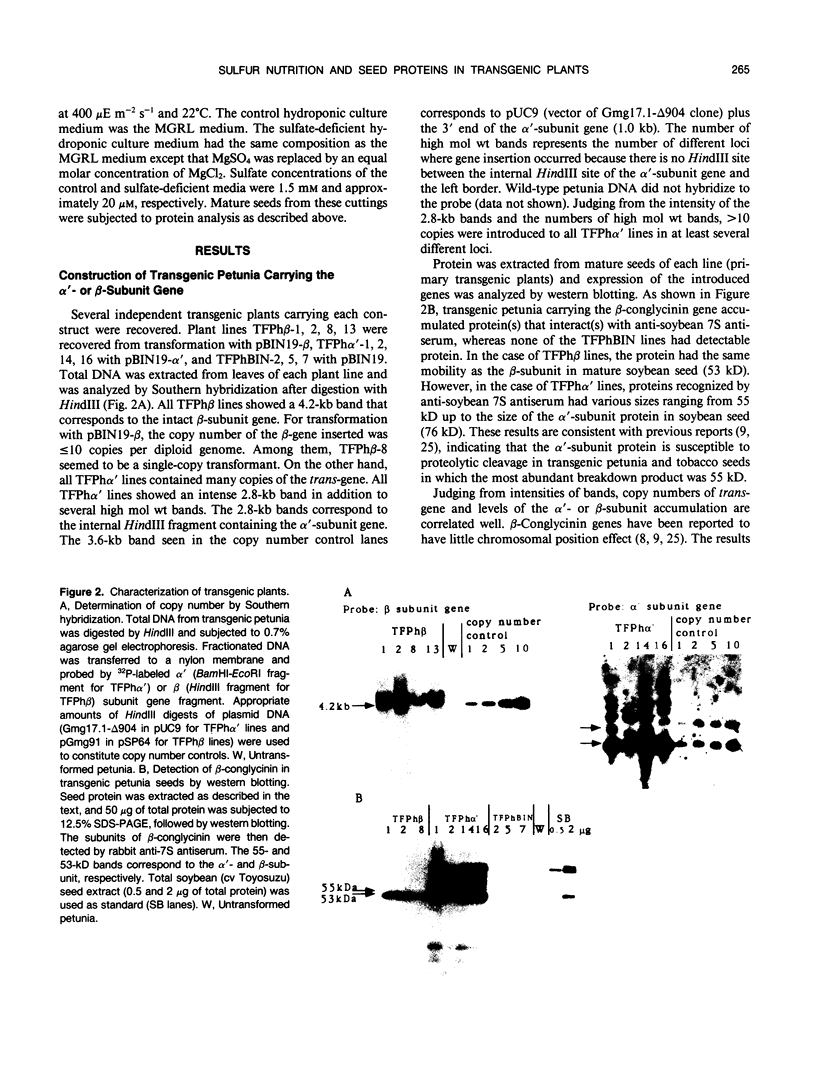
Abstract
The 7S seed storage protein (β-conglycinin) of soybean (Glycine max [L]. Merr.) has three major subunits; α, α′, and β. Accumulation of the β-subunit, but not the α- and α′-subunits, has been shown to be repressed by exogenously applied methionine to the immature cotyledon culture system (LP Holowach, JF Thompson, JT Madison [1984] Plant Physiol 74: 576-583) and to be enhanced under sulfate deficiency in soybean plants (KR Gayler, GE Sykes [1985] Plant Physiol 78: 582-585). Transgenic petunia (Petunia hybrida) harboring either the α′- or β-subunit gene were constructed to test whether the patterns of differential expression were retained in petunia. Petunia regulates these genes in a similar way as soybean in response to sulfur nutritional stimuli, i.e. (a) expression of the β-subunit gene is repressed by exogenous methionine in in vitro cultured seeds, whereas the α′-subunit gene expression is not affected; and (b) accumulation of the β-subunit is enhanced by sulfur deficiency. The pattern of accumulation of major seed storage protein of petunia was not affected by these treatments. These results indicate that this mechanism of gene regulation in response to sulfur nutrition is conserved in petunia even though it is not used to regulate its own major seed storage proteins.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barker S. J., Harada J. J., Goldberg R. B. Cellular localization of soybean storage protein mRNA in transformed tobacco seeds. Proc Natl Acad Sci U S A. 1988 Jan;85(2):458–462. doi: 10.1073/pnas.85.2.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach L. R., Spencer D., Randall P. J., Higgins T. J. Transcriptional and post-transcriptional regulation of storage protein gene expression in sulfur-deficient pea seeds. Nucleic Acids Res. 1985 Feb 11;13(3):999–1013. doi: 10.1093/nar/13.3.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beachy R. N., Chen Z. L., Horsch R. B., Rogers S. G., Hoffmann N. J., Fraley R. T. Accumulation and assembly of soybean beta-conglycinin in seeds of transformed petunia plants. EMBO J. 1985 Dec 1;4(12):3047–3053. doi: 10.1002/j.1460-2075.1985.tb04044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan M. Binary Agrobacterium vectors for plant transformation. Nucleic Acids Res. 1984 Nov 26;12(22):8711–8721. doi: 10.1093/nar/12.22.8711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z. L., Schuler M. A., Beachy R. N. Functional analysis of regulatory elements in a plant embryo-specific gene. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8560–8564. doi: 10.1073/pnas.83.22.8560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coates J. B., Medeiros J. S., Thanh V. H., Nielsen N. C. Characterization of the subunits of beta-conglycinin. Arch Biochem Biophys. 1985 Nov 15;243(1):184–194. doi: 10.1016/0003-9861(85)90787-8. [DOI] [PubMed] [Google Scholar]
- Creason G. L., Holowach L. P., Thompson J. F., Madison J. T. Exogenous methionine depresses level of mRNA for a soybean storage protein. Biochem Biophys Res Commun. 1983 Dec 28;117(3):658–662. doi: 10.1016/0006-291x(83)91647-9. [DOI] [PubMed] [Google Scholar]
- Deblaere R., Bytebier B., De Greve H., Deboeck F., Schell J., Van Montagu M., Leemans J. Efficient octopine Ti plasmid-derived vectors for Agrobacterium-mediated gene transfer to plants. Nucleic Acids Res. 1985 Jul 11;13(13):4777–4788. doi: 10.1093/nar/13.13.4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans I. M., Gatehouse J. A., Boulter D. Regulation of storage-protein synthesis in pea (Pisum sativum L.) cotyledons under conditions of sulphur deficiency. Biochem J. 1985 Nov 15;232(1):261–265. doi: 10.1042/bj2320261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figurski D. H., Helinski D. R. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gayler K. R., Sykes G. E. Effects of nutritional stress on the storage proteins of soybeans. Plant Physiol. 1985 Jul;78(3):582–585. doi: 10.1104/pp.78.3.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gayler K. R., Sykes G. E. beta-Conglycinins in Developing Soybean Seeds. Plant Physiol. 1981 May;67(5):958–961. doi: 10.1104/pp.67.5.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada J. J., Barker S. J., Goldberg R. B. Soybean beta-conglycinin genes are clustered in several DNA regions and are regulated by transcriptional and posttranscriptional processes. Plant Cell. 1989 Apr;1(4):415–425. doi: 10.1105/tpc.1.4.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holowach L. P., Madison J. T., Thompson J. F. Studies on the Mechanism of Regulation of the mRNA Level for a Soybean Storage Protein Subunit by Exogenous l-Methionine. Plant Physiol. 1986 Feb;80(2):561–567. doi: 10.1104/pp.80.2.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holowach L. P., Thompson J. F., Madison J. T. Effects of exogenous methionine on storage protein composition of soybean cotyledons cultured in vitro. Plant Physiol. 1984 Mar;74(3):576–583. doi: 10.1104/pp.74.3.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladin B. F., Tierney M. L., Meinke D. W., Hosángadi P., Veith M., Beachy R. N. Developmental Regulation of beta-Conglycinin in Soybean Axes and Cotyledons. Plant Physiol. 1987 May;84(1):35–41. doi: 10.1104/pp.84.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weising K., Schell J., Kahl G. Foreign genes in plants: transfer, structure, expression, and applications. Annu Rev Genet. 1988;22:421–477. doi: 10.1146/annurev.ge.22.120188.002225. [DOI] [PubMed] [Google Scholar]