Abstract
Type 1 CD4+-T-cell-mediated immunity is crucial for the resolution of chlamydial infection of the murine female genital tract. Previous studies demonstrating a correlation between CD4+-T-cell-mediated inhibition of chlamydial growth and gamma interferon (IFN-γ)-mediated induction of nitric oxide synthase suggested a potential role for the nitric oxide (NO) effector pathway in the clearance of Chlamydia from genital epithelial cells by the immune system. To clarify the role of this pathway, the growth levels of Chlamydia trachomatis organisms in normal (iNOS+/+) mice and in genetically engineered mice lacking the inducible nitric oxide synthase (iNOS) gene (iNOS−/− mice) were compared. There was no significant difference in the course of genital chlamydial infections in iNOS+/+ and iNOS−/− mice as determined by recovery of Chlamydia organisms shed from genital epithelial cells. Dissemination of Chlamydia to the spleen and lungs occurred to a greater extent in iNOS−/− than in iNOS+/+ mice, which correlated with a marginal increase in the susceptibility of macrophages from iNOS−/− mice to chlamydial infection in vitro. However, infections were rapidly cleared from all affected tissues, with no clinical signs of disease. The finding of minimal dissemination in iNOS−/− mice suggested that activation of the iNOS effector pathway was not the primary target of IFN-γ during CD4+-T-cell-mediated control of chlamydial growth in macrophages because previous reports demonstrated extensive and often fatal dissemination of Chlamydia in mice lacking IFN-γ. In summary, these results indicate that the iNOS effector pathway is not required for elimination of Chlamydia from epithelial cells lining the female genital tract of mice although it may contribute to the control of dissemination of C. trachomatis by infected macrophages.
The pathologic consequences of genital infection by Chlamydia trachomatis, including pelvic inflammatory disease, ectopic pregnancy, and infertility, have considerable psychological, public health, and economic implications. The high incidence of asymptomatic infections often precludes timely antibiotic therapy to control the sequelae of the infection, and so a vaccine has been recommended for controlling Chlamydia (37). Among other requirements for designing an effective vaccine is a detailed understanding of the pathogenesis and immunobiology of the disease, including host immune parameters that control Chlamydia, the immune effector mechanisms that function in chlamydial inhibition, and the chlamydial antigens that elicit protective immunity.
Experimental animal models have been useful tools for understanding the immunobiology of genital chlamydial disease (30, 36). Studies in the murine model have shown that T-cell-mediated immune responses are sufficient for conferring protective immunity against chlamydial infection in mice (18, 19, 30, 33, 34, 41). Because cell-mediated immunity is also important for chlamydial immunity in humans (3, 13), the murine model has furnished a reliable system for analyzing and understanding the mechanism of T-cell control of Chlamydia that could be extrapolated to humans. In this respect, T-cell-derived cytokines, especially gamma interferon (IFN-γ) and tumor necrosis factor alpha (TNF-α), have been implicated in chlamydial control in humans and in experimental animals (5, 9, 22, 31, 35, 39, 40, 43, 44). The biochemical basis of the antimicrobial action of IFN-γ includes the activation of phagocytes (e.g., macrophages) to rapidly ingest and destroy chlamydiae or infected cells (24) and the induction of indoleamine 2,3-dioxygenase (IDO) as demonstrated in human cells (6, 14). IDO is an enzyme that catalyzes the decyclization of l-tryptophan into N-formylkynurenine (6, 14, 27), thereby limiting the availability of this essential amino acid and inhibiting chlamydial growth in infected cells. IFN-γ can also induce intracellular iron deficiency that restricts microbial growth (4). Finally, as well established in murine cells, IFN-γ activates inducible nitric oxide synthase (iNOS), an enzyme that catalyzes the production of antimicrobial reactive nitrogen intermediates, including nitric oxide (NO) from l-arginine (43). Recent reports showing a correlation between T-cell effector inhibition of intracellular chlamydial growth and the induction of NO secretion (8, 16, 17, 20, 21, 43) suggested that chlamydial control in mice may involve the activation of the cytokine-iNOS system. In the present study, we sought to clearly define the role of NO in chlamydial control by employing genetically engineered iNOS knockout (iNOS−/−) mice to analyze the effect of NO on the in vivo and in vitro growth of Chlamydia. The results revealed that iNOS−/− mice could resolve genital chlamydial infections as readily as normal (iNOS+/+) mice. However, transient dissemination of chlamydiae to other tissues occurred to a greater extent in iNOS-deficient animals, suggesting that NO may play a partial role in preventing the systemic spread of Chlamydia.
MATERIALS AND METHODS
Animals.
Female iNOS−/− and iNOS+/+ mice on a (C57BL/6 × 129/J)F2 background, 5 to 8 weeks old, were obtained from Carl Nathan of Cornell University Medical Center, New York, N.Y., and The Jackson Laboratory, Bar Harbor, Maine. The animals were given food and water ad libitum and maintained in laminar-flow racks under pathogen-free conditions of 12 h of light and 12 h of darkness.
Chlamydia and infection of mice.
Stocks of C. trachomatis agent of mouse pneumonitis (MoPn) for infecting mice in vivo and peritoneal exudate macrophages (PEMs) in vitro were prepared by propagating elementary bodies in HeLa cells, as previously described (34). The titers of stocks were determined by infecting McCoy cells with various dilutions of elementary bodies, and the infectious titer was expressed as inclusion-forming units (IFU) per milliliter (34). Mice received medroxyprogesterone acetate (Depo-Provera; The Upjohn Co., Kalamazoo, Mich.) at 2.5 mg/mouse, and after 1 week, each mouse was infected intravaginally with 1,500 IFU of MoPn (equivalent to 100 50% infective doses) in a volume of 30 μl of phosphate-buffered saline (PBS). It has been established in previous studies that this infection regimen produces a 100% infection rate in mice (30). The course of the infection was monitored by periodic (every 3 days) cervico-vaginal swabbing of individual animals. Chlamydia was isolated from the swabs in tissue culture in accordance with standard methods, and inclusions were visualized and enumerated by immunofluorescence (32, 34). The mice were monitored for 6 weeks, a period that spans the course of MoPn infection in mice (30). Experiments were repeated two times to give 10 or 14 animals per experimental group.
Isolation of MoPn from the spleens and lungs was performed at different times after infection as follows. Mice were infected intravaginally as previously described (34). At the indicated time after infection, the entire spleen or lungs of each mouse were removed and teased with forceps and the tissue homogenates were collected in 1 ml of PBS. Chlamydia was isolated from the homogenate in tissue culture in accordance with a standard immunofluorescence method, as previously described (32, 34).
Cytokines, monoclonal antibodies, and other reagents.
Recombinant murine interleukin-1 (IL-1), TNF-α, and IFN-γ and fluoresceinated monoclonal antibodies against murine CD3 (clone KT3), major histocompatibility complex (MHC) class II (clone P7/7), and Mac-1 (clone M1/70.15.1) molecules were purchased from BioSource International, Camarillo, Calif., and Pharmingen, San Diego, Calif. Salmonella minnesota-derived lipopolysaccharide (LPS) and concanavalin A (ConA) were obtained from Atlanta Biologicals, Norcross, Ga. The l-arginine analog and inhibitor of nitric oxide synthase, l-NG-nitroarginine methyl ester (l-NAME), was supplied by CYCLO3PSS Biochemical Corp., Inc., Salt Lake City, Utah.
Preparation of PEMs.
Each mouse received 1 ml of 3% thioglycolate by the intraperitoneal route. The mice were sacrificed after 5 days by cervical dislocation, and the peritoneal cavities were washed with 10 ml of warmed PBS per mouse. The cells were washed three times and resuspended in complete Dulbecco’s modified Eagle’s medium (DMEM), which was composed of DMEM supplemented with 10 mM HEPES, 10% heat-inactivated fetal bovine serum, 1.0 mM sodium pyruvate, 0.1 mM nonessential amino acids, 2 mM glutamine, and 50 g of gentamicin per ml (all purchased from Atlanta Biologicals).
Induction of NO secretion and assessment of chlamydial multiplication in PEMs.
Monolayers of PEMs were established by seeding 2 × 105 cells into each well of 96-well tissue culture plates (Costar) and incubating the cells overnight in humidified incubators at 37°C and 5% CO2. Cultures to measure NO secretion were stimulated with optimum iNOS inducing agents, composed of 100 U of IFN-γ, 20 ng of TNF-α, 20 ng of IL-1, and 5 μg of LPS, each per ml (cytokines-LPS), as previously described (12, 25, 28, 29, 43).
Cultures to assess MoPn multiplication in PEMs were infected at a multiplicity of infection of 1 (MoPn/PEM ratio). Infected cells were centrifuged at 2,060 × g for 30 min to facilitate infection. Centrifugation did not affect the attachment of the PEMs as determined by microscopic observation. At the end of a 48-h incubation period, the productive growth of chlamydiae in PEMs in the presence or absence of cytokines-LPS or other reagents was determined by direct staining of wells with fluorescein isothiocyanate-labeled, genus-specific antichlamydial antibodies (Kallestad Diagnostics, Chaska, Minn.) to detect chlamydial inclusions by direct immunofluorescence (34). Cultures were established in triplicate, and the results were expressed as IFU per milliliter (mean ± standard deviation). Each set of experiments was repeated at least three times to obtain a quantifiable and consistent pattern of results. The percent inhibition was computed as follows: [mean IFU/ml of control cultures] − [mean IFU/ml of experimental cultures (with cytokines-LPS)] × 100 mean IFU/ml of control cultures
IFN-γ assay.
T-cell-enriched single spleen cells were prepared from naive mice by nylon wool purification, as previously described (19), and the cells were stimulated in vitro with 5 μg of ConA per ml for 72 h. The amount of IFN-γ secreted in cultures was measured by using a specific enzyme-linked immunosorbent assay kit (Cytoscreen immunoassay kit; BioSource International) in accordance with the supplier’s instructions. The amount of the cytokine in each sample was obtained by extrapolation from a standard calibration curve generated simultaneously. Data were calculated as the mean values (± standard deviations) of triplicate cultures for each experiment. The results were derived from at least three independent experiments.
Measurement of NO production.
The amount of NO induced in culture supernatants was quantitated by the Greiss reagent method (11). Briefly, equal volumes of twofold-diluted supernatants and the Greiss reagent (1% sulfanilamide, 0.1% naphthylethylene diamine dihydrochloride, 2.5% H3PO4) (50 μl each) were mixed in 96-well plates, and the absorbance associated with color change was measured within 10 min at a 550-nm wavelength in a Microplate Autoreader spectrophotometer (Bio-tex Instruments, Inc., Winooski, Vt.). Results represent the mean of quadruplicate wells for each set of samples obtained from different experiments.
Statistical analysis.
The levels of multiplication of Chlamydia in macrophages from iNOS−/− and iNOS+/+ mice and the differences between results from the two sets of animals were compared by performing a one- or two-tailed t test, and the relationship between different experimental groupings was assessed by analysis of variance. Minimal statistical significance was judged at a P of <0.05.
RESULTS
Comparison of levels of nitric oxide production by iNOS−/− and iNOS+/+ mice.
Genetically engineered iNOS knockout (iNOS−/−) and control (C57BL/6 × 129/J; NOS+/+) mice (26) were first analyzed for functional iNOS deficiency. PEMs from these mice were exposed in vitro to an optimum combination of iNOS inducers (cytokines-LPS as indicated in Materials and Methods) for 24, 48, and 72 h. Nitric oxide production was measured as nitrite in the culture supernatants by the standard Greiss reagent assay (11). PEMs from iNOS−/− mice produced no detectable NO, while PEMs from iNOS+/+ mice produced 32.3 ± 0.22, 34.5 ± 1.34, and 36.47 ± 1.1 μM NO after 24, 48, and 72 h of stimulation, respectively. The results corroborate previous reports demonstrating the absence of iNOS and NO in these mice (26) and indicate that these iNOS-deficient mice are suitable for testing the role of iNOS and NO in genital chlamydial disease in mice.
Comparison of critical immune response parameters in iNOS−/− and iNOS+/+ mice.
To ascertain that there were no overt immunologic deficiencies in the iNOS knockout mice besides the lack of NO-producing ability, the presence and integrity of certain critical immune response parameters were assessed and compared with those of control mice. The parameters investigated were the proportion of splenic CD3-bearing T cells, the amount of IFN-γ elaborated in response to ConA, and the proportion of Mac-1- and MHC class II-bearing splenocytes. The results presented in Table 1 show that there were no significant differences between iNOS−/− and iNOS+/+ mice in the proportion of cells expressing CD3, Mac-1, or MHC class II antigens or in the production of IFN-γ by cells. The results indicate that certain immunologic features, including the representation of mononuclear cells and the secretion of IFN-γ, are preserved in the absence of iNOS.
TABLE 1.
Splenic immune response parameters of iNOS-deficient and control micea
| Mouse type | CD3+ cells (% of total)b | IFN-γ production (pg/ml)c | Mac-1+ cells (% of total)d | MHC class II+ cells (% of total)e |
|---|---|---|---|---|
| iNOS−/− | 34.67 (1.2) | 888.33 (55.8) | 5.33 (0.3) | 53.75 (1.8) |
| iNOS+/+ | 38.0 (0.9) | 885.0 (48.3) | 5.33 (0.3) | 51.50 (2.1) |
Total splenic cells from either naive iNOS−/− or iNOS+/+ mice were stained with fluorescent-labeled monoclonal antibodies against the leukocyte antigen indicated. Nylon wool-purified T cells (97.6% CD3+) were stimulated with 5 μg of ConA per ml for 3 days, and the supernatants were assayed for IFN-γ as described in Materials and Methods. Results represent data from at least three independent experiments and are expressed as means with standard errors of the means shown in parentheses.
Results are significantly different at P = 0.059.
Results are significantly different at P = 0.965.
Results are significantly different at P = 1.0.
Results are significantly different at P = 0.664.
Chlamydial multiplication in PEMs from iNOS−/− and iNOS+/+ mice.
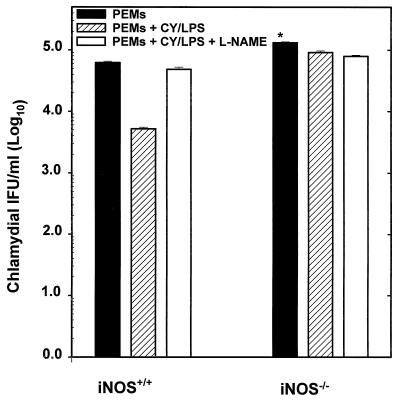
The analyses of chlamydial growth in macrophages have been useful for studying the mechanisms of intracellular survival, the biochemistry of chlamydial inhibition by certain cytokines, the pathogenesis of inflammation associated with chlamydial infection, and more recently the role of macrophages in the transportation, initiation, and development of Chlamydia pneumoniae-associated atherosclerotic plaques (23, 24). The capacity of C. trachomatis to multiply and form inclusions in PEMs from iNOS−/− mice was compared with that in PEMs from iNOS+/+ mice. The results presented in Fig. 1 show that twice as many inclusions were present at 48 h in PEMs from iNOS−/− mice than in PEMs from iNOS+/+ mice (P < 0.001). The chlamydial inclusions formed in PEMs from iNOS−/− mice were morphologically larger than those formed in PEMs from iNOS+/+ mice (data not shown). Treatment of PEMs from iNOS+/+ mice with cytokines-LPS caused a 12-fold reduction in the growth of MoPn in these macrophages. The presence of the NOS inhibitor l-NAME partially but significantly reversed the antichlamydial effect of cytokine-LPS treatment of PEMs from iNOS+/+ mice (P < 0.002). In contrast, there was minimal suppression of chlamydial growth in the PEMs from iNOS−/− mice following induction with cytokines-LPS and the addition of l-NAME had no effect. These results suggest that MoPn multiplies in PEMs from either iNOS-deficient or normal mice, although inclusions were larger and more numerous in macrophages derived from iNOS-deficient mice.
FIG. 1.
Chlamydial multiplication in PEMs from iNOS−/− and iNOS+/+ mice. PEMs were infected at a multiplicity of infection of 1 (MoPn/PEM ratio). At the end of a 48-h incubation period, the productive growth of chlamydiae in PEMs in the presence or absence of cytokines-LPS (CY/LPS) and l-NAME was determined by direct staining of wells with fluorescein-labeled, genus-specific antichlamydial antibodies to detect chlamydial inclusions by indirect immunofluorescence, as described in Materials and Methods. Results are expressed as the mean of values (IFU per milliliter) from at least three separate experiments. The asterisk indicates that the twofold increase in MoPn multiplication in PEMs from iNOS−/− mice relative to that in PEMs from iNOS+/+ mice was statistically significant (P < 0.001).
The course of MoPn genital infection in iNOS−/− and iNOS+/+ mice.
To investigate the effect of NO deficiency on chlamydial multiplication in vivo, we compared the durations and intensities of MoPn genital infections in iNOS−/− and iNOS+/+ mice. Groups of mice were genitally infected with MoPn and monitored for 6 weeks by isolation of chlamydiae from cervico-vaginal swabs in tissue culture. The results presented in Table 2 reveal that chlamydial burdens were similar in iNOS−/− and iNOS+/+ mice 1 week after infection. By 3 weeks postinfection, the rate of chlamydial clearance was slightly enhanced in iNOS-deficient mice as compared to that in controls in that the proportion of infected animals and the degree of chlamydial shedding were lower in iNOS−/− mice than in iNOS+/+ mice. Chlamydial clearance was complete in all mice by 5 weeks postinfection. These results indicate that possession of a functional pathway for generating NO is not required for efficient elimination of chlamydia from epithelial cells lining the female genital tract.
TABLE 2.
Clearance of Chlamydia from the female genital tract in iNOS knockout and immunocompetent control micea
| No. of wk postinfection | iNOS+/+ mice
|
iNOS−/− mice
|
||
|---|---|---|---|---|
| % Infected | No. of IFU (mean)b | % Infected | No. of IFU (mean)b | |
| 1 | 100 (12/12)c | 7.6 × 104 | 100 (12/12) | 1.6 × 105 |
| 2 | 100 (12/12) | 1.5 × 104 | 100 (12/12) | 7.0 × 103 |
| 3 | 91.7 (11/12) | 5.9 × 103 | 41.6 (5/12) | 1.3 × 101 |
| 4 | 8.3 (1/12) | 8.6 × 101 | 0 | 0 |
| 5 | 0 | 0 | 0 | 0 |
iNOS+/+ mice were (C57BL/6 × 129/J)F2; iNOS−/− mice were a third-generation random intercross of C57BL/6 mice homozygous for a 129/SvEv embryonic stem-derived mutant iNOS gene. Therefore, both iNOS+/+ and iNOS−/− mice are segregating for all other loci.
Mean number of recoverable chlamydial IFU from the indicated number of mice at selected time points following intravaginal infection with MoPn as described in Materials and Methods.
Values in parentheses indicate the number of mice infected relative to the total number of mice.
Dissemination of chlamydia in iNOS−/− and iNOS+/+ mice after genital infection.
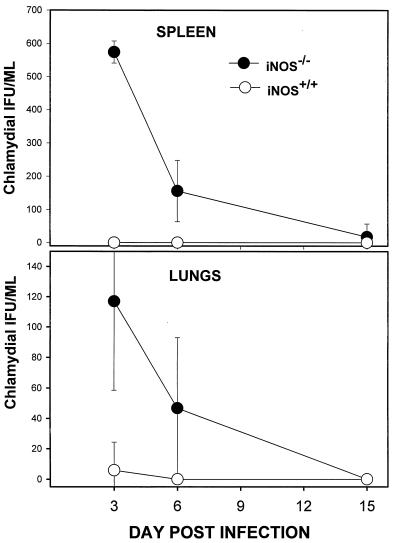
Previous studies revealed that mice genetically deficient in IFN-γ are also capable of clearing Chlamydia from the genital epithelium at a rate comparable to that in control animals (9, 31). However, dissemination of Chlamydia from the genital tract to systemic tissues such as the spleen and lung was noted with high frequency in IFN-γ knockout mice and attributed to secondary infection of macrophages (31). To determine the relative contribution of the IFN-γ-driven iNOS pathway to dissemination of Chlamydia from the genital mucosa, chlamydial burdens in systemic tissues of iNOS−/− and iNOS+/+ mice were compared. As shown in Fig. 2, small numbers of MoPn were detected in the spleens and lungs of iNOS-deficient mice but not in tissues from control mice for the first week following genital infection. The biologic significance of this transient and relatively low level of dissemination is unclear, however, since affected animals showed no overt signs of disease during this period.
FIG. 2.
Dissemination of Chlamydia in iNOS−/− and iNOS+/+ mice after genital infection. Tissue homogenates of spleens and lungs were prepared from genitally infected iNOS−/− and iNOS+/+ mice at the indicated time points postinfection. Chlamydial burdens in these tissues were assayed as described in Materials and Methods. Results represent the means ± standard errors from two independent experiments, each containing six animals per group.
DISCUSSION
An understanding of the various biochemical processes that mediate immune effector function against Chlamydia is a prerequisite for developing an appropriate vaccine or for designing other prophylactic measures to control the sequelae of chlamydial infection. T-cell-mediated immunity has been shown to be crucial for chlamydial control in humans as well as in experimental animal models of the disease (30, 36). Recent reports suggesting that T-cell control of Chlamydia in mice may involve the activation of the cytokine-iNOS system (8, 16, 17, 20, 21, 43) prompted the current investigations of chlamydial immunity in mice genetically deficient in iNOS activity. Specifically, these studies focused on defining the role of iNOS and the NO effector pathway in the elimination of C. trachomatis from epithelial cells lining the murine female genital tract. It was found that the courses of genital chlamydial infection in iNOS−/− and iNOS+/+ mice were essentially identical, which suggested that NO is not critical for chlamydial clearance from the genital mucosa of these mice. However, iNOS−/− mice were more susceptible to subclinical infections of the spleen and lungs than were control mice, which correlated with a modest increase in the in vitro susceptibility of macrophages from iNOS−/− mice to chlamydial infection. Considered together, the results suggest that NO is not critically required to control chlamydial infection in the genital epithelial cells but that NO does partially contribute to the resolution of chlamydial infection in macrophages. Ultimately, however, the ability of iNOS−/− mice to resolve infection in both epithelial cells and macrophages indicates that NO-independent immune mechanisms are more important for controlling C. trachomatis in the murine host.
Clearance of Chlamydia from the genital mucosa in the absence of a functional iNOS system was unexpected based on the results of previous studies using iNOS inhibitors in vitro (8, 16, 43) or in vivo (17). The capacity of NO synthase inhibitors to suppress chlamydial killing by cloned type 1 CD4+ T cells (17) predicted a greater contribution of the iNOS mechanism than was appreciated in the present study. A similar dichotomy is apparent in analyses of immunity to Toxoplasma gondii, an organism like Chlamydia that resides in parasitophorous vacuoles that are nonfusogenic with vesicles of the endosomal-lysosomal pathway (7, 15). Intracellular killing of tachyzoites was suppressed by treatment of normal macrophages with NOS inhibitors (1) or by use of macrophages from iNOS−/− donors (38). Yet the survival rate of iNOS−/− mice during acute Toxoplasma infections was similar to that of iNOS+/+ control mice (38). Attempts to reconcile these conflicting data by invoking immunological compensation or the effect of redundant antimicrobial mechanisms in mutant mice must be tempered by the enhanced susceptibility of iNOS−/− mice to lethal infection with Leishmania (42), which argues against the efficacy of compensatory effector pathways. Nevertheless, increases in local cytokine production have been documented previously (31a) and enhanced synthesis of IL-12p40 was suggested as a mechanism underlying the accelerated chlamydial clearance of iNOS−/− mice. However, since IL-12p40 functions as a regulatory rather than an effector cytokine, its effects are likely to be indirect, possibly involving the enhancement of other immune effectors. Future studies will address the possibility that other effector pathways (4, 10) could compensate for the iNOS deficiency.
Dissemination of Chlamydia from the site of inoculation to the spleen and lungs has been noted not only in iNOS−/− mice but also in IFN-γ−/− mice (9, 22, 31). Chlamydial burdens in involved tissues were substantially higher in IFN-γ−/− mice than in these iNOS−/− mice and were associated with significant clinical disease (9, 31). This would suggest that the iNOS pathway is not the sole mechanism for controlling chlamydial infection in macrophages. A review of the IFN-γ-induced genes that may contribute to bacterial resistance has been published elsewhere (2) and will not be reproduced here. Among the systems described, IFN-γ-mediated activation of the IDO pathway has documented relevance to the in vitro killing of Chlamydia (6, 14, 27). IDO mRNA is also up-regulated during murine chlamydial infection in vivo (10).
Recent studies indicated that the relative contributions of IFN-γ and IFN-γ-driven iNOS to the expression of chlamydial immunity may be even greater during infection with human C. trachomatis strains. Thus, mice deficient in IFN-γ receptor expression produced little or no NO and exhibited delayed genital tract clearance of primary C. trachomatis serovar D infection (22). In contrast, IFN-γ−/− mice displayed normal clearance of genital MoPn infections (9, 31). Differential susceptibilities to IFN-γ and, by inference, IFN-γ-driven pathways have also been demonstrated in vitro between C. trachomatis serovar D and MoPn in that MoPn was more resistant to the growth inhibitory effects of IFN-γ (unpublished observation). A testable hypothesis that can be advanced to reconcile the differential sensitivities of distinct chlamydial strains to IFN-γ is that strains may exhibit differential sensitivities to the toxic effects of nitrogen metabolites generated through the iNOS pathway or to depletion of tryptophan following IFN-γ-mediated induction of IDO. Thus, it appears that the definition of immune pathways relevant to chlamydial control may ultimately depend not only upon the cellular target of infection (i.e., macrophage or epithelial cell) but also upon the chlamydial strain under study and its sensitivity to IFN-γ-driven effector mechanisms, including the NO and IDO pathways. The common thread that unifies these potentially diverse killing strategies is the requirement for induction of IL-12-driven type 1 CD4+-T-cell immunity.
ACKNOWLEDGMENTS
This study was partly supported by PHS grants AI41231, RR03034, and RR115598 from the National Institutes of Health.
We thank Gordon B. Bailey and Carolyn Black for their constructive critiques and suggestions.
REFERENCES
- 1.Adams L B, Hibbs J B, Taintor R R, Krahenbuhl J L. Microbiostatic effect of murine-activated macrophages for Toxoplasma gondii: role of synthesis of inorganic nitrogen oxides from l-arginine. J Immunol. 1990;144:2725–2729. [PubMed] [Google Scholar]
- 2.Boehm U, Klamp T, Groot M, Howard J C. Cellular responses to interferon-gamma. Annu Rev Immunol. 1997;15:749–795. doi: 10.1146/annurev.immunol.15.1.749. [DOI] [PubMed] [Google Scholar]
- 3.Brunham R C, Martin D H, Kuo C-C, Wang S-P, Stevens C E, Hubbard T, Holmes K K. Cellular immune response during uncomplicated genital infection with Chlamydia trachomatis in humans. Infect Immun. 1981;34:98–104. doi: 10.1128/iai.34.1.98-104.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Byrd T F, Horwitz M A. Regulation of transferrin receptor expression and ferritin content in human mononuclear phagocytes. Coordinate upregulation by iron transferrin and downregulation by interferon gamma. J Clin Invest. 1993;91:969–976. doi: 10.1172/JCI116318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Byrne G I, Krueger D A. Lymphokine-mediated inhibition of Chlamydia replication in mouse fibroblasts is neutralized by anti-gamma interferon immunoglobulin. Infect Immun. 1983;42:1152–1158. doi: 10.1128/iai.42.3.1152-1158.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Byrne G I, Lehmann L K, Landry G J. Induction of tryptophan catabolism is the mechanism for gamma interferon-mediated inhibition of intracellular Chlamydia psittaci replication in T24 cells. Infect Immun. 1986;53:347–351. doi: 10.1128/iai.53.2.347-351.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang D-P, Fong D, Bray R S, editors. Biology of leishmania and leishmaniasis. Amsterdam, The Netherlands: Elsevier; 1985. [Google Scholar]
- 8.Chen B, Stout R, Campbell W F. Nitric oxide production: a mechanism of Chlamydia trachomatis inhibition in interferon-gamma-treated RAW264.7 cells. FEMS Immunol Med Microbiol. 1996;14:109–120. doi: 10.1111/j.1574-695X.1996.tb00277.x. [DOI] [PubMed] [Google Scholar]
- 9.Cotter T W, Ramsey K H, Miranpuri G S, Poulsen C E, Byrne G I. Dissemination of Chlamydia trachomatis chronic genital tract infection in gamma interferon gene knockout mice. Infect Immun. 1997;65:2145–2152. doi: 10.1128/iai.65.6.2145-2152.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Currier, A. R., and J. M. Carlin. Indoleamine dioxygenase and nitric oxide synthase mRNA expression during Chlamydia infection, abstr. D-15, p. 210. In Abstracts of the 97th General Meeting of the American Society for Microbiology 1997. American Society for Microbiology, Washington, D.C.
- 11.Ding A H, Nathan C F, Stuehr D J. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages: comparison of activating cytokines and evidence for independent production. J Immunol. 1988;141:2407–2412. [PubMed] [Google Scholar]
- 12.Green S J, Scheller L F, Marletta M A, Seguin M C, Klotz F W, Slayter M, Nelson B J, Nacy C A. Nitric oxide: cytokine-regulation of nitric oxide in host resistance to intracellular pathogens. Immunol Lett. 1994;43:87–94. doi: 10.1016/0165-2478(94)00158-8. [DOI] [PubMed] [Google Scholar]
- 13.Grifo J A, Jeremias J, Ledger W J, Witkin S S. Interferon-γ in the diagnosis and pathogenesis of pelvic inflammatory disease. Am J Obstet Gynecol. 1989;160:26–31. doi: 10.1016/0002-9378(89)90080-x. [DOI] [PubMed] [Google Scholar]
- 14.Gupta S L, Carlin J M, Pyati P, Dai W, Pfefferkorn E R, Murphy M J., Jr Antiparasitic and antiproliferative effects of indoleamine 2,3-dioxygenase enzyme expression in human fibroblasts. Infect Immun. 1994;62:2277–2284. doi: 10.1128/iai.62.6.2277-2284.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hackstadt, T. The diverse habitats of obligate intracellular parasites. Curr. Opin. Microbiol., in press. [DOI] [PubMed]
- 16.Igietseme J U. The molecular mechanism of T cell control of Chlamydia in mice: role of nitric oxide. Immunology. 1996;87:1–8. [PMC free article] [PubMed] [Google Scholar]
- 17.Igietseme J U. Molecular mechanism of T cell control of Chlamydia in mice: role of nitric oxide in vivo. Immunology. 1996;88:1–5. [PMC free article] [PubMed] [Google Scholar]
- 18.Igietseme J U, Magee D M, Williams D M, Rank R G. Role for CD8+ T cells in antichlamydial immunity defined by chlamydia-specific T lymphocyte clones. Infect Immun. 1994;62:5195–5197. doi: 10.1128/iai.62.11.5195-5197.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Igietseme J U, Ramsey K H, Magee D M, Williams D M, Kincy T J, Rank R G. Resolution of murine chlamydial genital infection by the adoptive transfer of a biovar-specific, Th1 lymphocyte clone. Reg Immunol. 1993;5:317–324. [PubMed] [Google Scholar]
- 20.Igietseme J U, Uriri I M, Hawkins R, Rank R G. Integrin-mediated epithelial-T cell interaction enhances nitric oxide production and increased intracellular inhibition of Chlamydia. J Leukocyte Biol. 1996;59:656–662. doi: 10.1002/jlb.59.5.656. [DOI] [PubMed] [Google Scholar]
- 21.Igietseme J U, Uriri M I, Chow M, Abe E, Rank R G. Inhibition of the intracellular multiplication of human strains of Chlamydia trachomatis by nitric oxide. Biochem Biophys Res Commun. 1997;232:595–601. doi: 10.1006/bbrc.1997.6335. [DOI] [PubMed] [Google Scholar]
- 22.Johansson M, Schon K, Ward M, Lycke N. Genital tract infection with Chlamydia trachomatis fails to induce protective immunity in gamma interferon receptor-deficient mice despite a strong local immunoglobulin A response. Infect Immun. 1997;65:1032–1044. doi: 10.1128/iai.65.3.1032-1044.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuo C C, Gown A M, Benditt E P, Grayston J T. Detection of Chlamydia pneumoniae in aortic lesions of atherosclerosis by immunocytochemical stain. Arterioscler Thromb. 1993;13:1501–1504. doi: 10.1161/01.atv.13.10.1501. [DOI] [PubMed] [Google Scholar]
- 24.La Verda D, Byrne G I. Interactions between macrophages and chlamydiae. Immunol Ser. 1994;60:381–399. [PubMed] [Google Scholar]
- 25.MacMicking J, Xie Q-W, Nathan C. Nitric oxide and macrophage function. Annu Rev Immunol. 1997;15:323–350. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- 26.MacMicking J D, Nathan C, Hom G, Chartrain N, Fletcher D S, Trumbauer M, Hutchinson N, Chen H, Mudgett J S. Altered responses to bacterial infection and endotoxic shock in mice lacking inducible nitric oxide synthase. Cell. 1995;81:641–650. doi: 10.1016/0092-8674(95)90085-3. [DOI] [PubMed] [Google Scholar]
- 27.Murray H W, Szuro-Sudol A, Wellner D, Oca M J, Granger A M, Libby D M, Rothermel C D, Rubin B Y. Role of tryptophan degradation in respiratory burst-independent antimicrobial activity of gamma interferon-stimulated human macrophages. Infect Immun. 1989;57:845–849. doi: 10.1128/iai.57.3.845-849.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nathan C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992;6:3051–3064. [PubMed] [Google Scholar]
- 29.Nussler A K, Billiar T R. Inflammation, immunoregulation, and inducible nitric oxide synthase. J Leukocyte Biol. 1993;54:171–178. [PubMed] [Google Scholar]
- 30.Patton D L, Rank R G. Animal models for the study of pelvic inflammatory disease. In: Quinn T C, editor. Sexually transmitted diseases. New York, N.Y: Raven Press; 1992. pp. 85–111. [Google Scholar]
- 31.Perry L L, Feilzer K, Caldwell H D. Immunity to Chlamydia trachomatis is mediated by T helper 1 cells through IFN-gamma-dependent and -independent pathways. J Immunol. 1997;158:3344–3352. [PubMed] [Google Scholar]
- 31a.Perry L L, Feilzer K, Caldwell H D. Neither interleukin-6 nor inducible nitric oxide synthase is required for clearance of Chlamydia trachomatis from the murine genital tract epithelium. Infect Immun. 1998;66:1265–1269. doi: 10.1128/iai.66.3.1265-1269.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramsey K H, Newhall W J, Rank R G. Humoral immune response to chlamydial genital infection of mice with the agent of mouse pneumonitis. Infect Immun. 1989;57:2441–2446. doi: 10.1128/iai.57.8.2441-2446.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ramsey K H, Rank R G. Resolution of chlamydial genital infection with antigen-specific T-lymphocyte lines. Infect Immun. 1991;59:925–931. doi: 10.1128/iai.59.3.925-931.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ramsey K H, Soderberg L S F, Rank R G. Resolution of chlamydial genital infection in B-cell-deficient mice and immunity to reinfection. Infect Immun. 1988;56:1320–1325. doi: 10.1128/iai.56.5.1320-1325.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rank R G, Ramsey K H, Pack E A, Williams D M. Effect of gamma interferon on resolution of murine chlamydial genital infection. Infect Immun. 1992;60:4427–4429. doi: 10.1128/iai.60.10.4427-4429.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rice P A, Schachter J. Pathogenesis of pelvic inflammatory disease. What are the questions? JAMA. 1991;266:2587–2593. [PubMed] [Google Scholar]
- 37.Schachter J. Overview of Chlamydia trachomatis infection and the requirements for a vaccine. Rev Infect Dis. 1985;7:713–716. doi: 10.1093/clinids/7.6.713. [DOI] [PubMed] [Google Scholar]
- 38.Scharton-Kersten T M, Yap G, Magram J, Sher A. Inducible nitric oxide is essential for host control of persistent but not acute infection with the intracellular pathogen Toxoplasma gondii. J Exp Med. 1997;185:1261–1273. doi: 10.1084/jem.185.7.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shemer Y, Sarov I. Inhibition of growth of Chlamydia trachomatis by human gamma interferon. Infect Immun. 1985;48:592–596. doi: 10.1128/iai.48.2.592-596.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Starnbach M N, Bevan M J, Lampe M F. Protective cytotoxic T lymphocytes are induced during murine infection with Chlamydia trachomatis. J Immunol. 1994;153:5183–5189. [PubMed] [Google Scholar]
- 41.Tuffrey M, Falder P, Taylor-Robinson D. Reinfection of the mouse genital tract with Chlamydia trachomatis: the relationship of antibody to immunity. Br J Exp Pathol. 1984;65:51–58. [PMC free article] [PubMed] [Google Scholar]
- 42.Wei X-Q, Charles I G, Smith A, Ure J, Feng G-J, Huang F-P, Xu D, Muller W, Moncada S, Liew F Y. Altered immune responses in mice lacking inducible nitric oxide synthase. Nature. 1995;375:408–411. doi: 10.1038/375408a0. [DOI] [PubMed] [Google Scholar]
- 43.Woods M L, Mayer J, Evans T G, Hibbs J B., Jr Antiparasitic effects of nitric oxide in an in vitro murine model of Chlamydia trachomatis infection and an in vivo model of Leishmania major infection. Immunol Ser. 1994;60:179–195. [PubMed] [Google Scholar]
- 44.Zhong G, Peterson E M, Czarniecki C W, Schreiber R D, de la Maza L M. Role of endogenous gamma interferon in host defense against Chlamydia trachomatis infections. Infect Immun. 1989;57:152–157. doi: 10.1128/iai.57.1.152-157.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]