ABSTRACT
Aims/Introduction
The aim was to examine the joint effect of metabolic syndrome (MetS) and insulin resistance (IR) with ideal cardiovascular health (iCVH) status on incident cardiovascular diseases (CVDs).
Materials and Methods
The study included 6,240 Iranian adults ≥30 years, free of prior cardiovascular disease. Ideal cardiovascular health was determined based on American Heart Association's Life Simple 7. Metabolic syndrome was defined according to the Joint Interim Statement Criteria, and insulin resistance was defined as HOMA‐IR ≥1.85 in women and ≥2.17 in men. Multivariable Cox proportional hazard ratios (HRs) were applied to examine the impact of metabolic syndrome, and insulin resistance at various levels of iCVH status.
Results
During the median follow‐up of 14.0 years, 909 cases of cardiovascular disease occurred. Metabolic syndrome and insulin resistance were significantly associated with incident cardiovascular disease events. In the poor and intermediate status, metabolic syndrome increased cardiovascular disease events with HRs of 1.83 and 1.57, respectively; the corresponding values for insulin resistance in the mentioned categories were 1.91 and 1.25, respectively (P values < 0.05). In the intermediate and poor iCVH status, hypertriglyceridemia was linked to a 40% and 35% higher risk of cardiovascular disease, the corresponding values for low HDL‐C was 20% and 60%, respectively (P values < 0.05). Although adding metabolic syndrome, its dyslipidemia and insulin resistance to iCVH status in both poor and intermediate status significantly improve the prediction of cardiovascular disease using net reclassification improvement (P values < 0.05), the value of C‐index did not change.
Conclusions
Metabolic syndrome and the dyslipidemia component had a negligible but significant improvement in the prediction of cardiovascular disease among individuals with non‐optimal iCVH status.
Keywords: Ideal cardiovascular health, Insulin resistance, Metabolic syndrome
Poor cardiovascular health status increased the risk of cardiovascular disease events by 5‐fold, compared with ideal CVH status. Intermediate CVH status increased the risk of cardiovascular disease events by 3‐fold, compared with the ideal CVH status. The presence of metabolic syndrome was associated with about a 2‐fold increased risk of cardiovascular disease. The presence of insulin resistance was associated with about a 2‐fold increased risk of cardiovascular disease. In the poor and intermediate status, metabolic syndrome increased cardiovascular disease events with the corresponding HRs of 1.83 (1.08–3.10) and 1.57 (1.34–1.84), respectively; the corresponding values for insulin resistance in the mentioned categories were 1.91 (1.13–3.23) and 1.25 (1.00–1.56), respectively.
INTRODUCTION
Cardiovascular disease (CVD) remains a significant global health concern, accounting for a substantial number of premature deaths worldwide 1 . The Middle East and North Africa (MENA) is a particularly affected region, due to the high burden of cardiovascular disease risk factors 2 .
To reduce premature cardiovascular disease mortality by 20% from 2010 to 2020, the American Heart Association (AHA) developed the AHA's Life Simple 7 to monitor population‐ and individual‐level cardiovascular health status 3 . This term was composed of seven modifiable factors and classical cardiometabolic risk factors, including smoking status, body mass index (BMI), physical activity, diet, total cholesterol (TC), blood pressure (BP), and fasting plasma glucose (FPG). The impact of maintaining ideal cardiovascular health (iCVH) status has been studied extensively and linked to various outcomes, including non‐communicable disorders, cardiovascular diseases, cancers, and mortalities 4 , 5 .
On the other hand, metabolic syndrome (MetS) is a cluster of interconnected risk factors 6 , 7 , that is widely considered a clinical manifestation of insulin resistance (IR) 7 . Insulin resistance has been shown to contribute independently to the progression of cardiovascular disease events 8 . Further, dyslipidemia in metabolic syndrome (high triglyceride (TG) and low HDL‐C) is shown to be strongly associated with insulin resistance 9 and consequent cardiovascular disease events 10 , 11 . These two lipid abnormalities were not addressed directly in the original AHA's Life Simple 7 3 . Furthermore, in 2022, Life's Essential 8 was released, which substituted total cholesterol with non‐HDL‐C (encompassing all atherogenic lipoproteins except HDL‐C) 12 .
To the best of our knowledge, this is the first study to investigate the joint impact of metabolic syndrome and iCVH status, as two closely related concepts to CVDs events over a decade‐long follow‐up period. In addition, we explored the collective risk of iCVH status and metabolic syndrome components that were not directly addressed in Life's Simple 7 (TG, HDL‐C, and waist circumference (WC)) 12 . We further analyzed the joint effect of insulin resistance using the Homeostatic Model Assessment of Insulin Resistance (HOMA‐IR) in a subgroup of the population with insulin data. Finally, the added value of metabolic syndrome, its dyslipidemia component, and insulin resistance into iCVH status for prediction of cardiovascular disease events was examined.
METHODS
Study design and population
The Tehran Lipid and Glucose Study (TLGS), which was established in 1999, is a population‐based prospective cohort aimed to identify predictors for non‐communicable diseases (NCDs) and related outcomes in the urban population of Tehran. Follow‐up visits are conducted at approximately 3‐year intervals, during which healthy lifestyle interventions are implemented 13 .
Following the primary examination conducted between 1999 and 2002, subsequent examination cycles were carried out as follows: phase 2 (2002–2005), phase 3 (2005–2008), phase 4 (2009–2011), phase 5 (2012–2015), and phase 6 (2015–2018). For the present study, phase 2 was considered the baseline, comprising 7,122 participants aged 30 years and above. The analysis excluded participants with prevalent cardiovascular disease (n = 520) and those without follow‐up or with missing anthropometric and laboratory data relevant to the diagnosis of iCVH status, metabolic syndrome components, and covariates (n = 362, accounting for overlapping groups). The final data analysis included 6,240 participants, of whom 2,704 were men, resulting in a response rate of 94.5%. Due to unavailable insulin data for the entire population, the combined impact of insulin resistance and iCVH status on cardiovascular disease events was assessed in a subsample of 3,433 participants.
The study protocol received approval from the Ethics Committee of the Research Institute for Endocrine Sciences (RIES) at Shahid Beheshti University of Medical Sciences in Tehran, Iran. Written informed consent was obtained from all participants.
Measurements
Information on lifestyle behaviors including smoking status, physical activity, educational level, medication use (such as antihypertensive, lipid‐lowering, and anti‐diabetes drugs), demographic status, and history of cardiovascular disease and premature cardiovascular disease in first‐degree relatives (i.e., parents and siblings under 55 years for males and under 65 years for females) was collected through a questionnaire. Weight was measured using a digital scale (Seca 707: range 0/0–150/0 kg; Seca GmbH, Hamburg, Germany) with a sensitivity of 0.1 kg, while participants were without shoes and wore minimal clothing. Height was measured in a standing position, with shoulders in a neutral alignment, without shoes, using a stadiometer (Seca225; Seca GmbH, Hamburg, Germany). The BMI was calculated by dividing weight (kg) by the square of height (m). Waist circumference was measured at the level of the umbilicus using a tape measure (accuracy, 0.5 cm). Blood pressure was measured twice on the right arm after a 15 min sitting rest period using a standard mercury sphygmomanometer to obtain systolic blood pressure (SBP) and diastolic blood pressure (DBP), and the average of the two measurements was used for calculating the participants’ blood pressure. Physical activity level was evaluated using the modifiable activity questionnaire (MAQ), which recorded various types of activities, including leisure time, work, and household activities, over the past year 14 . Physical activity levels were expressed in terms of metabolic equivalent (MET) minutes per week (MET‐min/week) 15 .
Biochemical assessments
Following an overnight fast of 12–14 h, venous blood samples were collected from all participants to assess fasting plasma glucose and lipid levels. Laboratory analyses were conducted on the same day of blood collection using commercially available kits (Pars Azmoon, Tehran, Iran) and an automated Selectra 2 analyzer (Vital Scientific, Spankeren, the Netherlands). Fasting plasma glucose levels were determined using an enzymatic colorimetric method employing glucose oxidase. Triglyceride and total cholesterol were measured using enzymatic colorimetric methods involving glycerol phosphate oxidase and cholesterol esterase‐cholesterol oxidase, respectively. The HDL‐C levels were quantified after precipitating apolipoprotein B‐containing lipoproteins with phosphotungstic acid. All samples were analyzed only if they met the acceptable criteria for internal quality control. The coefficients of variation for intra‐ and inter‐assay precision were below 2.2% for glucose, 1.9% for triglyceride, and 2.9% for HDL‐C.
Definition of term
Metabolic syndrome was defined based on the criteria outlined in the Joint Interim Statement (JIS) 16 . It required the presence of at least three of the following criteria: elevated fasting plasma glucose levels (≥5.6 mmol/L) or the use of medications to lower glucose levels, elevated serum triglyceride levels (≥1.7 mmol/L) or the use of lipid‐lowering drugs, reduced levels of HDL‐C (<1.03 mmol/L for men and <1.29 mmol/L for women), elevated blood pressure (≥130/85 mmHg) or the use of antihypertensive medications, and enlarged abdominal circumference (≥95 cm according to the population‐ and country‐specific cut‐off points for Iranian adults of both genders based on guidelines of the Iranian National Committee of Obesity) 17 , 18 . Insulin resistance was assessed by calculating the HOMA‐IR as fasting insulin (μU/mL) multiplied by FPG (mg/dL) divided by 405. Insulin resistance was defined as HOMA‐IR ≥1.85 in women and ≥2.17 in men 19 . The AHA defines iCVH status based on six factors, including three behavioral and three biological, each classified into ideal, intermediate, and poor levels 3 . Blood pressure was considered ideal (<120/80 mmHg if untreated), intermediate (120–139/80–89 mmHg or treated), or poor (≥140/90 mmHg); fasting plasma glucose was considered ideal (<100 mg/dL if untreated), intermediate (100–125 mg/dL or treated), or poor (≥126 mg/dL); total cholesterol was considered ideal (<200 mg/dL if untreated), intermediate (200–239 mg/dL or treated), or poor (>240 mg/dL); smoking status was considered ideal for those who never smoked or quit for more than 12 months, intermediate for former smokers or those who had abstained for ≤12 months, and poor for current smokers; BMI was considered ideal (<25.0 kg/m2), intermediate (25.0–29.9 kg/m2), or poor (≥30.0 kg/m2); and physical activity was considered ideal (≥1,500 MET‐min/week), intermediate (600–1,500 MET‐min/week), or poor (<600 MET‐min/week). A score of 1 was assigned to the ideal category and 0 to the intermediate or poor categories. The overall iCVH score was calculated by summing up the scores for the six iCVH metrics and classified as ideal (≥5), intermediate (3, 4), or poor (0–2).
Outcomes
During annual face‐to‐face visits, the TLGS participants were contacted to inquire about any hospitalizations that had occurred during the previous year, and these events were documented 20 . If participants reported hospitalization, a skilled nurse followed up to gather more information about the case, and a specialist physician collected additional data from medical records and home visits. A judging committee reviewed all the collected documents, and experts from various medical fields, such as cardiologists, internists, endocrinologists, and epidemiologists, evaluated the data to conclude the outcome. The definition of CVD included any fatal and non‐fatal strokes and coronary heart disease (CHD) events. Coronary heart disease was categorized as definite myocardial infarction (MI) (diagnostic electrocardiography and biomarkers), probable myocardial infarction (positive electrocardiograph findings plus cardiac symptoms or signs plus missing biomarkers or positive electrocardiograph findings plus equivocal biomarkers), and angiographic proven coronary heart disease. Incident stroke was defined as all cases of definite and a possible stroke and transient ischemic attack 21 . TLGS used ICD‐10 criteria and AHA classification for cardiovascular events (i.e., ischemic heart disease (ICD10 codes I20–I25), sudden cardiac death (I46.1), or stroke (ICD‐10 codes I60–I69)) 22 .
Statistical analysis
All statistical analyses were conducted using SPSS software version 18 (SPSS, Chicago, IL, USA) and STATA version 14 (StataCorp., College Station, TX, USA). The normality of variables was assessed using both the Kolmogorov–Smirnov test and histograms. Descriptive statistics were presented as mean ± standard deviation, median (interquartile range (IQR)), or percentage, as appropriate. Baseline characteristics of the study population were compared among the three groups of iCVH categories, stratified by the presence or absence of metabolic syndrome, using analysis of variance (anova). To estimate the hazard ratios (HRs) and 95% confidence intervals (CIs) of cardiovascular disease events associated with metabolic syndrome, insulin resistance, and iCVH status (with the ideal category as the reference), multivariable Cox proportional hazards regression analyses were performed.
Furthermore, using a joint classification approach, HRs for cardiovascular disease events were estimated based on the baseline status of metabolic syndrome, insulin resistance, and the three components of hypertriglyceridemia, low HDL‐C, and abdominal obesity at different levels of iCVH status (poor, intermediate, and ideal) in three models. The first model was unadjusted, the second model was adjusted for age and sex, and the third model was additionally adjusted for educational level, marital status, and history of premature cardiovascular disease.
To evaluate whether adding the metabolic syndrome, its dyslipidemia component and insulin resistance to iCVH status could improve the predictive value for cardiovascular disease, the Harrell's concordance statistic (C‐index) was calculated in poor and intermediate iCVH status. Additionally, net reclassification improvement (NRI) and integrated discrimination improvement (IDI) were also calculated for comparison of models and to further evaluate the incremental predictive value of these variables.
RESULTS
The study population included 6,240 individuals (56.6% women) with a mean age of 48.1 (12.5) years. Table 1 presents the baseline characteristics of the individuals according to the joint classification of iCVH and metabolic syndrome status. Individuals with ideal iCVH status and without metabolic syndrome had a lower mean age, BMI, WC, SBP, DBP, FPG, TC, and TG, and HOMA‐IR and a higher level of HDL‐C compared with those with metabolic syndrome and poor iCVH status. In addition, participants with ideal iCVH status and without metabolic syndrome were more likely to be female and insulin sensitive, less likely to be smokers, and more physically active than those with metabolic syndrome and poor iCVH status. Furthermore, the percentage of individuals with a higher education level and the percentage of participants using glucose and lipid‐lowering drugs and anti‐hypertensive drugs in the group with ideal iCVH status and without metabolic syndrome were lower than the group with metabolic syndrome and poor iCVH status. No significant difference was observed in other baseline characteristics, including marital status and premature cardiovascular disease.
Table 1.
Baseline characteristics of participants according to the joint classification of ideal cardiovascular health status and metabolic syndrome: Tehran Lipid and Glucose Study (TLGS), 2002–2018
| Poor/MetS | Poor/non‐MetS | Intermediate/MetS | Intermediate/Non‐MetS | Ideal/MetS | Ideal/Non‐MetS | P‐value | |
|---|---|---|---|---|---|---|---|
| Age (year) | 55.3 ± 11.9 | 53.7 ± 13.0 | 50.9 ± 11.9 | 45.9 ± 12.1 | 44.1 ± 10.1 | 41.4 ± 10.7 | <0.001 |
| BMI (kg/m2) | 30.9 ± 4.5 | 27.7 ± 2.8 | 30.0 ± 4.4 | 26.9 ± 3.8 | 28.6 ± 4.0 | 24.2 ± 3.5 | <0.001 |
| Waist (cm) | 103.3 ± 9.1 | 93.1 ± 8.3 | 99.8 ± 9.1 | 89.5 ± 9.3 | 98.5 ± 6.5 | 82.5 ± 9.0 | <0.001 |
| SBP (mmHg) | 134.8 ± 19.9 | 124.9 ± 13.8 | 126.4 ± 19.4 | 113.0 ± 15.2 | 112.1 ± 15.2 | 105.1 ± 10.7 | <0.001 |
| DBP (mmHg) | 82.3 ± 10.8 | 78.5 ± 7.5 | 80.2 ± 10.5 | 73.2 ± 8.9 | 73.8 ± 9.2 | 68.5 ± 7.2 | <0.001 |
| FPG (mg/dL) | 133.9 ± 52.2 | 105.6 ± 29.8 | 108.1 ± 38.4 | 90.6 ± 17.9 | 92.5 17.6 | 86.8 ± 13.1 | <0.001 |
| TC (mg/dL) | 230.4 ± 39.5 | 228.1 ± 28.4 | 204.7 ± 40.5 | 195.1 ± 37.5 | 177.8 ± 16.8 | 169.4 ± 27.0 | <0.001 |
| HDL cholesterol (mg/dL) | 36.8 ± 9.5 | 47.2 ± 12.2 | 35.2 ± 8.2 | 41.8 ± 11.3 | 29.5 ± 5.7 | 40.7 ± 10.3 | <0.001 |
| TG (mg/dL) | 216 (168–295) | 130 (99.2–164.5) | 192 (155–255) | 118 (89–148) | 203 (164–251) | 97 (72–129) | <0.001 |
| Men | 312 (50.0) | 48 (66.7) | 959 (43.9) | 1,057 (43.0) | 22 (52.4) | 306 (35.7) | <00.01 |
| Educational level (year) | |||||||
| ≤6 | 55 (8.8) | 7 (9.7) | 221 (10.1) | 397 (16.2) | 4 (9.5) | 169 (19.7) | <0.001 |
| 6–12 | 224 (35.9) | 33 (45.8) | 959 (43.9) | 1,316 (53.5) | 21 (50.0) | 511 (59.6) | |
| >12 | 345 (55.3) | 32 (44.4) | 1,006 (46.0) | 745 (30.3) | 17 (40.5) | 178 (20.7) | |
| Physical activity (MET‐min/week) | |||||||
| ≤600 | 364 (58.3) | 40 (55.6) | 752 (34.4) | 1,025 (41.7) | 0.0 (0) | 148 (17.3) | <0.001 |
| 600–1,500 | 202 (32.3) | 26 (36.1) | 457 (20.9) | 745 (30.3) | 0.0 (0) | 112 (13.1) | |
| ≥1,500 | 58 (9.4) | 6 (8.3) | 977 (44.7) | 688 (28.0) | 42 (100.0) | 598 (69.6) | |
| Smoking status | |||||||
| Current smoking | 170 (27.2) | 35 (48.6) | 221 (10.1) | 432 (17.5) | 0.0 (0) | 51 (5.9) | <0.001 |
| Former smokers | 101 (16.2) | 19 (26.4) | 161 (7.4) | 207 (8.4) | 0.0 (0) | 17 (1.9) | |
| Never smoked | 353 (56.6) | 18 (25.5) | 1,804 (82.5) | 1,819 (74.1) | 42 (100.0) | 790 (92.2) | |
| Marital status | |||||||
| Married | 536 (85.9) | 67 (93.1) | 1,907 (87.2) | 2,164 (88.0) | 42 (100.0) | 739 (86.1) | <0.001 |
| Divorced/widowed | 78 (12.5) | 4 (5.6) | 234 (10.7) | 176 (7.2) | 0.0 (0.0) | 37 (4.3) | |
| Single | 10 (1.6) | 1 (1.4) | 45 (2.1) | 118 (4.8) | 0 (0.0) | 82 (9.6) | |
| Glucose lowering drug use, yes | 131 (21.0) | 5 (6.9) | 192 (8.8) | 36 (1.5) | 1 (2.4) | 6 (0.7) | <0.001 |
| Anti‐hypertensive drug use, yes | 137 (22.0) | 6 (8.3) | 311 (14.2) | 84 (3.4) | 0 (0.0) | 4 (0.5) | <0.001 |
| Lipid‐lowering drug use, yes | 80 (12.8) | 0 (0) | 118 (5.4) | 24 (1.0) | 0 (0.0) | 2 (0.2) | <0.001 |
| Family history of CVD, yes | 130 (20.8) | 11 (15.3) | 426 (19.5) | 465 (18.9) | 10 (23.8) | 145 (16.9) | 0.364 |
| HOMA‐IR † | 3.0 (2.1–4.5) | 2.1 (1.5–3.2) | 2.4 (1.7–3.3) | 1.5 (1.1–2.2) | 2.2 (1.6–2.7) | 1.3 (0.9–1.7) | <0.001 |
| Insulin resistance † | 229 (78.7) | 19 (55.9) | 768 (65.0) | 443 (31.5) | 13 (68.4) | 106 (21.2) | <0.001 |
IR was defined as HOMA‐IR ≥1.85 in women and ≥2.17 in men.
Data were included for 3,433 participants.
BMI, body mass index; CVD, cardiovascular diseases; DBP, diastolic blood pressure; FPG, fasting plasma glucose; HDL cholesterol, high density lipoprotein; HOMA‐IR, Homeostatic Model Assessment of Insulin Resistance; SBP, systolic blood pressure; TC, total cholesterol; TG, triglyceride.
During the median (IQR) 14.0 (12.6–15.1) years of follow‐up, 909 (14.6%) new cases of cardiovascular disease were identified. Figure 1 presents the results of the individual assessment of the association of metabolic syndrome, insulin resistance, and iCVH status with cardiovascular disease events. Adjusted for age, sex, education, marital status, and premature cardiovascular disease, metabolic syndrome (HR: 2.01; 95% CI: 1.74–2.31) and insulin resistance (HR: 1.56; 95% CI: 1.29–1.89) were associated with an increased risk of cardiovascular disease. Moreover, the multivariable analysis revealed that the cardiovascular disease risk among poor and intermediate iCVH status was significantly higher than those with ideal iCVH status, with HRs of 5.81 (3.97–8.48) and 2.95 (2.06–4.24), respectively.
Figure 1.
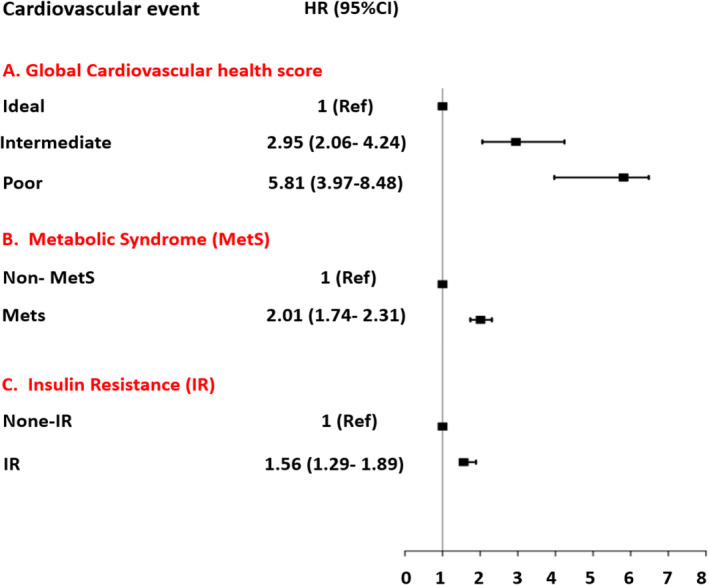
The hazard ratio (95% confidence interval) association of global cardiovascular health status, metabolic syndrome, and insulin resistance with the risk of cardiovascular event.
Table 2 presents the incident cardiovascular disease events according to the joint classification of iCVH status, metabolic syndrome, and insulin resistance, as well as the three metabolic syndrome components of hypertriglyceridemia, low HDL‐C, and abdominal obesity. In the poor and intermediate categories of iCVH metrics, metabolic syndrome was associated with a higher risk of cardiovascular disease events in all models compared with subjects without metabolic syndrome, with corresponding HRs of 1.83 (1.08–3.10) and 1.57 (1.34–1.84) in model 3, respectively. In the group with ideal iCVH status, no cardiovascular disease events were observed among the 42 cases of metabolic syndrome. A high triglyceride concentration resulted in a 42% increased risk of cardiovascular disease events in the poor status group (1.42, 1.01–2.00) and a 35% increased risk in the intermediate iCVH status group (1.35, 1.16–1.58), compared with a low triglyceride concentration, after adjusting for confounders in model 3. Additionally, in the group with poor and intermediate iCVH status, participants with a low HDL‐C had a higher risk of cardiovascular disease compared with those with a higher HDL‐C (HR: 1.63, 1.14–2.35; 1.21, 1.00–1.47, respectively). Furthermore, abdominal obesity only increased the risk of cardiovascular disease events in subjects with intermediate iCVH status (1.19, 1.02–1.40). Finally, insulin resistance as defined by HOMA‐IR increased the risk of cardiovascular disease events among those with poor and intermediate status by 90% (1.91, 1.13–3.23) and 25% (1.25, 1.00–1.56), respectively. In participants with ideal iCVH status, the presence of insulin resistance, high triglyceride, low HDL‐C, and abdominal obesity did not increase the risk of cardiovascular disease events.
Table 2.
Incidence of cardiovascular diseases according to joint classification of ideal cardiovascular health status, metabolic syndrome and its components, and insulin resistance: Tehran Lipid and Glucose Study, 2002–2018
| n/N | Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | ||
| Poor | |||||||
| Non‐MetS | 15/72 | 1.00 | 1.00 | 1.00 | |||
| MetS | 217/624 | 1.76 (1.04–2.98) | 0.033 | 1.83 (1.08–3.10) | 0.024 | 1.83 (1.08–3.10) | 0.024 |
| Intermediate | |||||||
| Non‐MetS | 241/2,458 | 1.00 | 1.00 | 1.00 | |||
| MetS | 405/2,186 | 1.99 (1.70–2.34) | <0.001 | 1.60 (1.36–1.88) | <0.001 | 1.57 (1.34–1.84) | <0.001 |
| Ideal | |||||||
| Non‐MetS | 31/858 | 1.00 | 1.00 | 1.00 | |||
| MetS | 0/42 | — | — | — | — | — | — |
| Poor | |||||||
| Low TG | 42/144 | 1.00 | 1.00 | 1.00 | |||
| High TG | 190/552 | 1.22 (0.87–1.70) | 0.242 | 1.39 (0.99–1.95) | 0.051 | 1.42 (1.01–2.00) | 0.039 |
| Intermediate | |||||||
| Low TG | 276/2,295 | 1.00 | 1.00 | 1.00 | |||
| High TG | 370/2,348 | 1.33 (1.13–1.55) | <0.001 | 1.37 (1.17–1.61) | <0.001 | 1.35 (1.16–1.58) | <0.001 |
| Ideal | |||||||
| Low TG | 24/724 | 1.00 | 1.00 | 1.00 | |||
| High TG | 7/176 | 1.11 (0.48–2.59) | 0.795 | 1.11 (0.47–2.61) | 0.801 | 0.92 (0.38–2.19) | 0.859 |
| Poor | |||||||
| High HDL‐C | 35/141 | 1.00 | 1.00 | 1.00 | |||
| Low HDL‐C | 195/551 | 1.47 (1.02–2.11) | 0.035 | 1.62 (1.13–2.33) | 0.008 | 1.63 (1.14–2.35) | 0.007 |
| Intermediate | |||||||
| High HDL‐C | 131/971 | 1.00 | 1.00 | 1.00 | |||
| Low HDL‐C | 514/3,668 | 1.00 (0.83–1.22) | 0.936 | 1.22 (1.01–1.48) | 0.041 | 1.21 (1.00–1.47) | 0.049 |
| Ideal | |||||||
| High HDL‐C | 10/223 | 1.00 | 1.00 | 1.00 | |||
| Low HDL‐C | 21/676 | 0.63 (0.29–1.34) | 0.231 | 0.90 (0.41–1.94) | 0.789 | 0.84 (0.39–1.83) | 0.672 |
| Poor | |||||||
| Non‐abdominal obesity | 34/124 | 1.00 | 1.00 | 1.00 | |||
| Abdominal obesity | 191/547 | 1.26 (0.87–1.81) | 0.213 | 1.18 (0.82–1.71) | 0.356 | 1.17 (0.81–1.69) | 0.400 |
| Intermediate | |||||||
| Non‐abdominal obesity | 261/2,255 | 1.00 | 1.00 | 1.00 | |||
| Abdominal obesity | 374/2,257 | 1.46 (1.25–1.71) | <0.001 | 1.22 (1.04–1.43) | 0.012 | 1.19 (1.02–1.40) | 0.026 |
| Ideal | |||||||
| Non‐abdominal obesity | 26/773 | 1.00 | 1.00 | 1.00 | |||
| Abdominal obesity | 4/111 | 1.04 (0.36–2.99) | 0.933 | 0.84 (0.29–2.42) | 0.757 | 0.75 (0.26–2.18) | 0.608 |
| Poor | |||||||
| Insulin sensitivity † | 17/77 | 1.00 | 1.00 | 1.00 | |||
| Insulin resistance | 87/248 | 1.76 (1.04–2.96) | 0.033 | 1.86 (1.10–3.15) | 0.020 | 1.91 (1.13–3.23) | 0.015 |
| Intermediate | |||||||
| Insulin sensitivity | 170/1,378 | 1.00 | 1.00 | 1.00 | |||
| Insulin resistance | 159/1,211 | 1.07 (0.86–1.33) | 0.499 | 1.26 (1.01–1.58) | 0.035 | 1.25 (1.00–1.56) | 0.046 |
| Ideal | |||||||
| Insulin sensitivity | 16/400 | 1.00 | 1.00 | 1.00 | |||
| Insulin resistance | 1/119 | 0.20 (0.03–1.52) | 0.122 | 0.31 (0.04–2.47) | 0.275 | 0.29 (0.04–2.31) | 0.247 |
Model 1: crude. Model 2: adjusted for age and sex. Model 3: Model 2 + further adjusted for educational level, marital status, and history of premature CVD. Global cardiovascular health status defined according to the number of ideal metrics: 0–2 (poor), 3–4 (intermediate), and 5–6 (ideal). Low TG (<150 mg/dL); High TG (≥150 mg/dL); Low HDL cholesterol (<50 mg/dL in women and <40 mg/dL in men); High HDL cholesterol (≥50 mg/dL in women and ≥40 mg/dL in men); non‐abdominal obesity (WC < 95 cm); abdominal obesity (WC ≥ 95 cm); insulin resistance (HOMA‐IR >1.85 in women and >2.17 men).
Data were included for 3,433 participants.
HDL‐C, high density lipoprotein cholesterol; MetS, metabolic syndrome; TG, triglyceride.
Bold font indicates statistical significance.
In terms of discrimination assessed by the C‐index, the addition of MetS, its dyslipidemia component, and insulin resistance to the iCVH status in both poor and intermediate status did not improve the predictive performance for the risk of cardiovascular disease. However, the result of the IDI and NRI showed that in a poor status, adding metabolic syndrome (IDI 0.006, P < 0.001; NRI 0.13, P = 0.015), high TG concentration (0.004, P < 0.001; 0.12, P = 0.014), low HDL‐C (0.005, P = 0.042; 0.09, P = 0.036), and IR (0.011, P = 0.045; 0.11, P = 0.042) to the iCVH status had an incremental effect on the predictive value for cardiovascular disease. Furthermore, in intermediate status, the addition of metabolic syndrome (0.002, P = 0.005; 0.14, P = 0.032), and low HDL‐C (0.0007, P = 0.003; 0.13, P = 0.012) to the iCVH status improved the risk prediction for cardiovascular disease (Table 3).
Table 3.
Predictive value of the metabolic syndrome, its components and insulin resistance for cardiovascular diseases
| Cox models | C statistic | Integrated discrimination improvement | Net reclassification index | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Index | 95% CI | P value | Index | 95% CI | P value | Index | 95% CI | P value | |
| Poor CVH status | |||||||||
| MetS | |||||||||
| Model 1 | 0.682 | 0.648–0.715 | <0.001 | ||||||
| Model 2 | 0.684 | 0.651–0.717 | <0.001 | 0.006 | 0.004–0.007 | <0.001 | 0.13 | 0.05–0.16 | 0.0154 |
| High TG | |||||||||
| Model 1 | 0.682 | 0.648–0.715 | <0.001 | ||||||
| Model 2 | 0.687 | 0.654–0.720 | <0.001 | 0.004 | 0.003–0.006 | <0.001 | 0.12 | 0.05–0.17 | 0.0140 |
| Low HDL‐C | |||||||||
| Model 1 | 0.682 | 0.648–0.715 | <0.001 | ||||||
| Model 2 | 0.686 | 0.653–0.719 | <0.001 | 0.005 | 0.0001–0.008 | 0.042 | 0.09 | 0.05–0.16 | 0.0362 |
| Abdominal obesity | |||||||||
| Model 1 | 0.682 | 0.648–0.715 | <0.001 | ||||||
| Model 2 | 0.679 | 0.645–0.713 | <0.001 | 0.005 | −0.003 to 0.013 | 0.241 | 0.09 | −0.19 to 0.18 | 0.1662 |
| Insulin resistance † | |||||||||
| Model 1 | 0.682 | 0.648–0.715 | <0.001 | ||||||
| Model 2 | 0.696 | 0.647–0.744 | <0.001 | 0.011 | 0.0002–0.021 | 0.045 | 0.11 | 0.06–0.18 | 0.04259 |
| Intermediate CVH status | |||||||||
| MetS | |||||||||
| Model 1 | 0.759 | 0.742–0.775 | <0.001 | ||||||
| Model 2 | 0.760 | 0.744–0.777 | <0.001 | 0.002 | 0.0007–0.004 | 0.005 | 0.14 | 0.10–0.21 | 0.03212 |
| High TG | |||||||||
| Model 1 | 0.759 | 0.742–0.775 | <0.001 | ||||||
| Model 2 | 0.759 | 0.743–0.776 | <0.001 | 0.0006 | −0.0004 to 0.001 | 0.265 | 0.07 | −0.12 to 0.22 | 0.0862 |
| Low HDL‐C | |||||||||
| Model 1 | 0.759 | 0.742–0.775 | <0.001 | ||||||
| Model 2 | 0.760 | 0.744–0.776 | <0.001 | 0.0007 | 0.0003–0.001 | 0.003 | 0.13 | 0.09–0.25 | 0.0123 |
| Abdominal obesity | |||||||||
| Model 1 | 0.759 | 0.742–0.775 | <0.001 | ||||||
| Model 2 | 0.756 | 0.739–0.772 | <0.001 | −0.0007 | −0.003 to 0.002 | 0.612 | 0.09 | −0.15 to 0.26 | 0.2652 |
| Insulin resistance | |||||||||
| Model 1 | 0.759 | 0.742–0.775 | <0.001 | ||||||
| Model 2 | 0.761 | 0.738–0.784 | <0.001 | 0.0006 | −0.001 to 0.002 | 0.478 | 0.10 | −0.17 to 0.17 | 0.2159 |
Data were included for 3,433 participants.
HDL‐C, high density lipoprotein cholesterol; MetS, metabolic syndrome; TG, triglyceride.
DISCUSSION
In this prospective study, we evaluated whether the coexistence of metabolic syndrome with iCVH status could affect the risk of cardiovascular disease. Our findings indicate that intermediate and poor iCVH status increased the risk of cardiovascular disease events by approximately 3‐fold and 5‐fold, respectively, compared with ideal iCVH status. Additionally, the presence of metabolic syndrome or insulin resistance status was associated with about a 2‐fold increased risk. Importantly, we found that the metabolic syndrome increased the risk of cardiovascular disease events among individuals with intermediate and poor iCVH status, but not among those with an ideal iCVH score. This issue is mainly attributable to the dyslipidemia component of metabolic syndrome. Importantly metabolic syndrome had a negligible but significant improvement in the prediction of cardiovascular disease applying C‐index and IDI/NRI.
Based on our data analysis, individuals with intermediate and poor iCVH status who exhibit insulin resistance, as diagnosed by either metabolic syndrome or HOMA‐IR, are at higher risk for cardiovascular disease events. In particular, those with poor iCVH status face a significantly greater cardiovascular disease risk of up to 100%. In previous prospective studies, the inclusion of metabolic syndrome did not add to the traditional risk factors in predicting cardiovascular disease in both US and MENA populations 23 , 24 , 25 . Contrary to these studies, use of MetS/IR an improvement in the risk prediction of cardiovascular disease was observed among individuals with coronary artery diseases 26 and type 2 diabetes 27 , 28 . It seems consistent with our findings that the diagnosis of metabolic syndrome and insulin resistance improved the predictive power of traditional risk factors for cardiovascular disease only among participants with a high risk of cardiometabolic diseases. Poor lifestyle habits can exacerbate the cardiovascular impact of insulin resistance by increasing the likelihood of developing type 2 diabetes at an earlier age 29 . Furthermore, some studies have suggested that the coexistence of metabolic disorders, such as dyslipidemia, may potentiate the vascular damage induced by insulin resistance 29 . Notably, as emphasized in a recent review study by Adeva‐Andany et al., 8 the impact of insulin resistance on subclinical atherosclerosis risk is independent of other cardiovascular predictors and diabetes status. Additionally, individuals who engage in risky behaviors, such as smoking, tend to experience more cardiovascular complications as a result of insulin resistance 30 . Overall, our findings suggest that early intervention for insulin resistance may be critical for reducing cardiovascular disease risk in individuals with intermediate and poor iCVH status.
The healthy cardiovascular index has been investigated extensively to date. To better account for the risk of cardiovascular disease, the updated version includes non‐HDL‐C as the main lipid component. Non‐HDL cholesterol is superior to LDL‐C for the prediction of major cardiovascular disease events and is responsible for half of all worldwide deaths 31 , 32 , 33 . The role of the management of non‐HDL cholesterol in the prevention of CVD events is unequivocal 34 , 35 , 36 . This index includes atherogenic lipoproteins, apolipoprotein β‐containing lipoproteins, including LDL‐C, very low‐density lipoprotein cholesterol, intermediate‐density lipoprotein cholesterol, lipoprotein (a), chylomicrons, and their TG‐rich remnants 37 . Triglyceride, which is also a component criterion of dyslipidemia in metabolic syndrome, is included in the iCVH scoring index as part of non‐HDL cholesterol in the updated version of Life's Essential 8. We found that hypertriglyceridemia, as a component of metabolic syndrome, was respectively associated with a 40% and 35% higher risk of cardiovascular disease among those with intermediate and poor iCVH status as defined by AHA's Life Simple 7. Triglyceride‐rich lipoprotein was also reported as a well‐established independent marker of cardiovascular events, which is supported by Mendelian randomization studies indicating the causality of triglyceride for cardiovascular disease events 38 . Furthermore, recent studies have shown that metabolic markers, including triglyceride (i.e., triglyceride‐glucose index, triglyceride to HDL‐C ratio), are becoming recognized as substitute predictors for atherosclerotic peripheral artery and cerebrovascular diseases 39 . It has been reported that among individuals with prior coronary artery disease with LDL‐C less than 70 mg/dL, lowering triglyceride to less than 200 mg/dL was associated with a lessening of coronary atheroma progression and a lowering of the residual risk of cardiovascular disease events 37 . In the current study, we also found that triglyceride is a useful measure, but with a slight predictive performance for cardiovascular disease and this predictive power was observed only among individuals with a poor status iCVH. Hence, we might speculate that interventions in the hypertriglyceridemia cut‐off point of metabolic syndrome could still provide clinical benefits to individuals with poor iCVH status defined by Life's simple 7 12 .
Herein, we also observed that individuals with intermediate and poor iCVH status had a greater proportion of cardiovascular disease attributed to low HDL‐C, at 20% and 60%, respectively. Furthermore, an improvement in risk prediction performance was observed with the inclusion of HDL‐C in non‐optimal iCVH status. Our findings are aligned with other previous studies showing that incorporation of HDL‐C into the basic risk prediction model led to a small improvement in risk discrimination 40 , 41 . This suggests that the risk of HDL‐C‐related cardiovascular disease is particularly elevated among individuals with non‐optimal iCVH status, rather than those adhering to healthy habits. Individuals with poor life habits, even in healthy status, are reported to be at a substantial risk of subclinical cardiovascular disease 42 . Accordingly, some studies suggested that low HDL‐C is an independent factor in the development of subclinical atherosclerosis in such cases 43 . The independent role of low HDL‐C on incident premature cardiovascular disease was also observed by a recent meta‐analysis with 12.7 million participants 44 . In high risk cardiovascular situations, the function of HDL‐C is disrupted, and its protective effect in preventing cardiovascular diseases is reduced 45 , 46 . However, Bian et al. 43 reported that among participants with risky behavior that for each 1 mm/L HDL‐C increase, the carotid plaque burden was significantly reduced by 67%. It seems that management of low HDL‐C in primary prevention among intermediate and poor healthy individuals may reduce the excess risk of subclinical atherosclerosis and subsequent clinical cardiovascular diseases. Meanwhile, further studies are required to better elucidate the conflicting protective role of HDL‐C on cardiovascular disease progression, particularly among those not having ideal status iCVH.
As a strength, this study is a novel contribution to the literature, as it is the first investigation to evaluate the joint impact of metabolic syndrome and its components and iCVH status in estimating the risk of cardiovascular disease events. Moreover, the precise measurement of metabolic syndrome components in the TLGS cohort, rather than self‐reported data, added to the validity of our findings. Additionally, the reasonable duration of follow‐up further strengthened the reliability of our results. This study should also be discussed in the context of limitations. First, data on insulin resistance, as captured by the HOMA‐IR criteria, were not available for the total population. Second, precise data on diet were not available during phase 2 of TLGS. However, we have previously demonstrated that the finding of adding the nutrition score to the total iCVH score in a subpopulation was similar to the result of the main analysis in the total population 47 , 48 . Third, due to low statistical power among ideal iCVH participants, the reported effect sizes were unstable. Therefore, further studies with larger samples are required to confirm the present findings. Fourth, despite reported gender differences in the impact of metabolic syndrome on cardiovascular disease events 49 , due to limited number of events in different subgroups, we did not address gender‐specific differences in the risk estimation of the corresponding joint model. Fifth, residual confounding factors, such as socio‐psycho‐economic metrics, may have affected the final findings. Last but not least, the present study was conducted in the Tehran metropolis; hence, the findings may not be extrapolated to rural regions.
CONCLUSION
In this prospective study of Iranian adults, we observed that the presence of insulin resistance could exacerbate the risk of cardiovascular disease events among individuals with intermediate and poor iCVH status. Additionally, dyslipidemia components of MetS were associated with an increased risk of cardiovascular disease. Incorporating metabolic syndrome, insulin resistance, and dyslipidemia into iCVD status results in slight but significant improvements in risk discrimination among individuals with non‐optimal iCVH status. In contrast, the diagnosis of insulin resistance among individuals with an ideal iCVH score did not significantly affect the cardiovascular disease risk detected by the AHA's Life Simple 7 metrics. Therefore, identifying and managing insulin resistance status and dyslipidemia components of metabolic syndrome may reduce the incidence of cardiovascular disease among individuals with poor and intermediate iCVH status.
DISCLOSURE
The authors declare no conflict of interest.
Approval of the research protocol: The study protocol was approved by the ethics committee of the Research Institute for Endocrine Sciences (RIES), Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Informed consent: Written informed consent was acquired from participants prior to their inclusion in the study.
Registry and the registration no. of the study/trial: 10 April 2023/ID: 43005540.
Animal studies: N/A.
ACKNOWLEDGMENT
This study was supported by Shahid Beheshti University of Medical Sciences. We express our appreciation to the participants of this study for their collaboration.
REFERENCES
- 1. Bray F, Laversanne M, Weiderpass E, et al. The ever‐increasing importance of cancer as a leading cause of premature death worldwide. Cancer 2021; 127: 3029–3030. [DOI] [PubMed] [Google Scholar]
- 2. Bhagavathula AS, Shehab A, Ullah A, et al. The burden of cardiovascular disease risk factors in the Middle East: A systematic review and meta‐analysis focusing on primary prevention. Curr Vasc Pharmacol 2021; 19: 379–389. [DOI] [PubMed] [Google Scholar]
- 3. Lloyd‐Jones DM, Hong Y, Labarthe D, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: The American Heart Association's strategic impact goal through 2020 and beyond. Circulation 2010; 121: 586–613. [DOI] [PubMed] [Google Scholar]
- 4. Michos ED, Khan SS. Further understanding of ideal cardiovascular health score metrics and cardiovascular disease. Expert Rev Cardiovasc Ther 2021; 19: 607–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Aekplakorn W, Neelapaichit N, Chariyalertsak S, et al. Ideal cardiovascular health and all‐cause or cardiovascular mortality in a longitudinal study of the Thai National Health Examination Survey IV and V. Sci Rep 2023; 13: 2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Park S, Lee S, Kim Y, et al. Altered risk for cardiovascular events with changes in the metabolic syndrome status: A nationwide population‐based study of approximately 10 million persons. Ann Intern Med 2019; 171: 875–884. [DOI] [PubMed] [Google Scholar]
- 7. Gami AS, Witt BJ, Howard DE, et al. Metabolic syndrome and risk of incident cardiovascular events and death: A systematic review and meta‐analysis of longitudinal studies. J Am Coll Cardiol 2007; 49: 403–414. [DOI] [PubMed] [Google Scholar]
- 8. Adeva‐Andany MM, Martínez‐Rodríguez J, González‐Lucán M, et al. Insulin resistance is a cardiovascular risk factor in humans. Diabetes Metab Syndr 2019; 13: 1449–1455. [DOI] [PubMed] [Google Scholar]
- 9. Bjornstad P, Eckel RH. Pathogenesis of lipid disorders in insulin resistance: A brief review. Curr Diab Rep 2018; 18: 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Raygor V, Khera A. New recommendations and revised concepts in recent guidelines on the management of dyslipidemia to prevent cardiovascular disease: The 2018 ACC/AHA and 2019 ESC/EAS guidelines. Curr Cardiol Rep 2020; 22: 87. [DOI] [PubMed] [Google Scholar]
- 11. Nelson RH. Hyperlipidemia as a risk factor for cardiovascular disease. Prim Care 2013; 40: 195–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lloyd‐Jones DM, Allen NB, Anderson CAM, et al. Life's essential 8: Updating and enhancing the American Heart Association's construct of cardiovascular health: A presidential advisory from the American Heart Association. Circulation 2022; 146: e18–e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Azizi F, Zadeh‐Vakili A, Takyar M. Review of rationale, design, and initial findings: Tehran lipid and glucose study. Int J Endocrinol Metab 2018; 16: e84777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Momenan AA, Delshad M, Sarbazi N, et al. Reliability and validity of the modifiable activity questionnaire (MAQ) in an Iranian urban adult population. Arch Iran Med 2012; 15: 279–282. [PubMed] [Google Scholar]
- 15. Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of physical activities: An update of activity codes and MET intensities. Med Sci Sports Exerc 2000; 32: S498–S504. [DOI] [PubMed] [Google Scholar]
- 16. Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009; 120: 1640–1645. [DOI] [PubMed] [Google Scholar]
- 17. Azizi F, Hadaegh F, Khalili D, et al. Appropriate definition of metabolic syndrome among Iranian adults: Report of the Iranian National Committee of Obesity. Arch Iran Med 2010; 13: 426–428. [PubMed] [Google Scholar]
- 18. Hadaegh F, Zabetian A, Sarbakhsh P, et al. Appropriate cutoff values of anthropometric variables to predict cardiovascular outcomes: 7.6 years follow‐up in an Iranian population. Int J Obes (Lond) 2005; 2009: 1437–1445. [DOI] [PubMed] [Google Scholar]
- 19. Ghasemi A, Tohidi M, Derakhshan A, et al. Cut‐off points of homeostasis model assessment of insulin resistance, beta‐cell function, and fasting serum insulin to identify future type 2 diabetes: Tehran Lipid and Glucose Study. Acta Diabetol 2015; 52: 905–915. [DOI] [PubMed] [Google Scholar]
- 20. Azizi F. Tehran lipid and glucose study: A national legacy. Int J Endocrinol Metab 2018; 16: e84774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fahimfar N, Khalili D, Mohebi R, et al. Risk factors for ischemic stroke; results from 9 years of follow‐up in a population based cohort of Iran. BMC Neurol 2012; 12: 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Azizi F, Ghanbarian A, Momenan AA, et al. Prevention of non‐communicable disease in a population in nutrition transition: Tehran Lipid and Glucose Study phase II. Trials 2009; 10: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hadaegh F, Zabetian A, Khalili D, et al. A new approach to compare the predictive power of metabolic syndrome defined by a joint interim statement versus its components for incident cardiovascular disease in Middle East Caucasian residents in Tehran. J Epidemiol Community Health 2012; 66: 427–432. [DOI] [PubMed] [Google Scholar]
- 24. Mozaffary A, Bozorgmanesh M, Sheikholeslami F, et al. Added value of different metabolic syndrome definitions for predicting cardiovascular disease and mortality events among elderly population: Tehran Lipid and Glucose Study. Eur J Clin Nutr 2014; 68: 853–858. [DOI] [PubMed] [Google Scholar]
- 25. Lorenzo C, Williams K, Hunt KJ, et al. The National Cholesterol Education Program ‐ Adult Treatment Panel III, International Diabetes Federation, and World Health Organization definitions of the metabolic syndrome as predictors of incident cardiovascular disease and diabetes. Diabetes Care 2007; 30: 8–13. [DOI] [PubMed] [Google Scholar]
- 26. Wu Z, Cui H, Li W, et al. Comparison of three non‐insulin‐based insulin resistance indexes in predicting the presence and severity of coronary artery disease. Front Cardiovasc Med 2022; 9: 918359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pan L, Zou H, Meng X, et al. Predictive values of metabolic score for insulin resistance on risk of major adverse cardiovascular events and comparison with other insulin resistance indices among Chinese with and without diabetes mellitus: Results from the 4C cohort study. J Diabetes Investig 2023; 14: 961–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mehta NN, Krishnamoorthy P, Martin SS, et al. Usefulness of insulin resistance estimation and the metabolic syndrome in predicting coronary atherosclerosis in type 2 diabetes mellitus. Am J Cardiol 2011; 107: 406–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Freeman AM, Pennings N. Insulin Resistance. StatPearls. Treasure Island, FL: StatPearls Publishing, 2022. [PubMed] [Google Scholar]
- 30. Artese A, Stamford BA, Moffatt RJ. Cigarette smoking: An accessory to the development of insulin resistance. Am J Lifestyle Med 2019; 13: 602–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. NCD Risk Factor Collaboration (NCD‐RisC) . Repositioning of the global epicentre of non‐optimal cholesterol. Nature 2020; 582: 73–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Brunner FJ, Waldeyer C, Ojeda F, et al. Application of non‐HDL cholesterol for population‐based cardiovascular risk stratification: Results from the Multinational Cardiovascular Risk Consortium. Lancet 2019; 394: 2173–2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Blaha MJ, Blumenthal RS, Brinton EA, et al. The importance of non‐HDL cholesterol reporting in lipid management. J Clin Lipidol 2008; 2: 267–273. [DOI] [PubMed] [Google Scholar]
- 34. Mach F, Baigent C, Catapano AL, et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur Heart J 2020; 41: 111–188. [DOI] [PubMed] [Google Scholar]
- 35. Wilson PWF, Polonsky TS, Miedema MD, et al. Systematic review for the 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: A report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Circulation 2019; 139: e1144–e1161. [DOI] [PubMed] [Google Scholar]
- 36. Silverman MG, Ference BA, Im K, et al. Association between lowering LDL‐C and cardiovascular risk reduction among different therapeutic interventions: A systematic review and meta‐analysis. JAMA 2016; 316: 1289–1297. [DOI] [PubMed] [Google Scholar]
- 37. Puri R, Nissen SE, Shao M, et al. Non‐HDL cholesterol and triglycerides: Implications for coronary atheroma progression and clinical events. Arterioscler Thromb Vasc Biol 2016; 36: 2220–2228. [DOI] [PubMed] [Google Scholar]
- 38. Nordestgaard BG, Varbo A. Triglycerides and cardiovascular disease. Lancet 2014; 384: 626–635. [DOI] [PubMed] [Google Scholar]
- 39. Sascău R, Clement A, Radu R, et al. Triglyceride‐rich lipoproteins and their remnants as silent promoters of atherosclerotic cardiovascular disease and other metabolic disorders: A review. Nutrients 2021; 13: 1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Paige E, Barrett J, Pennells L, et al. Use of repeated blood pressure and cholesterol measurements to improve cardiovascular disease risk prediction: An individual‐participant‐data meta‐analysis. Am J Epidemiol 2017; 186: 899–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Welsh C, Celis‐Morales CA, Brown R, et al. Comparison of conventional lipoprotein tests and apolipoproteins in the prediction of cardiovascular disease. Circulation 2019; 140: 542–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nonterah EA, Crowther NJ, Oduro A, et al. Poor cardiovascular health is associated with subclinical atherosclerosis in apparently healthy sub‐Saharan African populations: An H3Africa AWI‐Gen study. BMC Med 2021; 19: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bian L, Xia L, Wang Y, et al. Risk factors of subclinical atherosclerosis and plaque burden in high risk individuals: Results from a community‐based study. Front Physiol 2018; 9: 739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dugani SB, Hydoub YM, Ayala AP, et al. Risk factors for premature myocardial infarction: A systematic review and meta‐analysis of 77 studies. Mayo Clin Proc Innov Qual Outcomes 2021; 5: 783–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Vilahur G, Gutiérrez M, Casaní L, et al. Hypercholesterolemia abolishes high‐density lipoprotein‐related cardioprotective effects in the setting of myocardial infarction. J Am Coll Cardiol 2015; 66: 2469–2470. [DOI] [PubMed] [Google Scholar]
- 46. Judit Cubedo TP, Alonso R, Mata P, et al. ApoL1 levels in high density lipoprotein and cardiovascular event presentation in patients with familial hypercholesterolemia. J Lipid Res 2016; 57: 1059–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hadaegh F, Hosseinpour‐Niazi S, Deravi N, et al. Ideal cardiovascular health status and risk of cardiovascular disease and all‐cause mortality: Over a decade of follow‐up in the Tehran lipid and glucose study. Front Cardiovasc Med 2022; 9: 898681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Asgari S, Masrouri S, Hosseinpour‐Niazi S, et al. Association of ideal cardiovascular health metrics and incident type 2 diabetes mellitus among an urban population of Iran: One decade follow up in the Tehran Lipid and Glucose Study. J Diabetes Investig 2022; 13: 1711–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ramezankhani A, Azizi F, Hadaegh F. Gender differences in changes in metabolic syndrome status and its components and risk of cardiovascular disease: A longitudinal cohort study. Cardiovasc Diabetol 2022; 21: 227. [DOI] [PMC free article] [PubMed] [Google Scholar]


