Abstract
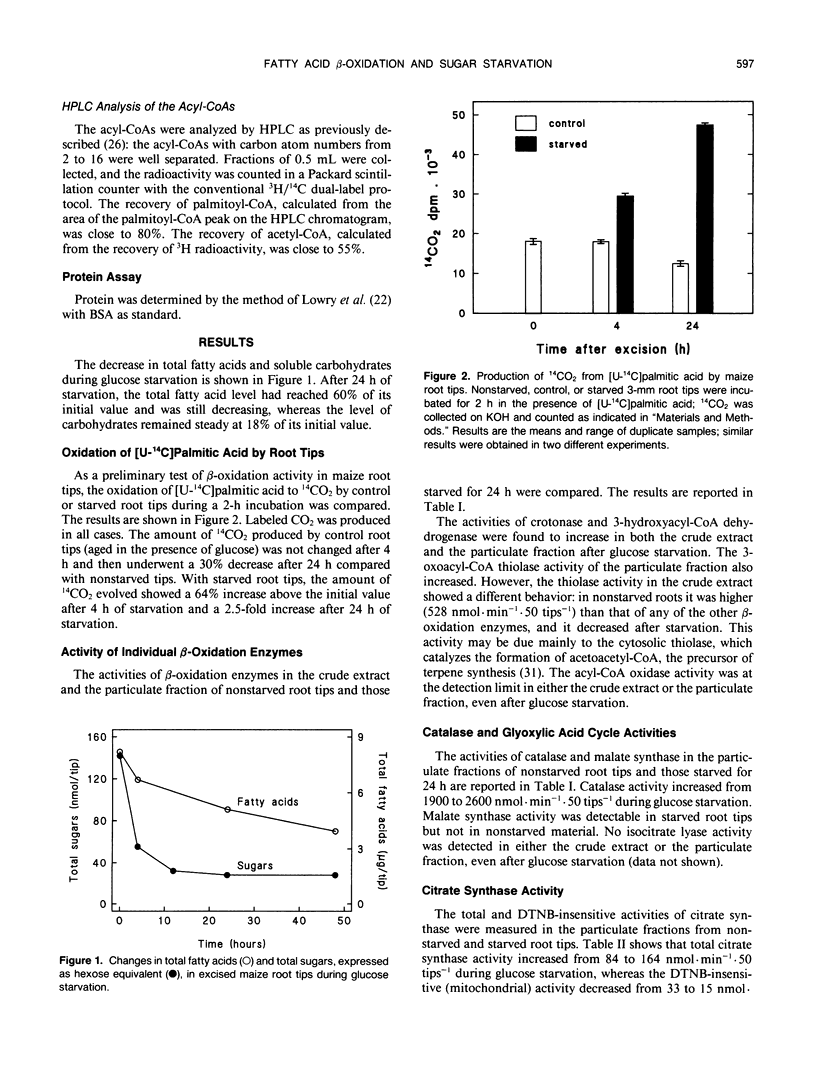
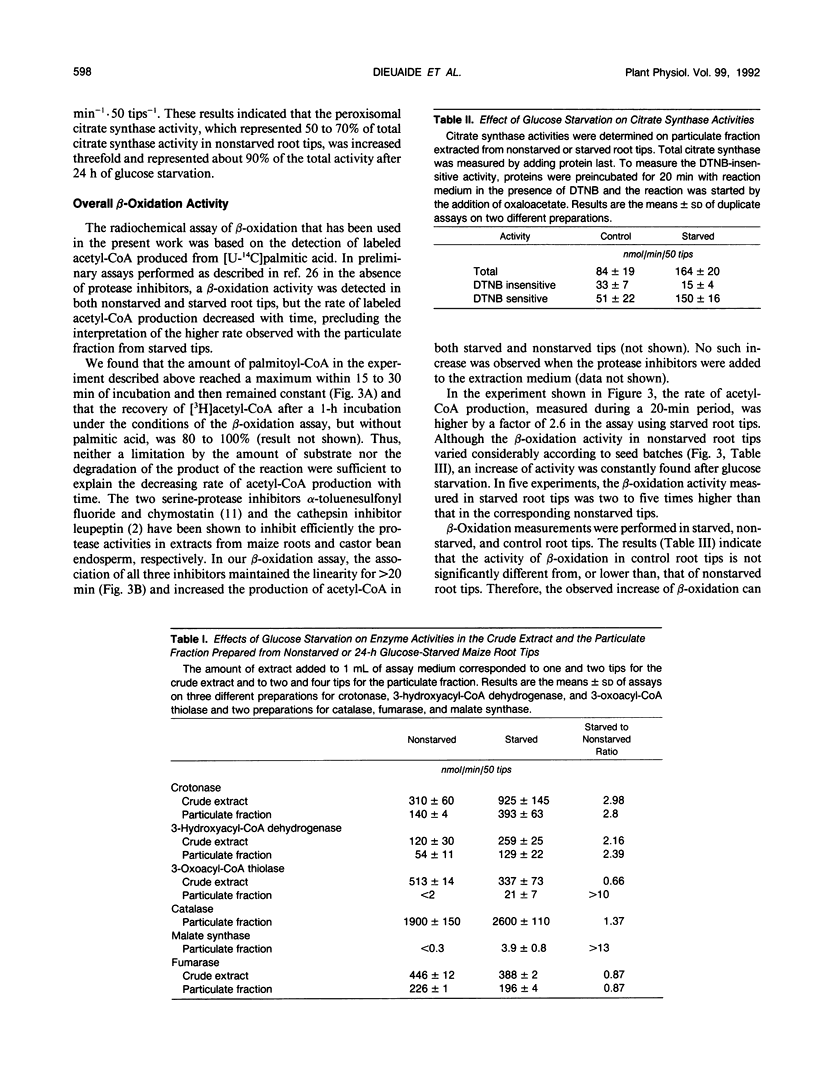
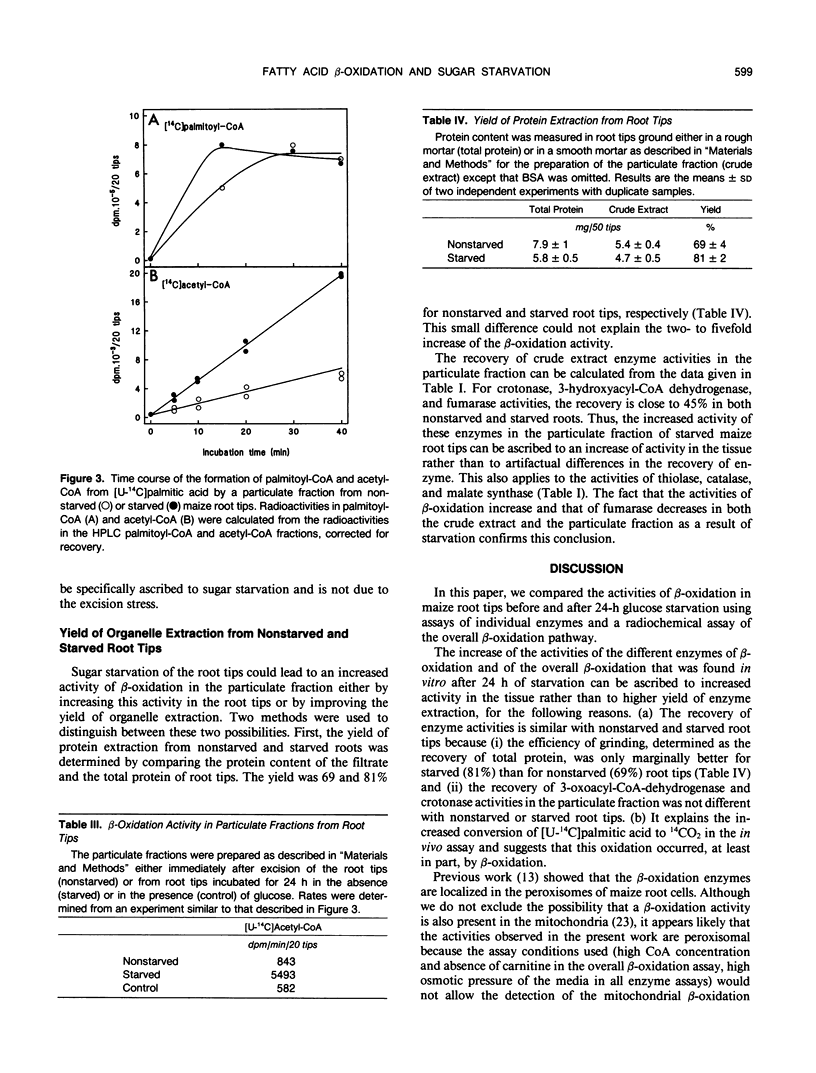
The effects of glucose starvation on the oxidation of fatty acids were studied in excised maize (Zea mays L.) root tips. After 24 hours of glucose starvation, the rate of oxidation of palmitic acid to CO2 by the root tips was increased 2.5-fold. Different enzyme activities were tested in a crude particulate fraction from nonstarved root tips and those starved for 24 hours. The activities of the β-oxidation enzymes crotonase, hydroxyacyl-coenzyme A (CoA) dehydrogenase, and thiolase and those of catalase, malate synthase, and peroxisomal citrate synthase were higher after starvation. However, no isocitrate lyase activity was detected, thus suggesting that the glyoxylate cycle does not operate. The overall β-oxidation activity was assayed as the formation of [14C]acetyl-CoA from [14C]palmitic acid after high-performance liquid chromatography analysis of the CoA derivatives. An activity was detected in sugar-fed root tips, and it was increased by two-to fivefold in starved roots. Because the recovery of enzyme activities is only marginally better in starved roots compared with nonstarved roots, these results indicate that the β-oxidation activity in the tissues is increased during sugar starvation. This increase is probably an essential part of the response to a situation in which lipids and proteins replace carbohydrates as the major respiratory substrates. These results are discussed in relation to the metabolic changes observed in senescing plant tissues.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alpi A., Beevers H. Proteinases and enzyme stability in crude extracts of castor bean endosperm. Plant Physiol. 1981 Mar;67(3):499–502. doi: 10.1104/pp.67.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker F. C., Schooley D. A. Analysis and purification of acyl coenzyme A thioesters by reversed-phase ion-pair liquid chromatography. Anal Biochem. 1979 Apr 15;94(2):417–424. doi: 10.1016/0003-2697(79)90384-1. [DOI] [PubMed] [Google Scholar]
- Baysdorfer C., Vanderwoude W. J. Carbohydrate Responsive Proteins in the Roots of Pennisetum americanum. Plant Physiol. 1988 Jul;87(3):566–570. doi: 10.1104/pp.87.3.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouquisse R., James F., Raymond P., Pradet A. Study of glucose starvation in excised maize root tips. Plant Physiol. 1991 Jun;96(2):619–626. doi: 10.1104/pp.96.2.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper T. G., Beevers H. Mitochondria and glyoxysomes from castor bean endosperm. Enzyme constitutents and catalytic capacity. J Biol Chem. 1969 Jul 10;244(13):3507–3513. [PubMed] [Google Scholar]
- Del Río L. A., Ortega M. G., López A. L., Gorgé J. L. A more sensitive modification of the catalase assay with the Clark oxygen electrode. Application to the kinetic study of the pea leaf enzyme. Anal Biochem. 1977 Jun;80(2):409–415. doi: 10.1016/0003-2697(77)90662-5. [DOI] [PubMed] [Google Scholar]
- Droillard M. J., Paulin A. Isozymes of Superoxide Dismutase in Mitochondria and Peroxisomes Isolated from Petals of Carnation (Dianthus caryophyllus) during Senescence. Plant Physiol. 1990 Nov;94(3):1187–1192. doi: 10.1104/pp.94.3.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fett W. F., Dunn M. F. Exopolysaccharides Produced by Phytopathogenic Pseudomonas syringae Pathovars in Infected Leaves of Susceptible Hosts. Plant Physiol. 1989 Jan;89(1):5–9. doi: 10.1104/pp.89.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher S. R., Carroll E. J., Leonard R. T. A sensitive diffusion plate assay for screening inhibitors of protease activity in plant cell fractions. Plant Physiol. 1986 Jul;81(3):869–874. doi: 10.1104/pp.81.3.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genix P., Bligny R., Martin J. B., Douce R. Transient accumulation of asparagine in sycamore cells after a long period of sucrose starvation. Plant Physiol. 1990 Oct;94(2):717–722. doi: 10.1104/pp.94.2.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr P. S., Rufty T. W., Huber S. C. Changes in Nonstructural Carbohydrates in Different Parts of Soybean (Glycine max [L.] Merr.) Plants during a Light/Dark Cycle and in Extended Darkness. Plant Physiol. 1985 Jul;78(3):576–581. doi: 10.1104/pp.78.3.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Miernyk J. A., Thomas D. R., Wood C. Partial purification and characterization of the mitochondrial and peroxisomal isozymes of enoyl-coenzyme a hydratase from germinating pea seedlings. Plant Physiol. 1991 Feb;95(2):564–569. doi: 10.1104/pp.95.2.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miernyk J. A., Trelease R. N. Role of malate synthase in citric Acid synthesis by maturing cotton embryos: a proposal. Plant Physiol. 1981 May;67(5):875–881. doi: 10.1104/pp.67.5.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saglio P. H., Pradet A. Soluble Sugars, Respiration, and Energy Charge during Aging of Excised Maize Root Tips. Plant Physiol. 1980 Sep;66(3):516–519. doi: 10.1104/pp.66.3.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saglio P. H., Raymond P., Pradet A. Metabolic Activity and Energy Charge of Excised Maize Root Tips under Anoxia: CONTROL BY SOLUBLE SUGARS. Plant Physiol. 1980 Dec;66(6):1053–1057. doi: 10.1104/pp.66.6.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salon C., Raymond P., Pradet A. Quantification of carbon fluxes through the tricarboxylic acid cycle in early germinating lettuce embryos. J Biol Chem. 1988 Sep 5;263(25):12278–12287. [PubMed] [Google Scholar]


