Abstract
BACKGROUND
Although genotyping allows family screening and influences risk-stratification in patients with nonischemic dilated cardiomyopathy (DCM) or isolated left ventricular systolic dysfunction (LVSD), its result is negative in a significant number of patients, limiting its widespread adoption.
OBJECTIVES
This study sought to develop and externally validate a score that predicts the probability for a positive genetic test result (G+) in DCM/LVSD.
METHODS
Clinical, electrocardiogram, and echocardiographic variables were collected in 1,015 genotyped patients from Spain with DCM/LVSD. Multivariable logistic regression analysis was used to identify variables independently predicting G+, which were summed to create the Madrid Genotype Score. The external validation sample comprised 1,097 genotyped patients from the Maastricht and Trieste registries.
RESULTS
A G+ result was found in 377 (37%) and 289 (26%) patients from the derivation and validation cohorts, respectively. Independent predictors of a G+ result in the derivation cohort were: family history of DCM (OR: 2.29; 95% CI: 1.73–3.04; P < 0.001), low electrocardiogram voltage in peripheral leads (OR: 3.61; 95% CI: 2.38–5.49; P < 0.001), skeletal myopathy (OR: 3.42; 95% CI: 1.60–7.31; P = 0.001), absence of hypertension (OR: 2.28; 95% CI: 1.67–3.13; P < 0.001), and absence of left bundle branch block (OR: 3.58; 95% CI: 2.57–5.01; P < 0.001). A score containing these factors predicted a G+ result, ranging from 3% when all predictors were absent to 79% when ≥4 predictors were present. Internal validation provided a C-statistic of 0.74 (95% CI: 0.71–0.77) and a calibration slope of 0.94 (95% CI: 0.80–1.10). The C-statistic in the external validation cohort was 0.74 (95% CI: 0.71–0.78).
CONCLUSIONS
The Madrid Genotype Score is an accurate tool to predict a G+ result in DCM/LVSD. (J Am Coll Cardiol 2022;80:1115–1126) © 2022 The Authors. Published by Elsevier on behalf of the American College of Cardiology Foundation.
Keywords: dilated cardiomyopathy, genetics, genotype, predictor, genetic variant
Nonischemic dilated cardiomyopathy (DCM) is characterized by left ventricular (LV) enlargement and systolic dysfunction that cannot be attributed to abnormal loading conditions or to coronary artery disease.1,2 It has an estimated prevalence of between 1 in 250 and 1 in 2,500 and has a major impact on public health systems.1–3
During the past decade, genetic testing in DCM has gained increasing attention, with recent reports suggesting that 30% to 40% of DCM cases are caused by pathogenic or likely pathogenic gene variants in >40 genes that have been described to be associated with the condition.3–5
The clinical spectrum of DCM is broader than previously recognized and includes both classical DCM (with dilatation) and hypokinetic nondilated DCM, an entity that includes patients with left ventricular systolic dysfunction (LVSD) without dilatation.2 Indeed, the genetic spectrum of patients with classical DCM and those with LVSD without dilatation has been shown to be very similar.6
Although genetic findings are increasingly used in clinical decision making, genetic testing is not part of routine clinical care for patients with DCM/LVSD in many centers, and has not been widely incorporated into clinical practice because of limitations that include a relatively low detection rate and the perception that genetic findings have limited influence on patients’ treatment.
Genetic testing is, however, helpful in DCM for diagnostic confirmation in ambiguous situations, to identify at-risk family members whenever a familial DCM-related genetic variant is known, and to recognize certain DCM/LVSD genotypes associated with increased progression of end-stage heart failure or that pose high arrhythmic risk and could benefit from close surveillance and early use of an implantable cardiac defibrillator.7–11 Moreover, recent data suggest that patients with genetic DCM have a worse prognosis than peers with a negative genetic test result, and it has been reported that genetic testing in DCM may have long-term implications in terms of cost-effectiveness by identifying family members with positive and negative genotypes, thus reducing the number of family members requiring serial clinical follow-up.11,12
On the basis of these considerations, the aim of our study was to construct and externally validate an easy-to-use clinical score to identify nonischemic DCM/LVSD patients with a high probability of a positive genetic result.
METHODS
STUDY POPULATION: DERIVATION AND VALIDATION COHORTS.
The derivation cohort included 1,015 unrelated probands from a multicenter cohort of patients with nonischemic DCM/LVSD, genetically evaluated from 2015 to 2020 at the inherited cardiac diseases and heart failure units of 20 Spanish hospitals. All patients were aged ≥15 years at the time of diagnosis and had undergone genetic testing with next-generation sequencing (NGS) as part of their routine clinical care, as described.11
Clinical diagnosis of DCM/LVSD was based on the 2-dimensional echocardiographic finding of a left ventricular ejection fraction (LVEF) <50% at diagnosis not explained by abnormal loading conditions or coronary artery disease.2 Additionally, patients did not have other identifiable causes of systolic disfunction (alcohol abuse, chemotoxicity, etc). Patients could exhibit concomitant skeletal myopathy but, in all cases, this was not the predominant manifestation of the disease and it should have not appeared in childhood.
All individuals were genetically tested using targeted NGS panels at participating institutions or at accredited genetics laboratories. Although panels could differ in the number of genes, all comprised >50 validated genes related to cardiomyopathies. All the genes classified as having definitive or strong evidence of implication in DCM according to the ClinGen DCM gene curation expert panel with the exception of FLNC were evaluated in 100% of individuals.13 FLNC was analyzed in 87% of probands.
Demographic, clinical, 12-lead electrocardiogram (ECG), and transthoracic echocardiogram data were gathered from clinical records using uniform methods, as described.11
The validation cohort was composed of 1,097 unrelated probands with nonischemic DCM or isolated LVSD, including 647 from the Maastricht Cardiomyopathy Registry (Maastricht University Medical Center, the Netherlands) and 450 from the Trieste Cardiomyopathy Registry (Azienda Sanitaria Universitaria Giuliano Isontina, University of Trieste), and all had been genetically tested using targeted NGS. Time frame for enrollment in the validation cohort was 2012 to 2020. The same inclusion and exclusion criteria for the derivation cohort were applied in the validation cohort to allow comparisons. Clinical data and the predictor variables included in the model were extracted from clinical records using the same methods as for the derivation cohort.
The study complies with the ethical principles of the Helsinki Declaration and was approved by the Hospital Universitario Puerta de Hierro ethics committee. The authors from each participating center guarantee the integrity of data.
VARIANT CLASSIFICATION.
The pathogenicity of the identified genetic variants, both in the derivation and the validation cohorts, was centrally analyzed at the time of this study, and variants were classified as pathogenic (P), likely pathogenic (LP), variant of unknown significance (VUS), or likely benign/benign after a systematic review by a cardiologist expert in cardiovascular genetics using modified criteria of the American College of Medical Genetics,14 as described in the Supplemental Methods. Regarding variants in the TTN gene, only variants affecting the A band and/or constitutive exons in the adult cardiac N2B isoform (>95% of exon usage—transcript incorporation—in human adult left ventricle) were considered.
A variant was considered disease-causing if it affected a DCM-related gene and was classified P/LP. Patients harboring P/LP variants were considered to have a positive genetic test result, and those harboring VUS/likely benign/benign variants were considered to have a negative genetic test result.
The frequencies of the variants in the general population were extracted from the gnomAD data-base version 2.1.1.15 To minimize the likelihood of incorrectly categorizing variants as disease causing, we additionally obtained a local control set of >5,254 European index cases with no evidence of structural cardiac disease (channelopathies and aortic diseases) sequenced by NGS at the Health in Code Molecular Genetics Laboratory (A Coruña, Spain) with a library that includes all of the genes with genotype-positive variants detected in this study.
CANDIDATE PREDICTOR VARIABLES.
A prespecified set of 18 clinical, electrocardiographic, and echocardiographic candidate variables that are easy to obtain during the first evaluation at the time of a new DCM diagnosis were selected for this analysis. There were no missing data among the selected variables in the derivation cohort.
Candidate variables included the following: age at diagnosis, sex, hypertension, diabetes, smoking history, hypercholesterolemia, skeletal muscle disease, family history of DCM, family history of sudden cardiac death (SCD) in a first-degree relative, family history of SCD in a non–first-degree relative, family history of skeletal myopathy, left bundle branch block (LBBB), atrioventricular block (any degree), atrial fibrillation, T-wave inversion, low QRS voltage in limb leads, low QRS voltage in precordial leads, and baseline LVEF. A more detailed definition of each variable can be found in the Supplemental Appendix.
MODEL DEVELOPMENT AND INTERNAL VALIDATION.
As a first step, the candidate predictor variables were compared in a univariable logistic regression analysis regarding their association with a positive genotype. Those variables with P values <0.10 were then selected for inclusion in a multivariable logistic regression analysis using an automatic backward selection strategy with a threshold of 0.05 in the Wald test for model retention. The goodness-of-fit for the final model was evaluated by means of discrimination by the C-statistic (equivalent to the area under the receiver-operating characteristic curve, with values ranging from 0.5 for no discrimination to 1.0 for perfect discrimination), and model calibration was assessed with the Hosmer-Lemeshow test.16
Internal validation was performed by 500 bootstrap resamples, as recommended in the TRIPOD guidelines.17 The automatic backward strategy for logistic regression using the full model was run in each resample. Variables were considered important if they appeared in >80% of the models. A calibration plot was generated showing the deciles of the observed and expected event risks. With perfect calibration, the line between the 2 risks would lie along the main diagonal of the plot. Discrimination was measured using the C-statistic. Coefficients, ORs, and their corresponding 95% CIs were estimated.
EXTERNAL VALIDATION.
The final predictive model was applied in the external validation cohort to determine the generalizability of the model. The performance measures in the assessment of external validity included calibration by means of a calibration plot and discrimination through the C-statistic.18
GENERAL STATISTICAL METHODS.
All statistical analyses were performed using Stata Statistics version 17 (StataCorp LLC). Variables are expressed as mean ± SD, median (IQR), or counts and percentages as appropriate.
RESULTS
CHARACTERISTICS OF THE DERIVATION COHORT.
The characteristics of patients included in the derivation cohort are summarized in Table 1. The mean age at diagnosis was 50 ± 14.5 years, and 695 (68.5%) were men. Mean LVEF was 32% ± 10.5% and mean indexed left ventricular end-diastolic diameter (LVEDD) was 32.7 ± 5.0 mm/m2. A DCM phenotype was observed in 536 (52.8%) patients, and 479 (47.2%) had isolated LVSD without LV dilatation. Family history of DCM was present in 480 (47.3%) patients, and 124 (12.2%) had a positive history of SCD in a first-degree relative. At baseline evaluation, 33.5% of patients (n = 340) had hypertension and 16.2% (n = 164) had diabetes. Only 3.6% (n = 36) showed skeletal myopathy.
TABLE 1.
Characteristics of the Derivation Sample According to Genetic Test Results
| Total (N = 1,015) | Positive Genetic test (n = 377) | Negative Genetic test (n = 638) | P Value | |
|---|---|---|---|---|
| Demographics | ||||
| Male | 695 (68.47) | 251 (66.58) | 444 (69.59) | 0.318 |
| Age at diagnosis, y | 50.06 ± 14.49 | 48.15 ± 14.35 | 51.20 ± 14.47 | <0.001 |
| Hypertension | 340 (33.50) | 85 (22.55) | 255 (39.97) | <0.001 |
| Diabetes | 164 (16.16) | 43 (11.41) | 121 (18.97) | 0.002 |
| Smoking history | 423 (41.67) | 140 (37.14) | 283 (44.36) | 0.024 |
| Hypercholesterolemia | 291 (28.67) | 89 (23.61) | 202 (31.66) | 0.006 |
| Skeletal muscle disease | 36 (3.55) | 23 (6.10) | 13 (2.04) | 0.001 |
| FH of DCM | 480 (47.29) | 231 (61.27) | 249 (39.03) | <0.001 |
| FH of SCD first-degree relative | 124 (12.22) | 62 (16.45) | 62 (9.72) | 0.002 |
| FH of SCD non–first degree relatives | 189 (18.62) | 87 (23.08) | 102 (15.99) | 0.005 |
| FH of skeletal myopathy | 25 (2.46) | 14 (3.71) | 11 (1.72) | 0.048 |
| NYHA functional class at first evaluation | 0.225 | |||
| I | 312 (30.74) | 116 (30.77) | 196 (30.72) | |
| II | 343 (33.79) | 114 (30.24) | 229 (35.89) | |
| III | 305 (30.05) | 125 (33.16) | 180 (28.21) | |
| IV | 55 (5.42) | 22 (5.84) | 33 (5.17) | |
| Baseline ECG | ||||
| Atrial fibrillation | 117 (11.53) | 42 (11.14) | 75 (11.76) | 0.767 |
| AV block (any degree) | 112 (11.03) | 42 (11.14) | 70 (10.97) | 0.934 |
| QRS duration, mm | 117.69 ± 29.11 | 108.98 ± 26.70 | 122.84 ± 29.27 | <0.001 |
| LBBB | 333 (32.81) | 60 (15.92) | 273 (42.79) | <0.001 |
| T-wave inversion | 368 (36.26) | 133 (35.28) | 235 (36.83) | 0.618 |
| Low QRS voltage limb leads | 131 (12.91) | 88 (23.34) | 43 (6.74) | <0.001 |
| Low QRS voltage precordial leads | 45 (4.43) | 29 (7.69) | 16 (2.51) | <0.001 |
| Baseline echocardiogram | ||||
| LVEF, % | 32.06 ± 10.45 | 32.22 ± 10.42 | 31.96 ± 10.48 | 0.741 |
| LVEF ≤35% | 639 (62.96) | 234 (62.07) | 405 (63.48) | 0.653 |
| iLVEDD, mm/m2 | 32.66 (4.97) | 32.95 (4.87) | 32.49 (5.03) | 0.179 |
| DCM phenotype | 536 (52.81) | 193 (51.19) | 343 (53.76) | 0.428 |
| iLVSD phenotype | 479 (47.19) | 184 (48.81) | 295 (46.24) | |
| MR moderate/severe | 339 (34.66) | 134 (37.02) | 205 (33.28) | 0.236 |
| RVSD (any degree) | 211 (23.16) | 90 (27.27) | 121 (20.83) | 0.027 |
Values are n (%) or mean ± SD.
AV = atrioventricular; DCM = dilated cardiomyopathy; FH = family history; iLVEDD = indexed left ventricular end-diastolic diameter; iLVSD = isolated left ventricular systolic dysfunction; LBBB = left bundle branch block; LVEF = left ventricular ejection fraction; MR = mitral regurgitation; NYHA = New York Heart Association; RVSD = right ventricular systolic dysfunction; SCD = sudden cardiac death.
Genetic testing identified a P or LP genetic variant in 377 individuals (37.2%), 250 (24.6%) carried a VUS, and 388 (38.2%) did not have any variant of interest. The distribution of the disease-causing genetic variants across genes and the criteria used to determine pathogenicity in each variant are shown in the Supplemental Appendix. As in other contemporary genotyped DCM cohorts, TTN, DSP, LMNA, BAG3, RBM20, and FLNC genes accounted for most of the P or LP variants found (Supplemental Table 1). P or LP genetic variants were identified in 8 different genes in 23 (66%) patients with skeletal myopathy, with LMNA and DMD as the most commonly affected genes (7 patients each). A complete list of genetic results in patients with skeletal myopathy can be found in the Supplemental Table 2.
Patients with a positive genetic test were significantly younger at diagnosis than peers with a negative genetic test result (48.2 ± 14.4 years vs 51.2 ± 14.5 years; P < 0.001); were more likely to have a family history of DCM (61.3% vs 39.0%; P < 0.001); and less frequently had a history of hypertension (22.6% vs 40.0%; P < 0.001), diabetes (11.4% vs 19.0%; P = 0.002), and hypercholesterolemia (23.6% vs 31.7%; P = 0.006). Patients with a positive genetic test result also less frequently had LBBB (15.9% vs 42.8%; P < 0.001) and more frequently had low voltage in peripheral leads (23.3% vs 6.7%; P < 0.001) on ECG. Baseline echocardiographic parameters were similar between groups, without differences in mean LVEF (32.2% ± 10.4% vs 32.0% ± 10.5%; P = 0.741) and mean indexed LVEDD (33.0 ± 4.9 mm/m2 vs 32.5 ± 5.0 mm/m2; P = 0.179).
MODEL DEVELOPMENT AND INTERNAL VALIDATION.
Univariable analysis identified significant demographic, clinical, and ECG variables predicting a positive genetic test result (Table 2). The following 13 candidate variables were included in the multivariable regression analysis: age at diagnosis, hypertension, diabetes, smoking history, hypercholesterolemia, skeletal muscle disease, family history of DCM, family history of SCD in a first-degree relative, family history of SCD in a non–first-degree relative, family history of skeletal myopathy, LBBB, low QRS voltage limb leads, and low QRS voltage precordial leads.
TABLE 2.
Associations Between Predictors for a Positive Genetic Test Result (Univariable and Multivariable Analysis)
| Univariable Analysis |
Full Multivariable Analysis |
|||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P Value | OR | 95% CI | P Value | |
| Age at diagnosis (per y) | 0.99 | 0.98–0.99 | 0.001 | 1.00 | 0.99–1.01 | 0.820 |
| Female | 1.15 | 0.87–1.51 | 0.318 | |||
| History of hypertension (absence of) | 2.29 | 1.71–3.05 | <0.001 | 2.09 | 1.48–2.95 | <0.001 |
| Diabetes | 0.55 | 0.38–0.80 | 0.002 | 0.69 | 0.44–1.09 | 0.110 |
| Smoking history | 0.74 | 0.57–0.96 | 0.024 | 0.81 | 0.61–1.09 | 0.173 |
| Hypercholesterolemia | 0.67 | 0.50–0.89 | 0.006 | 0.89 | 0.63–1.26 | 0.523 |
| Skeletal muscle disease | 3.12 | 1.56–6.24 | 0.001 | 3.11 | 1.33–7.28 | 0.009 |
| FH of DCM | 2.47 | 1.90–3.21 | <0.001 | 2.13 | 1.58–2.87 | <0.001 |
| FH of SCD first-degree relative | 1.83 | 1.25–2.67 | 0.002 | 1.29 | 0.83–2.01 | 0.263 |
| FH of SCD non–first-degree relatives | 1.58 | 1.15–2.17 | 0.005 | 1.12 | 0.77–1.63 | 0.545 |
| FH of skeletal myopathy | 2.20 | 0.99–4.89 | 0.054 | 1.13 | 0.42–3.08 | 0.809 |
| LBBB (absence of) | 3.95 | 2.88–5.43 | <0.001 | 3.55 | 2.52–4.99 | <0.001 |
| AV block (any degree) | 1.02 | 0.68–1.53 | 0.934 | |||
| Atrial fibrillation at baseline | 0.94 | 0.63–1.41 | 0.767 | |||
| T-wave inversion | 0.93 | 0.72–1.22 | 0.618 | |||
| Low QRS voltage limb leads | 4.21 | 2.85–6.23 | <0.001 | 3.64 | 2.30–5.76 | <0.001 |
| Low QRS voltage precordial leads | 3.24 | 1.74–6.05 | <0.001 | 1.14 | 0.54–2.40 | 0.725 |
| Baseline LVEF | 1.00 | 0.99–1.01 | 0.693 | |||
| Intercept | 0.08 | 0.04–0.18 | <0.001 | |||
Abbreviations as in Table 1. Bold indicates statistical significance.
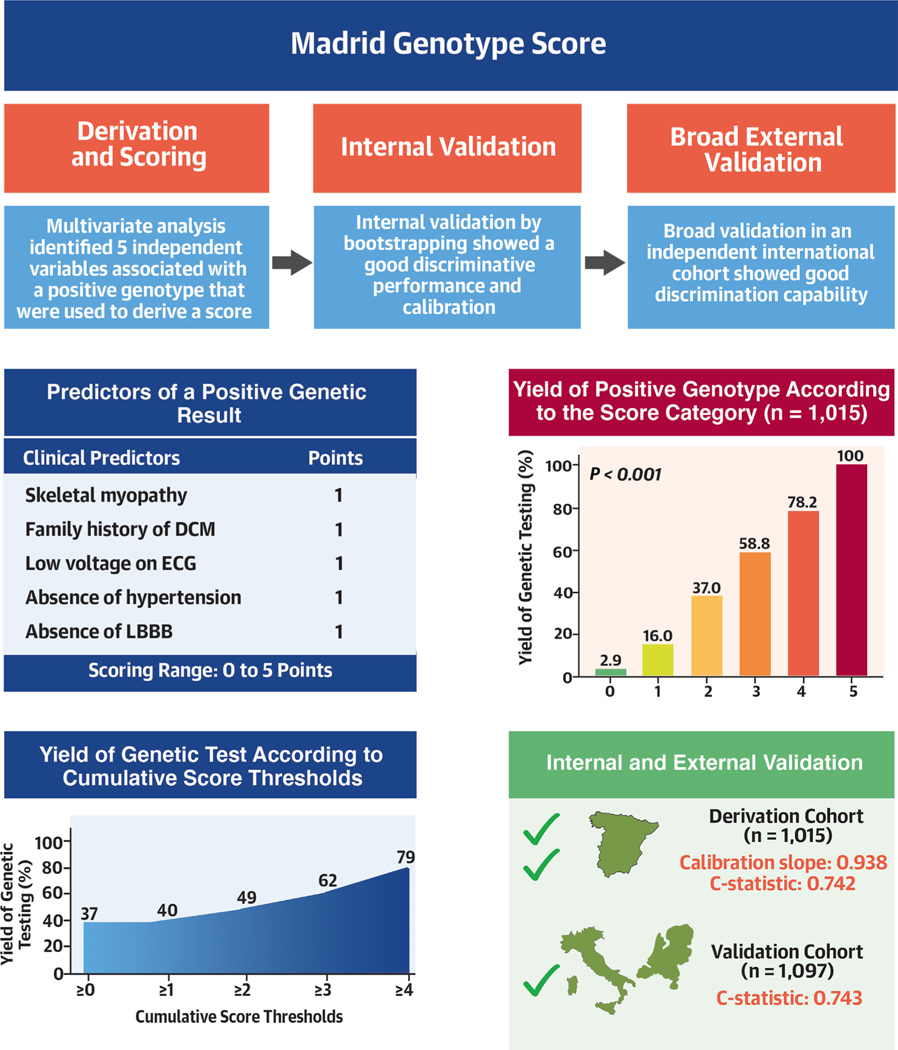
Multivariable regression analysis identified the following 5 independent variables independently associated with a positive genetic test result, all of which were included in the final model: family history of DCM, absence of hypertension, skeletal muscle disease, absence of LBBB, and low QRS voltage in limb leads. The ORs of the full multivariable analysis are presented in Table 2.
Two electrocardiographic parameters were found to be the strongest independent predictors of a positive genetic test result: absence of LBBB (OR: 3.58; 95% CI: 2.57–5.01; P < 0.001), and low QRS voltage in limb leads (OR: 3.61; 95% CI: 2.38–5.49; P < 0.001), followed by skeletal myopathy (OR: 3.42; 95% CI: 1.60–7.31; P = 0.001), absence of hypertension (OR: 2.28; 95% CI: 1.67–3.13; P < 0.001), and family history of DCM (OR: 2.29; 95% CI: 1.73–3.04; P < 0.001) (Table 3, Supplemental Table 3).
TABLE 3.
Clinical Predictors for a Positive Genetic Test in the Derivation and the Validation Samples in the Final Predictive Model
| Derivation Sample |
Validation Sample |
||||
|---|---|---|---|---|---|
| OR (95% CI) | Coefficient (95% CI) | P Value | OR (95% CI) | P Value | |
| FH of DCM | 2.29 (1.73–3.04) | 0.83 (0.55–1.11) | <0.001 | 5.15 (3.80–6.97) | <0.001 |
| Skeletal muscle disease | 3.42 (1.60–7.31) | 1.23 (0.47–1.99) | 0.001 | 4.09 (1.23–13.62) | 0.022 |
| LBBB (absence of) | 3.58 (2.57–5.01) | 1.28 (0.94–1.61) | <0.001 | 2.79 (1.88–4.13) | <0.001 |
| Low QRS voltage limb leads | 3.61 (2.38–5.49) | 1.28 (0.87–1.70) | <0.001 | 2.28 (1.48–3.52) | <0.001 |
| Hypertension (absence of) | 2.28 (1.67–3.13) | 0.83 (0.51–1.14) | <0.001 | 2.09 (1.45–3.02) | <0.001 |
| Intercept | 0.07 (0.05–0.11) | −2.65 (−3.07/−2.24) | <0.001 | 0.04 (0.03–0.07) | <0.001 |
Abbreviations as in Table 1.
Model presentation.
For an individual patient, the following equation, derived from the logit of the parsimonious model, allows calculation of the probability of a positive genetic test result:
Estimated probability of a positive genetic test result = 1/(1 + exp. —[—2.654365 + 0.8300685 × family history of DCM + 1.230924 × skeletal muscle disease + 1.27642 × absence of LBBB + 1.284825 × low QRS voltages in limb leads + 0.8263322 × absence of hypertension]).
Internal validation.
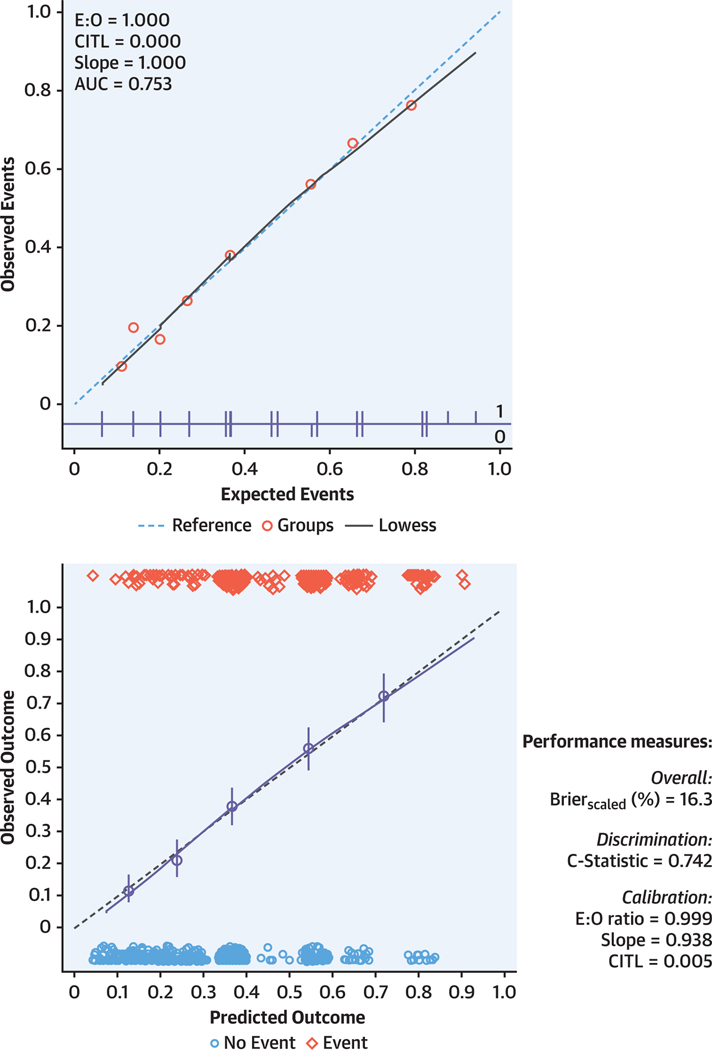
The model was well calibrated with a fit between predicted and observed frequencies (P = 0.899). The calibration slope was 1.000 (95% CI: 0.842–1.158) and the C-statistic was 0.753 (95% CI: 0.723–0.783). Internal validation with bootstrapping revealed an optimism-adjusted calibration slope of 0.938 (95% CI: 0.796–1.098) and a C-statistic of 0.742 (95% CI: 0.711–0.772).
Figure 1 displays a graphic representation of the model’s apparent performance and bootstrap performance (optimism-adjusted), showing good overall agreement between the predicted and the observed rates of a positive genotype.
FIGURE 1. Apparent Performance and Internal Validation by Bootstrapping of the Model.
Calibration plot assessing the apparent predictive performance of the model in the derivation cohort (top). Data points are mean predicted against mean observed frequencies of a positive genetic test result. The dashed blue line represents the line of equality. Internal validation by bootstrapping (bottom) showed good agreement between predicted (x-axis) and observed (y-axis) events (Gen+). Blue circles represent binned logistic regression estimates with 95% confidence for quintiles of the predicted outcome. The straight line is the continuous calibration (hazard regression). The dashed line represents perfect calibration. Red diamonds in the upper x-axis reflect the number of Gen— patients with a predicted outcome corresponding to the x-axis value, and blue circles in the lower x-axis reflect the number of patients with a positive genetic test result with a predicted outcome corresponding to the x-axis value.
We also analyzed the predictive nature of the risk score after removing 134 probands who harbor a common variant with at least 1 additional proband in the study to avoid a possible effect induced by possible endemic/over-represented variants and found that the performance measures of the score and the internal validation remained robust. The C-statistic was 0.740 (95% CI: 0.705–0.775) and calibration slope = 1.000 (95% CI: 0.813–1.187) for model performance, and C-statistic = 0.735 (95% CI: 0.700–0.771), calibration slope = 0.968 (95% CI: 0.803–1.155) for internal validation with bootstrapping.
CHARACTERISTICS OF THE VALIDATION COHORT.
The validation sample was composed of 1,097 genotyped patients with nonischemic DCM/LVSD. All patients had complete data. The mean age at diagnosis was 49.4 ± 15 years, and male sex predominated (67%). The mean baseline LVEF was 32.7% ± 10.9%, 30.4% (n = 333) had a family history of DCM, 28.9% (n = 317) had hypertension, 28.1% (n = 308) exhibited LBBB, 10.6% (n = 116) had low-voltages in limb leads, and 1.37% (n = 15) showed skeletal myopathy (Table 4).
TABLE 4.
Characteristics of the Derivation and the Validation Samples
| Derivation Sample (n = 1,015) |
Validation Sample (n = 1,097) |
|||||
|---|---|---|---|---|---|---|
| Positive Genetic Test (n = 377) | Negative Genetic Test (n = 638) | P Value | Positive Genetic Test (n = 289) | Negative Genetic Test (n = 808) | P Value | |
| Male | 251 (66.58) | 444 (69.59) | 0.318 | 209 (72.32) | 526 (65.10) | 0.025 |
| Age at diagnosis, y | 48.15 ± 14.35 | 51.20 ± 14.47 | <0.001 | 44.72 ± 15.50 | 51.03 ± 14.39 | <0.001 |
| Baseline LVEF | 32.22 ± 10.42 | 31.96 ± 10.48 | 0.741 | 33.13 ± 11.16 | 32.59 ± 10.84 | 0.548 |
| FH of DCM | 231 (61.27) | 249 (39.03) | <0.001 | 165 (57.09) | 168 (20.79) | <0.001 |
| Skeletal muscle disease | 23 (6.10) | 13 (2.04) | 0.001 | 10 (3.46) | 5 (0.62) | <0.001 |
| Hypertension | 85 (22.55) | 255 (39.97) | <0.001 | 50 (17.30) | 267 (33.04) | <0.001 |
| Left bundle branch block | 60 (15.92) | 273(42.79) | <0.001 | 39 (13.49) | 269 (33.29) | <0.001 |
| Low QRS voltage limb leads | 88 (23.34) | 43 (6.74) | <0.001 | 48 (16.61) | 68 (8.42) | <0.001 |
Values are n (%) or mean ± SD.
Abbreviations as in Table 1.
Genetic testing identified 289 individuals (26.3%) with a P/LP genetic variant, 216 (19.7%) carrying a VUS, and 592 (54%) who did not show any variant of interest. Distribution of the disease-causing genetic variants across genes and the criteria used to determine pathogenicity in each variant are provided in Supplemental Table 4. Genetic results of patients with skeletal myopathy can also be found in Supplemental Table 5.
External validation.
The model showed good discrimination in the external validation sample, with a C-statistic of 0.743 (95% CI: 0.711–0.775). The calibration slope in the validation cohort was 1.016 (Supplemental Figure 1).
Score performance.
Based on the results obtained, and to facilitate the use of the model in everyday practice, we derived a cumulative genotype predictive score for positive genetic testing with the 5 parameters that independently predicted a positive genetic test result. The score is obtained by adding the points generated by the 5 variables of the final model. Based on similar regression coefficients and ORs observed with the 5 parameters, each factor received the same weight in the predictive score (1 point), as previously described.19
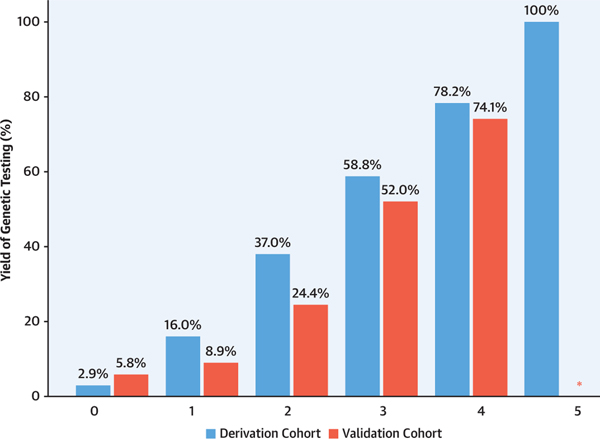
With this score, the probability of a positive genetic test result in the derivation cohort ranged from 2.9% for the 68 patients with 0 points, to 78.2% for the 55 patients with 4 points, and to 100% for the 2 patients with 5 points, with an incremental increase in yield between each score subgroup (P < 0.001) (Figure 2, Table 5).
FIGURE 2. Yield of Positive Genetic Test Result According to Score Value.
Percentage of observed individuals with a positive genetic test result in the derivation (blue bars) and validation (red bars) cohorts. *None of the patients in the validation cohort scored 5 points.
TABLE 5.
Genetic Yield for Each of the Scores in the Derivation and Validation Cohorts
| Derivation Sample |
Validation Sample |
|||||
|---|---|---|---|---|---|---|
| Total Score | Positive Genetic Test (n = 377) | Negative Genetic Test (n = 638) | Total (N = 1,015) | Positive Genetic Test (n = 289) | Negative Genetic Test (n = 808) | Total (N = 1,097) |
| 0 | 2 (2.94) | 66 (97.06) | 68 (6.70) | 5 (5.75) | 82 (94.25) | 87 (7.93) |
| 1 | 42 (15.97) | 221 (84.03) | 263 (25.91) | 26 (8.93) | 265 (91.07) | 291 (34.46) |
| 2 | 137 (37.03) | 233 (62.98) | 370 (36.45) | 108 (24.43) | 334 (75.57) | 442 (40.29) |
| 3 | 151 (58.75) | 106 (41.25) | 257 (25.32) | 130 (52.00) | 120 (48.00) | 250 (22.79) |
| 4 | 43 (78.18) | 12 (21.82) | 55 (5.42) | 20 (74.07) | 7 (25.93) | 27 (2.46) |
| 5 | 2 (100) | 0 | 2 (0.20) | 0 | 0 | 0 |
Values are n (%).
Similar findings were obtained when the predictive score was applied to the external validation cohort, with an incremental increase in yield between each score subgroup ranging from 5.8% for the 87 patients with 0 points to 74.1% for the 27 patients with 4 points (P < 0.001) (Figure 2, Table 5).
DISCUSSION
In the present study, we describe and validate a novel predictive tool to assess the probability of a positive genetic test result in patients with nonischemic DCM and isolated LVSD based on readily available clinical and ECG characteristics. The derivation and validation cohorts included 1,015 and 1,097 patients, respectively, representing 2 of the largest genotyped cohorts assessed in the literature. This large sample size allowed testing of an appropriate range of candidate variables to develop a score—The Madrid Genotype Score—which predicts a positive genetic testing result with good levels of discrimination, and its subsequent validation in an independent external cohort (Central Illustration).
CENTRAL ILLUSTRATION. Components, Validation, and Performance of the Madrid Genotype Score.
(Top left) Components of the Madrid Genotype Score and points attributed to each parameter. (Top right) Increase in the yield of positive genetic testing results according to increased score category of the Madrid Genotype Score. (Bottom left) Positive genetic testing result according to cumulative score threshold from ≥0 to ≥4 points of the Madrid Genotype Score. (Bottom right) Internal and external validation of the score showed a good overall agreement between the predicted and observed rate of a positive genetic testing result. DCM = dilated cardiomyopathy; ECG = electrocardiography; LBBB = left bundle branch block.
Genetic testing in patients with DCM has both prognostic and diagnostic value. Patients with positive genetic testing results have a significantly higher incidence of end-stage heart failure and ventricular arrhythmias, and lower rates of left ventricular reverse remodeling than patients with a negative genetic testing result.7–11 Furthermore, the results of genetic testing can inform cascade screening strategies for family members.12 Consequently, genetic testing can significantly influence clinical management in patients with DCM/LVSD and their relatives. The cost of genetic testing with high-throughput sequencing technologies has decreased considerably in recent years, and will likely continue to decrease, facilitating access to genotyping in these patients.
All of these arguments support the indication for genetic testing in all patients with nonischemic DCM/LVSD. However, genetic testing is still associated with substantial costs in certain settings, which has limited its widespread adoption. Moreover, the current yield of genetic testing for actionable variants in DCM is only around 30%, and VUS are found in >20% of cases,5,7,11,20 leading to significant challenges in variant interpretation and in communicating this information to patients and families.
In this context, the Madrid Genotype Score has been designed to predict a positive genetic test result in patients with DCM/LVSD to facilitate identification of those patients with a higher probability of having a positive result and who would derive the greatest benefit from genetic testing. The score relies on 5 predictors that are readily available in the clinic and provides reasonably accurate predictions.
The identified predictors (family history of DCM, absence of hypertension, skeletal muscle disease, absence of LBBB, and low QRS voltage in peripheral ECG leads) were all found to be strong independent predictors of a positive genetic test result. These findings have a biological explanation and are congruent with previous reports demonstrating higher detection rates of P/LP variants in familial DCM (in which a likely genetic etiology must be always suspected),21 skeletal myopathy (considered a strong red flag because of the shared genetic etiology among some forms of genetic DCM and skeletal muscle disease),22 low voltages on ECG (described as a common finding in certain genotypes such as PLN and desmosomal genes),23–26 and absence of LBBB (a potential role of desynchrony itself in LV systolic dysfunction).27 Interestingly, although LBBB and conduction disease are part of the phenotypic expression of certain forms of genetic DCM like LMNA-associated DCM, it was negatively associated with PV/LPVs both in the derivation and the validation cohorts. We think that the most likely explanation for this finding is that the frequency of LBBB as part of the phenotypic expression of a minority of individuals with certain DCM-causing variants gets diluted in a cohort of >1,000 individuals where the effect of the more frequent LBBB-induced DCM emerges. However, what appears to be underscored in the light of our findings is the relationship between hypertension and DCM/LVSD. In this case, a plausible biological explanation for a lower rate of positive genotype among patients with hypertension could be that hypertension precedes the diagnosis of LVSD and leads to cardiac remodeling.28 Of note, age did not predict a positive genetic testing result in our cohort, suggesting that age should not be considered when deciding which patients with DCM/LVSD should undergo genetic testing.
To assess the implications of our model in clinical practice, we explored the potential impact of using different score thresholds in the derivation cohort to guide generalization of genetic testing (Supplemental Figure 2). A score ≥1 (ie, if only patients with a Madrid Genotype Score of 1 or more points had been genotyped) would have resulted in a positive genotype yield of 39.6% and would have missed only 0.2% of patients with a positive genotype while reducing the total number of genotyped patients by 6.7% (Table 6). A threshold of ≥2 would have resulted in a positive genotype yield of almost 49%, after avoiding genotyping in almost one-third of patients (32.6%), and at a cost of missing a positive result in 4.3% of patients (Supplemental Figure 2).
TABLE 6.
Yield of Genetic Testing and Proportion of Individuals With a Positive Genetic Test Result Missed at Each Score Threshold in the Derivation Cohort
| Risk Category | Total, n (%) | Individuals With Positive Genetic Test Result Identified | Positive Genetic Test Yield, % | Individuals With Positive Genetic Test Result Missed | Percent Of Individuals With Positive Genetic Test Missed |
|---|---|---|---|---|---|
| ≥0 | 1,015 (100) | 377 | 37.14 | 0 | 0.00 |
| ≥1 | 947 (93.30) | 375 | 39.60 | 2 | 0.53 |
| ≥2 | 684 (67.39) | 333 | 48.68 | 44 | 11.67 |
| ≥3 | 314 (30.94) | 196 | 62.42 | 181 | 48.01 |
| ≥4 | 57 (5.62) | 45 | 78.95 | 332 | 88.06 |
Genotyping could potentially become the standard of care in all patients with DCM/LVSD in the future after rigorous exclusion of secondary causes and regardless of whether the disease has a familial presentation or not. Nevertheless, because genetic testing is currently not generalized, the proposed predictor genotype score provides a useful tool for selecting patients with a higher probability of a positive genetic test result to increase the yield of genetic testing.
Choosing any threshold of the score to undertake genetic testing is always arbitrary and should rely on local policies and the available resources. It is, therefore, the responsibility of the treating physician to decide when genetic testing should be pursued. We believe that the Madrid Genotype Score can assist physicians when addressing this decision in clinical practice, and we have made our model available online as a “prediction calculator.”29 The predicted probability for the combination of all sets is available in Supplemental Table 6. There will always be a trade-off between diagnostic sensitivity and specificity and the cost and effectiveness of genetic testing, but if appropriately used, the Madrid Genotype Score would be helpful in improving the cost-effective approach to decision-making on which patients should be genotyped.
STUDY LIMITATIONS.
Patients in the derivation cohort were mostly enrolled through Inherited Cardiac Diseases Units unlike patients from the validation cohort, who proceeded from tertiary DCM referral centers, potentially limiting the application of the score to other cohorts with other characteristics.
Although the main DCM genes were evaluated in all cases, not all patients underwent the same genetic analysis because the genes included in NGS panels varied between centers and over time, reflecting changes in the knowledge of genetics in the last few years. Moreover, the performance of the Madrid Genotype Score reflects current knowledge, as it was derived from classification of variants found in currently commercially available DCM-associated gene panels; however, all genetic variants in both the derivation and validation cohort were centrally evaluated. The classification of variants, as well as the genes associated with DCM, may both change over time as our understanding of the genetics of DCM/LVSD improves, therefore potentially limiting the predictive ability of the score in the future.
CONCLUSIONS
The Madrid Genotype Score predicts a positive genetic test result in nonischemic DCM/LVSD with good precision and discrimination. Thus, it represents an easy-to-use tool to direct genetic testing toward patients and families with a higher probability of a positive genetic test result to improve the diagnostic yield of genetic testing.
Supplementary Material
PERSPECTIVES.
COMPETENCY IN PATIENT CARE AND PROCEDURAL SKILLS:
A predictive score based on a family history of DCM, low electrocardiographic limb lead voltage, skeletal myopathy, absence of hypertension and absence of LBBB exhibited good performance in predicting a positive result of genetic testing in patients with DCM or isolated LVSD.
TRANSLATIONAL OUTLOOK:
The discovery of new disease causing genes and improved classification of genetic variants, along with extensive phenotyping, the results of advanced cardiac imaging, and artificial intelligence tools, should enhance identification of patients with genetic basis for DCM.
FUNDING SUPPORT AND AUTHOR DISCLOSURES
This work was supported by grants from the Instituto de Salud Carlos III (ISCIII) (PI17/01941, PI18/0004, PI19/01283, PI20/0320) (co-funded by European Regional Development Fund/European Social Fund “A way to make Europe”/”Investing in your future”). The CNIC is supported by the ISCIII, MCIN, the Pro-CNIC Foundation, and the Severo Ochoa Centers of Excellence program (CEX2020-001041-S). This study was also supported by the Netherlands Cardiovascular Research Initiative, an initiative with support from the Dutch Heart Foundation, DCVA Double Dosis 2020B005. The Hospital Universitario Puerta de Hierro, the Hospital Universitario Virgen de la Arrixaca and the Azienda Sanitaria Universitaria Giuliano-Isontina are members of the European Reference Network for rare, low-prevalence, and complex diseases of the heart (ERN GUARD-Heart). Dr Ochoa is an employee of Health in Code. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
ABBREVIATIONS AND ACRONYMS
- DCM
dilated cardiomyopathy
- LVEF
left ventricular ejection fraction
- LVSD
left ventricular systolic dysfunction
- NGS
next-generation sequencing
Footnotes
Ali Marian, MD, served as Guest Associate Editor for this paper. Christie Ballantyne, MD, served as Guest Editor-in-Chief for this paper.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
APPENDIX For an expanded Methods section as well as supplemental tables and figures, please see the online version of this paper.
REFERENCES
- 1.McKenna WJ, Judge DP. Epidemiology of the inherited cardiomyopathies. Nat Rev Cardiol. 2021;18:22–36. 10.1038/s41569-020-0428-2 [DOI] [PubMed] [Google Scholar]
- 2.Pinto YM, Elliott PM, Arbustini E, et al. Proposal for a revised definition of dilated cardiomyopathy, hypokinetic non-dilated cardiomyopathy, and its implications for clinical practice: a position statement of the ESC Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2016;37: 1850–1858. 10.1093/eurheartj/ehv727 [DOI] [PubMed] [Google Scholar]
- 3.Fatkin D, Huttner IG, Kovacic JC, Seidman JG, Seidman CE. Precision medicine in the management of dilated cardiomyopathy: JACC state-of-the-art review. J Am Coll Cardiol. 2019;74:2921–2938. 10.1016/j.jacc.2019.10.011 [DOI] [PubMed] [Google Scholar]
- 4.Hershberger RE, Givertz MM, Ho CY, et al. Genetic evaluation of cardiomyopathy: a clinical practice resource of the American College of Medical Genetics and Genomics (ACMG). Genet Med. 2018;20:899–909. 10.1038/s41436-018-0039-z [DOI] [PubMed] [Google Scholar]
- 5.Verdonschot JAJ, Hazebroek MR, Krapels IPC, et al. Implications of genetic testing in dilated cardiomyopathy. Circ Genomic Precis Med. 2020;13:476–487. 10.1161/CIRC-GEN.120.003031 [DOI] [PubMed] [Google Scholar]
- 6.Hazebroek MR, Krapels I, Verdonschot J, et al. Prevalence of pathogenic gene mutations and prognosis do not differ in isolated left ventricular dysfunction compared with dilated cardiomyopathy. Circ Heart Fail. 2018;11(3). e004682 10.1161/CIRCHEARTFAILURE.117.004682. PMID: 29540472. [DOI] [PubMed] [Google Scholar]
- 7.Dal Ferro M, Stolfo D, Altinier A, et al. Association between mutation status and left ventricular reverse remodelling in dilated cardiomyopathy. Heart. 2017;103:1704–1710. 10.1136/heartjnl-2016-311017 [DOI] [PubMed] [Google Scholar]
- 8.Kumar S, Baldinger SH, Gandjbakhch E, et al. Long-term arrhythmic and nonarrhythmic outcomes of lamin A/C mutation carriers. J Am Coll Cardiol. 2016;68:2299–2307. 10.1016/j.jacc.2016.08.058 [DOI] [PubMed] [Google Scholar]
- 9.Domínguez F, Cuenca S, Bilińska Z, et al. Dilated cardiomyopathy due to BLC2-associated athanogene 3 (BAG3) mutations. J Am Coll Cardiol. 2018;72:2471–2481. 10.1016/j.jacc.2018.08.2181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akhtar MM, Lorenzini M, Cicerchia M, et al. Clinical phenotypes and prognosis of dilated cardiomyopathy caused by truncating variants in the TTN Gene. Circ Heart Fail. 2020;13:496–508. 10.1161/CIRCHEARTFAILURE.119.006832 [DOI] [PubMed] [Google Scholar]
- 11.Escobar-Lopez L, Ochoa JP, Mirelis JG, et al. Association of genetic variants with outcomes in non-ischemic dilated cardiomyopathy. J Am Coll Cardiol. 2021;17:1682–1699. 10.1016/j.jacc.2021.08.039 [DOI] [PubMed] [Google Scholar]
- 12.Catchpool M, Ramchand J, Martyn M, et al. A cost-effectiveness model of genetic testing and periodical clinical screening for the evaluation of families with dilated cardiomyopathy. Genet Med. 2019;21:2815–2822. 10.1038/s41436-019-0582-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jordan E, Peterson L, Ai T, et al. Evidence-based assessment of genes in dilated cardiomyopathy. Circulation. 2021;144:7–19. 10.1161/CIRCULATIONAHA.120.053033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405–424. 10.1038/gim.2015.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karczewski KJ, Francioli LC, Tiao G, et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature. 2020;581: 434–443. 10.1038/s41586-020-2308-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cowley LE, Farewell DM, Maguire S, Kemp AM. Methodological standards for the development and evaluation of clinical prediction rules: a review of the literature. Diagnostic Progn Res. 2019;3:1–23. 10.1186/s41512-019-0060-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Collins GS, Reitsma JB, Altman DG, Moons KGM. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): The TRIPOD Statement. BMC Med. 2015;13:1–10. 10.1186/s12916-014-0241-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Altman DG, Vergouwe Y, Royston P, Moons KG. Prognosis and prognostic research: validating a prognostic model. BMJ. 2009;338: 1432–1435. 10.1136/bmj.b605 [DOI] [PubMed] [Google Scholar]
- 19.Mehta HB, Mehta V, Girman CJ, Adhikari D, Johnson ML. Regression coefficient-based scoring system should be used to assign weights to the risk index. J Clin Epidemiol. 2016;79:22–28. 10.1016/j.jclinepi.2016.03.031 [DOI] [PubMed] [Google Scholar]
- 20.Haas J, Frese KS, Peil B, et al. Atlas of the clinical genetics of human dilated cardiomyopathy. Eur Heart J. 2015;36:1123–1135. 10.1093/eurheartj/ehu301 [DOI] [PubMed] [Google Scholar]
- 21.Cuenca S, Ruiz-Cano MJ, Gimeno-Blanes JR, et al. Genetic basis of familial dilated cardiomyopathy patients undergoing heart transplantation. J Hear Lung Transplant. 2016;35:625–635. 10.1016/j.healun.2015.12.014 [DOI] [PubMed] [Google Scholar]
- 22.Harakalova M, Kummeling G, Sammani A, et al. A systematic analysis of genetic dilated cardiomyopathy reveals numerous ubiquitously expressed and muscle-specific genes. Eur J Heart Fail. 2015;17:484–493. 10.1002/ejhf.255 [DOI] [PubMed] [Google Scholar]
- 23.Finocchiaro G, Merlo M, Sheikh N, et al. The electrocardiogram in the diagnosis and management of patients with dilated cardiomyopathy. Eur J Heart Fail. 2020;22:1097–1107. 10.1002/ejhf.1815 [DOI] [PubMed] [Google Scholar]
- 24.Ortiz-Genga MF, Cuenca S, Dal Ferro M, et al. Truncating FLNC mutations are associated with high-risk dilated and arrhythmogenic cardiomyopathies. J Am Coll Cardiol. 2016;68(22): 2440–2451. 10.1016/j.jacc.2016.09.927 [DOI] [PubMed] [Google Scholar]
- 25.Akhtar MM, Lorenzini M, Cicerchia M, et al. Clinical phenotypes and prognosis of dilated cardiomyopathy caused by truncating variants in the TTN gene. Circ Hear Fail. 2020;13. e006832. [DOI] [PubMed] [Google Scholar]
- 26.Smith ED, Lakdawala NK, Papoutsidakis N, et al. Desmoplakin cardiomyopathy, a fibrotic and inflammatory form of cardiomyopathy distinct from typical dilated or arrhythmogenic right ventricular cardiomyopathy. Circulation. 2020;141: 1872–1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanna GD, Merlo M, Moccia E, et al. Left bundle branch block-induced cardiomyopathy: a diagnostic proposal for a poorly explored pathological entity. Int J Cardiol. 2020;15: 199–205. 10.1016/j.ijcard.2019.06.008 [DOI] [PubMed] [Google Scholar]
- 28.González A, Ravassa S, López B, et al. Myocardial remodeling in hypertension toward a new view of hypertensive heart disease. Hypertension. 2018;72:549–558. 10.1161/HYPERTENSIONAHA.118.11125 [DOI] [PubMed] [Google Scholar]
- 29.Madrid DCM Score. Accessed July 26, 2022. https://madriddcmscore.com/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.