Abstract
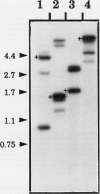
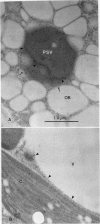
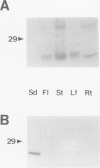
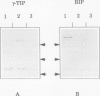
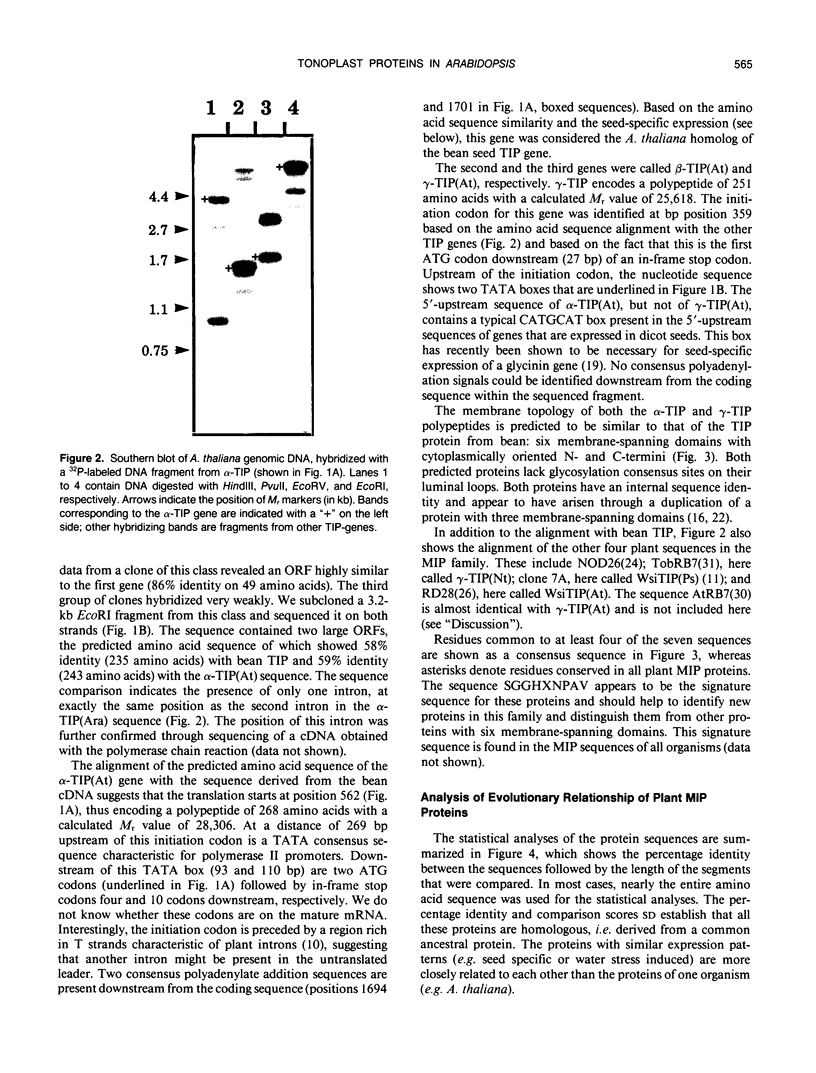
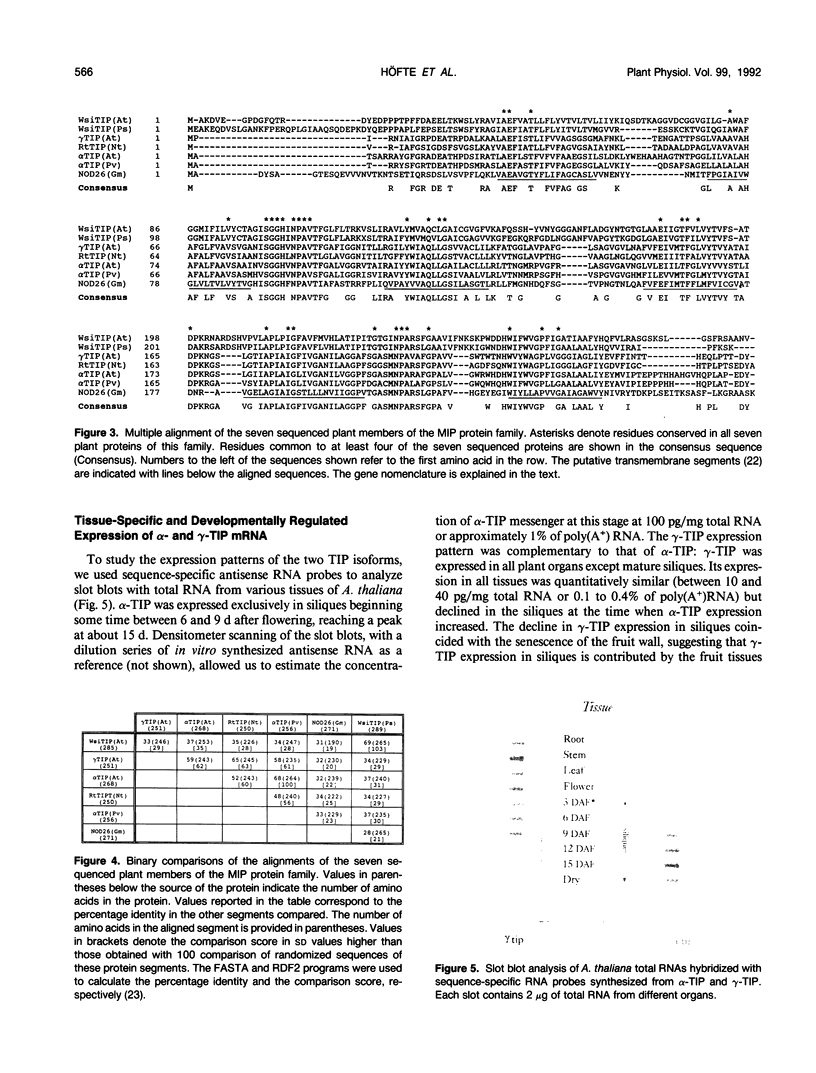
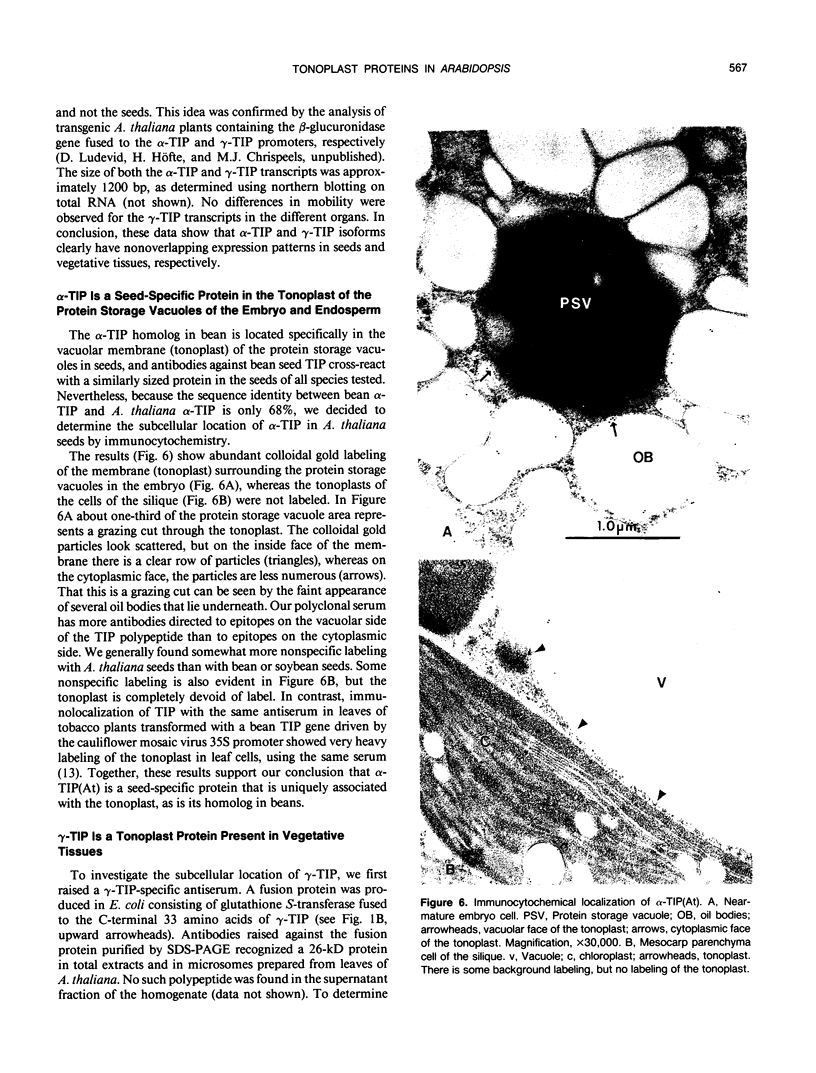
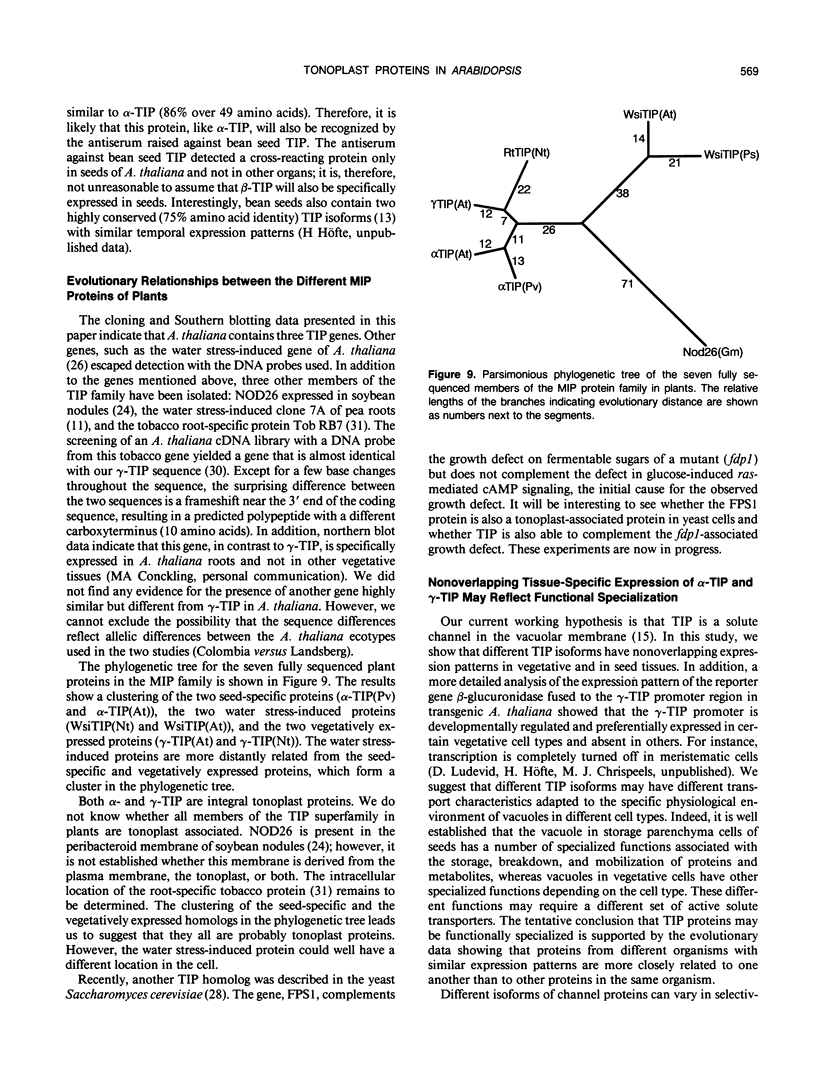
Reports from a number of laboratories describe the presence of a family of proteins (the major intrinsic protein family) in a variety of organisms. These proteins are postulated to form channels that function in metabolite transport. In plants, this family is represented by the product of NOD26, a nodulation gene in soybean that encodes a protein of the peribacteroid membrane, and tonoplast intrinsic protein (TIP), an abundant protein in the tonoplast of protein storage vacuoles of bean seeds (KD Johnson, H Höfte, MJ Chrispeels [1990] Plant Cell 2: 525-532). Other homologs that are induced by water stress in pea and in Arabidopsis thaliana and that are expressed in the roots of tobacco have been reported, but the location of the proteins they encode is not known. We now report the presence and derived amino acid sequences of two different TIP proteins in A. thaliana. α-TIP is a seed-specific protein that has 68% amino acid sequence identity with bean seed TIP; γ-TIP is expressed in the entire vegetative body of A. thaliana and has 58% amino acid identity with bean seed TIP. Both proteins are associated with the tonoplast. Comparisons of the derived amino acid sequences of the seven known plant proteins in the major intrinsic protein family show that genes with similar expression patterns (e.g. water stress-induced or seed specific) are more closely related to each other than the three A. thaliana homologs are related. We propose that the nonoverlapping gene expression patterns reported here, and the evolutionary relationships indicated by the phylogenetic tree, suggest a functional specialization of these proteins.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aerts T., Xia J. Z., Slegers H., de Block J., Clauwaert J. Hydrodynamic characterization of the major intrinsic protein from the bovine lens fiber membranes. Extraction in n-octyl-beta-D-glucopyranoside and evidence for a tetrameric structure. J Biol Chem. 1990 May 25;265(15):8675–8680. [PubMed] [Google Scholar]
- Boller T., Kende H. Hydrolytic enzymes in the central vacuole of plant cells. Plant Physiol. 1979 Jun;63(6):1123–1132. doi: 10.1104/pp.63.6.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doolittle R. F., Feng D. F. Nearest neighbor procedure for relating progressively aligned amino acid sequences. Methods Enzymol. 1990;183:659–669. doi: 10.1016/0076-6879(90)83043-9. [DOI] [PubMed] [Google Scholar]
- Ehring G. R., Zampighi G., Horwitz J., Bok D., Hall J. E. Properties of channels reconstituted from the major intrinsic protein of lens fiber membranes. J Gen Physiol. 1990 Sep;96(3):631–664. doi: 10.1085/jgp.96.3.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng D. F., Doolittle R. F. Progressive alignment and phylogenetic tree construction of protein sequences. Methods Enzymol. 1990;183:375–387. doi: 10.1016/0076-6879(90)83025-5. [DOI] [PubMed] [Google Scholar]
- Fitzgerald P. G., Bok D., Horwitz J. Immunocytochemical localization of the main intrinsic polypeptide (MIP) in ultrathin frozen sections of rat lens. J Cell Biol. 1983 Nov;97(5 Pt 1):1491–1499. doi: 10.1083/jcb.97.5.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodall G. J., Filipowicz W. The AU-rich sequences present in the introns of plant nuclear pre-mRNAs are required for splicing. Cell. 1989 Aug 11;58(3):473–483. doi: 10.1016/0092-8674(89)90428-5. [DOI] [PubMed] [Google Scholar]
- Guerrero F. D., Jones J. T., Mullet J. E. Turgor-responsive gene transcription and RNA levels increase rapidly when pea shoots are wilted. Sequence and expression of three inducible genes. Plant Mol Biol. 1990 Jul;15(1):11–26. doi: 10.1007/BF00017720. [DOI] [PubMed] [Google Scholar]
- Johnson K. D., Herman E. M., Chrispeels M. J. An abundant, highly conserved tonoplast protein in seeds. Plant Physiol. 1989 Nov;91(3):1006–1013. doi: 10.1104/pp.91.3.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. D., Höfte H., Chrispeels M. J. An intrinsic tonoplast protein of protein storage vacuoles in seeds is structurally related to a bacterial solute transporter (GIpF). Plant Cell. 1990 Jun;2(6):525–532. doi: 10.1105/tpc.2.6.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lelievre J. M., Oliveira L. O., Nielsen N. C. 5'CATGCAT-3' Elements Modulate the Expression of Glycinin Genes. Plant Physiol. 1992 Jan;98(1):387–391. doi: 10.1104/pp.98.1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeshima M. Characterization of the major integral protein of vacuolar membrane. Plant Physiol. 1992 Apr;98(4):1248–1254. doi: 10.1104/pp.98.4.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pao G. M., Wu L. F., Johnson K. D., Höfte H., Chrispeels M. J., Sweet G., Sandal N. N., Saier M. H., Jr Evolution of the MIP family of integral membrane transport proteins. Mol Microbiol. 1991 Jan;5(1):33–37. doi: 10.1111/j.1365-2958.1991.tb01823.x. [DOI] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandal N. N., Marcker K. A. Soybean nodulin 26 is homologous to the major intrinsic protein of the bovine lens fiber membrane. Nucleic Acids Res. 1988 Oct 11;16(19):9347–9347. doi: 10.1093/nar/16.19.9347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Van Aelst L., Hohmann S., Zimmermann F. K., Jans A. W., Thevelein J. M. A yeast homologue of the bovine lens fibre MIP gene family complements the growth defect of a Saccharomyces cerevisiae mutant on fermentable sugars but not its defect in glucose-induced RAS-mediated cAMP signalling. EMBO J. 1991 Aug;10(8):2095–2104. doi: 10.1002/j.1460-2075.1991.tb07742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson J. Q., Crawford N. M. Identification of the Arabidopsis CHL3 gene as the nitrate reductase structural gene NIA2. Plant Cell. 1991 May;3(5):461–471. doi: 10.1105/tpc.3.5.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y. T., Cheng C. L., Conkling M. A. Root-specific genes from tobacco and Arabidopsis homologous to an evolutionarily conserved gene family of membrane channel proteins. Nucleic Acids Res. 1990 Dec 25;18(24):7449–7449. doi: 10.1093/nar/18.24.7449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y. T., Taylor C. G., Acedo G. N., Cheng C. L., Conkling M. A. Characterization of cis-acting sequences regulating root-specific gene expression in tobacco. Plant Cell. 1991 Apr;3(4):371–382. doi: 10.1105/tpc.3.4.371. [DOI] [PMC free article] [PubMed] [Google Scholar]