Abstract
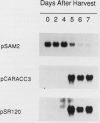
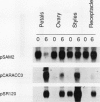
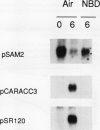
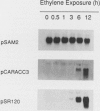
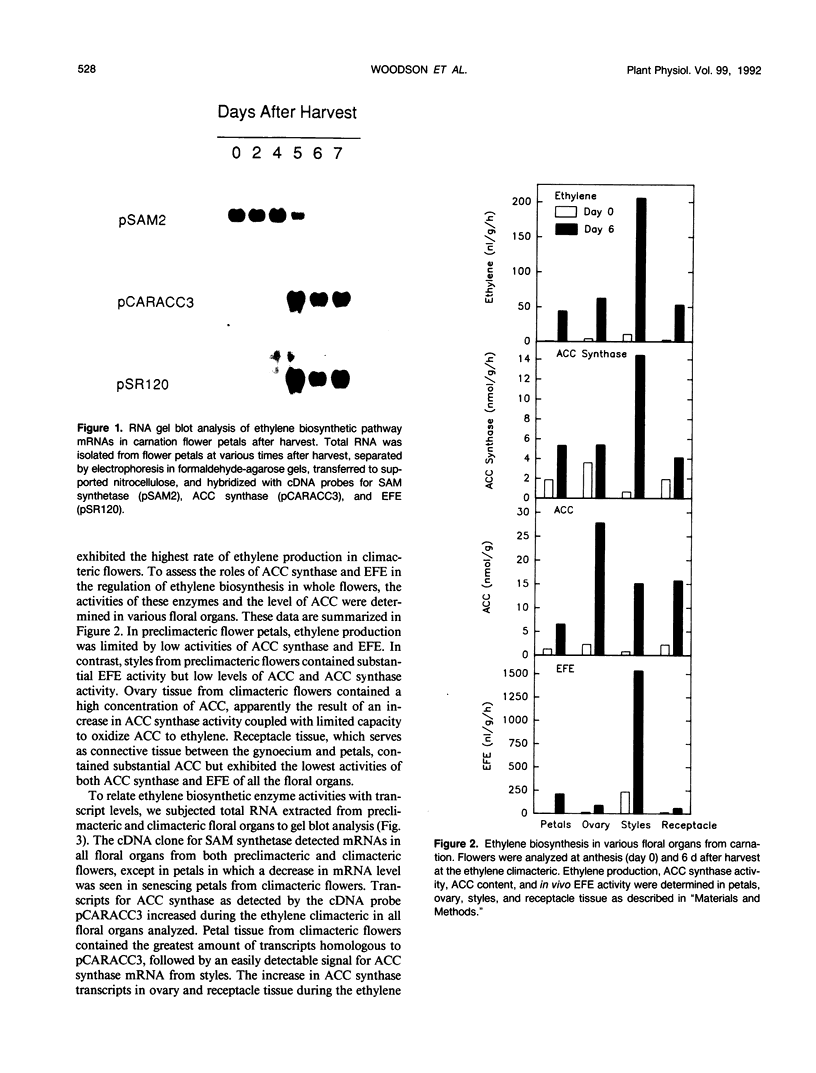
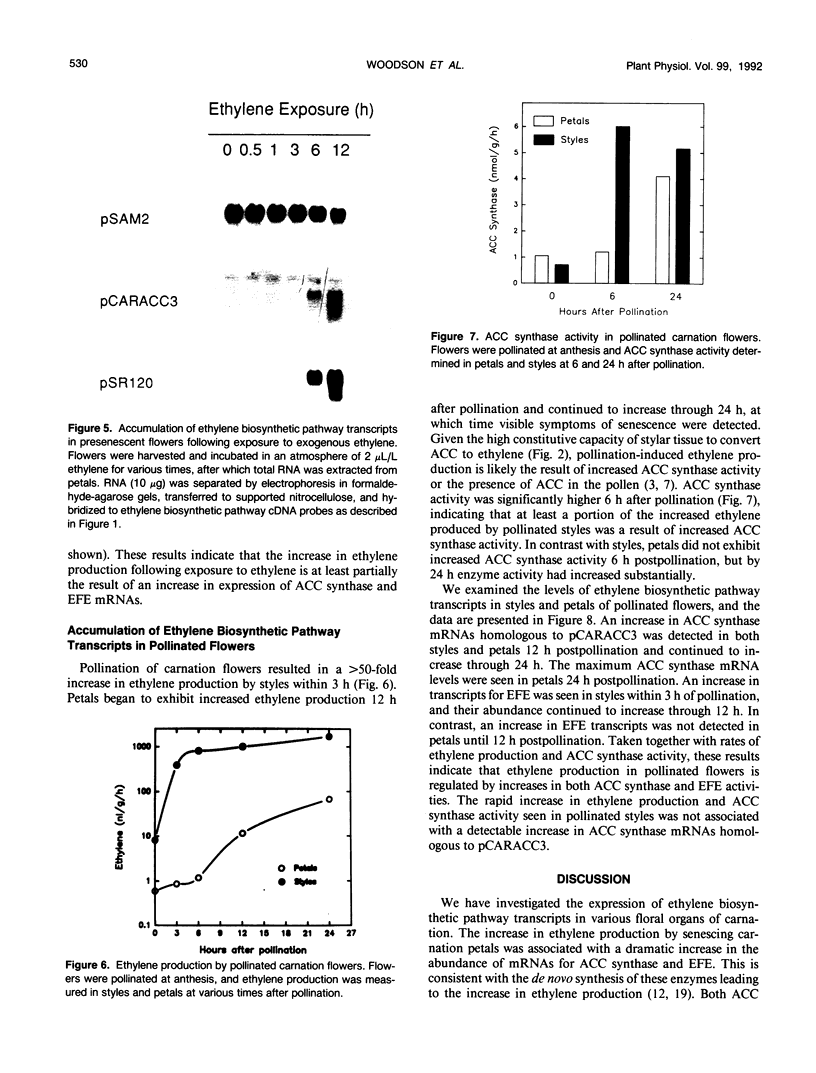
We have examined the expression of mRNAs for S-adenosylmethionine synthetase (EC 2.5.1.6), 1-aminocyclopropane-1-carboxylate (ACC) synthase (EC 4.4.1.14), and the ethylene-forming enzyme (EFE) in various floral organs of carnation (Dianthus caryophyllus) during the increase in ethylene biosynthesis associated with petal senescence. The abundance of ACC synthase and EFE mRNAs increased and S-adenosylmethionine synthetase transcripts decreased concomitantly with the ethylene climacteric in senescing petals. The increase in abundance of ACC synthase and EFE mRNAs in aging flowers was prevented by treatment with the ethylene action inhibitor 2,5-norbornadiene. Furthermore, an increase in ACC synthase and EFE transcripts was detected in petals from presenescent flowers within 3 to 6 hours of exposure to 2 microliters per liter of ethylene. The increase in ethylene production by senescing petals was associated with a concomitant increase in ethylene biosynthesis in styles, ovary, and receptacle tissues. In all tissues, this increase was associated with increased activities of ACC synthase and EFE. The increase in EFE activities by all floral organs examined was correlated with increased abundance of EFE transcripts. In contrast, the level of ACC synthase mRNA, as detected by the cDNA probe pCARACC3, did not always reflect enzyme activity. The combined tissues of the pistil exhibited high rates of ACC synthase activity but contained low levels of ACC synthase mRNAs homologous to pCARACC3. In addition, pollinated styles exhibited a rapid increase in ethylene production and ACC synthase activity but did not accumulate detectable levels of ACC synthase mRNA until several hours after the initiation of ethylene production. These results suggest that transcripts for ACC synthase leading to the early postpollination increase in ACC synthase activity and ethylene production are substantially different from the mRNA for the ethylene-responsive gene represented by pCARACC3.
Full text
PDF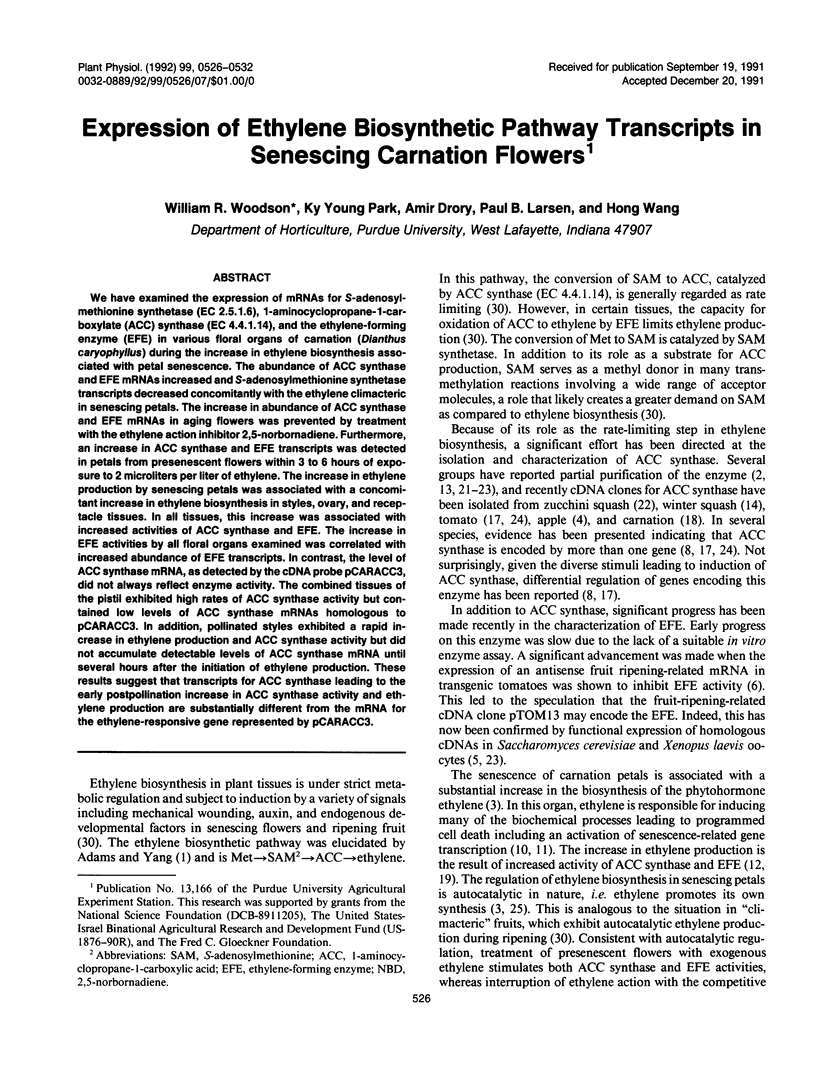




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. O., Yang S. F. Ethylene biosynthesis: Identification of 1-aminocyclopropane-1-carboxylic acid as an intermediate in the conversion of methionine to ethylene. Proc Natl Acad Sci U S A. 1979 Jan;76(1):170–174. doi: 10.1073/pnas.76.1.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleecker A. B., Kenyon W. H., Somerville S. C., Kende H. Use of monoclonal antibodies in the purification and characterization of 1-aminocyclopropane-1-carboxylate synthase, an enzyme in ethylene biosynthesis. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7755–7759. doi: 10.1073/pnas.83.20.7755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton A. J., Bouzayen M., Grierson D. Identification of a tomato gene for the ethylene-forming enzyme by expression in yeast. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7434–7437. doi: 10.1073/pnas.88.16.7434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekstra F. A., Weges R. Lack of Control by Early Pistillate Ethylene of the Accelerated Wilting of Petunia hybrida Flowers. Plant Physiol. 1986 Feb;80(2):403–408. doi: 10.1104/pp.80.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang P. L., Parks J. E., Rottmann W. H., Theologis A. Two genes encoding 1-aminocyclopropane-1-carboxylate synthase in zucchini (Cucurbita pepo) are clustered and similar but differentially regulated. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7021–7025. doi: 10.1073/pnas.88.16.7021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen P. B., Woodson W. R. Cloning and nucleotide sequence of a s-adenosylmethionine synthetase cDNA from carnation. Plant Physiol. 1991 Jul;96(3):997–999. doi: 10.1104/pp.96.3.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawton K. A., Huang B., Goldsbrough P. B., Woodson W. R. Molecular cloning and characterization of senescence-related genes from carnation flower petals. Plant Physiol. 1989 Jun;90(2):690–696. doi: 10.1104/pp.90.2.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawton K. A., Raghothama K. G., Goldsbrough P. B., Woodson W. R. Regulation of senescence-related gene expression in carnation flower petals by ethylene. Plant Physiol. 1990 Aug;93(4):1370–1375. doi: 10.1104/pp.93.4.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park K. Y., Drory A., Woodson W. R. Molecular cloning of an 1-aminocyclopropane-1-carboxylate synthase from senescing carnation flower petals. Plant Mol Biol. 1992 Jan;18(2):377–386. doi: 10.1007/BF00034964. [DOI] [PubMed] [Google Scholar]
- Peleman J., Boerjan W., Engler G., Seurinck J., Botterman J., Alliotte T., Van Montagu M., Inzé D. Strong cellular preference in the expression of a housekeeping gene of Arabidopsis thaliana encoding S-adenosylmethionine synthetase. Plant Cell. 1989 Jan;1(1):81–93. doi: 10.1105/tpc.1.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T., Oeller P. W., Theologis A. The 1-aminocyclopropane-1-carboxylate synthase of Cucurbita. Purification, properties, expression in Escherichia coli, and primary structure determination by DNA sequence analysis. J Biol Chem. 1991 Feb 25;266(6):3752–3759. [PubMed] [Google Scholar]
- Sato T., Theologis A. Cloning the mRNA encoding 1-aminocyclopropane-1-carboxylate synthase, the key enzyme for ethylene biosynthesis in plants. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6621–6625. doi: 10.1073/pnas.86.17.6621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanu P., Reinhardt D., Boller T. Analysis and cloning of the ethylene-forming enzyme from tomato by functional expression of its mRNA in Xenopus laevis oocytes. EMBO J. 1991 Aug;10(8):2007–2013. doi: 10.1002/j.1460-2075.1991.tb07730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Straeten D., Van Wiemeersch L., Goodman H. M., Van Montagu M. Cloning and sequence of two different cDNAs encoding 1-aminocyclopropane-1-carboxylate synthase in tomato. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4859–4863. doi: 10.1073/pnas.87.12.4859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Woodson W. R. A Flower Senescence-Related mRNA from Carnation Shares Sequence Similarity with Fruit Ripening-Related mRNAs Involved in Ethylene Biosynthesis. Plant Physiol. 1991 Jul;96(3):1000–1001. doi: 10.1104/pp.96.3.1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Woodson W. R. Reversible inhibition of ethylene action and interruption of petal senescence in carnation flowers by norbornadiene. Plant Physiol. 1989 Feb;89(2):434–438. doi: 10.1104/pp.89.2.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woltering E. J. Interorgan translocation of 1-aminocyclopropane-1-carboxylic Acid and ethylene coordinates senescence in emasculated cymbidium flowers. Plant Physiol. 1990 Mar;92(3):837–845. doi: 10.1104/pp.92.3.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodson W. R., Lawton K. A. Ethylene-induced gene expression in carnation petals : relationship to autocatalytic ethylene production and senescence. Plant Physiol. 1988 Jun;87(2):498–503. doi: 10.1104/pp.87.2.498. [DOI] [PMC free article] [PubMed] [Google Scholar]