Abstract
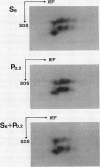
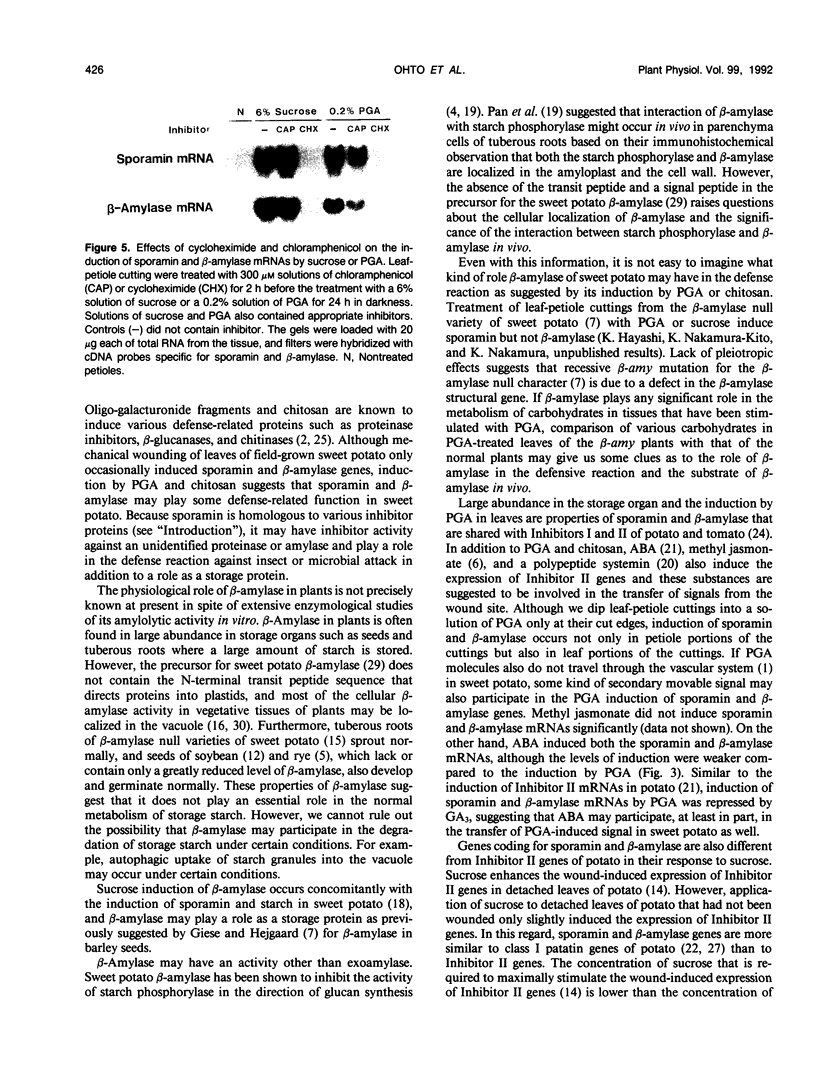
Sporamin and β-amylase are two major proteins of tuberous storage root of sweet potato (Ipomoea batatas) and their accumulation can be induced concomitantly with the accumulation of starch in leaves and petioles by sucrose (K Nakamura, M Ohto, N Yoshida, K Nakamura [1991] Plant Physiol 96: 902-909). Although mechanical wounding of leaves of sweet potato only occasionally induced the expression of sporamin and β-amylase genes, their expression could be reproducibly induced in leaf-petiole cuttings when these explants were dipped in a solution of polygalacturonic acid or chitosan at their cut edges. Polygalacturonic acid seemed to induce expression of the same genes coding for sporamin and β-amylase that are induced by sucrose. Because polygalacturonic acid and chitosan are known to mediate the induction of wound-inducible defense reactions, these results raise an interesting possibility that β-amylase, in addition to sporamin, may have some role in the defense reaction. Expression of sporamin and β-amylase genes could also be induced by abscisic acid, and this induction by abscisic acid, as well as induction by polygalacturonic acid or sucrose, was repressed by gibberellic acid. By contrast, methyl jasmonate did not cause the significant induction of either sporamin or β-amylase mRNAs. Induction of expression of sporamin and β-amylase genes by polygalacturonic acid or sucrose was inhibited by cycloheximide, suggesting that de novo synthesis of proteins is required for both of the induction processes.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowles D. J. Defense-related proteins in higher plants. Annu Rev Biochem. 1990;59:873–907. doi: 10.1146/annurev.bi.59.070190.004301. [DOI] [PubMed] [Google Scholar]
- Bradshaw H. D., Jr, Hollick J. B., Parsons T. J., Clarke H. R., Gordon M. P. Systemically wound-responsive genes in poplar trees encode proteins similar to sweet potato sporamins and legume Kunitz trypsin inhibitors. Plant Mol Biol. 1990 Jan;14(1):51–59. doi: 10.1007/BF00015654. [DOI] [PubMed] [Google Scholar]
- Chang T. C., Su J. C. Starch phosphorylase inhibitor from sweet potato. Plant Physiol. 1986 Feb;80(2):534–538. doi: 10.1104/pp.80.2.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer E. E., Ryan C. A. Interplant communication: airborne methyl jasmonate induces synthesis of proteinase inhibitors in plant leaves. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7713–7716. doi: 10.1073/pnas.87.19.7713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori T., Nakagawa S., Nakamura K. High-level expression of tuberous root storage protein genes of sweet potato in stems of plantlets grown in vitro on sucrose medium. Plant Mol Biol. 1990 Apr;14(4):595–604. doi: 10.1007/BF00027505. [DOI] [PubMed] [Google Scholar]
- Hattori T., Yoshida N., Nakamura K. Structural relationship among the members of a multigene family coding for the sweet potato tuberous root storage protein. Plant Mol Biol. 1989 Nov;13(5):563–572. doi: 10.1007/BF00027316. [DOI] [PubMed] [Google Scholar]
- Ishiguro S., Nakamura K. The nuclear factor SP8BF binds to the 5'-upstream regions of three different genes coding for major proteins of sweet potato tuberous roots. Plant Mol Biol. 1992 Jan;18(1):97–108. doi: 10.1007/BF00018460. [DOI] [PubMed] [Google Scholar]
- Johnson R., Ryan C. A. Wound-inducible potato inhibitor II genes: enhancement of expression by sucrose. Plant Mol Biol. 1990 Apr;14(4):527–536. doi: 10.1007/BF00027498. [DOI] [PubMed] [Google Scholar]
- Lin T. P., Spilatro S. R., Preiss J. Subcellular localization and characterization of amylases in Arabidopsis leaf. Plant Physiol. 1988 Jan;86(1):251–259. doi: 10.1104/pp.86.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K., Ohto M. A., Yoshida N., Nakamura K. Sucrose-Induced Accumulation of beta-Amylase Occurs Concomitant with the Accumulation of Starch and Sporamin in Leaf-Petiole Cuttings of Sweet Potato. Plant Physiol. 1991 Jul;96(3):902–909. doi: 10.1104/pp.96.3.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan S. M., Chang T. C., Juang R. H., Su J. C. Starch Phosphorylase Inhibitor Is beta-Amylase. Plant Physiol. 1988 Dec;88(4):1154–1156. doi: 10.1104/pp.88.4.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce G., Strydom D., Johnson S., Ryan C. A. A polypeptide from tomato leaves induces wound-inducible proteinase inhibitor proteins. Science. 1991 Aug 23;253(5022):895–897. doi: 10.1126/science.253.5022.895. [DOI] [PubMed] [Google Scholar]
- Pēna-Cortés H., Sánchez-Serrano J. J., Mertens R., Willmitzer L., Prat S. Abscisic acid is involved in the wound-induced expression of the proteinase inhibitor II gene in potato and tomato. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9851–9855. doi: 10.1073/pnas.86.24.9851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha-Sosa M., Sonnewald U., Frommer W., Stratmann M., Schell J., Willmitzer L. Both developmental and metabolic signals activate the promoter of a class I patatin gene. EMBO J. 1989 Jan;8(1):23–29. doi: 10.1002/j.1460-2075.1989.tb03344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida N., Nakamura K. Molecular cloning and expression in Escherichia coli of cDNA encoding the subunit of sweet potato beta-amylase. J Biochem. 1991 Aug;110(2):196–201. doi: 10.1093/oxfordjournals.jbchem.a123556. [DOI] [PubMed] [Google Scholar]
- Ziegler P., Beck E. Exoamylase activity in vacuoles isolated from pea and wheat leaf protoplasts. Plant Physiol. 1986 Dec;82(4):1119–1121. doi: 10.1104/pp.82.4.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]