Abstract
This study investigates whether ACA policies to increase access to breast pumps and lactation care were associated with innovation in the market for breast pumps.
Innovation in medical products for maternal health has been slow partly due to underfunding of research to advance scientific understanding of women’s health.1 The Patient Protection and Affordable Care Act (ACA),2 enacted in March 2010, required employers to provide employees who are breastfeeding a reasonable break time and appropriate location to express milk. A second provision, which became effective in August 2012, required all new insurance policies to provide coverage for preventive services, including breast pumps and lactation care, with no cost sharing. Beginning January 2014, this coverage was extended to insurance acquired through marketplace plans and in states that expanded Medicaid.3 This study investigated whether these ACA policies to increase access to breast pumps and lactation care were associated with innovation in the market for breast pumps.
Methods
We examined changes between 1994 and 2021 in 2 outcome variables: (1) number of patent filings and (2) number of 510(k) premarket notifications (PMNs) for breast pumps. Breast pumps are classified as Class II medical devices by the US Food and Drug Administration (FDA). While a patent filing requests exclusive right to an invention, a PMN seeks to demonstrate that the device is safe and effective. Firms are required to submit a PMN before marketing a breast pump in the US. Data on patent filings were obtained from the PatentsView dataset, US Patent and Trademark Office, and data on PMNs were obtained from the Premarket Notification 510(k) dataset from the FDA. Using the itsa package in Stata, version 16.1 (StataCorp), we specified interrupted time series analyses (ITSAs) to assess the statistical significance of the change in trends before ACA (1994-2009) vs after ACA (2010-2021) for outcomes. ITSA estimates coefficients with ordinary least-squares regression and produces Newey-West standard errors to correct for autocorrelation and possible heteroscedasticity. For patent filings, the model assumes 2 interruptions (ACA and COVID-19 pandemic). While patent filings occur in early-stage research that was disrupted by the COVID-19-pandemic,4 PMNs are part of product launch, which is less likely to be disrupted. We produced trendline plots with ITSA estimates and reported 95% CIs. P < .05 (2-sided) was considered statistically significant.
Results
There were 136 PMNs and 403 patent filings for breast pumps (Table) between 1994 and 2021. Of 136 PMNs, 121 (89.0%) were for new breast pumps. Before ACA, 20 of 25 firms (80.0%) submitted a PMN for the first time compared with 38 of 44 firms (86.4%) after ACA.
Table. Summary Statistics for Premarket Notifications and Patent Filings for Breast Pumps Before and After ACA, 1994-2021.
| Parameter | Before ACA (1994-2009) | After ACA (2010-2021) | ||
|---|---|---|---|---|
| Total No. | Yearly mean No. | Total No. | Yearly mean No. | |
| Patent filings for breast pumps | 152 | 9.50 (0.77) | 251 | 20.92 (3.60) |
| Premarket notifications for breast pumps | 50 | 3.13 (0.51) | 86 | 7.17 (0.85) |
Abbreviation: ACA, Patient Protection and Affordable Care Act.
Before ACA, the yearly mean number of PMNs was 3.13 (0.51), and the yearly mean number of patents was 9.50 (0.77). The trends in both outcomes did not change significantly from 1994 through 2009 (Figure). In 2010, there were no statistically significant changes in numbers of PMNs and patents. After ACA, there were significant increases, to a yearly mean of 7.17 PMNs (ITSA estimate for trend, 0.62; 95% CI, 0.16 to 1.08; P = .01) and 20.92 patents (ITSA estimate for trend, 1.42; 95% CI, 0.20-2.64; P = .02). In 2020, the number of patents decreased by 26.73 (95% CI, −33.29 to −20.17; P < .001) patents.
Figure. Interrupted Time Series Analyses (ITSAs) for Yearly Premarket Notifications (PMNs) and Patent Filings for Breast Pumps Before and After the Patient Protection and Affordable Care Act (ACA), 1994-2021.
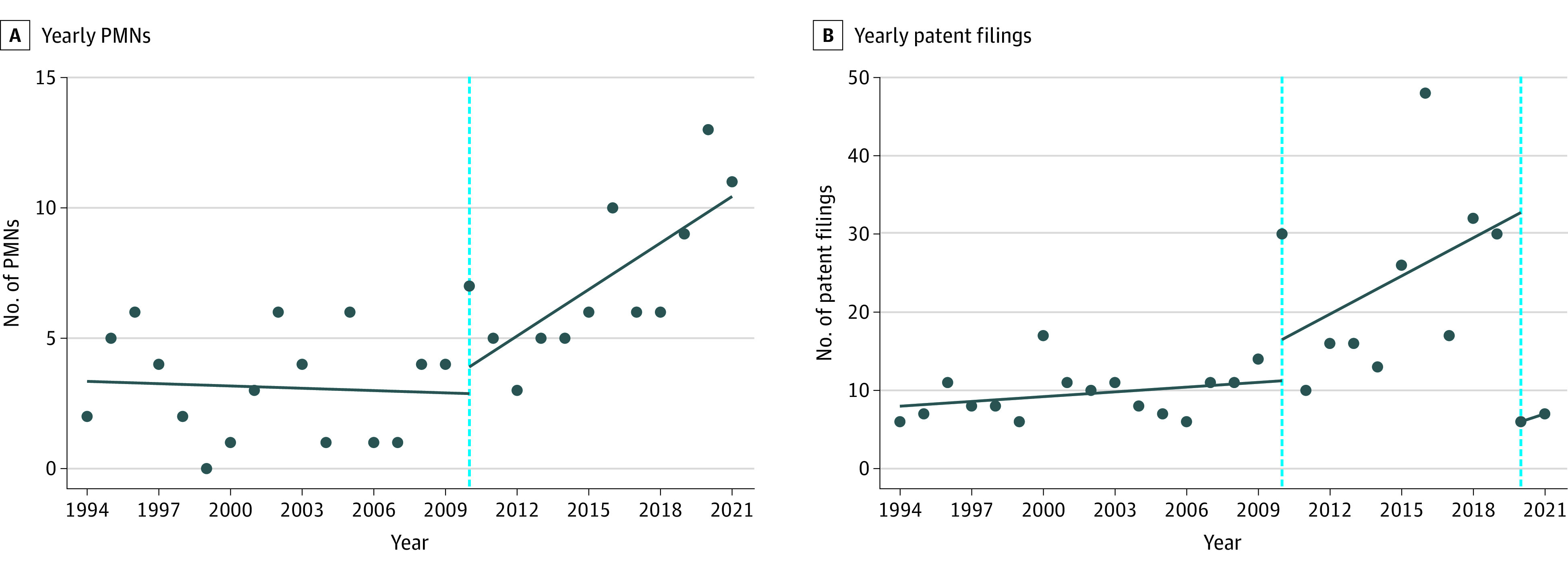
Trendline estimates were the ITSA regression with Newey-West 95% CIs. A, Interruption specified at 2010 (ACA) (dashed line). Pre-ACA trend (slope), −0.03 (95% CI, −0.22 to 0.16; P = .75). Change in number of PMNs in 2010, 1.02 (95% CI, −1.29 to 3.34; P = .37). Post-ACA trend (slope) compared with pre-ACA trend, 0.62 (95% CI, 0.16-1.08; P = .01). B, Interruption specified at 2010 (ACA) and 2020 (COVID-19 pandemic) (dashed lines). Pre-ACA trend (slope), 0.20 (95% CI, −0.04 to 0.44; P = .09). Change in number of patent filings in 2010, 5.27 (95% CI, −2.57 to 13.10; P = .18). Post-ACA trend (slope) compared with pre-ACA trend, 1.42 (95% CI, 0.20-2.64; P = .02). Change in number of patent filings in 2020, −26.73 (95% CI, −33.29 to −20.17; P < .001).
Discussion
This study’s findings suggest there has been an increase in innovation and competition in the breast pump market after ACA. In contrast, the potential market size for breast pumps, proxied by US births, decreased over the same period.5 The observed increase may have been driven by the anticipated increase in demand from expanded insurance coverage. ACA’s guaranteed reimbursement may have signaled reduced risk to suppliers, which might have incentivized private research and development.
While breastfeeding provisions of ACA have been associated with increased breastfeeding practices,6 this study is the first, to our knowledge, to show an association between ACA and indicators of innovation and competition in the breast pump market. Limitations of this study are that the extent of the innovation is unknown, and the analyses did not control for other factors beyond ACA. Whether increases in the numbers of PMNs and patent filings for breast pumps were associated with increased breastfeeding initiation and duration or reduced health disparities should be examined.
Section Editors: Kristin Walter, MD, and Jody W. Zylke, MD, Deputy Editors; Karen Lasser, MD, Senior Editor.
Data Sharing Statement
References
- 1.Fisk NM, Atun R. Systematic analysis of research underfunding in maternal and perinatal health. BJOG. 2009;116(3):347-356. doi: 10.1111/j.1471-0528.2008.02027.x [DOI] [PubMed] [Google Scholar]
- 2.Content details. 29 USC 207 - maximum hours. GovInfo. Accessed November 15, 2023. https://www.govinfo.gov/app/details/USCODE-2010-title29/USCODE-2010-title29-chap8-sec207
- 3.Hawkins SS, Dow-Fleisner S, Noble A. Breastfeeding and the Affordable Care Act. Pediatr Clin North Am. 2015;62(5):1071-1091. doi: 10.1016/j.pcl.2015.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lasch F, Psarelli EE, Herold R, et al. The impact of COVID-19 on the initiation of clinical trials in Europe and the United States. Clin Pharmacol Ther. 2022;111(5):1093-1102. doi: 10.1002/cpt.2534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Osterman MJK, Hamilton BE, Martin JA, Driscoll AK, Valenzuela CP. Births: final data for 2021. Natl Vital Stat Rep. 2023;72(1):1-53. [PubMed] [Google Scholar]
- 6.Hawkins SS, Horvath K, Noble A, Baum CF. ACA and Medicaid expansion increased breast pump claims and breastfeeding for women with public and private insurance. Womens Health Issues. 2022;32(2):114-121. doi: 10.1016/j.whi.2021.10.005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Sharing Statement


