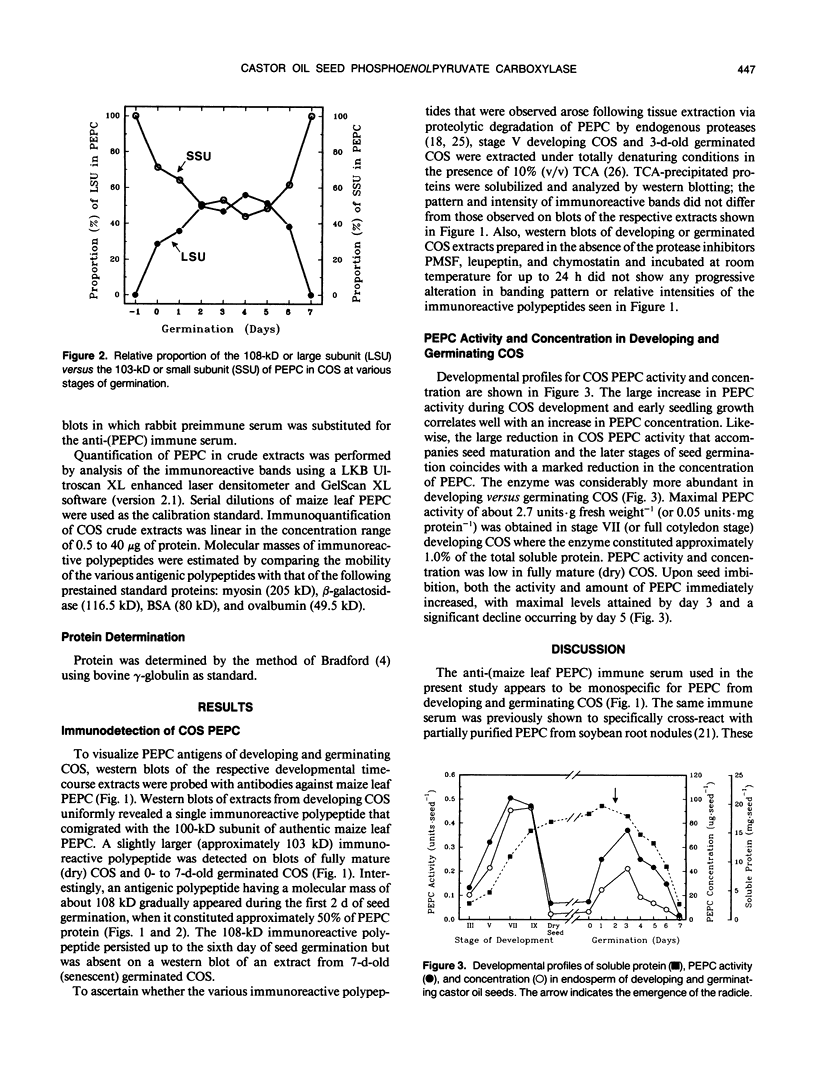
Abstract
Monospecific polyclonal antibodies against maize leaf phosphoenolpyruvate carboxylase (PEPC, EC 4.1.1.31) were utilized to examine the subunit composition and developmental profile of endosperm PEPC in developing and germinating castor oil seeds (Ricinus communis L. cv Baker 296). PEPC from developing endosperm consists of a single type of 100-kilodalton subunit, whereas the enzyme from 2- to 5-day germinated endosperm appears to contain equal proportions of immunologically related 103- and 108-kilodalton subunits. The maximal activity of PEPC in developing endosperms (2.67 micromoles oxaloacetate produced per minute per gram fresh weight) is approximately 20-fold and threefold greater than that of fully mature (dry seed) and germinating endosperms, respectively. The most significant increase in the activity and concentration of endosperm PEPC occurs during the middle cotyledon to full cotyledon stage of seed development; this period coincides with the most active phase of storage oil accumulation by ripening castor oil seeds. The data are compatible with the recent proposal (RG Smith, DA Gauthier, DT Dennis, DH Turpin [1992] Plant Physiol 1233-1238) that PEPC plays a fundamental role in vivo in the cytosolic production of an important substrate (malate) for fatty acid biosynthesis by developing castor oil seed leucoplasts. Immediately following seed imbibition, PEPC activity and concentration increase in parallel, with the greatest levels attained by the third day of germination. It is suggested that during this early phase of seed germination PEPC has a critical function to build up cellular dicarboxylic acid pools required to initiate significant activities of both the tricarboxylic acid and glyoxylate cycles.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benedict C. R., Beevers H. Formation of sucrose from malate in germinating castor beans. I. Conversion of malate to phosphoenol-pyruvate. Plant Physiol. 1961 Sep;36(5):540–544. doi: 10.1104/pp.36.5.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Doucet J. P., Trifaró J. M. A discontinuous and highly porous sodium dodecyl sulfate-polyacrylamide slab gel system of high resolution. Anal Biochem. 1988 Feb 1;168(2):265–271. doi: 10.1016/0003-2697(88)90317-x. [DOI] [PubMed] [Google Scholar]
- Gerhardt B. P., Beevers H. Developmental studies on glyoxysomes in Ricinus endosperm. J Cell Biol. 1970 Jan;44(1):94–102. doi: 10.1083/jcb.44.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudspeth R. L., Glackin C. A., Bonner J., Grula J. W. Genomic and cDNA clones for maize phosphoenolpyruvate carboxylase and pyruvate,orthophosphate dikinase: Expression of different gene-family members in leaves and roots. Proc Natl Acad Sci U S A. 1986 May;83(9):2884–2888. doi: 10.1073/pnas.83.9.2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao J. A., Chollet R. Posttranslational regulation of phosphoenolpyruvate carboxylase in c(4) and crassulacean Acid metabolism plants. Plant Physiol. 1991 Apr;95(4):981–985. doi: 10.1104/pp.95.4.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khayat E., Dumbroff E. B., Glick B. R. The synthesis of phosphoenolpyruvate carboxylase in imbibing sorghum seeds. Biochem Cell Biol. 1991 Feb-Mar;69(2-3):141–145. doi: 10.1139/o91-021. [DOI] [PubMed] [Google Scholar]
- Kobr M. J., Beevers H. Gluconeogenesis in the castor bean endosperm: I. Changes in glycolytic intermediates. Plant Physiol. 1971 Jan;47(1):48–52. doi: 10.1104/pp.47.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmo G. A., Nimmo H. G., Hamilton I. D., Fewson C. A., Wilkins M. B. Purification of the phosphorylated night form and dephosphorylated day form of phosphoenolpyruvate carboxylase from Bryophyllum fedtschenkoi. Biochem J. 1986 Oct 1;239(1):213–220. doi: 10.1042/bj2390213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura M., Beevers H. Isoenzymes of sugar phosphate metabolism in endosperm of germinating castor beans. Plant Physiol. 1981 Jun;67(6):1255–1258. doi: 10.1104/pp.67.6.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaxton W. C. Leucoplast Pyruvate Kinase from Developing Castor Oil Seeds : Characterization of the Enzyme's Degradation by a Cysteine Endopeptidase. Plant Physiol. 1991 Dec;97(4):1334–1338. doi: 10.1104/pp.97.4.1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaxton W. C. Molecular and immunological characterization of plastid and cytosolic pyruvate kinase isozymes from castor-oil-plant endosperm and leaf. Eur J Biochem. 1989 May 1;181(2):443–451. doi: 10.1111/j.1432-1033.1989.tb14745.x. [DOI] [PubMed] [Google Scholar]
- Schuller K. A., Plaxton W. C., Turpin D. H. Regulation of Phosphoenolpyruvate Carboxylase from the Green Alga Selenastrum minutum: Properties Associated with Replenishment of Tricarboxylic Acid Cycle Intermediates during Ammonium Assimilation. Plant Physiol. 1990 Aug;93(4):1303–1311. doi: 10.1104/pp.93.4.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuller K. A., Turpin D. H., Plaxton W. C. Metabolite regulation of partially purified soybean nodule phosphoenolpyruvate carboxylase. Plant Physiol. 1990 Nov;94(3):1429–1435. doi: 10.1104/pp.94.3.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. G., Gauthier D. A., Dennis D. T., Turpin D. H. Malate- and pyruvate-dependent Fatty Acid synthesis in leucoplasts from developing castor endosperm. Plant Physiol. 1992 Apr;98(4):1233–1238. doi: 10.1104/pp.98.4.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodorou M. E., Elrifi I. R., Turpin D. H., Plaxton W. C. Effects of Phosphorus Limitation on Respiratory Metabolism in the Green Alga Selenastrum minutum. Plant Physiol. 1991 Apr;95(4):1089–1095. doi: 10.1104/pp.95.4.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tully R. E., Beevers H. Proteases and Peptidases of Castor Bean Endosperm: Enzyme Characterization and Changes during Germination. Plant Physiol. 1978 Nov;62(5):746–750. doi: 10.1104/pp.62.5.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F. S., Wang M. Y. Extraction of proteins for sodium dodecyl sulfate-polyacrylamide gel electrophoresis from protease-rich plant tissues. Anal Biochem. 1984 May 15;139(1):100–103. doi: 10.1016/0003-2697(84)90394-4. [DOI] [PubMed] [Google Scholar]



