Abstract
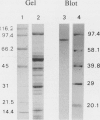
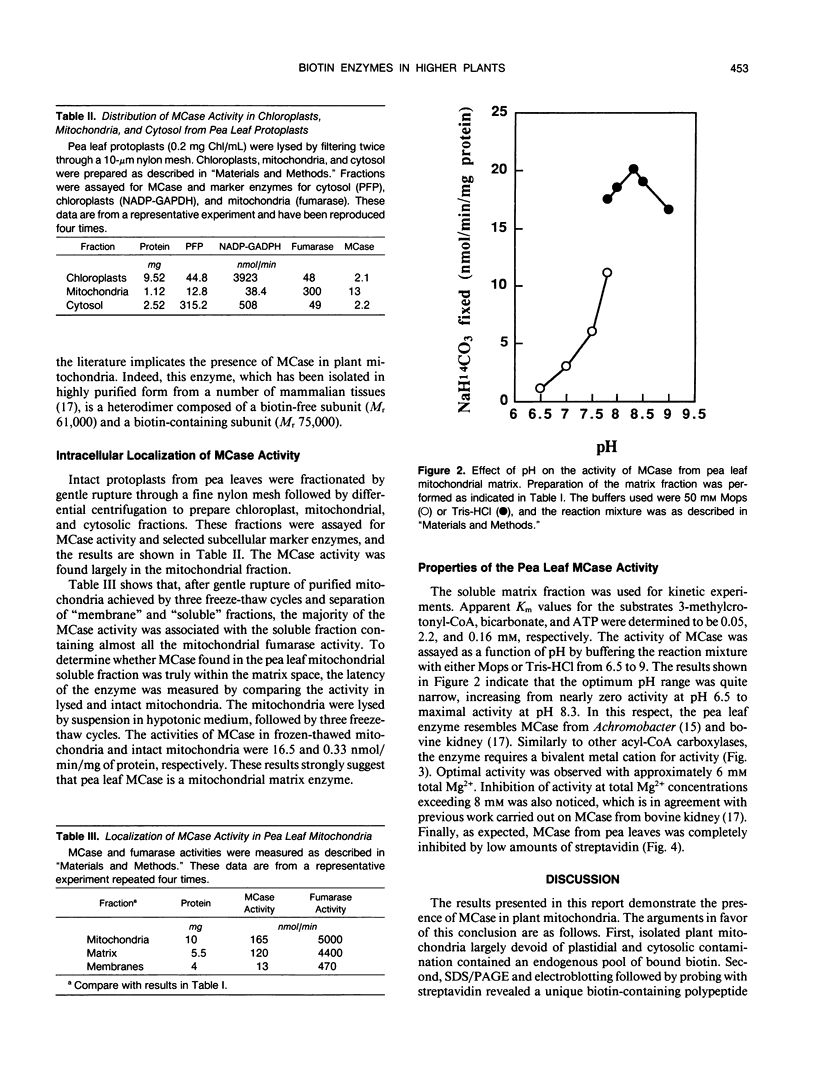
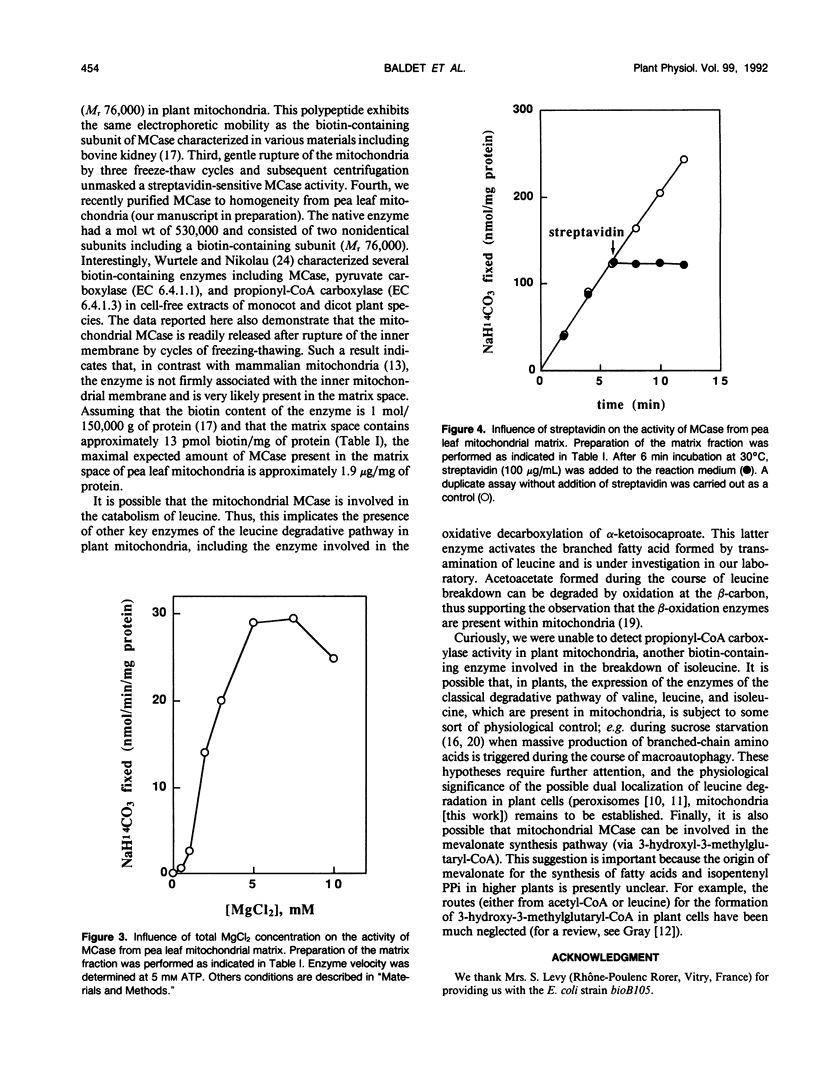
Mitochondria from green pea (Pisum sativum) leaves were purified free of peroxisomes and chlorophyll contamination and examined for their biotin content. The bulk of the bound biotin detected in plant mitochondria was shown to be associated with the matrix space to a concentration of about 13 micromolar, and no free biotin was detected. Western blot analysis of mitochondrial polypeptides using horseradish peroxidase-labeled streptavidin revealed a unique biotin-containing polypeptide with a molecular weight of 76,000. This polypeptide was implicated as being the biotinylated subunit of 3-methylcrotonyl-coenzyme A (CoA) carboxylase. Fractionation of pea leaf protoplasts demonstrated that this enzyme activity was located largely in mitochondria. The 3-methylcrotonyl-CoA carboxylase activity was latent when assayed in isotonic media. The majority of the enzyme activity was found in the soluble matrix of mitochondria. Maximal 3-methylcrotonyl-CoA carboxylase activity was found at pH 8.3 in the presence of Mg2+. Kinetic constants (apparent Km values) for the enzyme substrates were: 3-methylcrotonyl-CoA, 0.05 millimolar; ATP, 0.16 millimolar; HCO3−, 2.2 millimolar. The involvement of 3-methylcrotonyl-CoA carboxylase in the leucine degradation pathway in plant mitochondria is proposed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Douce R., Christensen E. L., Bonner W. D., Jr Preparation of intaintact plant mitochondria. Biochim Biophys Acta. 1972 Aug 17;275(2):148–160. doi: 10.1016/0005-2728(72)90035-7. [DOI] [PubMed] [Google Scholar]
- Eisenberg M. A. Mode of action of alpha-dehydrobiotin, a biotin analogue. J Bacteriol. 1975 Jul;123(1):248–254. doi: 10.1128/jb.123.1.248-254.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genix P., Bligny R., Martin J. B., Douce R. Transient accumulation of asparagine in sycamore cells after a long period of sucrose starvation. Plant Physiol. 1990 Oct;94(2):717–722. doi: 10.1104/pp.94.2.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerbling H., Gerhardt B. Oxidative decarboxylation of branched-chain 2-oxo Fatty acids by higher plant peroxisomes. Plant Physiol. 1988 Sep;88(1):13–15. doi: 10.1104/pp.88.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerbling H., Gerhardt B. Peroxisomal degradation of branched-chain 2-oxo acids. Plant Physiol. 1989 Dec;91(4):1387–1392. doi: 10.1104/pp.91.4.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIMES R. H., YOUNG D. L., RINGELMANN E., LYNEN F. The biochemical function of biotin. V. Further studies on beta-methylcrotonyl CoA carboxylase. Biochem Z. 1963;337:48–61. [PubMed] [Google Scholar]
- Hector M. L., Cochran B. C., Logue E. A., Fall R. R. Subcellular localization of 3-methylcrotonyl-coenzyme A carboxylase in bovine kidney. Arch Biochem Biophys. 1980 Jan;199(1):28–36. doi: 10.1016/0003-9861(80)90252-0. [DOI] [PubMed] [Google Scholar]
- Journet E. P., Bligny R., Douce R. Biochemical changes during sucrose deprivation in higher plant cells. J Biol Chem. 1986 Mar 5;261(7):3193–3199. [PubMed] [Google Scholar]
- Lau E. P., Cochran B. C., Fall R. R. Isolation of 3-methylcrotonyl-coenzyme A carboxylase from bovine kidney. Arch Biochem Biophys. 1980 Dec;205(2):352–359. doi: 10.1016/0003-9861(80)90117-4. [DOI] [PubMed] [Google Scholar]
- Miernyk J. A., Thomas D. R., Wood C. Partial purification and characterization of the mitochondrial and peroxisomal isozymes of enoyl-coenzyme a hydratase from germinating pea seedlings. Plant Physiol. 1991 Feb;95(2):564–569. doi: 10.1104/pp.95.2.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roby C., Martin J. B., Bligny R., Douce R. Biochemical changes during sucrose deprivation in higher plant cells. Phosphorus-31 nuclear magnetic resonance studies. J Biol Chem. 1987 Apr 15;262(11):5000–5007. [PubMed] [Google Scholar]
- Tolbert N. E., Yamazaki R. K., Oeser A. Localization and properties of hydroxypyruvate and glyoxylate reductases in spinach leaf particles. J Biol Chem. 1970 Oct 10;245(19):5129–5136. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurtele E. S., Nikolau B. J. Plants contain multiple biotin enzymes: discovery of 3-methylcrotonyl-CoA carboxylase, propionyl-CoA carboxylase and pyruvate carboxylase in the plant kingdom. Arch Biochem Biophys. 1990 Apr;278(1):179–186. doi: 10.1016/0003-9861(90)90246-u. [DOI] [PubMed] [Google Scholar]