Abstract
Background
Multiple sclerosis (MS) is a chronic immune‐mediated disorder characterized by the degradation of the myelin sheath in the central nervous system. Research indicates that individuals with MS exhibit a higher susceptibility to stroke compared to the general population. This association is rooted in shared underlying mechanisms, specifically involving neuroinflammatory processes.
Methodology
We performed an extensive search on PubMed, MEDLINE, Embase, Scopus, and Google Scholar using specific terms. The search terms included variations of “multiple sclerosis,” “stroke,” “cerebrovascular disease,” “vascular risk factors,” “disease‐modifying therapies,” and “neuroinflammation.” The search was limited to articles published from January 1, 2000, up to 31 May, 2023.
Results and Discussion
Stroke, a global health burden characterized by significant mortality and adult disability, underscores the critical importance of understanding the link between MS and stroke. Despite a growing body of research establishing an elevated risk of stroke in MS patients, notable information gaps persist. Limited prospective multicenter studies on stroke incidence in MS patients contribute to an incomplete understanding of the precise relationship between these two conditions.
Conclusion
In conclusion, this review underscores the critical need for a thorough understanding of the complex relationship between MS and stroke. The identified risk factors and the influence of MS DMTs on stroke risk necessitate further investigation to inform evidence‐based preventive and therapeutic strategies. Bridging the existing information gaps through prospective multicenter studies is imperative for a comprehensive understanding of this association. The development of targeted diagnostic and therapeutic approaches for acute stroke risk in MS patients is paramount to mitigate the impact of these debilitating conditions. Ultimately, this review serves as a foundation for future efforts to enhance preventative measures and therapeutic interventions, thereby improving the overall quality of life for individuals with MS susceptible to strokes.
Keywords: cerebrovascular disease, disease‐modifying therapies, multiple sclerosis, neuroinflammation, stroke, vascular risk factors
1. INTRODUCTION
Multiple sclerosis is an autoimmune condition affecting the central nervous system (CNS), characterized by its relapsing and progressive course, chronic inflammation, and demyelination. This disease affects millions of individuals worldwide, and its presentation is highly variable, ranging from a benign condition to a rapidly progressing disease affecting patients' quality of life. 1 , 2 Stroke is a significant cause of mortality and adult disability worldwide, particularly ischemic stroke. While many risk factors for stroke have been identified, a substantial percentage of stroke cases, especially in the young population, have no clear risk factors. Current studies indicate a relatively low incidence of stroke in MS patients, ranging from 0.4% to 7.0%. 1 , 2 , 3 However, further investigation is necessary to identify potential risk factors and prevention strategies. Ischemic stroke is brought about by the occlusion of an intracranial vessel, which causes a decrease in blood flow, causing death in the brain tissue and the symptoms that come along with the area affected. 3 While the primary pathology of MS primarily involves the white matter in the CNS, accumulating evidence suggests that MS may also affect the vascular system and contribute to an increased risk of stroke. 1 Understanding the association between MS and stroke is crucial for making sure the patient gets the appropriate treatment strategies and the prevention of stroke‐related complications in MS patients.
The emerging evidence linking MS and stroke suggests a potential shared pathophysiology and comorbidity. Proposed mechanisms include an interplay of immune‐mediated complications and mechanisms such as chronic inflammation, endothelial dysfunction, immune dysregulation, and vascular risk factors. Individuals diagnosed with MS exhibit a heightened susceptibility to cardiovascular comorbidities, including hypertension and hyperlipidemia. The presence of these vascular risk factors has been found to be correlated with the occurrence of stroke. The potential correlation between MS and stroke may be influenced by the presence of common vascular risk factors. Furthermore, it is worth noting that vascular dysfunction in MS has the potential to impact the regulation of cerebral blood flow, which could potentially heighten the vulnerability to cerebrovascular accidents. 3 , 4 Also, In both MS and stroke, inflammation plays a crucial role. The inflammation observed in MS is characterized by a chronic and autoimmune nature, whereas inflammation resulting from stroke is an immediate reaction triggered by the interruption of blood flow in the brain. The inquiry arises regarding the potential vulnerability of patients with MS, who already exhibit elevated levels of inflammation, to the inflammatory reaction observed in stroke cases. 5 Additionally, MS‐related disability and reduced mobility may exacerbate these risk factors and promote a sedentary lifestyle, amplifying the risk of stroke. This may be why some studies present that MS patients have increased stroke and stroke‐related complications. 3 , 4 , 5
Several studies have demonstrated that hospitalization rates among MS patients were either comparable to or lower than their healthy counterparts. However, stroke hospitalization risk was found to be higher among patients with MS when compared to control subjects. 6 , 7 , 8 , 9 In a retrospective analysis conducted on patients who were hospitalized for ischemic stroke, intracerebral hemorrhage (ICH), and unruptured aneurysms, an adjusted standardized prevalence ratio was calculated to determine the likelihood of an MS diagnosis. The results of the analysis revealed that the prevalence ratio for an MS diagnosis was 1.6 for patients with ischemic stroke, 1.3 for those with ICH, and 3.2 for patients with unruptured aneurysms. 7
The misinterpretation of relapses as cerebrovascular events or bias in monitoring may be responsible for the rise in cerebrovascular comorbidities in MS. The observed disparity in stroke risk between short‐term and long‐term data may potentially be attributed to the heightened frequency of neuroimaging assessments and medical consultations during the MS diagnosis and monitoring, particularly for relapsing‐remitting multiple sclerosis (RRMS) patients, with intervals as frequently as every 6 months with the first year after diagnosis is made. 8 , 10 However, the majority of related studies agree that in comparison to healthy subjects, individuals with MS face a heightened risk of experiencing cerebrovascular events, as well as a recurrence of IS or ICH. This elevated risk persists beyond the first year and continues to decrease over time, with some studies reporting follow‐up periods of up to 30 years, representing the longest duration of reported follow‐up to date. 7 , 8 , 11 One study reported that in the initial period after the diagnosis was made, MS patients were at a higher risk of developing ischemic stroke with a risk ratio (RR) of 2.02 and ICH with an RR of 2.65, compared to non‐MS patients. And even 10 years after the initial diagnosis and beginning of MS, the elevated risk remained, although diminished, with a relative risk of 1.29 for ischemic stroke.
The possible association between MS and the risk of stroke represents an exciting and clinically relevant research area due to the morbidity of either condition, let alone when both are present in the same patient. Understanding the underlying pathology and mechanisms, identifying modifiable risk factors, and implementing effective prevention and treatment strategies are paramount in optimizing patient care for individuals with MS, especially with the possible higher risk of stroke. 12 This paper aims to unravel the intricacies of this association and improve outcomes for MS patients regarding stroke prevention and overall vascular health.
2. METHODOLOGY
To conduct this review, we followed a comprehensive approach to gather relevant literature and extract pertinent information. The methodology comprises the following steps.
2.1. Literature search
We conducted an extensive literature search using online databases, including PubMed, MEDLINE, Embase, Scopus, and Google Scholar. The search terms included variations of “multiple sclerosis,” “stroke,” “cerebrovascular disease,” “vascular risk factors,” “disease‐modifying therapies,” and “neuroinflammation.” The search was limited to articles published from January 1, 2000, up to 31 May, 2023.
2.2. Inclusion and exclusion criteria
To ensure the relevance and reliability of the studies included in this review, we established specific inclusion and exclusion criteria. Included studies were required to meet the following criteria: (1) Published in peer‐reviewed journals. (2) Investigating the association between MS and stroke in human populations. (3) Studies reporting on the prevalence, incidence, risk factors, or outcomes of stroke in MS patients. (4) Articles written in English. Studies were excluded if they were: (1) Animal studies. (2) Conference abstracts. (3) Not directly relevant to the association between MS and stroke.
3. DISCUSSION
3.1. Pathophysiology of stroke in multiple sclerosis
The diverse and intricate interactions between genetic, environmental, and immunological variables that cause demyelination and tissue damage in MS are driven by a variety of different processes and may or may not be regulated by epigenetic mechanisms. 13 , 14 , 15 , 16
The pathophysiology of both stroke and MS involves several overlapping mechanisms, including dysfunction of the cerebral endothelium, disruption of the blood–brain barrier (BBB), disturbance of the CNS equilibrium, coagulation system dysregulation, and augmented migration of immune cell, all of which contribute to neuroinflammatory processes (Figure 1). 16 , 17 , 18 , 19 , 20 , 21 , 22 The association between stroke risk and various autoimmune conditions, such as psoriasis, rheumatoid arthritis, osteoarthritis, and bullous pemphigoid, has been established in prior research. 23 , 24 , 25 , 26 Systemic autoimmune disorders exhibit a heightened propensity for stroke relative to organ‐specific autoimmune disorders such as MS. This is attributed to the widespread inflammation that characterizes systemic autoimmune illnesses. 24 , 27 , 28
Figure 1.
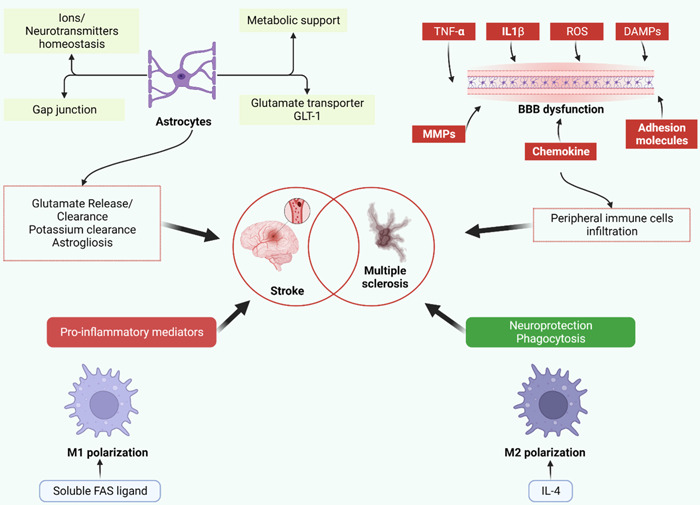
Depicts astrocytes, blood–brain barrier (BBB) failure, and M1/M2 polarization as common routes in the etiology of multiple sclerosis and ischemic stroke. Astrocytes have a role in ion and neurotransmitter balance as well as metabolic support. Glutamate uptake and clearance are enabled via gap junctions and glutamate transporters. Likewise, gap junctions facilitate potassium removal. Astrocytes may release stored glutamate, which may be responsible for delayed lesions. Similarly, astrogliosis has both negative and positive outcomes. It reduces neurogenesis in ischemic tissue while protecting preserved tissue from inflammation. Tumor necrosis factor (TNF)‐ and interleukin‐1 (IL‐1)‐, reactive oxygen species, danger associated molecular patterns (DAMPs, molecules released from necrotic cells), chemokines, matrix metalloproteinases (MMPs), and adhesion molecules all contribute to BBB disruption and peripheral immune cell infiltration. Immune cell M1 polarization is related to an increase in pro‐inflammatory mediators and negative effects on neural lesions. M2 polarization, on the other hand, is connected with neuroprotection and dead cell phagocytosis, allowing for a reduction in inflammation (original figure, made with Biorender).
The implication of ferroptosis/oxytocin‐induced damage has been observed in the context of inflammatory demyelinating conditions, including but not limited to stroke and MS. 29 , 30 , 31 , 32 , 33 , 34 And whether the early neurodegenerative event in the brain is the cause of the autoimmune response, or the autoimmune pathology in MS is the initial pathology, remains unclear. 35 Preclinical studies have revealed a link between the neuroinflammatory process, coagulation system, neuroimmunology, and neurodegenerative symptoms of MS. 36 In MS, platelet activation works as a mediator of neuroinflammation by causing an increase in adhesion and aggregation, as well as the production of pro‐thrombotic microparticles. 37
Proteomics has verified the link between MS and coagulation factors and shown that MS patients have higher levels of platelet activation indicators. 37 Patients with MS were shown to have Brain tissue changes resembling acute white matter strokes, which may indicate that a hypoxia‐like event might contribute to demyelination and neurodegeneration in MS. 32 One of the causes of neuronal malfunction and mortality in patients diagnosed with MS and ischemic stroke is oxidative stress which also damages platelets and is associated with impairment in progressive MS. 30 In fact, a few antioxidant indicators can be utilized to diagnose MS with accuracy. In addition to being linked to stroke and MS, the nitro‐oxidative stress pathway additionally forecast MS disability and poor outcomes in stroke. 32 Immunological assays and metabolomic analyses have demonstrated substantial differences in the glycerophospholipid pathway and the metabolism of linoleic acid in individuals with multiple sclerosis. 38
Additionally, both clinical and preclinical research have linked genes involved in immune responses and cell formation to MS and stroke. 31 Because of the same underlying mechanisms, the vascular comorbidity may appear after the MS's clinical symptoms onset or may be brought on by MS's biological processes. 39
3.2. Risk factors contributing to stroke in multiple sclerosis
The incidence of stroke episodes among women is typically higher than that of males, which may be attributed to their longer life expectancy and a greater likelihood of experiencing a stroke at advanced ages, despite the fact that males have greater age‐related stroke rates. The distribution of stroke risk variables, such as comorbidities, stroke variants, degree of severity, and consequences varied between men and women. 40 Women are more likely than males to develop MS, especially between the ages of 40 and 60. The limited number of studies investigating the incidence of stroke in individuals with MS and the disproportionate representation of females in these studies precludes the establishment of definitive conclusions. 6 , 39 Multiple risk factors have been identified in the literature to describe the intricate connection between MS and stroke (Table 1).
Table 1.
Risk factors and biomarkers that play a role in the association of stroke and multiple sclerosis.
| Risk factors and biomarkers | Association with multiple sclerosis (MS) | Further notes |
|---|---|---|
| Gender | Stroke incidence is higher in females with MS compared to men | Age‐related stroke rates in men contribute to the complex relationship between gender and stroke in MS |
| Comorbidities | A higher risk of comorbidities is seen in MS patients which may account as a risk factor for stroke | Hypertension, hyperlipidemia, and others, is common in MS patients |
| Platelet abnormalities | MS patients experience platelet abnormalities due to disease‐modifying therapies and immune thrombocytopenic purpura | DMTs affect blood clotting and increase the risk of stroke |
| Smoking and obesity | More frequently encountered in MS patients | |
| Low levels of Vitamin D | Low vitamin D levels have been linked to the progression and outcomes of MS | Vitamin D has also been linked to the risk of developing infection‐related stroke |
| Infections | MS have higher infection, hospitalization, and infection‐related mortality rates | Infections can trigger or exacerbate stroke risk in MS patients |
| Antiphospholipid (APL) syndrome | APL have been identified in MS patients and is linked to vascular thrombosis | APL antibodies in MS patients increase the risk of vascular events and neurological symptoms |
| Uric acid levels | Altered levels of uric acid in MS patients may have implications for Stroke risk | This is especially true in progressive MS subtypes |
| Nitric oxide and endothelin‐1 | Higher levels of NO and endothelin‐1 are expressed in MS. | NO, and endothelin‐1 have significant implications for cerebral microcirculation and inflammation in MS patients |
| Gut dysbiosis | Growing evidence links gut dysbiosis to stroke etiology | The gut‐brain axis and its influence on stroke etiology and outcomes in MS are areas of active research. |
When compared to matched controls, comorbidities that are recognized as stroke risk factors are more frequent in patients diagnosed with MS, which may be the reason for the higher risk for stroke. 41 , 42 , 43 Hypertension and hyperlipidemia are the most commonly documented cardiovascular comorbidities in MS, with prevalence rates exceeding 10% and rising with advancing age. 41 , 42 Moreover, MS patients typically experience platelet abnormalities that may be related to DMT; in contrast, immune thrombocytopenic purpura patients have higher MS risk development. Due to the inflammatory effects of platelet microparticles, Immune thrombocytopenia, which is connected to ischemic stroke, increases bleeding episodes such that they occur more frequently. Considering modifiable risk factors for stroke, it is more frequent to smoke for patients diagnosed with MS and also be obese than the general population, however, there is a dearth of information on alcohol intake. 11 , 42 , 44 , 45 Even in the early stages of MS when disability is moderate, the reduced physical activity of MS patients might activate coagulation pathways, increasing the risk for venous thromboembolism (VTE) and subclinical atherosclerosis. 42 Low levels of vitamin D and infections are among the additional risk factors that exhibit independent associations with the progression and outcomes of MS or the risk for stroke caused by bacteria or viruses. 13 , 45 Patients with MS had greater rates of infection incidence, hospitalization associated with infection, and infection‐related mortality. 46 , 47 A lack of vitamin D increases immunological responses in MS and increases the risk of relapses. 31 , 33
Antiphospholipid (APL) antibodies of many different types have been identified in MS patients. The autoimmune condition antiphospholipid syndrome (APS) has been linked to the presence of these antibodies in conjunction with recurrent thrombosis. The primary APS consequences include vascular events and/or pregnancy difficulties when paired with APLs. 48 Due to dysfunction in thrombosis and the immune system, transient and persistent ischemia episodes are the predominant neurological symptoms of APS, especially in young patients. 49 Because APS may develop as a result of MS and because its neurological symptoms may be difficult to identify from MS itself in brain MRI, misdiagnosis and inappropriate management of stroke caused by APS rather than MS are real possibilities. 50 Several MS clinical subtypes have a wide variety of APL antibodies. Anticardiolipin (ACL), anti‐2‐glycoprotein‐i (A2GPI), and lupus anticoagulant (LA) are three of the APL antibodies that are frequently used to diagnose APS. Patients with MS have a higher incidence of APLs antibodies in their blood and cerebrospinal fluid (CSF) than healthy controls and is noticeably greater during MS exacerbations. 51 Further research is being done on APL antibodies in MS patients, but more research is needed to understand how they affect thrombosis. Rarely have LA antibodies been found in MS patients.
Severe findings in MRI and worsening in clinical features were noted among MS patients receiving IFN‐β (interferon β protein) who are diagnosed with MS. Antiannexin antibodies (AANV) are considered to have a larger role in vascular thrombosis in CNS, in APS. Research findings reveal that acute myocardial infarction patients who did not possess traditional cardiovascular risk factors exhibited AANV antibodies along with decreased plasma levels of annexin v. Based on clinical investigations, a strong correlation was observed between the elevated levels of total cholesterol and low‐density lipoprotein (LDL) cholesterol in various types of MS. Additionally, research findings suggest that AANV titers are associated with disease progression patients with RRMS subtype. 51
Alterations in the levels of specific metabolites or biomarkers have also been related to MS, and they are thought to have a role in stroke. For instance, elevated ferritin levels brought on by oxidative damage from poor iron metabolism are linked to inflammation and neurodegeneration in both MS and stroke. 33 It has also been noted that MS patients have a ferritin shortage that strongly corresponds with depressive disorders. 52 Uric acid, which has an antioxidant activity that can have a neuronal protection function against oxidative damage, has been linked to increasing both ischemic and hemorrhagic stroke risk, as well as a possible exacerbation of stroke outcomes. There exist differences in the extent of reduction among secondary‐progressive multiple sclerosis (SPMS), RRMS, or primary‐progressive multiple sclerosis (PPMS) patients. However, it is observed that individuals diagnosed with MS exhibit decreased levels of serum uric acid (UA) in comparison to healthy individuals. In particular, SPMS patients exhibit lower serum uric acid levels compared to RRMS or PPMS patients. Furthermore, a poor MS prognosis has been related to low uric acid levels. Low uric acid levels are not only associated with MS disease outcomes but also with other neurological disorders and dysfunctional BBB in MS.
The reduced likelihood of gout in MS patients suggests a link between the level of metabolism of uric acid and MS, which is a sign of elevated levels of uric acid. This connection is further strengthened by the levels of uric acid in MS patients. The potential advantages of elevated uric acid levels in individuals with MS are subject to the intricacies of the endogenous neurologically protective mechanisms linked with uric acid.
It is common to report hyperhomocysteinemia in neurological illnesses, which is a well‐known risk factor for diseases affecting endothelium and is connected to a higher risk of atherosclerosis and myocardial infarction. The increases in Homocysteine levels in patients with MS are linked with inflammation, dysfunction in cognition, impaired neuronal homeostasis, dysfunction of endothelial cells, and risk of increased recurrent endothelial dysfunction of the vasculature. They also correspond with the progression of the disease and decreases in cognition. 6 , 32 Regardless of sex or age, MS patients with progressive rather than RRMS had higher levels of homocysteine. They also had higher levels of circulating d‐dimer.
In the context of an acute clinical episode, it has been observed that MS patients exhibit elevated concentrations of nitric oxide (NO) and endothelin‐1. These molecules are known to play a significant role in the pathophysiology of MS and are essential for maintaining cerebral microcirculation. 53 , 54 , 55 It has been observed that individuals who aren't diagnosed with MS have exhibited a surge in the levels of plasma endothelin‐1 in the aftermath of an ischemic stroke, which persists for a duration of up to 24 h. Nevertheless, endothelin‐1‐mediated cerebral hypoperfusion appears to be reversible. 55 Moreover, the levels of NO have been reported to be greater in patients diagnosed with MS compared to healthy subjects and in MS patients undergoing DMT compared to those with MS not on DMT. Despite other factors, including CSF glutamate levels, that are associated with the development of a stroke, higher NO levels are linked to more severe brain damage and early neurological decline after stroke. 32 In one study, Serum glutamate and levels of NO in patients with MS who are relapsing were substantially higher than those with stable disease or healthy controls. Also, it was noted that MS patients' serum glutamate levels were considerably greater than those of healthy controls. As compared to healthy controls, the serum of those with MS along with CSF analysis showed higher levels of Nitric oxide and interleukin‐10 during flare‐ups.
Although there is no obvious causal link between stroke and autonomic nervous system dysfunction, cardiac autonomic dysfunction (CAD), which increases the risk factors that make people more susceptible to stroke, is frequently seen in patients who have already experienced a stroke. 56 , 57 , 58 CAD is also seen in progressive variations of MS, because of the disparity of the output of both sympathetic along with parasympathetic systems to the cardiovascular system. Dysfunction in the parasympathetic system, as opposed to the sympathetic, is linked with the increases in disability and disease progression and may even be the cause of MS. On the other hand, dysfunction in the sympathetic system is correlated to the clinical and inflammation activity of MS and may have an impact on the development of the disease. As well as causing severe metabolic and structural abnormalities in the venous system, CAD may accelerate MS's inflammatory and neurodegenerative pathways. 8
There is growing evidence linking gut dysbiosis to stroke etiology, and this may have an impact on stroke outcomes if it occurs before IS. It's noteworthy to note that gastrointestinal issues can be seen in MS patients before the onset of classic MS symptoms. Moreover, the development of experimental autoimmune encephalomyelitis (EAE) in healthy mice was caused by the passive transfer of the gut microbiota from animals with EAE. It is still unclear if MS's neuroinflammation results from or is caused by gut dysbiosis, and its impact on the BBB is being studied. 59
3.3. Preventive strategies for stroke in multiple sclerosis
The use of an adjuvant preventive medication for stroke prevention in patients with MS at risk has been considered. However, a comprehensive analysis of the ratio between benefits and risks is necessary. Anticoagulant medication, either alone or as an addition to DMT therapy, has not yet been the subject of any placebo‐controlled research to see if patients diagnosed with MS can benefit. Aspirin antiplatelet therapy has been suggested to reduce inflammation and improve MS symptoms, but it may potentially raise the chance of ICH or other bleeding events. Nonetheless, anticoagulant has been linked to a higher ICH incidence in patients diagnosed with MS compared to antiplatelet therapy. 9 For the treatment of APS‐related IS, antiplatelet or anticoagulant treatments are recommended.
Additionally, MS patients with APL positive should have routine testing for APL titers because it is still unclear how APLs contribute to the disease etiology. A subset of MS patients who were treated with acetylsalicylic acid for an average of 20.8 months and occasionally with brief courses of steroids experienced a slow pace of development of their condition. These patients had atypical clinical features and elevated ACL antibody levels consistently. The identification of subgroups of MS patients exhibiting chronically elevated levels of APL presents an opportunity for the implementation of adjunctive therapies aimed at reducing the associated risk. Specifically, the administration of antiplatelet, anticoagulant, or statin treatments may prove beneficial in mitigating the aforementioned risk, while more research is needed in this area.
As a result of similar underlying processes, immunotherapy for stroke utilizing medications approved for MS has been employed in medication repurposing trials to reduce neuroinflammation and for the maintenance of BBB integrity. 18 The extension of treatment indications for stroke has been the subject of clinical studies, with a focus on the efficacy of fingolimod and natalizumab (NTZ), based on encouraging preclinical data. Improvements in functional outcomes and a reduction in infarct volume growth were seen after fingolimod treatment in pilot investigations in IS or ICH. In double‐blind clinical trials, the administration of NTZ less than 24 h following the beginning of acute ischemic stroke had no positive impact on patient outcomes.
Statins have been effectively utilized to reduce the permeability of the BBB, limiting the migration of leukocytes through the BBB, especially in the initial disease course. Supplementing MS patients' diets with dietary antioxidants that lower their risk of stroke, including vitamin C, may also be helpful. 59 The evidence for routine vitamin delivery in MS patients is weak, with the exception of vitamin D, and controlled clinical research will provide proof of the potential therapeutic value of this intervention. 60
3.4. Impact of multiple sclerosis treatment on stroke
The goal of treatment for MS patients is to either stop the disease's progression, control its symptoms, or prevent relapses. Currently, there are no drugs that totally eradicate relapses in MS, however, the use of DMTs is of great value to reduce the activity of MRI and the rate of relapsing. These DMTs have different mechanisms of action and administration approaches. 15 They can include monoclonal antibodies, glatiramer acetate (GA), interferons, and sphingosine 1‐phosphate receptor modulators.
The availability of ocrelizumab as the only medication for patients with RRMS and PPMS has significantly transformed the treatment of MS, including both relapsing and progressive forms. 61 Current clinical studies are exploring the potential of other monoclonal antibodies in this regard. A reduction in the rate of annual recurrence by 29%–68% is observed when treated with the currently available DMTs as compared to placebo or active comparators. 62 The possibility for treating MS patients differently is made possible by the wide variety of DMTs that are readily available. 63 To treat MS and inflammation as best as possible, switching between DMTs is frequently employed in clinical practice. DMTs may enhance the risk of cerebrovascular illness in vulnerable patients by amplifying risk factors of stroke. 64 It has been noted that the usage of IFN‐β and GA and stroke risk factors are positively correlated. IFN‐β therapy has an impact on blood pressure (BP), lipid balance, and perhaps infection risk in MS patients. 65 Similar to fingolimod, dimethyl fumarate (DMF) medication has been linked to higher HDL levels in MS patients, while it is unknown if this is related to vascular morbidity. Hematological problems may also result from MS therapy. Alternately, hematological diseases may present as a consequence of cerebral ischemia or a brief ischemic event. 49 In one study, female patients and those who had been exposed previously to natalizumab were more likely to experience lymphopenia when taking fingolimod as compared to DMF for a minimum of a year. On the other hand, low absolute lymphocyte count was associated with DMF therapy. The objective of this investigation was to ascertain the factors that contribute to the hematological irregularities commonly observed in association with these therapies. Several reports have surfaced regarding the incidence of stroke and suboptimal neurological outcomes among patients diagnosed with MS who have undergone alemtuzumab therapy for a minimum of two doses at least. 66 , 67 They were perhaps linked to hypertension, hence MS patients on alemtuzumab must have frequent arterial pressure monitoring, with special attention paid to increases in systolic BP of more than 20 mmHg, or 20%.
Thrombotic microangiopathy, a rare but severe case report of MS patients treated with IFN‐Β, has been linked to thrombocytopenia, hemolytic anemia, and microvascular occlusions. 14 According to adjusted studies, individuals receiving high doses of IFN‐Β therapy had an eightfold higher chance of developing thrombocytopenia than those receiving low doses or no IFN‐Β therapy for their MS. 68 Additional MS investigations revealed that compared to NTZ and fingolimod, the administration of therapy involving GA or IFN‐β has been found to be associated with a significantly increased incidence of stroke, with a relative risk of approximately 10 and 50 times higher, respectively. The potential association between IFN‐β and heightened platelet adherence to the endothelium may be attributed to its impact on arterial wall competence or its ability to upregulate the expression of the mean corpuscular hemoglobin (MHC) molecule in platelets and other cellular entities. As an alternative, the potential exacerbator effects of IFN‐β on coexisting autoimmune conditions, such as vasculitis, have been proposed in the literature. Fingolimod prevents astrocytes from oxygen‐glucose deprivation damage and can help with acute ischemic stroke or cerebral edema after ICH by lowering infiltration of lymphocytes, minimizing disruption in BBB, and enhancing blood flow to the cerebrum. 69 There are a number of documented instances of reversible cerebral vasoconstriction syndrome induced by fingolimod in patients with multiple sclerosis that are considered rare; these studies most likely included doses of more than 0.5 mg daily. 70
In blocking the entry of immune cells over the BBB into the CNS, clinical trials have shown the antiatherogenic potential of natalizumab. 64 Moreover, NTZ has been linked to extremely low rates of thrombosis and stroke (0.2% each). In a modest short‐term follow‐up study (12.9, 6.2 months), NTZ therapy also resulted in statistically significant elevated UA levels. Rare safety hazards of NTZ that may exacerbate stroke include the emergence of viral infections and inflammatory illnesses. 71 Nonsteroidal anti‐inflammatory medicines, which may be used to treat MS‐related symptomatic pain, have been linked in a number of studies to a probable increased risk of stroke due to thrombosis, however, the evidence is still ambiguous. A myocardial infarction, stroke, and cerebrovascular illness (cerebral thrombophlebitis) may all be made more likely by systemic glucocorticoids, especially when used in large dosages.
The presence of certain risk factors such as disability, spasticity, recent antidepressant use, or systemic glucocorticoid usage in individuals with MS may increase the likelihood of VTE while also potentially confounding the results of studies investigating this phenomenon. As a result, there exists a notable scarcity of dependable evidence in this area. 72 The administration of high‐dosage intravenous glucocorticoids subsequent to lumbar puncture has been linked, albeit in a limited number of case reports, to cerebral venous thrombosis in both adult and pediatric patients diagnosed with multiple sclerosis. As a result, the prophylactic prescription of anticoagulants is warranted under these circumstances.
The higher prevalence of stroke risk factors in MS patients and potential side effects of MS therapy, as well as the interaction between inflammation and the cerebral microenvironment that occurs in chronic neurodegenerative disorders, are all factors that contribute to a greater likelihood of stroke in MS patients. 73 While there is a possibility of an overestimation of the short‐term risk for stroke due to increased monitoring bias following the diagnosis of MS, the majority of research studies concur that the elevated risk for stroke remains significant over the long term, with a duration of up to 30 years post‐MS diagnosis. Further investigation into multiple sclerosis patients that takes into consideration the risk factors associated with stroke is imperative in comprehending the efficacy of this approach.
Ponesimod, a drug that recently got the FDA‐approval for RRMS, is a rapidly reversible sphingosine phosphate (S1P) receptor modulator which recently approved in both the United States and the European Union for treatment of relapsing forms of multiple sclerosis. Its mechanism of action to eliminate the lymphocytes from the lymphoid organs, which in turn lead to restrict the autoreactive cells to enter the CNS. 74
Ponesimod has been shown temporary increasing of blood enzymes during therapy despite the limited experience with its usage, for example, elevation of serum ALT were common (in up to 23% of recipients) but were almost mild and with any clinical manifestations, returning to its baseline values even with its continuation of using or some months after cessation of drug. Also serum aminotransferase increase above three times upper limit of normal were discovered in 17% of patients who taking this drug, although there were no any approved case of acute hepatitis or clinically apparent liver injury with jaundice. 75
This drug shows wide spectrum of side effects such as lymphopenia, headache, dizziness, dizziness, diarrhea, cough, rhinorrhea, peripheral edema as well as back and abdominal pain, other severe adverse effects include viral, bacterial, or fungal infections, atrial arrhythmia, and bradycardia, macular edema, decrease in pulmonary function, progressive multifocal leukoencephalopathy, and embryonal‐fetal toxicity, that's why patients should be monitored for any infectious or complications and other cardiopulmonary and ophthalmologic conditions. 75
4. CONCLUSION
This review article explores the association between stroke and multiple sclerosis, focusing on the immune‐mediated nature of MS and its effects on the CNS. It emphasizes that research on the venous aspect of MS, particularly in terms of clinical, pharmacological, and pathological investigation, is relatively underdeveloped. Differentiating between stroke prevalence and incidence, as well as distinguishing between ischemic stroke and ICH, is crucial due to the heterogeneity in clinical findings. Several risk factors for stroke in MS are identified, including comorbidities like hypertension and hyperlipidemia, platelet abnormalities, vitamin D deficiency, and autoimmune factors such as APL antibodies. Preventive strategies for stroke in MS include the use of anticoagulants or antiplatelet therapy and potential treatment repurposing using medications approved for MS. There is a need for further research to enhance understanding of the link between stroke and MS, identify risk factors, and develop evidence‐based strategies for prevention and treatment. Multiple large‐scale longitudinal studies to track a cohort of MS patients over an extended period, collecting detailed information on their medical history, disease progression, and the occurrence of strokes can be attempted. Additionally, exploring the potential biomarkers in blood or CSF that could indicate an increased risk of stroke in MS patients.
5. LIMITATIONS
The review identified limitations in the literature, including a lack of distinction between stroke prevalence and incidence, and insufficient differentiation between ischemic stroke and ICH. Furthermore, variations in clinical settings (hospitalized vs. outpatient) were not consistently considered. To address these gaps, larger prospective studies are warranted to enhance our understanding of stroke risk in MS patients and to develop targeted prevention strategies tailored to this specific population.
AUTHOR CONTRIBUTIONS
Mohammed Dheyaa Marsool Marsool: Methodology; resources; validation; visualization; writing—original draft; writing—review and editing. Priyadarshi Prajjwal: Conceptualization; methodology; writing—original draft; writing—review and editing. Jobby John: Validation; visualization; writing—original draft. Harshada S. Keluskar: Conceptualization; methodology; writing—original draft. Venu V. Sivarajan: Validation; visualization; writing—original draft. Khadijah A. Kundiri: Validation; writing—original draft. Justin R. Lam: Writing—original draft. Sachi Chavda: Writing—original draft. Hundaol G. Atew: Writing—original draft. Ali Dheyaa Marsool Marsool: Writing—original draft; Writing—review and editing. Al‐Tuaama A. Z. Hameed: Writing—review and editing. Omniat A. Hussin: Writing—review and editing.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
TRANSPARENCY STATEMENT
The lead author Omniat Amir Hussin affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Marsool MDM, Prajjwal P, John J, et al. Association of multiple sclerosis with stroke: a comprehensive review. Health Sci Rep. 2024;7:e1837. 10.1002/hsr2.1837
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Dahlöf B. Prospects for the prevention of stroke. J Hypertens. 2006;24(2):S3‐S9. 10.1097/01.hjh.0000220097.04531.6f [DOI] [PubMed] [Google Scholar]
- 2. Tallantyre EC, Morgan PS, Dixon JE, et al. A comparison of 3T and 7T in the detection of small parenchymal veins within MS lesions. Invest Radiol. 2009;44(9):491‐494. 10.1097/RLI.0b013e3181b4c144 [DOI] [PubMed] [Google Scholar]
- 3. Donnan GA, Fisher M, Macleod M, Davis SM. Stroke. Lancet. 2008;371(9624):1612‐1623. 10.1016/S0140-6736(08)60694-7 [DOI] [PubMed] [Google Scholar]
- 4. Meschia JF, Bushnell C, Boden‐Albala B, et al. Guidelines for the primary prevention of stroke: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(12):3754‐3832. 10.1161/STR.0000000000000046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Straus SE, Majumdar SR, McAlister FA. New evidence for stroke prevention: scientific review. JAMA. 2002;288(11):1388‐1395. 10.1001/jama.288.11.1388 [DOI] [PubMed] [Google Scholar]
- 6. Jadidi E, Mohammadi M, Moradi T. High risk of cardiovascular diseases after diagnosis of multiple sclerosis. Multiple Sclerosis Journal. 2013;19(10):1336‐1340. 10.1177/1352458513475833 [DOI] [PubMed] [Google Scholar]
- 7. Thormann A, Magyari M, Koch‐Henriksen N, Laursen B, Sørensen PS. Vascular comorbidities in multiple sclerosis: a nationwide study from Denmark. J Neurol. 2016;263(12):2484‐2493. 10.1007/s00415-016-8295-9 [DOI] [PubMed] [Google Scholar]
- 8. Roshanisefat H, Bahmanyar S, Hillert J, Olsson T, Montgomery S. Multiple sclerosis clinical course and cardiovascular disease risk ‐ Swedish cohort study. Eur J Neurol. 2014;21(11):1353‐e88. 10.1111/ene.12518 [DOI] [PubMed] [Google Scholar]
- 9. Jakimovski D, Topolski M, Genovese AV, Weinstock‐Guttman B, Zivadinov R. Vascular aspects of multiple sclerosis: emphasis on perfusion and cardiovascular comorbidities. Expert Rev Neurother. 2019;19(5):445‐458. 10.1080/14737175.2019.1610394 [DOI] [PubMed] [Google Scholar]
- 10. Tseng CH, Huang WS, Lin CL, Chang YJ. Increased risk of ischaemic stroke among patients with multiple sclerosis. Eur J Neurol. 2015;22(3):500‐506. 10.1111/ene.12598 [DOI] [PubMed] [Google Scholar]
- 11. Hong Y, Tang HR, Ma M, Chen N, Xie X, He L. Multiple sclerosis and stroke: a systematic review and meta‐analysis. BMC Neurol. 2019;19(1):139. 10.1186/s12883-019-1366-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rodés‐Cabau J, Noël M, Marrero A, et al. Atherosclerotic burden findings in young cryptogenic stroke patients with and without a patent foramen ovale. Stroke. 2009;40(2):419‐425. 10.1161/STROKEAHA.108.527507 [DOI] [PubMed] [Google Scholar]
- 13. Celarain N, Tomas‐Roig J. Aberrant DNA methylation profile exacerbates inflammation and neurodegeneration in multiple sclerosis patients. J Neuroinflammation. 2020;17(1):21. 10.1186/s12974-019-1667-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Koudriavtseva T. Thrombotic processes in multiple sclerosis as manifestation of innate immune activation. Front Neurol. 2014;5:119. 10.3389/fneur.2014.00119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Petersen MA, Ryu JK, Akassoglou K. Fibrinogen in neurological diseases: mechanisms, imaging and therapeutics. Nat Rev Neurosci. 2018;19(5):283‐301. 10.1038/nrn.2018.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhao Z, Nelson AR, Betsholtz C, Zlokovic BV. Establishment and dysfunction of the blood‐brain barrier. Cell. 2015;163(5):1064‐1078. 10.1016/j.cell.2015.10.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sheikh MH, Henson SM, Loiola RA, et al. Immuno‐metabolic impact of the multiple sclerosis patients' sera on endothelial cells of the blood‐brain barrier. J Neuroinflammation. 2020;17(1):153. 10.1186/s12974-020-01810-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Levard D, Buendia I, Lanquetin A, Glavan M, Vivien D, Rubio M. Filling the gaps on stroke research: focus on inflammation and immunity. Brain Behav Immun. 2021;91:649‐667. 10.1016/j.bbi.2020.09.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Plantone D, Inglese M, Salvetti M, Koudriavtseva T. A perspective of coagulation dysfunction in multiple sclerosis and in experimental allergic encephalomyelitis. Front Neurol. 2019;9:1175. 10.3389/fneur.2018.01175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ziliotto N, Bernardi F, Jakimovski D, Zivadinov R. Coagulation pathways in neurological diseases: multiple sclerosis. Front Neurol. 2019;10:409. 10.3389/fneur.2019.00409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Koike H, Katsuno M. Macrophages and autoantibodies in demyelinating diseases. Cells. 2021;10(4):844. 10.3390/cells10040844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhao J, Roberts A, Wang Z, Savage J, Ji RR. Emerging role of PD‐1 in the central nervous system and brain diseases. Neurosci Bull. 2021;37(8):1188‐1202. 10.1007/s12264-021-00683-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Baena‐Díez JM, Garcia‐Gil M, Comas‐Cufí M, et al. Association between chronic immune‐mediated inflammatory diseases and cardiovascular risk. Heart. 2018;104(2):119‐126. 10.1136/heartjnl-2017-311279 [DOI] [PubMed] [Google Scholar]
- 24. Packer M. Potential role of atrial myopathy in the pathogenesis of stroke in rheumatoid arthritis and psoriasis: a conceptual framework and implications for prophylaxis. J Am Heart Assoc. 2020;9(3):e014764. 10.1161/JAHA.119.014764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tarazona MJM, Mota ANCM, Gripp AC, Unterstell N, Bressan AL. Bullous pemphigoid and neurological disease: statistics from a dermatology service. An Bras Dermatol. 2015;90(2):280‐282. 10.1590/abd1806-4841.20153334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zha AM, Di Napoli M, Behrouz R. Prevention of stroke in rheumatoid arthritis. Curr Neurol Neurosci Rep. 2015;15(12):77. 10.1007/s11910-015-0600-y [DOI] [PubMed] [Google Scholar]
- 27. Boehncke WH. Systemic inflammation and cardiovascular comorbidity in psoriasis patients: causes and consequences. Front Immunol. 2018;9:579. 10.3389/fimmu.2018.00579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wiseman SJ, Ralston SH, Wardlaw JM. Cerebrovascular disease in rheumatic diseases: a systematic review and meta‐analysis. Stroke. 2016;47(4):943‐950. 10.1161/STROKEAHA.115.012052 [DOI] [PubMed] [Google Scholar]
- 29. Maher P, Currais A, Schubert D. Using the oxytosis/ferroptosis pathway to understand and treat age‐associated neurodegenerative diseases. Cell Chem Biol. 2020;27(12):1456‐1471. 10.1016/j.chembiol.2020.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ren JX, Li C, Yan XL, Qu Y, Yang Y, Guo ZN. Crosstalk between oxidative stress and ferroptosis/oxytosis in ischemic stroke: possible targets and molecular mechanisms. Oxid Med Cell Longevity. 2021;2021:6643382. 10.1155/2021/6643382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Alfieri DF, Lehmann MF, Flauzino T, et al. Immune‐inflammatory, metabolic, oxidative, and nitrosative stress biomarkers predict acute ischemic stroke and short‐term outcome. Neurotoxic Res. 2020;38(2):330‐343. 10.1007/s12640-020-00221-0 [DOI] [PubMed] [Google Scholar]
- 32. Flauzino T, Simão ANC, de Carvalho Jennings Pereira WL, et al. Disability in multiple sclerosis is associated with age and inflammatory, metabolic and oxidative/nitrosative stress biomarkers: results of multivariate and machine learning procedures. Metab Brain Dis. 2019;34(5):1401‐1413. 10.1007/s11011-019-00456-7 [DOI] [PubMed] [Google Scholar]
- 33. Reiche EMV, Gelinksi JR, Alfieri DF, et al. Immune‐inflammatory, oxidative stress and biochemical biomarkers predict short‐term acute ischemic stroke death. Metab Brain Dis. 2019;34(3):789‐804. 10.1007/s11011-019-00403-6 [DOI] [PubMed] [Google Scholar]
- 34. Tan Q, Fang Y, Gu Q. Mechanisms of modulation of ferroptosis and its role in central nervous system diseases. Front Pharmacol. 2021;12:657033. 10.3389/fphar.2021.657033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sen MK, Almuslehi MSM, Shortland PJ, Coorssen JR, Mahns DA. Revisiting the pathoetiology of multiple sclerosis: has the tail been wagging the mouse? Front Immunol. 2020;11:572186. 10.3389/fimmu.2020.572186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ramagopalan SV, Wotton CJ, Handel AE, Yeates D, Goldacre MJ. Risk of venous thromboembolism in people admitted to hospital with selected immune‐mediated diseases: record‐linkage study. BMC Med. 2011;9:1. 10.1186/1741-7015-9-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dziedzic A, Miller E, Bijak M, Przyslo L, Saluk‐Bijak J. Increased pro‐thrombotic platelet activity associated with thrombin/PAR1‐dependent pathway disorder in patients with secondary progressive multiple sclerosis. Int J Mol Sci. 2020;21(20):7722. 10.3390/ijms21207722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Stoessel D, Stellmann JP, Willing A, et al. Metabolomic profiles for primary progressive multiple sclerosis stratification and disease course monitoring. Front Hum Neurosci. 2018;12:226. 10.3389/fnhum.2018.00226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hauer L, Perneczky J, Sellner J. A global view of comorbidity in multiple sclerosis: a systematic review with a focus on regional differences, methodology, and clinical implications. J Neurol. 2021;268(11):4066‐4077. 10.1007/s00415-020-10107-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Arboix A, Cartanyà A, Lowak M, et al. Gender differences and woman‐specific trends in acute stroke: results from a hospital‐based registry (1986‐2009). Clin Neurol Neurosurg. 2014;127:19‐24. 10.1016/j.clineuro.2014.09.024 [DOI] [PubMed] [Google Scholar]
- 41. Edwards NC, Munsell M, Menzin J, Phillips AL. Comorbidity in US patients with multiple sclerosis. Patient Relat Outcome Meas. 2018;9:97‐102. 10.2147/PROM.S148387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Marrie RA, Reider N, Cohen J, et al. A systematic review of the incidence and prevalence of cardiac, cerebrovascular, and peripheral vascular disease in multiple sclerosis. Multi Scler J. 2015;21(3):318‐331. 10.1177/1352458514564485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Marrie RA, Rudick R, Horwitz R, et al. Vascular comorbidity is associated with more rapid disability progression in multiple sclerosis. Neurology. 2010;74(13):1041‐1047. 10.1212/WNL.0b013e3181d6b125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mincu RI, Magda LS, Florescu M, et al. Cardiovascular dysfunction in multiple sclerosis. Maedica. 2015;10(4):364‐370. [PMC free article] [PubMed] [Google Scholar]
- 45. Caprio MG, Russo C, Giugliano A, et al. vascular disease in patients with multiple sclerosis: a review. vasc. Med Surg. 2016;4(2):1000259. [Google Scholar]
- 46. Marrie RA, Elliott L, Marriott J, et al. Effect of comorbidity on mortality in multiple sclerosis. Neurology. 2015;85(3):240‐247. 10.1212/WNL.0000000000001718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Murtonen A, Kurki S, Hänninen K, Soilu‐Hänninen M, Sumelahti ML. Common comorbidities and survival in MS: risk for stroke, type 1 diabetes and infections. Multi Scler Rel Disord. 2018;19:109‐114. 10.1016/j.msard.2017.10.019 [DOI] [PubMed] [Google Scholar]
- 48. Martins FF, Campos TML. Evaluation of frequency, clinical correlation, and antibodies confirmation profile in patients with suspected antiphospholipid syndrome. TH Open. 2021;05(4):e470‐e478. 10.1055/s-0041-1736289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Arboix A, Jiménez C, Massons J, Parra O, Besses C. Hematological disorders: a commonly unrecognized cause of acute stroke. Expert Rev Hematol. 2016;9(9):891‐901. 10.1080/17474086.2016.1208555 [DOI] [PubMed] [Google Scholar]
- 50. Fleetwood T, Cantello R, Comi C. Antiphospholipid syndrome and the neurologist: from pathogenesis to therapy. Front Neurol. 2018;9:1001. 10.3389/fneur.2018.01001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mandoj C, Renna R, Plantone D, et al. Anti‐annexin antibodies, cholesterol levels and disability in multiple sclerosis. Neurosci Lett. 2015;606:156‐160. 10.1016/j.neulet.2015.08.054 [DOI] [PubMed] [Google Scholar]
- 52. Knyszyńska A, Radecka A, Zabielska P, Łuczak J, Karakiewicz B, Lubkowska A. The role of iron metabolism in fatigue, depression, and quality of life in multiple sclerosis patients. Int J Environ Res Public Health. 2020;17(18):6818. 10.3390/ijerph17186818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wang Q, Wen X, Kong J. Recent progress on uric acid detection: a review. Crit Rev Anal Chem. 2020;50(4):359‐375. 10.1080/10408347.2019.1637711 [DOI] [PubMed] [Google Scholar]
- 54. Guerrero AL, Martín‐Polo J, Laherrán E, et al. Variation of serum uric acid levels in multiple sclerosis during relapses and immunomodulatory treatment. Eur J Neurol. 2008;15(4):394‐397. 10.1111/j.1468-1331.2008.02087.x [DOI] [PubMed] [Google Scholar]
- 55. D'haeseleer M, Beelen R, Fierens Y, et al. Cerebral hypoperfusion in multiple sclerosis is reversible and mediated by endothelin‐1. Proc Natl Acad Sci USA. 2013;110(14):5654‐5658. 10.1073/pnas.1222560110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Steensig K, Olesen KKW, Thim T, et al. CAD is an independent risk factor for stroke among patients with atrial fibrillation. J Am Coll Cardiol. 2018;72(20):2540‐2542. 10.1016/j.jacc.2018.08.1046 [DOI] [PubMed] [Google Scholar]
- 57. Paolicelli D, Manni A, Direnzo V, et al. Long‐term cardiac safety and tolerability of fingolimod in multiple sclerosis: a postmarketing study. J Clin Pharmacol. 2015;55(10):1131‐1136. 10.1002/jcph.519 [DOI] [PubMed] [Google Scholar]
- 58. Pröbstel AK, Baranzini SE. The role of the gut microbiome in multiple sclerosis risk and progression: towards characterization of the “MS microbiome”. Neurotherapeutics. 2018;15(1):126‐134. 10.1007/s13311-017-0587-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ramírez‐Salazar SA, Herren C, McCartney J, Ortiz García JG. Dietary insights in neurological diseases. Curr Neurol Neurosci Rep. 2021;21(10):55. 10.1007/s11910-021-01143-w [DOI] [PubMed] [Google Scholar]
- 60. Evans E, Piccio L, Cross AH. Use of vitamins and dietary supplements by patients with multiple sclerosis: a review. JAMA Neurol. 2018;75(8):1013‐1021. 10.1001/jamaneurol.2018.0611 [DOI] [PubMed] [Google Scholar]
- 61. Prajjwal P, Marsool MDM, Asharaf S, et al. Comparison of recent updates in genetics, immunology, biomarkers, and neuroimaging of primary‐progressive and relapsing‐remitting multiple sclerosis and the role of ocrelizumab in the management of their refractory cases. Health Sci Rep. 2023;6(7):e1422. 10.1002/hsr2.1422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ghezzi A. European and American guidelines for multiple sclerosis treatment. Neurol Ther. 2018;7(2):189‐194. 10.1007/s40120-018-0112-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wingerchuk DM, Weinshenker BG. Disease modifying therapies for relapsing multiple sclerosis. BMJ. 2016;354:i3518. 10.1136/bmj.i3518 [DOI] [PubMed] [Google Scholar]
- 64. Sternberg Z, Leung C, Sternberg D, Yu J, Hojnacki D. Disease modifying therapies modulate cardiovascular risk factors in patients with multiple sclerosis. Cardiovasc Ther. 2014;32(2):33‐39. 10.1111/1755-5922.12049 [DOI] [PubMed] [Google Scholar]
- 65. Wijnands JMA, Zhu F, Kingwell E, et al. Disease‐modifying drugs for multiple sclerosis and infection risk: a cohort study. J Neurol Neurosurg Psychiat. 2018;89(10):1050‐1056. 10.1136/jnnp-2017-317493 [DOI] [PubMed] [Google Scholar]
- 66. Alnahdi MA, Aljarba SI, Al Malik YM. Alemtuzumab‐induced simultaneous onset of autoimmune haemolytic anaemia, alveolar haemorrhage, nephropathy, and stroke: a case report. Multi Scler Relat Disord. 2020;41:102141. 10.1016/j.msard.2020.102141 [DOI] [PubMed] [Google Scholar]
- 67. Sabidó M, Venkatesh S, Hayward B, Aldridge J, Gillett A. Subcutaneous iInterferon‐β1a does not increase the risk of stroke in patients with multiple sclerosis: analysis of pooled clinical trials and post‐marketing surveillance. Adv Ther. 2018;35(11):2041‐2053. 10.1007/s12325-018-0790-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Koudriavtseva T, Plantone D, Renna R, Mandoj C, Giannarelli D, Mainero C. Interferon‐β therapy and risk of thrombocytopenia in multiple sclerosis patients. Neurol Sci. 2015;36(12):2263‐2268. 10.1007/s10072-015-2348-1 [DOI] [PubMed] [Google Scholar]
- 69. Fu Y, Zhang N, Ren L, et al. Impact of an immune modulator fingolimod on acute ischemic stroke. Proc Natl Acad Sci USA. 2014;111(51):18315‐18320. 10.1073/pnas.1416166111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Belliston S, Sundararajan J, Hammond N, Newell K, Lynch S. Reversible cerebral vasoconstriction syndrome in association with fingolimod use. Int J Neurosci. 2017;127(9):831‐834. 10.1080/00207454.2016.1257991 [DOI] [PubMed] [Google Scholar]
- 71. Hamidi V, Couto E, Ringerike T, Klemp M. A multiple treatment comparison of eleven disease‐modifying drugs used for multiple sclerosis. J Clin Med Res. 2018;10(2):88‐105. 10.14740/jocmr3168w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Peeters PJHL, Bazelier MT, Uitdehaag BMJ, Leufkens HGM, De Bruin ML, de Vries F. The risk of venous thromboembolism in patients with multiple sclerosis: the clinical practice research datalink. J Thromb Haemostasis. 2014;12(4):444‐451. 10.1111/jth.12523 [DOI] [PubMed] [Google Scholar]
- 73. Perry VH. Contribution of systemic inflammation to chronic neurodegeneration. Acta Neuropathol. 2010;120(3):277‐286. 10.1007/s00401-010-0722-x [DOI] [PubMed] [Google Scholar]
- 74. Ianniello A, Pozzilli C. Ponesimod to treat multiple sclerosis. Drugs Today. 2021;57(12):745‐758. 10.1358/dot.2021.57.12.3353166 [DOI] [PubMed] [Google Scholar]
- 75. Ponesimod. LiverTox: Clinical and Research Information on Drug‐Induced Liver Injury. National Institute of Diabetes and Digestive and Kidney Diseases; 2021. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


