Abstract
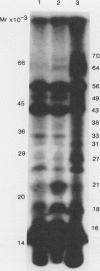
The ability of the cyanobacterium Synechocystis PCC6803 to transport inorganic carbon in the form of bicarbonate rapidly decreased following a shift from bicarbonate-limited growth to either excess bicarbonate supply or to photoheterotrophic growth on glucose. Nonmetabolizable analogs of glucose did not exert this effect. The rate at which the bicarbonate uptake rate declined was too rapid to be accounted for by dilution of the activity by culture growth and suggested that posttranslational modification may be involved. Several proteins that were unphosphorylated during bicarbonate-limited growth became phosphorylated during the shifts to high CO2 conditions and to photoheterotrophic growth. A similar alteration in the profile of phosphopolypeptides was observed following a shift into the dark. The changes in protein phosphorylation were not blocked by chloramphenicol or rifampicin.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Badger M. R., Andrews T. J. Photosynthesis and Inorganic Carbon Usage by the Marine Cyanobacterium, Synechococcus sp. Plant Physiol. 1982 Aug;70(2):517–523. doi: 10.1104/pp.70.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzone A. J. Protein phosphorylation in prokaryotes. Annu Rev Microbiol. 1988;42:97–125. doi: 10.1146/annurev.mi.42.100188.000525. [DOI] [PubMed] [Google Scholar]
- Der-Vartanian M., Joset-Espardellier F., Astier C. Contributions of Respiratory and Photosynthetic Pathways during Growth of a Facultative Photoautotrophic Cyanobacterium, Aphanocapsa 6714. Plant Physiol. 1981 Oct;68(4):974–978. doi: 10.1104/pp.68.4.974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores E., Schmetterer G. Interaction of fructose with the glucose permease of the cyanobacterium Synechocystis sp. strain PCC 6803. J Bacteriol. 1986 May;166(2):693–696. doi: 10.1128/jb.166.2.693-696.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foyer C. H. Stromal protein phosphorylation in spinach (Spinacia oleracea) chloroplasts. Biochem J. 1985 Oct 1;231(1):97–103. doi: 10.1042/bj2310097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manaï M., Cozzone A. J. Endogenous protein phosphorylation in Escherichia coli extracts. Biochem Biophys Res Commun. 1982 Aug;107(3):981–988. doi: 10.1016/0006-291x(82)90619-2. [DOI] [PubMed] [Google Scholar]
- Marcus Y., Zenvirth D., Harel E., Kaplan A. Induction of HCO(3) Transporting Capability and High Photosynthetic Affinity to Inorganic Carbon by Low Concentration of CO(2) in Anabaena variabilis. Plant Physiol. 1982 May;69(5):1008–1012. doi: 10.1104/pp.69.5.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. G., Canvin D. T. Na-Stimulation of Photosynthesis in the Cyanobacterium Synechococcus UTEX 625 Grown on High Levels of Inorganic Carbon. Plant Physiol. 1987 May;84(1):118–124. doi: 10.1104/pp.84.1.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. G., Espie G. S., Canvin D. T. Active Transport of CO(2) by the Cyanobacterium Synechococcus UTEX 625 : Measurement by Mass Spectrometry. Plant Physiol. 1988 Mar;86(3):677–683. doi: 10.1104/pp.86.3.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroney J. V., Kitayama M., Togasaki R. K., Tolbert N. E. Evidence for Inorganic Carbon Transport by Intact Chloroplasts of Chlamydomonas reinhardtii. Plant Physiol. 1987 Mar;83(3):460–463. doi: 10.1104/pp.83.3.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omata T., Ogawa T. Biosynthesis of a 42-kD Polypeptide in the Cytoplasmic Membrane of the Cyanobacterium Anacystis nidulans Strain R2 during Adaptation to Low CO(2) Concentration. Plant Physiol. 1986 Feb;80(2):525–530. doi: 10.1104/pp.80.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelroy R. A., Bassham J. A. Photosynthetic and dark carbon metabolism in unicellular blue-green algae. Arch Mikrobiol. 1972;86(1):25–38. doi: 10.1007/BF00412397. [DOI] [PubMed] [Google Scholar]
- Price G. D., Badger M. R. Expression of Human Carbonic Anhydrase in the Cyanobacterium Synechococcus PCC7942 Creates a High CO(2)-Requiring Phenotype : Evidence for a Central Role for Carboxysomes in the CO(2) Concentrating Mechanism. Plant Physiol. 1989 Oct;91(2):505–513. doi: 10.1104/pp.91.2.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price G. D., Badger M. R. Isolation and Characterization of High CO(2)-Requiring-Mutants of the Cyanobacterium Synechococcus PCC7942 : Two Phenotypes that Accumulate Inorganic Carbon but Are Apparently Unable to Generate CO(2) within the Carboxysome. Plant Physiol. 1989 Oct;91(2):514–525. doi: 10.1104/pp.91.2.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C. C., Durand M. C., Jeanjean R., Joset F. Molecular and genetical analysis of the fructose-glucose transport system in the cyanobacterium Synechocystis PCC6803. Mol Microbiol. 1989 Sep;3(9):1221–1229. doi: 10.1111/j.1365-2958.1989.tb00272.x. [DOI] [PubMed] [Google Scholar]