Abstract
Background:
Posterior cruciate ligament (PCL) injuries to the knee are uncommon, and ideal surgical management of these injuries is unclear. Current surgical techniques include PCL reconstruction with remnant debridement, remnant-preserving techniques, and primary PCL repair. Augmentation of PCL repairs and reconstructions has been proposed to protect repairs or grafts in the postoperative period.
Purpose:
To describe PCL repair with the hamstring autograft augmentation technique and examine our preliminary midterm outcomes from a sequential cohort of patients.
Study Design:
Case series; Level of evidence, 4.
Methods:
The authors identified patients at their institution who underwent remnant-preserving primary PCL repair with hamstring autograft augmentation for both isolated tears and tears associated with multiligament knee injury (MLKI). Patient-reported outcomes were evaluated at a minimum 2-year follow-up using the International Knee Documentation Committee (IKDC) subjective knee form, the 12-item Short Form Survey, and a custom return-to-play questionnaire. Patient-reported outcomes data were summarized, and the predictors of outcomes from the descriptive data and clinical measures were further examined.
Results:
A total of 23 patients with a mean follow-up of 5.3 years met the inclusion criteria. Of these patients, 87% were associated with MLKI. The mean IKDC score was 87.7. Approximately 83% of patients were able to successfully return to their sport or occupation. Among 19 athletes, only 2 reported being unable to return to their preinjury level of sport because of limitations from their PCL surgery. Patient-reported outcome scores and return to sport or occupation did not have a statistically significant association with age, sex, body mass index, time from injury to surgery, or follow-up time.
Conclusion:
Outcomes of our cohort with remnant-preserving primary PCL repairs with hamstring autograft augmentation demonstrated comparable clinical outcomes to previously published PCL data. The advantages of remnant preservation, primary repair, and augmentation with an independent hamstring autograft reconstruction are combined within this technique.
Keywords: augmentation, multiligament, posterior cruciate ligament, posterior cruciate ligament reconstruction, posterior cruciate ligament repair
Posterior cruciate ligament (PCL) injuries to the knee are uncommon, and the ideal management of these injuries is unclear. 3 They may occur in isolation or association with other ligamentous injuries about the knee, constituting a multiligament knee injury (MLKI). Previous studies suggested low- and midgrade partial PCL tears heal reliably with nonoperative treatment, bracing, and quadriceps strengthening.37-39 In contrast, high-grade injuries may warrant surgical intervention in high-level athletes, in patients with MLKI, or patients with continued instability after nonoperative treatment.3,26 There is much debate surrounding the surgical treatment of high-grade PCL injuries that largely focuses on the timing of surgery, graft choice, and specific surgical technique.3,26
A variety of techniques have been described to address PCL injuries, with no consensus on a superior approach.3,26 Single-bundle PCL reconstruction with PCL remnant debridement remains a cornerstone technique advocated by many.3,6,29,32,49 Transtibial and inlay reconstruction techniques are largely equivalent, as both use 4-strand hamstring autografts and patellar tendon autografts, with good functional and return-to-play outcomes.3,6,29,32,49 However, loss of a grade of posterior drawer stability (ie, from 0 to 1+, or from 1+ to 2+) is common, with only 52% of patients with PCL reconstruction retaining ligament stability during mechanical testing. 49 The use of semitendinosus, tibialis, and Achilles tendon allografts have also demonstrated good functional results. However, there is a known risk of tunnel widening, cyst formation, effusions, delayed healing, and increased graft laxity with allografts compared with autografts.4,24 Other authors recommended preserving the PCL remnant during reconstruction, citing improved graft maturation and earlier healing.12,25,41,48 However, reconstruction with remnant preservation can be challenging because of decreased arthroscopic visibility.25,41,48 Still, others advocate for primary PCL repair, citing the PCL's ability to heal and the importance of preserving native anatomic landmarks as guiding principles.44,45,52 These repairs may be difficult to protect from shear stresses in the postoperative period, and loss of stability is similarly a concern. 34
Augmentation of PCL repairs and reconstructions has been proposed to protect the ligament or the graft from excess strain and is frequently accomplished by adding a graft or a multifilament, ultrahigh strength suture tape to the surgical construct.20,35,43,54 Biomechanical studies have demonstrated decreased anteroposterior translation with suture tape augmentation compared with traditional PCL reconstruction techniques.16,42 Nevertheless, there is a theoretical concern that ligament augmentation with artificial materials can lead to ligament stress shielding, knee overconstraint, and/or synovitis postoperatively.18,23,33,47
With these considerations in mind, for high-grade PCL injuries warranting surgical intervention, we prefer a technique that integrates portions of each of the aforementioned techniques, incorporating a remnant-preserving primary PCL repair with hamstring autograft augmentation. In this study, we detail this technique and examine our preliminary midterm outcomes from a sequential cohort of patients.
Methods
We followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines in the development, conduct, and reporting of this manuscript. 46 Before the initiation of this study, institutional review board approval was obtained, and informed consent was acquired appropriately. To identify potential study participants, we conducted a retrospective billing code database search of PCL procedures between June 2013 and July 2020 at a single institution (Current Procedural Terminology codes 29889, 27428, 27407, and 27557; all included patients had procedures coded as 29889). We included potential patients in the study if they (1) underwent a primary PCL repair with hamstring autograft augmentation; (2) were between the ages of 14 and 65 years at the time of surgery; and (3) were at least 2 years postoperative at the time of follow-up patient-reported outcomes data collection. We excluded potential patients from the study if they (1) had a history of major knee ligament surgery—including PCL, anterior cruciate ligament (ACL), medial collateral ligament (MCL), or lateral collateral ligament (LCL) repair or reconstruction on the ipsilateral side before the index PCL repair; (2) had advanced osteoarthritis—Kellgren-Lawrence (KL) grade 3 or 4—on radiographs at clinical presentation/time of surgery; (3) had >2 years of skeletal growth remaining on standard hand radiographs at clinical presentation/time of surgery—as determined by the Sanders classification 36 ; or (4) had a body mass index (BMI) >40 kg/m2 at the time of surgery.
Patient Selection, Surgical Technique, and Postoperative Rehabilitation
Patients with acute high-grade PCL tears were identified and selected for surgery according to activity level, subjective instability elicited on patient history, positive posterior drawer ≥2+, positive PCL sag sign, and/or positive PCL quadriceps active test. Both isolated PCL injuries and PCL injuries associated with MLKI were included. All surgeries were performed in a single setting in the supine position using a thigh tourniquet. An examination under anesthesia was performed for each case to confirm the posterior drawer ≥2+. A midline utility incision was utilized from the inferior pole of the patella to approximately 2 cm below the tibial tubercle, allowing for hamstring tendon graft harvest, bone–patellar tendon–bone graft harvest (if needed for ACL reconstruction), and portal placement. A diagnostic arthroscopy was quickly performed to confirm the examination under anesthesia, identify meniscal and capsular injury, and characterize the PCL tear, noting the quality of tissue and location of rupture. Collateral ligament and corner injuries were addressed via open approaches to the medial or lateral side as needed. A hamstring autograft was harvested with an atraumatic tendon stripper (Semi-T Stripper; Stryker) and prepared over a cortical button construct. A standard 4-strand graft of doubled-over semitendinosus and gracilis was typically used for the PCL augmentation. If significant high-quality native PCL tissue was identified on diagnostic arthroscopy, then a 2-strand semitendinosus graft was used, as long as the diameter of the doubled semitendinosus was ≥6 mm. Using standard anteromedial and anterolateral portals, a fat pad was debrided to allow for visualization of the intercondylar notch. A transpatellar tendon portal was created, and a cannula was inserted for later suture passage. In the case of ACL rupture, the ACL remnant was debrided, and a notchplasty was performed. The PCL tissue was meticulously preserved for later repair. The medial Gillquist interval 14 between the PCL and the medial femoral condyle was enlarged slightly with a shaver, removing minimal synovium and preserving as much PCL tissue as possible.
At this stage, if the PCL injury was a femoral-sided avulsion, 2 permanent No. 2 sutures (Maxbraid No. 2; Zimmer) were passed in grasping figure-of-8 fashion through the ligament stump for later repair, and suture limbs were retrieved through the transpatellar tendon portal cannula (Figure 1A). If the PCL injury was a midsubstance rupture, 2 permanent No. 2 sutures were passed in grasping figure-of-8 fashion through both ligament stumps and retrieved for later repair. The arthroscope was then directed into the medial Gillquist interval to visualize the posteromedial space, and a standard posteromedial portal was created under direct visualization. A radiofrequency ablator was then inserted through the posteromedial portal and used to establish a tibial starting point at the medial margin of the native PCL insertion at the posterior aspect of the tibia. 53 The arthroscope was then placed in the posteromedial portal, and the tibial insertion of the PCL was visualized. If the PCL injury was a tibial-sided avulsion, 2 permanent No. 2 sutures (Maxbraid) were passed in grasping figure-of-8 fashion through the ligament stump for later repair, and the suture limbs were retrieved through the transpatellar tendon portal. A PCL drill guide was then inserted through the medial Gillquist interval and positioned at the tibial starting point, and a 2.4-mm guidewire was drilled into place (Figure 1B). Wire position was confirmed by direct visualization and/or fluoroscopy. This was overreamed with a cannulated drill bit of the surgeon's preference to a diameter equal to the harvested hamstring tendon graft. A bone-cutting shaver was used to remove bone debris, smooth the tibial tunnel, and blunt the “killer turn.” 21 A passing suture (FiberStick; Arthrex) was then retrieved from the tibial tunnel out through the transpatellar tendon cannula.
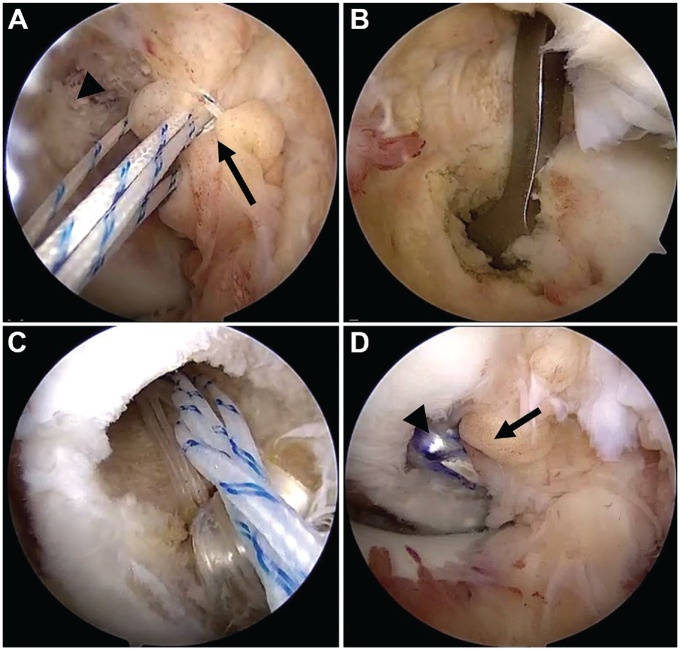
Figure 1.
Intraoperative arthroscopic images demonstrating (A) placement of repair sutures within the residual PCL stump (arrow) avulsed from the femoral origin (arrowhead); (B) drilling of the tibial tunnel with the placement of the drill guide through the medial Gillquist interval; (C) suture configuration during graft passage, with graft, cortical button construct (white sutures), and repair sutures (white/blue stripes) being advanced into the femoral tunnel; and (D) the final construct, with augmentation graft (arrowhead) and native PCL repair (arrow). PCL, posterior cruciate ligament.
The arthroscope was then switched to an anterior portal, and the femoral origin of the PCL was visualized. A socket 20 mm in length was created at the most anterior and distal aspect of the PCL origin using either a FlipCutter (Arthrex) retrograde drilling technique or an anterolateral portal drilling technique, with care taken not to violate the medial femoral condylar cartilage. A passing suture (FiberStick) was then retrieved from the femoral tunnel out through the transpatellar tendon cannula (Figure 1C). The previously placed PCL repair sutures were then passed through the femoral or tibial tunnel as appropriate. The hamstring tendon graft augment was passed through the tibial tunnel into the knee and docked into the femoral blind tunnel. The cortical button was visualized directly as lying on bone through a small incision over the medial femoral condyle. Isometry of the graft was examined, and the graft was cycled. The PCL augment was then set using an Intrafix screw and sheath system (Depuy Mitek), with an anterior drawer force applied to the knee at 90° of flexion with the tibia 1 cm anterior to the distal femoral condyles.5,27 PCL repair sutures from the native PCL stump were then tied (ie, over the femoral cortical button in the case of femoral-sided avulsion, over the Intrafix screw in the case of tibial-sided avulsion, and to the opposite stump's repair sutures in the case of midsubstance rupture) (Figure 1D). The order of fixation in the case of MLKI was as follows: collateral ligament and corner repair, followed by PCL augmentation, followed by PCL repair, and followed by ACL reconstruction.
Postoperative rehabilitation included physical therapy beginning on postoperative day 1 for range of motion, weightbearing as tolerated with 2 crutches (unless nonweightbearing was dictated by collateral or corner surgery), knee immobilization in a hinged knee brace locked in extension for 6 weeks, and dynamic strengthening beginning around the end of week 6. Before return to sports participation for athletes, isokinetic strength testing was performed at 60, 180, and 300 deg/s (Biodex) for the quadriceps and hamstrings and analyzed for limb symmetry (compared with the contralateral/uninvolved limb) as well as for the hamstring to quadriceps ratio. Patients were permitted to start agility exercises and begin return-to-sports interval programs when clinically indicated, when quadriceps and hamstring strength was within 15% of the contralateral leg (limb symmetry >85%), and when the hamstring to quadriceps ratio was >0.7.
Clinical and Outcomes Data Collection
For the included patients, we collected descriptive and clinical data from both clinic and operative notes that were housed within the electronic health record—including age, sex, BMI, time between injury and PCL repair, injury setting, primary sport, concomitant knee procedures alongside PCL repair, PCL tear location, and occurrence of same-institution revision knee ligament surgery. We utilized an electronic data repository—the Outcomes Based Electronic Research Database (OBERD) (Universal Research Solutions)—to collect patient-reported outcomes data. The OBERD system regularly distributed outcomes surveys electronically to enrolled PCL repair patients using automated emails and/or short message service (ie, text message). Patients who did not respond to the electronic survey request were contacted via telephone, and survey question responses were obtained from them verbally.
Postoperative outcomes were evaluated at a minimum 2-year follow-up. The International Knee Documentation Committee (IKDC) subjective knee form was used to evaluate the knee-related function, and the 12-item Short Form Survey (SF-12) was used to assess overall health-related quality of life. The IKDC subjective knee form is a 10-item knee-specific measure of symptoms, function, and sports activity. 22 In addition to the overall IKDC score, we included the patient-rated evaluation of current function—with a score of 0 indicating an inability to perform daily activities and a score of 10 indicating normal function. The SF-12 is reliable and valid 50 and includes both physical and mental quality of life components, which are scored based on a United States general population–centered mean score of 50. We also assessed the proportion of patients who were able to successfully return to preinjury sport (athletes) or occupation (nonathletes) with the use of our return-to-play questionnaire (available separately as Supplemental Material).
Statistical Analyses
Summary statistics for the baseline and follow-up descriptive, clinical, surgical, and outcomes data were calculated. As a secondary analysis and because a large proportion of our cohort participated in football before the injury, we compared outcome scores (IKDC and SF-12) between football athletes and other athletes/nonathletes using independent t tests. Last, we examined the association between preinjury characteristics and injury variables (age, follow-up time, sex, days between injury and surgery, and BMI at surgery) and the IKDC score at the follow-up or successful return to preinjury sport or occupation using linear and logistic regression, respectively. All regression analyses were univariate models with only 1 independent variable entered per model— linear regression for the IKDC score at the follow-up and logistic regression for a successful return to preinjury sport or occupation. For all analyses, P < .05 was considered statistically significant. We performed all statistical analyses using SPSS software (version 28.0; IBM; Chicago, IL, USA).
Results
A total of 85 PCL procedures were identified with our retrospective billing query. Among these, there were 29 PCL primary repairs with hamstring autograft augmentation procedures. Two patients were excluded for having undergone previous major ligament knee surgery, leaving 27 patients who met the inclusion criteria. Data on 2-year follow-up outcomes were collected for 23 (85.2%) of the 27 potential patients. The mean follow-up length was 5.3 years, and the mean time from injury to surgery was 20.6 days. Descriptive and clinical data for the cohort are shown in Table 1.
Table 1.
Descriptive and Clinical Data for the Study Cohort (N = 23) a
| Characteristic | Value |
|---|---|
| Age at surgery, y | 22.2 ± 7.5 (14.9-44.3) |
| Age at follow-up, y | 27.5 ± 8.2 (20.6-52.8) |
| Follow-up time, y | 5.3 ± 2.0 (2.1-8.7) |
| Time between injury and surgery, d | 20.6 ± 22.7 (4-113) |
| Sex | |
| Male | 21 (91.3) |
| Female | 2 (8.7) |
| BMI, kg/m2 | 28.3 ± 4.6 (20-37) |
| Injury setting | |
| Sport competition | 15 (65.2) |
| Sport practice | 4 (17.4) |
| Nonathletic trauma | 3 (13) |
| Work/occupation | 1 (4.3) |
| Primary sport | |
| Football | 11 (52.2) |
| Soccer | 3 (13) |
| Softball | 2 (8.7) |
| Basketball | 1 (4.3) |
| Baseball | 1 (4.3) |
| Nonathlete | 4 (17.4) |
| Concomitant knee procedures b | |
| ACL reconstruction | 11 (47.8) |
| MCL/PMC repair | 8 (34.8) |
| LCL/PLC repair | 6 (26.1) |
| Meniscus repair | 5 (21.7) |
| Partial meniscectomy | 5 (21.7) |
| PCL tear location | |
| Femoral | 15 (65) |
| Midsubstance | 6 (26) |
| Tibial | 2 (9) |
Data are reported as mean ± SD (range) or n (%). ACL, anterior cruciate ligament; BMI, body mass index; LCL, lateral collateral ligament; MCL, medial collateral ligament; PCL, posterior cruciate ligament; PLC, posterolateral corner; PMC, posteromedial corner.
Concomitant procedures could occur in combination.
No patients were excluded based on radiographic signs of osteoarthritis (KL grade 3 or 4) at the time of surgery, and no patients were included who had radiographic symptoms of osteoarthritis at the time of surgery. Two patients (8.7%) had physes <2 years from skeletal maturity, while the remainder of the cohort (91.3%) were skeletally mature. Three patients (13%) had isolated PCL injuries, whereas 20 patients (87%) had MLKI requiring repair or reconstruction of another major knee ligament. Eleven patients (47.8%) also underwent ACL reconstruction with bone–patellar tendon–bone autografts, 8 patients (34.8%) underwent MCL or posteromedial corner repair, and 6 patients (26.1%) underwent LCL or posterolateral corner repair. Four patients (17.4%) underwent medial meniscus repair, 2 patients (8.7%) underwent partial medial meniscectomy, 3 patients (13%) underwent lateral meniscus repair, and 4 patients (17.4%) underwent partial lateral meniscectomy. There were no high-grade cartilage lesions requiring treatment beyond abrasion chondroplasty in this cohort. PCL ruptures were femoral-sided in 15 patients (65.2%), tibial-sided in 2 patients (8.7%), and midsubstance in 6 patients (26.1%). There were no patients who required revision ligament repair or reconstruction surgery at the time of the latest contact.
Outcomes data for the cohort are shown in Table 2. The mean IKDC score was 87.7. The mean SF-12 physical summary score was 55.3, and the mean SF-12 mental summary score was 61 (United States population-centered 50 mean, 50). Also, 19 (82.6%) patients were able to return to their preinjury sport or occupation, with 13 out of 19 (68.4%) athletes returning to their preinjury level of sport or competition (Table 2). Of those athletes unable to successfully return to their preinjury sport level (n = 6), only 2 reported that their inability was due to limitations from their PCL surgery (Table 2).
Table 2.
Outcomes at Minimum 2-Year Follow-up for the Study Cohort a
| Variable | Value |
|---|---|
| IKDC score | 87.7 ± 9.8 (55.2-98.9) |
| Current knee function (0-10) | 8.1 ± 1.4 (5-10) |
| SF-12 physical score b | 55.3 ± 3.2 (47.9-59.3) |
| SF-12 mental score b | 61 ± 5.6 (39.8-66.7) |
| Able to return to sports or occupations | 19/23 (82.6) c |
| Athletes able to return to preinjury level of sports d | 13/19 (68.4) |
Data are reported as mean ± SD (range) or n (%). IKDC, International Knee Documentation Committee subjective knee form; SF-12, 12-item Short Form Survey.
A score of 50 represents the mean of the United States population.
Includes 4 nonathletes.
Of the 6 athletes unable to successfully return to their preinjury sport level, 2 reported that this was because of limitations from their PCL surgery, 2 reported that they had graduated and were not talented enough for the next level, and 2 reported that they did not return for personal reasons (eg, loss of interest).
Subgroup analysis revealed no significant differences between football players and the rest of the cohort in the IKDC, SF-12 mental, or SF-12 physical scores (all P > .12). Age, sex, and BMI were not significantly associated with the IKDC score at the final follow-up (P = .44, P = .19, and P = .18, respectively). The time from injury to surgery and the follow-up length were not significantly associated with the follow-up IKDC score (P = .15 and P = .69, respectively). Similarly, in examining return to sport or work, we found no significant associations between the patient's ability to return to their preinjury sport or occupation and their age, sex, BMI, time from injury to surgery, and follow-up time (all P≥ .62). In comparing femoral-sided PCL ruptures with all other ruptures, there were no significant differences between the groups in IKDC scores (P = .07).
Regarding postoperative complications, 1 patient underwent debridement of a distal wound, arthroscopy with lysis of adhesions, and manipulation under anesthesia at 10 weeks, followed by repeat arthroscopy, trochlear chondroplasty, and open excision of heterotopic ossification about the MCL at 28 weeks.
Discussion
The findings demonstrate excellent patient-reported outcome scores for our unique PCL repair with augmentation technique in a moderately sized cohort with midterm follow-up. Specifically, IKDC and SF-12 scores were excellent—we demonstrated a high proportion of patients returning to their preinjury sport or occupation, and there were no same-institution revision surgeries. Specifically, at the follow-up, we found a mean IKDC score of 87.7, a mean SF-12 physical score of 55.3, and a mean SF-12 mental score of 61.
These outcome scores are comparable to those of other cohorts in the current literature. Four-year outcomes data from Lahner et al, 28 investigating isolated PCL single-bundle reconstruction with hamstring autografts, demonstrated a mean postoperative IKDC score of 65.1. Other series of isolated PCL reconstruction reported mean IKDC scores ranging from 66.3 to 92.5 in their cohorts.7,13,17,31 Regarding SF-12 scores, whereas to our knowledge, no previous studies have evaluated health-related quality of life in PCL patients, large cohort data from Webster and Feller 51 on patients who had isolated ACL reconstruction reported a mean SF-12 physical component summary score of 54.4 and a mean mental component summary score of 54.8. In a cohort of MLKI, Alentorn-Geli et al 1 reported a mean IKDC score of 62.7, a mean SF-12 physical score of 44.8, and a mean SF-12 mental score of 51.8 at a 2-year follow-up. Other MLKI studies 17 reported mean IKDC scores at follow-ups ranging from 71.8 to 92.4. Godin et al 15 in a unique series of adolescent MLKIs, reported a mean SF-12 physical score of 56.1 and a mean SF-12 mental score of 56.9.
Regarding return to sport, studies evaluating isolated PCL injuries have reported return-to-sport proportions ranging from 50% to 82% of athletes. In contrast, studies of athletes with MLKIs reported 59% successful return, with approximately 64% being elite football players.2,10,17 For return-to-sport outcomes, our present data meet or exceed the current results found in the literature. We found that approximately 83% of patients were able to return to either sport or occupation (for nonathletes), and examining only athletes, approximately 68% were able to successfully return to their preinjury level of sport. Among these, only 2 of the 19 athletes (10.5%) reported that they were unable to return because of limitations from their PCL surgery. In contrast, others did not return because they were not talented enough for the next level or because of other personal reasons.
Two recent systematic reviews comparing PCL remnant-sparing augmentation to traditional reconstruction demonstrated similar or better functional scores, ligament laxity, and complication rates with augmentation.9,40 There are several potential advantages to our PCL repair with the graft augmentation technique. Remnant preservation retains ligament tissue that may have the potential to heal if protected from excessive stress. This remnant may also lend blood supply to the graft along its length, possibly resulting in earlier incorporation and maturation of the graft. 12 Primary repair of the PCL allows for the reduction of native tissue to anatomic landmarks and possibly more rapid healing and ligamentization compared with reconstruction.11,19 The addition of hamstring autograft augmentation may further protect the primary repair by maintaining the tibiofemoral relationship as an independent PCL reconstruction and by adding collagen to the construct without any of the complications of artificial material augmentation, namely, stress shielding, overconstraint, synovitis, and/or failure. As the augmentation graft experiences stress, it may mature and remodel with the repaired remnant tissue to create a robust PCL. We prefer autografts over allografts because of the known risk of tunnel widening, cyst formation, effusions, delayed healing, and increased graft laxity with allografts.4,24 Disadvantages of this technique include difficulty with arthroscopic visualization.25,41,48
Limitations
There are several limitations to consider pertinent to our study. As with most PCL studies, the size of the cohort was reduced by the rarity of the injury as well as limited indications for operative treatment. In light of this, some of our secondary analyses may have been underpowered; specifically, we found a trend toward differences in IKDC scores among PCL tear location subgroups that did not reach statistical significance (femoral-sided vs midsubstance vs tibial-sided; P = .07). However, the largest magnitude group difference among these 3 subgroups was 8.5 points (does not exceed recently reported IKDC minimal clinically important difference values of 16.8 and 16.7 from studies of Liu et al 30 and Chen et al, 8 respectively). Further, patient outcomes data in a cohort with a high proportion of MLKI, as in the present cohort, are vulnerable to confounding because of concomitant injuries. In addition, inherent biases are present in single-arm cohort and outcomes-based research studies, including the lack of a comparison group either not undergoing PCL surgery (eg, nonoperative management) or undergoing PCL surgery with a different technique. Moreover, it is important to note that we did not record clinical PCL failures that did not undergo revision surgery, nor did we track revision surgeries outside of our institution.
Conclusion
Outcomes from our cohort of remnant-preserving primary PCL repairs with hamstring autograft augmentation demonstrated comparable clinical results to previously published PCL and MLKI surgical outcomes data. The approach detailed in this paper remains our preferred method for acute PCL surgery, as the advantages of remnant preservation, primary repair, and augmentation with an independent hamstring autograft reconstruction are all combined within this technique.
Supplemental Material
Supplemental material, sj-pdf-1-ojs-10.1177_23259671231213988 for Technique and Outcomes of Posterior Cruciate Ligament Repair With Augmentation by Alexander E. Loeb, Matthew P. Ithurburn, Ariel Kidwell-Chandler, Andrew Atkinson and E. Lyle Cain in Orthopaedic Journal of Sports Medicine
Footnotes
Final revision submitted May 25, 2023; accepted June 19, 2023.
One or more of the authors has declared the following potential conflict of interest or source of funding: A.E.L. has received a grant from Arthrex and DJO; education payments from Smith & Nephew and Supreme Orthopedic Systems; and hospitality payments from Zimmer Biomet Holdings. E.L.C. has received education payments from Prime Surgical and Zimmer Biomet Holdings; consulting fees from Arthrex, Zimmer Biomet Holdings, DJO, Smith & Nephew; nonconsulting fees from Arthrex, Medical Device Business Services, and Smith & Nephew; royalties from Arthrex; and hospitality payments from Encore Medical. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
Ethical approval for this study was obtained from Sterling Institutional Review Board (9487).
Supplemental Material: Supplemental Material for this article is available at https://journals.sagepub.com/doi/full/10.1177/23259671231213988#supplementary-materials.
References
- 1. Alentorn-Geli E, Lazarides AL, Utturkar GM, et al. Factors predictive of poorer outcomes in the surgical repair of multiligament knee injuries. Knee Surg Sports Traumatol Arthrosc. 2019;27(2):445-459. [DOI] [PubMed] [Google Scholar]
- 2. Bakshi NK, Khan M, Lee S, et al. Return to play after multiligament knee injuries in National Football League athletes. Sports Health. 2018;10(6):495-499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bedi A, Musahl V, Cowan JB. Management of posterior cruciate ligament injuries: an evidence-based review. J Am Acad Orthop Surg. 2016;24(5):277-289. [DOI] [PubMed] [Google Scholar]
- 4. Belk JW, Kraeutler MJ, Purcell JM, McCarty EC. Autograft versus allograft for posterior cruciate ligament reconstruction: an updated systematic review and meta-analysis. Am J Sports Med. 2018;46(7):1752-1757. [DOI] [PubMed] [Google Scholar]
- 5. Burns WC, II, Draganich LF, Pyevich M, Reider B. The effect of femoral tunnel position and graft tensioning technique on posterior laxity of the posterior cruciate ligament-reconstructed knee. Am J Sports Med. 1995;23(4):424-430. [DOI] [PubMed] [Google Scholar]
- 6. Chan YS, Yang SC, Chang CH, et al. Arthroscopic reconstruction of the posterior cruciate ligament with use of a quadruple hamstring tendon graft with 3- to 5-year follow-up. Arthroscopy. 2006;22(7):762-770. [DOI] [PubMed] [Google Scholar]
- 7. Chen B, Gao S. Double-bundle posterior cruciate ligament reconstruction using a non-hardware suspension fixation technique and 8 strands of autogenous hamstring tendons. Arthroscopy. 2009;25(7):777-782. [DOI] [PubMed] [Google Scholar]
- 8. Chen YJ, Yang CP, Ho CS, et al. Midterm outcomes after revision posterior cruciate ligament reconstruction with a single-bundle transtibial autograft. Orthop J Sports Med. 2022;10(8):23259671221115423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Del Buono A, Radmilovic J, Gargano G, Gatto S, Maffulli N. Augmentation or reconstruction of PCL? A quantitative review. Knee Surg Sports Traumatol Arthrosc. 2013;21(5):1050-1063. [DOI] [PubMed] [Google Scholar]
- 10. Everhart JS, Du A, Chalasani R, et al. Return to work or sport after multiligament knee injury: a systematic review of 21 studies and 524 patients. Arthroscopy. 2018;34(5):1708-1716. [DOI] [PubMed] [Google Scholar]
- 11. Ferretti A, Monaco E, Annibaldi A, et al. The healing potential of an acutely repaired ACL: a sequential MRI study. J Orthop Traumatol. 2020;21(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fujimoto E, Sasashige Y, Masuda Y, et al. Serial magnetic resonance imaging study of posterior cruciate ligament reconstruction or augmentation using hamstring tendons. Orthop Traumatol Surg Res. 2014;100(7):755-760. [DOI] [PubMed] [Google Scholar]
- 13. Garofalo R, Jolles BM, Moretti B, Siegrist O. Double-bundle transtibial posterior cruciate ligament reconstruction with a tendon-patellar bone-semitendinosus tendon autograft: clinical results with a minimum of 2 years’ follow-up. Arthroscopy. 2006;22(12):1331-1338. [DOI] [PubMed] [Google Scholar]
- 14. Gillquist J, Hagberg G, Oretorp N. Arthroscopic examination of the posteromedial compartment of the knee joint. Int Orthop. 1979;3(1):13-18. [DOI] [PubMed] [Google Scholar]
- 15. Godin JA, Cinque ME, Pogorzelski J, et al. Multiligament knee injuries in older adolescents: a 2-year minimum follow-up study. Orthop J Sports Med. 2017;5(9):2325967117727717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Grotting JA, Nelson TJ, Banffy MB, et al. Biomechanical evaluation of PCL reconstruction with suture augmentation. Knee. 2020;27(2):375-383. [DOI] [PubMed] [Google Scholar]
- 17. Hammoud S, Reinhardt KR, Marx RG. Outcomes of posterior cruciate ligament treatment: a review of the evidence. Sports Med Arthrosc Rev. 2010;18(4):280-291. [DOI] [PubMed] [Google Scholar]
- 18. Heusdens CHW, Blockhuys K, Roelant E, et al. Suture tape augmentation ACL repair, stable knee, and favorable PROMs, but a re-rupture rate of 11% within 2 years. Knee Surg Sports Traumatol Arthrosc. 2021;29(11):3706-3714. [DOI] [PubMed] [Google Scholar]
- 19. Hofbauer M, Soldati F, Szomolanyi P, et al. Hamstring tendon autografts do not show complete graft maturity 6 months postoperatively after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2019;27(1):130-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hopper GP, Irfan A, Jenkins JM, Wilson WT, Mackay GM. Posterior cruciate ligament repair with suture tape augmentation: a case series with minimum 2-year follow-up. J Exp Orthop. 2021;8(1):28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Huang TW, Wang CJ, Weng LH, Chan YS. Reducing the "killer turn" in posterior cruciate ligament reconstruction. Arthroscopy. 2003;19(7):712-716. [DOI] [PubMed] [Google Scholar]
- 22. Irrgang JJ, Anderson AF, Boland AL, et al. Development and validation of the international knee documentation committee subjective knee form. Am J Sports Med. 2001;29(5):600-613. [DOI] [PubMed] [Google Scholar]
- 23. Itoh S, Muneta T, Shinomiya K, Ichinose S. Electron microscopic evaluation of the effects of stress-shielding on maturation of the mid-substance and ligament-bone junction of the reconstructed anterior cruciate ligament in rabbits. J Mater Sci Mater Med. 1999;10(3):185-190. [DOI] [PubMed] [Google Scholar]
- 24. Johnson P, Mitchell SM, Gortz S. Graft considerations in posterior cruciate ligament reconstruction. Curr Rev Musculoskelet Med. 2018;11(3):521-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jung YB, Jung HJ, Song KS, et al. Remnant posterior cruciate ligament-augmenting stent procedure for injuries in the acute or subacute stage. Arthroscopy. 2010;26(2):223-229. [DOI] [PubMed] [Google Scholar]
- 26. Kew ME, Miller MD. Posterior cruciate ligament reconstruction in the multiple ligament injured knee. J Knee Surg. 2020;33(5):421-430. [DOI] [PubMed] [Google Scholar]
- 27. Komatsu T, Kadoya Y, Nakagawa S, Yoshida G, Takaoka K. Movement of the posterior cruciate ligament during knee flexion—MRI analysis. J Orthop Res. 2005;23(2):334-339. [DOI] [PubMed] [Google Scholar]
- 28. Lahner M, Vogel T, Schulz MS, Strobel MJ. Outcome 4 years after isolated single-bundle posterior cruciate ligament reconstruction. Article in German. Orthopade. 2012;41(3):206-211. [DOI] [PubMed] [Google Scholar]
- 29. Lin YC, Chen SK, Liu TH, Cheng YM, Chou PP. Arthroscopic transtibial single-bundle posterior cruciate ligament reconstruction using patellar tendon graft compared with hamstring tendon graft. Arch Orthop Trauma Surg. 2013;133(4):523-530. [DOI] [PubMed] [Google Scholar]
- 30. Liu CH, Chiu CH, Chang SS, et al. Clinical and functional outcomes of isolated posterior cruciate ligament reconstruction in patients over the age of 40 years. BMC Musculoskelet Disord. 2022;23(1):210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Luo Y, Wang ZG, Li ZJ, Wei M. Arthroscopic reconstruction of the posterior cruciate ligament with a ligament-advanced reinforcement system and hamstring tendon autograft: a retrospective study. Curr Med Sci. 2021;41(5):930-935. [DOI] [PubMed] [Google Scholar]
- 32. MacGillivray JD, Stein BE, Park M, et al. Comparison of tibial inlay versus transtibial techniques for isolated posterior cruciate ligament reconstruction: minimum 2-year follow-up. Arthroscopy. 2006;22(3):320-328. [DOI] [PubMed] [Google Scholar]
- 33. Mackenzie CEA, Huntington LS, Tulloch S. Suture tape augmentation of anterior cruciate ligament reconstruction increases biomechanical stability: a scoping review of biomechanical, animal, and clinical studies. Arthroscopy. 2022;38(6):2073-2089. [DOI] [PubMed] [Google Scholar]
- 34. Mariani PP, Santoriello P, Iannone S, Condello V, Adriani E. Comparison of surgical treatments for knee dislocation. Am J Knee Surg. 1999;12(4):214-221. [PubMed] [Google Scholar]
- 35. McDonald LK, Cosic F, Joseph S. The use of the ligament augmentation and reconstruction system for posterior cruciate ligament reconstruction in isolated and multiligament knee injuries: a systematic review. Knee. 2021;30:322-336. [DOI] [PubMed] [Google Scholar]
- 36. Sanders JO, Khoury JG, Kishan S, et al. Predicting scoliosis progression from skeletal maturity: a simplified classification during adolescence. J Bone Joint Surg Am. 2008;90(3):540-553. [DOI] [PubMed] [Google Scholar]
- 37. Shelbourne KD, Clark M, Gray T. Minimum 10-year follow-up of patients after an acute, isolated posterior cruciate ligament injury treated nonoperatively. Am J Sports Med. 2013;41(7):1526-1533. [DOI] [PubMed] [Google Scholar]
- 38. Shelbourne KD, Davis TJ, Patel DV. The natural history of acute, isolated, nonoperatively treated posterior cruciate ligament injuries. A prospective study. Am J Sports Med. 1999;27(3):276-283. [DOI] [PubMed] [Google Scholar]
- 39. Shelbourne KD, Jennings RW, Vahey TN. Magnetic resonance imaging of posterior cruciate ligament injuries: assessment of healing. Am J Knee Surg. 1999;12(4):209-213. [PubMed] [Google Scholar]
- 40. Song JG, Kim HJ, Han JH, et al. Clinical outcome of posterior cruciate ligament reconstruction with and without remnant preservation. Arthroscopy. 2015;31(9):1796-1806. [DOI] [PubMed] [Google Scholar]
- 41. Surendran S, Choi NY, Yoon KJ, Han CW. Arthroscopic posterior cruciate ligament augmentation using an autogenous hamstring tendon graft and the posterior-posterior triangulation technique. Arthroscopy. 2007;23(4):444.e1-6. [DOI] [PubMed] [Google Scholar]
- 42. Trasolini NA, Hatch GF, Wright D, et al. Posterior cruciate ligament reconstruction with internal brace augmentation reduces posterior tibial translation under cyclic loading. Orthopedics. 2021;44(4):235-240. [DOI] [PubMed] [Google Scholar]
- 43. van der List JP, DiFelice GS. Arthroscopic primary posterior cruciate ligament repair with suture augmentation. Arthrosc Tech. 2017;6(5):e1685-e1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Vermeijden HD, van der List JP, DiFelice GS. Arthroscopic posterior cruciate ligament primary repair. Sports Med Arthrosc Rev. 2020;28(1):23-29. [DOI] [PubMed] [Google Scholar]
- 45. Vermeijden HD, van der List JP, DiFelice GS. Arthroscopic primary repair of the posterior cruciate ligament. J Knee Surg. 2021;34(5):478-485. [DOI] [PubMed] [Google Scholar]
- 46. von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453-1457. [DOI] [PubMed] [Google Scholar]
- 47. Waly AH, ElShafie HI, Morsy MG, et al. All-inside anterior cruciate ligament reconstruction with suture tape augmentation: button tie-over technique (BTOT). Arthrosc Tech. 2021;10(11):e2559-e2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wang CJ, Chan YS, Weng LH. Posterior cruciate ligament reconstruction using hamstring tendon graft with remnant augmentation. Arthroscopy. 2005;21(11):1401. [DOI] [PubMed] [Google Scholar]
- 49. Wang CJ, Chen HS, Huang TW. Outcome of arthroscopic single bundle reconstruction for complete posterior cruciate ligament tear. Injury. 2003;34(10):747-751. [DOI] [PubMed] [Google Scholar]
- 50. Ware J, Jr, Kosinski M, Keller SD. A 12-item short-form health survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220-233. [DOI] [PubMed] [Google Scholar]
- 51. Webster KE, Feller JA. Use of the short form health surveys as an outcome measure for anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2014;22(5):1142-1148. [DOI] [PubMed] [Google Scholar]
- 52. Wheatley WB, Martinez AE, Sacks T, et al. Arthroscopic posterior cruciate ligament repair. Arthroscopy. 2002;18(7):695-702. [DOI] [PubMed] [Google Scholar]
- 53. Zhao JZ, Huang-Fu XQ, He YH, Yang XG. Single-bundle posterior cruciate ligament reconstruction with remnant preservation: lateral versus medial-sided augmentation technique. Orthop Surg. 2009;1(1):66-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zhao X, Duan MY, Chen SQ, et al. Posterior cruciate ligament reconstruction with independent internal brace reinforcement: surgical technique and clinical outcomes with a minimum two year follow-up. Int Orthop. 2022;46(9):2019-2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-ojs-10.1177_23259671231213988 for Technique and Outcomes of Posterior Cruciate Ligament Repair With Augmentation by Alexander E. Loeb, Matthew P. Ithurburn, Ariel Kidwell-Chandler, Andrew Atkinson and E. Lyle Cain in Orthopaedic Journal of Sports Medicine