Abstract
BACKGROUND:
Per- and polyfluoroalkyl substances (PFAS) are a growing class of manufactured chemical compounds found in a variety of consumer products. PFAS are ubiquitous in the environment and were found in many humans sampled in the United States (U.S.). Yet, significant gaps in understanding statewide levels of exposure to PFAS remain.
OBJECTIVE:
The goals of this study are to establish a baseline of exposure at the state level by measuring PFAS serum levels among a representative sample of Wisconsin residents and compare to United States National Health and Nutrition Examination Survey (NHANES).
METHODS:
The study sample included 605 adults (18+ years of age) selected from the 2014–2016 sample of the Survey of the Health of Wisconsin (SHOW). Thirty-eight PFAS serum concentrations were measured using high-pressure liquid chromatography coupled with tandem mass spectrometric detection (HPLC-MS/MS) and geometric means were presented. Weighted geometric mean serum values of eight PFAS analytes from SHOW were compared to U.S. national levels from the NHANES 2015–2016 sample (PFOS, PFOA, PFNA, PFHxS, PFHpS, PFDA, PFUnDA), and the 2017–2018 sample for Me-PFOSA, PFHPS using the Wilcoxon rank-sum test.
RESULTS:
PFOS, PFHxS, PFHpS, PFDA, PFNA, and PFOA were detected in over 96% of SHOW participants. In general, SHOW participants had lower serum levels across all PFAS when compared to NHANES. Serum levels increased with age and were higher among males and whites. Similar trends were seen in NHANES, except non-whites had higher PFAS levels at higher percentiles in NHANES.
IMPACT STATEMENT:
The present study conducts biomonitoring of 38 PFAS among representative sample of residents in the state of Wisconsin. Results suggest that while the majority of Wisconsin residents tested have detectable levels of PFAS in their blood serum, they may have a lower body burden of some PFAS compared to a nationally representative sample. Older adults, males, and whites may have a higher body burden of PFAS relative to other groups, both in Wisconsin and the wider United States.
Keywords: Exposure assessment, PFAS, Per and Poly fluoroalkyl substances, Population-based
INTRODUCTION
Per- and polyfluoroalkyl substances (PFAS) are a large family of human-made, fluorinated compounds produced for a variety of consumer and industrial products. PFAS are persistent due to their long half-lives and accumulation potential in the environment, and in their bioaccumulation potential in blood and tissues of animals and humans [1-3].
PFAS are highly stable compounds that not only repel water, oils, and lipids, but bind to proteins, making them desirable for use as flame retardants and other common consumer products [2, 4]. A variety of perflourinated compounds have been manufactured. A defining characteristic of PFAS include multiple carbon-fluorine bonds. The most studied long-chain PFAS, including PFOA, PFOS, PFHxS, and PFNA [5], do not readily degrade, which makes them both highly stable in the environment and equally challenging to remove from environmental media. Environmental contamination sites for PFAS, often arise from industrial production, agricultural practices using contaminated fertilizer, or from historical use of class B aqueous film forming foam (AFFF) at military or fire training sites [3, 6]. Some PFAS compounds can leach into groundwater, surface water, and soil, remaining within all trophic levels for years or decades [3, 7].
Widespread detection of PFAS in the environment and potential population exposure has increased public health concerns, especially as PFAS research finds more associations between PFAS exposure and adverse human health effects [8, 9]. According to findings from the National Health and Nutrition Examination Survey (NHANES) survey (2011–2018) nearly all the n = 7991 U.S. residents have detectable levels of one or more long-chain PFAS [5]. Major exposure pathways for PFAS in humans include consumption of contaminated drinking water and food, especially fish and red meat [10]. Other sources of human exposure to PFAS include contact with consumer products and ingestion of contaminated dust particles [2]. Exposure to PFAS has been associated with several adverse health outcomes [3] including kidney and testicular cancers [11], dyslipidemia [12], liver disease [13], lower infant birth weight [14], decreased pediatric vaccine response [15], increased risk of gestational hypertension or preeclampsia [16], and immune suppression [17].
While PFOS and PFOA have been manufactured for decades, it was not until 2002 when the U.S. Environmental Protection Agency (EPA) began to regulate PFAS, requiring manufacturers to notify the EPA of the manufacture or import of 13, and later 75, of the compounds [18]. Long-chain PFAS are still used in imported products from developing countries [19, 20]. In June 2022, the EPA set a non-enforceable lifetime health advisory for PFOS and PFOA in drinking water to 0.02 and 0.004 parts per trillion, respectively [21]. A handful of states, including Wisconsin, have adopted the 2016 standard as the statewide regulatory standard for at least one PFAS compound [22]. This is a rapidly evolving regulatory landscape, with some states making recommendations more stringent than the EPA standard, and others making no recommendations at all [22]. However, there are an estimated 4000 PFAS compounds [18] produced by industry, no biomonitoring or epidemiological research on the vast majority of PFAS. Although long-chain, “legacy” PFASs (PFOA and PFAS) have been phased out of production in the U.S. and Canada, they are replaced by “emerging” PFAS shorter carbon chains or fluoroethers in industrial production [23]. Replacement chemicals have a lower tendency to bioaccumulate but also have very similar chemical properties to legacy PFAS. Less is known about population exposure to these emerging contaminants and additional biomonitoring and research are needed to understand how short-chain PFAS affect human health [24].
While the CDC has conducted biomonitoring of PFAS in the U.S. general population through the National Health and Nutrition Examination Survey, little population-based biomonitoring has occurred in the Upper Midwest and central region of the U.S. [25-29]. With NHANES data, the CDC established baseline exposure data for PFAS in the U.S., but the scale of the survey lacks the granularity needed to understand PFAS within smaller regions and demographic strata at the state or local level. The other community-based and localized biomonitoring studies to-date are not sampled in such a way to provide representation of PFAS exposure across an entire state. This study begins to fill this data gap by providing baseline PFAS levels representative of residents in the state of Wisconsin. The study uses serum samples from The Survey of the Health of Wisconsin (SHOW) cohort, the only statewide representative health survey in the U.S. modeled after NHANES.
In Wisconsin, PFAS have been found in groundwater supplies exceeding health-based recommendations [30]. There are known contamination sites in Madison, Marinette, and Peshtigo communities due to production and testing of AFFF [31], and there is potential concern of exposure from agricultural use of wastewater sludge on crop fields [32, 33]. PFAS has been detected in not only groundwater, but in milk raised and produced in Wisconsin, the second largest dairy producing state [32]. Eighteen different PFAS compounds were detected in well samples in Madison, Wisconsin in domestic, municipal, and agricultural wells [34]. Furthermore, Wisconsin’s variable geography (forested, agricultural, and varying levels of urbanicity) and demographics are reasonably comparable to that of the broader United States in age structure, gender, and race [35].
In 2020, the Wisconsin State Laboratory of Hygiene (WSLH) developed a new method to detect PFAS compounds at lower concentrations which increases the number of compounds available for assessment than have been used by many previous biomonitoring studies. The goal of this study is to provide a baseline prevalence of PFAS among a representative sample of adults in the state of Wisconsin and compare PFAS prevalence by demographics to nationally representative sample estimates from NHANES. In this study, we examine an expanded list of PFAS compounds, analyzed using high-pressure liquid chromatography coupled with tandem mass spectrometric detection (HPLC-MS/MS). While other studies have drawn comparisons to NHANES PFAS levels, to our knowledge, no other statewide representative samples have compared PFAS levels to NHANES.
METHODS
Survey of the Health of Wisconsin (SHOW)
A subset of (n = 605) participants were selected at random the 2014–2016 Survey of the Health of Wisconsin (SHOW) sample of adults ages 18 and older (n = 1957). Details on the SHOW sampling frame, recruitment, and methods are described elsewhere [36]. In brief, the SHOW 2014–2016 cohort was designed as a three-year, statewide representative sample using a three-stage cluster-sampling approach. One county per strata was randomly selected within the strata of county mortality rates, followed by random selection of census block groups by poverty status strata. Then 30–35 residential households were randomly selected from commercially available U.S. Postal Service residential and mailing address listings (including postal office (P.O.) boxes and rural routes (R.R.)) using simple random sampling. Selected households are sent an advanced letter and recruitment occurs with a household visit by a trained field interviewer.
SHOW is unique in that it is the only statewide household-based examination survey in the United States and offers an abundance of survey and physical measurement data upon which to characterize PFAS exposure within our sample. Modeled after the CDC’s NHANES, its survey data includes demographics (age, gender, education, income, race), behaviors (smoking, diet), occupation (military service, firefighter), housing (type and age of residence), drinking water characteristics (private well vs. municipal, depth of private well, consumption pattern, treatment/filter use), diet (fish, dairy) and chronic health conditions (cancer, diabetes, hypertension, obesity). In addition, participant households span rural, urban and suburban settings and are geocoded to allow for linkages to contextual environmental data, including potential PFAS sources (landfills, industrial and municipal wastewater facilities, agricultural fields).
Like NHANES, SHOW includes both objective and subjective data collection using several methods. Interviewers conduct in-home visits upon which they gather health information via computer assisted personal interview (CAPI). Participants also complete a self-administered paper questionnaire. Following an in-home visit, participants visit a collection site near their home where phlebotomists measure blood pressure, weight, height, waist and hip circumference, respiratory function, and collect venous blood and urine samples. Several tubes of venous blood (about 55–60 ml in total) are immediately processed for serum and plasma, aliquoted into cryovials and frozen at −80 C. SHOW participants consent to the use of biospecimens for unspecified research. The core SHOW study and this study is approved by the University of Wisconsin Health Sciences Institutional Review Board and all biosecurity and institutional safety procedures are HIPAA compliant.
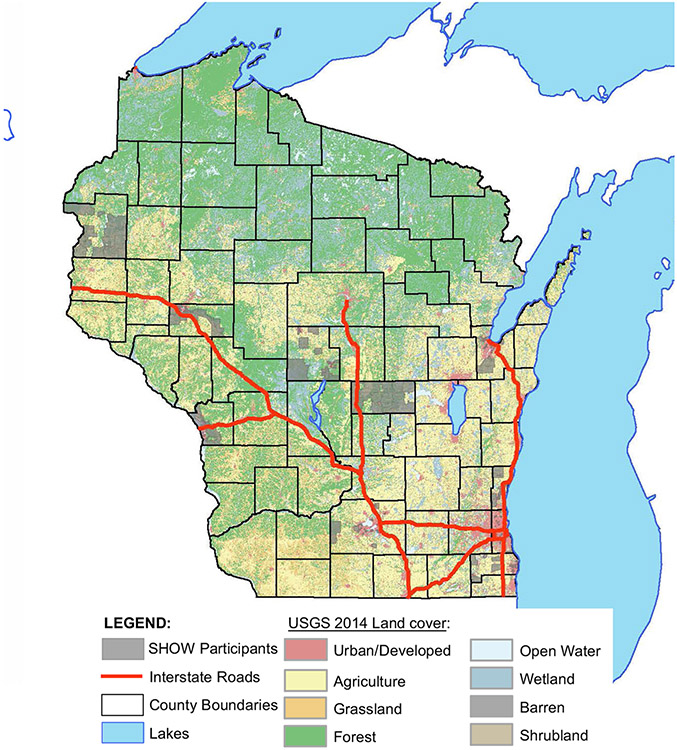
For this pilot study and retrospective sample analysis, a single random sample of adults 18+ (n = 50; stratified by race, sex, age), was pulled from each of 12 counties representing each of 5 health regions in the state. Only individuals with stored serum were included when selecting the study sample. This allows for the assessment of historic PFAS exposures across Wisconsin and accelerates the ability to observe time-dependent trends in exposures when prospective sampling is performed. An additional sample was pulled per health region as substitution in case any samples were not viable for PFAS analyses. Serum samples were extracted from SHOW’s freezer and sent to the Wisconsin State Laboratory of Hygiene on dry ice for PFAS analyses. Figure 1 depicts the geographic distribution of participants throughout the state by census block group.
Fig. 1. United States Geological Survey (USGS) land cover map of Wisconsin, USA, depicting SHOW participants by the shaded U.S.
Census Block Group they reside in.
PFAS sample analysis
The PFAS analyses were performed by the Wisconsin State Lab of Hygiene (WSLH) using high-pressure liquid chromatography coupled with tandem mass spectrometric detection (HPLC-MS/MS). The 38 PFAS compounds tested were selected based on several considerations, including, (1) cover the spectrum of compounds to look for class-specific bioaccumulation, (2) include compounds most often used in products and tested by CDC in NHANES, (3) include both short and long chain PFAS to assess differences by type, (4) include emerging compound classes for which we have little data, (5) consider potential toxicity estimates, and (6) limited by the availability of suitable materials to assure reliable measurement.
The WSLH test method was adapted primarily from a method developed by Minnesota Department of Public Health [37], with elements from CDC Method 6304.08 [38], the New York State Department of Health [39], and the Michigan Department of Community Health [40]. Several of these documents are laboratory manuals, some shared privately between colleagues. Sample preparation involved spiking aliquots of serum with an isotopically labeled PFAS mixture. Acetonitrile was added to precipitate proteins, followed by vortex mixing. Samples were then centrifuged, the supernatant transferred to a 96-well plate, and evaporated to a volume of 100–200 mL.
Prepared samples were injected on an Agilent 1290 Infinity UPLC (Santa Clara, CA) equipped with a BEH C18 1.7 mm 2.1 X 50 mm Column (Waters, Milford, MA). Good chromatographic separation was achieved using a reverse phase gradient, with a 20-minute run time at 45 °C. Sample detection and quantification was achieved with a Sciex 4500 MS/MS system (Toronto, CA), employing multiple reaction mode (MRM) scanning with negative polarity turbospray ionization. Q1 and Q3 masses, declustering Potential (DP), collision energy (CE) and collision cell exit potential (CXP) values for each MRM transition were optimized by parameter ramping experiments with direct standard solution infusion to the source. Sciex Analyst version 1.6.3 was used for data acquisition and results calculation.
Method quality control characteristics included a method blank and seven standard linear calibration standards (r ≥ 0.995). Standards were verified using a second source material. Calibration curves were generated in serum and standards were first made in ACN (levels 1–7) and then spiked into blank fetal bovine serum and run like a normal sample. Limits of detection and quantitation were calculated by doing a LOD study by spiking eight blank serum samples with the lowest level calibrator. Analytical signal/noise for S1 was required to be ≥10. Ion confirmation ratio for samples was required to fall within ±30% of the mean ratio of the standards, with exceptions for PFOS, FOSA, PFDA, PFNS, 11Cl-PF3OUdS, PFHxA, PFTrDA, PFDS, PFTeDA, and 8:2 diPAP, which were widened to ±40% based on poor confirmation ion sensitivity. Method detection limits varied considerably by compound, as did the upper quantification limits, but compare favorably with NHANES quantification thresholds for those compounds. Three levels of analytical controls were measured in every analytical run, and acceptable control values bracketed all test samples. Method validation also included precision, analytical measurement range, and spike recovery assessment.
Additional details on the analytical method area and observations on compounds not comparable with NHANES are in preparation. The internal standards (IS) that were used were: M3-PFPeA, M4-PFHpA, M3-PFBS, M3-N-MeFOSAA, M8-FOSA, M4-6:2 diPAP, M4-PFBA, M2-PFHxA, M4-PFOA, M5-PFNA, M2-PFDA, M2-PFUnDA, M2-PFDoA, M2-PFHxS, M4-PFOS, M7-MeFOSE, M9-EtFOSE, M2-PFTeDA, M2-PFHxDA, M5-EtFOSA, M3-MeFOSA, Cl-PFHxDA, M5-EtFOSAA, M2-8:2 diPAP, M4-4:2 FTSA, M2-6:2 FTSA, M2-8:2 FTSA, and M4-10:2 FTSA. Since there were not matching IS for every compound, IS were used based on very chemically similar compounds (ex. M4-PFOS was used to quantify PFNS).
For this study, all PFAS values were reported that were above the calculated LOD. Ion ratio checks were performed for hits above the LOD. If a compound with a confirmation ion had a hit for the quantitation ion but did not have a hit for the confirmation ion it was considered not detectable. If a compound had a hit for the quantitation ion and a hit for the confirmation ion, but the ion ratio did not match with the average of the ion ratios of the standards it was flagged for, it was considered a failed ion ratio confirmation and deemed not detectable. If there were hits for both quantitation and the confirmation ion, and it passed an ion ratio check, then it was deemed detectable.
National Health and Nutrition Examination Survey (NHANES)
NHANES is a nationally representative, repeated, cross-sectional survey administered by the National Center of Health Statistics (NCHS) within the CDC. NHANES uses a multistage cluster sampling approach to examine a study population that is representative of the United States’ non-institutionalized population. Blood samples are taken from all NHANES participants 12 years of age and older. Approximately 2000 of these samples were analyzed for several PFAS compounds in each cycle. The NHANES 2015-2016 sample intentionally oversamples Hispanic, non-Hispanic black, Non-Hispanic Asian, Non-Hispanic white and others at or below 185 percent of the Department of Health and Human Services (HHS) poverty guidelines, and non-Hispanic white and other people aged 80 years and older. NHANES releases publicly available laboratory and demographic data on these participants. Education and smoking status for adolescents (age 12–19) is considered sensitive data and not included in public use datasets. Documentation for NHANES data includes detailed laboratory methods [41]. For this study, SHOW study sample PFAS serum levels were compared with those from the National Health and Nutrition Examination Survey (NHANES) 2015–2016 sample (n = 1829) for all corresponding samples. However, given that NHANES did not sample the entire suite of compounds in this study, the 2017–2018 (n = 1862) samples were compared for select compounds (ME-PFOSA and PFHPS).
Demographics
Self-reported demographics data for gender, age, race/ethnicity, highest education level attained, and income were collected by CAPI. Smoking status data were obtained through a self-administered questionnaire (SAQ) for the Wisconsin Sample and personal interview for NHANES. Income/poverty ratio was calculated by dividing the midpoint of reported household income range by HHS poverty guidelines, which is calculated by the number of people supported by that income [42]. Body mass index (BMI) (kg/m2) was calculated using WHO standards by dividing measured height (cm) by 100, and squaring that value, and then dividing weight (kg) by that value. SHOW and NHANES participants were stratified by gender, age, and race for analysis of PFAS serum levels. Age groups were determined by generational changes (18–39, 40–59, 60+), and race was grouped by non-Hispanic white and non-white. Minors in the NHANES samples were excluded from the final analysis.
Statistical analyses
All statistical analyses were performed using SAS v9.4. All thirty-eight PFAS compounds were reported for SHOW using the lower limit of detection (LLOD) available from the WSLH assays. However, only compounds analyzed in both SHOW and available in NHANES public use laboratory data were included in comparative data analysis. Furthermore, only compounds detected above the LLOD in more than 50% of individuals in the SHOW sample could be reliably compared to NHANES. Thus, weighted geometric means for 8 PFAS compounds, as well as weighted geometric means for the 50th, 75th, 90th, and 95th percentiles and their corresponding 95% confidence intervals were calculated for SHOW and NHANES (see Table 2 for complete list of PFAS). The LLOD for all compounds in NHANES was 0.1 ng/mL, with values lower than the LLOD set to 0.1 divided by the square root of 2, or approximately 0.07. The LLODs for SHOW participants were different from 0.1, but to make direct comparisons, all PFAS compounds within SHOW were assigned the same LLOD as NHANES for comparison analyses. Therefore, weighted geometric means differ for SHOW in the comparison tables with NHANES, when compared to findings from SHOW with the LLOD from WSLH, unaltered to match NHANES.
Table 2.
Serum PFAS (ng/mL) lab results summary among SHOW 2014–2016 (n = 605) serum for 38 PFAS compounds tested.
| Analyte | Abbreviation | CAS-RNb | Detection Limit |
n with a detection result |
% with a detection result |
Weighted Geometric Mean (ng/mL) |
Min result (ng/mL) |
Max Result (ng/mL) |
|---|---|---|---|---|---|---|---|---|
| N-ethyl perfluorooctanesulfonamidoacetic acid | NEtFOSAA | 2991-50-6 | 0.184 | 13 | 2.2 | 0.073 | 0.195 | 2.02 |
| N-methyl perfluorooctanesulfonamidoacetic acid | NMeFOSAA | 2355-31-9 | 0.145 | 294 | 48.6 | 0.137 | 0.147 | 2.8 |
| Perfluoro-n-dodecanoic acid | PFDoA | 307-55-1 | 0.053 | 23 | 3.8 | 0.071 | 0.057 | 0.295 |
| Perfluoro-n-hexanoic acid | PFHxA | 307-24-4 | 0.043 | 2 | 0.3 | 0.071 | 0.046 | 0.071 |
| Perfluoro-n-undecanoic acid | PFUnDA | 2058-94-8 | 0.042 | 460 | 76.0 | 0.094 | 0.042 | 1.36 |
| Perfluoro-n-octanesulfonic acid | PFOS | 1763-23-1 | 0.036 | 602 | 99.5 | 4.513 | 0.056 | 32.9 |
| Perfluoro-n-hexanesulfonic acid | PFHxS | 355-46-4 | 0.028 | 599 | 99.0 | 1.138 | 0.033 | 30 |
| Perfluoro-n-heptanesulfonic acid | PFHpS | 375-92-8 | 0.027 | 583 | 96.4 | 0.168 | 0.027 | 2.58 |
| Perfluoro-n-octanoic acid | PFOA | 335-67-1 | 0.025 | 603 | 99.7 | 1.196 | 0.038 | 19.1 |
| Perfluoro-n-decanoic acid | PFDA | 335-76-2 | 0.024 | 598 | 98.8 | 0.142 | 0.024 | 4.51 |
| Perfluoro-n-butanesulfonic acid | PFBS | 375-73-5 | 0.022 | 78 | 12.9 | 0.0708 | 0.022 | 0.158 |
| Perfluoro-n-nonanoic acid | PFNA | 375-95-1 | 0.021 | 601 | 99.3 | 0.452 | 0.022 | 12 |
| 4,8-Dioxa-3H-perfluorononanoic acid | DONA | 919005-14-4 | 0.012 | 0 | 0 | N/A | N/A | N/A |
| 9-Chlorohexadecafluoro-3-oxanonane-1-sulfonic acid | 9Cl-PF3ONS | 756426-58-1 | 0.009 | 252 | 41.7 | 0.071 | 0.009 | 0.293 |
| Perfluoro-n-tridecanoic acid | PFTriA | 72629-94-8 | 0.391 | 0 | 0 | N/A | N/A | N/A |
| N-methyl perfluorooctanesulfonamide | NMeFOSA | 31506-32-8 | 0.246 | 0 | 0 | N/A | N/A | N/A |
| Perfluoro-n-octadecanoic acid | PFODA | 16517-11-6 | 0.164 | 0 | 0 | N/A | N/A | N/A |
| 6:2 Fluorotelomer sulfonic acid | 6:2 FTSA | 27619-97-2 | 0.149 | 0 | 0 | N/A | N/A | N/A |
| N-Ethyl perfluorooctanesulfonamidoethanol | N-EtFOSE | 1691-99-2 | 0.139 | 0 | 0 | N/A | N/A | N/A |
| 8:2 di-subs. polyfluoroalkyl phosphate. | 8:2 diPAP | 678-41-1 | 0.134 | 3 | 0.5 | 0.071 | 0.254 | 0.296 |
| 8:2 Fluorotelomer sulfonic acid | 8:2 FTSA | 39108-34-4 | 0.101 | 26 | 4.3 | 0.072 | 0.102 | 0.227 |
| N-Methyl perfluorooctanesulfonamidoethanol | N-MeFOSE | 24448-09-7 | 0.099 | 1 | 0.2 | 0.071 | 0.128 | 0.128 |
| Perfluoro-n-hexadecanoic acid | PFHxDA | 67905-19-5 | 0.085 | 1 | 0.2 | 0.071 | 0.097 | 0.097 |
| 10:2 Fluorotelomer sulfonic acid | 10:2 FTSA | 120226-60-0 | 0.081 | 33 | 5.5 | 0.071 | 0.082 | 0.122 |
| 6:2 di-subs. polyfluoroalkyl phosphate. | 6:2 diPAP | 57677-95-9 | 0.077 | 23 | 3.8 | 0.073 | 0.078 | 0.451 |
| 4:2 Fluorotelomer sulfonic acid | 4:2 FTSA | 757124-72-4 | 0.067 | 50 | 8.3 | 0.072 | 0.066 | 0.151 |
| N-ethyl perfluorooctanesulfonamide | NEtFOSA | 4151-50-2 | 0.064 | 3 | 0.5 | 0.071 | 0.085 | 0.102 |
| Perfluoro-n-decanesulfonic acid | PFDS | 335-77-3 | 0.049 | 16 | 2.6 | 0.071 | 0.05 | 0.188 |
| Perfluoro-n-butanoic acid | PFBA | 375-22-4 | 0.039 | 173 | 28.6 | 0.074 | 0.039 | 0.546 |
| Perfluoro-n-tetradecanoic acid | PFTeA | 376-06-7 | 0.032 | 2 | 0.3 | 0.071 | 0.035 | 0.041 |
| Perfluoro-n-heptanoic acid | PFHpA | 375-85-9 | 0.031 | 352 | 58.2 | 0.075 | 0.031 | 2.85 |
| Perfluorooctanesulfonamide | FOSA | 754-91-6 | 0.029 | 1 | 0.2 | 0.071 | 0.055 | 0.055 |
| Perfluoro-2-methoxypropanoic acid. | PFMPA | 377-73-1 | 0.019 | 0 | 0 | N/A | N/A | N/A |
| Perfluorononanesulfonic acid | PFNS | 68259-12-1 | 0.018 | 1 | 0.2 | 0.071 | 0.021 | 0.021 |
| Perfluoro-2-methoxybutanoic acid. | PFMBA | 863090-89-5 | 0.017 | 0 | 0 | N/A | N/A | N/A |
| Perfluoropentanesulfonic acid | PFPeS | 2706-91-4 | 0.015 | 351 | 58.0 | 0.074 | 0.015 | 0.523 |
| Perfluoro-n-pentanoic acid | PFPeA | 2706-90-3 | 0.015 | 39 | 6.5 | 0.072 | 0.016 | 1.11 |
| 11-chloroeicosafluoro-3-oxaundecane-1-sulfonic acid | 11Cl-PF3OUdS | 763051-92-9 | 0.011 | 2 | 0.3 | 0.071 | 0.13 | 0.14 |
Highlighted rows indicate inclusion in both SHOW and NHANES analyses. Compounds with percentages of positive results less than 15% in SHOW were not included in comparisons as we are unable to draw reliable conclusions.
CAS-RN chemical abstracts service registry number.
All NHANES LOD were 0.1 ng/mL.
PFOS and PFOA were analyzed as the sum of their linear and branched isomers, consistent with NHANES methods. Data from minors ages 12–17 in the NHANES sample were used to validate data analysis methods against published NHANES data tables, but minors were not included in the final analysis as all SHOW participants were over the age of 18. Geometric means of serum levels and corresponding 95% confidence intervals were calculated for all compounds and compared between SHOW and NHANES. Both NHANES and SHOW geometric means were calculated using subsample weights, and domains of gender, age, and race.
While overall and demographic strata comparisons of PFAS serum levels in SHOW vs. NHANES are weighted, due to the different complexities of sampling in both SHOW and NHANES, unweighted comparisons of PFAS serum concentrations were evaluated using the Wilcoxon rank-sum test to test statistical difference.
RESULTS
Study sample
Descriptive characteristics comparing the SHOW 2014–2016 subsample to NHANES 2015–2016 and NHANES 2017–2018 samples are presented in Table 1. The SHOW study sample is comparable to NHANES in terms of gender and age distribution. There are slightly more females (50.7%) than males in SHOW;similar in NHANES (51.5–51.6%). The SHOW sample differs from NHANES most in terms of racial diversity. The SHOW consists of 81.3% non-Hispanic white, much higher when compared with NHANES where 62.3–62.9% are non-Hispanic white. SHOW also had fewer participants with <13 income to poverty ratio, fewer smokers, and more with a BMI > 30 (Table 1). SHOW participants also tended to be more educated than NHANES participants on average, with 27.1% of SHOW participants reporting high school/GED or less in SHOW, compared with 34.2% in NHANES. Figure 1 is a map of Wisconsin depicting the residential locations by census block group of the SHOW 2014–2016 subsample included in this study. While participants span urban and rural areas across the state, they are clustered in 10 of 72 total counties in the state.
Table 1.
Demographics characteristics of SHOW 2014–2016 compared with NHANES 2015–2016, and NHANES 2017–2018 cohorts.
| SHOW 2014–2016 | NHANES 2015–2016 | NHANES 2017–2018 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| (n = 605) | (n = 1829) | (n = 1862) | |||||||
| n | Column %a | 95% CI (%) | n | Column %b | 95% CI (%) | n | Column %b | 95% CI | |
| Gender | |||||||||
| Male | 257 | 49.3 | 44.8, 53.8 | 865 | 48.4 | 45.1, 51.6 | 922 | 48.5 | 45.1, 52.0 |
| Female | 346 | 50.7 | 46.2, 55.2 | 964 | 51.6 | 48.4, 54.9 | 940 | 51.5 | 48.0, 54.9 |
| Age (in years) | |||||||||
| 18–39 | 162 | 36.0 | 31.5, 40.6 | 674 | 37.6 | 34.6, 40.7 | 641 | 38.5 | 35.2, 41.8 |
| 40–59 | 208 | 36.4 | 32.1, 40.7 | 581 | 35.1 | 31.8, 38.3 | 558 | 33.4 | 29.9, 36.9 |
| 60–94 | 233 | 27.6 | 24.1, 31.2 | 574 | 27.3 | 24.4, 30.2 | 663 | 28.1 | 25.1, 31.0 |
| Race | |||||||||
| White (non-Hispanic) | 502 | 81.3 | 77.5, 85.0 | 563 | 62.9 | 60.2, 65.7 | 644 | 62.3 | 59.4, 65.3 |
| Non-white | 100 | 18.7 | 15.0, 22.5 | 1266 | 37.1 | 34.3, 39.8 | 1218 | 37.7 | 34.7, 40.6 |
| Education* | |||||||||
| H.S./GED or less | 151 | 27.1 | 23.0, 30.5 | 778 | 34.2 | 31.3, 37.2 | 771 | 38.2 | 34.9, 41.6 |
| Some college | 217 | 36.1 | 31.8, 40.4 | 547 | 34.8 | 31.6, 37.9 | 574 | 30.1 | 27.0, 33.1 |
| Bachelors or higher | 235 | 36.8 | 32.6, 41.1 | 424 | 31.0 | 27.8, 34.2 | 415 | 31.7 | 28.1, 35.3 |
| Income/Poverty Ratioc | |||||||||
| <1 | 44 | 14.0 | 10.7, 17.3 | 395 | 21.0 | 18.7, 23.2 | 351 | 22.6 | 20.1, 25.2 |
| 1–3 | 220 | 38.9 | 34.5, 43.3 | 708 | 33.2 | 30.3, 36.1 | 717 | 31.7 | 28.8, 34.6 |
| >3 | 311 | 47.1 | 42.6, 51.5 | 445 | 45.9 | 42.5, 49.2 | 422 | 45.7 | 42.1, 49.2 |
| Smoking Status* | |||||||||
| Current | 73 | 15.0 | 11.5, 18.6 | 356 | 19.6 | 17.1, 22.1 | 334 | 17.3 | 14.8, 19.8 |
| Former | 146 | 23.7 | 19.9, 27.6 | 389 | 23.8 | 21.0, 26.7 | 408 | 21.3 | 18.4, 24.1 |
| Never | 345 | 61.2 | 56.7, 65.6 | 1081 | 56.6 | 53.3, 59.8 | 1120 | 61.4 | 58.1, 64.8 |
| BMI | |||||||||
| <25 | 161 | 26.6 | 22.7, 30.5 | 537 | 31.3 | 28.3, 34.3 | 541 | 29.0 | 25.8, 32.1 |
| 25–30 | 200 | 31.4 | 27.3, 35.4 | 548 | 29.4 | 26.4, 32.3 | 577 | 31.5 | 28.2, 34.8 |
| >30 | 237 | 42.1 | 37.6, 46.5 | 744 | 39.3 | 36.2, 42.5 | 744 | 39.5 | 36.2, 42.9 |
Met Min/wk metabolic minutes per week, HS high school, GED general education diploma, BMI body mass index.
18 and 19 year olds excluded from education and smoking data in NHANES sample.
SHOW sample weights used for statewide representation.
NHANES sample weights used for national representation.
Income/Poverty Ratio is calculated by dividing total family income by the poverty guidelines from the Department of Health and Human Services (HHS) for the number of people supported by that income.
Prevalence of detectable serum PFAS levels
Table 2 depicts the detection limit and summary statistics for the number and percent of individuals with detectable levels, geometric mean, minimum and maximums for all 38 PFAS compounds. Nine of the 38 PFAS compounds were detected in at least 50% of SHOW participants (PFOS, PFOA, PFNA, PFHxS, PFDA, PFHpS, PFUnDA, PFHpA, PFPeS) (Table 2). More than 96% of SHOW participants had serum levels above the lower limit of detection for six PFAS analytes, PFOS, PFOA, PFNA, PFHxS, PFDA, and PFHpS with geometric mean values being 4.51, 1.20, 0.45, 1.14, 0.14, and 0.17 ng/mL, respectively. Eight PFAS compounds were below the limit of detection in 100% of the study sample (NMeFOSA, N-EtFOSE, 6:2 FTSA, PFTriA, PFODA, DONA, PFMPA, PFMBA). SHOW participants had much higher levels of PFOS compared to other compounds, with a whole sample geometric mean of 4.51 ng/mL (Table 2).
PFAS comparison of SHOW to NHANES by demographics
Table 3 depicts comparisons in geometric means and percentiles between the entire SHOW and NHANES samples where LLOD has been adjusted for SHOW to match NHANES. Tables 4-6 (and Supplementary Tables 1-5) depict geometric means and percentiles comparing SHOW and NHANES by demographic strata. Weighted geometric means of PFAS serum levels were slightly higher among NHANES study sample compared to SHOW for PFOS, PFOA, PFNA, PFHxS, PFDA, and PFHPS. However, only PFOA and PFNA were statistically higher among NHANES compared SHOW (p < 0.001) (Tables 5 and 6). Most notable differences in weighted geometric means were seen for PFOA, PFNA, and PFHPS, where NHANES serum samples were 32.5%, 31.1%, and 35.3%, higher than in SHOW, respectively (Tables 5, 6 and Supplementary Table 3). PFOS, PFDA, and PFHxS weighted geometric mean serum levels were 10.2%, 14.3%, 7.0% higher in NHANES when compared to SHOW (Table 4, and Supplementary Tables 1 and 2). PFOS weighted geometric means (95% CI) were highest of all PFAS compounds in both study samples;4.51 (4.18–4.87) ng/mL in SHOW compared to 4.97 (4.71–5.25) ng/mL in NHANES (Table 4).
Table 3.
Geometric Means and Percentile Comparisons (in ng/mL) between SHOW 2014–2016 and NHANES 2015–2016 for 6 PFAS compounds.
| PFAS | Sample | n | Geometric mean (95% CI) | 50th (95% CI) | 75th (95% CI) | 90th (95% CI) | 95th (95% CI) | p-trenda |
|---|---|---|---|---|---|---|---|---|
| PFOS | SHOW | 605 | 4.51 (4.18–4.87) | 5.01 (4.54–5.49) | 8.19 (7.53–8.85) | 11.28 (10.61–12.43) | 14.97 (13.32–16.77) | 0.54 |
| NHANES | 1829 | 4.97 (4.71–5.25) | 5.20 (4.80–5.40) | 8.60 (8.00–9.40) | 13.80 (12.90–15.40) | 19.00 (16.80–21.50) | ||
| PFOA | SHOW | 605 | 1.20 (1.12–1.27) | 1.30 (1.20–1.40) | 1.90 (1.80–1.90) | 2.60 (2.40–2.90) | 3.30 (2.90–3.50) | <0.0001 |
| NHANES | 1829 | 1.59 (1.52–1.67) | 1.60 (1.50–1.70) | 2.50 (2.20–2.50) | 3.40 (3.20–3.60) | 4.20 (3.80–4.70) | ||
| PFNA | SHOW | 605 | 0.45 (0.43–0.48) | 0.50 (0.40–0.50) | 0.70 (0.60–0.70) | 1.00 (0.90–1.20) | 1.40 (1.20–1.70) | <0.0001 |
| NHANES | 1829 | 0.59 (0.56–0.62) | 0.50 (0.40–0.50) | 0.90 (0.80–0.90) | 1.40 (1.20–1.40) | 1.90 (1.50–2.10) | ||
| PFHxS | SHOW | 605 | 1.14 (1.04–1.24) | 1.30 (1.20–1.40) | 2.10 (1.80–2.30) | 3.30 (3.00–4.00) | 4.60 (4.00–5.60) | 0.58 |
| NHANES | 1829 | 1.22 (1.15–1.29) | 1.20 (1.10–1.30) | 2.10 (1.90–2.20) | 3.40 (3.20–3.80) | 5.00 (4.10–5.80) | ||
| PFDA | SHOW | 605 | 0.14 (0.13–0.15) | 0.10 (0.10–0.20) | 0.20 (0.20–0.20) | 0.30 (0.30–0.40) | 0.50 (0.40–0.60) | 0.023 |
| NHANES | 1829 | 0.16 (0.15–0.17) | 0.10 (0.10–0.10) | 0.20 (0.20–0.20) | 0.40 (0.30–0.40) | 0.70 (0.50–0.70) | ||
| PFHPS | SHOW | 605 | 0.17 (0.16–0.18) | 0.17 (0.16–0.19) | 0.28 (0.26–0.30) | 0.37 (0.35–0.40) | 0.46 (0.42–0.49) | <0.0001 |
| NHANES | 2133 | 0.23 (0.22–0.25) | 0.19 (0.10–0.19) | 0.34 (0.28–0.34) | 0.60 (0.50–0.60) | 0.95 (0.69–1.40) |
CI confidence interval.
Chi-Square test for significance.
Table 4.
Comparison of geometric means and percentiles of serum PFOS (in ng/mL) in SHOW (2014–2016) and NHANES (2015–2016) by age, sex and race.
| Sample | Characteristic | n | Geometric mean (95% CI) | 50th (95% CI) | 75th (95% CI) | 90th (95% CI) | 95th (95% CI) |
|---|---|---|---|---|---|---|---|
| Age | |||||||
| SHOW | 18–39 | 162 | 3.13 (2.71–3.62) | 3.70 (3.30–4.10) | 5.50 (4.60–6.20) | 7.60 (6.30–8.80) | 8.80 (7.70–10.20) |
| NHANES | 18–39 | 674 | 3.49 (3.20–3.79) | 3.60 (3.20–3.80) | 5.90 (5.30–6.50) | 9.50 (8.20–10.60) | 11.30 (10.40–14.10) |
| SHOW | 40–59 | 208 | 4.46 (3.97–5.00) | 4.90 (4.20–5.50) | 8.20 (7.00–9.10) | 10.80 (9.60–12.30) | 12.60 (11.10–16.50) |
| NHANES | 40–59 | 581 | 5.20 (4.77–5.66) | 5.30 (4.60–5.70) | 8.20 (7.10–9.70) | 13.60 (11.40–16.00) | 17.40 (15.70–22.60) |
| SHOW | 60+ | 233 | 7.39 (6.72–8.312) | 7.80 (7.20–8.70) | 11.20 (10.40–12.80) | 16.60 (14.10–18.70) | 19.70 (17.20–25.30) |
| NHANES | 60+ | 574 | 7.56 (6.83–8.37) | 7.90 (7.10–8.50) | 12.70 (11.10–13.60) | 19.10 (16.70–22.10) | 26.30 (21.80–30.50) |
| Sex | |||||||
| SHOW | Male | 257 | 5.08 (4.49–5.74) | 5.70 (5.20–6.40) | 8.90 (8.20–9.30) | 12.20 (10.70–14.30) | 16.40 (13.60–18.50) |
| NHANES | Male | 865 | 6.59 (6.16–7.05) | 6.60 (6.00–7.10) | 10.40 (9.60–11.10) | 16.10 (14.10–18.90) | 22.00 (19.00–24.50) |
| SHOW | Female | 346 | 4.02 (3.67–4.41) | 4.10 (3.70–4.60) | 7.20 (6.40–8.00) | 10.90 (9.70–12.20) | 13.60 (11.60–16.40) |
| NHANES | Female | 964 | 3.81 (3.53–4.12) | 3.80 (3.40–4.10) | 6.50 (5.90–7.20) | 11.10 (10.20–12.60) | 15.80 (13.20–17.80) |
| Race | |||||||
| SHOW | White (Non-Hispanic) | 502 | 4.91 (4.54–5.31) | 5.45 (4.92–5.87) | 8.50 (7.82–9.10) | 12.14 (11.02–13.46) | 15.59 (13.49–16.81) |
| NHANES | White (Non-Hispanic) | 563 | 5.16 (4.77–5.58) | 5.30 (4.74–5.62) | 9.03 (7.90–10.17) | 13.41 (12.30–15.88) | 17.64 (15.97–21.94) |
| SHOW | Non-White | 100 | 3.16 (2.55–3.91) | 3.57 (2.93–4.19) | 5.75 (4.43–8.03) | 9.25 (7.66–11.06) | 10.48 (8.75–18.06) |
| NHANES | Non-White | 1266 | 4.65 (4.38–4.95) | 4.73 (4.40–5.10) | 8.34 (7.74–8.85) | 14.51 (12.99–15.99) | 21.79 (18.29–23.54) |
CI confidence interval.
Table 6.
Comparison of geometric means and percentiles of serum PFNA (in ng/mL) in SHOW (2014–2016) and NHANES (2015–2016) by age, sex and race.
| Sample | Characteristic | n | Geometric mean (95% CI) | 50th (95% CI) | 75th (95% CI) | 90th (95% CI) | 95th (95% CI) |
|---|---|---|---|---|---|---|---|
| Age | |||||||
| SHOW | 18–39 | 162 | 0.36 (0.32–0.41) | 0.40 (0.30–0.40) | 0.60 (0.50–0.70) | 0.80 (0.70–1.10) | 1.10 (0.80–1.40) |
| NHANES | 18–39 | 674 | 0.46 (0.43–0.50) | 0.40 (0.30–0.40) | 0.70 (0.60–0.70) | 1.00 (0.90–1.00) | 1.30 (1.10–1.50) |
| SHOW | 40–59 | 208 | 0.44 (0.40–0.48) | 0.40 (0.40–0.50) | 0.60 (0.60–0.70) | 0.90 (0.80–1.20) | 1.30 (1.00–1.70) |
| NHANES | 40–59 | 581 | 0.60 (0.55–0.66) | 0.50 (0.50–0.60) | 0.90 (0.80–1.00) | 1.30 (1.20–1.50) | 1.90 (1.40–2.30) |
| SHOW | 60+ | 233 | 0.62 (0.56–0.67) | 0.60 (0.60–0.70) | 0.90 (0.80–0.90) | 1.40 (1.20–1.80) | 2.10 (1.50–2.60) |
| NHANES | 60+ | 574 | 0.78 (0.71–0.85) | 0.70 (0.60–0.80) | 1.10 (1.00–1.30) | 1.70 (1.50–2.00) | 2.30 (1.90–2.60) |
| Sex | |||||||
| SHOW | Male | 257 | 0.47 (0.43–0.51) | 0.50 (0.40–0.50) | 0.70 (0.60–0.80) | 1.00 (0.90–1.20) | 1.40 (1.10–2.10) |
| NHANES | Male | 865 | 0.64 (0.60–0.69) | 0.50 (0.40–0.50) | 0.90 (0.80–1.00) | 1.40 (1.10–1.40) | 1.90 (1.50–2.10) |
| SHOW | Female | 346 | 0.44 (0.40–0.48) | 0.40 (0.40–0.50) | 0.70 (0.60–0.80) | 1.10 (0.90–1.30) | 1.40 (1.20–2.00) |
| NHANES | Female | 964 | 0.54 (0.50–0.58) | 0.40 (0.30–0.40) | 0.80 (0.70–0.80) | 1.30 (1.10–1.40) | 1.90 (1.40–2.40) |
| Race | |||||||
| SHOW | White (Non-Hispanic) | 502 | 0.48 (0.45–0.51) | 0.50 (0.40–0.50) | 0.70 (0.70–0.80) | 1.10 (0.90–1.30) | 1.50 (1.30–2.10) |
| NHANES | White (Non-Hispanic) | 563 | 0.58 (0.54–0.62) | 0.50 (0.40–0.50) | 0.90 (0.70–0.90) | 1.30 (1.10–1.40) | 1.90 (1.40–2.30) |
| SHOW | Non-White | 100 | 0.36 (0.31–0.41) | 0.30 (0.30–0.40) | 0.60 (0.50–0.70) | 0.80 (0.60–1.10) | 1.00 (0.80–1.50) |
| NHANES | Non-White | 1266 | 0.60 (0.57–0.63) | 0.50 (0.40–0.50) | 0.90 (0.80–1.00) | 1.40 (1.30–1.50) | 1.90 (1.60–2.00) |
CI confidence interval.
Table 5.
Comparison of geometric means and percentiles of serum PFOA (in ng/mL) in SHOW (2014–2016) and NHANES (2015–2016) by age, sex and race.
| Sample | Characteristic | n | Geometric mean (95% CI) | 50th (95% CI) | 75th (95% CI) | 90th (95% CI) | 95th (95% CI) |
|---|---|---|---|---|---|---|---|
| Age | |||||||
| SHOW | 18–39 | 162 | 0.93 (0.82–1.05) | 1.10 (0.90–1.20) | 1.60 (1.40–1.80) | 2.00 (1.90–2.30) | 2.40 (2.00–3.20) |
| NHANES | 18–39 | 674 | 1.31 (1.21–1.40) | 1.40 (1.20–1.40) | 2.00 (1.80–2.20) | 2.80 (2.50–3.00) | 3.50 (2.90–3.90) |
| SHOW | 40–59 | 208 | 1.28 (1.16–1.41) | 1.30 (1.20–1.50) | 1.80 (1.70–2.10) | 2.60 (2.40–3.10) | 3.30 (2.70–4.60) |
| NHANES | 40–59 | 581 | 1.64 (1.53–1.77) | 1.60 (1.40–1.80) | 2.40 (2.10–2.50) | 3.30 (3.10–3.60) | 3.90 (3.50–5.20) |
| SHOW | 60+ | 233 | 1.51 (1.38–1.65) | 1.50 (1.40–1.80) | 2.30 (2.10–2.50) | 3.30 (2.80–3.50) | 3.50 (3.40–4.20) |
| NHANES | 60+ | 574 | 2.01 (1.81–2.22) | 2.10 (1.90–2.20) | 2.80 (2.60–3.10) | 4.20 (3.60–4.90) | 5.20 (4.60–6.90) |
| Sex | |||||||
| SHOW | Male | 257 | 1.25 (1.13–1.37) | 1.40 (1.30–1.50) | 1.90 (1.80–2.00) | 2.60 (2.40–3.00) | 3.20 (2.90–3.60) |
| NHANES | Male | 865 | 1.85 (1.75–1.96) | 1.90 (1.70–2.00) | 2.60 (2.50–2.70) | 3.40 (3.20–3.60) | 4.10 (3.60–5.01) |
| SHOW | Female | 346 | 1.15 (1.06–1.25) | 1.20 (1.10–1.20) | 1.80 (1.70–2.00) | 2.70 (2.40–3.10) | 3.50 (2.90–4.20) |
| NHANES | Female | 964 | 1.39 (1.29–1.49) | 1.40 (1.30–1.50) | 2.30 (2.00–2.40) | 3.40 (2.90–3.90) | 4.30 (3.90–6.10) |
| Race | |||||||
| SHOW | White (Non-Hispanic) | 502 | 1.28 (1.20–1.37) | 1.40 (1.30–1.50) | 1.90 (1.80–2.00) | 2.80 (2.50–3.10) | 3.40 (3.10–4.00) |
| NHANES | White (Non-Hispanic) | 563 | 1.72 (1.60–1.84) | 1.80 (1.60–1.90) | 2.60 (2.40–2.70) | 3.50 (3.20–3.80) | 4.60 (3.90–5.60) |
| SHOW | Non-White | 100 | 0.90 (0.77–1.05) | 1.00 (0.80–1.10) | 1.40 (1.20–1.80) | 2.00 (1.60–2.50) | 2.40 (2.00–3.00) |
| NHANES | Non-White | 1266 | 1.40 (1.34–1.47) | 1.40 (1.30–1.40) | 2.10 (2.00–2.20) | 3.10 (2.80–3.30) | 4.00 (3.60–4.10) |
CI confidence interval.
Serum levels increased with age in both SHOW and NHANES for all 6 PFAS compounds. In SHOW, 18–39-year-olds had a PFOS geometric mean of 3.13 (2.71–3.62) ng/mL compared to 60+ year-olds 7.39 (6.72–8.31) ng/mL;whereas the 95th percentile was 8.80 (7.70–10.20) ng/mL for 18–39-year-olds and 26.3 (21.8–30.5) ng/mL for 60+ year-olds. Similar trends held for NHANES (Table 4).
In SHOW, males had higher serum levels of PFOS, PFOA, PFNA, PFHxS, and PFHPS than females; a difference in weighted geometric means of 1.06, 0.1, 0.03, and 0.5 ng/mL, respectively (Tables 4-6, and Supplementary Tables 2 and 3). Levels did not differ by sex for PFDA (Supplementary Table 1); these trends by sex were seen in both the NHANES and SHOW samples for all PFAS.
Weighted geometric mean serum levels were higher among whites (non-Hispanic) in SHOW and NHANES for PFOA, PFOS, PFHxS and PFHPS when compared to nonwhites. These trends held in the 50–95th percentiles for SHOW, but for NHANES, these trends were opposite at the higher percentiles (90th and 95th) for PFHPS and PFOS. PFHPS was 40% higher, and PFOS was 20% higher, among nonwhites compared to whites (non-Hispanic) in NHANES at the 95th percentile (Tables 4, 5 and Supplementary Tables 2, 3).
SHOW and NHANES samples were largely comparable for other PFAS compounds, (see Supplementary Tables 1-5), albeit low levels of detection were seen for both SHOW and NHANES for these additional PFAS compounds.
DISCUSSION
To our knowledge this is the first study to characterize PFAS serum levels among a statewide representative sample and compare levels to those of NHANES. This study expanded biomonitoring to a panel of 38 PFAS compounds and detected concentrations in serum with LLOD lower than many prior studies. The study leveraged the SHOW cohort, the only statewide adult cohort in the U.S. modeled after NHANES to provide baseline data on health for the state of Wisconsin. Beyond NHANES, current understanding of human serum PFAS levels come from specific sub-population cohorts (i.e. occupational, maternal-child cohorts) [25, 43], high-risk or localized communities near contamination [26-28]. While baseline PFAS levels are known at a national scale using NHANES, those data lack the granularity needed at the state level and within subregions and strata in a state. SHOW’s statewide cohort provides PFAS serum prevalence for the state, and within subpopulations and communities, spanning different demographics, socio-economic backgrounds, and neighborhood environments. This study fills a data gap and allows for identification of previously unknown high-risk areas or subpopulations upon which researchers and state health officials can target additional biomonitoring and testing.
Among the 38 PFAS analytes tested, six were widely prevalent among SHOW;more than 96% of SHOW participants had detectable serum levels of PFOS, PFOA, PFNA, PFHxS, PFDA, and PFHpS. These six PFAS compounds are further classified as long chained perfluoroalkyl sulfonic acids (PFSAs), indicating they have a sulfonic function head and include more than five carbons [9, 11, 44, 45]. Long-chain PFAS have longer half-lives and great bioaccumulation in the environment and mammalian blood and tissue than short chain PFAS. Hence, it is not surprising short-chain compounds tested were less often detected compared to long-chain PFAS, and these findings align with findings in other studies. For example, Yu and colleagues conducted statewide testing in New Jersey from remnant sera from clinical labs and blood banks (n = 1030) and of the 12 PFAS compounds tested, PFOA, PFNA, PFOS, and PFHxS were the ones detected in over 99% of the study population with geometric means greater than 0.5 μg/L [43]. Even among high-risk or communities near contamination sites, the same PFAS compounds were the most widespread, and with the highest geometric means. Among n = 192 claimants from class-action lawsuit in Paulsboro, New Jersey, who lived near a contamination site, of 13 PFAS compounds tested, PFOS, PFOA, PFNA and PFHxS had the highest prevalence, with over 70% with detectable levels [26]. This held true in other states, such as the communities tested near military bases in Pennsylvania [46] where PFOS, PFOA, PFHxS, and PFNA were highest and most prevalent of the 11 PFAS tested, and those in the Annison Community Health Survey living near a high-risk manufacturing site in Alabama, where among the 8 PFAS tested, PFOS, PFNA, PFOA, and PFHxS were detected in >96% [28].
Overall, geometric means across demographic strata among SHOW participants were similar to those seen in NHANES. Yet, NHANES consistently had slightly higher geometric means for all 6 main PFAS compounds analyzed (PFOS, PFOA, PFNA, PFDA, PFHxS, PFHPS), even though only PFHPS, PFDA, and PFNA were statistically significantly different at 0.05 level. While we are unsure why this is the case, it may be that Wisconsin has lower environmental levels of PFAS and/or slightly lower use of PFAS-containing products. PFOS was among the most prevalent of the PFAS compounds measured among the SHOW sample and had the highest geometric mean and percentile concentrations. This was also true among NHANES, as was seen among nearly all prior biomonitoring studies, including those who tested high-risk populations such as firefighters and communities near contamination sites [26-28, 47, 48]. Higher serum concentrations levels of PFOS are not surprising due its (and PFOA’s) U.S. production since the 1940s, which peaked between 1970 and 2002. PFOS and PFOA were the most widely used PFAS compounds in consumer products up until recently [11].
PFAS levels were higher in males compared to females, and in older individuals compared to younger ones. This held true across all PFAS compounds analyzed in SHOW, as well as in NHANES and other studies. For example, communities tested near military bases in Pennsylvania [46] found PFOS, PFOA, and PFHxS were all higher in males, and all increased with age. Those tested living nearby a PFAS manufacturing facility in New Jersey saw similar increases in PFAS concentration with age and were higher among males [26]. Olsen et al. similarly found sex and age trends as seen in NHANES [49]. Lower concentrations in females may be explained by greater excretion rate through menstrual blood loss and lactation [50]. We would expect PFAS concentrations to be higher among older adults compared to younger adults due to the bioaccumulation that occurs as one ages, in conjunction with potentially higher exposure >10 years ago before PFAS started being manufactured overseas and phased out of products.
Both SHOW and NHANES saw similar trends in PFAS concentrations by race, with non-Hispanic whites having higher geometrics means compared to non-whites. While in SHOW, this trend remained across percentiles, the NHANES sample saw opposite trends at the 90th and 95th percentile for some compounds (PFHPS, PFDA, PFNA, PFOS), where non-whites had higher PFAS concentrations than non-Hispanic whites. This difference may be due to the small non-white sample in SHOW and therefore an inability to adequately capture potential racial differences in exposure and risk. Non-whites only account for 18.7% of the SHOW sample, compared with approximately 35% in each NHANES sample. While the SHOW study oversampled non-whites in 2019, they did not conduct oversampling of non-whites during their statewide sampling years (2014–2016) used in this study. Due to Wisconsin’s proportionately small non-white population, the non-white sample in the SHOW study sample is small. This likely resulted in little variability within the non-white subpopulation which may have resulted in an unrepresentative substratum of non-whites in the state. As such, those who were included are weighted to represent a larger proportion of their racial/ethnic subpopulation. Racial and ethnic minorities, and those of lower socio-economic and education attainment, are more likely to reside near industrial and contamination sites that are at greater risk of exposure to environmental contaminants of concern, such as PFAS. This was seen in biomonitoring of a high-risk community due to manufacturing in Alabama where PFOS levels were ~2 times higher in African Americans compared to whites in the study [28]. Other studies have found the opposite, those with higher education status and income level had higher levels of PFAS [51, 52]. These results suggest that proximity to contaminated PFAS sites rather than race may be a more important predictor of PFAS concentration, but additional research is needed. Furthermore, as mentioned above, short-chain PFAS may be in the environment but may not be detectable in human serum. Furthermore, as mentioned above, short-chain PFAS may be in the environment but may not be detectable in human serum.
The SHOW’s relatively small substratum of non-whites was a limitation of the study. This may have resulted in a less balanced comparison to NHANES and limited the study’s ability to identify racial disparities relating to PFAS that may exist in Wisconsin. While race may be an important predictor of PFAS exposure, there are other demographic differences between SHOW and NHANES that may have contributed to differences in the comparison of results. On average, SHOW participants had a slightly higher education level and income to poverty ratio than NHANES participants in all sampling years. It is well-known that lower income status is associated with increased adverse environmental exposures [53], which may have contributed to the lower levels of PFAS in SHOW participants than may exist in the state’s population. There is evidence that higher PFAS exposure is associated with lower educational attainment [47].
While the SHOW 2014–2016 sample is representative of the state, its primary sampling unit was at the county level, and only 10 of 72 counties were represented. Selection criteria used for SHOW’s sampling frame to ensure representation was based on socioeconomic status and population density; geographical representation beyond Census urban rural stratification was not a factor. This is a significant limitation of the study as northern regions of the state, and more isolated areas, are not represented. Future PFAS biomonitoring in the state should include oversampling of non-whites and geographical representation to adequately capture varied land use throughout the state; ensuring unique areas in central sands regions and northeast areas with karst geology are included, due to potentially vulnerable groundwater contamination. In addition, data on education and smoking status were unavailable for 18- and 19-year-olds for NHANES, so some demographic comparisons do not include these individuals. Systematic differences in PFAS concentrations may also be due to different laboratory procedures between SHOW and NHANES, which may explain some of the overall differences in exposure metrics. Lastly, this study was cross-sectional, with serum concentrations from over 6 years ago. Hence, not only can causality not be inferred, but changes over time are not captured, and current PFAS concentrations among the state’s population today may be different than seen in this study. Future PFAS biomonitoring in Wisconsin should consider repeat testing, especially as short chain PFAS become more widely used and long chain PFAS are phased out.
While this study has several limitations, it has many strengths. This is the first statewide representative cohort for which we have baseline PFAS serum concentrations. Studies-to-date have conducted biomonitoring on specific cohorts (i.e. California Teachers Study), among high-risk occupational workers (Firefighters), or communities residing near a contamination site. While Yu et al. conducted statewide biomonitoring, they relied convenience testing from clinical and lab sera [43], whereas SHOW relied on probability sampling to produce a statewide representative sample with weights. This study also tested a wider range of PFAS compounds with LLOD for many. This was an important contribution to the field and increased our understanding of the extent other PFAS compounds are in the environment and in our bodies.
These data suggest that Wisconsin residents may not be disproportionately burdened by PFAS contamination compared to the wider US population. However, there are known pockets of environmental contamination found in regions not captured with this statewide representative sample and more research is needed to determine the extent of PFAS exposure in Wisconsin which ensures geographical variation and adequate oversampling of non-whites. This is the beginning of our understanding of PFAS exposure among Wisconsin residents, but importantly, it offers a baseline prevalence of PFAS. Future directions include utilizing the rich SHOW survey data to better characterize PFAS serum levels based on diet, housing, and other factors. These findings can help state agencies in resource allocation for additional PFAS biomonitoring, and direct resources where most needed. In addition, residential address and residential history is known for SHOW participants, which increases the capacity to study cumulative exposure to PFAS through neighborhood-level contextual factors like industrial sites and nearby land uses through the adult life course. Finally, longitudinal follow-up should be conducted in the future to track changes in PFAS burden in the population over time.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank all the SHOW investigators who made this work possible, WSLH staff who contributed to the creation of this data, and the SHOW participants. Special acknowledgement to the WSLH, NS for conceptualization and BS for sample analysis.
FUNDING
Funding for SHOW comes from the Wisconsin Division of Public Health, the Wisconsin Partnership Program (PERC) Award (223 PRJ 25DJ), the National Institutes of Health’s Clinical and Translational Science Award (5UL 1RR025011), and the National Heart Lunch and Blood Institute (1 RC2 HL101468) and a core grant to the Center for Demography and Ecology at the University of Wisconsin-Madison (P2C HD047873). Additional funding for this analysis came from the Association of Public Health Laboratories (APHL).
Footnotes
COMPETING INTERESTS
The authors declare no competing interests.
ETHICAL APPROVAL
The SHOW protocol and informed consent documents are approved by the Health Sciences Institutional Review Board of the University of Wisconsin-Madison. Participants in SHOW gave consent to their information being used for research prior to this study.
Supplementary information The online version contains supplementary material available at https://doi.org/10.1038/s41370-023-00593-3.
DATA AVAILABILITY
The datasets generated during and analyzed during the current study are not publicly available due to HIPAA protections for SHOW participants but may be available from the corresponding author on reasonable request with IRB approval.
REFERENCES
- 1.Ghisi R, Vamerali T, Manzetti S. Accumulation of perfluorinated alkyl substances (PFAS) in agricultural plants: a review. Environ Res. 2019;169:326–41. [DOI] [PubMed] [Google Scholar]
- 2.Sunderland EM, Hu XC, Dassuncao C, Tokranov AK, Wagner CC, Allen JG. A review of the pathways of human exposure to poly- and perfluoroalkyl substances (pfass) and present understanding of health effects. J Exposure Sci Environ Epidemiol. 2018;29:131–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Panieri E, Baralic K, Djukic-Cosic D, Buha Djordjevic A, Saso L. PFAS molecules: a major concern for the human health and the environment. Toxics. 2022;10:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haukås M, Berger U, Hop H, Gulliksen B, Gabrielsen GW. Bioaccumulation of per- and polyfluorinated alkyl substances (PFAS) in selected species from the Barents Sea Food web. Environ Pollut. 2007;148:360–71. [DOI] [PubMed] [Google Scholar]
- 5.Per- and polyfluorinated substances (PFAS) factsheet [Internet]. Centers for Disease Control and Prevention. Centers for Disease Control and Prevention; 2022. Available from: https://www.cdc.gov/biomonitoring/PFAS_FactSheet.html. [Google Scholar]
- 6.Hu XC, Andrews DQ, Lindstrom AB, Bruton TA, Schaider LA, Grandjean P, et al. Detection of poly- and perfluoroalkyl substances (pfass) in U.S. drinking water linked to industrial sites, military fire training areas, and wastewater treatment plants. Environ Sci Technol Lett. 2016;3:344–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Faithfull NS, Weers JG. Perfluorocarbon compounds. Vox Sanguinis. 1998;74:243–8. [DOI] [PubMed] [Google Scholar]
- 8.Sinclair GM, Long SM, Jones OAH. What are the effects of PFAS exposure at environmentally relevant concentrations? Chemosphere 2020;258:127340. [DOI] [PubMed] [Google Scholar]
- 9.Fenton SE, Ducatman A, Boobis A, DeWitt JC, Lau C, Ng C, et al. Per- and polyfluoroalkyl substance toxicity and human health review: current state of knowledge and strategies for informing future research. Environ Toxicol Chem. 2020;40:606–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kurwadkar S, Dane J, Kanel SR, Nadagouda MN, Cawdrey RW, Ambade B, et al. Per- and polyfluoroalkyl substances in water and wastewater: a critical review of their global occurrence and distribution. Sci Total Environ. 2022;809:151003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeWitt JC. Toxicological effects of perfluoroalkyl and polyfluoroalkyl substances. Cham, New York: Springer International Publishing; 2015. [Google Scholar]
- 12.Nelson JW, Hatch EE, Webster TF. Exposure to polyfluoroalkyl chemicals and cholesterol, body weight, and insulin resistance in the general U.S. population. Environ Health Perspect. 2010;118:197–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bassler J, Ducatman A, Elliott M, Wen S, Wahlang B, Barnett J, et al. Environmental perfluoroalkyl acid exposures are associated with liver disease characterized by apoptosis and altered serum adipocytokines. Environ Pollut. 2019;247:1055–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wikström S, Lin P-I, Lindh CH, Shu H, Bornehag C-G. Maternal serum levels of perfluoroalkyl substances in early pregnancy and offspring birth weight. Pediatr Res. 2019;87:1093–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.von Holst H, Nayak P, Dembek Z, Buehler S, Echeverria D, Fallacara D, et al. Perfluoroalkyl substances exposure and immunity, allergic response, infection, and asthma in children: review of epidemiologic studies. Heliyon. 2021;7:e08160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borghese MM, Walker M, Helewa ME, Fraser WD, Arbuckle TE. Association of perfluoroalkyl substances with gestational hypertension and preeclampsia in the MIREC study. Environ Int. 2020;141:105789. [DOI] [PubMed] [Google Scholar]
- 17.Granum B, Haug LS, Namork E, Stølevik SB, Thomsen C, Aaberge IS, et al. Pre-natal exposure to perfluoroalkyl substances may be associated with altered vaccine antibody levels and immune-related health outcomes in early childhood. J Immunotoxicol. 2013;10:373–9. [DOI] [PubMed] [Google Scholar]
- 18.Brennan NM, Evans AT, Fritz MK, Peak SA, von Holst HE. Trends in the regulation of per- and polyfluoroalkyl substances (PFAS): a scoping review. Int J Environ Res Public Health. 2021;18:10900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen J, Tang L, Chen W-Q, Peaslee GF, Jiang D. Flows, stock, and emissions of poly- and perfluoroalkyl substances in California carpet in 2000–2030 under different scenarios. Environ Sci Technol. 2020;54:6908–18. [DOI] [PubMed] [Google Scholar]
- 20.Podder A, Sadmani AHMA, Reinhart D, Chang N-B, Goel R. Per and polyfluoroalkyl substances (PFAS) as a contaminant of emerging concern in Surface Water: a transboundary review of their occurrences and toxicity effects. J Hazard Mater. 2021;419:126361. [DOI] [PubMed] [Google Scholar]
- 21.US EPA. Lifetime Drinking Water Health Advisories for Four Perfluoroalkyl Substances. 2022; 87 FRL 9855–01–OW. (To be codified and 87 C.F.R. pts. 36848, 36849).
- 22.Kidd J, Fabricatore E, Jackson D. Current and future federal and state sampling guidance for per- and polyfluoroalkyl substances in environmental matrices. Sci Total Environ. 2022;836:155523. [DOI] [PubMed] [Google Scholar]
- 23.Chang CJ, Ryan PB, Smarr MM, Kannan K, Panuwet P, Dunlop A, et al. Serum per- and polyfluoroalkyl substance (PFAS) concentrations and predictors of exposure among pregnant African American women in the Atlanta area, Georgia. Environ Res. 2021;157:106843. Available from: https://www.sciencedirect.com/science/article/pii/S0160412021004682?via%3Dihub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Birnbaum LS, Grandjean P. Alternatives to pfass: perspectives on the science. Environ Health Perspect. 2015;123:A104–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aylward LL, Seiber JN, Hays SM. California biomonitoring data: comparison to NHANES and interpretation in a risk assessment context. Regulatory Toxicol Pharmacol. 2015;73:875–84. [DOI] [PubMed] [Google Scholar]
- 26.Graber JM, Alexander C, Laumbach RJ, Black K, Strickland PO, Georgopoulos PG, et al. Per and polyfluoroalkyl substances (PFAS) blood levels after contamination of a community water supply and comparison with 2013–2014 NHANES. J Exposure Sci Environ Epidemiol. 2018;29:172–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Graber JM, Black TM, Shah NN, Caban-Martinez AJ, Lu SE, Brancard T, et al. Prevalence and predictors of per- and polyfluoroalkyl substances (PFAS) serum levels among members of a suburban US volunteer fire department. Int J Environ Res Public Health. 2021;18:3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Petriello MC, Mottaleb MA, Serio TC, Balyan B, Cave MC, Pavuk M, et al. Serum concentrations of legacy and emerging per- and polyfluoroalkyl substances in the Anniston Community Health Surveys (ACHS I and ACHS II). Environ Int. 2022;158:106907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu CH, Weisel CP, Alimokhtari S, Georgopoulos PG, Fan ZT. Biomonitoring: a tool to assess PFNA body burdens and evaluate the effectiveness of drinking water intervention for communities in New Jersey. Int J Hyg Environ Health. 2021;235:113757. [DOI] [PubMed] [Google Scholar]
- 30.Multiple state agencies responding to PFAS contamination on French Island [Internet]. Wisconsin Department of Natural Resources. Wisconsin Department of Natural Resources; 2021. Available from: https://dnr.wisconsin.gov/newsroom/release/42441. [Google Scholar]
- 31.Pfas contamination in the Marinette and Peshtigo Area [Internet]. PFAS Contamination in the Marinette and Peshtigo Area ∣ ∣ Wisconsin DNR. Wisconsin Department of Natural Resources; 2022. Available from: https://dnr.wisconsin.gov/topic/PFAS/Marinette.html. [Google Scholar]
- 32.Jha G, Kankarla V, McLennon E, Pal S, Sihi D, Dari B, et al. Per- and polyfluoroalkyl substances (PFAS) in integrated crop–livestock systems: environmental exposure and human health risks. Int J Environ Res Public Health. 2021;18:12550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnson GR. Pfas in soil and groundwater following historical land application of Biosolids. Water Res. 2022;211:118035. [DOI] [PubMed] [Google Scholar]
- 34.Water utility [Internet]. City of Madison, Wisconsin. Available from: https://www.cityofmadison.com/water/water-quality/water-quality-testing/perfluorinated-compounds. [Google Scholar]
- 35.Staff AC. Wisconsin population increased 3.6% since 2010 [Internet]. Census.gov. United States Census Bureau; 2021. Available from: https://www.census.gov/library/stories/state-by-state/wisconsin-population-change-between-census-decade.html. [Google Scholar]
- 36.Malecki KM, Nikodemova M, Schultz AA, LeCaire TJ, Bersch AJ, Cadmus-Bertram L, et al. The survey of the Health of Wisconsin (show) program: an infrastructure for advancing population health. Front Public Health. 2022;10:818777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Minnesota Dept. of Public Health, Public Health Laboratory Division. Document DOC-563, Detection of PFAS in serum and Plasma via LC-MS/MS. Unpublished methods.
- 38.Centers for Disease Control & Prevention, National Center for Environmental Health. Laboratory Procedure manual NHANES 2015-16, method 6304.08. Perfluoroalkyl and Polyfluoroalkyl Substances. [Google Scholar]
- 39.New York State Dept. of Health, Division of Environmental Health Sciences, Laboratory of Organic Analytical Chemistry. Analysis of Perfluoroalkyl Substances In Human Serum by Solid Phase Extraction and Ultra High Performance Liquid Chromatography Tandem Mass Spectrometry. Unpublished methods.
- 40.Michigan Dept. of Community Health, Bureau of Laboratories. Document AC-54. Analysis of serum for PFAS by RP-HPLC-MRM-MS/MS. 2018. Unpublished methods.
- 41.Centers for Disease Control and Prevention. Nhanes - about the National Health and Nutrition Examination Survey. 2017. https://www.cdc.gov/nchs/nhanes/about_nhanes.htm (accessed 2 May 2022).
- 42.Prior HHS Poverty Guidelines and Federal Register references [Internet]. ASPE. 2022. Available from: https://aspe.hhs.gov/topics/poverty-economic-mobility/poverty-guidelines/prior-hhs-poverty-guidelines-federal-register-references. [Google Scholar]
- 43.Yu CH, Riker CD, Lu SE, Fan ZT. Biomonitoring of emerging contaminants, perfluoroalkyl and polyfluoroalkyl substances (PFAS), in New Jersey Adults in 2016–2018. Int J Hyg Environ Health. 2020;223:34–44. [DOI] [PubMed] [Google Scholar]
- 44.Brunn H, Arnold G, Körner W, Rippen G, Steinhäuser KG, Valentin I. PFAS: forever chemicals—persistent, bioaccumulative and mobile. Reviewing the status and the need for their phase out and remediation of contaminated sites. Environ Sci Eur. 2023;35:1–50. [Google Scholar]
- 45.Yao Y, Burgess J, Volchek K, Brown C. Short-Chain PFAS Their Sources, Properties, Toxicity, Environmental Fate, and Treatment. In: Kempisty D, Xing Y, Racz L, editors. Perfluoroalkyl Substances in the Environment Theory, Practice, and Innovation [Internet]. Boca Raton: CRC Press; 2018. p. 447–67. Available from: https://www.taylorfrancis.com/chapters/edit/10.1201/9780429487125-20/short-chain-pfas-yuan-yao-justin-burgess-konstantin-volchek-carl-brown?context=ubx&refId=afa9c083-21ea-4dae-9c6e-4050e54eb255. [Google Scholar]
- 46.Nair AS, Ma ZQ, Watkins SM, Wood SS. Demographic and exposure characteristics as predictors of serum per- and polyfluoroalkyl substances (pfass) levels – a community-level biomonitoring project in Pennsylvania. Int J Hyg Environ Health. 2021;231:113631. [DOI] [PubMed] [Google Scholar]
- 47.Sagiv SK, Rifas-Shiman SL, Webster TF, Mora AM, Harris MH, Calafat AM, et al. Sociodemographic and perinatal predictors of early pregnancy per- and polyfluoroalkyl substance (PFAS) concentrations. Environ Sci Technol. 2015;49:11849–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wattigney WA, Savadatti SS, Liu M, Pavuk M, Lewis-Michl E, Kannan K, et al. Biomonitoring of per- and polyfluoroalkyl substances in minority angler communities in Central New York State. Environ Res. 2022;204:112309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Olsen GW, Mair DC, Lange CC, Harrington LM, Church TR, Goldberg CL, et al. Per- and polyfluoroalkyl substances (PFAS) in American Red Cross Adult Blood Donors, 2000–2015. Environ Res. 2017;157:87–95. [DOI] [PubMed] [Google Scholar]
- 50.Ding N, Harlow SD, Batterman S, Mukherjee B, Park SK. Longitudinal trends in perfluoroalkyl and polyfluoroalkyl substances among multiethnic midlife women from 1999 to 2011: The study of women′s health across the nation. Environ Int. 2020;135:105381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.DeLuca NM, Thomas K, Mullikin A, Slover R, Stanek LW, Pilant AN, et al. Geographic and demographic variability in serum PFAS concentrations for pregnant women in the United States. J Exp Sci Environ Epidemiol. 2023:1–15. 10.1038/s41370-023-00520-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chang CJ, Ryan PB, Smarr MM, Kannan K, Panuwet P, Dunlop AL, et al. Serum per- and polyfluoroalkyl substance (PFAS) concentrations and predictors of exposure among pregnant African American women in the Atlanta area, Georgia. Environ Res. 2021;198:110445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Evans GW, Kantrowitz E. Socioeconomic status and health: The potential role of environmental risk exposure. Annu Rev Public Health. 2002;23:303–31. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and analyzed during the current study are not publicly available due to HIPAA protections for SHOW participants but may be available from the corresponding author on reasonable request with IRB approval.