Abstract
Background
Achilles tendinopathy is a common condition, often with significant functional consequences. As a wide range of injection treatments are available, a review of randomised trials evaluating injection therapies to help inform treatment decisions is warranted.
Objectives
To assess the effects (benefits and harms) of injection therapies for people with Achilles tendinopathy.
Search methods
We searched the following databases up to 20 April 2015: the Cochrane Bone, Joint and Muscle Trauma Group Specialised Register, the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, AMED, CINAHL and SPORTDiscus. We also searched trial registers (29 May 2014) and reference lists of articles to identify additional studies.
Selection criteria
We included randomised and quasi‐randomised controlled trials evaluating injection therapies in adults with an investigator‐reported diagnosis of Achilles tendinopathy. We accepted comparison arms of placebo (sham) or no injection control, or other active treatment (such as physiotherapy, pharmaceuticals or surgery). Our primary outcomes were function, using measures such as the VISA‐A (Victorian Institute of Sport Assessment‐Achilles questionnaire), and adverse events.
Data collection and analysis
Two review authors independently extracted data from the included studies. We assessed treatment effects using mean differences (MDs) and 95% confidence intervals (CIs) for continuous variables and risk ratios (RRs) and 95% CIs for dichotomous variables. For follow‐up data, we defined short‐term as up to six weeks, medium‐term as up to three months and longer‐term as data beyond three months. We performed meta‐analysis where appropriate.
Main results
We included 18 studies (732 participants). Seven trials exclusively studied athletic populations. The mean ages of the participants in the individual trials ranged from 20 years to 50 years. Fifteen trials compared an injection therapy with a placebo injection or no injection control, four trials compared an injection therapy with active treatment, and one compared two different concentrations of the same injection. Thus no trials compared different injection therapies. Two studies had three trial arms and we included them twice in two different categories. Within these categories, we further subdivided injection therapies by mode of action (injury‐causing versus direct repair agents).
The risk of bias was unclear (due to poor reporting) or high in six trials published between 1987 and 1994. Improved methodology and reporting for the subsequent trials published between 2004 and 2013 meant that these were at less risk of bias.
Given the very low quality evidence available from each of four small trials comparing different combinations of injection therapy versus active treatment and the single trial comparing two doses of one injection therapy, only the results of the first comparison (injection therapy versus control) are presented.
There is low quality evidence of a lack of significant or clinically important differences in VISA‐A scores (0 to 100: best function) between injection therapy and control groups at six weeks (MD 0.79, 95% CI ‐4.56 to 6.14; 200 participants, five trials), three months (MD ‐0.94, 95% CI ‐6.34 to 4.46; 189 participants, five trials) or between six and 12 months (MD 0.14, 95% CI ‐6.54 to 6.82; 132 participants, three trials). Very low quality evidence from 13 trials showed little difference between the two groups in adverse events (14/243 versus 12/206; RR 0.97, 95% CI 0.50 to 1.89), most of which were minor and short‐lasting. The only major adverse event in the injection therapy group was an Achilles tendon rupture, which happened in a trial testing corticosteroid injections. There was very low quality evidence in favour of the injection therapy group in short‐term (under three months) pain (219 participants, seven trials) and in the return to sports (335 participants, seven trials). There was very low quality evidence indicating little difference between groups in patient satisfaction with treatment (152 participants, four trials). There was insufficient evidence to conclude on subgroup differences based on mode of action given that only two trials tested injury‐causing agents and the clear heterogeneity of the other 13 trials, which tested seven different therapies that act directly on the repair pathway.
Authors' conclusions
There is insufficient evidence from randomised controlled trials to draw conclusions on the use, or to support the routine use, of injection therapies for treating Achilles tendinopathy. This review has highlighted a need for definitive research in the area of injection therapies for Achilles tendinopathy, including in older non‐athletic populations. This review has shown that there is a consensus in the literature that placebo‐controlled trials are considered the most appropriate trial design.
Keywords: Adult; Humans; Middle Aged; Young Adult; Achilles Tendon; Adrenal Cortex Hormones; Adrenal Cortex Hormones/administration & dosage; Aprotinin; Aprotinin/administration & dosage; Athletes; Fibroblasts; Fibroblasts/transplantation; Glycosaminoglycans; Glycosaminoglycans/administration & dosage; Hemodialysis Solutions; Hemodialysis Solutions/administration & dosage; Injections, Intralesional; Injections, Intralesional/adverse effects; Injections, Intralesional/methods; Platelet Transfusion; Polidocanol; Polyethylene Glycols; Polyethylene Glycols/administration & dosage; Randomized Controlled Trials as Topic; Sodium Chloride; Sodium Chloride/administration & dosage; Tendinopathy; Tendinopathy/therapy
Plain language summary
Injection treatment for painful Achilles tendons in adults
Background and aim of the review
The Achilles tendon connects the calf muscles to the heel bone. Painful and stiff Achilles tendons are common overuse injuries in people undertaking sports, such as running, but also occur for other reasons in inactive people. The underlying cause is an imbalance between the damage and repair processes in the tendon. Painful Achilles tendons are often disabling and can take a long time to get better. Many treatments exist for this condition and this review set out to find out whether treatment with an injection, with a variety of agents, decreases pain and allows people to return to their previous activities.
Results of the search
We searched medical databases up to 20 April 2015 for studies that compared injection therapy with a placebo injection or no injection, or with an active treatment such as exercises, or different doses or types of injection therapy. We found 18 studies, which included 732 participants. Seven studies included athletes only. Study participants in the individual studies were mainly young to middle aged adults.
Key results
In 15 studies, patients had been assigned randomly to receive an injection therapy (such as a steroid), a placebo injection, or no injection at all. There were several different types of injection agents used and so we separated them into those agents that acted by causing damage to the tendon and those that acted to repair the tendon directly. However, there were not enough data to distinguish between these two types of injection therapies and so we only report the overall results for all injection therapies.
The review of the evidence from these studies found no clinically important difference between the injection therapy or placebo or no injection groups in patient function scores at six weeks, three months or subsequently. Similar numbers of minor adverse events, such as pain during the injection, occurred in both groups. The only serious adverse event in the injection therapy group was an Achilles tendon rupture, which happened in a study testing steroid injections. There was some evidence that injection therapy may help get patients back to sporting activities and decrease pain in the short term, but there was no evidence indicating a difference between groups in patient satisfaction with treatment.
The evidence for the other comparisons, such as injection therapy versus exercises, made by single studies was too limited to report here.
Quality of the evidence
Most of the studies had some aspects that could undermine the reliability of their results. We decided the evidence was of low or very low quality for all outcomes. Thus, the findings remain uncertain and further research may provide evidence that could change our conclusions.
Conclusions
The currently available evidence is insufficient to support the routine use of injection therapies for painful Achilles tendons in adults. Future studies are needed to provide definitive evidence for this potentially important treatment.
Summary of findings
Summary of findings for the main comparison. Summary of findings: Injection therapies versus placebo or no injection control.
| Injection therapies versus placebo injection or no injection control for people with Achilles tendinopathy | ||||||
|
Population: individuals with an investigator‐reported diagnosis of Achilles tendinopathy (or related terminology, e.g. tendinitis). We excluded trials focusing on the treatment of individuals with systemic conditions (e.g. rheumatoid arthritis and diabetes)1 Setting: primary or secondary care Intervention: injection therapies for Achilles tendinopathy Comparison: no injection control or placebo (sham) treatment | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| No injection control placebo injection | Injection therapy | |||||
| VISA‐A (score 0 to 100; 100 = no problems) At 6 weeks | The mean VISA‐A scores across control groups ranged from 57 to 71 | The mean VISA‐A in the intervention groups was 0.8 points higher (4.6 points lower to 6.1 points higher) |
MD 0.79 (‐4.56 to 6.14) |
200 (5 RCTs) | ⊕⊕⊝⊝ low2 | These results do not include the putative MCID of 12 points3 |
| VISA‐A (score 0 to 100; 100 = no problems) At 3 months | The mean VISA‐A scores across control groups ranged from 61 to 84 | The mean VISA‐A in the intervention groups was 0.9 points lower (6.3 points lower to 4.5 points higher) | MD ‐0.94 (‐6.34 to 4.46) | 189 (5 RCTs) | ⊕⊕⊝⊝ low2 | These results do not include the putative MCID of 12 points3 |
| VISA‐A (score 0 to 100; 100 = no problems) After 3 months (6 to 12 months) | The mean VISA‐A scores across control groups ranged from 73 to 82 | The mean VISA‐A in the intervention groups was 0.1 points lower (6.5 points lower to 6.8 points higher) |
MD 0.14 (‐6.54 to 6.82) |
132 (3 RCTs) | ⊕⊕⊝⊝ low4 | These results do not include the putative MCID of 12 points3 |
|
Adverse events At final follow‐up |
46 per 10005 | 45 per 1000 (23 to 87) |
RR 0.97 (0.50 to 1.89) |
449 (13 RCTs) | ⊕⊝⊝⊝ very low6 | The only major adverse event of injection therapy was a tendon rupture in a trial testing local steroid injection |
|
Pain (VAS; score 0 to 100; 0 = no pain) Follow‐up to 3 months |
The mean pain scores across control groups ranged from 10 to 78 | The mean pain score in the intervention groups was 22.9 points lower (37.5 to 8.4 points lower) | MD ‐22.94 (‐37.53 to ‐8.36) | 219 (7 RCTs) | ⊕⊝⊝⊝ very low7 | The mean values were extracted from graphs and the SDs imputed for 5 of the 67 RCTs (73% of the weight) 5 RCTs (172 participants (78.5%)) were in athletes |
|
Return to sports At final follow‐up |
563 per 10008 | 783 per 1000 (563 to 1000) | RR 1.39 (1.00 to 1.94) | 335 (7 RCTs) | ⊕⊝⊝⊝ very low9 | 4 RCTs (266 participants (79.4%)) were in athletes |
|
Patient satisfaction (number of participants satisfied with their treatment) At final follow‐up |
584 per 10008 | 613 per 1000 (444 to 859) | RR 1.05 (0.76 to 1.47) | 152 (4 RCTs) | ⊕⊝⊝⊝ very low10 | — |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MCID: minimum clinically important difference; MD: mean difference; RCT: randomised controlled trial; RR: risk ratio; SD: standard deviation; VISA‐A = Victorian Institute of Sport Assessment‐Achilles questionnaire | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Of the 15 studies (600 participants) making this comparison, 7 studies included athletes only. Study participants in the individual trials were mainly young to middle aged adults (mean ages of studies ranged from 20 to 50 years).
2We downgraded the evidence one level for limitations in the design and implementation (4 of the 5 trials were at risk of bias, either performance bias or other bias) and one level for imprecision: we imputed the SDs for 3 of the 5 trials (> 36% of the weight).
3The MCID of 12 points was proposed in De Vos 2010.
4We downgraded the evidence one level for limitations in the design and implementation (2 of the 3 trials were at risk of bias, either performance bias or other bias) and one level for imprecision: there were fewer participants at this time point and we imputed the SDs for 1 of the 3 trials (18% of the weight).
5This is the mean event rate. Ten of 13 RCTs had no events in the control group and thus the median event rate was 0.
6We downgraded the evidence two levels for serious imprecision (few or zero events in individual trials) and one level for indirectness (the majority of adverse events were minor and their impact was not stated).
7We downgraded the evidence one level for limitations in the design and implementation (e.g. 4 of the 7 RCTs failed to document or include random sequence generation or allocation concealment), one level for inconsistency (the studies were significantly heterogeneous; I2 = 65%), and one level for imprecision reflecting the wide confidence interval.
8The basis for the assumed risk was the median control group risk across studies.
9We downgraded the evidence one level for limitations in the design and implementation (5 of the 7 RCTs failed to document or include random sequence generation, allocation concealment or blinding of outcome assessment), one level for inconsistency (the studies were significantly heterogeneous; I2 = 84%), and one level for indirectness relating to the timing of the pain outcome, which was too early in several trials to represent final outcome.
10We downgraded the evidence one level for limitations in the design and implementation (3 of the 4 RCTs were at high risk of bias for one domain) and one level for imprecision, reflecting the wide confidence interval.
Background
Description of the condition
The Achilles tendon connects the calf muscles (gastrocnemius, soleus and plantaris) to the heel bone (calcaneus). It transmits muscular forces that effect plantarflexion of the ankle against resistance (such as when standing on tiptoes). It has a fundamental role in walking and locomotion in general.
The tendon is composed of a parallel alignment of collagen, which consists of long stranded molecules called 'tropocollagen' organised into small overlapping bundles. The molecular strands are cross linked to each other, like a rung of ladders, that have a crimped configuration at rest, but straighten when under tension (Evans 2000)
When the Achilles tendon is subject to greatly increased forces (e.g. sudden increase in intensity of an exercise activity) or repetitive submaximal forces over a prolonged duration, such as in long‐distance running, these cross‐links begin to fail across the length of the tendon. This process is followed by a period of remodelling and repair of the damaged tendon. When there is an imbalance between damage and repair, the tendon may begin to exhibit characteristics associated with Achilles tendinopathy, which is sometimes known as 'Achilles tendinitis'. The term tendinitis is less frequently used because it is a term that implies there is in underlying inflammation. Although the role of inflammation has long been debated, it is accepted that tendinopathy is a degenerative condition that subsequently predisposes to other injuries such as Achilles tendon rupture (Narici 2008; Riley 2008).
Achilles tendinopathy occurs at either the heel bone (insertion) or mid‐portion (3 cm to 6 cm from the heel bone). The insertion of the Achilles tendon is thought to be predisposed to developing tendinopathy because of the excessive shear and compressive forces that occur at this site. Tendinopathy at the mid‐portion (also called the 'mid‐substance'), where the calf muscles attach, has been attributed to decreased vascularity as the tendon fibres spiral laterally through 90 degrees at this point (Riley 2008).
Factors associated with Achilles tendinopathy include biomechanical faults (hyperpronation of the foot), systemic diseases (such as diabetes), smoking, age, activity level (exercise intensity and alteration in intensity) and obesity. However, the aetiology is probably multifactorial rather than the result of any one of these considerations (Kraemer 2012; Van Sterkenburg 2011).
Common features of Achilles tendinopathy include pain and stiffness, particularly over the lower portion of the calf. There may also be thickening of the tendon and swelling. Although classically worse in the morning, the pain may be constant or intermittent and aggravated either during or after weight‐bearing exercise (Maffulli 2010). Pain on weight bearing in previously active people may cause considerable disruption to activities of daily living, work and sports.
One study in the Netherlands estimated the annual incidence of symptoms attributable to Achilles tendinopathy in the general population at 2.01 per 1000 people (De Jonge 2011). The annual incidence for mid‐portion Achilles tendinopathy was 1.85 per 1000 people. The annual median age at presentation for mid‐portion tendinopathy was 43.4 years; in 34.6% of cases, a specific relationship to sporting activities was noted. However, this study used Dutch general practitioner (GP) practice records and is likely to have underestimated the true incidence as people may have presented to other healthcare practitioners (e.g. physiotherapists) or not presented at all.
Description of the intervention
There is a large array of non‐surgical (conservative) interventions available for the management of Achilles tendinopathy (Andres 2008; Kearney 2010; Sussmilch‐Leitch 2012). Examples include eccentric exercises, cryotherapy, extracorporeal shockwave therapy, low‐level laser therapy, ultrasound, orthotics, splints, topical nitroglycerin, injections and non‐steroidal anti‐inflammatory drugs (NSAIDS). Our review focuses on injection therapies, of which there are a growing number in use (Coombes 2010).
Injection therapies include a range of options such as corticosteroids, high‐volume saline, prolotherapy, autologous blood, platelet‐rich plasma, aprotinin, botulinum toxin, sodium hyaluronate, polysulphated glycosaminoglycan and polidocanol (Coombes 2010).
Injection therapies can be guided by real‐time ultrasound imaging or unguided; they can be administered in isolation or in combination with any of the above interventions; they can be administered in a single dose or consist of a course; and they can be injected locally into the tendon or targeted at specific sites (such as areas of vascular ingrowth). There is no consensus on many of these factors and the exact intervention is at the discretion of the responsible clinician (Maffulli 2010).
How the intervention might work
All injection therapies are used to deliver a drug directly to the damaged tendon. In general, these substances are thought to act either pharmacologically (e.g. corticosteroids) or mechanically (e.g. high‐volume saline to disrupt neovascular growth).
The injection therapies reported in previous systematic reviews (Coombes 2010; DTB 2012) are listed below together with a brief description of their proposed mechanism of action. In broad terms, they have been classified into two groups. Firstly those stimulating repair activity through causing injury and/or destruction of new vascular ingrowth, which is thought to be a source of pain as this new vascular ingrowth is often accompanied by the proliferation of nerve endings. Secondly those targeting the promotion of repair activity through the introduction of substances to act directly on the repair pathway.
Agents causing injury or disrupting vascular ingrowth to promote repair activity
High‐volume saline: a saline solution is injected along the surface of the Achilles tendon, with or without local anaesthetic. The injection produces a mechanical effect on the new vascular ingrowth associated with tendinopathy, resulting in the new blood vessels stretching and breaking.
Polidocanol: targeted disruption of new vasculature by administration of a scelerosant to precipitate blood vessel fibrosis.
Prolotherapy: hypertonic glucose injected locally to initiate repair activity by causing local tissue trauma.
Agents acting directly on the repair pathway
Autologous blood: injected locally to promote repair activity through the administration of growth factors (present in a person's own blood) directly to the site of injury.
Platelet‐rich plasma: injected locally to promote repair activity through the administration of concentrated growth factors (present in a person's own blood that has been spun at a high speed to separate out the platelet‐rich plasma layer) directly to the injury site.
Aprotinin: injected locally to inhibit collagenase, which would otherwise break down collagen and has been found to be increased in tendinopathy.
Polysulphated glycosaminoglycan: injected locally to prevent destruction and facilitate repair through inhibiting metalloproteinase enzyme activity.
Botulinum toxin: injected locally to decrease tensile stress through the tendon and inhibit substance P, which is increased in tendinopathy.
Sodium hyaluronate: injected locally to absorb mechanical stress and provide a protective buffer for tissues.
Corticosteroid: injected locally to down regulate (acting to decrease) inflammation in the affected tendon.
Injection therapies have a common suite of potential adverse effects, including local infection, bleeding, swelling and tendon rupture. Adverse effects may be the consequence of the injection itself (e.g. local bleeding and weakening of the tendon) or the injected substance.
Why it is important to do this review
Achilles tendinopathy is a common condition, often with significant functional consequences. A review of the evidence from randomised trials of injection therapies to help inform treatment decisions is warranted in the light of the wide range of available treatments, together with an exponential increase in their use (Kaux 2011). A synthesis of the available evidence may also help to direct future research in this area.
Objectives
To assess the effects (benefits and harms) of injection therapies for people with Achilles tendinopathy.
We compared injection therapy versus no treatment, placebo (sham) treatment, no injection control or other active treatment (injection or any other treatment including surgery, physiotherapy or pharmacology). Use of supplementary conservative treatments across study groups was acceptable.
Methods
Criteria for considering studies for this review
Types of studies
Randomised and quasi‐randomised (using a method of allocating participants to a treatment that is not strictly random, e.g. by hospital number) controlled clinical trials evaluating injection therapies for Achilles tendinopathy.
Types of participants
People with an investigator‐reported diagnosis of Achilles tendinopathy (or related terminology, e.g. tendinitis). We excluded trials focusing on the treatment of individuals with systemic conditions (e.g. rheumatoid arthritis and diabetes).
We excluded mixed population trials, including other conditions, unless the proportion of the population with other conditions was small and comparable between the intervention groups, or separate data were available for people with Achilles tendinopathy.
Types of interventions
As described above, there are many different types of injection therapies. In the first instance, we grouped the therapies by the following modes of action:
Injection therapies that cause injury to promote repair
Injection therapies acting directly on the repair pathway
Our main comparisons were injection therapy versus no treatment, placebo (sham) treatment or no injection control; and injection therapy versus other active treatment (such as exercises, orthoses or surgery). All active treatments were accepted, without exclusion. Use of supplementary conservative treatments across study groups was acceptable.
We also compared different injection therapies, again attempting to group these by mode of action; and different doses or number of injections for the same injection therapy.
No single injection therapy is well established or in common use as a treatment for Achilles tendinopathy. This makes it difficult to choose a meaningful control intervention when comparing different injection therapies. However, we adopted the following rules when selecting the control intervention in any comparison: this will be the older, more traditional therapy (e.g. corticosteroid would be selected for a comparison of platelet‐rich plasma versus corticosteroid); the less destructive; or the less intensive of the interventions being tested.
Types of outcome measures
The review focused on functional recovery, together with reported adverse events.
Primary outcomes
Function measured by a validated patient‐reported measure for Achilles tendinopathy (e.g. VISA‐A: an Achilles tendinopathy specific questionnaire, which contains eight questions that cover three domains of pain, function and activity. An asymptomatic person would score 100; the lower the score, the greater the disability (Robinson 2001)).
-
Adverse events:
Serious: e.g. tendon rupture
Non‐serious: e.g. post injection discomfort
Secondary outcomes
Patient‐reported quality of life (e.g. EQ‐5D, 12‐Item Short Form Health Survey)
Non‐validated patient‐reported functional outcomes for Achilles tendinopathy
Pain (e.g. as measured by a visual analogue scale (VAS))
Return to previous level of activity
Patient rating of acceptability or satisfaction
Resource use
Some included articles reported multiple measures of pain (e.g. pain on palpation, pain on walking, pain on resting). Where this was the case, we accepted the patient‐reported pain score during activity and where no description of the pain score was provided, we assumed it to refer to pain on activity. All articles used either a 10‐point pain scale or a 100‐point pain scale. To allow comparison in data analyses, the authors transformed all 10‐point scales to 100‐point scales by multiplying the outcomes by 10.
Regarding the outcome of return to previous level of activity, some articles reported binary yes/no data; others provided further categories such as returned to sport pain free/returned to sport with pain/return to some sport/return to no sport. In these instances the authors recorded all participants that had returned to full sporting activities in one group and collated the remaining responses into the not returned to sport group. The same scenario presented with the outcome of patient rating of acceptability/or satisfaction; again the authors recorded all participants who were satisfied with their treatment in one group and placed all other responses in the not satisfied category.
Timing of outcome measurement
Functional outcome scores were reported at multiple time points. We performed separate analyses representing short (last data point up to six weeks), medium (last data point up to three months) and long‐term follow‐up (last data point after three months) on the primary outcome measure only. Most secondary outcome measures (e.g. patient rating of satisfaction and return to sports) were reported at the final time point only, therefore we performed a single time point analysis on these outcome measures.
Search methods for identification of studies
Electronic searches
The searches were run in two stages. We initially searched the Cochrane Bone, Joint and Muscle Trauma Group Specialised Register (24 February 2014), the Cochrane Central Register of Controlled Trials (CENTRAL 2014, Issue 1), MEDLINE (1946 to February Week 2 2014), MEDLINE In‐Process & Other Non‐Indexed Citations (19 February 2014), EMBASE (1974 to 2014 Week 07), Allied and Complementary Medicine Database (AMED) (1985 to February 2014), Cumulative Index to Nursing and Allied Health Literature (CINAHL) (1981 to 28 February 2014) and SPORTDiscus (1985 to 28 February 2014). We also searched the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) and ISRCTN registry for ongoing and recently completed studies (29 May 2014). We did not apply any restrictions based on language or publication status.
In MEDLINE (Ovid Online), we combined a subject‐specific strategy with the sensitivity‐maximising version of the Cochrane Highly Sensitive Search Strategy for identifying randomised trials (Lefebvre 2011). Search strategies for CENTRAL, MEDLINE, EMBASE, AMED, CINAHL, SPORTDiscus, the WHO ICTRP and the ISRCTN registry are shown in Appendix 1.
Subsequently, we conducted a search update on 20 April 2015 of the Group's Specialised Register, CENTRAL (2015, Issue 3), MEDLINE, EMBASE, CINAHL, AMED and SPORTDiscus.
Searching other resources
We searched reference lists of articles retrieved from the electronic searches and contacted experts in the field for any additional published or unpublished articles.
Data collection and analysis
Selection of studies
Two review authors (RK and DM) independently screened search results for potentially eligible studies, for which we obtained full‐text reports. The same two review authors independently selected articles for inclusion based on the inclusion criteria listed above. We resolved any disagreements through discussion, with arbitration by a third review author (MC) as required.
Data extraction and management
Two review authors (RK and DM) independently extracted data using a piloted data extraction form. Disagreements were resolved through discussion, with arbitration by a third review author (MC) as required. The review statistician (NP), who was independent from the study selection discussions, collated and managed the data.
Assessment of risk of bias in included studies
Two review authors (RK and DM) independently assessed the risk of bias using Cochrane's 'Risk of bias' tool (Higgins 2011). This tool includes the assessment of selection bias (random allocation and allocation concealment), performance bias (blinding of participants and personnel), detection bias (blinding of outcome assessment), attrition bias (incomplete outcome data), reporting bias (selective reporting) and other sources of bias, such as sponsorship from industry. We determined the risk of bias from blinding of subjective and objective outcome measures separately. We resolved any disagreements through discussion and consensus between those conducting the review.
Measures of treatment effect
For continuous data, such as functional scores, we calculated mean differences with 95% confidence intervals (CI). We planned to use standardised mean differences where the same outcome measure was measured using different scoring systems but decided that this was unnecessary in this version of the review. For dichotomous outcomes, such as adverse events, we calculated risk ratios with 95% CI.
Unit of analysis issues
We planned to analyse the data by individual participant. We anticipated that studies would exclude cases of bilateral Achilles tendinopathy and thus unit of analysis issues associated with a disparity between unit of randomisation (person) and analysis (feet) would not arise. As this was not the case, we recorded all exceptions that arose (see Characteristics of included studies). However, the number of participants with cases of bilateral Achilles tendinopathy included in such studies constituted a very small number overall and, as this group of patients could not be separated from the unilateral cases, we did not conduct sensitivity analysis.
We anticipated simple parallel‐group designs, which was the case. However, in the unlikely event that future trials report cross‐over designs, we intend to analyse only the first phase of the results.
Dealing with missing data
Where there were missing data for binary outcomes, we categorised them as failures, providing an overall conservative analysis. For continuous data, we analysed data available and explored the effect of missing data through sensitivity analyses as appropriate.
We endeavoured to acquire missing data directly from the study authors. Finally, where standard deviations were not available, we calculated these from exact P values, CIs, or standard errors. If it was not possible to calculate the standard deviations, then we imputed them in cases where standard deviations for the same outcome measure at the same outcome time point were available from other studies in the review.
Assessment of heterogeneity
We assessed statistical heterogeneity between studies by visual inspection of the overlap of the CIs on the forest plots, and consideration of the Chi² test (P value < 0.1 was interpreted as significant heterogeneity) and the I² statistic. We interpreted the I² results as suggested in Higgins 2011: 0% to 40% might not be important, 30% to 60% may represent moderate heterogeneity, 50% to 90% may represent substantial heterogeneity and 75% to 100% may represent considerable (very substantial) heterogeneity.
Assessment of reporting biases
Where at least 10 studies contributed data to a meta‐analysis, we planned to generate a funnel plot to explore the potential for publication bias.
Data synthesis
We pooled results of comparable groups of trials using both fixed‐effect and random‐effects models. The choice of the model to report was guided by a careful consideration of the extent of heterogeneity and whether it could be explained, in addition to other factors such as the number and size of studies. If there was substantial unexplained heterogeneity (I² > 75%) we considered whether we should still perform a meta‐analysis but instead present a narrative description.
Subgroup analysis and investigation of heterogeneity
Our primary planned subgroup analysis was by mode of action. We also planned subgroup analysis for the following groups.
Insertional versus mid substance tendinopathy
Athletes versus non‐athletes
Smokers versus non‐smokers
Aged over 65 versus aged 65 years or younger
We planned to investigate whether the results of subgroups were significantly different by inspecting the overlap of CIs and performing the test for subgroup differences available in Review Manager 5 (RevMan 2014). If the heterogeneity statistic was large and indicated that one or more of the studies was a clear outlier, then we planned to conduct a meta‐analysis with and without the outliers and document all such decisions. It was also likely that the actual substance injected may be a key determinant of outcome and great source of heterogeneity. Therefore we also planned to explore clinical heterogeneity according to the substance injected.
Sensitivity analysis
When appropriate we performed sensitivity analyses to examine various aspects of the trial and review methodology. This included the effects of missing data (seeDealing with missing data); results at different time points (seeTypes of outcome measures); including trials at high or unclear risk of bias (seeAssessment of risk of bias in included studies); the selection of a statistical model for pooling (seeAssessment of heterogeneity); and including and excluding study outliers.
'Summary of findings' tables
We prepared a 'Summary of findings' table for the main comparison. We used the GRADE approach to assess the quality of evidence related to each of the key outcomes listed in the Types of outcome measures (Chapter 12.2, Higgins 2011).
Results
Description of studies
SeeCharacteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification; Characteristics of ongoing studies.
Results of the search
From the results of our first search (run between February 2014 and May 2014), we screened a total of 677 records from the following databases: Cochrane Bone, Joint and Muscle Trauma Group Specialised Register (37 records); CENTRAL (33), MEDLINE (134), EMBASE (93), AMED (62), CINAHL (148), SPORTDiscus (56), the WHO ICTRP (95) and Current Controlled Trials (19). There were no potentially eligible studies from other sources.
From our subsequent search update on 20 April 2015 of the Group's Specialised Register, CENTRAL, MEDLINE, EMBASE, CINAHL, AMED and SPORTDiscus, we screened a total of 97 records. A trial registration document was also identified for an included study (Kearney 2013).
The search identified a total of 23 articles for potential inclusion and five registered studies, for which we obtained full reports where possible. Upon study selection, 21 articles were included in 18 studies (Alfredson 2005; Alfredson 2007; Bell 2013; Brown 2006; Capasso 1993; Chouchane 1989; DaCruz 1988; De Vos 2010 (published in three articles); Fabbro 2012; Fredberg 2004; Kearney 2013 (published in two articles); Larsen 1987; Obaid 2012; Pearson 2012; Pforringer 1994; Sundqvist 1987; Willberg 2008; Yelland 2011). We excluded one article (Ferrero 2012), four registered studies were ongoing (NCT01343836; NCT01954108; ISRCTN85334402; NCT01583504), and one further registered study (EUCTR2010‐020513‐87), and one study reported only as a conference abstract (Petrella 2013), await classification. A flow diagram summarising the study selection process is shown in Figure 1.
1.
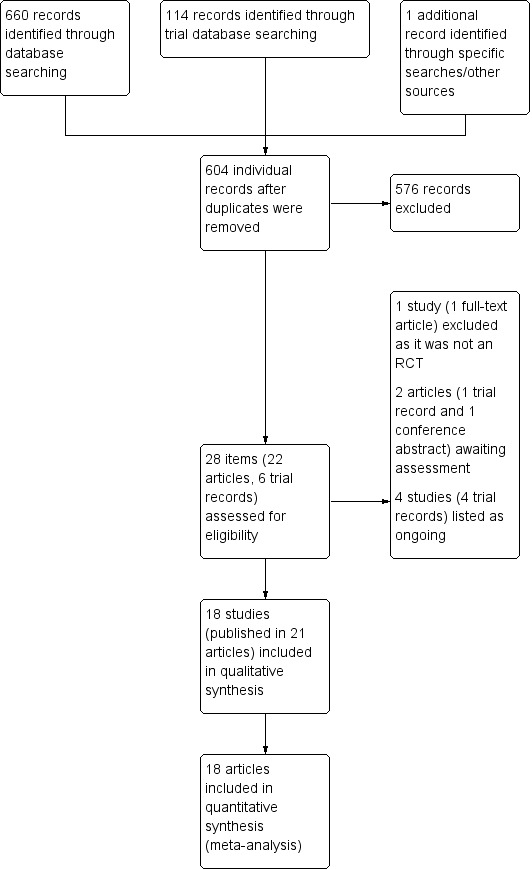
Study flow diagram
Included studies
All included studies were full reports (not abstracts). For further details, please see the Characteristics of included studies.
Design and comparisons
We included 18 randomised controlled trials evaluating injection therapies for Achilles tendinopathy published between 1987 and 2013. Sixteen studies had two groups. The remaining two studies were three‐arm randomised controlled trials, each contributing data to two of the three comparisons tested by the included trials (Fabbro 2012; Yelland 2011).
Fifteen trials (600 participants) compared an injection therapy with a placebo injection or no injection control (Alfredson 2005; Bell 2013; Brown 2006; Capasso 1993; Chouchane 1989; DaCruz 1988; De Vos 2010; Fabbro 2012; Fredberg 2004; Larsen 1987; Obaid 2012; Pearson 2012; Pforringer 1994; Sundqvist 1987; Yelland 2011). Two of these compared injections that cause injury to promote repair to a placebo injection or no injection control (49 participants in total) (Alfredson 2005; Yelland 2011), with the remainder comparing injections that act directly on the repair pathway.
In the second comparison, four studies (105 participants) compared an injection therapy with an active treatment (Alfredson 2007; Fabbro 2012; Kearney 2013; Yelland 2011). Two studies tested injury‐causing agents (Alfredson 2007; Yelland 2011), and the other two studies tested direct repair agents (Fabbro 2012; Kearney 2013). The active treatments were surgery (Alfredson 2007), eccentric loading exercises (Kearney 2013; Yelland 2011), and dry needling (Fabbro 2012).
In a third comparison, one study with 48 participants compared two different concentrations (high versus low dose) of the same injection (polidocanol) (Willberg 2008).
Setting
Studies were conducted within sports medicine clinics (Alfredson 2005; Alfredson 2007; Bell 2013; De Vos 2010; Fredberg 2004; Pearson 2012; Willberg 2008), private practices (Brown 2006), accident and emergency departments (DaCruz 1988), orthopaedic departments (Kearney 2013; Obaid 2012; Pforringer 1994; Sundqvist 1987), primary care centres (Yelland 2011), and occupational medical centres (Larsen 1987), and not stated in three (Capasso 1993; Chouchane 1989; Fabbro 2012). All but Yelland 2011 were conducted in single centres.
Four trials were completed in Sweden (Alfredson 2005; Alfredson 2007; Sundqvist 1987; Willberg 2008), four in Australasia (Bell 2013; Brown 2006; Pearson 2012; Yelland 2011), three in the UK (DaCruz 1988; Kearney 2013; Obaid 2012), two in Denmark (Fredberg 2004; Larsen 1987), one in the Netherlands (De Vos 2010), one in France (Chouchane 1989), and one in Germany (Pforringer 1994), and the country was not stated in two (Capasso 1993; Fabbro 2012).
Six included articles received funding to complete the trials. Industry sources of funding were: Biomet Biologics LLC, which funded De Vos 2010; Innovacell, which funded Obaid 2012, and Leo Pharmaceutical Products, which funded Larsen 1987. Public or profession‐based sources of funding were cited in Kearney 2013 (Chartered Society of Physiotherapy), Willberg 2008 (Swedish Research Council for Sports), and Yelland 2011 (Musculoskeletal Research Foundation of Australia, the Australian Podiatry Education and Research Foundation and the Griffith University Office of Research).
Participants
A total of 732 participants were included in the 18 included trials. Study samples ranged from 20 (Alfredson 2005; Alfredson 2007; Kearney 2013; Larsen 1987) to 97 (Capasso 1993).
The mean age of the participants in the individual trials ranged from 20 years (Larsen 1987) to 50 years (Alfredson 2005; Pearson 2012; Willberg 2008). Of the 17 trials reporting on gender, 11 reported a higher ratio of male to female participants (Bell 2013; Brown 2006; Capasso 1993; Chouchane 1989; DaCruz 1988; Fabbro 2012; Fredberg 2004; Larsen 1987; Obaid 2012; Sundqvist 1987; Willberg 2008). Seven trials exclusively evaluated injection therapies in recreational/professional athletes (Capasso 1993; Chouchane 1989; Fabbro 2012; Fredberg 2004; Larsen 1987; Pforringer 1994; Sundqvist 1987). None of the 18 trials recorded the smoking status of included patients.
Eight trials included participants with bilateral symptoms (Brown 2006; DaCruz 1988; Kearney 2013; Larsen 1987; Obaid 2012; Pearson 2012; Willberg 2008; Yelland 2011). Of these, four trials explicitly randomised each Achilles tendon as a separate unit (i.e. one patient was randomised twice for each tendon) (Brown 2006; DaCruz 1988; Obaid 2012; Pearson 2012), and the remaining four trials seemed to have randomised the patient as one unit (i.e. one patient was randomised once for both tendons). Only one study described the inclusion of participants with pain at the insertion of the Achilles tendon (Capasso 1993). The remaining studies all assessed mid portion tendinopathy.
Interventions
The following injection therapies that cause injury or disrupting vascular ingrowth to promote repair activity were evaluated in the included studies:
Polidocanol: Alfredson 2005; Alfredson 2007; Willberg 2008
Prolotherapy: Yelland 2011
The following injection therapies acting directly on the repair pathway were evaluated in the included studies:
Autologous blood: Bell 2013; Pearson 2012
Platelet‐rich plasma: De Vos 2010; Kearney 2013
Deproteinised haemodialysate: Pforringer 1994
Aprotinin: Brown 2006; Capasso 1993
Polysulphated glycosaminoglycan: Larsen 1987; Sundqvist 1987
Corticosteroid: Chouchane 1989; DaCruz 1988; Fabbro 2012; Fredberg 2004
Skin derived fibroblasts: Obaid 2012
Outcomes
This review considered two primary outcome measures, function measured by a validated patient‐reported measure and adverse events. Of the 18 trials, seven reported the VISA‐A (Bell 2013; Brown 2006; De Vos 2010; Kearney 2013; Pearson 2012; Yelland 2011; Obaid 2012), and all but Pforringer 1994 reported adverse events. This review also considered the following secondary outcome measures, which were reported by the following studies:
Patient‐reported quality of life: Kearney 2013
Non‐validated patient‐reported outcomes: Brown 2006; Pforringer 1994
Pain: Alfredson 2005; Alfredson 2007; Chouchane 1989; DaCruz 1988; Fabbro 2012; Fredberg 2004; Larsen 1987; Obaid 2012; Pforringer 1994; Willberg 2008; Yelland 2011
Return to previous activities: Bell 2013; Brown 2006; Capasso 1993; DaCruz 1988; De Vos 2010; Pforringer 1994
Other adverse events: reported by all except Pforringer 1994
Patient rating of satisfaction: Alfredson 2005; Alfredson 2007; Bell 2013; De Vos 2010; Willberg 2008; Yelland 2011
Resource use: Fabbro 2012; Yelland 2011
Excluded studies
SeeCharacteristics of excluded studies.
Ferrero 2012 evaluated the effectiveness of platelet‐rich plasma in chronic Achilles tendinopathy but, on further analysis, proved not to be a randomised controlled trial and was subsequently excluded.
Ongoing studies
SeeCharacteristics of ongoing studies.
We identified four ongoing studies (NCT01343836; NCT01954108; ISRCTN85334402; NCT01583504). These studies include evaluation of autologous tenocyte implantation, hyaluronan, cell therapy based on PRP and high‐volume saline injections.
Studies awaiting classification
SeeCharacteristics of studies awaiting classification.
Two studies are awaiting classification (EUCTR2010‐020513‐87; Petrella 2013). We were unable to determine the status or obtain further information on EUCTR2010‐020513‐87, which is reported only in a trial registration document. Petrella 2013, which compares hyaluronan versus placebo injection in 35 people with chronic Achilles tendinopathy, is currently insufficiently reported in a conference abstract only.
Risk of bias in included studies
See Characteristics of included studies and Figure 2.
2.
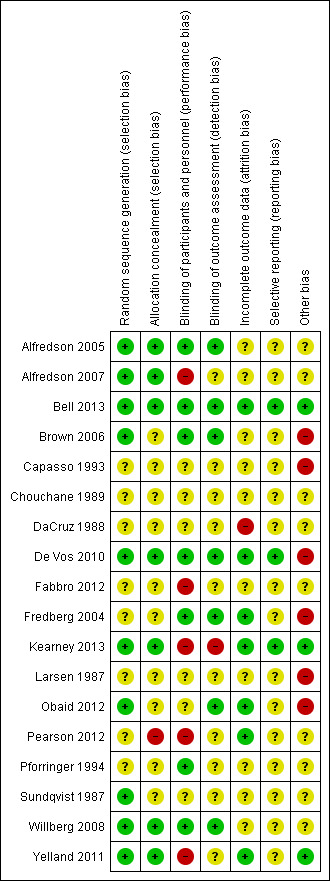
'Risk of bias' summary: review authors' judgements about each risk of bias item for each study.
Figure 2 highlights the variability amongst the articles regarding reporting of key methodological considerations. In particular, trials reported up to 1994 consistently lacked sufficient detailed reporting to make valid judgements on several risk of bias domains.
Allocation
Eight studies did not describe the random sequence generation (Capasso 1993; Chouchane 1989; DaCruz 1988; Fabbro 2012; Fredberg 2004; Larsen 1987; Pearson 2012; Pforringer 1994). The 10 remaining studies all used methods describing a random component in the sequence. Only seven of these 10 also reported adequate concealment of allocation, including methods of central randomisation (Kearney 2013; Yelland 2011) and sealed, opaque envelopes (Alfredson 2005; Alfredson 2007; Bell 2013; De Vos 2010; Willberg 2008). We judged these seven as being at low risk of selection bias. We judged one study at high risk of bias because participants could foresee their allocation (Pearson 2012).
Blinding
Six of the trials reported blinding of participants and personnel and outcome assessment (Alfredson 2005; Bell 2013; Brown 2006; De Vos 2010; Fredberg 2004; Willberg 2008), and we scored these at low risk of performance and detection bias. Seven studies did not describe this component in their methods section (Alfredson 2005; Capasso 1993; Chouchane 1989; DaCruz 1988; Fabbro 2012; Larsen 1987; Sundqvist 1987). Three studies reported that there was no blinding of participants, personnel or outcome assessment (Kearney 2013; Pearson 2012; Yelland 2011). Obaid 2012 reported blinding of the outcome measure assessment only and Pforringer 1994 reported blinding of the participants and personnel only. We judged all four trials comparing injection therapy versus an active treatment (Alfredson 2007; Fabbro 2012; Kearney 2013; Yelland 2011) and Pearson 2012 at high risk of performance bias. We judged only Kearney 2013 to be at high risk of detection bias.
Incomplete outcome data
Alfredson 2005; Alfredson 2007; Bell 2013; Capasso 1993; Chouchane 1989; Fabbro 2012; Larsen 1987; Pforringer 1994; Sundqvist 1987 and Willberg 2008 did not discuss missing data or its handling in their final results. We judged Bell 2013; De Vos 2010; Fredberg 2004; Kearney 2013; Obaid 2012; Pearson 2012 and Yelland 2011 as being a low risk as they either reported no missing data or reasons for data being missing with appropriate analysis methods. This is in contrast to DaCruz 1988, which we judged as being at high risk of attrition bias.
Selective reporting
Only three studies provided evidence of publication of prior protocols in trials databases (Bell 2013; De Vos 2010; Kearney 2013).
Other potential sources of bias
We judged six studies as having a high risk of another potential source of bias. Brown 2006 carried out their study in a private practice and provided all participants with free treatment and follow‐up in the private clinic. The lack of details on randomisation and absence of an explanation for the imbalance in numbers in the intervention and control groups of Capasso 1993 mean that we cannot rule out that data from non‐randomised patients were included. Fredberg 2004 had a high number of participants crossing over within the study period. Three were supported with industry funding (De Vos 2010; Larsen 1987; Obaid 2012).
Effects of interventions
See: Table 1
Where available, the primary outcome data (Victorian Institute of Sport Assessment‐Achilles questionnaire (VISA‐A)) are presented for short (last data point up to six weeks), medium (last data point up to three months) and long‐term (last data point after three months) time points. Where available, the secondary outcome data are reported at final follow‐up for individual trials. We made an exception for pain scores because of the distribution of these.
The inclusion of 10 studies only occurred in one analysis of adverse events (Analysis 1.5), however we did not generate a funnel plot in this case due to the low number of events.
1.5. Analysis.
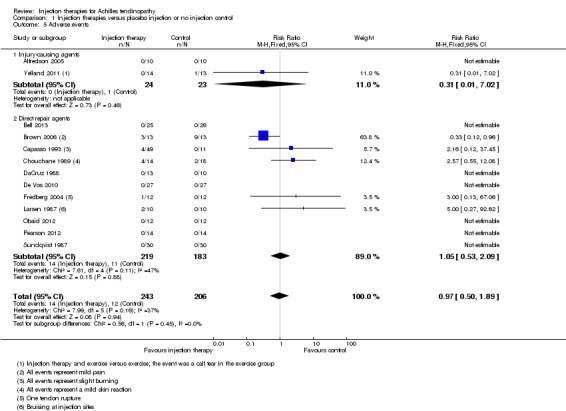
Comparison 1 Injection therapies versus placebo injection or no injection control, Outcome 5 Adverse events.
Comparison I: Injection therapies versus placebo injection or no injection control
Fifteen studies compared injection therapies versus a placebo injection or no injection control (Alfredson 2005; Bell 2013; Brown 2006; Capasso 1993; Chouchane 1989; DaCruz 1988; De Vos 2010; Fabbro 2012; Fredberg 2004; Larsen 1987; Obaid 2012; Pforringer 1994; Pearson 2012; Sundqvist 1987; Yelland 2011). We subgrouped these by mode of action. Two studies, which evaluated polidocanol and prolotherapy, were in the subgroup of injection therapies (Alfredson 2005; Yelland 2011). The other 13 studies were in the subgroup of injection therapies that act directly on the repair pathway; these included injection therapies of autologous blood, platelet‐rich plasma, deproteinised haemodialysate, aprotinin, polysulphated glycosaminoglycan, corticosteroid and skin‐derived fibroblasts). Due to the large range of injection types and outcomes reported, we did not undertake separate subgroup analyses by individual injection therapies.
Primary outcome measures
VISA‐A
The VISA‐A was reported by five studies at six weeks and three months (Bell 2013; Brown 2006; De Vos 2010; Pearson 2012; Yelland 2011; 200 patients) and by three studies beyond three months (Bell 2013; De Vos 2010; Yelland 2011; 132 patients). At each time point, we considered heterogeneity to be unimportant (overall I² below 40% for all time points).
The pooled analysis at all three time points shows that the injection group is no better than placebo and/or no injection control (six weeks: mean difference (MD) 0.79, 95% confidence interval (CI) ‐4.56 to 6.14; three months: MD ‐0.94, 95% CI ‐6.34 to 4.46; after three months: MD 0.14, 95% CI ‐6.54 to 6.82). When we divided the data into subgroups of those injections that cause injury to promote repair (Yelland 2011), and those that act directly on the repair pathway (Bell 2013; Brown 2006; De Vos 2010; Pearson 2012), again at no time points did the injection therapy group demonstrate superiority (seeAnalysis 1.1; Analysis 1.2; Analysis 1.3). We extracted data for mean scores for Yelland 2011 from a graph and we imputed standard deviations for three trials (Brown 2006; Pearson 2012; Yelland 2011) from data from similar studies. Yelland 2011 presented an intention‐to‐treat analysis for the number of participants who had achieved the minimum clinically important increase of 20 points in VISA‐A scores from baseline over time; this also did not show a difference between the two groups at any of the three follow‐up times (seeAnalysis 1.4).
1.1. Analysis.
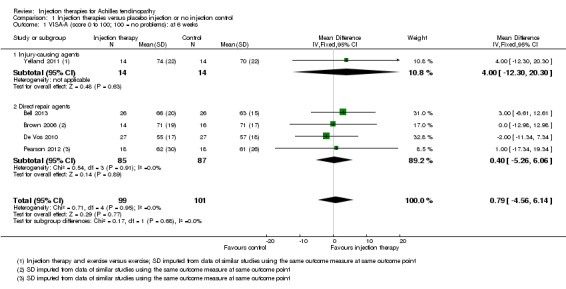
Comparison 1 Injection therapies versus placebo injection or no injection control, Outcome 1 VISA‐A (score 0 to 100; 100 = no problems): at 6 weeks.
1.2. Analysis.
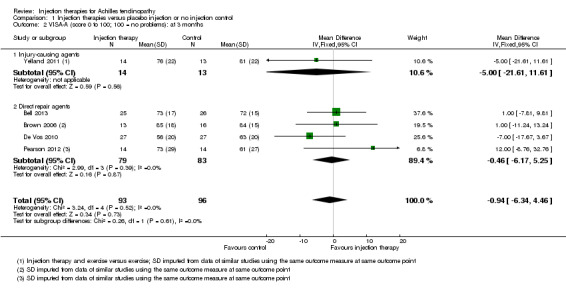
Comparison 1 Injection therapies versus placebo injection or no injection control, Outcome 2 VISA‐A (score 0 to 100; 100 = no problems): at 3 months.
1.3. Analysis.
Comparison 1 Injection therapies versus placebo injection or no injection control, Outcome 3 VISA‐A (score 0 to 100; 100 = no problems): after 3 months.
1.4. Analysis.
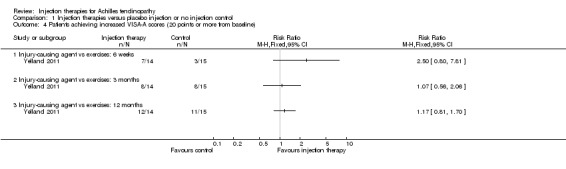
Comparison 1 Injection therapies versus placebo injection or no injection control, Outcome 4 Patients achieving increased VISA‐A scores (20 points or more from baseline).
Obaid 2012 reported VISA‐A at six weeks, three months and six months. However, the data reported were median and ranges and therefore we did not include them in the pooled analysis. At each time point the VISA‐A results for the 12 patients in the interventional arm were 50 (range 15 to 85), 50 (range 30 to 90) and 80 (range 35 to 90). For the control arm the results were 35 (range 10 to 50), 36 (range 20 to 55) and 34 (range 22 to 58).
Adverse events
Adverse events were reported by all but one study (Pforringer 1994). Data split by injection therapy and control were available for 13 studies (14/243 versus 12/206; risk ratio (RR) 0.97, 95% CI 0.50 to 1.89; seeAnalysis 1.5). The overall I² indicated insignificant heterogeneity (less than 40%), with no evidence of subgroup differences based on mode of action. Adverse events in the injection groups included reports of increased mild pain (Brown 2006), slight burning (Capasso 1993), slight skin reaction (Chouchane 1989), tendon rupture (Fredberg 2004), and bruising at injection sites (Larsen 1987). The tendon rupture was the only serious adverse event and occurred during a trial of local steroid injection. Adverse events in the placebo/no injection control included one calf tear (Yelland 2011), mild pain (Brown 2006), and slight skin reaction (Chouchane 1989). Fabbro 2012, which compared injection therapy plus dry needling versus dry needling only, reported only three minor complications, such as "mild pain after the procedure" but did not identify the group(s) in which these occurred.
Secondary outcome measures
Patient‐reported quality of life
Not reported.
Non‐validated patient‐reported functional outcomes
Not reported.
Pain (visual analogue scale (VAS) 0 to 100: worst pain)
Seven studies reported pain outcomes, totalling 219 participants (Alfredson 2005; Chouchane 1989; Fabbro 2012; Fredberg 2004; Larsen 1987; Pforringer 1994; Yelland 2011). Of these we subgrouped Alfredson 2005 and Yelland 2011 into injections that cause injury to promote repair (47 participants) and we subgrouped the remaining studies into injections that act directly on the repair pathway (172 participants). We extracted data for mean scores from graphs for five studies (Fabbro 2012; Fredberg 2004; Larsen 1987; Pforringer 1994; Yelland 2011), and we imputed standard deviations for these five trials from data from similar studies. Pain results for periods up to three months are presented in Analysis 1.6. The individual trial and pooled results are all in favour of the injection group (MD ‐22.94, 95% CI ‐37.53 to ‐8.36), but there was very significant heterogeneity in the results for the injection therapies that act directly on the repair pathway (I² = 86%). DaCruz 1988 also reported a pain score within their trial; however, the article contained insufficient data to report any summary statistics.
1.6. Analysis.
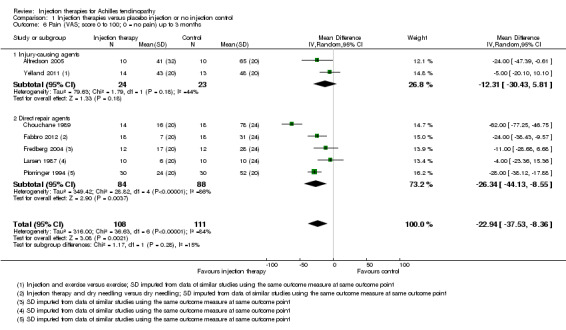
Comparison 1 Injection therapies versus placebo injection or no injection control, Outcome 6 Pain (VAS; score 0 to 100; 0 = no pain) up to 3 months.
Three trials, which did not include cross‐over to the active intervention for participants allocated placebo, reported on longer‐term results (Fabbro 2012; Obaid 2012; Yelland 2011). By 12 months follow‐up in Fabbro 2012, the mean VAS pain scores in both the steroid injection plus dry needling group and the dry needling group had dropped to zero. Obaid 2012 reported median and ranges only of 40 (range 30 to 60) for the injection therapy group and 10 (range 0 to 20) for the placebo group at six months. Pain scores in Yelland 2011 declined over time in both groups but to a lesser extent in the exercises only group; the mean pain scores at 12 months were 12.5 in the prolotherapy plus exercises group versus 31 in the exercises group.
Return to previous level of activity
Seven studies reported return to sport as an outcome, including 335 participants in total (Bell 2013; Brown 2006; Capasso 1993; DaCruz 1988; De Vos 2010; Larsen 1987; Pforringer 1994). The seven studies included six different injection therapies (autologous blood, platelet‐rich plasma, aprotinin, corticosteroid, heparin and deproteinised haemodialysate). The pooled data for the number of participants returning to sport or military training (Larsen 1987) favour injection therapy (RR 1.39, 95% CI 1.00 to 1.94), but there is significant and substantial heterogeneity (I² = 65%; seeAnalysis 1.7).
1.7. Analysis.
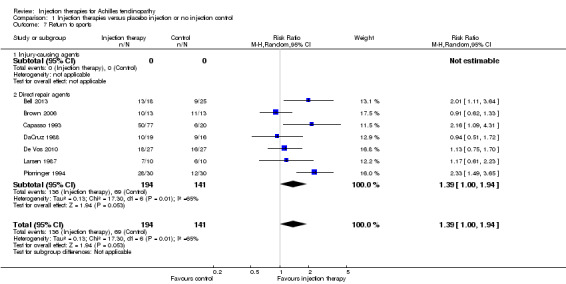
Comparison 1 Injection therapies versus placebo injection or no injection control, Outcome 7 Return to sports.
Patient rating of acceptability or satisfaction
Four studies reported this outcome (Alfredson 2005; Bell 2013; De Vos 2010; Yelland 2011; 152 participants). We subgrouped Alfredson 2005 and Yelland 2011 into injury‐causing agents (47 participants) and we subgrouped Bell 2013 and De Vos 2010 into direct repair agents (105 participants).
The pooled analysis shows no significant result (53/76 versus 48/76 were satisfied; RR 1.05, 95% CI 0.76 to 1.47), with no indication of subgroup differences (I² = 0%) (Analysis 1.8).
1.8. Analysis.
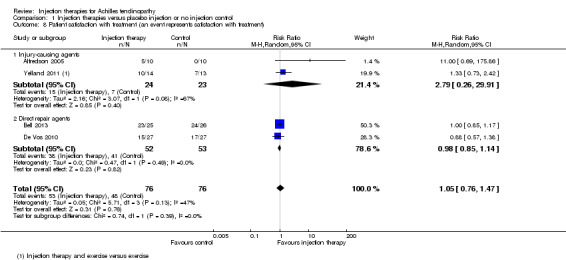
Comparison 1 Injection therapies versus placebo injection or no injection control, Outcome 8 Patient satisfaction with treatment (an event represents satisfaction with treatment).
Resource use
Fabbro 2012 reported the cost of the intervention to be EUR 70; no further data were presented. Yelland 2011 also reported the cost of the interventions only, reporting the combined injection and exercise to be AUD 591 and exercise only AUD 400 per patient. This cost difference was based on the sum of health insurance for the respective treatments, additional GP and specialist visits, allied health professional visits, pharmaceutical costs and 'other' costs. No other studies discussed resource use.
Comparison II: Injection therapies versus active treatment
Four studies (105 participants in this comparison) compared injection therapies versus an active treatment (Alfredson 2007; Fabbro 2012; Kearney 2013; Yelland 2011). Two studies tested injury‐causing agents (Alfredson 2007; Yelland 2011), respectively polidocanol and prolotherapy, and the other two studies tested direct repair agents (Fabbro 2012; Kearney 2013), respectively corticosteroid and platelet‐rich plasma. The active treatments were surgery (Alfredson 2007), eccentric loading exercises (Kearney 2013; Yelland 2011), and dry needling (Fabbro 2012). Given the disparity between the active treatments, we have presented the results grouped by comparison.
Primary outcome measures
VISA‐A (0 to 100: best score)
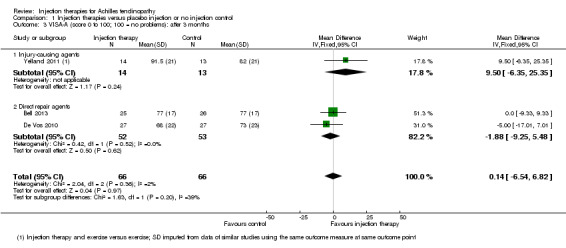
This outcome was reported by Kearney 2013 and Yelland 2011 at each time point. Although favouring injection therapy, none of the differences between the two groups in Kearney 2013 were significant at any of the three time points (seeAnalysis 2.1). Mean scores for Yelland 2011, extracted from a graph, showed little difference between the two groups at three time points: 71 for injection therapy versus 70 for exercises (six weeks); 81 versus 80 (three months); and 86 versus 82 (12 months). Yelland 2011 presented an intention‐to‐treat analysis for the number of participants who had achieved the minimum clinically important increase of 20 points in VISA‐A scores from baseline over time; this also did not show a difference between the two groups at any of the three follow‐up times (seeAnalysis 2.2).
2.1. Analysis.
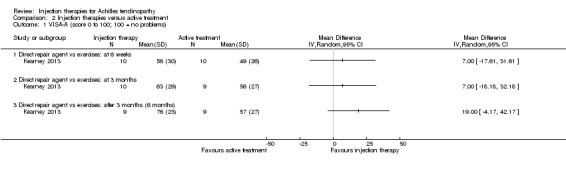
Comparison 2 Injection therapies versus active treatment, Outcome 1 VISA‐A (score 0 to 100; 100 = no problems).
2.2. Analysis.
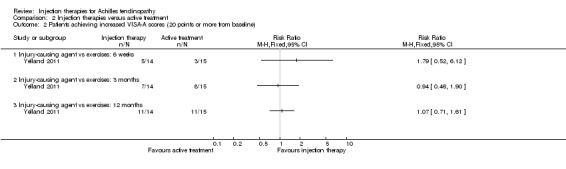
Comparison 2 Injection therapies versus active treatment, Outcome 2 Patients achieving increased VISA‐A scores (20 points or more from baseline).
Adverse events
All four studies (102 participants) reported on adverse events. Alfredson 2007 reported one deep wound infection in the surgical group, Kearney 2013 reported there were no complications in either group and Yelland 2011 reported one calf tear in their eccentric loading exercises group (seeAnalysis 2.3). Fabbro 2012 reported only three minor complications, such as "mild pain after the procedure" but did not identify the group(s) in which these occurred.
2.3. Analysis.
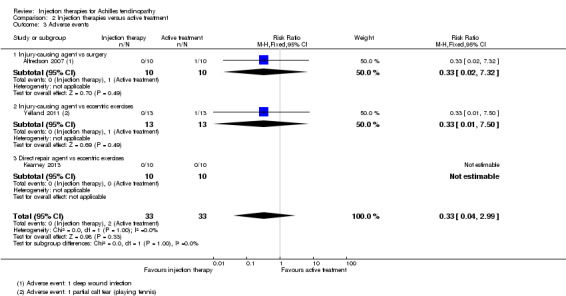
Comparison 2 Injection therapies versus active treatment, Outcome 3 Adverse events.
Secondary outcome measures
Patient‐reported quality of life
This was only reported by Kearney 2013, who found no significant difference in EQ‐5D scores (0 to 1: best quality of life) at six months (MD 0.08, 95% CI ‐0.25 to 0.41; seeAnalysis 2.4).
2.4. Analysis.
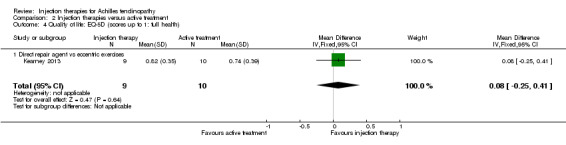
Comparison 2 Injection therapies versus active treatment, Outcome 4 Quality of life: EQ‐5D (scores up to 1: full health).
Non‐validated patient‐reported functional outcomes for Achilles tendinopathy
Not reported.
Pain (VAS 0 to 10: worst pain)
There were no usable data for Alfredson 2007, who reported pain outcomes for subgroups only. Mean pain scores were presented graphically for both Fabbro 2012 and Yelland 2011. By 12 months follow‐up in Fabbro 2012, the mean VAS pain score in the steroid injection therapy group had increased from a low point of less than 0.5 points at 14 days to approximately 5.1 points, while that for the dry needling group had dropped to zero. Pain scores in Yelland 2011 declined over time in both groups but to a lesser extent in the exercises group; the mean pain scores at 12 months were 1.25 in the prolotherapy group versus 3.1 in the exercises group. Yelland 2011 reported that the decreases in pain scores from baseline for the exercises group "were significantly less by a clinically important difference than for prolotherapy at 6 months (difference 2.3; 95% Wald CI 0.3 to 4.4; p=0.028)".
Return to previous level of activity
Not reported.
Patient rating of acceptability or satisfaction
Two trials reported patient satisfaction with treatment (Alfredson 2007; Yelland 2011) (seeAnalysis 2.5). In Alfredson 2007, fewer (6/10) participants were satisfied with the injection therapy compared with those in the surgery group (10/10): RR 0.62, 95% CI 0.37 to 1.03. Yelland 2011 found slightly more satisfied participants given injection therapy compared with those given eccentric exercises: 9/13 versus 7/13; RR 1.29, 95% CI 0.69 to 2.39.
2.5. Analysis.
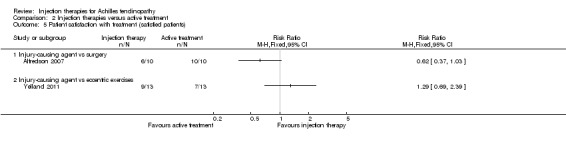
Comparison 2 Injection therapies versus active treatment, Outcome 5 Patient satisfaction with treatment (satisfied patients).
Resource use
No studies conducted a health economic analysis. However, Yelland 2011 reported that prolotherapy cost an additional AUD 90 in total compared with the eccentric exercises. This cost difference was based on the sum of health insurance for the respective treatments, additional GP and specialist visits, allied health professional visits, pharmaceutical costs and 'other' costs.
Comparison III: High‐dose versus low‐dose injection therapy
One study compared high‐dose (10 mg/ml) with low‐dose (5 mg/ml) polidocanol in 48 participants with 52 affected tendons (Willberg 2008). No adverse events were reported in either trial arm (seeAnalysis 3.1). There was no difference between the two doses in the pain scores after one to three treatments (treatments were six to eight weeks apart) measured on a VAS (0 to 100: higher scores mean worse pain): MD ‐1.00, 95% CI ‐17.06 to 15.06; 52 tendons (seeAnalysis 3.2). Similar numbers of participants were satisfied with the treatment of their tendon after a maximum of three treatments (19/26 versus 20/26; RR 0.95, 95% CI 0.69 to 1.30; seeAnalysis 3.3); all 13 dissatisfied participants accepted the offer of another injection and all participants were reported as being ultimately satisfied with their treatment after a maximum of five injections.
3.1. Analysis.
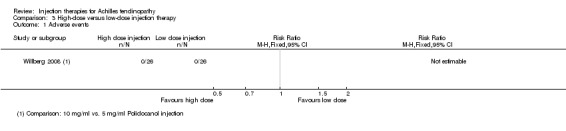
Comparison 3 High‐dose versus low‐dose injection therapy, Outcome 1 Adverse events.
3.2. Analysis.
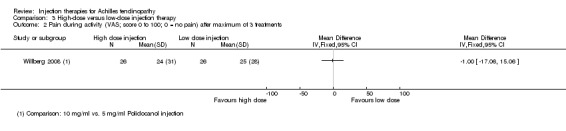
Comparison 3 High‐dose versus low‐dose injection therapy, Outcome 2 Pain during activity (VAS; score 0 to 100; 0 = no pain) after maximum of 3 treatments.
3.3. Analysis.
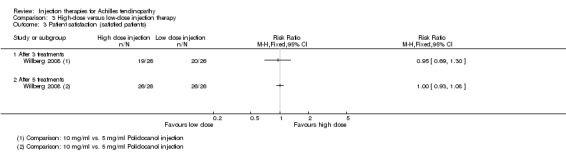
Comparison 3 High‐dose versus low‐dose injection therapy, Outcome 3 Patient satisfaction (satisfied patients).
Comparison IV: Injection therapy versus injection therapy
No studies were included.
Subgroup analyses
There were either insufficient or no available data to conduct any of the four pre‐planned subgroup analyses relating to participant characteristics (seeSubgroup analysis and investigation of heterogeneity). Of particular note is that only Capasso 1993 included participants with insertional tendinopathy.
Discussion
Summary of main results
This review, which covers injection therapies for Achilles tendinopathy, includes 18 small trials involving a total of 732 participants. Sixteen trials had two groups. The other two trials had three groups, and contributed data to two of the three main comparisons tested by the included trials. Seven of the included trials reported the primary outcome measure of interest, Victorian Institute of Sport Assessment‐Achilles questionnaire (VISA‐A). These trials were all published from 2006 onwards, which probably reflects the timeline between the outcome measure development (Robinson 2001) and uptake in clinical trials. All but one trial reported on adverse events. The 18 studies evaluated nine different injection therapies, two of which were injury‐causing agents (polidocanol, prolotherapy), and the other seven of which were direct repair agents (autologous blood, platelet‐rich plasma, deproteinised haemodialysate, aprotinin, polysulphated glycosaminoglycan, corticosteroid and skin‐derived fibroblasts). Consistent with our protocol and given the small number of trials and limitation of the outcome data, we subgrouped injection therapy by mode of action rather than different injection therapies.
Fifteen trials compared one of nine different injection therapies with a placebo injection or no injection control, four trials compared an injection therapy with active treatment, and one trial compared two different concentrations of the same injection. No trials compared different injection therapies.
Comparison I: Injection therapies versus placebo injection or no injection control
The findings for this comparison, tested by 15 trials, are summarised in Table 1. There is low quality evidence of a lack of clinically important differences in VISA‐A scores between injection therapy and control groups at six weeks (200 participants, five trials), three months (189 participants, five trials) or between six and 12 months (132 participants, three trials). Very low quality evidence showed little difference between the two groups in adverse events (449 participants, 13 trials), most of which were minor and short‐lasting. The only major adverse event in the injection therapy group was an Achilles tendon rupture, which happened in a trial testing corticosteroid injections. There was very low quality evidence in favour of the injection therapy group in short‐term (under three months) pain (219 participants, seven trials) and in return to sports (335 participants, seven trials) There was very low quality evidence indicating little difference between groups in patient satisfaction with treatment (152 participants, four trials). There was insufficient evidence to conclude on subgroup differences based on mode of action given that only two trials tested injury‐causing agents and the clear heterogeneity of the other 13 trials, which tested therapies that act directly on the repair pathway.
The review authors identified three ongoing (or not fully characterized) studies in this category, evaluating autologous tenocyte implantation, hyaluronan with botulinus toxin, and platelet‐rich plasma. None of these studies is a large multi‐centre study that is likely to provide future definitive evidence on this group of therapies.
Comparison II: Injection therapies versus active treatment
Four small studies compared an injection therapy versus an active treatment. While presented together in one section, each trial provided low or very low quality and generally incomplete evidence for a different comparison. One trial, Alfredson 2007 with 20 participants, comparing an injury‐causing agent versus surgery, reported a deep wound infection in the surgery group but found that all 10 participants in this group were satisfied with their treatment compared with six of 10 treated with injection therapy. One trial, Fabbro 2012 with 36 participants, comparing a direct repair agent versus dry needling, reported three minor adverse events (mild pain post procedure) but did not identify the treatment group in which these occurred. It found significantly higher pain scores in the steroid group compared with the exercise group at 12 months. One pilot study, Kearney 2013, with 20 participants comparing a direct repair agent versus eccentric exercises, found no significant difference between the two interventions in VISA‐A scores at six weeks, and three and six months. The study reported no complications and minimal between‐group difference in quality of life. One study, Yelland 2011 with 29 participants, comparing an injury‐causing agent versus eccentric exercises, found no significant difference between the two interventions in VISA‐A results at six weeks, and three and 12 months. It reported that one participant in the eccentric exercise group suffered a calf muscle tear during sport, and found greater pain in the exercise group at 12 months, and slightly but not significantly greater patient satisfaction in the injection group.
We identified one ongoing but small study in this category that is comparing hyaluronan versus extracorporeal shock wave therapy (NCT01954108).
Comparison III: High‐dose versus low‐dose injection therapy
The evidence from one study with 48 participants (52 tendons) that compared polidocanol 10 mg/ml versus polidocanol 5 mg/ml was of very low quality. The trial reported no adverse events, and no difference in pain or in the numbers of participants who were satisfied after a maximum of three treatments.
Comparison IV: Injection therapy versus injection therapy
We included no studies in this category. We identified one ongoing study that is evaluating high‐volume injection therapy. However, this is a small study that is unlikely to provide definitive evidence.
Overall completeness and applicability of evidence
The main comparison of this review was evaluated in 600 people with Achilles tendinopathy by 15 small trials. However, data were available for the key primary outcome (VISA‐A) for a maximum of only 200 participants in five trials. Exact mean values for final VISA‐A scores could be calculated for only four trials and actual standard deviations were only available for two trials (106 participants). This illustrates the incompleteness of the data for this review. Although adverse outcome data could be pooled from 13 trials (449 participants), the rarity of serious adverse events means that a far greater population size would be required to appreciate a true picture. Follow‐up was too short in several trials, in particular to measure outcomes such as recurrence. This is largely reflective of clinical practice, whereby after a period of six months it would not be unreasonable to trial a different treatment modality if the one initially administered was ineffective.
We kept the inclusion criteria for this review broad in an attempt to ensure that the final results were applicable to everyday practice. However, seven studies evaluated injection therapies, all acting directly on the repair pathway, exclusively in an athletic population (Capasso 1993; Chouchane 1989; Fabbro 2012; Fredberg 2004; Larsen 1987; Pforringer 1994; Sundqvist 1987). This finding is in keeping with the study settings, of which just under half took place in sports medicine clinics. Furthermore, the mean age of participants in all 18 trials was under 50 years; therefore the applicability of the results to an older non‐athletic group in a secondary or primary care setting is limited. The studies, however, were conducted across several countries (Australasia, Denmark, France, Germany, the Netherlands, Sweden, UK) and therefore not specific to one particular healthcare system.
Although the data were insufficient to draw any conclusion on the relative effects of injection therapies that involve injury‐causing agents and those that involve direct repair agents, it should be noted that the majority of the evidence was for injection therapies that act directly on the repair pathway. The results of these were often heterogeneous, potentially due to the range of injection treatments. There is, however, insufficient evidence from different injection therapies to draw any conclusions on individual therapies. Nonetheless, it can be observed that the sole serious event, a tendon rupture, occurred after injection therapy involving a corticosteroid.
When interpreting the outcome measures it is important to consider that, of these, only the VISA‐A is a validated score with clinically meaningful interpretation (Robinson 2001). The definition and interpretation of pain, return to sports and patient satisfaction scores are more difficult to interpret clinically due to the differences in scoring systems and absolute definitions. For example, some of the included articles reported return to sport as a binary yes/no response; others further sub‐categorised the responses to return to sport without pain and return to sport with pain.
Quality of the evidence
The risk of bias amongst all trials up to 1994 was unclear or high. It is encouraging that the subsequent series of trials between 2004 and 2013 are of higher quality, as shown in Figure 2. Despite the improvement in quality, only Bell 2013 scored positively on all parameters. It is also important to note that, although the majority of studies were placebo‐controlled randomised controlled trials, considered to be the gold standard in trial design, the majority failed to describe blinding procedures. Furthermore, the largest trial sample was 97 (Capasso 1993), and so this review is comprised of trials with predominantly small sample sizes.
The review is also limited by the large range of different injections evaluated. Although the authors have attempted to group the injections by comparator arm and mode of action, this introduces clinical heterogeneity. There was also large statistical heterogeneity amongst some analyses, which may be due in part to the problems highlighted above with inconsistent definitions of outcome measures and timing of outcome reporting. In these cases we removed clear outliers for further sensitivity analysis. Consequently, the heterogeneity and quality of the 18 included studies precludes the drawing of robust conclusions.
We assessed the evidence for the outcomes of the comparison of injection therapy versus placebo or no injection control tested by 15 small studies as being either of low quality (VISA‐A results) or very low quality (adverse events, pain, return to sports, patient satisfaction); see details in Table 1. As well as for limitations in study design and implementation, we downgraded the evidence further for imprecision, indirectness and inconsistency. As noted in Table 1, the interpretation of 'low quality' evidence is that "Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate". That of very low quality evidence is that "We are very uncertain about the estimate".
We assessed the evidence for all the available outcomes from the four small studies testing four different comparisons in the injection therapy versus active treatment category as being of very low quality. We downgraded the evidence one level for study limitations, including performance bias from lack of blinding of care providers, and two levels for serious imprecision given the few data available for each comparison.
We assessed the evidence for the study comparing two doses of the same injection therapy as being of very low quality. We downgraded it two levels for indirectness of evidence and one for imprecision of results: only one study included, at a single centre, using a single operator to administer one type of injection therapy not in common use in a small study sample.
Potential biases in the review process
We have searched the published literature using a comprehensive search strategy, as outlined in Appendix 1. We are therefore confident that we have not missed any large body of definitive evidence that would change clinical practice. However, it is possible that we have failed to identify trials, particularly those of non‐English publication, abstract only publications or those not published, e.g. commercially sponsored with negative results. Additionally, although we searched trial registries, it is likely that we have missed ongoing studies that have not been registered. Where data were missing we made efforts to contact authors. We also strived to make the most of the data that were available, such as by reading mean VISA‐A and pain scores off graphs and imputing missing standard deviations. However, the validity of these data is questionable and we downgraded the quality of the evidence with this in mind.
Agreements and disagreements with other studies or reviews
Our results are consistent with previous systematic reviews that have also discussed the large range of injection therapies reporting inconsistent outcome measures at multiple time points across a large range of injection types (Coombes 2010; DTB 2012). Neither of these reviews found sufficient clinical evidence to recommend injection therapies for Achilles tendinopathy.
Authors' conclusions
Implications for practice.
There is insufficient evidence from randomised controlled trials to draw conclusions on the use of injection therapies for treating Achilles tendinopathy. Since this review does not add support to the wider clinical use of injection therapies for Achilles tendinopathy, the use of injection therapies should be considered in research settings in the first instance to address this lack of evidence.
Implications for research.
This review has highlighted a need for definitive research in the area of injection therapies for Achilles tendinopathy. It has also highlighted the need for research in primary and secondary care settings amongst an older non‐athletic population in addition to those who are younger and more active. Discussion in the research community, with consumer and other stakeholder input, is required to prioritise the choice of injection therapies and research questions. This review has shown that a placebo‐controlled/no injection control trial is largely considered the most appropriate trial design to answer the question of treatment efficacy of this intervention. Follow‐up of at least six months is required as well as comprehensive reporting of trial methods and final outcome, including of final function using validated outcome measures.
Notes
Future updates
A future update on this topic will consider the following:
Inclusion of recurrence of tendinopathy where longer‐term follow‐up is available.
Subgroup analysis per injection type.
Acknowledgements
We would like to thank the external referees (Nicola Maffulli, Stephen Pribut, Haris S Vasiliadis), the two editors (Nigel Hanchard and Helen Handoll) and editorial staff (Joanne Elliott, Lindsey Elstub and Laura MacDonald) of the Cochrane Bone, Joint and Muscle Trauma Group for help and feedback on the protocol and final review.
This project was supported by the National Institute for Health Research via Cochrane Infrastructure funding to the Cochrane Bone, Joint and Muscle Trauma Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Appendices
Appendix 1. Search strategies
CENTRAL (Wiley Online Library)
2014, Issue 1
#1 MeSH descriptor: [Achilles Tendon] this term only (201) #2 Achilles or calcan*:ti,ab,kw (Word variations have been searched) (730) #3 #1 or #2 (730) #4 [mh Tendinopathy] or [mh ^"Athletic Injuries"] or [mh ^"Tendon Injuries"] or [mh ^"Soft Tissue Injuries"] (945) #5 tend?nitis or tenosynovitis or tendinopath* or tendinosis or paratend?nitis or peritend?nitis:ti,ab,kw (Word variations have been searched) (603) #6 #4 or #5 (1249) #7 #3 and #6 (171) #8 [mh ^Injections] or [mh ^"Injections, Intralesional"] (2476) #9 injection*:ti,ab,kw (Word variations have been searched) (40536) #10 MeSH descriptor: [Adrenal Cortex Hormones] explode all trees and with qualifier(s): [Administration & dosage ‐ AD, Pharmacology ‐ PD, Therapeutic use ‐ TU] (5341) #11 MeSH descriptor: [Steroids] explode all trees and with qualifier(s): [Administration & dosage ‐ AD, Pharmacology ‐ PD, Therapeutic use ‐ TU] (25397) #12 MeSH descriptor: [Anti‐Inflammatory Agents] this term only (4585) #13 glucocorticoid* or corticoster* or methylprednisolone or prednisolone or betamethasone or triamcinolone or cortisone or hydrocortisone:ti,ab,kw (Word variations have been searched) (22661) #14 "high volume":ti,ab,kw (Word variations have been searched) (499) #15 prolotherapy or "proliferation therapy":ti,ab,kw (Word variations have been searched) (30) #16 autologous near/3 blood:ti,ab,kw (Word variations have been searched) (1230) #17 MeSH descriptor: [Blood Transfusion, Autologous] this term only (602) #18 ((platelet rich near/3 (plasma or therap*)) or PRP):ti,ab,kw (Word variations have been searched) (889) #19 MeSH descriptor: [Platelet‐Rich Plasma] this term only (142) #20 MeSH descriptor: [Aprotinin] this term only (528) #21 Aprotinin:ti,ab,kw (Word variations have been searched) (816) #22 MeSH descriptor: [Botulinum Toxins] explode all trees (829) #23 "botulinum toxin":ti,ab,kw (Word variations have been searched) (1374) #24 "sodium hyaluronate":ti,ab,kw (Word variations have been searched) (393) #25 MeSH descriptor: [Glycosaminoglycans] this term only (215) #26 Glycosaminoglycan*:ti,ab,kw (Word variations have been searched) (367) #27 [mh ^"Sclerosing Solutions"] or [mh ^Sclerotherapy] (638) #28 MeSH descriptor: [Polyethylene Glycols] this term only (1675) #29 polidocanol:ti,ab,kw (Word variations have been searched) (164) #30 lauromacrogol:ti,ab,kw (Word variations have been searched) (2) #31 "hyperosmolar dextrose":ti,ab,kw (Word variations have been searched) (3) #32 {or #8‐#31} (82892) #33 #7 and #32 (33) [Trials]
The top‐up search in April 2015 found 46 records (no date restrictions were applied to this search)
MEDLINE (Ovid Online)
1946 to February 2014
1 Achilles Tendon/ (5825) 2 (Achilles or calcan*).tw. (14547) 3 1 or 2 (15986) 4 exp Tendinopathy/ or Athletic Injuries/ or Tendon Injuries/ or Soft Tissue Injuries/ (38022) 5 (Tend#nitis or tenosynovitis or tendinopath* or tendinosis or paratend#nitis or peritend#nitis).tw. (6355) 6 4 or 5 (40263) 7 3 and 6 (3052) 8 Injections/ or Injections, Intralesional/ (37991) 9 injection*.tw. (436022) 10 exp Adrenal Cortex Hormones/ad, dt, pd, tu [Administration & Dosage, Drug Therapy, Pharmacology, Therapeutic Use] (198119) 11 exp Steroids/ (690880) 12 Anti‐Inflammatory Agents/ (54257) 13 (glucocorticoid* or corticoster* or methylprednisolone or prednisolone or betamethasone or triamcinolone or cortisone or hydrocortisone).tw. (185628) 14 "high volume".tw. (7066) 15 (prolotherapy or "proliferation therapy").tw. (105) 16 (autologous adj3 blood).tw. (8067) 17 Blood Transfusion, Autologous/ (6584) 18 ((platelet rich adj3 (plasma or therap*)) or PRP).tw. (13910) 19 Platelet‐Rich Plasma/ (1410) 20 Aprotinin/ (6164) 21 Aprotinin.tw. (4080) 22 exp Botulinum Toxins/ (12118) 23 "botulinum toxin".tw. (8901) 24 "sodium hyaluronate".tw. (1328) 25 Glycosaminoglycans/ (21879) 26 glycosaminoglycan.tw. (10105) 27 Sclerosing Solutions/ or Sclerotherapy/ (7508) 28 Polyethylene Glycols/ (36754) 29 polidocanol.tw. (520) 30 lauromacrogol.tw. (6) 31 "hyperosmolar dextrose".tw. (16) 32 or/8‐31 (1347665) 33 7 and 32 (346) 34 Randomized controlled trial.pt. (363145) 35 Controlled clinical trial.pt. (87554) 36 randomized.ab. (283334) 37 placebo.ab. (149893) 38 Drug therapy.fs. (1663527) 39 randomly.ab. (205978) 40 trial.ab. (292168) 41 groups.ab. (1317336) 42 or/34‐41 (3254461) 43 exp Animals/ not Humans/ (3880949) 44 42 not 43 (2788526) 45 33 and 44 (134)
The top‐up search in April 2015 found 14 records.
EMBASE (Ovid Online)
1974 to February 2014
1 Achilles Tendinitis/ (688) 2 Achilles Tendon/ (6523) 3 (Achill* or calcan*).tw. (19770) 4 2 or 3 (21334) 5 Tendinitis/ or Tenosynovitis/ or Sport Injury/ or Tendon Injury/ or Soft Tissue Injury/ (46247) 6 (tend#nitis or tenosynovitis or tendinopath* or tendinosis or paratend#nitis or peritend#nitis).tw. (8323) 7 5 or 6 (48590) 8 4 and 7 (3272) 9 1 or 8 (3549) 10 Injection/ or Intralesional Drug Administration/ (70292) 11 injection*.tw. (551885) 12 exp Corticosteroid/ (729149) 13 exp Antiinflammatory Agent/ (1232211) 14 (glucocorticoid* or corticoster* or methylprednisolone or prednisolone or betamethasone or triamcinolone or cortisone or hydrocortisone).tw. (244765) 15 "high volume".tw. (10915) 16 (prolotherapy or "proliferation therapy").tw. (164) 17 (autologous adj3 blood).tw. (10388) 18 exp Blood Transfusion/ (130039) 19 ((platelet rich adj3 (plasma or therap*)) or PRP).tw. (17170) 20 Plasma Transfusion/ or Thrombocyte Rich Plasma/ (7628) 21 Aprotinin/ (12571) 22 aprotinin.tw. (5030) 23 Botulinum Toxin/ (11498) 24 "botulinum toxin".tw. (12192) 25 "sodium hyaluronate".tw. (1897) 26 Glycosaminoglycan/ (26125) 27 glycosaminoglycan.tw. (11628) 28 Sclerosing Agent/ or Sclerotherapy/ (11224) 29 Macrogol derivative/ (12133) 30 Polidocanol/ (3278) 31 polidocanol.tw. (803) 32 lauromacrogol.tw. (22) 33 "hyperosmolar dextrose".tw. (20) 34 or/10‐33 (2156429) 35 and/9,34 (770) 36 exp Randomized Controlled Trial/ or exp Single Blind Procedure/ or exp Double Blind Procedure/ or Crossover Procedure/ (417550) 37 (random* or RCT or placebo or allocat* or crossover* or 'cross over' or trial or (doubl* adj1 blind*) or (singl* adj1 blind*)).ti,ab. (1288581) 38 36 or 37 (1369001) 39 (exp Animal/ or animal.hw. or Nonhuman/) not (exp Human/ or Human cell/ or (human or humans).ti.) (5569950) 40 38 not 39 (1204310) 41 35 and 40 (93)
The top‐up search in April 2015 found 12 records
AMED (Ovid Online)
1985 to February 2014
1 Achilles Tendon/ (592) 2 (Achilles or calcan*).tw. (2085) 3 1 or 2 (2085) 4 Tendinopathy/ or Tenosynovitis/ or exp Athletic Injuries/ or Tendon Injuries/ (4234) 5 (tend#nitis or tenosynovitis or tendinopath* or tendinosis or paratend#nitis or peritend#nitis).tw. (705) 6 4 or 5 (4548) 7 3 and 6 (517) 8 Randomized controlled trial.pt. (2853) 9 Controlled clinical trial.pt. (70) 10 Randomized Controlled Trials/ (1649) 11 Random Allocation/ (311) 12 Double‐Blind Method/ (500) 13 or/8‐12 (5129) 14 exp Animals/ not Humans/ (7399) 15 13 not 14 (5100) 16 clinical trial.pt. (1158) 17 exp Clinical trials/ (3352) 18 (clinic$ adj25 trial$).tw. (5818) 19 ((singl$ or doubl$ or trebl$ or tripl$) adj (mask$ or blind$)).tw. (2324) 20 Placebos/ (545) 21 placebo$.tw. (2635) 22 random$.tw. (14004) 23 exp Research design/ (17849) 24 (latin adj square).tw. (24) 25 or/16‐24 (31349) 26 25 not 14 (30812) 27 26 not 15 (25847) 28 7 and 27 (62)
The top‐up search in April 2015 found 2 records
CINAHL (EBSCO)
1981 to February 2014
S1 (MH "Achilles Tendinopathy") (406) S2 (MH "Achilles Tendon") (1,557) S3 TI ( Achill* or calcan* ) OR AB ( Achill* or calcan* ) (3,618) S4 S2 OR S3 (3,968) S5 (MH "Tendinopathy") OR (MH "Tenosynovitis") OR (MH "Athletic Injuries") OR (MH "Tendon Injuries") OR (MH "Soft Tissue Injuries") (16,600) S6 TX tendinitis ot tendonitis or tenosynovitis or tendinopath* or tendinosis or paratendinitis or paratendonitis or peritendinitis or peritendonitis (2,761) S7 S5 OR S6 (17,415) S8 S4 AND S7 (1,103) S9 S1 AND S8 (304) S10 (MH "Injections") OR (MH "Injections, Intralesional") (7,118) S11 TI injection* OR AB injection* (21,165) S12 (MH "Adrenal Cortex Hormones+") (19,001) S13 (MH "Antiinflammatory Agents") (5,385) S14 TX (glucocorticoid* or corticoster* or methylprednisolone or prednisolone or betamethasone or triamcinolone or cortisone or hydrocortisone) (21,247) S15 TX "high volume" (1,170) S16 TX (prolotherapy or "proliferation therapy") (141) S17 TX (autologous n3 blood) (1,185) S18 (MH "Blood Transfusion, Autologous") (814) S19 ((platelet rich n3 (plasma or therap*)) or PRP) (962) S20 (MH "Platelet‐Rich Plasma") (107) S21 (MH "Aprotinin") (354) S22 TX aprotinin (435) S23 (MH "Botulinum Toxins") (3,163) S24 TX "botulinum toxin" (2,060) S25 TX "sodium hyaluronate" (105) S26 (MH "Glycosaminoglycans") (505) S27 TX glycosaminoglycan* (700) S28 (MH "Sclerosing Solutions") OR (MH "Sclerotherapy") (759) S29 (MH "Polyethylene Glycols") (754) S30 TX polidocanol (39) S31 TX lauromacrogol (0) S32 TX "hyperosmolar dextrose" (6) S33 S3 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 (65,862) S34 S9 AND S33 (297) S35 (MH "Clinical Trials+") (171,321) S36 (MH "Evaluation Research+") (20,176) S37 (MH "Comparative Studies") (75,438) S38 (MH "Crossover Design") (11,400) S39 PT Clinical Trial (75,447) S40 (MH "Random Assignment") (36,644) S41 S35 or S36 or S37 or S38 or S39 or S40 (271,532) S42 TX ((clinical or controlled or comparative or placebo or prospective or randomi?ed) and (trial or study)) (476,924) S43 TX (random* and (allocat* or allot* or assign* or basis* or divid* or order*)) (65,094) S44 TX ((singl* or doubl* or trebl* or tripl*) and (blind* or mask*)) (716,865) S45 TX ( crossover* or 'cross over' ) or TX cross n1 over (14,250) S46 TX ((allocat* or allot* or assign* or divid*) and (condition* or experiment* or intervention* or treatment* or therap* or control* or group*)) (81,724) S47 S42 or S43 or S44 or S45 or S46 (1,103,137) S48 S41 or S47 (1,168,734) S49 S34 AND S48 (148)
The top‐up search in April 2015 found 21 records
SPORTDiscus (EBSCO)
1985 to February 2014
S1 DE "ACHILLES tendinitis" (220) S2 (DE "ACHILLES tendon") OR (DE "ACHILLES tendon ‐‐ Wounds & injuries") (2,098) S3 TX Achill* or calcan* (4,537) S4 S2 OR S3 (4,537) S5 (DE "TENDINITIS") OR (DE "TENOSYNOVITIS") OR (DE "SOFT tissue injuries") OR (DE "SPORTS injuries") (8,224) S6 TX tendinitis ot tendonitis or tenosynovitis or tendinopath* or tendinosis or paratendinitis or paratendonitis or peritendinitis or peritendonitis (1,729) S7 S5 OR S6 (9,108) S8 S4 AND S7 (972) S9 S1 OR S8 (1,015) S10 DE "INJECTIONS" (875) S11 TX injection* (6,591) S12 DE "ANTI‐inflammatory agents" (752) S13 TX (glucocorticoid* or corticoster* or methylprednisolone or prednisolone or betamethasone or triamcinolone or cortisone or hydrocortisone) (4,038) S14 TX "high volume" (425) S15 TX (prolotherapy or "proliferation therapy") (55) S16 TX (autologous n3 blood) (94) S17 TX ((platelet rich n3 (plasma or therap*)) or PRP) (320) S18 TX aprotinin (44) S19 DE "BOTULINUM toxin" (486) S20 TX "botulinum toxin" (581) S21 TX "sodium hyaluronate" (24) S22 TX glycosaminoglycan* (173) S23 TX sclerosing solution* or sclerotherap* (54) S24 TX polyethylene glycol* (89) S25 TX polidocanol (48) S26 TX lauromacrogol (0) S27 TX hyperosmolar dextrose (5) S28 S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 (11,840) S29 S9 AND S28 (149) S30 TX ( (clinic* N3 trial) or (controlled N3 trial) or (comparative N3 trial) or (placebo N3 trial) or (prospective N3 trial) or (randomi?ed N3 trial) ) or TX ( (clinic* N3 study) or (controlled N3 study) or (comparative N3 study) or (placebo N3 study) or (prospective N3 study) or (randomi?ed N3 study) ) (63,486) S31 (random* N7 allot*) or (random* N7 assign*) or (random* N7 basis*) or (random* N7 divid*) or (random* N7 order*) (8,164) S32 TX ( (singl* N7 blind*) or (doubl* N7 blind*) or (trebl* N7 blind*) or (tripl* N7 blind*) ) or TX ( (singl* N7 mask*) or (doubl* N7 mask*) or (trebl* N7 mask*) or (tripl* N7 mask*) ) (5,124) S33 TX (cross#over*) or TX (cross N1 over*) (3,545) S34 TX randomi?ed control* trial* (7,589) S35 TX ( (allocat* N3 condition*) or (allocat* N3 experiment*) or (allocat* N3 intervention*) or (allocat* N3 treatment*) or (allocat* N3 therap*) or (allocat* N3 control*) or (allocat* N3 group*) ) or TX ( (allot* N3 condition*) or (allot* N3 experiment*) or (allot* N3 intervention*) or (allot* N3 treatment*) or (allot* N3 therap*) or (allot* N3 control*) or (allot* N3 group*) ) or TX ( (assign* N3 condition*) or (assign* N3 experiment*) or (assign* N3 intervention*) or (assign* N3 treatment*) or (assign* N3 therap*) or (assign* N3 control*) or (assign* N3 group*) ) or TX ( (divid* N3 condition*) or (divid* N3 experiment*) or (divid* N3 intervention*) or (divid* N3 treatment*) or (divid* N3 therap*) or (divid* N3 control*) or (divid* N3 group*) )( 8,484) S36 TX placebo* (7,506) S37 S30 or S31 or S32 or S33 or S34 or S35 or S36 (77,885) S38 S29 AND S37 (56)
The top‐up search in April 2015 found 2 records
ISRCTN registry
May 2014
1. Achilles (19)
WHO ICTRP
May 2014
1. Achilles (95)
Data and analyses
Comparison 1. Injection therapies versus placebo injection or no injection control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 VISA‐A (score 0 to 100; 100 = no problems): at 6 weeks | 5 | 200 | Mean Difference (IV, Fixed, 95% CI) | 0.79 [‐4.56, 6.14] |
| 1.1 Injury‐causing agents | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | 4.0 [‐12.30, 20.30] |
| 1.2 Direct repair agents | 4 | 172 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [‐5.26, 6.06] |
| 2 VISA‐A (score 0 to 100; 100 = no problems): at 3 months | 5 | 189 | Mean Difference (IV, Fixed, 95% CI) | ‐0.94 [‐6.34, 4.46] |
| 2.1 Injury‐causing agents | 1 | 27 | Mean Difference (IV, Fixed, 95% CI) | ‐5.0 [‐21.61, 11.61] |
| 2.2 Direct repair agents | 4 | 162 | Mean Difference (IV, Fixed, 95% CI) | ‐0.46 [‐6.17, 5.25] |
| 3 VISA‐A (score 0 to 100; 100 = no problems): after 3 months | 3 | 132 | Mean Difference (IV, Fixed, 95% CI) | 0.14 [‐6.54, 6.82] |
| 3.1 Injury‐causing agents | 1 | 27 | Mean Difference (IV, Fixed, 95% CI) | 9.5 [‐6.35, 25.35] |
| 3.2 Direct repair agents | 2 | 105 | Mean Difference (IV, Fixed, 95% CI) | ‐1.88 [‐9.25, 5.48] |
| 4 Patients achieving increased VISA‐A scores (20 points or more from baseline) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 Injury‐causing agent vs exercises: 6 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Injury‐causing agent vs exercises: 3 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Injury‐causing agent vs exercises: 12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Adverse events | 13 | 449 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.50, 1.89] |
| 5.1 Injury‐causing agents | 2 | 47 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.01, 7.02] |
| 5.2 Direct repair agents | 11 | 402 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.53, 2.09] |
| 6 Pain (VAS; score 0 to 100; 0 = no pain) up to 3 months | 7 | 219 | Mean Difference (IV, Random, 95% CI) | ‐22.94 [‐37.53, ‐8.36] |
| 6.1 Injury‐causing agents | 2 | 47 | Mean Difference (IV, Random, 95% CI) | ‐12.31 [‐30.43, 5.81] |
| 6.2 Direct repair agents | 5 | 172 | Mean Difference (IV, Random, 95% CI) | ‐26.34 [‐44.13, ‐8.55] |
| 7 Return to sports | 7 | 335 | Risk Ratio (M‐H, Random, 95% CI) | 1.39 [1.00, 1.94] |
| 7.1 Injury‐causing agents | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 Direct repair agents | 7 | 335 | Risk Ratio (M‐H, Random, 95% CI) | 1.39 [1.00, 1.94] |
| 8 Patient satisfaction with treatment (an event represents satisfaction with treatment) | 4 | 152 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.76, 1.47] |
| 8.1 Injury‐causing agents | 2 | 47 | Risk Ratio (M‐H, Random, 95% CI) | 2.79 [0.26, 29.91] |
| 8.2 Direct repair agents | 2 | 105 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.85, 1.14] |
Comparison 2. Injection therapies versus active treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 VISA‐A (score 0 to 100; 100 = no problems) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 Direct repair agent vs exercises: at 6 weeks | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Direct repair agent vs exercises: at 3 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Direct repair agent vs exercises: after 3 months (6 months) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Patients achieving increased VISA‐A scores (20 points or more from baseline) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Injury‐causing agent vs exercises: 6 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Injury‐causing agent vs exercises: 3 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Injury‐causing agent vs exercises: 12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Adverse events | 3 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.04, 2.99] |
| 3.1 Injury‐causing agent vs surgery | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.02, 7.32] |
| 3.2 Injury‐causing agent vs eccentric exercises | 1 | 26 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.50] |
| 3.3 Direct repair agent vs eccentric exercises | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Quality of life: EQ‐5D (scores up to 1: full health) | 1 | 19 | Mean Difference (IV, Fixed, 95% CI) | 0.08 [‐0.25, 0.41] |
| 4.1 Direct repair agent vs eccentric exercises | 1 | 19 | Mean Difference (IV, Fixed, 95% CI) | 0.08 [‐0.25, 0.41] |
| 5 Patient satisfaction with treatment (satisfied patients) | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 Injury‐causing agent vs surgery | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Injury‐causing agent vs eccentric exercises | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 3. High‐dose versus low‐dose injection therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Pain during activity (VAS; score 0 to 100; 0 = no pain) after maximum of 3 treatments | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Patient satisfaction (satisfied patients) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 After 3 treatments | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 After 5 treatments | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Alfredson 2005.
| Methods | Randomised controlled trial | |
| Participants | Setting: Sports medicine unit, Sweden Sample: 20 participants, referred from general practitioners, with clinically diagnosed mid portion Achilles tendinopathy Characteristics: 9 men and 11 women, mean (range) age 50 years (unknown). Unilateral tendinopathy only | |
| Interventions | In all participants the injections were directly into areas of local neo visualisation and after 14 days free activity with full tendon loading was allowed. A maximum of 2 treatments 3 to 6 weeks apart were administered Intervention: ultrasound and doppler‐guided polidocanol (5 mg/ml) Control: ultrasound and doppler‐guided lidocaine hydrochloride (5 mg/ml) and adrenaline (5 µg/ml) |
|
| Outcomes | All patients followed up at 3 months Primary: pain during Achilles tendon loading activities (VAS 0 to 100) and presence or absence of neo visualisation Secondary: patent satisfaction with the treatment (interview) | |
| Notes | The trial authors describe this as a trial of injection therapy with a substance that has a sclerosing and an anaesthetic effect versus injection with a substance that has an anaesthetic effect only. The comparator was categorised as a control rather than an active treatment in this trial by the review authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...the patients selected an envelope allocating themselves to either treatment..." Comment: the investigators describe a random component in the sequence generation process |
| Allocation concealment (selection bias) | Low risk | Quote: "...box with 20 opaque envelopes..." Comment: the investigators' assignment envelopes were used with safeguards (e.g. non‐opaque) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "...The radiologist...and patients were blinded to the substance that was injected..." Comment: blinding of patients and personnel ensured |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "...The radiologist, who performed all ultrasound and doppler examinations and the patients were blinded..." Comment: blinding of outcome assessment ensured |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: no missing outcome data reported or discussed |
| Selective reporting (reporting bias) | Unclear risk | Comment: no published protocol available |
| Other bias | Unclear risk | Comment: insufficient information presented to assess whether an important risk of bias exists |
Alfredson 2007.
| Methods | Randomised controlled trial | |
| Participants | Setting: Sports medicine unit, Sweden
Sample: 20 participants, referred from general practitioners, with clinically diagnosed mid portion Achilles tendinopathy Characteristics: 9 men and 11 women, mean (range) 46 years (unknown). Unilateral tendinopathy only |
|
| Interventions | Intervention: ultrasound and doppler‐guided polidocanol (10 mg/ml) injected into areas of local neovascularisation. After 14 days free activity with full tendon loading was allowed. Additional treatments offered if pain persisted Control: surgical treatment. Achilles tendon released from ventral soft tissue, followed by haemostasis using diathermia. After 14 days free activity with full tendon loading was allowed |
|
| Outcomes | All patients were followed up at 3 and 6 months Primary: pain during Achilles tendon loading activity (VAS 0 to 100) Secondary: patient‐reported satisfaction (satisfied or not satisfied) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...the patients selected an envelope allocating themselves to either treatment..." Comment: the investigators describe a random component in the sequence generation process |
| Allocation concealment (selection bias) | Low risk | Quote: "...box with 20 opaque envelopes..." Comment: the investigators' assignment envelopes were used with safeguards |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: investigators do not report blinding procedures but blinding of care providers is unlikely given the interventions under comparison |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: investigators do not report blinding procedures |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: no missing outcome data reported or discussed |
| Selective reporting (reporting bias) | Unclear risk | Comment: no published protocol available |
| Other bias | Unclear risk | Comment: insufficient information presented to assess whether an important risk of bias exists |
Bell 2013.
| Methods | Randomised controlled trial | |
| Participants | Setting: Sports medicine clinic, New Zealand
Sample: 53 participants with clinically diagnosed mid portion Achilles tendinopathy. Unilateral tendinopathy only Characteristics: 28 men and 25 women mean age (SD) 51.2 years (10.6) in the intervention group and 47.2 (9.7) in the control group |
|
| Interventions | All participants received 2 unguided peritendinous injections at the site of maximal tenderness at baseline and 1 month later. All had 3 ml of their own blood taken from the antecubital fossa. All had a standardised injection through a single puncture site. All completed a 12‐week eccentric loading programme following the injection Intervention: patients received the 3 ml of blood Control: no substance injected ('dry needling', no anaesthesia) |
|
| Outcomes | All patients were followed up at 1, 2, 3 and 6 months Primary: VISA‐A Secondary: 6‐point Likert score at final follow‐up to assess perceived rehabilitation; return to sport and adherence to eccentric loading programme |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...each participant underwent simple randomisation into one of the two groups by selecting sealed envelope from the box..." Comment: the investigators describe a random component in the sequence generation process |
| Allocation concealment (selection bias) | Low risk | Quote: "...equal numbers of opaque envelopes..." Comment: the investigators' assignment envelopes were used with safeguards |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "...participants lay prone with a screen over their legs to block any view of the intervention taking place... participants completed the questionnaire under supervision from the blinded assessor..." Comment: blinding of participants and study personnel ensured |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "...participants completed the questionnaire under supervision from the blinded assessor..." Comment: blinding of outcome assessment ensured |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "...we used intention to treat analysis via last observation carried forward for the three participants lost to follow up, with their final recorded outcome being brought forward for the remaining missed data points..." Comment: missing data have been imputed using appropriate methods |
| Selective reporting (reporting bias) | Low risk | The study protocol is available and all of the study's pre‐specified outcomes have been reported |
| Other bias | Low risk | None |
Brown 2006.
| Methods | Randomised controlled trial | |
| Participants | Setting: private practice, Australia Sample: 26 participants with clinically diagnosed mid portion Achilles tendinopathy. Bilateral tendinopathy included: 33 tendons Characteristics: 17 men and 9 women, mean age (range) 46 years (30 to 73) | |
| Interventions | All patients received 3 injections 1 week apart Intervention: 12‐week eccentric loading programme and aprotinin injection (3 ml aprotinin and 1 ml xylocaine 1%) Control: 12‐week eccentric loading programme and placebo injection (3 ml saline and 1 ml xylocaine) | |
| Outcomes | All patients were followed up at 2 weeks, 1 month, 3 month and 12 months Primary: VISA‐A Secondary: patient rating of improvement and return to full activities |
|
| Notes | Achilles tendinopathy randomised as per tendon; as evidenced: "This patient received bilateral injections (one aprotinin injection and one placebo injection)." Data analysis: SD imputed for VISA‐A scores from other trials in the same analysis category |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...allocation of patients was organised by AH, using a random number selection..." Comment: the investigators describe a random component in the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Comment: the investigators do not report the method of allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "...patients and examiners were blinded to their allocation.." Comment: blinding of patients and study personnel ensured |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "...the evaluating authors were blinded to the treatment groups..." Comment: blinding of outcome assessment ensured |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: no missing outcome data reported or discussed |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of low risk or high risk |
| Other bias | High risk | Patients who chose to enrol received free treatment and follow‐up in the private clinic |
Capasso 1993.
| Methods | Randomised controlled trial | |
| Participants | Setting: not stated Sample: 97 participants, professional and amateur sports people with mid portion and insertional Achilles tendinopathy. Unilateral tendinopathy only Characteristics: 65 men and 32 women, age not reported | |
| Interventions | All patients were advised to rest throughout the treatment period. All patients had between 4 and 6 injections Intervention: 2.5 ml of aprotinin Control: apyrogenic double distilled water | |
| Outcomes | No time points described Patient satisfaction, symptoms (spontaneous or provoked pain, local swelling, limitation of function), ultrasound or thermography, time to return to sports | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: no description |
| Allocation concealment (selection bias) | Unclear risk | Comment: no description |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: the authors describe this as a placebo‐controlled trial, but no description of blinding procedures is provided |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no description |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: no description of missing data or how this was handled |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of low risk or high risk |
| Other bias | High risk | Comment: the trial is poorly reported and only described in outline. It has not been possible to exclude other types of bias from this report and the lack of any details of the randomisation method and the unexplained imbalance in treatment allocation could include the strong possibility that some non‐randomised participants treated with aprotinin were included in the analysis |
Chouchane 1989.
| Methods | Randomised controlled trial | |
| Participants | Setting: only country stated, France Sample: 32 participants with tendinopathy secondary to sports overuse. Unilateral tendinopathy only Characteristics: 20 men and 12 women, average age 38 years | |
| Interventions | All patients administered 2 injections, twice a day for 7 days Intervention: 2 ml percutalgine Control: placebo (substance not stated) | |
| Outcomes | All outcomes at 7 days Local pain on VAS (0 to 10), pain during mobilisation, calf raises, overall effectiveness was assessed by a doctor on a 4‐point scale and adverse effects | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: no description |
| Allocation concealment (selection bias) | Unclear risk | Comment: no description |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: no description |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no description |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: no description |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of low risk or high risk |
| Other bias | Unclear risk | Comment: the trial is poorly reported and only described in outline. It has not been possible to exclude other types of bias from this report |
DaCruz 1988.
| Methods | Randomised controlled trial | |
| Participants | Setting: Accident and Emergency Department, UK Sample: 36 participants, presenting to an accident and emergency department, with clinically diagnosed mid portion Achilles tendinopathy Characteristics: 18 men and 10 women (8 unknown), mean (range) 28 years (22 to 46). Bilateral tendinopathy included (6 of 28 in analysis) | |
| Interventions | All patients received a 4‐week period of physiotherapy including ice application, therapeutic ultrasound and felt heel inserts Intervention: 40 mg methyl prednisolone acetate in 1 ml of bupivacaine hydrochloride 0.25% Control: 2 ml of 0.25% bupivacaine hydrochloride alone |
|
| Outcomes | All patients were followed up at 3 weeks, 6 weeks and 12 weeks Outcomes: 10 cm linear analogue scale in response to the question 'How bad is your pain when it is at its worse?'; tendon thickness; activity level score; tenderness |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "...all patients were randomised..." Comment: insufficient information about the sequence generation to permit judgement of low or high risk |
| Allocation concealment (selection bias) | Unclear risk | Quote: "...all patients were randomised..." Comment: method of concealment is not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "...follow up was conducted on a double blind basis..." Comment: no further information is provided regarding how or who was blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "...follow up was conducted on a double blind basis..." Comment: no further information is provided regarding how or who was blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "...A total of 36 patients were enrolled but six of these failed to attend for physiotherapy and two more refused further injection when they came to cross over..." Comment: no information on handling of missing data presented |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information to permit judgement |
| Other bias | Unclear risk | Comment: insufficient information to assess whether an important risk of bias exists |
De Vos 2010.
| Methods | Randomised controlled trial | |
| Participants | Setting: Sports medicine outpatient department, Netherlands Sample: 54 participants, recruited through advertisements on websites, folders and regional radio to health professionals and the public. Aged 18 to 70 years, all had clinically diagnosed mid portion Achilles tendinopathy. Unilateral tendinopathy Characteristics: 26 men and 28 women, mean (SD) 49 years (8.1) in the intervention group and 50 years (9.4) in the control group | |
| Interventions | All patients received 2 ml of 0.5% bupivacaine hydrochloride in the skin and subcutaneous tissue. All injections were ultrasound‐guided into several sites in the degenerative area of the main body of the tendon. After 1 week all patients completed an additional 12‐week eccentric loading programme. After 4 weeks all patients could return to full sporting activities Intervention: 54 ml of whole blood with 6 ml of citrate centrifuged for 15 minutes; 4 ml PRP layer extracted and added to 0.3 ml of 8.4% sodium bicarbonate buffer Control: 4 ml isotonic saline |
|
| Outcomes | All patients followed up at 6, 12 and 24 weeks Primary: VISA‐A Secondary: patient satisfaction (poor, fair, good, excellent), return to sports, ultrasonographic structure and adherence to the eccentric exercises |
|
| Notes | 1‐year follow‐up data available in follow‐up studies published by de Jonge 2011 and de Vos 2011 The study was funded by Biomet Biologics LLC |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...patients were randomised into 1 of 2 treatment groups by choosing a closed envelope. To ensure balance in the number of patiens between the groups, a block randomisation was performed..." Comment: the investigators describe a random component in the sequence generation process |
| Allocation concealment (selection bias) | Low risk | Quote: "...randomisation was performed using sealed opaque, identical envelopes..." Comment: the investigators' assignment envelopes were used with safeguards |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "...One unblinded sports medicine physician selected the correct injection and blinded the injection with the use of a covering sheath surrounding the syringe and hub of the needle. To ensure concealment of allocation, data on allocation were stored in a secret location. The content on the injection was blinded for the treating sports medicine physician, research and patients..." Comment: blinding of patients and personnel ensured |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: outcomes were patient‐reported; all patients were blinded to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "...There were no patients lost to follow up and there were no missing data..." Comment: no missing outcome data |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol is available and all of the study's pre‐specified (primary and secondary) outcomes have been reported in the pre‐specified way |
| Other bias | High risk | Comment: the study was funded by Biomet Biologics LLC |
Fabbro 2012.
| Methods | Randomised controlled trial | |
| Participants | Setting: not stated Sample: 54 patients referred for ultrasound‐guided treatment of mid portion Achilles tendinopathy Characteristics: 18 participants (11 males, mean (SD) 50.7 years (10.0)) in the steroid injection group, 18 (9 males, mean (SD) 47.2 years (11.8)) in the dry needling only group, and 18 participants (9 males, mean (SD) 45.7 years (8.6)) in the dry needling and steroid injection group | |
| Interventions | Intervention 1: Steroid injection comprising ultrasound‐guided injection of 1 ml 40 mg/ml triamcinolone acetonide into the peritendinous soft tissues, deliberately avoiding the tendon substance Control: dry needling comprising ultrasound‐guided injection of local anaesthetic (5 ml 2% lidocaine) into the peritendinous soft tissues and tendon body. Dry needling (around 20 punctures) was performed on the degenerated portion of the tendon. Post‐intervention use of appropriate orthotics for 1 week Intervention 2: dry needling (as in control group) followed by peritendinous steroid injection |
|
| Outcomes | Follow‐up was at days 7, 14, 30, 90, 180 and 360 The sole outcome measure was use of a visual analogue scoring system although it is not stated what this explicitly refers to (pain on activity, disability, satisfaction, etc.) | |
| Notes | Data analysis: SD imputed for pain scores from other trials in the same analysis category | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: no description of the randomisation process. The only mention of randomisation is in the manuscript title, which describes the study as a "randomised controlled trial" |
| Allocation concealment (selection bias) | Unclear risk | Comment: no description of the randomisation process. The only mention of randomisation is in the manuscript title, which describes the study as a "randomised controlled trial" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: investigators do not report blinding procedures but blinding of care providers is unlikely given the interventions under comparison |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no statement about blinding of participants or personnel |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: it is impossible to determine from the report whether or not patients were lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | The protocol was not published beforehand and so it is impossible to determine whether it was changed after recruitment commenced |
| Other bias | Unclear risk | The trial is poorly reported and only described in outline. It has not been possible to exclude other types of bias from this report |
Fredberg 2004.
| Methods | Randomised controlled trial | |
| Participants | Setting: single hospital rheumatology and sports medicine service, Denmark Sample: 24 amateur or professional athletes referred for surgery because of symptomatic unilateral Achilles tendinopathy. Unilateral tendinopathy Characteristics: 15 men and 9 women, mean (range) 43.7 years (24 to 55) | |
| Interventions | Intervention: 3.5 ml 10 mg/ml lidocaine and 0.5 ml Kenalog (containing 20 mg triamcinolone, a corticosteroid) was injected peri‐tendinously under ultrasound guidance on both sides of the thickest point of the tendon. Injections were administered at days 0, 7 and 21. The third injection was not given to patients who were asymptomatic following 2 injections. 4 days of rest was advised following each injection, after which patients could return to normal activities limited only by pain Control: 3.5 ml 1% lidocaine and 0.5 ml 20% intralipid (intralipid was added in order to make the placebo look like the milky Kenalog solution). The injection schedule was as per the intervention group | |
| Outcomes | Participants were followed up at days 0, 7, 21, 28, 6 months, and by telephone at 2 years Outcome measures included tendon diameter as measured by ultrasound, pressure‐pain detection threshold as measured by pressure algometry, walking pain as reported on a 0 to 10 numerical rating scale, and reported side effects. No primary outcome measure was identified | |
| Notes | The study combined patients with Achilles and patellar tendinopathy but has been included as the populations were analysed and reported separately Data analysis: SD imputed for pain scores from other trials in the same analysis category |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The athletes were randomised in four blocks of six athletes" Comment: no further details are given as to how the randomised blocks were achieved |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information pertaining to allocation concealment is provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "It was not possible to tell the difference between placebo and active treatment by colour or viscosity. All the injections were administered by the same investigator under blind conditions" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "One person was responsible for the randomisation and preparation of the injected medicine, however, the same person had nothing to do with diagnostic procedures or monitoring of effects" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: follow‐up data provided for all cases up until 2 years |
| Selective reporting (reporting bias) | Unclear risk | Comment: the trial protocol was not registered beforehand and so it is not possible to identify any changes to the protocol that occurred during or after the trial |
| Other bias | High risk | Comment: there was 100% cross‐over of patients from the placebo to the intervention groups at 6 months follow‐up due to lack of symptomatic improvement Quote: "In both placebo groups... treatment regimen was discontinued because the athletes did not feel sufficient improvement in all cases except one... In this way, all 24 athletes who were primarily treated with placebo were subsequently administered steroid treatment." |
Kearney 2013.
| Methods | Pilot randomised controlled trial | |
| Participants | Setting: single outpatient orthopaedic department, UK Sample: 20 patients with a clinical diagnosis of mid‐substance Achilles tendinopathy. Bilateral tendinopathy included (number not stated) Characteristics: 7 men and 13 women, mean (range) 48.9 years (35 to 66) | |
| Interventions | Intervention: 52 ml venous blood was drawn, combined with anticoagulant citrate, then centrifuged. The platelet layer was then extracted and injected into the Achilles tendon. Patients were advised to return to normal activities as pain allowed Control: eccentric loading programme involving 2 exercises: (1) Patient in a standing position with the heel over the edge of a step and legs straight. The heels are then lowered beyond the level of the step. (2) Same exercises with the knee slightly flexed to maximise use of soleus. Both exercises were performed twice daily for 12 weeks before being progressed from double‐leg to single‐leg then with added weight. A single session included 3 sets of 15 repetitions of each exercise |
|
| Outcomes | Follow‐up at 6 weeks, 3 months and 6 months Primary: VISA‐A questionnaire Secondary: EuroQol 5‐Dimension questionnaire (EQ‐5D) | |
| Notes | Bilateral cases randomised as 1 unit Chartered Society Research Foundation provided funding for this pilot study |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Treatment allocation was determined using a computer‐generated random number sequence and administered by an independent trial co‐ordinator" |
| Allocation concealment (selection bias) | Low risk | Quote: "Treatment allocation was determined using a computer‐generated random number sequence and administered by an independent trial co‐ordinator" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "It was not possible to blind the clinician administering the intervention or the patient receiving the intervention" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "The primary data was patient‐reported" and "It was not possible to blind... the patient receiving the intervention" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "One [patient] was lost to follow‐up" |
| Selective reporting (reporting bias) | Low risk | Quote: "The trial was registered on the current controlled trials database ISRCTN95369715" before recruitment commenced Comment: the final trial protocol did not differ substantially from that published in advance |
| Other bias | Low risk | Quote: "Chartered Society Research Foundation provided funding for this pilot study. They did not have a role in study design, collection, analysis/interpretation of data, writing of the manuscript or in the decision to submit the manuscript for publication" |
Larsen 1987.
| Methods | Randomised controlled trial | |
| Participants | Setting: medical centre for Royal Life Guards, Denmark Sample: 20 participants with clinical findings of tendinopathy. Bilateral tendinopathy included (no data on whether this occurred) Characteristics: all male. Mean age 20 years (SD 1 year) | |
| Interventions | All participants were advised to rest Intervention: 5 injections of heparin (5000 IU) Control: 5 isotonic saline injections (5000 IU) | |
| Outcomes | All outcomes were collected at day 5, 8 and 15
The main outcome measure was an investigator derived "total symptom score" Pain (0 to 10 VAS) on resting and during exercise Return to military training Adverse events |
|
| Notes | Bilateral tendinopathy randomised as one unit Data analysis: SD imputed for pain scores from other trials in the same analysis category |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomised packages of heparin and of the placebo were provided by Leo Pharmaceuticals" Comment: insufficient information provided |
| Allocation concealment (selection bias) | Unclear risk | Comment: no description provided by the authors |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: no description provided by the authors |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no description provided by the authors |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: incomplete data and methods for handling not described |
| Selective reporting (reporting bias) | Unclear risk | Comment: the trial protocol was not registered beforehand and so it is not possible identify any changes to the protocol that occurred during or after the trial |
| Other bias | High risk | Quote: "..Leo Pharmaceutical Products are thanked for their assistance" Comment: the trial is poorly reported and only described in outline. It has not been possible to exclude other types of bias from this report |
Obaid 2012.
| Methods | Randomised controlled trial | |
| Participants | Setting: single specialist orthopaedic hospital, UK Sample: 32 participants with a clinical and sonographic diagnosis of non‐insertional Achilles tendinosis. Bilateral tendinopathy included Characteristics: 32 participants (20 male, 12 female), 8 of which had bilateral Achilles tendinopathy, mean (range) 45.2 years (22 to 67) | |
| Interventions | Intervention: injection of 5 ml 0.25% bupivacaine hydrochloride onto the ventral surface of the Achilles tendon at its midsection with subsequent re‐positioning of the needle and injection with a combination of skin‐derived fibroblasts and autologous platelet‐rich plasma. Participants were advised to rest for 48 hours before commencing a programme of eccentric‐loading physiotherapy Control: injection of 5 ml 0.25% bupivacaine hydrochloride onto the ventral surface of the Achilles tendon at its midsection. Advice and physiotherapy were administered as in the intervention group |
|
| Outcomes | Follow‐up was at 6 weeks after physiotherapy, at the time of harvesting fibroblasts from skin, at cell implantation and at 6 weeks, 3 months and 6 months post intervention Outcomes included score on the VISA‐A questionnaire, patient‐reported level of health using a VAS score, and ultrasound assessment | |
| Notes | Bilateral tendinopathy randomised per tendon The study was funded by an Austrian biotechnology company, Innovacell |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The patients were randomised with use of a sequence of random numbers from a computer‐generated sequence" |
| Allocation concealment (selection bias) | Unclear risk | Comment: no explicit statement that allocation to groups was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: no explicit statement that participants and personnel were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Blinding was carried out at all evaluations" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: all included cases followed up according to the study flow diagram |
| Selective reporting (reporting bias) | Unclear risk | Comment: the protocol was not published beforehand and so it is impossible to determine whether it was changed after recruitment commenced |
| Other bias | High risk | Comment: the study was funded by an Austrian biotech company, Innovacell. There was no explicit statement as to the involvement of this company in the study design, data collection, analysis or decision to publish |
Pearson 2012.
| Methods | Randomised controlled trial | |
| Participants | Setting: private sports medicine clinic, New Zealand Sample: 33 participants with 40 clinical and sonographic diagnosis of Achilles tendinopathy. Bilateral tendinopathy included Characteristics: treatment group: 8 male and 12 female, age 49 years (range 34 to 65); control group: 7 male and 13 female, age 51 years (range 42 to 70) | |
| Interventions | Intervention: 1 ml lignocaine 1% at the point of maximal tenderness and 3 ml of autologous blood, followed by an eccentric loading programme within 48 hours Control: eccentric loading programme | |
| Outcomes | Follow‐up was at 6 and 12 weeks Primary: VISA‐A Secondary: perceived discomfort on a Likert scale | |
| Notes | Patients with bilateral tendinopathy were randomised per tendon Pacific radiology performed the ultrasounds free of charge to the patients Data analysis: SD imputed for VISA‐A scores from other trials in the same analysis category |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: no description provided |
| Allocation concealment (selection bias) | High risk | Quote: "Bilateral tendinopathy cases were randomly allocated with one tendon to the treatment group and one to the control group" Comment: participants and investigators could foresee assignments in these cases |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "No placebo injection was performed; hence neither patients nor treatment providers were blind to the treatment allocation" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: the study did not address this outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: missing data balanced in numbers across intervention groups, with similar reasons for missing data across groups |
| Selective reporting (reporting bias) | Unclear risk | Comment: the protocol was not published beforehand and so it is impossible to determine whether it was changed after recruitment commenced |
| Other bias | Unclear risk | Quote: "Pacific Radiology performed the ultrasounds free of charge to the patients" |
Pforringer 1994.
| Methods | Randomised controlled trial | |
| Participants | Setting: single orthopaedic clinic in Munich, Germany Sample: 60 recreational and professional athletes with a clinical diagnosis of Achilles tendon pain and thickening of the tendon on ultrasound examination. Unilateral tendinopathy only Characteristics: mean (SD) 31.0 years (7.5) in the treatment and 34.0 (10.4) in the placebo arm | |
| Interventions | Intervention: paratendinous injection with 5 ml 1% local anaesthetic (mepivacaine hydrochloride) with 5 ml of the study preparation (haemodialysate). Further injections were administered after 3 to 4 days and 9 to 10 days. All patients were also given a soft pad heel support Control: as in the intervention group, although the study preparation was substituted for 5 ml 0.9% saline solution | |
| Outcomes | Follow‐up was 3 time points; at 3 to 4, 9 to 10 and 20 to 23 days
Primary: tendon diameter and density as determined by ultrasound Secondary: patient‐reported pain on walking, running and full activity ("no symptoms", "mild symptoms", "severe symptoms"), pain whilst standing on tiptoes, squatting and on palpation ("no pain", "mild pain", "moderate pain", "severe pain") and overall patient‐reported pain on a 0 to 10 scale |
|
| Notes | Data analysis: SD imputed for pain scores from other trials in the same analysis category | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: unclear description Quote: "The patients were allocated to the treatment groups according to a randomization list, which the manufacture of the coded medications was based" |
| Allocation concealment (selection bias) | Unclear risk | Comment: no clear statement as to whether the allocation was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: the placebo solution had "identical appearance to the drug" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: although described in the manuscript as a "double‐blind" trial, there was no explicit statement that the assessors were blinded to the study group |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: it is not possible to determine from the report whether any patients were lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Comment: the protocol was not published beforehand and so it is impossible to determine whether it was changed after recruitment commenced |
| Other bias | Unclear risk | Comment: no statement as to sources of study funding or conflicts of interest |
Sundqvist 1987.
| Methods | Randomised controlled trial | |
| Participants | Setting: department of orthopaedic surgery, Sweden Sample: 60 recreational/competitive athletes with clinically diagnosed tendinopathy. Unilateral tendinopathy only Characteristics: 51 males and 8 females (1 participant excluded with no additional data). Mean age 33 years (range 21 to 52) | |
| Interventions | All were prescribed a period of restricted training for at least 14 days and provided with a stretching programme and orthotics as required Intervention: 6 local injections of glycosaminoglycan polysulphate (50 mg/ml, 3 injections a week) combined with 3 x 1 placebo tablets Control: 6 placebo injections (1 ml saline) combined with 3 x 50 mg high‐dose indomethacin | |
| Outcomes | All were assessed at week 2, week 4, month 6 and month 12 Outcomes included symptoms, pain on palpation, physicians' evaluation of therapeutic effect and the patients' opinions on how much the injury impeded his/her sports training | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "By using random number code the patiens were allocated to one of two treatment groups" Comment: the investigators describe a random component in the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information presented to permit judgement of low or high risk |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: insufficient information presented on blinding procedures |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: insufficient information presented on blinding procedures |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: missing data not reported |
| Selective reporting (reporting bias) | Unclear risk | Comment: the protocol was not published beforehand and so it is impossible to determine whether it was changed after recruitment commenced |
| Other bias | Unclear risk | Comment: the trial is poorly reported and only described in outline. It has not been possible to exclude other types of bias from this report |
Willberg 2008.
| Methods | Randomised controlled trial | |
| Participants | Setting: a single sports medicine clinic in Stockholm, Sweden
Sample: 48 patients with 52 symptomatic mid portion tendinopathy referred to a single clinic by primary care practitioners Characteristics: mean (SD) 51.8 years (12.4) in the 10 mg/ml group and 47.4 years (7.8) in the 5 mg/ml group. Male/female ratio (by tendon) was 20/6 in the 10 mg/ml group and 15/11 in the 5 mg/ml group. Bilateral tendinopathy included (4 participants) |
|
| Interventions | Intervention (high‐dose): ultrasound‐guided injection of small volumes of polidocanol 10 mg/ml into areas of local neovascularisation outside the tendon. Full Achilles tendon loading was permitted 14 days after each treatment. 3 treatments (at 6 to 8‐week intervals) were given before the first evaluation, after which participants with persisting symptoms were offered further injections Intervention (low‐dose): as in the high‐dose intervention but using polidocanol 5 mg/ml. Participants with persisting symptoms were offered further injections but of 10 mg/ml after the third treatment | |
| Outcomes | Follow‐up time points not specified; mean follow‐up 14 months (range 2 to 35 months) Outcome measures included pain on activity scored on a visual analogue scale, self reported patient satisfaction, number of treatments needed to restore patients to the pre‐injury Achilles tendon loading activities, total volume of polidocanol injections before achieving this result, and adverse events | |
| Notes | Funding for the study has been achieved through the Swedish Research Council for Sports | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The patients selected an envelope (52 opaque envelopes), allocating themselves to either treatment with Polidocanol 5 or 10mg/ml" |
| Allocation concealment (selection bias) | Low risk | Quote: "The patients selected an envelope (52 opaque envelopes), allocating themselves to either treatment with Polidocanol 5 or 10mg/ml" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The chosen envelope was opened in a separate room by an assistant and the substance was prepared by the assistant for injection. There were no visible differences (colour, density, etc) between the substances" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The patients, the treating orthopaedic surgeon, the sonographer, who performed all ultrasound and colour Doppler examinations and treatments were blinded to the substance that was injected" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "Participants left the study at the point at which they became asymptomatic." Comment: departure from the study was therefore collected as an outcome measure. There were no participants remaining in either group after the fifth treatment |
| Selective reporting (reporting bias) | Unclear risk | Comment: the trial protocol was not registered beforehand and so it is not possible identify any changes to the protocol that occurred during or after the trial |
| Other bias | Unclear risk | Quote: "Funding for the study has been achieved through the Swedish Research Council for Sports". There was no explicit statement as to the involvement of this funding body |
Yelland 2011.
| Methods | Randomised controlled trial | |
| Participants | Setting: 5 Australian primary care centres Sample: 43 patients aged > 18 with mid‐portion Achilles tendinosis. The participants were recruited from clinician referrals and advertising in newspapers, brochures and online Characteristics: mean age (range) 46 years (40 to 58) in the eccentric loading exercises group, 48 years (41 to 54) in the prolotherapy group, and 46 years (40 to 57) in the combined treatment group. Bilateral tendinopathy included (15 participants). No information on gender distribution | |
| Interventions | Intervention: injection of tender points in the subcutaneous tissues adjacent to the affected tendon with 20% glucose, 0.1% lignocaine and 0.1% ropivacaine weekly for 4 to 12 weeks. Treatment ceased when the patient reported pain‐free activity or requested to stop receiving injections Control: standardised eccentric loading exercises (3 sets of 15 repetitions each with the knee straight then flexed) twice daily for 12 weeks Combined interventions: protocols for injection and eccentric loading exercise groups implemented concurrently | |
| Outcomes | Follow‐up was at 6 weeks and month 3, 6 and 12 Primary: VISA‐A questionnaire by telephone at 6 weeks, 3 months, 6 months and 12 months. The criterion for treatment success was set a priori as an increase in 20 points on the VISA‐A score Secondary: 7‐point Likert scale for treatment satisfaction, the Patient Global Impression of Change scale, and 0 to 10 scales for worst pain in the last week, usual morning stiffness and limitation of normal activities | |
| Notes | Bilateral tendinopathy randomised per unit The trial was funded by grants from the Musculoskeletal Research Foundation of Australia, the Australian Podiatry Education and Research Foundation and the Griffith University Office of Research Data analysis: SD imputed for VISA‐A scores from other trials in the same analysis category |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomisation schedule was generated and administered by telephone independently by the National Health and Medical Research Council Clinical Trials Centre in Sydney, Australia" |
| Allocation concealment (selection bias) | Low risk | Quote: "The randomisation schedule was generated and administered by telephone independently by the National Health and Medical Research Council Clinical Trials Centre in Sydney, Australia" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no explicit statement, however participants must have known whether they were receiving an exercise regimen, injections or both |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no statement as to whether the assessors were blinded as to the study group. The primary outcome measure was patient‐reported and the patient could not have been blinded as to the arm to which they were randomised |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "The proportion of missing final outcome measurements is small (<3% for the primary outcome measure), and they are imputed by carrying the last value forward method" |
| Selective reporting (reporting bias) | Unclear risk | Comment: the protocol was not published beforehand and so it is impossible to determine whether it was changed after recruitment commenced. |
| Other bias | Low risk | Quote: "The trial was funded by grants from the Musculoskeletal Research Foundation of Australia, the Australian Podiatry Education and Research Foundation and the Griffith University Office of Research. The funding bodies had no role in the study design; in the collection, analysis and interpretation of data; in the writing of the report or in the decision to submit the paper for publication". |
PRP = platelet‐rich plasma SD = standard deviation VAS = visual analogue scale VISA‐A = Victorian Institute of Sport Assessment‐Achilles questionnaire
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ferrero 2012 | Prospective cohort study without any attempt to randomise patients or compare injection therapies with a control intervention |
Characteristics of studies awaiting assessment [ordered by study ID]
EUCTR2010‐020513‐87.
| Methods | Randomised, placebo‐controlled, double‐blind trial |
| Participants | Inclusion criteria: Aged 18 to 70 years
Exclusion criteria:
|
| Interventions | Intervention was injection with hyaluronic acid. Control arm not described |
| Outcomes | Primary outcome measure using the VISA‐A questionnaire. Secondary outcome measures include:
|
| Notes | https://www.clinicaltrialsregister.eu/ctr‐search/search?query=eudract_number:2010‐020513‐87. Reported completed but not published |
Petrella 2013.
| Methods | Randomised placebo‐controlled trial (use of computerised random number generator) |
| Participants | 35 participants with a chronic recalcitrant (> 6 months) non‐insertional Achilles tendinopathy |
| Interventions | Hyaluronan (2.8 cc, 730 to 1300 kDa) or normal saline (2.8 cc) was injected peri‐tendinously under ultrasound guidance at baseline and 7 days |
| Outcomes | Follow‐up: days 7, 14, 30 and 90 Primary outcome measure: VISA‐A (Victorian Institute of Sport Assessment ‐ Achilles) score Secondary outcomes: pain VAS on weight bearing (0 to 100 mm), patients' global assessment of Achilles injury (5‐point categorical scale), patients' assessment of normal function/activity (5‐point categorical scale), physician's global assessment of Achilles injury (5‐point categorical scale), patients/physician satisfaction assessment (10‐point categorical scale), time to return to pain‐free and disability‐free sport and adverse events as per WHO definition |
| Notes | Reported in abstract form only |
BMI = body mass index VAS = visual analogue scale VISA‐A = Victorian Institute of Sport Assessment ‐ Achilles scale WHO = World Health Organization
Characteristics of ongoing studies [ordered by study ID]
ISRCTN85334402.
| Trial name or title | A trial evaluating the efficacy of cell therapy based on autologous platelet rich plasma (PRP) for the treatment of Achilles and patellar tendinopathies |
| Methods | Double‐blind randomised controlled trial |
| Participants | 128 patients (64 with Achilles and 64 with patellar tendinopathy) |
| Interventions | Intervention was ultrasound‐guided injection of platelet‐rich plasma. The control group will receive an ultrasound‐guided injection of platelet‐poor plasma |
| Outcomes | Victorian Institute of Sport Assessment Questionnaire (VISA)‐A for Achilles tendinopathy and ‐P for patellar tendinopathy. Foot and Ankle Ability Measure. Visual analogue scale (VAS) 0 to 10. Participant‐reported overall satisfaction and response to treatment at 4 weeks, 2 months, 4 months and 12 months |
| Starting date | 8 October 2013 |
| Contact information | Dr Ilias Petrou, Regenerative Therapy Unit (UTR), Service of Plastic and Reconstructive Surgery, Department of Musculoskeletal Medicine DAL, CHUV‐EPCR/Croisettes 22, Epalinges, Switzerland |
| Notes | — |
NCT01343836.
| Trial name or title | Autologous tenocyte implantation in patients with chronic Achilles tendinopathy (ATI) |
| Methods | Double‐blind randomised controlled trial |
| Participants | Participants aged 18 to 55 years with symptoms of > 2 months duration that include pain on palpation 2 to 7 cm proximal from the tendon insertion. Exclusion criteria are:
|
| Interventions | Intervention was ultrasound‐guided intratendinous autologous tenocyte implantation with eccentric exercises. The control arm received ultrasound‐guided intratendinous saline injection with eccentric exercises |
| Outcomes | Primary outcome measure VISA‐A score at 24 weeks post‐intervention Secondary outcome measures are ultrasonographic tendon repair |
| Starting date | April 2011 |
| Contact information | Dr S. de Jonge, Sports Medicine Department Medical Center, The Hague Leidschendam, Zuid‐Holland, Netherlands, 2262 BA |
| Notes | — |
NCT01583504.
| Trial name or title | A double blind, randomised controlled trial of high volume saline injections for chronic midportion Achilles tendinopathy |
| Methods | Randomised, double‐blind, controlled trial |
| Participants | Participants aged 18+ with more than 13 weeks of pain in the Achilles tendon area, completed eccentric tendon loading programme with a physiotherapist, Achilles tendon tender to palpation in the midportion, tendon diameter greater than 0.7 cm on ultrasound scan, evidence of neovascularisation on doppler ultrasound scan, sufficient English language to complete questionnaires and consent. Exclusion criteria are ultrasound evidence or previous history of partial or full tendon tear, another co‐existing significant foot or ankle pathology, taking anticoagulant medication, i.e. warfarin, clopidogrel, dipyridamole, a medical condition that would affect safety of injection, i.e. diabetic neuropathy, peripheral vascular disease, previous Achilles tendon surgery, unable to give informed consent |
| Interventions | Intervention group is ultrasound‐guided injection of steroid, local anaesthetic and high‐volume saline. Control arm received ultrasound‐guided injection of steroid and local anaesthetic only |
| Outcomes | Primary outcome measure 100 mm visual analogue scale (VAS) at 6 weeks post‐injection Secondary outcome measures include Foot and Ankle Outcome Score, EQ5D‐3L, ultrasound measurement of Achilles tendon diameter, neovascularisation grading at 6, 12 and 40 weeks |
| Starting date | March 2012 |
| Contact information | Ms Marie Hoddell, Leeds Musculoskeletal and Rehabilitation Service, Leeds, West Yorkshire, LS7 4SA |
| Notes | — |
NCT01954108.
| Trial name or title | Hyaluronan in the treatment of painful Achilles tendinopathy |
| Methods | Randomised, single‐blind trial |
| Participants | Participants aged 18 to 75. Inclusion criteria:
Exclusion criteria:
|
| Interventions | Intervention group was extracorporeal shock wave therapy versus a second arm that received hyaluronic acid injections |
| Outcomes | VISA‐A scores at regular intervals post‐intervention, clinical parameters (redness, warmth, swelling, tenderness, crepitus, fluid accumulation) on a 5‐point scale at days 7, 28, 90 and 180, and adverse events |
| Starting date | December 2013 |
| Contact information | Dr Petra Dobner, dobner@trbchemedica.de |
| Notes | Estimated completion date April 2015 |
ESWT = extracorporeal shock wave therapy VAS = visual analogue scale VISA‐A = Victorian Institute of Sport Assessment ‐ Achilles scale
Differences between protocol and review
The current protocol differs from the originally published protocol in three ways:
Inclusion of a surgical management comparison arm.
Combining serious and non‐serious adverse events into the same analysis.
Imputation of standard deviations in cases where standard deviations for the same outcome measure at the same outcome time point were available from other studies in the review.
Contributions of authors
RK: is responsible for the conception, design, database searching, interpretation of the data, writing of the review and final approval of the document. She is guarantor of the review. NP: is the review statistician. He is responsible for the conception, design, data management, analysis plan, interpretation of the data, critical commentary and final approval of the document. DM: is responsible for conception, design, database searching, interpretation of the data, critical commentary and final approval of the document. MC: is responsible for conception, design, interpretation of the data, critical commentary and final approval of the document.
Sources of support
Internal sources
-
University of Warwick, UK.
Salaries for the authors to support the development of the protocol.
External sources
No sources of support supplied
Declarations of interest
Rebecca S Kearney, Nick Parsons, David Metcalfe and Matthew L Costa: the authors' institution, University of Warwick, has received research grants and PRP (platelet‐rich plasma) materials at cost price for studies related to the treatment of Achilles tendinopathy and rupture, including injection studies.
Rebecca S Kearney, Nick Parsons and Matthew L Costa were authors on one of the included study (Kearney 2013). Risk of bias for this trial was independently assessed by David Metcalfe, who had no involvement in this earlier study.
New
References
References to studies included in this review
Alfredson 2005 {published data only}
- Alfredson H, Ohberg L. Sclerosing injections to areas of neo‐vascularisation reduce pain in chronic Achilles tendinopathy: a double blind randomised controlled trial. Knee Surgery, Sports, Traumatology, Arthroscopy 2005;13(4):338‐44. [DOI] [PubMed] [Google Scholar]
Alfredson 2007 {published data only}
- Alfredson H, Ohberg L, Zeisig E, Lorentzon R. Treatment of midportion Achilles tendinosis: similar clinical results with US and CD guided surgery outside the tendon and sclerosing polidocanol injections. Knee, Surgery, Sports, Traumatology, Arthroscopy 2007;15(12):1504‐9. [DOI] [PubMed] [Google Scholar]
Bell 2013 {published data only}
- Bell KJ, Fulcher ML, Rowlands DS, Kerse N. Impact of autologous blood injections in treatment of mid‐portion Achilles tendinopathy: double blind randomised controlled trial. BMJ 2013;346:f2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brown 2006 {published data only}
- Brown R, Orchard J, Kinchington M, Hooper A, Nalder G. Aprotinin in the management of Achilles tendinopathy: a randomised controlled trial. British Journal Sports Medicine 2006;40(3):275‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Capasso 1993 {published data only}
- Capasso G, Maffulli N, Testa V, Sgambato. Preliminary results with peritendinous protease inhibitor injections in the management of Achilles tendinitis. Journal of Sports Traumatology and Related Research 1993;15(1):37–43. [Google Scholar]
Chouchane 1989 {published data only}
- Chouchane A, Commandree F, Fournier S, Plas JN, Viani JL, Zakarian H. Percutaneous treatment of tendinopathy with dexamethasone‐salicyles. Cinesiologie 1989;28:366‐71. [Google Scholar]
DaCruz 1988 {published data only}
- DaCruz D, Geeson M, Allen MJ, Phair I. Achilles paratendonitis: an evaluation of steroid injection. British Journal of Sports Medicine 1988;22(2):64‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
De Vos 2010 {published data only}
- Jonge S, Vos RJ, Weir A, Schie HT, Bierma‐Zeinstra S, Verhaar J, et al. One‐year follow‐up of platelet‐rich plasma treatment in chronic Achilles tendinopathy: a double‐blind randomized placebo‐controlled trial. American Journal of Sports Medicine 2011;39(8):1623‐9. [DOI] [PubMed] [Google Scholar]
- Vos R, Weir A, Bierma‐Zeinstra S, Verhaar J, Tol J. Platelet‐rich plasma injection for chronic Achilles tendinopathy: a randomised controlled trial. JAMA 2010;303(2):144‐9. [DOI] [PubMed] [Google Scholar]
- Vos R, Weir A, Verhaar J, Weinans H, Schie H. No effects of PRP on ultrasonographic tendon structure and neovascularisation in chronic midportion Achilles tendinopathy. British Journal of Sports Medicine 2011;45(5):387‐92. [DOI] [PubMed] [Google Scholar]
Fabbro 2012 {published data only}
- Fabbro E, Sconfienza LM, Ferrero G, Orlandi D, Lacelli F, Serafini G, et al. One year outcome of ultrasound (US)‐guided percutaneous treatment of Achilles tendinopathy: results of a randomized controlled trial. European Congress of Radiology; 2012 Mar 1‐5; Vienna, Austria. European Society of Radiology Electronic Presentation Online System, 2012:Poster C‐1850. [DOI: 10.1594/ecr2012/C-1850] [DOI]
Fredberg 2004 {published data only}
- Fredberg U, Bolvig L, Pfeiffer‐Jensen M, Clemmensen D, Jakobsen BW, Stengaard‐Pedersen K. Ultrasonography as a tool for diagnosis, guidance of local steroid injection and, together with pressure algometry, monitoring of the treatment of athletes with chronic jumper's knee and Achilles tendinitis: a randomized, double‐blind, placebo‐controlled study. Scandinavian Journal of Rheumatology 2004;33(2):94‐101. [DOI: 10.1080/03009740310004126] [DOI] [PubMed] [Google Scholar]
Kearney 2013 {published data only}
- Costa M. Achilles tendinopathy management: platelet‐rich plasma versus eccentric loading programme. http://www.isrctn.com/ISRCTN95369715 (accessed 17 April 2015).
- Kearney RS, Parsons N, Costa ML. Achilles tendinopathy management: a pilot randomised controlled trial comparing platelet‐rich plasma injection with an eccentric loading programme. Bone and Joint Research 2013;2(10):227‐32. [DOI: 10.1302/2046-3758.210.2000200] [DOI] [PMC free article] [PubMed] [Google Scholar]
Larsen 1987 {published data only}
- Larsen AI, Egfjord M, Jelsdorff HM. Low‐dose heparin in the treatment of calcaneal peritendinitis. Scandinavian Journal of Rheumatology 1987;16(1):47‐51. [PubMed] [Google Scholar]
Obaid 2012 {published data only}
- Obaid H, Clarke A, Rosenfeld P, Leach C, Connell D. Skin‐derived fibroblasts for the treatment of refractory Achilles tendinosis: preliminary short‐term results. Journal of Bone and Joint Surgery ‐ American Volume 2012;94(3):193‐200. [DOI: 10.2106/JBJS.J.00781] [DOI] [PubMed] [Google Scholar]
Pearson 2012 {published data only}
- Pearson J, Rolands D, Highet R. Autologous blood injection to treat Achilles tendinopathy? A randomized controlled trial. Journal of Sport Rehabilitation 2012;21(3):218‐24. [DOI] [PubMed] [Google Scholar]
Pforringer 1994 {published data only}
- Pforringer W, Pfister A, Kuntz G. The treatment of Achilles paratendinitis: results of a double‐blind, placebo‐controlled study with a deproteinized hemodialysate. Clinical Journal of Sports Medicine 1994;4(2):92‐9. [Google Scholar]
Sundqvist 1987 {published data only}
- Sundqvist H, Forsskahl B, Kvist M. A promising therapy for Achilles peritendinitis: double‐blind comparison of glycosaminoglycan polysulfate and high dose indomethacin. International Journal of Sports Medicine 1987;8(4):298‐303. [DOI] [PubMed] [Google Scholar]
Willberg 2008 {published data only}
- Willberg L, Sunding K, Ohberg L, Forssblad M, Fahlstrom M, Alfredson H. Sclerosis injections to treat midportion Achilles tendinosis: a randomised controlled study evaluating two different concentrations of Polidocanol. Knee Surgery, Sports Traumatology, Arthroscopy 2008;16(9):859‐64. [DOI: 10.1007/s00167-008-0579-x] [DOI] [PubMed] [Google Scholar]
Yelland 2011 {published data only}
- Yelland MJ, Sweeting KR, Lyftogt JA, Ng SK, Scuffham PA, Evans KA. Prolotherapy injections and eccentric loading exercises for painful Achilles tendinosis: a randomised trial. British Journal of Sports Medicine 2011;45(5):421‐8. [DOI: 10.1136/bjsm.2009.057968] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Ferrero 2012 {published data only}
- Ferrero G, Fabbro E, Orlandi D, Martini C, Lacelli F, Serafini G, et al. Ultrasound‐guided injection of platelet‐rich plasma in chronic Achilles and patellar tendinopathy. Journal of Ultrasound 2012;15(4):260‐6. [DOI: 10.1016/j.jus.2912.09.006] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
EUCTR2010‐020513‐87 {published data only}
- FIDIA. A randomized, placebo‐controlled, double‐blind study on the intensity and duration of efficacy of sodium hyaluronate therapy (500‐730 KDa) (HYALGAN) in the conservative treatment of Achilles tendinopathy. https://www.clinicaltrialsregister.eu/ctr‐search/trial/2010‐020513‐87/IT (accessed 17 November 2014).
Petrella 2013 {published data only}
- Petrella R, Petrella M, Decaria J. Hyaluronan alone, combined with botulinus toxin or placebo injection therapy for treatment of chronic noninsertional achilles tendinopathy. Annals of the Rheumatic Diseases 2013;72(Suppl 3):A354. [Google Scholar]
References to ongoing studies
ISRCTN85334402 {published data only}
- Petrou I. A trial evaluating the efficacy of cell therapy based on autologous platelet‐rich plasma (PRP) for the treatment of Achilles and Patellar tendinopathies. http://www.controlled‐trials.com/isrctn/pf/85334402 (accessed 17 November 2014).
NCT01343836 {published data only}
- Jonge S. Autologous tenocyte implantation in patients with chronic achilles tendinopathy (ATI). http://clinicaltrials.gov/show/NCT01343836 (accessed 17 November 2014).
NCT01583504 {published data only}
- Smith M. High volume saline injections for Achilles tendinopathy. http://clinicaltrials.gov/show/NCT01583504 (accessed 17 November 2014).
NCT01954108 {published data only}
- Vroey T, Lynen N. Hyaluronan in the treatment of painful Achilles tendinopathy. http://clinicaltrials.gov/show/NCT01954108 (accessed 17 November 2014).
Additional references
Andres 2008
- Andres BM, Murrell GAC. Treatment of tendinopathy: what works, what does not, and what is on the horizon. Clinical Orthopaedics and Related Research 2008;466(7):1539‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Coombes 2010
- Coombes BK, Bisset L, Vicenzino B. Efficacy and safety of corticosteroid injections and other injections for management of tendinopathy: a systematic review of randomised controlled trials. Lancet 2010;376(9754):1751‐67. [DOI] [PubMed] [Google Scholar]
De Jonge 2011
- Jonge S, Berg C, Vos R, Heide H, Weir A, Verhaar J, et al. Incidence of midportion Achilles tendinopathy in the general population. British Journal of Sports Medicine 2011;45(13):1026‐8. [DOI] [PubMed] [Google Scholar]
DTB 2012
- Anonymous. Management of chronic Achilles tendinopathy. Drugs and Therapy Bulletin 2012;50(8):93‐6. [DOI] [PubMed] [Google Scholar]
Evans 2000
- Evans NA, Stanish WD. The basic science of tendon injuries. Current Orthopaedics 2000;14(6):403‐12. [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Kaux 2011
- Kaux JF, Forthomme B, Goff C, Crielaard JM, Croiser JL. Current opinions on tendinopathy. Journal of Sports Science and Medicine 2011;10:238‐53. [PMC free article] [PubMed] [Google Scholar]
Kearney 2010
- Kearney RS, Costa ML. Insertional Achilles tendinopathy management: a systematic review. Foot and Ankle International 2010;31(8):689‐94. [DOI] [PubMed] [Google Scholar]
Kraemer 2012
- Kraemer R, Wuerfel W, Lorenzen J, Busche M, Vogt PM, Knobloch K. Analysis of hereditary and medical risk factors in Achilles tendinopathy and Achilles tendon ruptures: a matched pair analysis. Archives of Orthopaedic and Trauma Surgery 2012;132:847‐53. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Maffulli 2010
- Maffulli N, Longo UG, Denaro V. Novel approaches for the management of tendinopathy. Journal of Bone and Joint Surgery ‐ American Volume 2010;92(15):2604‐13. [DOI] [PubMed] [Google Scholar]
Narici 2008
- Narici MV, Maffulli N, Maganaris CN. Ageing of human muscles and tendons. Disability and Rehabilitation 2008;30:1548‐54. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Riley 2008
- Riley G. Tendinopathy ‐ from basic science to treatment. Nature Clinical Practice Rheumatology 2008;4(2):82‐9. [DOI] [PubMed] [Google Scholar]
Robinson 2001
- Robinson JM, Cook JL, Purdam C, Visentini PJ, Ross J, Maffulli N, et al. The VISA‐A questionnaire: a valid and reliable index of the clinical severity of Achilles tendinopathy. British Journal of Sports Medicine 2001;35(5):335‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sussmilch‐Leitch 2012
- Sussmilch‐Leitch S, Collins N, Bialocerkowski A, Warden S, Crossley K. Physical therapies for Achilles tendinopathy: systematic review and meta‐analysis. Journal of Foot and Ankle Research 2012;5(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Sterkenburg 2011
- Sterkenburg MN, Dijk CN. Mid‐portion Achilles tendinopathy: why painful? An evidence‐based philosophy. Knee Surgery, Sports Traumatology, Arthroscopy 2011;19(8):1367‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Kearney 2014
- Kearney RS, Parsons N, Metcalfe D, Costa ML. Injection therapies for Achilles tendinopathy. Cochrane Database of Systematic Reviews 2014, Issue 2. [DOI: 10.1002/14651858.CD010960] [DOI] [PMC free article] [PubMed] [Google Scholar]


