Abstract
Cancer drug resistance has become one of the main challenges for the failure of chemotherapy, greatly limiting the selection and use of anticancer drugs and dashing the hopes of cancer patients. The emergence of supramolecular host-guest nanosystems has brought the field of supramolecular chemistry into the nanoworld, providing a potential solution to this challenge. Compared with conventional chemotherapeutic platforms, supramolecular host-guest nanosystems can reverse cancer drug resistance by increasing drug uptake, reducing drug efflux, activating drugs, and inhibiting DNA repair. Herein, we summarize the research progress of supramolecular host-guest nanosystems for overcoming cancer drug resistance and discuss the future research direction in this field. It is hoped that this review will provide more positive references for overcoming cancer drug resistance and promoting the development of supramolecular host-guest nanosystems.
Keywords: Supramolecular nanosystems, host-guest interaction, cancer drug resistance
INTRODUCTION
With the number of cancer cases increasing each year, cancer has become the second leading cause of death worldwide[1]. Although chemotherapy remains the primary method of cancer treatment, its effectiveness is severely limited by cancer drug resistance[2-5]. The occurrence of cancer drug resistance is associated with multiple factors, including the overexpression of multidrug resistance gene (MDR1), anti-apoptotic protein (BCL-2), multidrug resistance-associated protein (MRP), and the enhanced activity of glutathione S-transferase (GST) and DNA repair enzyme[6-8]. These factors can lead to decreased drug uptake, increased drug efflux, DNA damage repair, abnormal drug metabolism, and dysfunctional apoptosis, resulting in cancer drug resistance[9,10]. Nanosystems have been widely used to overcome cancer drug resistance due to their ability to alter the way drugs enter cells, increase drug uptake, and improve drug stability[11,12]. Common nanosystems used to overcome cancer drug resistance include liposomes, polymeric nanoparticles, and metal nanoparticles. However, there are still some problems in the application of these nanosystems. For example, drugs loaded in liposomes tend to leak in the circulatory system before reaching the tumor; polymeric nanoparticles have a high burst release effect; and metal nanoparticles have poor biocompatibility. These problems have led to the limited role of these nanosystems in overcoming cancer drug resistance[13,14]. Therefore, it is urgent to develop a class of novel nanosystems to reverse cancer drug resistance.
Supramolecular chemistry is “chemistry beyond the molecule”[15]. Supramolecules generally refer to organized aggregates formed by non-covalent interactions of two or more molecules, including electrostatic interaction, hydrogen bond, van der Waals force, and π-π interaction[16-21]. By introducing supramolecules into the nanosystem, it is possible to construct a more promising new drug delivery system, supramolecular host-guest nanosystem, which provides a potential solution for cancer drug resistance[22-28]. Compared with traditional nanomaterials constructed by covalent interactions, supramolecular host-guest nanomaterials constructed by non-covalent interactions have excellent dynamic reversibility and responsiveness to various stimuli (such as weak acidity, specific enzymes, and different redox environments)[29-33]. Based on these advantages, supramolecular host-guest nanosystems can increase drug uptake, accurately release drugs, inhibit drug efflux, and protect the activity of drugs, which provide great possibilities for eliminating cancer drug resistance and promoting the progress of cancer treatment[34-37].
In this review, we summarize the research progress of supramolecular host-guest nanosystems for overcoming cancer drug resistance over the past few years, including cyclodextrins, calixarenes, cucurbiturils, and pillararenes [Scheme 1]. Moreover, the challenges and prospects of supramolecular host-guest nanosystems for overcoming cancer drug resistance are discussed extensively. This review aims to provide valuable insights and contribute to the development of more effective ways to reverse cancer drug resistance.
Scheme 1.
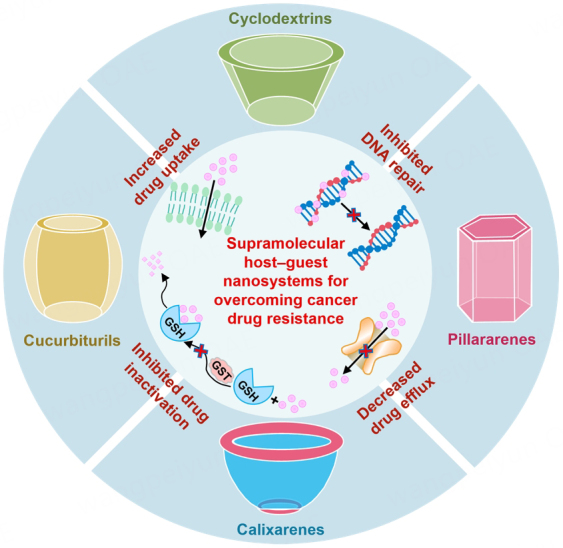
Supramolecular host-guest nanosystems for overcoming cancer drug resistance. GSH: Glutathione; GST: glutathione S-transferase.
CYCLODEXTRINS-BASED HOST-GUEST NANOSYSTEMS FOR OVERCOMING CANCER DRUG RESISTANCE
Cyclodextrins (CDs), a class of natural oligosaccharides obtained from the degradation of starch, are linked by glucopyranose units through α-1,4-glycosidic bonds [Figure 1][38,39]. The most common CDs contain six, seven, and eight glucopyranose units, respectively, known as α, β, and γ-CDs[40,41]. CDs have hydrophobic cavities, which can encapsulate hydrophobic drug molecules to form host-guest complexes[42-45]. In addition, these complexes can self-assemble into nanoparticles, greatly improving the efficiency of the drug (such as good water solubility, high stability, and low physiological toxicity)[46-48]. Therefore, CDs-based host-guest nanosystems have the potential to reverse cancer drug resistance by increasing drug uptake and decreasing drug efflux[49-51]. For example, Yang et al. constructed three nanomedicines based on β-CDs that enhanced the drug uptake and the toxicity of drug-resistant cells[52]. Das et al. prepared a dual-responsive nanocarrier by embedding carbon nanotubes into β-CDs-based polymers, enabling the combination of cocktail chemotherapy with photothermal therapy, which was conducive to multidrug resistance reversal[53].
Figure 1.
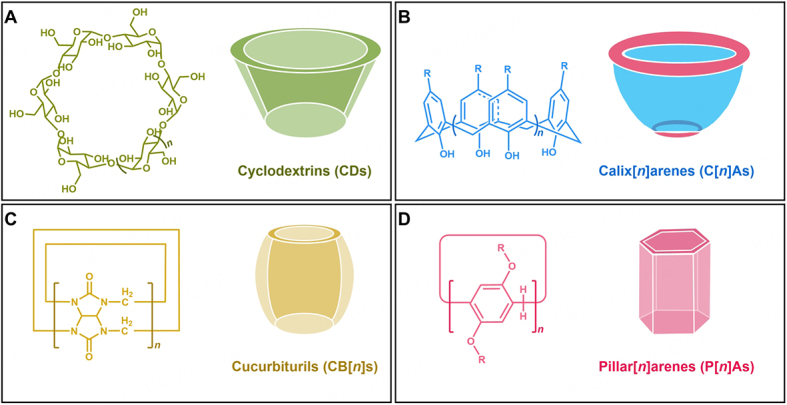
Schematic illustration of structures of (A) CDs; (B) calixarenes (C[n]As); (C) cucurbiturils (CB[n]s); and (D) pillararenes (P[n]As).
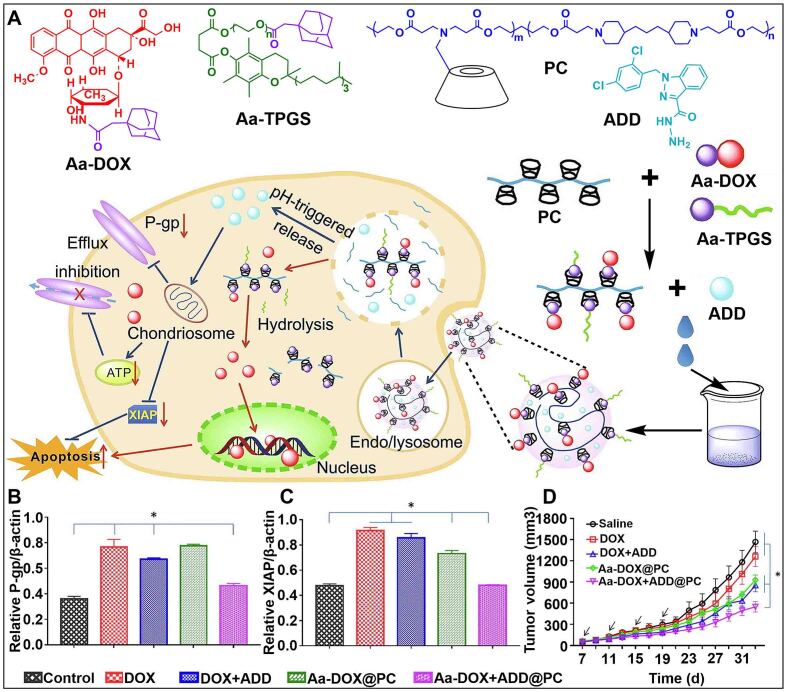
P-glycoprotein (P-gp) is an energy-dependent efflux pump located on the cell membrane[54,55]. P-gp depends on the energy produced by ATP hydrolysis within the mitochondria to keep intracellular drug concentrations low by transporting drug molecules outside the cell, resulting in drug resistance[56-58]. Therefore, drug resistance can be effectively reversed by inducing mitochondrial dysfunction. Wang et al. constructed a nanosystem (Aa-DOX + ADD@PC) based on a pH-sensitive graft copolymer (PBAE-g-β-CD) to achieve co-loading of the anticancer drug doxorubicin (DOX) and mitochondrial inhibitor (ADD) [Figure 2A][59]. When Aa-DOX + ADD@PC was endocytosed by tumor cells, DOX and ADD were released in the acidic environment for combined chemotherapy. Western blot assay was used to study the expression levels of P-gp and X-linked inhibitor of apoptosis protein (XIAP), and it was found that Aa-DOX + ADD@PC showed the best inhibitory effect on P-gp and XIAP [Figures 2B and C]. Moreover, the therapeutic effect of Aa-DOX + ADD@PC was better than that of free DOX, significantly inhibiting the growth of drug-resistant tumors [Figure 2D]. In this work, the effective loading of mitochondrial inhibitors by CDs was used to successfully reverse drug resistance by decreasing drug efflux, providing a new therapeutic platform for overcoming multidrug resistance (MDR).
Figure 2.
(A) Schematic illustration of dual-drug co-loaded nanoparticle (Aa-DOX + ADD@PC) for overcoming cancer drug resistance; The expression levels of (B) P-gp and (C) XIAP in MCF-7/ADR cells with different treatments; (D) Tumor growth inhibition curves of tumor-bearing mice after various formulations. (*P < 0.05) This figure is quoted with permission from Wang et al.[59]. ADD: Mitochondrial inhibitor; DOX: doxorubicin; XIAP: X-linked inhibitor of apoptosis protein.
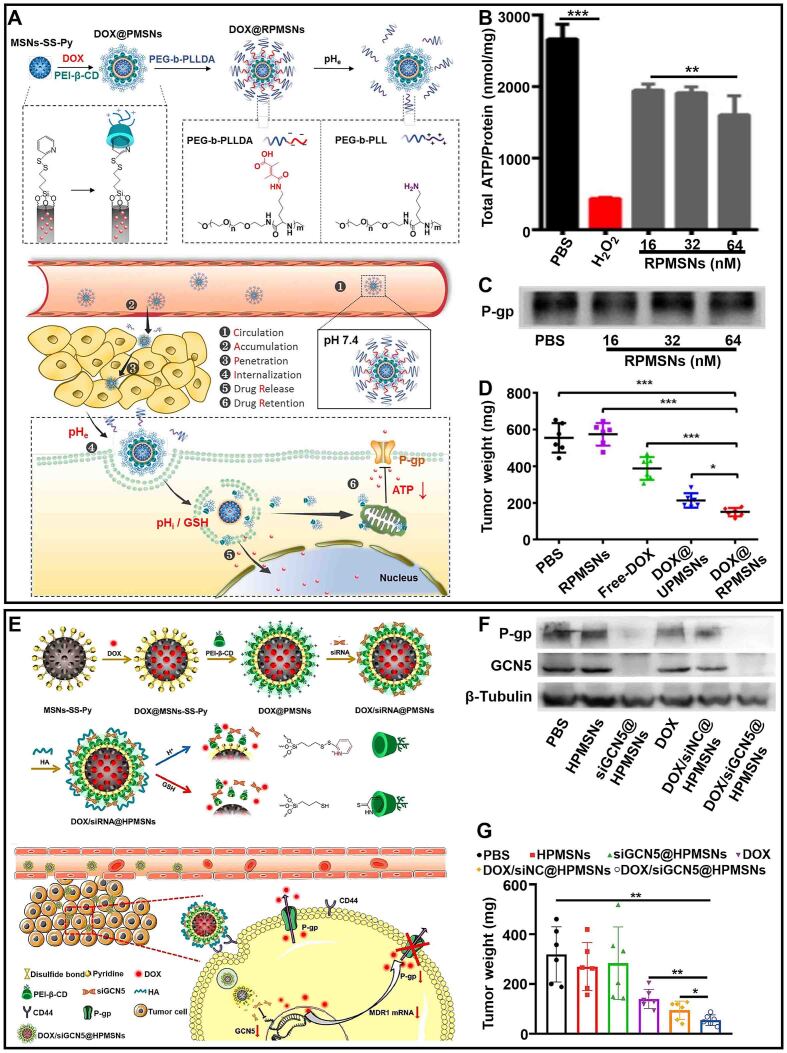
Furthermore, compared with free drugs, tumor cells can effectively take up nanomedicine, which is conducive to the reversal of drug resistance caused by low intracellular drug concentration[60,61]. Liu et al. developed pH/redox dual-responsive DOX delivery nanosystems (DOX@RPMSNs) based on cationic β-cyclodextrin-PEI (PEI-β-CD) to overcome drug resistance of tumor cells [Figure 3A][62]. The poly (ethylene glycol) amine derivative shell (PEG-b-PLLDA) of DOX@RPMSNs could protect DOX@RPMSNs from safely reaching the vicinity of tumor cells, increasing the absorption of drugs by tumor cells. PEI-β-CD and DOX were sequentially released in response to the action of acid and glutathione (GSH) in tumor cells. DOX was used to kill tumor cells, and PEI-β-CD acted as an inhibitor to downregulate the expression of drug resistance-related P-gp by reducing ATP [Figure 3B]. Compared with other formulations, DOX@RPMSNs significantly inhibited tumor growth [Figures 3C and D]. These results indicated that DOX@RPMSNs successfully improved drug resistance reversal.
Figure 3.
(A) Schematic illustration of the construction of sequentially responsive nanosystem (DOX@RPMSNs) and dual-responsive drug release; (B) Total ATP concentrations of MCF7/ADR cells treated with H2O2 and different doses of RPMSNs; (C) The expression levels of P-gp in tumor cells with different treatments; (D) Changes of tumor volume in tumor-bearing mice with different treatments (*P < 0.05, **P < 0.01, ***P < 0.001). This figure is quoted with permission from Liu et al.[62]; (E) Schematic illustration of the construction of co-delivery nanosystem (HPMSNs) and dual-responsive drug release; (F) The expression levels of P-gp and GCN5 in tumor cells with different treatments; (G) Changes of tumor weight in tumor-bearing mice with different treatments (*P < 0.05, **P < 0.01). This figure is quoted with permission from Yuan et al.[68]. DOX: Doxorubicin; PEG-b-PLLDA: poly (ethylene glycol) amine derivative shell.
Histone-acetyltransferase (GCN5) is a silencing protein closely related to drug-resistance genes. Drug resistance caused by efflux can be reversed by down-regulating the expression of GCN5[63,64]. RNA interference (RNAi) is a therapeutic technique that specifically targets mRNA and regulates the expression of silencing proteins[65-67]. Yuan et al. exploited a nanosystem (DOX/siRNA@HPMSNs) to combine RNAi and DOX, which could knockout drug-resistance genes (Figure 3E)[68]. The hyaluronan (HA) shell of DOX/siRNA@HPMSNs could prolong the circulation time of DOX/siRNA@HPMSNs in vivo and target tumor cells, which promoted the accumulation of antitumor drugs. In the microenvironment of tumor cells, the effective release of siRNA could downregulate the expression of GCN5 to reduce the efflux of DOX caused by P-gp [Figure 3F]. Additionally, the inhibition rate of DOX/siRNA@HPMSNs on the growth of drug-resistance tumors was higher than DOX by evaluating the chemotherapeutic effects of different drug delivery systems [Figure 3G]. The two pH/redox dual-responsive nanosystems reduced drug efflux caused by the overexpression of P-gp in different ways, providing more possibilities for reversing MDR.
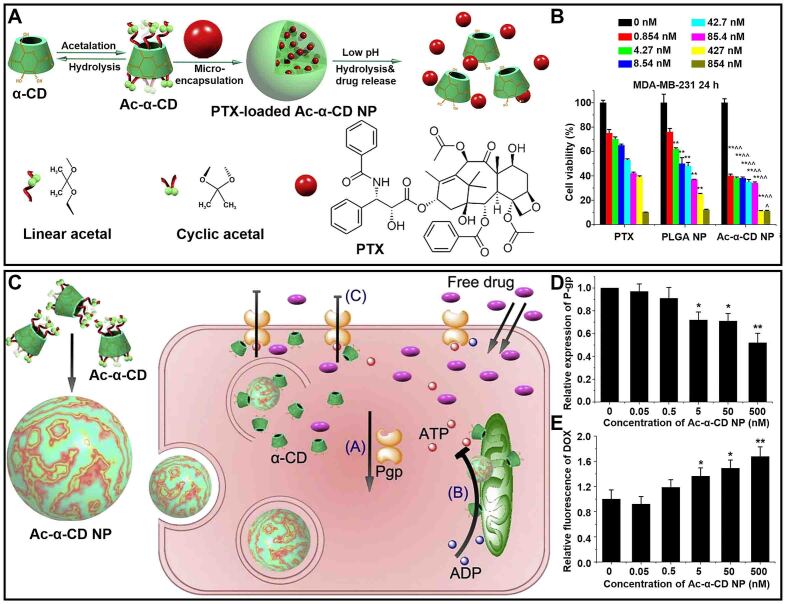
The acidic tumor microenvironment commonly found in solid tumors can reduce the endocytosis of free drugs and dissociate drug molecules[69,70]. Therefore, pH-responsive supramolecular host-guest nanosystems have been widely developed to enhance cell internalization and protect drugs from dissociation[71-73]. He et al. prepared a pH-responsive nanoparticle (Ac-α-CD NP) based on acetylated α-CD (Ac-α-CD), which could stably encapsulate paclitaxel (PTX) [Figure 4A][74]. Ac-α-CD NP had a stronger inhibitory effect on the viability of breast cancer drug-resistant cells (MDA-MB-231) compared with free PTX and PLGA NPs [Figure 4B]. Moreover, Ac-α-CD NP exhibited good drug activity at a low concentration (0.854 nM), indicating its potential to kill drug-resistant cells.
Figure 4.
(A) Schematic illustration of the formation of pH-sensitive nanosystems (Ac-α-CD NP); (B) The cell viability of MDA-MB-231 cells treated with different doses of PTX, PLGA NP, and Ac-α-CD NP (**P < 0.01). This figure is quoted with permission from He et al.[74]; (C) Schematic illustration of the antitumor progress of Ac-α-CD NP in MCF-7/ADR cells; (D) The P-gp expression level; and (E) the accumulation of DOX with different doses of Ac-α-CD NP in MCF-7/ADR cells (*P < 0.05, **P < 0.01). This figure is quoted with permission from Shi et al.[75]. CD: Cyclodextrin; DOX: doxorubicin; NP: nanoparticle; PTX: paclitaxel.
Additionally, further studies showed that Ac-α-CD NP could also enhance the uptake and sensitivity of drug-resistant cells to DOX [Figure 4C][75]. α-CD was released by pH-induced hydrolysis of Ac-α-CD NP to inhibit the expression of P-gp and decrease the activity of ATPase, eliminating drug resistance caused by drug efflux [Figure 4D]. The changes in drug concentrations indicated that the downregulation of P-gp expression directly increased the accumulation of DOX in drug-resistant cells, thus achieving the purpose of inhibiting cancer drug resistance [Figure 4E]. Such pH-responsive supramolecular nanoparticles could not only inhibit the viability of drug-resistant cells at low concentrations but also increase the uptake and sensitization of drug-resistant cells to DOX, successfully reversing drug resistance from multiple angles.
Various star-shaped polymers can be obtained by modifying β-CD with different polymer chains[76,77]. These polymers can further self-assemble into stable supramolecular host-guest nanoparticles after loading anticancer drugs[78-80]. Compared with free drugs, nanoparticles are more easily internalized by tumor cells, reducing the efflux of drugs[81-83]. These factors work together to eliminate cancer drug resistance[84,85]. Chen et al. constructed a cationic β-CD-based nanocarrier that co-delivered PTX and Nur77 gene (an orphan nuclear receptor) to eliminate cancer drug resistance[86]. In addition, they reported a nanoparticle based on a PEGylated star-shaped copolymer successfully reversed MDR1-induced drug resistance[87].
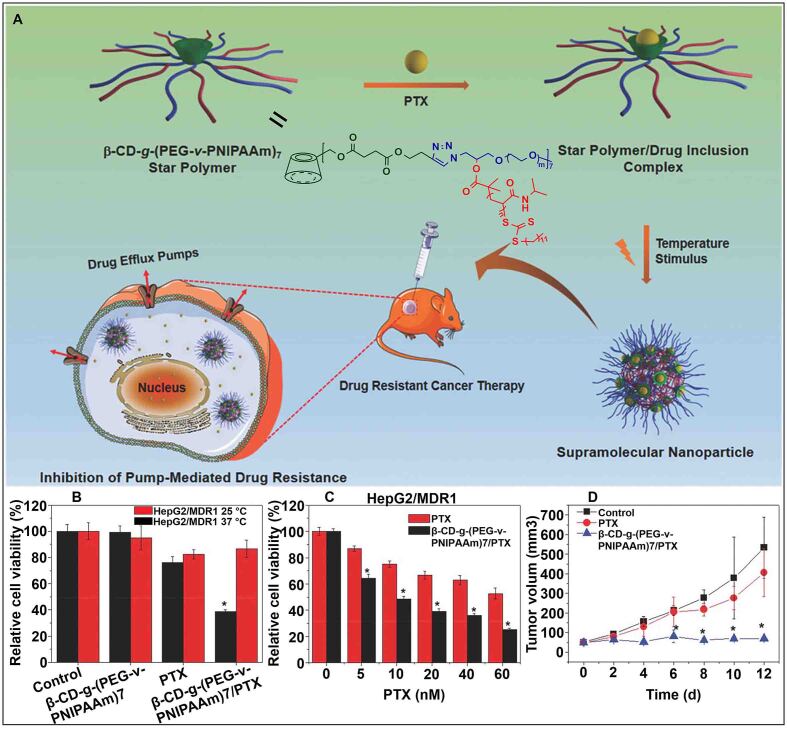
Subsequently, they designed a new type of thermosensitive star-shaped polymer β-CD-g-(PEG-v-PNIPAAm)7 with “V”-shaped arms, which encapsulated PTX through the cavity of β-CD [Figure 5A][88]. The drug-loaded polymer further self-assembled into a stable supramolecular host-guest nanomedicine at 37 °C, greatly enhancing the retention of drugs in cells. Compared with other drugs, this supramolecular host-guest nanomedicine was more sensitive to the change of temperature, causing a sharp decline in cell viability at 37 °C [Figure 5B]. When the drug-resistance tumor was transplanted into mice and treated with PTX and nanomedicine, respectively, β-CD-g-(PEG-v-PNIPAAm)7/PTX was more prominent in reducing cell viability [Figure 5C]. Additionally, there was no obvious change in tumor volume after treatment with β-CD-g-(PEG-v-PNIPAAm)7/PTX [Figure 5D]. These results indicated that β-CD-based temperature-responsive nanomedicine had a good therapeutic efficacy against drug-resistant tumors.
Figure 5.
(A) Schematic illustration of supramolecular nanoparticle for inhibiting pump-mediated drug resistance; (B) Cell viability of HepG2/MDR1 cells treated with β-CD-g-(PEG-v-PNIPAAm)7, PTX, and β-CD-g-(PEG-v-PNIPAAm)7/PTX at different temperatures; (C) Changes in cell viability of HepG2/MDR1 cells treated with different doses of PTX and β-CD-g-(PEG-v-PNIPAAm)7/PTX; (D) Tumor growth inhibition curves of tumor-bearing mice after various treatments (*P < 0.05). This figure is quoted with permission from Fan et al.[88]. CD: Cyclodextrin; MDR1: multidrug resistance gene; PEG: poly (ethylene glycol); PTX: paclitaxel.
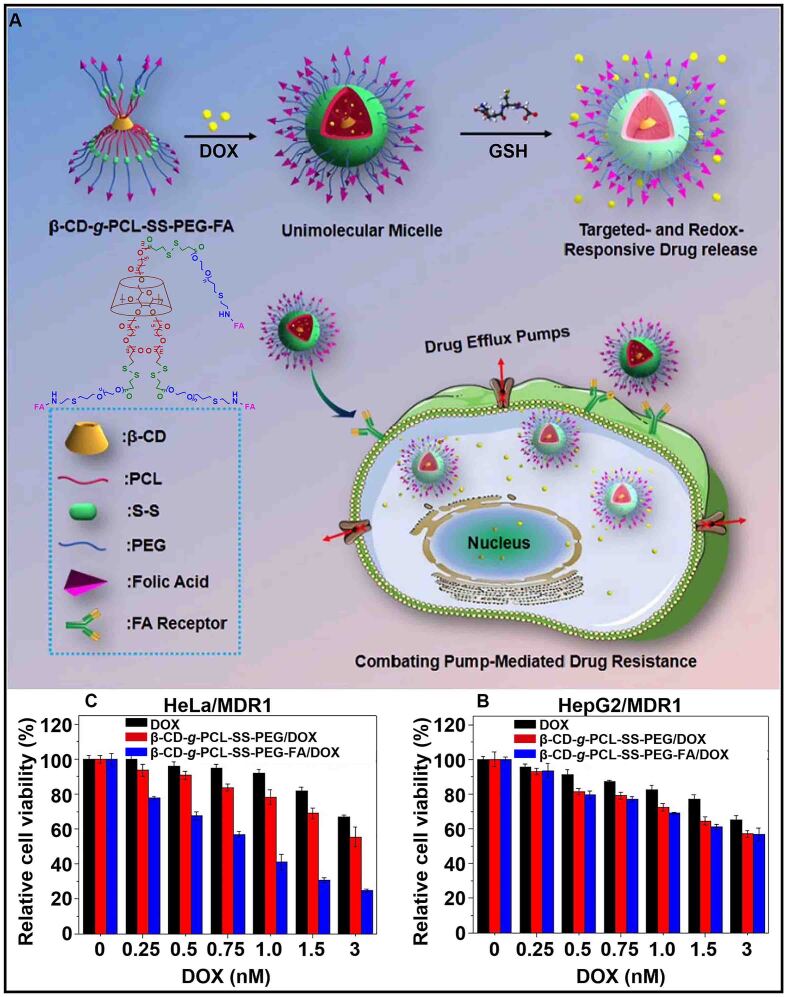
Moreover, Li et al. constructed a unimolecular micelle based on a star-shaped polymer (β-CD-g-PCL-SS-PEG-FA) that stably encapsulated DOX [Figure 6A][89]. The folic acid (FA) in the unimolecular micelle could target and penetrate tumor cells to increase the accumulation of DOX, inhibiting the cancer drug resistance caused by decreased drug uptake. The drug loaded in the unimolecular micelle could be released in response to GSH. MTT assay analysis indicated that β-CD-g-PCL-SS-PEG-FA had a better inhibitory effect on cell viability compared with free DOX [Figure 6B and C]. In addition, the overexpression of folate receptors on cervical cancer drug-resistant cells (HeLa/MDR1) accelerated the uptake of β-CD-g-PCL-SS-PEG-FA, enhancing the therapeutic effect of DOX on drug-resistant cells. Such β-CD-based stimuli-responsive supramolecular host-guest nanoparticles showed exciting results in overcoming cancer drug resistance due to the precise targeting, effective uptake, and controlled release of drugs.
Figure 6.
(A) Schematic illustration of a unimolecular micelle for inhibiting pump-mediated drug resistance; Cell viability of (B) HepG2/MDR1 cells and (C) HeLa/MDR1 cells treated with different doses of DOX, β-CD-g-PLC-SS-PEG/DOX, and β-CD-g-PLC-SS-PEG-FA/DOX. This figure is quoted with permission from Li et al.[89]. CD: Cyclodextrin; DOX: doxorubicin; FA: folic acid; GSH: glutathione; MDR1: multidrug resistance gene; PEG: poly (ethylene glycol).
CALIXARENES-BASED HOST-GUEST NANOSYSTEMS FOR OVERCOMING CANCER DRUG RESISTANCE
Calixarenes are a class of cyclic oligomers formed by methylene-bridging ortho-phenolic hydroxyl groups [Figure 1B][90-92]. Due to their molecular shapes similar to the Sangreal, they are named calix[n]arenes (C[n]As) by Gutsche[93]. By introducing hydrophilic and hydrophobic groups at the upper and lower rims of C[n]As, respectively, amphiphilic C[n]As can be designed, which are easy to self-assemble into vesicles, nanoparticles, or other aggregates[94-99]. Host-guest nanosystems based on C[n]As have low toxicity and good biocompatibility, becoming a new research hotspot in the field of cancer drug resistance[100-102].
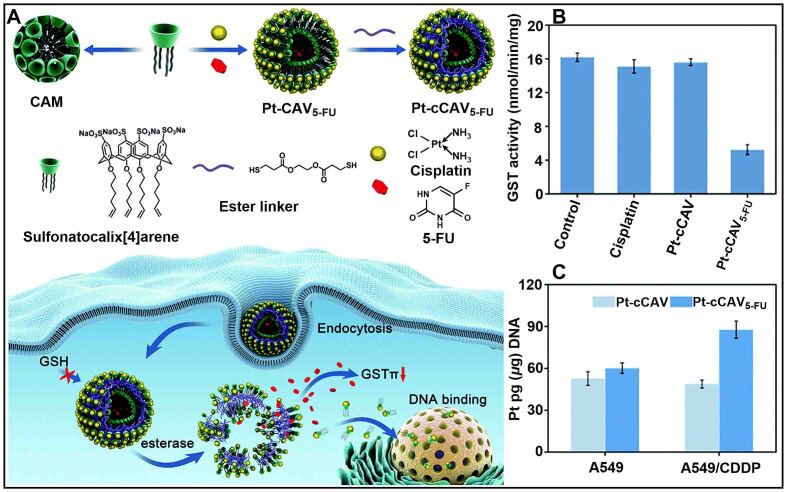
The close coordination between GSH and GST can initiate detoxification mechanisms within tumor cells, leading to the formation of drug resistance[103-105]. For example, GST can catalyze the binding of GSH to electrophilic antitumor drugs, accelerating the degradation of drugs[106-108]. Therefore, drug resistance can be reversed by regulating the GST. Recently, Dai et al. designed a nanomedicine (Pt-cCAV5-FU) based on sulfonatocalix[4]arene for overcoming GST-induced cancer drug resistance [Figure 7A][109]. The GST regulator (5-FU) was encased into the hydrophilic core of Pt-cCAV5-FU self-assembled from a host-guest complex of sulfonatocalix[4]arene with cisplatin. Pt-cCAV5-FU actively released cisplatin and 5-FU during the hydrolysis process caused by esterase. 5-FU could downregulate GST activity, prompting cisplatin to damage DNA rather than binding to GSH [Figure 7B]. In addition, the endocytosis of cisplatin resistance cells A549/CDDP against Pt-cCAV5-FU was stronger than that of A549, which increased the accumulation of cisplatin in cancer cells [Figure 7C]. All of these factors ultimately made Pt-cCAV5-FU more toxic to drug-resistance cells. This work developed a novel nanomedicine, laying the foundation for C[n]As-based host-guest nanosystems to reverse cisplatin resistance.
Figure 7.
(A) Schematic illustration of Pt-cCAV5-FU for overcoming cisplatin resistance in A549/CDDP cells; (B) The GST activity of A549/CDDP cells with different treatments; (C) Platinum content in the genomic DNA of A549 and A549/CDDP cells after incubation with Pt-cCAV and Pt-cCAV5-FU for 12 h. This figure is quoted with permission from Dai et al.[109]. GSH: Glutathione; GST: glutathione S-transferase.
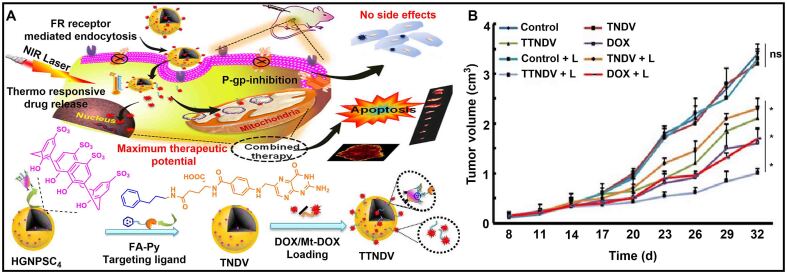
Furthermore, although free DOX can kill the nuclear DNA (n-DNA) of tumor cells, the undamaged mitochondrial DNA (Mt-DNA) can trigger drug resistance[110-112]. Therefore, the design of a drug to destroy synchronously n-DNA and Mt-DNA can promote the reversal of drug resistance[113]. Nair et al. constructed a gold nanotherapy platform (TTNDV) based on sulfonatocalix[4]arene [Figure 8A][114]. The nanoplatform could encapsulate DOX and mitochondrion-targeted analogue (Mt-DOX) in an optimal ratio of 1:100 to reverse cancer drug resistance caused by mitochondrial escape. In vitro and in vivo experiments showed that TTNDV had less toxic side effects than free DOX. In addition, TTNDV had a stimulating response to temperature. Under near-infrared irradiation, drugs embedded in TTNDV were released simultaneously to kill n-DNA and Mt-DNA, successfully overcoming DOX resistance and improving the chemotherapeutic effect of DOX [Figure 8B]. This work solved the problem of DOX resistance by killing Mt-DNA to induce apoptosis, providing a feasible strategy for reversing cancer drug resistance.
Figure 8.
(A) Schematic illustration of TTNDV to overcome cancer drug resistance by killing n-DNA and Mt-DNA; (B) Tumor growth inhibition curves of tumor-bearing mice after different formulations (*P < 0.05). This figure is quoted with permission from Nair et al.[114]. DOX: Doxorubicin; FA: folic acid.
CUCURBITURILS-BASED HOST-GUEST NANOSYSTEMS FOR OVERCOMING CANCER DRUG RESISTANCE
Cucurbiturils (CB[n]s) are a kind of macrocyclic hosts constructed by condensation of glycoluril with formaldehyde under acid conditions, which are the fourth macrocyclic hosts after crown ethers, cyclodextrins, and calixarenes [Figure 1C][115-117]. According to the different number of glycoluril units, different types of CB[n]s can be obtained, and common cucurbiturils include CB[6], CB[7], and CB[8][118,119]. Due to their unique structures of hydrophobic cavity and hydrophilic port, CB[n]s are easy to form host-guest complexes with drug molecules and are promising materials for reducing side effects and enhancing the stability of antitumor drugs[120-124]. In addition, CB[n]s-based supramolecular nanosystems can be used to effectively reverse cancer drug resistance[125-128].
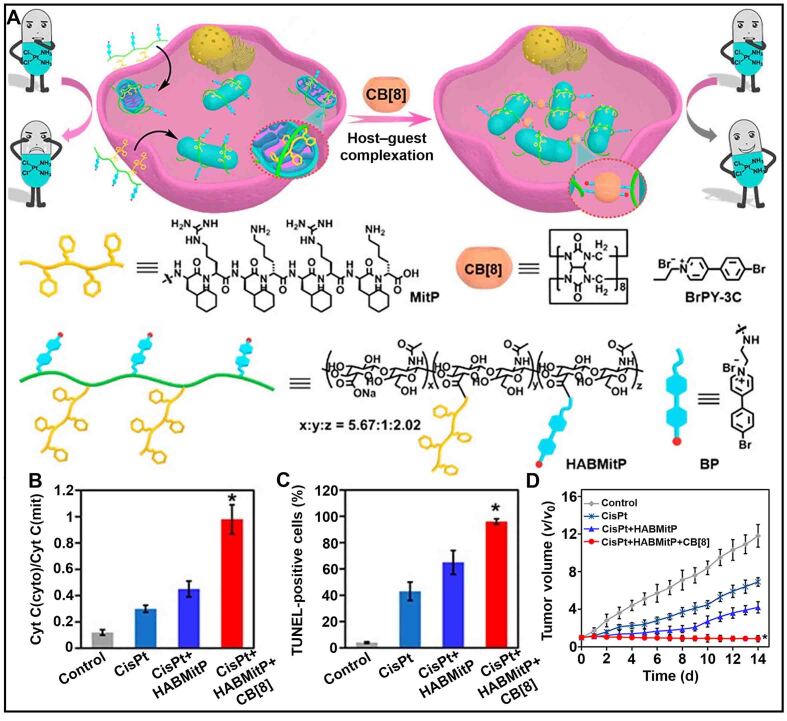
Cancer drug resistance is closely related to the inhibition of tumor apoptosis[129,130]. Mitochondria serves as the center for regulating tumor cell apoptosis[131,132]. Therefore, the destruction of mitochondria is also an effective way to overcome cancer drug resistance[133-135]. Recently, Dai et al. synthesized a multivalent supramolecular polymer (HABMitP) by modifying HA with mitochondrial targeting peptide and 4-bromophenylpyridium [Figure 9A][136]. The combination of HABMitP, cisplatin, and CB[8] could promote mitochondrial aggregation, which led to the deterioration of mitochondria to release apoptosis-inducing factor (cytochrome C), thereby activating the apoptosis of tumor cells [Figure 9B and C]. Moreover, cisplatin-resistant tumors did not grow treated with CisPt + HABMitP + CB[8] for 14 days, indicating that assembly-induced mitochondrial aggregation significantly improved the antitumor efficacy of cisplatin [Figure 9D]. This study showed that the regulation of mitochondrial behavior was beneficial to the reversal of drug resistance, which provided a broad prospect for overcoming tumor drug resistance.
Figure 9.
(A) Schematic illustration of mitochondrial aggregation progress after treatment with multivalent supramolecular polymer (HABMitP) and CB[8]; (B) The ratio of cytosol cytochrome C [Cyt C(cyto)] to mitochondrial cytochrome C [Cyt C(mit)] and (C) The apoptosis percentage of TUNEL-positive cells with different treatments; (D) Changes of tumor volume in tumor-bearing mice with different formulations (*P < 0.05). This figure is quoted with permission from Dai et al.[136].
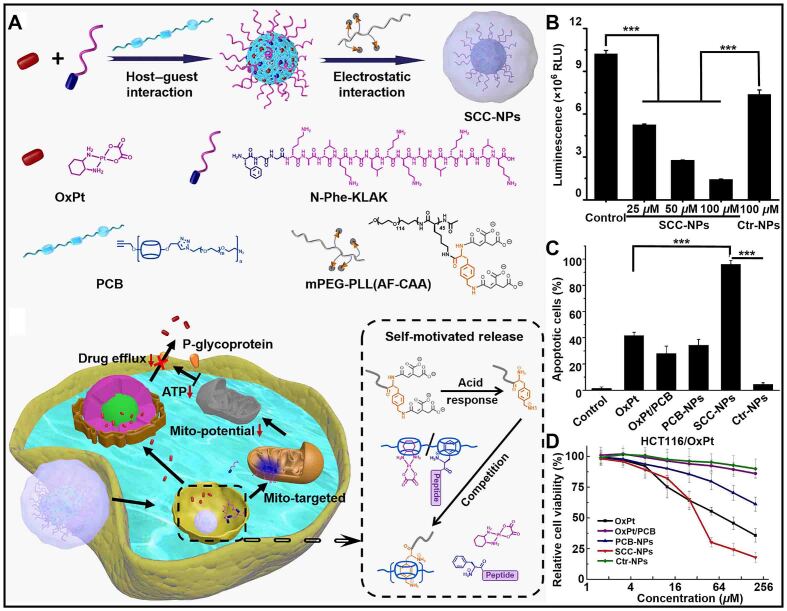
The inhibition of P-gp expression by reducing ATP concentration can reduce drug resistance in tumor cells[137,138]. Wang et al. reported nanoparticles (SCC-NPs) based on CB[7], which encapsulated the anticancer drug oxaliplatin (OxPt) and mitochondria-targeting peptide (N-Phe-KLAK) by the excellent host-guest properties of CB[7] [Figure 10A][139]. Due to the special acid responsiveness and competitiveness of the polymeric shell, SCC-NPs were used for self-motivated supramolecular combination chemotherapy. In acidic tumor environments, the amidomethyl phenylamine moieties on the polymeric shell were restored to form host-guest complexes with CB[7], competing to replace and release OxPt and N-Phe-KLAK. The released N-Phe-KLAK could effectively inhibit the production of ATP, resulting in the damage of energy-dependent drug efflux pump [Figure 10B]. Additionally, the accumulation of OxPt in cells directly led to an increase in the number of apoptotic cancer cells, which successfully inhibited the viability of drug-resistance cells [Figure 10C and D). Self-motivated supramolecular combination chemotherapy provided a new strategy for addressing the issue of cancer drug resistance.
Figure 10.
(A) Schematic illustration of the preparation and mechanism of self-motivated nanoparticles (SCC-NPs) in overcoming drug resistance; (B) ATP levels in HCT116/OxPt cells treated with Ctr NPs and SCC NPs at different doses; (C) The number of apoptotic cells treated with different formulations; (D) Cell viability of HCT116/OxPt cells after incubating with different treatments (***P < 0.001). This figure is quoted with permission from Wang et al.[139]. PEG: Poly (ethylene glycol).
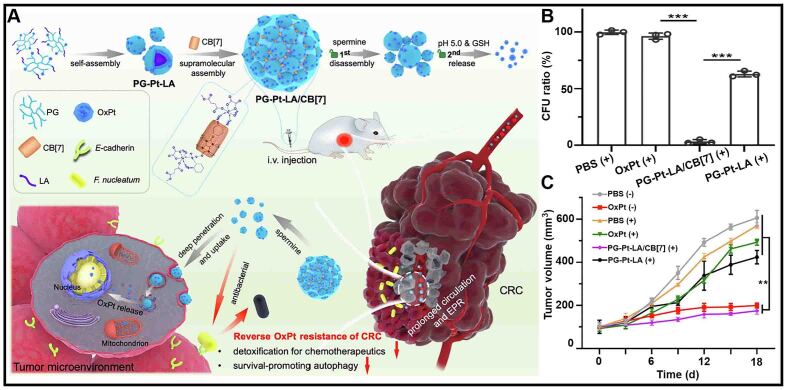
The Fusobacterium nucleatum (F. nucleatum) with apoptosis-inhibiting effect can trigger drug resistance in colorectal cancer (CRC) cells[140-143]. To address this issue, Yan et al. constructed a CB[7]-based nanomedicine (PG-Pt-LA/CB[7]) by multiple assemblies to overcome drug resistance [Figure 11A][144]. PG-Pt-LA/CB[7] targeted and penetrated cancer cells and released OxPt in response to the GSH. The efficient uptake and stable release of drugs increased the accumulation of OxPt in CRC cells. In addition, PG-Pt-LA/CB[7] showed the best inhibition effect on F. nucleatum compared to OxPt and PG-Pt-LA, successfully overcoming the drug resistance of CRC cells caused by F. nucleatum [Figure 11B]. A negligible growth in tumor volume was observed after 18 d of incubating tumors with PG-Pt-LA/CB[7], showing that PG-Pt-LA/CB[7] improved the chemotherapeutic effect of OxPt on CRC cells [Figure 11C]. PG-Pt-LA/CB[7] was expected to be a good material for improving the effect of OxPt on CRC cells.
Figure 11.
(A) Schematic illustration of the preparation and mechanism of CB[7]-based nanomedicine (PG-Pt-LA/CB[7]) in overcoming drug resistance of CRC cells; (B) Changes of F. nucleatum levels in CRC cells with different treatments; (C) Changes of tumor volume in tumor-bearing mice with different formulations (**P < 0.01, ***P < 0.001). This figure is quoted with permission from Yan et al.[144]. CRC: Colorectal cancer.
PILLARARENES-BASED HOST-GUEST NANOSYSTEMS FOR OVERCOMING CANCER DRUG RESISTANCE
Pillararenes (P[n]As) are a new type of macrocyclic hosts that bridge hydroquinone units through methylene discovered by Ogoshi et al. [Figure 1D][145]. These hydroquinone units are generally 5-10, with P[5]A and P[6]A being the most common[146-151]. P[n]As are widely used in various fields such as drug delivery, ion recognition, adsorptive separation, sensors, and optoelectronic materials due to the characteristics of symmetrical rigid skeleton, adjustable electron-rich cavity, and easy functionalization[152-154]. In addition, because of the highly attractive host-guest properties of P[n]As, more and more attention has been paid to the construction of P[n]As-based host-guest nanosystems to overcome cancer drug resistance[155-157]. Liu et al. prepared a novel carboxylatopillar[5]arene-based supramolecular quaternary ammonium nanoparticle to overcome the drug resistance generated during the chemotherapy of CRC[158]. Chang et al. constructed a redox-responsive cationic vesicle based on amphiphilic pillar[5]arene, successfully overcoming the drug resistance of tumors[159].
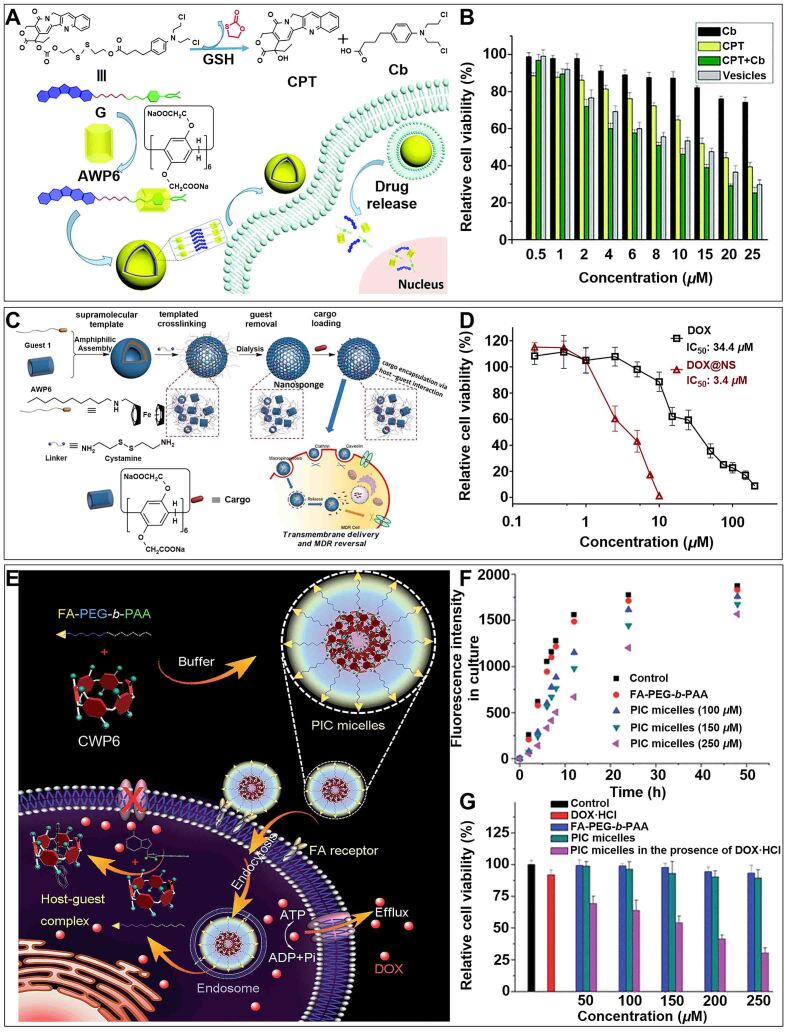
The water-soluble pillar[6]arene (WP6) not only forms stable host-guest complexes with a variety of guest molecules but also exhibits good biocompatibility and stimuli-responsiveness, which offers the possibility for constructing supramolecular host-guest nanoplatforms to reverse cancer drug resistance[160-166]. Shao et al. reported a host-guest complex (AWP6 G) containing anionic WP6 (AWP6) and prodrug (G), which further self-assembled to form nanovesicles for inhibiting cancer drug resistance [Figure 12A][167]. The nanovesicles released camptothecin (CPT) and chlorambucil (Cb) under the action of GSH to achieve combination chemotherapy. The dual-drug co-loaded nanovesicles showed a better inhibition effect on drug-resistance cells compared to the single drug [Figure 12B]. This study showed that P[n]As-based supramolecular host-guest nanosystems were expected to be ideal materials for inhibiting MDR.
G) containing anionic WP6 (AWP6) and prodrug (G), which further self-assembled to form nanovesicles for inhibiting cancer drug resistance [Figure 12A][167]. The nanovesicles released camptothecin (CPT) and chlorambucil (Cb) under the action of GSH to achieve combination chemotherapy. The dual-drug co-loaded nanovesicles showed a better inhibition effect on drug-resistance cells compared to the single drug [Figure 12B]. This study showed that P[n]As-based supramolecular host-guest nanosystems were expected to be ideal materials for inhibiting MDR.
Figure 12.
(A) Schematic illustration of the formation of nanovesicle and its internalization progress; (B) Cell viability of MCF-7 cells after incubation with Cb, CPT, Cb + CPT mixture, and vesicles for 24 h. This figure is quoted with permission from Shao et al.[167]; (C) Schematic illustration of water-solution pillar[6]arene nanosponges (NS) in overcoming MDR; (D) Cell viability of MCF-7/ADR cells after incubation with DOX and DOX@NS. This figure is quoted with permission from Liu et al.[168]; (E) Schematic illustration of the preparation of PIC micelles and their application in inhibiting drug efflux; (F) Changes of extracellular fluorescence intensity after incubating with FA-PEG-b-PAA and different concentrations of PIC micelles; (G) Cell viability of MCF-7/ADR cells after incubation with different treatments. This figure is quoted with permission from Yu et al.[170]. AWP6: Anionic WP6; CPT: camptothecin; DOX: doxorubicin; FA: folic acid; GSH: glutathione; MDR: multidrug resistance; NS: nanosponge; PEG: poly (ethylene glycol); PIC: polyion complex.
Subsequently, Liu et al. prepared a nanosponge (NS) based on AWP6 using a “bottom-up” template preparation technique [Figure 12C][168]. Through the host-guest interaction, antitumor drugs and dyes were stably encapsulated in AWP6 to overcome MDR. The IC50 of DOX@NS (3.4 μM) was significantly lower than that of free DOX (34.4 μM) when different doses of free DOX and DOX-loaded NS were incubated in drug-resistance cells [Figure 12D]. Mechanistic studies indicated that the effective loading and stable encapsulation of DOX based on host-guest interaction were the main reasons for overcoming MDR. This work showed that the delivery of anticancer drugs through host-guest interaction was a promising way to overcome MDR.
Additionally, cationic WP6 (CWP6) can encapsulate ATP, blocking the energy of drug efflux[169]. Yu et al. prepared a polyion complex (PIC) micelle by modifying CWP6 with functionalized diblock copolymer (FA-PEG-b-PAA) [Figure 12E][170]. PIC micelles could specifically target and penetrate cancer cells overexpressed with FA receptors. The decrease in extracellular fluorescence intensity indicated that CWP6 successfully blocked the energy source of calcein (model drug) efflux [Figure 12F]. In addition, PIC micelles significantly enhanced the inhibitory effect of DOX·HCl on cell viability compared with free DOX·HCl [Figure 12G]. These results suggested that the supramolecular nanomicelle endocytosed by drug-resistance cells and released CWP6 to selectively form a host-guest complex with ATP, which provided a new method for blocking the energy of drug efflux and was expected to become an ideal material for overcoming cancer drug resistance.
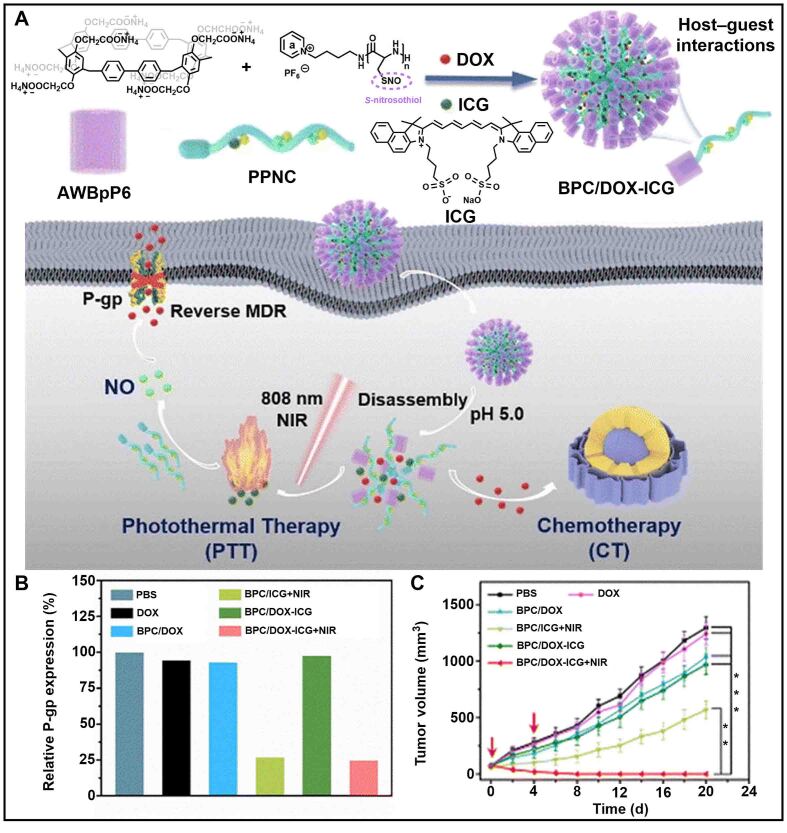
In addition to blocking the energy source of P-gp expression, nitric oxide (NO) can also downregulate the expression level of P-gp, reversing the drug resistance of cancer[171-173]. To achieve stable delivery and selective release of NO in tumor cells, Ding et al. designed supramolecular peptide nanomedicine (BPC/DOX-ICG) based on the host-guest complexation of anionic water-soluble [2]biphenyl-extended-pillar[6]arene (AWBpP6) with pyridinium-terminal-modified polypeptide (PPNC) [Figure 13A][174]. DOX and indocyanine green (ICG) loaded in BPC/DOX-ICG were used to simultaneously treat cancer cells with chemotherapy and photothermal therapy. S-nitrosothiol on PPNC released NO to downregulate the expression level of P-gp after near-infrared (NIR) irradiation. Western Blot analysis showed that the P-gp level in MCF-7/ADR cells was significantly reduced to 24.9% when treated with NIR irradiation and BPC/DOX-ICG [Figure 13B]. The reduced P-gp could greatly enhance the efficacy of chemotherapeutic drugs, inhibiting tumor growth [Figure 13C]. Therefore, P[n]As-based nanocarriers could effectively deliver NO to downregulate P-gp expression, providing a promising approach to eliminate cancer drug resistance.
Figure 13.
(A) Schematic illustration of synergistic PTT and CT using supramolecular polypeptide nanomedicine (BPC/DOX-ICG); (B) The P-gp expression levels in MCF-7/ADR cells with different treatments; (C) Changes of tumor volume in tumor-bearing mice with different formulations (**P < 0.01, ***P < 0.001). This figure is quoted with permission from Ding et al.[174]. DOX: Doxorubicin; ICG: indocyanine green; MDR: multidrug resistance; NIR: near-infrared; PPNC: pyridinium-terminal-modified polypeptide.
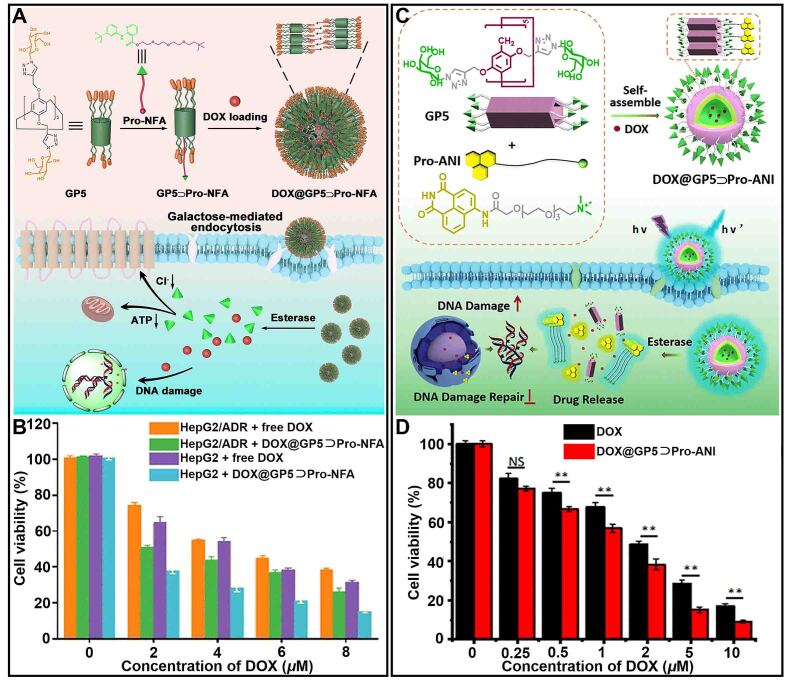
Chloride channel protein is highly expressed in various cancer cells and has a significant correlation with tumor drug resistance[175,176]. Yang et al. reported a supramolecular nanoprodrug (DOX@GP5 Pro-NFA) based on the host-guest complexation between galactose-modified pillar[5]arene (GP5) and chloride channel inhibitor prodrug (Pro-NFA) to reverse drug resistance [Figure 14A][177]. DOX@GP5
Pro-NFA) based on the host-guest complexation between galactose-modified pillar[5]arene (GP5) and chloride channel inhibitor prodrug (Pro-NFA) to reverse drug resistance [Figure 14A][177]. DOX@GP5 Pro-NFA was hydrolyzed under the action of esterase to release DOX and NFA, which could effectively block chloride ion channels, and reverse cancer drug resistance. Additionally, the inhibitory effect of DOX@GP5
Pro-NFA was hydrolyzed under the action of esterase to release DOX and NFA, which could effectively block chloride ion channels, and reverse cancer drug resistance. Additionally, the inhibitory effect of DOX@GP5 Pro-NFA on tumor cells, especially drug-resistance cells, was significantly higher than that of free DOX [Figure 14B]. Moreover, poly(ADP ribose)polymerase (PARP) can repair DNA to directly lead to drug resistance[178,179]. Yang et al. designed a nanoparticle (DOX@GP5
Pro-NFA on tumor cells, especially drug-resistance cells, was significantly higher than that of free DOX [Figure 14B]. Moreover, poly(ADP ribose)polymerase (PARP) can repair DNA to directly lead to drug resistance[178,179]. Yang et al. designed a nanoparticle (DOX@GP5 Pro-ANI) based on GP5 to load PARP inhibitor prodrug (Pro-ANI) that could inhibit DNA repair [Figure 14C][180]. DOX@GP5
Pro-ANI) based on GP5 to load PARP inhibitor prodrug (Pro-ANI) that could inhibit DNA repair [Figure 14C][180]. DOX@GP5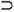 Pro-ANI overcame tumor drug resistance by inhibiting the expression of PARP, effectively reducing the viability of drug-resistance cells [Figure 14D]. These studies showed that P[n]As could effectively load anticancer prodrugs, which opened up broad prospects for inhibiting the expression of proteins associated with drug resistance.
Pro-ANI overcame tumor drug resistance by inhibiting the expression of PARP, effectively reducing the viability of drug-resistance cells [Figure 14D]. These studies showed that P[n]As could effectively load anticancer prodrugs, which opened up broad prospects for inhibiting the expression of proteins associated with drug resistance.
Figure 14.
(A) Schematic illustration of the preparation of supramolecular nanoprodrugsDOX@GP5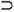 Pro-NFA and their applications in overcoming cancer drug resistance; (B) Cell viability of HepG2 cells and HepG2/ADR cells treated with free DOX and DOX@GP5
Pro-NFA and their applications in overcoming cancer drug resistance; (B) Cell viability of HepG2 cells and HepG2/ADR cells treated with free DOX and DOX@GP5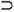 Pro-NFA, respectively. This figure is quoted with permission from Yang et al.[177]; (C) Schematic illustration of the preparation of supramolecular nanoprodrugs DOX@GP5
Pro-NFA, respectively. This figure is quoted with permission from Yang et al.[177]; (C) Schematic illustration of the preparation of supramolecular nanoprodrugs DOX@GP5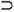 Pro-ANI and their applications in overcoming cancer drug resistance; (D) Cell viability of HepG2/ADR cells treated with free DOX and DOX@GP5
Pro-ANI and their applications in overcoming cancer drug resistance; (D) Cell viability of HepG2/ADR cells treated with free DOX and DOX@GP5 Pro-ANI (**P < 0.01). This figure is quoted with permission from Yang et al.[180]. DOX: Doxorubicin; GP5: galactose-modified pillar[5]arene.
Pro-ANI (**P < 0.01). This figure is quoted with permission from Yang et al.[180]. DOX: Doxorubicin; GP5: galactose-modified pillar[5]arene.
CONCLUSION
In summary, we reviewed the application of supramolecular host-guest nanosystems based on cyclodextrins, calixarenes, cucurbiturils, and pillararenes in overcoming cancer drug resistance. Compared with traditional small molecule drugs, nanosystems can effectively reduce the side effects of drugs and improve the accumulation of drugs in tumors. However, traditional nanosystems also have some drawbacks, such as lack of stimuli-responsiveness, difficulty in preparation and synthesis, and slow degradation in vivo. The emergence of supramolecular nanosystems complements the drawbacks of these traditional nanosystems. Due to their dynamic and reversible host-guest interactions, supramolecular nanosystems are endowed with rich stimuli-responsiveness to release drugs within tumors. Furthermore, supramolecular host-guest nanosystems have some advantages, such as high drug loading, low side effects, and good biocompatibility, which can co-deliver multiple drugs to inhibit cancer drug resistance by damaging mitochondrial function, blocking the energy source, inhibiting DNA repair, and reducing the level of GSH. However, supramolecular host-guest nanosystems still face some challenges in overcoming cancer drug resistance:
(i) Further improve the targeting. Tumor cells differ from healthy cells in many ways, such as acidity, hypoxia, and metabolism. Thus, more specific supramolecular host-guest nanosystems should be developed by focusing on the tumor microenvironment to target and penetrate tumor cells, reversing cancer drug resistance caused by drug uptake; (ii) Optimize drug loading strategy. Although multidrug-loaded supramolecular host-guest nanosystems can inhibit drug resistance, their effects are difficult to predict due to the different pharmacokinetics of each drug. Therefore, it is necessary to optimize the combination and dosage of loaded drugs and design supramolecular host-guest nanosystems with excellent performances; (iii) Improve stability. Supramolecular host-guest nanosystems have rich stimuli-responsiveness, but at the same time, there are problems of poor stability. In future studies, the stimuli-responsiveness of supramolecule and the stability of macromolecule can be better combined to prepare supramolecular polymeric nanosystems to overcome cancer drug resistance; (iv) Promote the development of cancer synergistic therapy. At present, most supramolecular host-guest nanosystems used to overcome drug resistance of tumors remain at the level of drug delivery, which greatly limits the inhibitory effect on drug-resistance cells. Introducing other therapeutic methods (such as photodynamic therapy, gene therapy, and immunotherapy) into supramolecular host-guest nanosystems will help establish more accurate and personalized strategies to combat cancer drug resistance; (v) Enhance the clinical translation. While some preliminary studies have shown that supramolecular host-guest nanosystems can be used to overcome drug resistance in cancer, there is still a long way to go before clinical translation. Firstly, the pharmacokinetics, biodistribution, metabolic behavior, and toxicological characteristics of supramolecular host-guest nanosystems are still in the research stage, and there are still unpredictable risks to their safety. Secondly, in vitro and in vivo experiments of supramolecular host-guest nanosystems cannot completely mimic the complex microenvironment of tumors in the body, resulting in lower clinical therapeutic effects than expected. Thirdly, the large-scale production of supramolecular host-guest nanosystems is a bottleneck in clinical applications, and small changes in the manufacturing process can cause significant changes in their physicochemical properties, which will affect their safety and biological effects. Therefore, more basic research and clinical trials are needed to assess their safety, efficacy, and feasibility.
The development of supramolecular host-guest nanosystems offers new hope to alleviate drug resistance in cancer, although innovation and progress are still required in many aspects. We believe that with continued research efforts, supramolecular host-guest nanosystems will make further progress in reversing cancer drug resistance, and bring new breakthroughs for cancer treatment and even human health.
DECLARATIONS
Authors’ contributions
Conceptualization: Wu S, Zhou J
Original draft preparation: Wu S
Review and editing: Zhou J, Yan M, Liang M, Yang W, Chen J
Supervision: Zhou J
Availability of data and materials
Not applicable.
Financial support and sponsorship
We thank the National Natural Science Foundation of China (22101043), the Fundamental Research Funds for the Central Universities (N2205013, N232410019), the Open Fund of Guangdong Provincial Key Laboratory of Functional Supramolecular Coordination Materials and Applications (2022A07), and Northeastern University for financial support.
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2023.
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Bukowski K, Kciuk M, Kontek R. Mechanisms of multidrug resistance in cancer chemotherapy. Int J Mol Sci. 2020;21:3233. doi: 10.3390/ijms21093233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ward RA, Fawell S, Floc’h N, Flemington V, McKerrecher D, Smith PD. Challenges and opportunities in cancer drug resistance. Chem Rev. 2021;121:3297–351. doi: 10.1021/acs.chemrev.0c00383. [DOI] [PubMed] [Google Scholar]
- 4.Vasan N, Baselga J, Hyman DM. A view on drug resistance in cancer. Nature. 2019;575:299–309. doi: 10.1038/s41586-019-1730-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tiek D, Cheng SY. DNA damage and metabolic mechanisms of cancer drug resistance. Cancer Drug Resist. 2022;5:368–79. doi: 10.20517/cdr.2021.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu G, Wilson G, George J, Liddle C, Hebbard L, Qiao L. Overcoming treatment resistance in cancer: current understanding and tactics. Cancer Lett. 2017;387:69–76. doi: 10.1016/j.canlet.2016.04.018. [DOI] [PubMed] [Google Scholar]
- 7.Aleksakhina SN, Kashyap A, Imyanitov EN. Mechanisms of acquired tumor drug resistance. Biochim Biophys Acta Rev Cancer. 2019;1872:188310. doi: 10.1016/j.bbcan.2019.188310. [DOI] [PubMed] [Google Scholar]
- 8.Robey RW, Pluchino KM, Hall MD, Fojo AT, Bates SE, Gottesman MM. Revisiting the role of ABC transporters in multidrug-resistant cancer. Nat Rev Cancer. 2018;18:452–64. doi: 10.1038/s41568-018-0005-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oxnard GR. The cellular origins of drug resistance in cancer. Nat Med. 2016;22:232–4. doi: 10.1038/nm.4058. [DOI] [PubMed] [Google Scholar]
- 10.Mullard A. Stemming the tide of drug resistance in cancer. Nat Rev Drug Discov. 2020;19:221–3. doi: 10.1038/d41573-020-00050-y. [DOI] [PubMed] [Google Scholar]
- 11.Liu J, Chen C, Wei T, et al. Dendrimeric nanosystem consistently circumvents heterogeneous drug response and resistance in pancreatic cancer. Exploration. 2021;1:21–34. doi: 10.1002/exp.20210003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mura S, Nicolas J, Couvreur P. Stimuli-responsive nanocarriers for drug delivery. Nat Mater. 2013;12:991–1003. doi: 10.1038/nmat3776. [DOI] [PubMed] [Google Scholar]
- 13.Su Z, Dong S, Zhao SC, et al. Novel nanomedicines to overcome cancer multidrug resistance. Drug Resist Updat. 2021;58:100777. doi: 10.1016/j.drup.2021.100777. [DOI] [PubMed] [Google Scholar]
- 14.Coombes R. Cancer drug resistance needs urgent attention, says research chief. BMJ. 2019;365:l1934. doi: 10.1136/bmj.l1934. [DOI] [PubMed] [Google Scholar]
- 15.Lehn JM. Supramolecular chemistry: Where from? Where to? Chem Soc Rev. 2017;46:2378–9. doi: 10.1039/c7cs00115k. [DOI] [PubMed] [Google Scholar]
- 16.Zhou J, Yu G, Li Q, Wang M, Huang F. Separation of benzene and cyclohexane by nonporous adaptive crystals of a hybrid[3]arene. J Am Chem Soc. 2020;142:2228–32. doi: 10.1021/jacs.9b13548. [DOI] [PubMed] [Google Scholar]
- 17.Tang R, Ye Y, Zhu S, Wang Y, Lu B, Yao Y. Pillar[6]arenes: from preparation, host-guest property to self-assembly and applications. Chinese Chem Lett. 2023;34:107734. doi: 10.1016/j.cclet.2022.08.014. [DOI] [Google Scholar]
- 18.Yang L, Tan X, Wang Z, Zhang X. Supramolecular polymers: historical development, preparation, characterization, and functions. Chem Rev. 2015;115:7196–239. doi: 10.1021/cr500633b. [DOI] [PubMed] [Google Scholar]
- 19.Yu G, Jie K, Huang F. Supramolecular amphiphiles based on host-guest molecular recognition motifs. Chem Rev. 2015;115:7240–303. doi: 10.1021/cr5005315. [DOI] [PubMed] [Google Scholar]
- 20.Zhang W, Yang W, Zhou J. Biphenarenes, versatile synthetic macrocycles for supramolecular chemistry. Molecules. 2023;28:4422. doi: 10.3390/molecules28114422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu XY, Gao J, Chen FY, Guo DS. A host-guest drug delivery nanosystem for supramolecular chemotherapy. J Control Release. 2020;324:124–33. doi: 10.1016/j.jconrel.2020.05.008. [DOI] [PubMed] [Google Scholar]
- 22.Wang H, Monroe M, Leslie F, Flexner C, Cui H. Supramolecular nanomedicines through rational design of self-assembling prodrugs. Trends Pharmacol Sci. 2022;43:510–21. doi: 10.1016/j.tips.2022.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang D, Yu C, Xu L, et al. Nucleoside analogue-based supramolecular nanodrugs driven by molecular recognition for synergistic cancer therapy. J Am Chem Soc. 2018;140:8797–806. doi: 10.1021/jacs.8b04556. [DOI] [PubMed] [Google Scholar]
- 24.Chang R, Zou Q, Zhao L, Liu Y, Xing R, Yan X. Amino-acid-encoded supramolecular photothermal nanomedicine for enhanced cancer therapy. Adv Mater. 2022;34:2200139. doi: 10.1002/adma.202200139. [DOI] [PubMed] [Google Scholar]
- 25.Yan M, Zhou J. Methylene-bridged naphthotubes: new macrocyclic arenes with great potential for supramolecular chemistry. Org Chem Front. 2023;10:2340–5. doi: 10.1039/d3qo00258f. [DOI] [Google Scholar]
- 26.Zhou J, Rao L, Yu G, Cook TR, Chen X, Huang F. Supramolecular cancer nanotheranostics. Chem Soc Rev. 2021;50:2839–91. doi: 10.1039/d0cs00011f. [DOI] [PubMed] [Google Scholar]
- 27.Li Z, Song N, Yang Y. Stimuli-responsive drug-delivery systems based on supramolecular nanovalves. Matter. 2019;1:345–68. doi: 10.1016/j.matt.2019.05.019. [DOI] [Google Scholar]
- 28.Wang L, Li L, Fan Y, Wang H. Host-guest supramolecular nanosystems for cancer diagnostics and therapeutics. Adv Mater. 2013;25:3888–98. doi: 10.1002/adma.201301202. [DOI] [PubMed] [Google Scholar]
- 29.Zhou J, Yu G, Li Y, et al. [2]Pseudorotaxane-based supramolecular optical indicator for the visual detection of cellular cyanide excretion. Chemistry. 2019;25:14447–53. doi: 10.1002/chem.201903577. [DOI] [PubMed] [Google Scholar]
- 30.Yang X, Wu B, Zhou J, et al. Controlling intracellular enzymatic self-assembly of peptide by host-guest complexation for programming cancer cell death. Nano Lett. 2022;22:7588–96. doi: 10.1021/acs.nanolett.2c02612. [DOI] [PubMed] [Google Scholar]
- 31.Onishi Y, Eshita Y, Ji RC, et al. Supermolecular drug challenge to overcome drug resistance in cancer cells. Drug Discov Today. 2018;23:1556–63. doi: 10.1016/j.drudis.2018.05.037. [DOI] [PubMed] [Google Scholar]
- 32.Xu S, Zhu X, Huang W, Zhou Y, Yan D. Supramolecular cisplatin-vorinostat nanodrug for overcoming drug resistance in cancer synergistic therapy. J Control Release. 2017;266:36–46. doi: 10.1016/j.jconrel.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 33.Wang Q, Xiao M, Wang D, et al. In situ supramolecular self-assembly of Pt(IV) prodrug to conquer cisplatin resistance. Adv Funct Mater. 2021;31:2101826. doi: 10.1002/adfm.202101826. [DOI] [Google Scholar]
- 34.Yang K, Qi S, Yu X, et al. A hybrid supramolecular polymeric nanomedicine for cascade-amplified synergetic cancer therapy. Angew Chem Int Ed Engl. 2022;61:e202203786. doi: 10.1002/anie.202203786. [DOI] [PubMed] [Google Scholar]
- 35.Yan M, Zhou J. Suprasomes: an emerging platform for cancer theranostics. Sci China Chem. 2023;66:613–4. doi: 10.1007/s11426-022-1477-x. [DOI] [Google Scholar]
- 36.Sun X, Zhao P, Lin J, Chen K, Shen J. Recent advances in access to overcome cancer drug resistance by nanocarrier drug delivery system. Cancer Drug Resist. 2023;6:390–415. doi: 10.20517/cdr.2023.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hu T, Gong H, Xu J, Huang Y, Wu F, He Z. Nanomedicines for overcoming cancer drug resistance. Pharmaceutics. 2022;14:1606. doi: 10.3390/pharmaceutics14081606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ikuta D, Hirata Y, Wakamori S, et al. Conformationally supple glucose monomers enable synthesis of the smallest cyclodextrins. Science. 2019;364:674–7. doi: 10.1126/science.aaw3053. [DOI] [PubMed] [Google Scholar]
- 39.Szejtli J. Introduction and general overview of cyclodextrin chemistry. Chem Rev. 1998;98:1743–54. doi: 10.1021/cr970022c. [DOI] [PubMed] [Google Scholar]
- 40.Valle E. Cyclodextrins and their uses: a review. Process Biochem. 2004;39:1033–46. doi: 10.1016/s0032-9592(03)00258-9. [DOI] [Google Scholar]
- 41.Utzeri G, Matias PMC, Murtinho D, Valente AJM. Cyclodextrin-based nanosponges: overview and opportunities. Front Chem. 2022;10:859406. doi: 10.3389/fchem.2022.859406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gandhi S, Shende P. Cyclodextrins-modified metallic nanoparticles for effective cancer therapy. J Control Release. 2021;339:41–50. doi: 10.1016/j.jconrel.2021.09.025. [DOI] [PubMed] [Google Scholar]
- 43.Wankar J, Kotla NG, Gera S, Rasala S, Pandit A, Rochev YA. Recent advances in host-guest self-assembled cyclodextrin carriers: implications for responsive drug delivery and biomedical engineering. Adv Funct Mater. 2020;30:1909049. doi: 10.1002/adfm.201909049. [DOI] [Google Scholar]
- 44.Wang M, Zhou J. Discovery of non-classical complex models between a cationic water-soluble pillar[6]arene and naphthalenesulfonate derivatives and their self-assembling behaviors. Soft Matter. 2019;15:4127–31. doi: 10.1039/c9sm00659a. [DOI] [PubMed] [Google Scholar]
- 45.Fang G, Yang X, Chen S, Wang Q, Zhang A, Tang B. Cyclodextrin-based host-guest supramolecular hydrogels for local drug delivery. Coord Chem Rev. 2022;454:214352. doi: 10.1016/j.ccr.2021.214352. [DOI] [Google Scholar]
- 46.Liu Z, Liu Y. Multicharged cyclodextrin supramolecular assemblies. Chem Soc Rev. 2022;51:4786–827. doi: 10.1039/d1cs00821h. [DOI] [PubMed] [Google Scholar]
- 47.Davis ME, Brewster ME. Cyclodextrin-based pharmaceutics: past, present and future. Nat Rev Drug Discov. 2004;3:1023–35. doi: 10.1038/nrd1576. [DOI] [PubMed] [Google Scholar]
- 48.Yuan Y, Nie T, Fang Y, You X, Huang H, Wu J. Stimuli-responsive cyclodextrin-based supramolecular assemblies as drug carriers. J Mater Chem B. 2022;10:2077–96. doi: 10.1039/d1tb02683f. [DOI] [PubMed] [Google Scholar]
- 49.Yang B, Dong X, Lei Q, Zhuo R, Feng J, Zhang X. Host-guest interaction-based self-engineering of nano-sized vesicles for co-delivery of genes and anticancer drugs. ACS Appl Mater Interfaces. 2015;7:22084–94. doi: 10.1021/acsami.5b07549. [DOI] [PubMed] [Google Scholar]
- 50.Wang H, Li S, Yang Y, Zhang L, Zhang Y, Wei T. Perspectives of metal-organic framework nanosystem to overcome tumor drug resistance. Cancer Drug Resist. 2022;5:954–70. doi: 10.20517/cdr.2022.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yu G, Zhao X, Zhou J, et al. Supramolecular polymer-based nanomedicine: high therapeutic performance and negligible long-term immunotoxicity. J Am Chem Soc. 2018;140:8005–19. doi: 10.1021/jacs.8b04400.s001. [DOI] [PubMed] [Google Scholar]
- 52.Yang C, Qin Y, Tu K, Xu C, Li Z, Zhang Z. Star-shaped polymer of β-cyclodextrin-g-vitamin E TPGS for doxorubicin delivery and multidrug resistance inhibition. Colloid Surface B. 2018;169:10–9. doi: 10.1016/j.colsurfb.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 53.Das M, Nariya P, Joshi A, et al. Carbon nanotube embedded cyclodextrin polymer derived injectable nanocarrier: a multiple faceted platform for stimulation of multi-drug resistance reversal. Carbohydr Polym. 2020;247:116751. doi: 10.1016/j.carbpol.2020.116751. [DOI] [PubMed] [Google Scholar]
- 54.Mirzaei S, Gholami MH, Hashemi F, et al. Advances in understanding the role of P-gp in doxorubicin resistance: molecular pathways, therapeutic strategies, and prospects. Drug Discov Today. 2022;27:436–55. doi: 10.1016/j.drudis.2021.09.020. [DOI] [PubMed] [Google Scholar]
- 55.Zhang H, Xu H, Ashby CR Jr, Assaraf YG, Chen ZS, Liu HM. Chemical molecular-based approach to overcome multidrug resistance in cancer by targeting P-glycoprotein (P-gp) Med Res Rev. 2021;41:525–55. doi: 10.1002/med.21739. [DOI] [PubMed] [Google Scholar]
- 56.Halder J, Pradhan D, Kar B, Ghosh G, Rath G. Nanotherapeutics approaches to overcome P-glycoprotein-mediated multi-drug resistance in cancer. Nanomedicine. 2022;40:102494. doi: 10.1016/j.nano.2021.102494. [DOI] [PubMed] [Google Scholar]
- 57.Gottesman MM, Pastan IH. The role of multidrug resistance efflux pumps in cancer: revisiting a JNCI publication exploring expression of the MDR1 (P-glycoprotein) gene. J Natl Cancer Inst. 2015;107:djv222. doi: 10.1093/jnci/djv222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang L, Li Y, Hu C, et al. CDK6-PI3K signaling axis is an efficient target for attenuating ABCB1/P-gp mediated multi-drug resistance (MDR) in cancer cells. Mol Cancer. 2022;21:103. doi: 10.1186/s12943-022-01524-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang Q, Zou C, Wang L, et al. Doxorubicin and adjudin co-loaded pH-sensitive nanoparticles for the treatment of drug-resistant cancer. Acta Biomater. 2019;94:469–81. doi: 10.1016/j.actbio.2019.05.061. [DOI] [PubMed] [Google Scholar]
- 60.de Almeida M, Susnik E, Drasler B, Taladriz-Blanco P, Petri-Fink A, Rothen-Rutishauser B. Understanding nanoparticle endocytosis to improve targeting strategies in nanomedicine. Chem Soc Rev. 2021;50:5397–434. doi: 10.1039/d0cs01127d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tang G, He J, Liu J, Yan X, Fan K. Nanozyme for tumor therapy: surface modification matters. Exploration. 2021;1:75–89. doi: 10.1002/exp.20210005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu J, Zhao L, Shi L, et al. A sequentially responsive nanosystem breaches cascaded bio-barriers and suppresses P-glycoprotein function for reversing cancer drug resistance. ACS Appl Mater Interfaces. 2020;12:54343–55. doi: 10.1021/acsami.0c13852. [DOI] [PubMed] [Google Scholar]
- 63.Brownell JE, Zhou J, Ranalli T, et al. Tetrahymena histone acetyltransferase A: a homolog to yeast Gcn5p linking histone acetylation to gene activation. Cell. 1996;84:843–51. doi: 10.1016/s0092-8674(00)81063-6. [DOI] [PubMed] [Google Scholar]
- 64.Sun NK, Kohli A, Huang SL, Chang TC, Chao CCK. Androgen receptor transcriptional activity and chromatin modifications on the ABCB1/MDR gene are critical for taxol resistance in ovarian cancer cells. J Cell Physiol. 2019;234:8760–75. doi: 10.1002/jcp.27535. [DOI] [PubMed] [Google Scholar]
- 65.Hu B, Zhong L, Weng Y, et al. Therapeutic siRNA: state of the art. Signal Transduct Target Ther. 2020;5:101. doi: 10.1038/s41392-020-0207-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Balwani M, Sardh E, Ventura P, et al. Phase 3 trial of RNAi therapeutic givosiran for acute intermittent porphyria. N Engl J Med. 2020;382:2289–301. doi: 10.1056/NEJMoa1913147. [DOI] [PubMed] [Google Scholar]
- 67.Adams D, Gonzalez-Duarte A, O’Riordan WD, et al. Patisiran, an RNAi therapeutic, for hereditary transthyretin amyloidosis. N Engl J Med. 2018;379:11–21. doi: 10.1056/nejmoa1716153. [DOI] [PubMed] [Google Scholar]
- 68.Yuan Y, Liu J, Yu X, et al. Tumor-targeting pH/redox dual-responsive nanosystem epigenetically reverses cancer drug resistance by co-delivering doxorubicin and GCN5 siRNA. Acta Biomater. 2021;135:556–66. doi: 10.1016/j.actbio.2021.09.002. [DOI] [PubMed] [Google Scholar]
- 69.Koltai T. The complex relationship between multiple drug resistance and the tumor pH gradient: a review. Cancer Drug Resist. 2022;5:277–303. doi: 10.20517/cdr.2021.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xing S, Hu K, Wang Y. Tumor immune microenvironment and immunotherapy in non-small cell lung cancer: update and new challenges. Aging Dis. 2022;13:1615–32. doi: 10.14336/ad.2022.0407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shi B, Jie K, Zhou Y, Zhou J, Xia D, Huang F. Nanoparticles with near-infrared emission enhanced by pillararene-based molecular recognition in water. J Am Chem Soc. 2016;138:80–3. doi: 10.1021/jacs.5b11676. [DOI] [PubMed] [Google Scholar]
- 72.Sa P, Sahoo SK, Dilnawaz F. Responsive role of nanomedicine in the tumor microenvironment and cancer drug resistance. Curr Med Chem. 2023;30:3335–55. doi: 10.2174/0929867329666220922111336. [DOI] [PubMed] [Google Scholar]
- 73.Wu Y, Li J, Zhong X, et al. A pH-sensitive supramolecular nanosystem with chlorin e6 and triptolide co-delivery for chemo-photodynamic combination therapy. Asian J Pharm Sci. 2022;17:206–18. doi: 10.1016/j.ajps.2021.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.He H, Chen S, Zhou J, et al. Cyclodextrin-derived pH-responsive nanoparticles for delivery of paclitaxel. Biomaterials. 2013;34:5344–58. doi: 10.1016/j.biomaterials.2013.03.068. [DOI] [PubMed] [Google Scholar]
- 75.Shi Q, Zhang L, Liu M, et al. Reversion of multidrug resistance by a pH-responsive cyclodextrin-derived nanomedicine in drug resistant cancer cells. Biomaterials. 2015;67:169–82. doi: 10.1016/j.biomaterials.2015.07.023. [DOI] [PubMed] [Google Scholar]
- 76.Zhou Z, Guo F, Wang N, Meng M, Li G. Dual pH-sensitive supramolecular micelles from star-shaped PDMAEMA based on β-cyclodextrin for drug release. Int J Biol Macromol. 2018;116:911–9. doi: 10.1016/j.ijbiomac.2018.05.092. [DOI] [PubMed] [Google Scholar]
- 77.Adeli F, Abbasi F, Babazadeh M, Davaran S. Thermo/pH dual-responsive micelles based on the host-guest interaction between benzimidazole-terminated graft copolymer and β-cyclodextrin-functionalized star block copolymer for smart drug delivery. J Nanobiotechnology. 2022;20:91. doi: 10.1186/s12951-022-01290-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yao X, Mu J, Zeng L, et al. Stimuli-responsive cyclodextrin-based nanoplatforms for cancer treatment and theranostics. Mater Horiz. 2019;6:846–70. doi: 10.1039/c9mh00166b. [DOI] [Google Scholar]
- 79.Ren JM, McKenzie TG, Fu Q, et al. Star Polymers. Chem Rev. 2016;116:6743–836. doi: 10.1021/acs.chemrev.6b00008. [DOI] [PubMed] [Google Scholar]
- 80.Song X, Zhang Z, Zhu J, et al. Thermoresponsive hydrogel induced by dual supramolecular assemblies and its controlled release property for enhanced anticancer drug delivery. Biomacromolecules. 2020;21:1516–27. doi: 10.1021/acs.biomac.0c00077.s001. [DOI] [PubMed] [Google Scholar]
- 81.Kost B, Brzeziński M, Cieślak M, et al. Stereocomplexed micelles based on polylactides with β-cyclodextrin core as anti-cancer drug carriers. Eur Polym J. 2019;120:109271. doi: 10.1016/j.eurpolymj.2019.109271. [DOI] [Google Scholar]
- 82.Zhou J, Yu G, Yang J, et al. Polymeric nanoparticles integrated from discrete organoplatinum(II) metallacycle by stepwise post-assembly polymerization for synergistic cancer therapy. Chem Mater. 2020;32:4564–73. doi: 10.1021/acs.chemmater.0c00615.s001. [DOI] [Google Scholar]
- 83.Zhang Y, Yang D, Chen H, et al. Reduction-sensitive fluorescence enhanced polymeric prodrug nanoparticles for combinational photothermal-chemotherapy. Biomaterials. 2018;163:14–24. doi: 10.1016/j.biomaterials.2018.02.023. [DOI] [PubMed] [Google Scholar]
- 84.Pawar CS, Rajendra Prasad N, Yadav P, et al. Enhanced delivery of quercetin and doxorubicin using β-cyclodextrin polymer to overcome P-glycoprotein mediated multidrug resistance. Int J Pharm. 2023;635:122763. doi: 10.1016/j.ijpharm.2023.122763. [DOI] [PubMed] [Google Scholar]
- 85.Zhang W, Yang W, Chen J, Wang Y, Yan M, Zhou J. An amphiphilic water-soluble biphen[3]arene with a tunable lower critical solution temperature behavior. New J Chem. 2022;46:21453–7. doi: 10.1039/d2nj03918d. [DOI] [Google Scholar]
- 86.Chen X, Qiu YK, Owh C, Loh XJ, Wu YL. Supramolecular cyclodextrin nanocarriers for chemo- and gene therapy towards the effective treatment of drug resistant cancers. Nanoscale. 2016;8:18876–81. doi: 10.1039/c6nr08055c. [DOI] [PubMed] [Google Scholar]
- 87.Cheng H, Fan X, Wang X, et al. Hierarchically self-assembled supramolecular host-guest delivery system for drug resistant cancer therapy. Biomacromolecules. 2018;19:1926–38. doi: 10.1021/acs.biomac.7b01693. [DOI] [PubMed] [Google Scholar]
- 88.Fan X, Cheng H, Wang X, et al. Thermoresponsive supramolecular chemotherapy by “V”-shaped armed β-cyclodextrin star polymer to overcome drug resistance. Adv Healthc Mater. 2018;7:1701143. doi: 10.1002/adhm.201701143. [DOI] [PubMed] [Google Scholar]
- 89.Li W, Xu C, Li S, et al. Cyclodextrin based unimolecular micelles with targeting and biocleavable abilities as chemotherapeutic carrier to overcome drug resistance. Mater Sci Eng C Mater Biol Appl. 2019;105:110047. doi: 10.1016/j.msec.2019.110047. [DOI] [PubMed] [Google Scholar]
- 90.Kumar R, Sharma A, Singh H, et al. Revisiting fluorescent calixarenes: from molecular sensors to smart materials. Chem Rev. 2019;119:9657–721. doi: 10.1021/acs.chemrev.8b00605. [DOI] [PubMed] [Google Scholar]
- 91.Feng HT, Li Y, Duan X, et al. Substitution activated precise phototheranostics through supramolecular assembly of AIEgen and calixarene. J Am Chem Soc. 2020;142:15966–74. doi: 10.1021/jacs.0c06872. [DOI] [PubMed] [Google Scholar]
- 92.Cao S, Zhang H, Zhao Y, Zhao Y. Pillararene/Calixarene-based systems for battery and supercapacitor applications. eScience. 2021;1:28–43. doi: 10.1016/j.esci.2021.10.001. [DOI] [Google Scholar]
- 93.Gutsche CD. Calixarenes. Acc Chem Res. 1983;16:161–70. doi: 10.1021/ar00089a003. [DOI] [Google Scholar]
- 94.Wang J, Ding X, Guo X. Assembly behaviors of calixarene-based amphiphile and supra-amphiphile and the applications in drug delivery and protein recognition. Adv Colloid Interface Sci. 2019;269:187–202. doi: 10.1016/j.cis.2019.04.004. [DOI] [PubMed] [Google Scholar]
- 95.Zhou J, Yang J, Zhang Z, Yu G. A cationic water-soluble biphen[3]arene: synthesis, host-guest complexation and fabrication of a supra-amphiphile. RSC Adv. 2016;6:77179–83. doi: 10.1039/c6ra18691b. [DOI] [Google Scholar]
- 96.Zhou J, Yu G, Huang F. Supramolecular chemotherapy based on host-guest molecular recognition: a novel strategy in the battle against cancer with a bright future. Chem Soc Rev. 2017;46:7021–53. doi: 10.1039/c6cs00898d. [DOI] [PubMed] [Google Scholar]
- 97.Chen C, Ni X, Tian HW, Liu Q, Guo DS, Ding D. Calixarene-based supramolecular AIE dots with highly inhibited nonradiative decay and intersystem crossing for ultrasensitive fluorescence image-guided cancer surgery. Angew Chem Int Ed Engl. 2020;59:10008–12. doi: 10.1002/anie.201916430. [DOI] [PubMed] [Google Scholar]
- 98.Zhang Z, Yue Y, Li Q, et al. Design of calixarene-based ICD inducer for efficient cancer immunotherapy. Adv Funct Mater. 2023;33:2213967. doi: 10.1002/adfm.202213967. [DOI] [Google Scholar]
- 99.Xu L, Chai J, Wang Y, et al. Calixarene-integrated nano-drug delivery system for tumor-targeted delivery and tracking of anti-cancer drugs in vivo. Nano Res. 2022;15:7295–303. doi: 10.1007/s12274-022-4332-4. [DOI] [Google Scholar]
- 100.Liu Q, Zhang TX, Zheng Y, et al. Calixarene-embedded nanoparticles for interference-free gene-drug combination cancer therapy. Small. 2021;17:2006223. doi: 10.1002/smll.202006223. [DOI] [PubMed] [Google Scholar]
- 101.Fan X, Guo X. Development of calixarene-based drug nanocarriers. J Mol Liq. 2021;325:115246. doi: 10.1016/j.molliq.2020.115246. [DOI] [Google Scholar]
- 102.Rahimi M, Karimian R, Noruzi EB, et al. Needle-shaped amphoteric calix[4]arene as a magnetic nanocarrier for simultaneous delivery of anticancer drugs to the breast cancer cells. Int J Nanomedicine. 2019;14:2619–36. doi: 10.2147/ijn.s194596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tew KD. Glutathione-associated enzymes in anticancer drug resistance. Cancer Res. 2016;76:7–9. doi: 10.1158/0008-5472.can-15-3143. [DOI] [PubMed] [Google Scholar]
- 104.Cheng X, Xu HD, Ran HH, Liang G, Wu FG. Glutathione-depleting nanomedicines for synergistic cancer therapy. ACS Nano. 2021;15:8039–68. doi: 10.1021/acsnano.1c00498. [DOI] [PubMed] [Google Scholar]
- 105.Xiao X, Wang K, Zong Q, Tu Y, Dong Y, Yuan Y. Polyprodrug with glutathione depletion and cascade drug activation for multi-drug resistance reversal. Biomaterials. 2021;270:120649. doi: 10.1016/j.biomaterials.2020.120649. [DOI] [PubMed] [Google Scholar]
- 106.Gorrini C, Mak TW. Glutathione metabolism: an achilles’ heel of ARID1A-deficient tumors. Cancer Cell. 2019;35:161–3. doi: 10.1016/j.ccell.2019.01.017. [DOI] [PubMed] [Google Scholar]
- 107.Xiong Y, Xiao C, Li Z, Yang X. Engineering nanomedicine for glutathione depletion-augmented cancer therapy. Chem Soc Rev. 2021;50:6013–41. doi: 10.1039/d0cs00718h. [DOI] [PubMed] [Google Scholar]
- 108.Ding Y, Dai Y, Wu M, Li L. Glutathione-mediated nanomedicines for cancer diagnosis and therapy. Chem Eng J. 2021;426:128880. doi: 10.1016/j.cej.2021.128880. [DOI] [Google Scholar]
- 109.Dai X, Zhou X, Liao C, Yao Y, Yu Y, Zhang S. A nanodrug to combat cisplatin-resistance by protecting cisplatin with p-sulfonatocalix[4]arene and regulating glutathione S-transferases with loaded 5-fluorouracil. Chem Commun. 2019;55:7199–202. doi: 10.1039/c9cc03012c. [DOI] [PubMed] [Google Scholar]
- 110.Jin P, Jiang J, Zhou L, et al. Mitochondrial adaptation in cancer drug resistance: prevalence, mechanisms, and management. J Hematol Oncol. 2022;15:97. doi: 10.1186/s13045-022-01313-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yu G, Wu D, Li Y, et al. A pillar[5]arene-based [2]rotaxane lights up mitochondria. Chem Sci. 2016;7:3017–24. doi: 10.1039/c6sc00036c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sansone P, Savini C, Kurelac I, et al. Packaging and transfer of mitochondrial DNA via exosomes regulate escape from dormancy in hormonal therapy-resistant breast cancer. Proc Natl Acad Sci U S A. 2017;114:E9066–75. doi: 10.1073/pnas.1704862114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Luo X, Gong X, Su L, et al. Activatable mitochondria-targeting organoarsenic prodrugs for bioenergetic cancer therapy. Angew Chem Int Ed Engl. 2021;60:1403–10. doi: 10.1002/anie.202012237. [DOI] [PubMed] [Google Scholar]
- 114.Nair JB, Joseph MM, Arya JS, Sreedevi P, Sujai PT, Maiti KK. Elucidating a thermoresponsive multimodal photo-chemotherapeutic nanodelivery vehicle to overcome the barriers of doxorubicin therapy. ACS Appl Mater Interfaces. 2020;12:43365–79. doi: 10.1021/acsami.0c08762. [DOI] [PubMed] [Google Scholar]
- 115.Mukhopadhyay RD, Kim K. Cucurbituril curiosities. Nat Chem. 2023;15:438. doi: 10.1038/s41557-023-01141-0. [DOI] [PubMed] [Google Scholar]
- 116.Hu J, Huang Y, Redshaw C, Tao Z, Xiao X. Cucurbit[n]uril-based supramolecular hydrogels: synthesis, properties and applications. Coord Chem Rev. 2023;489:215194. doi: 10.1016/j.ccr.2023.215194. [DOI] [Google Scholar]
- 117.Ghosh SK, Dhamija A, Ko YH, et al. Superacid-mediated functionalization of hydroxylated cucurbit[n]urils. J Am Chem Soc. 2019;141:17503–6. doi: 10.1021/jacs.9b09639. [DOI] [PubMed] [Google Scholar]
- 118.Fahmy SA, Nematallah KA, Mahdy NK, El-Askary HI, Meselhy MR, El-Said Azzazy HM. Enhanced antioxidant, antiviral, and anticancer activities of the extract of fermented egyptian rice bran complexed with hydroxypropyl-β-cyclodextrin. ACS Omega. 2022;7:19545–54. doi: 10.1021/acsomega.2c01281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wu D, Li Y, Yang J, et al. Supramolecular nanomedicine constructed from cucurbit[8]uril-based amphiphilic brush copolymer for cancer therapy. ACS Appl Mater Interfaces. 2017;9:44392–401. doi: 10.1021/acsami.7b16734. [DOI] [PubMed] [Google Scholar]
- 120.Wang Z, Sun C, Yang K, Chen X, Wang R. Cucurbituril-based supramolecular polymers for biomedical applications. Angew Chem Int Ed Engl. 2022;61:e202206763. doi: 10.1002/anie.202206763. [DOI] [PubMed] [Google Scholar]
- 121.You Y, Zhou K, Guo B, et al. Measuring binding constants of cucurbituril-based host-guest interactions at the single-molecule level with nanopores. ACS Sens. 2019;4:774–9. doi: 10.1021/acssensors.9b00408. [DOI] [PubMed] [Google Scholar]
- 122.Shukla S, Sagar B, Sood AK, Gaur A, Batra S, Gulati S. Supramolecular chemotherapy with cucurbit[n]urils as encapsulating hosts. ACS Appl Bio Mater. 2023;6:2089–101. doi: 10.1021/acsabm.3c00244. [DOI] [PubMed] [Google Scholar]
- 123.Redondo-Gómez C, Padilla-Lopátegui S, Mata A, Azevedo HS. Peptide amphiphile hydrogels based on homoternary cucurbit[8]uril host-guest complexes. Bioconjug Chem. 2022;33:111–20. doi: 10.1021/acs.bioconjchem.1c00441. [DOI] [PubMed] [Google Scholar]
- 124.Barooah N, Mohanty J, Bhasikuttan AC. Cucurbituril-based supramolecular assemblies: prospective on drug delivery, sensing, separation, and catalytic applications. Langmuir. 2022;38:6249–64. doi: 10.1021/acs.langmuir.2c00556. [DOI] [PubMed] [Google Scholar]
- 125.Liu Z, Lin W, Liu Y. Macrocyclic supramolecular assemblies based on hyaluronic acid and their biological applications. Acc Chem Res. 2022;55:3417–29. doi: 10.1021/acs.accounts.2c00462. [DOI] [PubMed] [Google Scholar]
- 126.Li Q, Sun J, Zhou J, Hua B, Shao L, Huang F. Barium cation-responsive supra-amphiphile constructed by a new twisted cucurbit[15]uril/paraquat recognition motif in water. Org Chem Front. 2018;5:1940–4. doi: 10.1039/c8qo00323h. [DOI] [Google Scholar]
- 127.Alabrahim OAA, Fahmy SA, Azzazy HME. Stimuli-responsive cucurbit[n]uril-based supramolecular nanocarriers for delivery of chemotherapeutics. ACS Appl Nano Mater. 2023;6:3139–58. doi: 10.1021/acsanm.2c05391. [DOI] [Google Scholar]
- 128.Yu Q, Deng T, Lin FC, Zhang B, Zink JI. Supramolecular assemblies of heterogeneous mesoporous silica nanoparticles to co-deliver antimicrobial peptides and antibiotics for synergistic eradication of pathogenic biofilms. ACS Nano. 2020;14:5926–37. doi: 10.1021/acsnano.0c01336. [DOI] [PubMed] [Google Scholar]
- 129.Safa AR. Drug and apoptosis resistance in cancer stem cells: a puzzle with many pieces. Cancer Drug Resist. 2022;5:850–72. doi: 10.20517/cdr.2022.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Neophytou CM, Trougakos IP, Erin N, Papageorgis P. Apoptosis deregulation and the development of cancer multi-drug resistance. Cancers. 2021;13:4363. doi: 10.3390/cancers13174363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sun M, He L, Fan Z, Tang R, Du J. Effective treatment of drug-resistant lung cancer via a nanogel capable of reactivating cisplatin and enhancing early apoptosis. Biomaterials. 2020;257:120252. doi: 10.1016/j.biomaterials.2020.120252. [DOI] [PubMed] [Google Scholar]
- 132.Janzen DM, Tiourin E, Salehi JA, et al. Retraction note: an apoptosis-enhancing drug overcomes platinum resistance in a tumour-initiating subpopulation of ovarian cancer. Nat Commun. 2020;11:2218. doi: 10.1038/s41467-020-15721-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Rainho MA, Siqueira PB, de Amorim ÍSS, Mencalha AL, Thole AA. Mitochondria in colorectal cancer stem cells - a target in drug resistance. Cancer Drug Resist. 2023;6:273–83. doi: 10.20517/cdr.2022.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chen W, Shi K, Chu B, Wei X, Qian Z. Mitochondrial surface engineering for multidrug resistance reversal. Nano Lett. 2019;19:2905–13. doi: 10.1021/acs.nanolett.8b05188. [DOI] [PubMed] [Google Scholar]
- 135.Zhou J, Hua B, Shao L, Feng H, Yu G. Host-guest interaction enhanced aggregation-induced emission and its application in cell imaging. Chem Commun. 2016;52:5749–52. doi: 10.1039/c6cc01860b. [DOI] [PubMed] [Google Scholar]
- 136.Dai XY, Zhang B, Yu Q, Liu Y. In situ coassembly induced mitochondrial aggregation activated drug-resistant tumor treatment. J Med Chem. 2022;65:7363–70. doi: 10.1021/acs.jmedchem.2c00372. [DOI] [PubMed] [Google Scholar]
- 137.Kroll T, Prescher M, Smits SHJ, Schmitt L. Structure and function of hepatobiliary ATP binding cassette transporters. Chem Rev. 2021;121:5240–88. doi: 10.1021/acs.chemrev.0c00659. [DOI] [PubMed] [Google Scholar]
- 138.Goebel J, Chmielewski J, Hrycyna CA. The roles of the human ATP-binding cassette transporters P-glycoprotein and ABCG2 in multidrug resistance in cancer and at endogenous sites: future opportunities for structure-based drug design of inhibitors. Cancer Drug Resist. 2021;4:784–804. doi: 10.20517/cdr.2021.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wang H, Wu H, Yi Y, et al. Self-motivated supramolecular combination chemotherapy for overcoming drug resistance based on acid-activated competition of host-guest interactions. CCS Chem. 2021;3:1413–25. doi: 10.31635/ccschem.021.202100964. [DOI] [Google Scholar]
- 140.Zhao T, Wang X, Fu L, Yang K. Fusobacterium nucleatum: a new player in regulation of cancer development and therapeutic response. Cancer Drug Resist. 2022;5:436–50. doi: 10.20517/cdr.2021.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Brennan CA, Garrett WS. Fusobacterium nucleatum - symbiont, opportunist and oncobacterium. Nat Rev Microbiol. 2019;17:156–66. doi: 10.1038/s41579-018-0129-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wang N, Fang JY. Fusobacterium nucleatum, a key pathogenic factor and microbial biomarker for colorectal cancer. Trends Microbiol. 2023;31:159–72. doi: 10.1016/j.tim.2022.08.010. [DOI] [PubMed] [Google Scholar]
- 143.Gmeiner WH, Okechukwu CC. Review of 5-FU resistance mechanisms in colorectal cancer: clinical significance of attenuated on-target effects. Cancer Drug Resist. 2023;6:257–72. doi: 10.20517/cdr.2022.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Yan X, Ma F, Chen Q, et al. Construction of size-transformable supramolecular nano-platform against drug-resistant colorectal cancer caused by Fusobacterium nucleatum. Chem Eng J. 2022;450:137605. doi: 10.1016/j.cej.2022.137605. [DOI] [Google Scholar]
- 145.Ogoshi T, Kanai S, Fujinami S, Yamagishi TA, Nakamoto Y. para-Bridged symmetrical pillar[5]arenes: their Lewis acid catalyzed synthesis and host-guest property. J Am Chem Soc. 2008;130:5022–3. doi: 10.1021/ja711260m. [DOI] [PubMed] [Google Scholar]
- 146.Sun J, Hua B, Li Q, Zhou J, Yang J. Acid/base-controllable FRET and self-assembling systems fabricated by rhodamine B functionalized pillar[5]arene-based host-guest recognition motifs. Org Lett. 2018;20:365–8. doi: 10.1021/acs.orglett.7b03612. [DOI] [PubMed] [Google Scholar]
- 147.Yan M, Zhou J. Pillararene-based supramolecular polymers for cancer therapy. Molecules. 2023;28:1470. doi: 10.3390/molecules28031470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Song N, Kakuta T, Yamagishi T, Yang Y, Ogoshi T. Molecular-scale porous materials based on pillar[n]arenes. Chem. 2018;4:2029–53. doi: 10.1016/j.chempr.2018.05.015. [DOI] [Google Scholar]
- 149.Wang J, Wang D, Cen M, et al. GOx-assisted synthesis of pillar[5]arene based supramolecular polymeric nanoparticles for targeted/synergistic chemo-chemodynamic cancer therapy. J Nanobiotechnology. 2022;20:33. doi: 10.1186/s12951-021-01237-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wang C, Li H, Dong J, et al. Pillararene-based supramolecular vesicles for stimuli-responsive drug delivery. Chemistry. 2022;28:e202202050. doi: 10.1002/chem.202202050. [DOI] [PubMed] [Google Scholar]
- 151.Zhu W, Li E, Zhou J, Zhou Y, Sheng X, Huang F. Highly selective removal of heterocyclic impurities from toluene by nonporous adaptive crystals of perethylated pillar[6]arene. Mater Chem Front. 2020;4:2325–9. doi: 10.1039/d0qm00334d. [DOI] [Google Scholar]
- 152.Wang M, Fang S, Yang S, et al. Separation of ethyltoluene isomers by nonporous adaptive crystals of perethylated and perbromoethylated pillararenes. Mater Today Chem. 2022;24:100919. doi: 10.1016/j.mtchem.2022.100919. [DOI] [Google Scholar]
- 153.Yang W, Zhang W, Chen J, Zhou J. Mono-functionalized pillar[n]arenes: syntheses, host-guest properties and applications. Chinese Chem Lett. 2024;35:108740. doi: 10.1016/j.cclet.2023.108740. [DOI] [Google Scholar]
- 154.Wang Y, Wang D, Wang J, et al. Pillar[5]arene-derived covalent organic materials with pre-encoded molecular recognition for targeted and synergistic cancer photo- and chemotherapy. Chem Commun. 2022;58:1689–92. doi: 10.1039/d1cc07072j. [DOI] [PubMed] [Google Scholar]
- 155.Zhou J, Yu G, Shao L, Hua B, Huang F. A water-soluble biphen[3]arene: synthesis, host-guest complexation, and application in controllable self-assembly and controlled release. Chem Commun. 2015;51:4188–91. doi: 10.1039/c5cc00225g. [DOI] [PubMed] [Google Scholar]
- 156.Zhou L, Cao S, Liu C, Zhang H, Zhao Y. Pillar[n]arene-based polymeric systems for biomedical applications. Coord Chem Rev. 2023;491:215260. doi: 10.1016/j.ccr.2023.215260. [DOI] [Google Scholar]
- 157.Li Q, Li X, Ning L, Tan CH, Mu Y, Wang R. Hyperfast water transport through biomimetic nanochannels from peptide-attached (pR)-pillar[5]arene. Small. 2019;15:1804678. doi: 10.1002/smll.201804678. [DOI] [PubMed] [Google Scholar]
- 158.Liu H, Yang J, Yan X, et al. A dendritic polyamidoamine supramolecular system composed of pillar[5]arene and azobenzene for targeting drug-resistant colon cancer. J Mater Chem B. 2021;9:9594–605. doi: 10.1039/d1tb02134f. [DOI] [PubMed] [Google Scholar]
- 159.Chang Y, Yang K, Wei P, et al. Cationic vesicles based on amphiphilic pillar[5]arene capped with ferrocenium: a redox-responsive system for drug/siRNA co-delivery. Angew Chem Int Ed Engl. 2014;53:13126–30. doi: 10.1002/anie.201407272. [DOI] [PubMed] [Google Scholar]
- 160.Wang M, Zhou J, Li E, Zhou Y, Li Q, Huang F. Separation of monochlorotoluene isomers by nonporous adaptive crystals of perethylated pillar[5]arene and pillar[6]arene. J Am Chem Soc. 2019;141:17102–6. doi: 10.1021/jacs.9b09988. [DOI] [PubMed] [Google Scholar]
- 161.Wang Y, Pei Z, Feng W, Pei Y. Stimuli-responsive supramolecular nano-systems based on pillar[n]arenes and their related applications. J Mater Chem B. 2019;7:7656–75. doi: 10.1039/c9tb01913h. [DOI] [PubMed] [Google Scholar]
- 162.Liu X, Jia K, Wang Y, et al. Dual-responsive bola-type supra-amphiphile constructed from water-soluble pillar[5]arene and naphthalimide-containing amphiphile for intracellular drug delivery. ACS Appl Mater Interfaces. 2017;9:4843–50. doi: 10.1021/acsami.7b00643.s001. [DOI] [PubMed] [Google Scholar]
- 163.Wang M, Li Q, Li E, Liu J, Zhou J, Huang F. Vapochromic behaviors of a solid-state supramolecular polymer based on exo-wall complexation of perethylated pillar[5]arene with 1,2,4,5-tetracyanobenzene. Angew Chem Int Ed Engl. 2021;133:8196–201. doi: 10.1002/anie.202013701. [DOI] [PubMed] [Google Scholar]
- 164.Yang K, Wen J, Chao S, et al. A supramolecular photosensitizer system based on the host-guest complexation between water-soluble pillar[6]arene and methylene blue for durable photodynamic therapy. Chem Commun. 2018;54:5911–4. doi: 10.1039/c8cc02739k. [DOI] [PubMed] [Google Scholar]
- 165.Bai Y, Liu C, Yang J, Liu C, Shang Q, Tian W. Supramolecular self-assemblies based on water-soluble pillar[6]arene and drug-drug conjugates for the combination of chemotherapy. Colloid Surface B. 2022;217:112606. doi: 10.1016/j.colsurfb.2022.112606. [DOI] [PubMed] [Google Scholar]
- 166.Yu G, Zhou J, Chi X. Pillar[10]arene-based size-selective host-guest complexation and its application in tuning the LCST behavior of a thermoresponsive polymer. Macromol Rapid Commun. 2015;36:23–30. doi: 10.1002/marc.201400570. [DOI] [PubMed] [Google Scholar]
- 167.Shao W, Liu X, Sun G, Hu XY, Zhu JJ, Wang L. Construction of drug-drug conjugate supramolecular nanocarriers based on water-soluble pillar[6]arene for combination chemotherapy. Chem Commun. 2018;54:9462–5. doi: 10.1039/c8cc05180a. [DOI] [PubMed] [Google Scholar]
- 168.Liu Y, Liao Y, Li P, Li ZT, Ma D. Cross-linked pillar[6]arene nanosponges fabricated by the use of a supra-amphiphilic template: cargo encapsulation and overcoming multidrug resistance. ACS Appl Mater Interfaces. 2020;12:7974–83. doi: 10.1021/acsami.9b22066. [DOI] [PubMed] [Google Scholar]
- 169.Zhou J, Chen M, Diao G. Synthesis of the first amphiphilic pillar[6]arene and its enzyme-responsive self-assembly in water. Chem Commun. 2014;50:11954–6. doi: 10.1039/c4cc05621c. [DOI] [PubMed] [Google Scholar]
- 170.Yu G, Zhou J, Shen J, Tang G, Huang F. Cationic pillar[6]arene/ATP host-guest recognition: selectivity, inhibition of ATP hydrolysis, and application in multidrug resistance treatment. Chem Sci. 2016;7:4073–8. doi: 10.1039/c6sc00531d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Yao X, Yang B, Xu J, He Q, Yang W. Novel gas-based nanomedicines for cancer therapy. VIEW. 2022;3:20200185. doi: 10.1002/viw.20200185. [DOI] [Google Scholar]
- 172.Deng Y, Jia F, Chen X, Jin Q, Ji J. ATP suppression by pH-activated mitochondria-targeted delivery of nitric oxide nanoplatform for drug resistance reversal and metastasis inhibition. Small. 2020;16:2001747. doi: 10.1002/smll.202001747. [DOI] [PubMed] [Google Scholar]
- 173.Zhou J, Yang J, Hua B, Shao L, Zhang Z, Yu G. The synthesis, structure, and molecular recognition properties of a [2]calix[1]biphenyl-type hybrid[3]arene. Chem Commun. 2016;52:1622–4. doi: 10.1039/c5cc09088a. [DOI] [PubMed] [Google Scholar]
- 174.Ding Y, Ma Y, Zhu L, et al. Nitric oxide-containing supramolecular polypeptide nanomedicine based on [2]biphenyl-extended-pillar[6]arenes for drug resistance reversal. J Mater Chem B. 2022;10:6181–6. doi: 10.1039/d2tb01127a. [DOI] [PubMed] [Google Scholar]
- 175.Valverde MA, Díaz M, Sepúlveda FV, Gill DR, Hyde SC, Higgins CF. Volume-regulated chloride channels associated with the human multidrug-resistance P-glycoprotein. Nature. 1992;355:830–3. doi: 10.1038/355830a0. [DOI] [PubMed] [Google Scholar]
- 176.Gill DR, Hyde SC, Higgins CF, Valverde MA, Mintenig GM, Sepúlveda FV. Separation of drug transport and chloride channel functions of the human multidrug resistance P-glycoprotein. Cell. 1992;71:23–32. doi: 10.1016/0092-8674(92)90263-c. [DOI] [PubMed] [Google Scholar]
- 177.Yang K, Ma K, Yang M, Lv Y, Pei Y, Pei Z. Supramolecular nanoprodrug based on a chloride channel blocker and glycosylated pillar[5]arenes for targeted chemoresistance cancer therapy. Chem Commun. 2023;59:3779–82. doi: 10.1039/d3cc00233k. [DOI] [PubMed] [Google Scholar]
- 178.Waardenburg RCAM, Yang ES. Targeting DNA repair pathways to overcome cancer drug resistance. Cancer Drug Resist. 2021;4:837–41. doi: 10.20517/cdr.2021.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Nickoloff JA, Taylor L, Sharma N, Kato TA. Exploiting DNA repair pathways for tumor sensitization, mitigation of resistance, and normal tissue protection in radiotherapy. Cancer Drug Resist. 2021;4:244–63. doi: 10.20517/cdr.2020.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Yang M, Yang K, Gao B, et al. A supramolecular nano-delivery system based on AIE PARP inhibitor prodrug and glycosylated pillar[5]arene for drug-resistance therapy. Chem Commun. 2022;58:11147–50. doi: 10.1039/d2cc04238j. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.