1038/s41467-022-35245-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schwartzberg LS, Li G, Tolba K, et al. Complementary roles for tissue and blood based comprehensive genomic profiling for detection of actionable driver alterations in advanced NSCLC. JTO Clin Res Rep. 2022;3(9):100386. doi: 10.1016/j.jtocrr.2022.100386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beaubier N, Bontrager M, Huether R, et al. Integrated genomic profiling expands clinical options for patients with cancer. Nat Biotechnol. 2019;37(11):1351-1360. doi: 10.1038/s41587-019-0259-z [DOI] [PubMed] [Google Scholar]
- 40.Beaubier N, Tell R, Lau D, et al. Clinical validation of the Tempus xT next-generation targeted oncology sequencing assay. Oncotarget. 2019;10(24):2384-2396. doi: 10.18632/oncotarget.26797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bidard FC, Kaklamani VG, Neven P, et al. Elacestrant (oral selective estrogen receptor degrader) versus standard endocrine therapy for estrogen receptor-positive, human epidermal growth factor receptor 2–negative advanced breast cancer: results from the randomized phase III EMERALD trial. J Clin Oncol. 2022;40(28):3246-3256. doi: 10.1200/JCO.22.00338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bardia A, Chandarlapaty S, Linden HM, et al. AMEERA-1 phase 1/2 study of amcenestrant, SAR439859, in postmenopausal women with ER-positive/HER2-negative advanced breast cancer. Nat Commun. 2022;13(1):4116. doi: 10.1038/s41467-022-31668-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baird R, Oliveira M, Gil EMC, et al. Abstract PS11-05: updated data from SERENA-1: a phase 1 dose escalation and expansion study of the next generation oral SERD AZD9833 as a monotherapy and in combination with palbociclib, in women with ER-positive, HER2-negative advanced breast cancer. Cancer Res. 2021;81(4)(suppl):PS11-PS05. doi: 10.1158/1538-7445.SABCS20-PS11-05 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods.
eReferences.
eTable 1. Overview of Cohort Demographics and Clinical Features for Individual Cancer Types and the Combined Cohort
eTable 2. List of Indication-Matched, Guidelines-Based Variants Used to Assess Actionability
eTable 3. Overview of Actionable Variant Counts per Patient for Each Cancer Type and in the Combined Cohort
eTable 4. Overview of Actionable Variant Counts Within an Individual Gene at the Patient-Level for Each Cancer Type, Highlighting That Many Patients Have More Than One Actionable Variant Identified Within the Same Gene
eFigure 1. (A) Positive Percent Agreement (PPA) for Different Thresholds of ctDNA Burden. (B) Distributions of ctDNA Burden Tumor Shedding Values for Concordant, ctDNA-Unique, and Solid-Unique Variants. Proportion of all Concordant (C), ctDNA-Unique (D), and Solid-Unique (E) Variants at Varying ctDNA Burden Thresholds
eFigure 2. Distributions of Solid-Tumor Purity Values for Concordant, Solid-Unique, and ctDNA-Unique Variants
eFigure 3. Comparison of VAFs by Assay Concordance
eFigure 4. (A) Gene-Specific Actionable Variant Prevalence in the Actionable NSCLC Cohort. (B) Concordance Values Between ctDNA and Solid-Tissue at the Individual Gene Level in the NSCLC Cohort. ctDNA Burden Values for Individual Variant Types Stratified According to Gene: (C) KRAS, (D) EGFR, (E) MET, (F) BRAF, (G) ERBB2, and (H) ALK
eFigure 5. (A) Gene-Specific Actionable Variant Prevalence in the Actionable Breast Cancer Cohort. (B) Concordance Values Between ctDNA and Solid-Tissue at the Individual Gene Level in the Breast Cancer Cohort. ctDNA Burden Values for Individual Variant Types Stratified According to Gene/Biomarker: (C) PIK3CA, (D) HER2, (E) BRCA1, (F) BRCA2 and (G) MSI
eFigure 6. (A) Actionable Variants in CRC by Type. (B) Proportion of All ctDNA-Unique and Solid-Unique Variants Detected in the Indicated Genes. (C) Variants Detected in the CRC Cohort Are Classified as Either Concordant or Assay-Unique. (D) Percent Increase in the Number of Patients With an Actionable Finding Identified by Concurrent Testing Compared to Solid-Tissue Testing Alone
eFigure 7. (A) Gene-Specific Actionable Variant Prevalence in the Actionable CRC Cohort. (B) Concordance Values Between ctDNA and Solid-Tissue at the Individual Gene Level in the CRC Cohort. ctDNA Burden Values for Individual Variant Types Stratified According to Gene/Biomarker: (C) KRAS, (D) NRAS, (E) ERBB2, (F) BRAF, (G) NTRK1 and (H) MSI
eFigure 8. (A) Actionable Variants in Prostate Cancer by Type. (B) Proportion of all ctDNA-Unique and Solid-Unique Variants Detected in the Indicated Genes. (C) Variants Detected in the Prostate Cancer Cohort Are Classified as Either Concordant or Assay-Unique. (D) Percent Increase in the Number of Patients With an Actionable Finding Identified by Concurrent Testing Compared to Solid-Tissue Testing Alone
eFigure 9. (A) Gene-Specific Actionable Variant Prevalence in the Actionable Prostate Cancer Cohort. (B) Concordance Values Between ctDNA and Solid-Tissue at the Individual Gene Level in the Prostate Cancer Cohort. ctDNA Burden Values for Individual Variant Types Stratified According to Gene/Biomarker: (C) ATM, (D) BRCA1, (E) BRCA2, (F) PALB2, (G) RAD51C and (H) MSI
Data Sharing Statement