Abstract
We have used a mouse model of vaginal candidiasis to determine the effect of neutrophil depletion on (a) the clearance of Candida albicans and (b) the degree of inflammation associated with infection. No differences in recoverable yeast number or rate of clearance were observed between normal and neutrophil-depleted mice; however, vaginal inflammation was significantly decreased in neutrophil-depleted animals.
Vaginal candidiasis is a common infection in women with up to 75% of women experiencing at least one infection during adult life (4, 5). Factors which predispose women to vaginal Candida infection include oral contraceptive use, diabetes, pregnancy, and antibiotic use (12). Approximately 5% of women, however, experience chronic recurrent infections not attributable to these factors (11). In all infections the most common causative agent is Candida albicans, a commensal organism which can be routinely isolated from up to 50% of the female population (1). Chronic recurrent infections have been attributed to a variety of factors including T-lymphocyte dysfunction, macrophage defects, changes in normal vaginal flora, and defects in innate immune function; however, no one hypothesis has found broad acceptance (3, 7, 13, 15).
Neutrophils provide the major defense against systemic Candida infections in animal models, and yeast infections are a common sequelae of neutropenia in humans (6, 8). The role of neutrophils in protection against Candida in the vagina is, however, unclear. Sobel observed that in humans polymorphonuclear cells (PMNs) rarely migrated through the cornified epithelium into the vaginal lumen (11), whereas Monif found numerous PMNs in the vaginal lumen of some patients with vulvovaginal candidiasis (9). In this group of patients the presence of PMNs was associated with significant mycotic replication. In a rat model of experimental vaginal candidiasis very few PMNs were found in the vaginal lumen (14); however, in a mouse model of Candida vaginitis, PMN migration into the vaginal lumen is common (unpublished data). We have used this model to determine if neutrophils play a significant role in vaginal clearance of yeast by depleting PMN in vivo prior to infection.
Female specific-pathogen-free CBA/CaH mice obtained from the Animal Resource Center (Murdoch, Australia) were maintained under specific-pathogen-free conditions and provided with sterile rodent chow and water ad libitum. RB6-C85 monoclonal antibody (generously provided by Bob Coffman, DNAX, Palo Alto, Calif.) was purified from ascites by ammonium sulfate precipitation and was dialyzed extensively against normal saline. PMNs were depleted by intravenous injection of 100 μg of antibody (10). In RB6-C85-treated mice neutrophils were undetectable in vaginal washes and vaginal sections, were depleted (>98%) in bone marrow, and constituted <2% of total leukocytes (data not shown).
Candida vaginitis was induced by a modification of the method of Fidel et al. (2). Briefly, on day 0 mice were subcutaneously given 5 μg of estrogen (Schering, Alexandria, Australia) in 50 μl of sunflower oil to induce estrus. Estrus was verified by examination of wet mounts of vaginal washes collected on day 2, which consisted entirely of large squamous epithelia. On day 2.5 animals received RB6-C85 antibody or phosphate-buffered saline (PBS) diluent. On day 3 mice were infected intravaginally with 106 C. albicans in 20 μl of PBS. The strain of C. albicans used was originally isolated from a patient with recurrent vaginitis and was routinely typed by the rapid yeast plus test (Innovative Diagnostic Systems Inc, Norcross, Ga.) and analyzed for germ tube formation in serum. On day 8 mice were washed vaginally with 40 μl of PBS. The washes were serially diluted, plated on Sabouraud dextrose agar plates containing chloramphenicol, and incubated for 24 h at 37°C, and the colonies were enumerated. For evaluation of inflammation, vaginal tissues (day 8) were fixed in buffered formalin and 2-μm sections of the entire vagina were stained with hematoxylin and eosin (H&E) and periodic acid-Schiff base (PAS) and scored for inflammation and extent of infection. The grading scale for the level of fungal burden was 0, no detectable yeast in vaginal section by PAS; 1, mild to moderate infection—sporadic (1 to 50) pseudohyphae and/or blastoconidia in the vaginal lumen and/or cornified epithelial layer; and 2, florid infection—high levels of detectable yeast (50+ CFU) in the vaginal lumen as well as pseudohyphae and yeast in the deeper cornified epithelial layers. The level of inflammation was scored according to influx of neutrophils and degree of ultrastructural change. The scale for neutrophilic influx was 0, no neutrophils present; 1, mild—1 to 10 neutrophils present in the lumen and one to five microabcesses present in the epithelial layer; 2, moderate—numerous neutrophils in the lumen and numerous microabcesses and neutrophils present in submucosa; and 3, severe—large abscess formation, microabcesses coating the cornified epithelial layer, and numerous neutrophils in submucosa. The scale for ultrastructural changes was 0, no change—well-defined circular blood vessels and layered submucosal fibroblasts; 1, moderate—vessels were constricted or ovoid from edema, and fibroblasts were arranged chaotically; and 2, severe—fibroblasts were depleted and necrosis was present in submucosa.
The significance of differences in vaginal yeast burden and inflammation between experimental and control groups was determined by Mann-Whitney analysis.
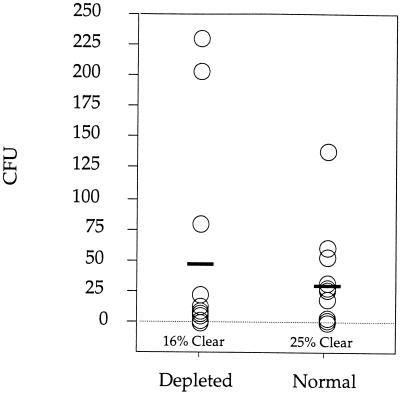
RB6-C85-induced PMN depletion is maintained for only 5 days (16) so the numbers of recoverable yeast cells in vaginal washes were determined at 5 days postinfection. No significant difference in the number of recoverable Candida was seen between control and PMN-depleted animals (Fig. 1). Twenty-five percent of the control animals had completely cleared the infection compared to 16% of the PMN-depleted animals, but this difference was not significant. In both control (Fig. 2A) and PMN-depleted (Fig. 2C) mice infection was limited to the cornified epithelial layers and consisted primarily of pseudohyphae. In PMN-depleted animals pseudohyphae appeared less filamentous and more “stubby.” Blastoconidia were common in vaginal sections from control animals but were rarely seen in vaginal sections from PMN-depleted mice. Numerous neutrophils in both the lumen and lamina propria were obvious in control animals, and microabscesses were frequently observed (Fig. 2B). Lumenal PMNs in control mice appear to have emigrated from the cervix. PMNs were not observed in RB6-C85-treated animals (Fig. 2D). In noninfected, estrogen-treated animals PMN infiltration into the vagina was minimal and microabscesses were not observed (Fig. 2E), demonstrating that the observed effects were due to Candida infection rather than to the induction of estrus.
FIG. 1.
Neutrophil depletion does not affect infection. Vaginal washes at day 5 postinfection were plated, and the numbers of recoverable yeast cells (CFU) were counted. Neither recoverable yeast numbers nor clearance rates (shown as percentages) were significantly different between control and neutrophil-depleted mice. CFU for individual mice are shown (n = 12). These data are representative of two separate experiments. Horizontal bars indicate average values.
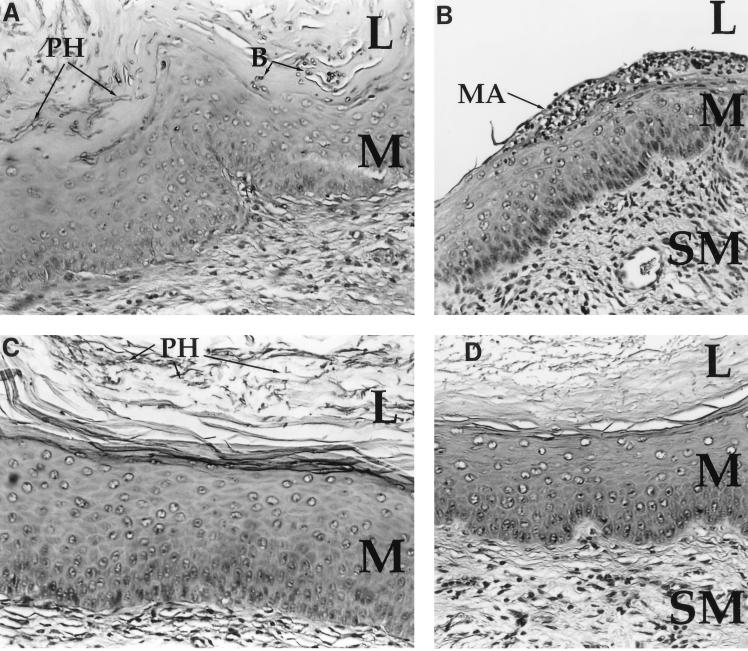
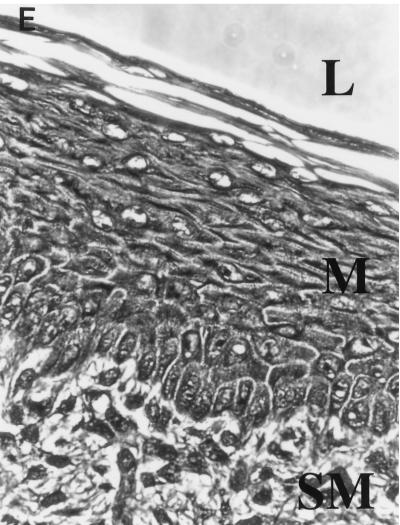
FIG. 2.
Vaginal histology of C. albicans-infected mice. (A) PAS stain of a vaginal section from a control mouse shows a florid infection with pseudohyphae (PH) and blastoconidia (B) penetrating the cornified epithelium. The lumen (L), mucosa (M), and submucosa (SM) are denoted for orientation. Magnification, ×83. (B) H&E stain of a vaginal section from the same mouse as in panel A. Neutrophils are present in the mucosa and have formed a microabscess (MA) in the cornified epithelium. Significant edema and submucosal fibroblastic disorganization are also present. Magnification, ×83. (C) PAS stain of a vaginal section from a neutrophil-depleted mouse. Pseudohyphae (PH) are present in the cornified epithelium in numbers similar to those of control mice. No blastoconidia are seen, and the pseudohyphae form short stubby chains. Magnification, ×83. (D) H&E stain of a vaginal section from a neutrophil-depleted mouse shows a profound lack of neutrophils in either the cornified epithelium or mucosa. Some edema has occurred, but the severity of inflammation is significantly less than that in the control animals. Magnification, ×83. (E) H&E stain of a vaginal section from a noninfected mouse 3 days after estrogen administration. No microabscesses are evident, and PMN infiltration is minimal. Magnification, ×83.
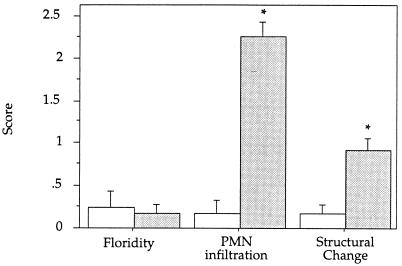
Histological grading of vaginal sections from PMN-depleted and control mice (Fig. 3) showed no difference in the floridity of infection, supporting the counts of recoverable yeast cells shown in Fig. 1 and the expected absence of PMN infiltration in RB6-C85-treated animals. Interestingly, structural changes associated with inflammation were significantly decreased in PMN-depleted animals (P < 0.05), suggesting that the neutrophil influx associated with infection contributes to inflammation.
FIG. 3.
Neutrophil depletion decreases vaginal inflammation. Histologic sections were graded as described in the text. No statistical difference in floridity between control and neutrophil-depleted mice was observed; however, both the level of PMN infiltration (P < 0.001) and the degree of inflammation (P < 0.05) were significantly decreased in the neutrophil-depleted mice. The average scores ± standard errors (indicated by error bars) are graphed (n = 2). Results are representative of two separate experiments. Open bars, neutrophil-depleted mice; shaded bars, control mice. Asterisks indicate significant differences.
Our data show that while neutrophils are recruited into the mouse vaginal mucosa during induced estrus they appear not to play a major role in clearance of C. albicans from this site. Depletion of PMNs did, however, result in decreased inflammation in the vagina, suggesting that neutrophils contribute to the symptomatology of vaginitis. As mice are only infectable during induced estrus it may be that PMNs recruited into the mucosa cannot enter the lumen due to the cornified epithelium. Hence, degranulation at this site would enhance inflammation but be ineffective in clearing lumenal Candida. Mice will, however, spontaneously clear the infection if allowed to cycle normally. Thus, the growth of C. albicans in this model is favored by conditions found at estrus where the cornified epithelium not only provides dead epithelial cells to which yeast can adhere and invade but may also isolate the Candida from the innate immune system.
Acknowledgments
These studies were supported by Cortecs plc Ltd.
REFERENCES
- 1.Drake T E, Maibach H I. Candida and candidiasis: cultural conditions, epidemiology and pathogenesis. Postgrad Med. 1973;53:83–87. doi: 10.1080/00325481.1973.11713368. [DOI] [PubMed] [Google Scholar]
- 2.Fidel P J, Lynch M E, Sobel J D. Candida-specific Th1-type responsiveness in mice with experimental vaginal candidiasis. Infect Immun. 1993;61:4202–4207. doi: 10.1128/iai.61.10.4202-4207.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fidel P J, Sobel J D. The role of cell-mediated immunity in candidiasis. Trends Microbiol. 1994;2:202–206. doi: 10.1016/0966-842x(94)90112-i. [DOI] [PubMed] [Google Scholar]
- 4.Hurley R. Candida vaginitis. Postgrad Med J. 1979;55:645–657. doi: 10.1136/pgmj.55.647.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hurley, R. 1977. Trends in candidal vaginitis. Proc. R. Soc. Med. 70(Suppl. 4):1–8. [DOI] [PMC free article] [PubMed]
- 6.Hurtrel B, Lagrange P H, Michel J C. Systemic candidiasis in mice. II. Main role of polymorphonuclear leukocytes in resistance to infection. Ann Immunol. 1980;13C:105–118. [PubMed] [Google Scholar]
- 7.Kinsman O S, Collard A E. Hormonal factors in vaginal candidiasis in rats. Infect Immun. 1986;53:498–504. doi: 10.1128/iai.53.3.498-504.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meyers J, editor. Infections in marrow transplant recipients. 3rd ed. New York, N.Y: Churchill-Livingstone; 1990. [Google Scholar]
- 9.Monif G R G. Classification and pathogenesis of vulvovaginal candidiasis. Am J Obstet Gynecol. 1985;152:935–939. doi: 10.1016/s0002-9378(85)80004-1. [DOI] [PubMed] [Google Scholar]
- 10.Rogers H, Unanue E. Neutrophils are involved in acute, nonspecific resistance to Listeria monocytogenes in mice. Infect Immun. 1993;61:5090–5096. doi: 10.1128/iai.61.12.5090-5096.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sobel J D. Genital candidiasis. In: Bodey G P, editor. Candidiasis: pathogenesis, diagnosis and treatment. New York, N.Y: Raven Press; 1993. pp. 225–247. [Google Scholar]
- 12.Sobel J D, editor. Pathogenesis of Candida vulvovaginitis. Stuttgart, Germany: Springer-Verlag; 1989. [Google Scholar]
- 13.Sobel J D. Vaginitis and vaginal flora: controversies abound. Curr Opin Infect Dis. 1996;9:42–47. [Google Scholar]
- 14.Sobel J D, Muller G, McCormick J F. Experimental chronic vaginal candidosis in rats. Sabouraudia. 1985;23:199–206. doi: 10.1080/00362178585380301. [DOI] [PubMed] [Google Scholar]
- 15.Taschdjian C L, Reiss F, Kozinn P J. Experimental vaginal candidiasis in mice; its implications for superficial candidasis in humans. J Investig Dermatol. 1960;34:89–93. [PubMed] [Google Scholar]
- 16.Wozniak, A., A. Vander Pol, and B. D. McColl. Role of neutrophils in dextran sulphate-induced colitis in mice. Submitted for publication.