Abstract
Objective
Long pentraxin‐3 (PTX‐3) is an acute phase protein associated with cardiovascular disease, lung injury, and mortality. We evaluated the association between computed tomography (CT)‐measurements of adipose tissue and plasma levels of PTX‐3.
Methods
We performed a cross‐sectional analysis of community‐dwelling adults enrolled in the multi‐center Multiethnic Study of Atherosclerosis who underwent cardiac or abdominal CT and had available PTX‐3 measurements.
Results
There was a U‐shaped association between pericardial adipose tissue volume (PAT), abdominal visceral adipose tissue area (VAT), hepatic attenuation, and PTX‐3 levels, with extremes of adiposity associated with greater PTX‐3 levels. Using multivariable‐adjusted piecewise regression models, among participants with low PAT, every 1% increase in PAT volume was associated with a 13.8% decrease in PTX‐3 (95% confidence interval [CI] −21.6 to −6.0); among participants with high PAT, every 1% increase in PAT volume was associated with a 6.0% increase in PTX‐3 (95% CI −0.4 to 12.5). Results were similar for abdominal VAT and hepatic attenuation.
Conclusions
In a cohort of community‐dwelling adults, we demonstrated a “U‐shaped” association between pericardial, abdominal visceral, and hepatic adiposity with PTX3 levels, suggesting that extreme adiposity is associated with greater circulating levels of PTX3. Further work is required to identify the mechanisms linking adiposity and PTX‐3.
Keywords: liver attenuation, long pentraxin‐3, pericardial adipose, visceral adipose
We demonstrated a non‐linear association between the adipose tissue and long pentraxin‐3 levels. This may explain the inconsistent findings in the prior literature evaluating this association. This association can now be used to justify additional investigations into whether long pentraxin‐3 could mediate the association between adiposity and cardiovascular, lung injury, or cancer.
1. INTRODUCTION
The prevalence of obesity is rising worldwide and is expected to affect 42% of the United States population by 2030. 1 Obesity is a risk factor for cardiovascular disease, lung injury, and cancer. A better understanding of the ways that excess adipose tissue contributes to these diseases, could identify novel targets for treatment and disease prevention.
During times of energy excess, fatty acids are stored in adipose tissue in either newly differentiated or pre‐existing adipocytes. 2 The addition of fatty acids to existing adipocytes results in adipocyte hypertrophy, increased production of inflammatory cytokines, and recruitment of pro‐inflammatory macrophages. 2 , 3 , 4 Levels of long pentraxin‐3 (PTX‐3), an acute phase protein produced by multiple cells including adipocytes, increase after 10–20 weeks of high fat diet in mice and PTX‐3 knock‐out mice have fewer inflammatory adipose tissue macrophages. 5 PTX‐3 may impair angiogenesis in adipose tissue, impeding the recruitment of pre‐adipocytes, and resulting in adipocyte hypertrophy with macrophage infiltration. 5 Whether these PTX‐3‐mediated changes in adipose tissue result in detectable changes in circulating levels of PTX‐3 is unclear.
The association between obesity and circulating PTX‐3 is inconsistent. Small studies demonstrated higher PTX‐3 levels in patients with obesity. 6 , 7 The presence of a haplotype of PTX‐3 that is associated with lower circulating levels was also associated with less central adiposity in both mice and humans. 5 In contrast, a large epidemiologic study in older Scandinavian men, demonstrated that more visceral adipose tissue on MRI appeared to be associated with lower PTX‐3 levels. 8 The association between adiposity and PTX‐3 levels may be modified by age and sex, thus limiting interpretation of these small studies, and those limited to older men. 9 , 10 Many of these studies also dichotomized obesity, missing potentially non‐linear relationships which are often present in analyses of body composition. 11
We therefore investigated the association between pericardial adipose tissue volume, abdominal visceral and subcutaneous adipose tissue cross‐sectional areas and attenuation, and ectopic adipose deposition in the liver with circulating PTX‐3 levels in a large multi‐center multi‐ethnic study of community‐dwelling adults. We hypothesized that increased adipose depot size and greater lipid deposition in the liver would be associated with higher levels of PTX‐3.
2. METHODS
2.1. Study participants
The Multi‐Ethnic Study of Atherosclerosis (MESA, ClinicalTrials.gov identifier NCT00005487) is an NHLBI‐funded multi‐center prospective cohort study of 6814 adults initially enrolled between 2000 and 2002. At the time of enrollment, patients were aged 45–84 years without clinically evident cardiovascular disease. Individuals were followed longitudinally with five additional follow‐up exams. MESA and all ancillary studies were approved by individual site Institutional Review Boards. Informed consent was obtained from all participants.
2.2. Pericardial adipose tissue volume and liver attenuation
The primary exposure of interest is PAT as measured on cardiac computed tomography (CT) scan at MESA exam 1 (2000–2002). PAT was quantified using previously published and validated techniques (Supporting Information S1: Methods). 12 , 13 PAT was operationalized in primary analyses as volume (cm3) and in secondary analyses as the PAT index (PAT volume/height2) to account for total body size and exposure. 14 Sensitivity analyses were performed with PAT volume measured at MESA exam 3 (2004–2005) since abdominal measures had been obtained at later exams. PAT attenuation was not measured. We evaluated correlations between PAT measures at exams 1 and 3 using Pearson correlations.
Cardiac CT scans contained images of the liver, which were used to quantify hepatic fat content. 15 Using previously described methods, three regions of interest (ROI) of at least 100 mm2 in size were identified (two in the right lobe and one in the left lobe), and mean attenuation in each ROI was recorded. 15
2.3. Abdominal adipose tissue area and attenuation
A random subset of 1947 individuals underwent abdominal CT scan between exams 2–4 (2002–2007). 16 Secondary exposures quantified on abdominal CT scan include abdominal visceral adipose tissue area (VAT) and its mean attenuation (lower attenuation indicating greater lipid content), abdominal subcutaneous adipose tissue area (SAT) and its mean attenuation. Measures obtained at the level of L3/L4 were included in the primary analysis. In sensitivity analyses, VAT was operationalized as the VAT index (VAT area/height2) to account for total body size and at the level of L4/L5. 14 Methods for quantifying adipose have been previously described. 17 As abdominal SAT was incompletely imaged on a subset of scans, imputation methods were used to estimate these missing values. 18 Imputed SAT values were used in SAT analyses.
2.4. Long pentraxin‐3
A sub‐group of 2880 patients, selected to achieve balanced race/ethnicity, underwent measures of PTX‐3 levels on plasma obtained at exam 1 (2000–2002). PTX‐3 was measured using a sandwich enzyme‐linked immune‐sorbent assay with the PTX‐3 (human) detection set (Alexis Biochemicals, San Diego, CA; coefficient of variation 10.2%) 19 at the Laboratory for Clinical Biochemistry Research at the University of Vermont.
2.5. Statistical analysis
PTX‐3 levels and all adipose depot measures were highly skewed and were log‐transformed. Changes in PTX‐3 levels are reported as percent‐change per 1% increase in adipose depot size. Adipose, and liver attenuation were normally distributed. Changes in PTX‐3 levels are reported per 1‐HU increase in attenuation.
Given the known quadratic associations between adiposity and various outcomes, 11 , 20 , 21 we investigated non‐linear associations between adipose measures and PTX‐3 using three methods: (1) generalized additive models with the “gam” function in R, (2) the Wald p‐value for the predictor2 term, and (3) the “utest” function for quadratic relationships in STATA. 11 , 14 , 22 We concluded there was a non‐linear association if findings were consistent across all three methods. We analyzed non‐linear associations using piecewise regression, which allows us to evaluate the association between the predictor and the outcomes in different intervals of the predictor, with a separate line fit for each interval. We defined two intervals with the cut‐point between these intervals defined as the extreme value quantified by the Utest function. 22 When there was insufficient evidence to support a non‐linear association, we used linear regression models. Analyses were adjusted for potential confounders including age, sex, race/ethnicity, estimated glomerular filtration rate, smoking status, physical activity, hypertension, and coronary artery disease measured by the Agatston coronary artery calcium score. We adjusted for the Agatston score given the known associations between PTX‐3 and vascular inflammation. 23 Physical activity was self‐reported as the amount of moderate and vigorous physical activity per week in Exam 1 using the MESA physical activity Questionnaire. 24 Analyses of liver attenuation were further adjusted for self‐reported alcohol use. 25 Subgroup analyses were defined a priori, by sex and age (<65 or ≥65).
All analyses were performed using STATA/SE version 16.1 (Statacorp, LP College Station, TX), and R version 3.3.1 (R Foundation for statistical computing).
3. RESULTS
3.1. Study participants
There were 6814 adults enrolled in MESA, of whom 2838 had available measures of PTX‐3 (Figure 1). Of the 2826 individuals with available measures of both PAT volume and PTX‐3, median age (IQR) was 61 (53–70), 46% were male, 25% were Caucasian, 25% were Asian, 25% were African‐American, median BMI (IQR) was 27.1 kg/m2 (23.9–30.7), median (IQR) PAT volume was 69.8 cm3 (49.8–87.3), and median (IQR) PTX3 level was 1.9 ng/mL (1.4–2.5) (Table S1). Eight‐hundred and twenty‐nine individuals had available measures of abdominal VAT, while 2809 had available measures of liver attenuation (Figure 1). Those with available measures of VAT and liver attenuation were similar in characteristics to the PAT cohort and full MESA cohort (Table S1). In the PAT sub‐cohort, individuals with high PAT were older, more likely to be male, less likely to be African‐American and had significantly higher weight and BMI (Table 1).
FIGURE 1.
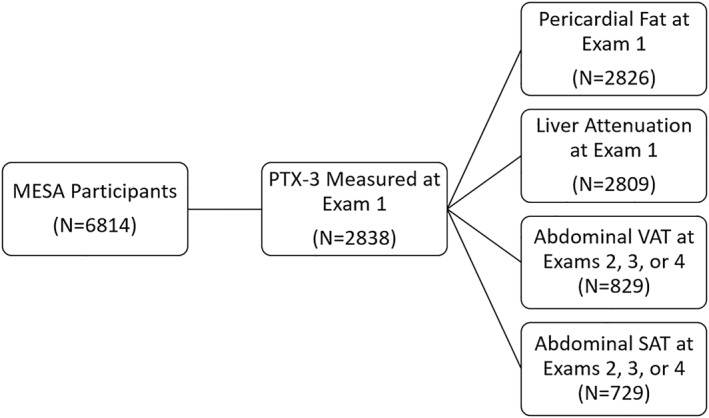
Flowchart for study inclusion.
TABLE 1.
Baseline Characteristics by volume of pericardial adipose tissue.
| Low PAT volume (<62.5 cm3) (N = 1170) | High PAT volume (>62.5 cm3) (N = 1656) | p‐value | |
|---|---|---|---|
| Age | 57 (51–66) | 64 (55–71) | <0.001 |
| Male | 420 (36) | 885 (53) | <0.001 |
| Race | <0.001 | ||
| Caucasian | 281 (24) | 433 (26) | |
| Asian | 299 (26) | 406 (25) | |
| African‐American | 364 (31) | 338 (20) | |
| Hispanic | 226 (19) | 479 (29) | |
| Weight (kg) | 68 (59–77) | 79 (68–93) | <0.001 |
| Height (cm) | 164 (158–170) | 166 (158–173) | <0.001 |
| BMI (kg/m2) | 24.8 (22.3–27.9) | 28.8 (25.8–32.4) | <0.001 |
| BMI category | <0.001 | ||
| <18.5 | 37 (3) | 1 (0) | |
| 18.5–25.0 | 563 (48) | 311 (19) | |
| 25.0–30.0 | 410 (35) | 683 (41) | |
| 30–35 | 113 (10) | 422 (25) | |
| >35 | 47 (4) | 239 (14) | |
| Smoking status | <0.001 | ||
| Never smoker | 694 (60) | 838 (51) | |
| Former smoker | 321 (28) | 581 (35) | |
| Current smoker | 151 (13) | 233 (14) | |
| Estimated GFR | 82 (73–94) | 79 (68–91) | <0.001 |
| Agatston calcium score | 0 (0–26) | 6 (0–112) | <0.001 |
| PAT volume (cm3) | 46.2 (36.9–54.8) | 91.6 (74.8–116.3) | <0.001 |
| VAT area (cm2) | 101.3 (71.2–142.2) | 188.8 (138.9–259.3) | <0.001 |
| SAT area (cm2) | 157.6 (106.5–215.3) | 190.7 (135.1–269.7) | <0.001 |
Note: Continuous variables reported as median (interquartile range); Categorical variables reported as N (%); Missing values: Smoking status on 8 individuals.
Abbreviations: BMI, body mass index; PAT, pericardial adipose tissue; SAT, abdominal subcutaneous adipose tissue; VAT, abdominal visceral adipose tissue.
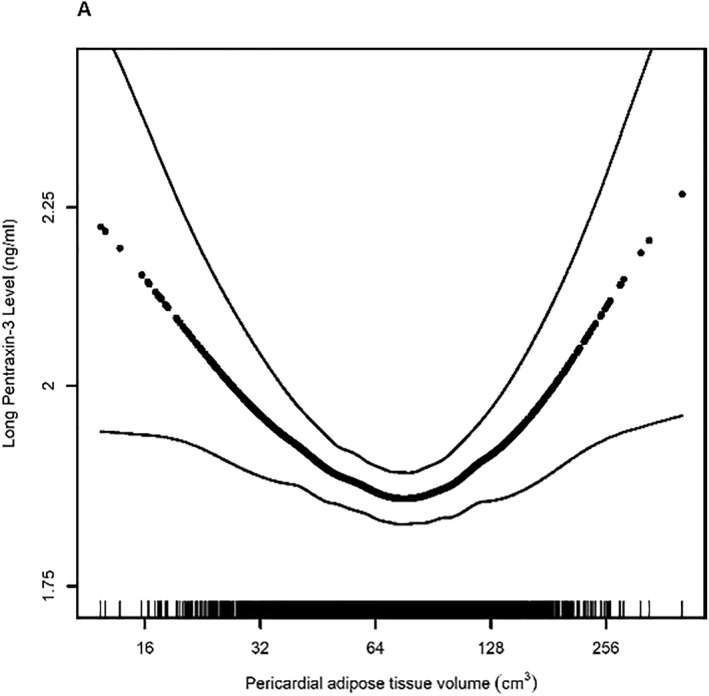
3.2. Pericardial adipose
There was a U‐shaped association between PAT volume and PTX‐3 as demonstrated by a significant association between the PAT 2 term and PTX‐3, the U‐test function, and the test of non‐linearity in the generalized additive model (Table S2, Figure 2A). The U‐test estimated an extreme point of the curve at a value of 62.5 cm3, which we then used to divide the cohort into two groups for piecewise regression analysis: those with PAT volume ≤62.5 cm3 (low PAT) and those with PAT volume >62.5 cm3 (high PAT). In adjusted analyses, individuals with low PAT had a significant decrease of 13.8% (95% CI −21.6 to −6.0, p = 0.001, Table 2) in PTX‐3 levels for every 1% increase in PAT volume. Individuals with high PAT volume had an increase of 6.0% (95% confidence interval [CI] −0.4 to 12.5, p = 0.067) in PTX‐3 levels for every 1% increase in PAT volume. Similar associations were present in unadjusted analyses, as well as analyses performed using the PAT index (Table S3). PAT volume measured at Exam 3 was strongly correlated with values from Exam 1 (r = 0.90, p < 0.0001) and demonstrated a similar association with PTX‐3 (Table S4).
FIGURE 2.
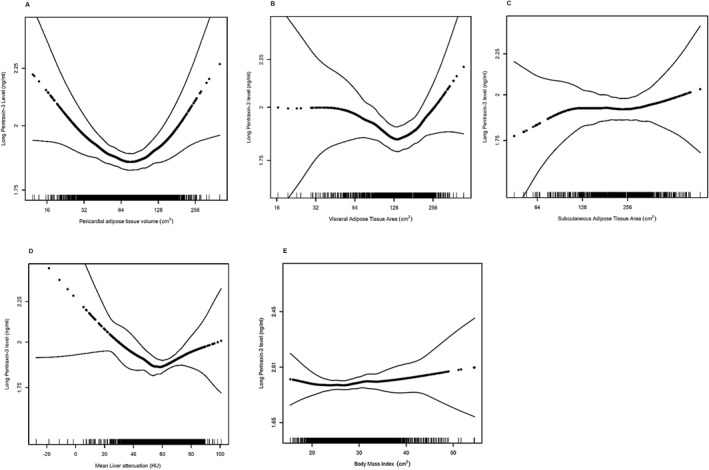
Association between (A) pericardial adipose volume (p for non‐linearity<0.001), (B) visceral adipose tissue area (p for non‐linearity = 0.02), (C) subcutaneous adipose tissue area (p for non‐linearity = 0.49), (D) liver attenuation (p for non‐linearity = 0.02), (E) body mass index (p for non‐linearity = 0.55) and long pentraxin‐3 levels. Models are adjusted for age and sex. Dark dotted black line represents the effect estimates. Surrounding thin lines represent 95% confidence bands. Vertical lines along the x‐axis each represent a single study individual.
TABLE 2.
Associations of adipose tissue depots with long pentraxin‐3 levels in piecewise regression models.
| PAT | N | Low PAT volume (≤62.5 cm3) | High PAT volume (>62.5 cm3) | ||||
|---|---|---|---|---|---|---|---|
| % change in PTX‐3 per 1% increase in PAT volume | 95% CI | p‐value | % change in PTX‐3 per 1% increase in PAT volume | 95% CI | p‐value | ||
| Model 1 | 2826 | −9.8% | −17.6 to–2.1 | 0.013 | 10.4% | 4.1 to 16.6 | 0.001 |
| Model 2 | 2826 | −13.6% | −21.4 to −5.8 | 0.001 | 8.3% | 1.9 to 14.7 | 0.011 |
| Model 3 | 2815 | −13.8% | −21.6 to −6.0 | 0.001 | 6.0% | −0.4 to 12.5 | 0.067 |
| VAT | N | Low VAT area (≤122.1 cm2) | High VAT area (>122.1 cm2) | ||||
|---|---|---|---|---|---|---|---|
| % change in PTX‐3 per 1% increase in VAT area | 95% CI | p‐value | % change in PTX‐3 per 1% increase in VAT area | 95% CI | p‐value | ||
| Model 1 | 820 | −7.3% | −17.3 to 2.7 | 0.15 | 11.4% | 1.1 to 21.8 | 0.03 |
| Model 2 | 795 | −8.3% | −18.4 to 1.9 | 0.11 | 7.9% | −3.1 to 19.0 | 0.16 |
| Model 3 | 790 | −8.8% | −19.0 to 1.4 | 0.09 | 5.7% | −5.5 to 16.8 | 0.32 |
| Liver attenuation | N | Low liver attenuation (≤54.2 HU) | High liver attenuation (>54.2 HU) | ||||
|---|---|---|---|---|---|---|---|
| % change in PTX‐3 per 1‐HU increase in attenuation | 95% CI | p‐value | % change in PTX‐3 per 1‐HU increase in attenuation | 95% CI | p‐value | ||
| Model 1 | 2809 | −0.49% | −0.9 to −0.08 | 0.02 | 0.38% | 0.03 to 0.73 | 0.03 |
| Model 2 | 2809 | −0.52% | −0.9 to −0.10 | 0.01 | 0.29% | −0.05 to 0.64 | 0.10 |
| Model 3 | 2075 | −0.36% | −0.7 to −0.001 | 0.046 | 0.22% | −0.06 to 0.49 | 0.12 |
Note: Model 1: Unadjusted. Model 2: Adjusted for age, sex, race. Model 3: Model 2 + smoking status, estimated glomerular filtration rate, physical activity, Agatston coronary calcium score, hypertension; Liver attenuation models are additionally adjusted for self‐reported current alcohol use.
Abbreviations: CI, confidence interval; PAT, pericardial adipose tissue; PTX‐3, long pentraxin‐3; SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue.
3.3. Visceral adipose
A similar U‐shaped association was noted between abdominal VAT cross‐sectional area and PTX‐3 levels, as demonstrated by a significant association by all three measures of non‐linearity (Table S2, Figure 2B). Using an extreme point of 122.1 cm2, individuals with low VAT area had a non‐significant decrease in PTX‐3 levels by 8.8% (95% CI −19.0 to 1.4, p = 0.09, Table 2) for every 1% increase in VAT area while individuals with high VAT area had an increase in PTX‐3 levels by 5.7% (95% CI −5.5 to 16.8, p = 0.32) for every 1% increase in VAT area. Similar associations were present in unadjusted analyses, analyses performed using the VAT index, and analyses at L4/L5 (Tables S3 and S5).
There was no evidence of a non‐linear or linear association between VAT attenuation and PTX‐3 levels (Tables S1 and S6, Figure S1A).
3.4. Liver attenuation
Liver attenuation and PTX‐3 levels had a significant U‐shaped relationship by all three measures of non‐linearity (Table S1, Figure 2D). Individuals with low liver attenuation (indicating greater lipid content) demonstrated a 0.36% decrease (95% CI −0.7 to −0.001) in PTX‐3 level with every 1‐HU increase in attenuation; individuals with higher liver attenuation (indicating less lipid content), had a 0.22% (95% CI −0.06 to 0.49, p = 0.12) increase in PTX‐3 level per 1‐HU increase in attenuation. There was a moderate correlation between mean liver attenuation at exams 1 and 3 (r = 0.57, p < 0.001). In analyses performed with liver attenuation measured at Exam 3 (Table S2), there were no significant linear or non‐linear associations between liver attenuation and PTX‐3 levels (Table S7).
3.5. Subcutaneous adipose
There was no evidence of any significant association between the abdominal subcutaneous adipose tissue cross‐sectional area or abdominal subcutaneous adipose tissue attenuation and PTX‐3 levels in unadjusted or fully adjusted models (Tables S2, S6 and S8, Figure 2D and Figure S1B). Similarly, there was no significant association between body mass index and PTX‐3 levels (Figure 2E).
3.6. Subgroup analyses
Age may modify the association between PAT and PTX‐3 (p‐for interaction 0.01, Table 3). Among those with low PAT, every 1% increase in PAT volume was associated with a 27.4% (95% CI −43.2% to −11.7%, p = 0.001) decrease in PTX‐3 among those over age 65, but only an 8.6% decrease (95% CI −17.5% to 0.3%, p = 0.059) among those less than 65 years of age. Age did not significantly modify the association between VAT and hepatic attenuation and PTX‐3 though effect estimates demonstrate similar trends (Table 3).
TABLE 3.
Associations between PAT volume, abdominal VAT area, liver attenuation and PTX‐3 levels in sub‐groups defined by age.
| PAT | N | Low PAT volume (≤62.5 cm3) | High PAT volume (>62.5 cm3) | p For interaction | ||||
|---|---|---|---|---|---|---|---|---|
| % change in PTX‐3 per 1% increase in PAT volume | 95% CI | p‐value | % change in PTX‐3 per 1% increase in PAT volume | 95% CI | p‐value | |||
| 0.02 | ||||||||
| Age <65 | 1662 | −9.0% | −17.9 to −9.06 | 0.048 | 6.3% | −2.3 to 14.9 | 0.15 | |
| Age ≥65 | 1153 | −27.7% | −43.4 to −12.0 | 0.001 | 7.8% | −2.1 to 17.7 | 0.12 | |
| VAT | N | Low VAT area (≤122.1 cm2) | High VAT area (>122.1 cm2) | p For interaction | ||||
|---|---|---|---|---|---|---|---|---|
| % change in PTX‐3 per 1% increase in VAT area | 95% CI | p‐value | % change in PTX‐3 per 1% increase in VAT area | 95% CI | p‐value | |||
| 0.70 | ||||||||
| Age <65 | 491 | −3.8% | 016.8 to 9.2 | 0.57 | −8.3% | −23.0 to 6.4 | 0.57 | |
| Age ≥65 | 299 | −15.9% | −32.0 to 0.2 | 0.052 | 22.9% | 6.2 to 39.7 | 0.007 | |
| Liver attenuation | N | Low liver attenuation (≤54.2 HU) | High liver attenuation (>54.2 HU) | p For interaction | ||||
|---|---|---|---|---|---|---|---|---|
| % change in PTX‐3 per 1‐HU increase in attenuation | 95% CI | p‐value | % change in PTX‐3 per 1‐HU increase in 51attenuation | 95% CI | p‐value | |||
| 0.99 | ||||||||
| Age <65 | 1268 | −0.2% | −0.6 to 0.2 | 0.29 | 0.2 | −0.1 to 0.6 | 0.21 | |
| Age ≥65 | 807 | −0.8% | −1.5 to −0.02 | 0.04 | 0.3% | −0.1 to 0.7 | 0.18 | |
Note: Analyses are adjusted for sex, race/ethnicity, smoking status, estimated glomerular filtration rate, Agatston coronary calcium score, physical activity, hypertension; liver attenuation analyses are additionally adjusted for self‐reported alcohol use.
Abbreviations: CI, confidence interval; HU, Hounsfield Units; PAT, pericardial adipose tissue volume; PTX‐3, long pentraxin 3; VAT, visceral adipose tissue area.
The association between VAT and PTX‐3 may be modified by sex (p‐for interaction 0.02, Table 4) though there was no similar effect modification for PAT or hepatic attenuation.
TABLE 4.
Associations between PAT volume, abdominal VAT area, liver attenuation and PTX‐3 levels within sub‐groups defined by sex.
| PAT | N | Low PAT volume (≤62.5 cm3) | High PAT volume (>62.5 cm3) | p For interaction | ||||
|---|---|---|---|---|---|---|---|---|
| % change in PTX‐3 per 1% increase in PAT volume | 95% CI | p‐value | % change in PTX‐3 per 1% increase in PAT volume | 95% CI | p‐value | |||
| 0.51 | ||||||||
| Male | 1299 | −14.8% | −27.9 to 1.6 | 0.03 | 7.5% | −0.8 to 15.8 | 0.08 | |
| Female | 1516 | −15.2% | −25.1 to 05.3 | 0.003 | 5.4% | −5.4 to 16.2 | 0.33 | |
| VAT | N | Low VAT area (≤122.1 cm2) | High VAT area (>122.1 cm2) | p For interaction | ||||
|---|---|---|---|---|---|---|---|---|
| % change in PTX‐3 per 1% increase in VAT area | 95% CI | p‐value | % change in PTX‐3 per 1% increase in VAT area | 95% CI | p‐value | |||
| 0.01 | ||||||||
| Male | 382 | 4.4% | −18.6 to 27.4 | 0.71 | 12.5% | −1.9 to 26.9 | 0.09 | |
| Female | 408 | −8.9% | −20.5 to 2.5 | 0.13 | −13.0% | −32.0 to 6.0 | 0.18 | |
| Liver attenuation | N | Low liver attenuation (≤54.2 HU) | High liver attenuation (>54.2 HU) | p For interaction | ||||
|---|---|---|---|---|---|---|---|---|
| % change in PTX‐3 per 1‐HU increase in attenuation | 95% CI | p‐value | % change in PTX‐3 per 1‐HU increase in attenuation | 95% CI | p‐value | |||
| 0.52 | ||||||||
| Male | 1120 | −0.2% | −0.8 to 0.3 | 0.39 | 0.3% | −0.1 to 0.6 | 0.17 | |
| Female | 955 | −0.4% | −0.9 to 0.03 | 0.064 | 0.1% | −0.2 to 0.6 | 0.41 | |
Note: Analyses are adjusted for age, race/ethnicity, smoking status, estimated glomerular filtration rate, Agatston coronary calcium score, physical activity, hypertension; liver attenuation analyses are additionally adjusted for self‐reported alcohol use.
Abbreviations: CI, confidence interval; HU, Hounsfield Units; PAT, pericardial adipose tissue volume; PTX‐3, long pentraxin 3; VAT, visceral adipose tissue area.
4. DISCUSSION
In this study of community‐dwelling adults, we found a relationship between PAT, VAT, and hepatic attenuation with circulating PTX‐3 levels. This relationship was U‐shaped with the highest PTX‐3 levels in those with high and low adiposity. Importantly, this relationship may be significantly modified by age. Our findings suggest that PTX‐3 may be a relevant biomarker of the extremes of adiposity.
Prior studies evaluating the association between adiposity and PTX‐3 have been inconsistent. Our finding of a U‐shaped association between adipose depot size and PTX‐3 may explain these prior seemingly contradictory reports. 5 , 6 , 7 , 8 , 26 , 27 Linear modeling used in prior studies allows individuals at the extremes to define the overall direction of effect. Body mass index, which was used in multiple reports 7 , 8 , 28 but had no significant association with PTX‐3 level in our study, varies significantly by race‐ethnicity and is a poor measure of adiposity. 29 , 30 The absence of an association between BMI and PTX‐3 in our study further emphasizes the limitations of the use of BMI in the study of adiposity. The largest prior study identified a significant association between low PTX‐3 levels and high VAT on MRI in 287 Scandinavian men over the age of 70. 8 We identified effect modification by age, with a stronger association between low PAT and PTX‐3 among those over age 65. The inverse association found in the Scandinavian study may reflect the steeper downward slope of the association among those over age 65. Notably, our finding of significant effect modification by sex, with a direct relationship between VAT area and PTX‐3 levels in men, still contradicts the Scandinavian study. Whether this reflects differences between DEXA scan and CT imaging, predominant effects of aging, or the limitations of our study design, is unclear.
Long pentraxin‐3 belongs to the pentraxin family of acute phase proteins, the best known of which is c‐reactive protein (CRP). While CRP is produced centrally in the liver, PTX‐3 is produced in the peripheral tissues by a multitude of cell types including adipocytes, endothelial cells, fibroblasts, alveolar epithelial cells, neutrophils, and macrophages. 31 For this reason, it has often been invoked as a marker of local rather than systemic inflammation, and extensively investigated as a marker of atherosclerotic disease, vasculitis, right ventricular function, and lung injury. 23 , 32 , 33 , 34 , 35 However, the promiscuous sources of PTX‐3 suggest that it may not reflect inflammation or injury in any single tissue but rather reflect cumulative inflammation or injury across multiple tissues. This is consistent with recent work identifying an association between higher PTX‐3 and an increased risk of death in sepsis 34 and COVID‐19 36 and increased cardiovascular and all‐cause mortality in older adults, 19 heart failure, 37 and hospitalized patients. 38 Furthermore, greater PTX‐3 is associated with higher levels of CRP and interleukin‐6 in the MESA cohort. 39 Our work adds to this literature by suggesting that PTX‐3 may reflect states of chronic cumulative systemic inflammation associated with extremes of adiposity. Furthermore, while obesity has been associated with CRP, PTX‐3 could potentially be an important distinct biomarker of disease given (1) different sources, 31 (2) effects on neutrophil function, 40 and (3) antagonistic effects on CRP in specific tissues. 27
Patients with very little adipose tissue may have greater systemic inflammation due to malnutrition or chronic disease. 35 , 37 , 41 , 42 Greater PTX‐3 has been previously associated with aging, 43 loss of body weight, 44 and lower cognitive performance. 44 Higher PTX‐3 was strongly associated with lower albumin levels and lower BMI in end‐stage renal disease, further suggesting it may be a marker of protein‐calorie malnutrition. 45 PTX‐3 levels are increased in chronic diseases including cardiovascular disease and cancer. 6 , 23 , 35 , 37 , 41 This association may also explain our demonstrated effect modification by age with a stronger association between PAT and PTX‐3 levels among those over age 65 with low PAT. Whether the association between PAT and PTX‐3 in those under age 65 can also be explained by malnutrition or chronic disease, is unknown. Reassuringly, our findings were unchanged in models adjusted for hypertension, renal function, and coronary artery disease, suggesting that the association between low adiposity and PTX‐3 levels is independent of these comorbidities. Further work is required to evaluate whether the association between low adiposity and PTX‐3 levels reflects malnutrition states.
The association between high PAT and greater PTX‐3 levels may similarly reflect greater systemic inflammation due to excess adipose tissue or obesity‐associated chronic disease. Excess energy, in the form of free fatty acids, can be stored in adipose tissue in either newly differentiated pre‐adipocytes or in existing adipocytes. The addition of fatty acids to existing adipocytes can result in adipocyte hypertrophy, production of inflammatory cytokines, and recruitment of pro‐inflammatory adipose tissue macrophages. 3 , 4 PTX‐3 may impair angiogenesis contributing to decreased recruitment of new pre‐adipocytes, increased adipocyte hypertrophy, and increased adipose tissue macrophages. 5 This is consistent with demonstrated increases in serum PTX‐3 levels in mice after 10–20 weeks on a high fat diet, 5 suggesting that excess fat intake may result in adipose expansion and greater production of PTX‐3. PTX‐3 may also be elevated in chronic diseases including left ventricular diastolic dysfunction and vascular inflammation. 28 , 42 We found some attenuation of effect among high adiposity groups when adjusting for hypertension and coronary artery disease, suggesting that these comorbidities may partially explain the association between high adiposity and PTX‐3 though whether these diseases are simply associated with greater PTX‐3 or result in pathologic changes that produce PTX‐3 is unknown. Furthermore, while PTX‐3 may be a marker of inflammation or injury, its local effects may be immune‐modulatory. 23 , 46
This study has important strengths. We demonstrated a consistent association between adipose tissue and PTX‐3 across multiple adipose depots using well‐validated measurement techniques. This association is present in a large multi‐ethnic cohort of community‐dwelling adults without known cardiovascular disease. Finally, we performed extensive sensitivity analyses and demonstrated consistent associations.
There are also several limitations to this study. First, PTX‐3 levels are only available at exam 1, while measures of abdominal adipose are available at later exams. Reassuringly, our primary analysis involves pericardial adipose tissue measured at exam 1. Second, PTX‐3 levels are low in this cohort with only half of the individuals with a level above the lower limit of normal (2 ng/mL), suggesting that even patients with “high PTX‐3” still have relatively low concentrations. However, the ability to identify significant differences in PTX‐3 levels by adiposity, even within a narrow range of values, further supports its potential to identify even small changes in inflammation. Third, despite an acceptable analytical coefficient of variation, 47 there is a possibility of mismeasurement. Fourth, we performed a cross‐sectional analysis of observational data preventing us from establishing a causal association between adipose tissue and PTX‐3. Fifth, whether PTX‐3 haplotypes modify the association between adiposity and circulating levels of PTX‐3 is unknown. Future work should evaluate the association between adiposity and PTX‐3 in patients with different PTX‐3 haplotypes. Fifth, adipose distribution may vary by race‐ethnicity. Future work could evaluate how environmental, socioeconomic, and genetic factors modify this association. Sixth, we used the data to identify inflection points for our analysis. Similar thresholds have been associated with the risk of clinical outcomes in prior work, 48 , 49 , 50 , 51 , 52 , 53 though absolute thresholds are difficult to compare across studies due to differences in measurement protocols, scanner models, and frequent reports from small cohorts at single centers.
In conclusion, we demonstrated a non‐linear “U‐shaped” association between pericardial, abdominal visceral, and hepatic adiposity with PTX3 levels suggesting that extremes of adiposity are associated with greater circulating levels of PTX3. Further work is required to identify mechanisms linking adiposity and PTX including the roles of frailty and malnutrition.
AUTHOR CONTRIBUTIONS
Conception and Design: Michaela R. Anderson, J.C. Acquisition, analysis, and interpretation of data: all authors. First Draft: Michaela R. Anderson. Drafting or revising the manuscript for important intellectual content: all authors. Statistical Analysis: Michaela R. Anderson. Final approval of submission: all authors.
CONFLICT OF INTEREST STATEMENT
JSK receives grant support from the NIH/NHLBI and serves on the Data, Safety, and Monitoring Board for the University of Virginia trial of convalescent plasma.
Supporting information
Supporting Information S1
ACKNOWLEDGMENTS
MRA, JC, and JD conceived of and designed this study. MRA wrote the first draft of the manuscript. All authors contributed to analysis and interpretation of data and drafting the manuscript. All authors approved the submission of this manuscript. Multi‐Ethnic Study of Atherosclerosis (MESA) is supported by the National Heart, Lung, and Blood Institute (NHLBI) in collaboration with MESA investigators. Support for MESA is provided by contracts 75N92020D00001, HHSN268201500003sI, N01‐HC‐95159, 75N92020D00005, N01‐HC‐95160, 75N92020D00002, N01‐HC‐95161, 75N92020D00003, N01‐HC‐95162, 75N92020D00006, N01‐HC‐95163, 75N92020D00004, N01‐HC‐95164, 75N92020D00007, N01‐HC‐95165, N01‐HC‐95166, N01‐HC‐95167, N01‐HC‐95168 and N01‐HC‐95169 from the National Heart, Lung, and Blood Institute, and by grants UL1‐TR‐000040, UL1‐TR‐001079, and UL1‐TR‐001420 from the National Center for Advancing Translational Sciences (NCATS). This work was additionally supported by NIH K23 HL140199, K23 HL 150280, K23 HL 150301, and the Pulmonary Fibrosis Foundation.
Anderson MR, Kim JS, Podolanczuk A, et al. Nonlinear associations between computed tomography‐measures of adiposity and long pentraxin‐3 in the Multi‐Ethnic Study of Atherosclerosis. Obes Sci Pract. 2024;e708. 10.1002/osp4.708
REFERENCES
- 1. Finkelstein EA, Khavjou OA, Thompson H, et al. Obesity and severe obesity forecasts through 2030. Am J Prev Med. 2012;42(6):563‐570. 10.1016/j.amepre.2011.10.026 [DOI] [PubMed] [Google Scholar]
- 2. Vishvanath L, Gupta RK. Contribution of adipogenesis to healthy adipose tissue expansion in obesity. J Clin Invest. 2019;129(10):4022‐4031. 10.1172/JCI129191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dalmas E, Clement K, Guerre‐Millo M. Defining macrophage phenotype and function in adipose tissue. Trends Immunol. 2011;32(7):307‐314. 10.1016/j.it.2011.04.008 [DOI] [PubMed] [Google Scholar]
- 4. Curat CA, Miranville A, Sengenes C, et al. From blood monocytes to adipose tissue‐resident macrophages: induction of diapedesis by human mature adipocytes. Diabetes. 2004;53(5):1285‐1292. 10.2337/diabetes.53.5.1285 [DOI] [PubMed] [Google Scholar]
- 5. Bonacina F, Moregola A, Porte R, et al. Pentraxin 3 deficiency protects from the metabolic inflammation associated to diet‐induced obesity. Cardiovasc Res. 2019;115(13):1861‐1872. 10.1093/cvr/cvz068 [DOI] [PubMed] [Google Scholar]
- 6. Zanetti M, Bosutti A, Ferreira C, et al. Circulating pentraxin 3 levels are higher in metabolic syndrome with subclinical atherosclerosis: evidence for association with atherogenic lipid profile. Clin Exp Med. 2009;9(3):243‐248. 10.1007/s10238-009-0039-z [DOI] [PubMed] [Google Scholar]
- 7. Barazzoni R, Palmisano S, Gortan Cappellari G, et al. Gastric bypass‐induced weight loss alters obesity‐associated patterns of plasma pentraxin‐3 and systemic inflammatory markers. Surg Obes Relat Dis. 2016;12(1):23‐32. 10.1016/j.soard.2015.04.013 [DOI] [PubMed] [Google Scholar]
- 8. Witasp A, Carrero JJ, Michaelsson K, et al. Inflammatory biomarker pentraxin 3 (PTX3) in relation to obesity, body fat depots and weight loss. Obesity. 2014;22(5):1373‐1379. 10.1002/oby.20695 [DOI] [PubMed] [Google Scholar]
- 9. Slusher AL, Zuniga TM, Acevedo EO. Inflamm‐aging is associated with lower plasma PTX3 concentrations and an impaired capacity of PBMCs to express hTERT following LPS stimulation. Mediat Inflamm. 2019;2019:2324193. 10.1155/2019/2324193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yamasaki K, Kurimura M, Kasai T, Sagara M, Kodama T, Inoue K. Determination of physiological plasma pentraxin 3 (PTX3) levels in healthy populations. Clin Chem Lab Med. 2009;47(4):471‐477. 10.1515/CCLM.2009.110 [DOI] [PubMed] [Google Scholar]
- 11. Anderson MR, Kolaitis NA, Gao Y, et al. A nonlinear relationship between visceral adipose tissue and frailty in adult lung transplant candidates. Am J Transpl. 2019;19(11):3155‐3161. 10.1111/ajt.15525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Miao C, Chen S, Ding J, et al. The association of pericardial fat with coronary artery plaque index at MR imaging: the Multi‐Ethnic Study of Atherosclerosis (MESA). Radiology. 2011;261(1):109‐115. 10.1148/radiol.11110346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Anderson MR, Kim JS, Allison M, et al. Adiposity and interstitial lung abnormalities in community dwelling adults: the MESA cohort study. Chest. 2021;160(2):582‐594. 10.1016/j.chest.2021.03.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Anderson MR, Udupa JK, Edwin E, et al. Adipose tissue quantification and primary graft dysfunction after lung transplantation: the Lung Transplant Body Composition study. J Heart Lung Transpl. 2019;38(12):1246‐1256. 10.1016/j.healun.2019.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zeb I, Li D, Nasir K, Katz R, Larijani VN, Budoff MJ. Computed tomography scans in the evaluation of fatty liver disease in a population based study: the multi‐ethnic study of atherosclerosis. Acad Radiol. 2012;19(7):811‐818. 10.1016/j.acra.2012.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vella CA, Allison MA. Associations of abdominal intermuscular adipose tissue and inflammation: the Multi‐Ethnic Study of Atherosclerosis. Obes Res Clin Pract. 2018;12(6):534‐540. 10.1016/j.orcp.2018.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shah AD, Kandula NR, Lin F, et al. Less favorable body composition and adipokines in South Asians compared with other US ethnic groups: results from the MASALA and MESA studies. Int J Obes. 2016;40(4):639‐645. 10.1038/ijo.2015.219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mongraw‐Chaffin M, Allison MA, Burke GL, et al. CT‐derived body fat distribution and incident cardiovascular disease: the multi‐ethnic study of atherosclerosis. J Clin Endocrinol Metab. 2017;102(11):4173‐4183. 10.1210/jc.2017-01113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jenny NS, Arnold AM, Kuller LH, Tracy RP, Psaty BM. Associations of pentraxin 3 with cardiovascular disease and all‐cause death: the Cardiovascular Health Study. Arterioscler Thromb Vasc Biol. 2009;29(4):594‐599. 10.1161/ATVBAHA.108.178947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cheng FW, Gao X, Mitchell DC, et al. Body mass index and all‐cause mortality among older adults. Obesity. 2016;24(10):2232‐2239. 10.1002/oby.21612 [DOI] [PubMed] [Google Scholar]
- 21. Anderson MR, Geleris J, Anderson DR, et al. Body mass index and risk for intubation or death in SARS‐CoV‐2 infection: a retrospective cohort study. Ann Intern Med. 2020;173(10):782‐790. 10.7326/M20-3214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lind JT, Mehlum H. With or without U? The appropriate test for a U‐shaped relationship. Oxf Bull Econ Stat. 2009;72(1):109‐118. 10.1111/j.1468-0084.2009.00569.x [DOI] [Google Scholar]
- 23. Norata GD, Marchesi P, Pulakazhi Venu VK, et al. Deficiency of the long pentraxin PTX3 promotes vascular inflammation and atherosclerosis. Circulation. 2009;120(8):699‐708. 10.1161/CIRCULATIONAHA.108.806547 [DOI] [PubMed] [Google Scholar]
- 24. Jones SA, Li Q, Aiello AE, O'Rand AM, Evenson KR. Physical activity, sedentary behavior, and retirement: the multi‐ethnic study of atherosclerosis. Am J Prev Med. 2018;54(6):786‐794. 10.1016/j.amepre.2018.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ishak KG, Zimmerman HJ, Ray MB. Alcoholic liver disease: pathologic, pathogenetic and clinical aspects. Alcohol Clin Exp Res. 1991;15(1):45‐66. 10.1111/j.1530-0277.1991.tb00518.x [DOI] [PubMed] [Google Scholar]
- 26. Miyaki A, Maeda S, Choi Y, et al. Association of plasma pentraxin 3 with arterial stiffness in overweight and obese individuals. Am J Hypertens. 2013;26(10):1250‐1255. 10.1093/ajh/hpt103 [DOI] [PubMed] [Google Scholar]
- 27. Ogawa T, Kawano Y, Imamura T, et al. Reciprocal contribution of pentraxin 3 and C‐reactive protein to obesity and metabolic syndrome. Obesity. 2010;18(9):1871‐1874. 10.1038/oby.2009.507 [DOI] [PubMed] [Google Scholar]
- 28. Kim J, Gozal D, Bhattacharjee R, Kheirandish‐Gozal L. TREM‐1 and pentraxin‐3 plasma levels and their association with obstructive sleep apnea, obesity, and endothelial function in children. Sleep. 2013;36(6):923‐931. 10.5665/sleep.2726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Romero‐Corral A, Somers VK, Sierra‐Johnson J, et al. Accuracy of body mass index in diagnosing obesity in the adult general population. Int J Obes. 2008;32(6):959‐966. 10.1038/ijo.2008.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Heymsfield SB, Peterson CM, Thomas DM, Heo M, Schuna JM, Jr . Why are there race/ethnic differences in adult body mass index‐adiposity relationships? A quantitative critical review. Obes Rev. 2016;17(3):262‐275. 10.1111/obr.12358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Vilahur G, Badimon L. Biological actions of pentraxins. Vasc Pharmacol. 2015;73:38‐44. 10.1016/j.vph.2015.05.001 [DOI] [PubMed] [Google Scholar]
- 32. Diamond JM, Meyer NJ, Feng R, et al. Variation in PTX3 is associated with primary graft dysfunction after lung transplantation. Am J Respir Crit Care Med. 2012;186(6):546‐552. 10.1164/rccm.201204-0692OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Liu S, Qu X, Liu F, Wang C. Pentraxin 3 as a prognostic biomarker in patients with systemic inflammation or infection. Mediat Inflamm. 2014;2014:421429. 10.1155/2014/421429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mauri T, Bellani G, Patroniti N, et al. Persisting high levels of plasma pentraxin 3 over the first days after severe sepsis and septic shock onset are associated with mortality. Intensive Care Med. 2010;36(4):621‐629. 10.1007/s00134-010-1752-5 [DOI] [PubMed] [Google Scholar]
- 35. Leary PJ, Jenny NS, Barr RG, et al. Pentraxin‐3 and the right ventricle: the multi‐ethnic study of atherosclerosis‐right ventricle study. Pulm Circ. 2014;4(2):250‐259. 10.1086/675988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Brunetta E, Folci M, Bottazzi B, et al. Macrophage expression and prognostic significance of the long pentraxin PTX3 in COVID‐19. Nat Immunol. 2021;22(1):19‐24. 10.1038/s41590-020-00832-x [DOI] [PubMed] [Google Scholar]
- 37. Latini R, Gullestad L, Masson S, et al. Pentraxin‐3 in chronic heart failure: the CORONA and GISSI‐HF trials. Eur J Heart Fail. 2012;14(9):992‐999. 10.1093/eurjhf/hfs092 [DOI] [PubMed] [Google Scholar]
- 38. Bastrup‐Birk S, Munthe‐Fog L, Skjoedt MO, et al. Pentraxin‐3 level at admission is a strong predictor of short‐term mortality in a community‐based hospital setting. J Intern Med. 2015;277(5):562‐572. 10.1111/joim.12294 [DOI] [PubMed] [Google Scholar]
- 39. Jenny NS, Blumenthal RS, Kronmal RA, Rotter JI, Siscovick DS, Psaty BM. Associations of pentraxin 3 with cardiovascular disease: the multi‐ethnic study of atherosclerosis. J Thromb Haemostasis. 2014;12(6):999‐1005. 10.1111/jth.12557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cunha C, Aversa F, Lacerda JF, et al. Genetic PTX3 deficiency and aspergillosis in stem‐cell transplantation. N Engl J Med. 2014;370(5):421‐432. 10.1056/NEJMoa1211161 [DOI] [PubMed] [Google Scholar]
- 41. Infante M, Allavena P, Garlanda C, et al. Prognostic and diagnostic potential of local and circulating levels of pentraxin 3 in lung cancer patients. Int J Cancer. 2016;138(4):983‐991. 10.1002/ijc.29822 [DOI] [PubMed] [Google Scholar]
- 42. Matsubara J, Sugiyama S, Nozaki T, et al. Pentraxin 3 is a new inflammatory marker correlated with left ventricular diastolic dysfunction and heart failure with normal ejection fraction. J Am Coll Cardiol. 2011;57(7):861‐869. 10.1016/j.jacc.2010.10.018 [DOI] [PubMed] [Google Scholar]
- 43. Anuurad E, Enkhmaa B, Gungor Z, et al. Age as a modulator of inflammatory cardiovascular risk factors. Arterioscler Thromb Vasc Biol. 2011;31(9):2151‐2156. 10.1161/ATVBAHA.111.232348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yano Y, Matsuda S, Hatakeyama K, et al. Plasma Pentraxin 3, but not high‐sensitivity C‐reactive protein, is a useful inflammatory biomarker for predicting cognitive impairment in elderly hypertensive patients. J Gerontol A Biol Sci Med Sci. 2010;65(5):547‐552. 10.1093/gerona/glq030 [DOI] [PubMed] [Google Scholar]
- 45. Valente MJ, Rocha S, Coimbra S, et al. Long pentraxin 3 as a broader biomarker for multiple risk factors in end‐stage renal disease: association with all‐cause mortality. Mediat Inflamm. 2019;2019:3295725. 10.1155/2019/3295725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Salio M, Chimenti S, De Angelis N, et al. Cardioprotective function of the long pentraxin PTX3 in acute myocardial infarction. Circulation. 2008;117(8):1055‐1064. 10.1161/CIRCULATIONAHA.107.749234 [DOI] [PubMed] [Google Scholar]
- 47. Bioanalytical method validation guidance for industry. In: Services USDoHaH, Administration FaD, (CDER) CfDEaR, (CVM) CfVM, eds. Silver Spring, MD. 2018. [Google Scholar]
- 48. Faria G, Goncalves A, Cunha R, et al. Beyond central adiposity: liver fat and visceral fat area are associated with metabolic syndrome in morbidly obese patients. Int J Surg. 2015;14:75‐79. 10.1016/j.ijsu.2014.12.028 [DOI] [PubMed] [Google Scholar]
- 49. Kodama Y, Ng CS, Wu TT, et al. Comparison of CT methods for determining the fat content of the liver. AJR Am J Roentgenol. 2007;188(5):1307‐1312. 10.2214/AJR.06.0992 [DOI] [PubMed] [Google Scholar]
- 50. Boyce CJ, Pickhardt PJ, Kim DH, et al. Hepatic steatosis (fatty liver disease) in asymptomatic adults identified by unenhanced low‐dose CT. AJR Am J Roentgenol. 2010;194(3):623‐628. 10.2214/AJR.09.2590 [DOI] [PubMed] [Google Scholar]
- 51. Birnbaum BA, Hindman N, Lee J, Babb JS. Multi‐detector row CT attenuation measurements: assessment of intra‐ and interscanner variability with an anthropomorphic body CT phantom. Radiology. 2007;242(1):109‐119. 10.1148/radiol.2421052066 [DOI] [PubMed] [Google Scholar]
- 52. Ding J, Kritchevsky SB, Harris TB, et al. The association of pericardial fat with calcified coronary plaque. Obesity. 2008;16(8):1914‐1919. 10.1038/oby.2008.278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Shah RV, Anderson A, Ding J, et al. Pericardial, but not hepatic, fat by CT is associated with CV outcomes and structure: the multi‐ethnic study of atherosclerosis. JACC Cardiovasc Imaging. 2017;10(9):1016‐1027. 10.1016/j.jcmg.2016.10.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information S1


