Abstract
In the present study, neonatal ZU.ICR mice and their mothers were infected with trophozoites of Giardia lamblia clone GS/M-83-H7 expressing the variant surface protein (VSP) H7. The infection experiments included a detailed analysis of the specificities of anti-Giardia immunoglobulin A (IgA) antibodies in mother’s milk and a determination of the effects of the milk antibodies on both the growth of the parasite during in vitro cultivation and colonization of the parasite within the intestine of suckling offspring. These investigations revealed that transiently emerging milk IgA antibodies against a variant-specific 314-amino-acid N-terminal region of VSP H7 exhibit a strong parasiticidal effect on VSP H7-type trophozoites both in vitro and in vivo. These findings indicated that parasiticidal effects of local IgA antibodies against the N-terminal part of VSP H7 select for new variant types within the intestinal parasite population of suckling mice. The selective influence of such antibodies promotes in vivo antigenic variation of G. lamblia clone GS/M-83-H7 and modulates the early course of parasite infection in these animals.
The protozoan parasite Giardia lamblia is an intestinal parasite of humans and various animals. Manifestations of disease vary from asymptomatic carriage to severe diarrhea and malabsorption. The development of the disease is thought to be substantially influenced by the immune system of an infected host, but the relevant immunological mechanisms combating the parasite infection are still not completely understood (3, 4). In the past decade, the immunobiology of G. lamblia was especially investigated in terms of the parasite’s capability to undergo antigenic variation. In G. lamblia, antigenic variation is mediated by a unique family of cysteine-rich proteins, the variant surface proteins (VSPs) and was demonstrated to be associated with a diversification of the intestinal parasite population into a complex mixture of different variant antigen types (10). In vitro studies revealed that antigenic variation can occur spontaneously (11) and that immunological (11) or physiological (12) factors can select for, or against, different VSP types. As demonstrated in experimental infections of immunocompetent and B-cell (and antibody)-deficient adult mice, in vivo antigenic variation of G. lamblia is promoted by the intestinal anti-VSP immunoglobulin A (IgA) antibody response (14).
In one of our recent studies, neonatal mice were infected with trophozoites of G. lamblia clone GS/M-83-H7 (human isolate) and were subsequently investigated for their serum antibody response directed against the major surface antigen (VSP H7) of the parasite (9). Recombinant polypeptides representing overlapping segments of VSP H7 and native Giardia proteins were used as antigenic reagents to examine the antigenic substructure of VSP H7 and the extent of antigenic variation in vivo. The data indicated that VSP H7 consists of two antigenically distinct parts: (i) a unique, variant-specific 314-amino-acid (aa) N-terminal region which elicits a low antibody response preferentially detectable during the early phase of the infection and (ii) a 171-aa C-terminal region containing relatively conserved epitopes which elicit a high-level antibody response during the later and regressive phase of the infection. Further investigations indicated that antigenic variation of the intestinal parasite population was associated with a diversification into at least six to nine new variant antigen types. None of these proteins shared antigenic epitopes with the N-terminal portion, but several of them cross-reacted with antibodies specific to the C-terminal portion of VSP H7. These results indicated the following. (i) Due to its high variability, the N-terminal part of VSP stimulates only a transient and consequently low-level antibody response. (ii) The semiconserved epitopes of the C-terminal part stimulate a persistent and consequently high-level antibody response during the parasite infection. Serum antibodies from mice during the early phase of infection, directed against the variable epitopes of the N-terminal region, caused detachment and aggregation of trophozoites and exhibited a complement-independent cytotoxic effect towards the parasite (15). In contrast, serum antibodies from the late phase of infection directed against the semiconserved epitopes of the C-terminal region, did not have a cytotoxic effect and provoked only transient parasite detachment and aggregation.
In order to find out whether the local anti-Giardia response generates a heterogeneous repertoire of anti-VSP H7 antibodies resembling the systemic antibody response, we have now performed an additional series of experimental G. lamblia (clone GS/M-83-H7) infections using the combined mother-offspring mouse model. These experiments included an investigation of the maternal production of secretory anti-VSP H7 IgA in milk and a characterization of the growth-selective consequences of these antibodies on the parasite population in suckling mice. Analyses revealed that ingestion by offspring of transiently emerging milk IgA antibodies against the variable N-terminal part of VSP H7 causes a direct parasiticidal effect on trophozoites of the original inoculum. By selecting for new variant antigen types within the intestinal parasite population, this antibody-mediated parasiticidal mechanism seems to initiate the process of antigenic diversification of G. lamblia clone GS/M-83-H7 populations in the intestine of the murine host.
MATERIALS AND METHODS
Animals.
Gravid 10- to 12-week-old outbred ZU.ICR mice were obtained from the Institut für Labortierkunde, University of Zürich, Zürich, Switzerland. Animals were kept according to Swiss regulations for animal experiments with free access to germfree food and sterile water.
Parasite.
The origin, axenization, and cloning of G. lamblia clone GS/M-83-H7 has been described by Aggarwal and coworkers (1). This clone expresses a major 72-kDa antigen (VSP H7) on its surface which is recognized by monoclonal antibody (MAb) G10/4. G. lamblia trophozoites were cultivated in TYI-S-33 medium with antibiotics as previously described (8).
Experimental parasite infection and sample collection.
A group of eight gravid ZU.ICR mice (designated as preinfected mothers) was infected three times (at days 5, 10, and 15 postfertilization of the animals) with G. lamblia clone GS/M-83-H7 by using a blunt-end needle for peroral inoculation of 200 μl of parasite suspension containing 106 trophozoites. The parasite inoculum was prepared by washing in vitro-cultivated trophozoites twice with sterile phosphate-buffered saline (PBS) and subsequent resuspension of the cells in 0.3 M NaHCO3 buffer. In parallel, a group of eight gravid ZU.ICR mice (designated as nonpreinfected mothers) was inoculated with 200 μl of 0.3 M NaHCO3 buffer. Animals from litters (8 to 12 mice per litter) of preinfected and nonpreinfected mothers were infected postpartum by intragastric inoculation of 5 × 104 G. lamblia trophozoites (clone GS/M-83-H7) as described previously (6).
Milk from the mammary gland was collected according to the method of Parr and coworkers (13) upon euthanasia (with CO2) of the mothers. Milk ingested by offspring was obtained by isolating the stomach content (about 200 to 500 μl per mouse), with the subsequent addition of the same volume of PBS and final homogenization of the samples by vortexing for 2 min. Milk samples were collected and stored at −20°C. After thawing of the samples, insoluble components were removed by centrifugation for 10 min at 10,000 × g, and milk supernatants were then used for further investigations (see below).
The course of the G. lamblia infection within mothers and offspring was determined by quantifying the parasite burden through microscopical examination of adherent trophozoites from intestinal washes and by additionally confirming the presence of viable intestinal parasites through a 6-day proliferation of such adherent trophozoites in TYI-S-33 culture medium containing antibiotics (8). In particular, sections of about 0.5 (section 1) and 1 (section 2) cm from the upper part of the duodenum were slit longitudinally. Section 1 was placed in a petri dish, supplemented with 2 drops of PBS, and examined microscopically for the presence of trophozoites. Where section 1 showed a high level of infection with the parasite (i.e., several hundred to several thousand parasites per microscopical field at a 100-fold magnification), section 2 was further investigated by first incubating the section for 20 min in 1 ml of PBS on ice to detach trophozoites from the intestinal surface and subsequently using a hemocytometer to determine the number of detached trophozoites in the PBS supernatant. Where section 1 showed a low level of infection (less than 10 trophozoites per microscopical field), the second section was handled in the same way as described above but detached trophozoites were subsequently readhered to the bottom of a well from a 24-well tissue culture plate (Sarstedt, Newton, N.C.) during a 30-min incubation of the trophozoite suspension at 37°C. Readhered trophozoites were counted by microscopical examination (at a 100-fold magnification) using a transparent 1-mm2 grid pattern for better orientation. The cumulative number of trophozoites in 10 separate microscopical fields (size, ≈3 mm2) was taken for extrapolation of the total number of adherent cells per well (bottom size, ≈200 mm2).
Immunofluorescence assays.
The kinetics of expression of the major surface antigen on trophozoites isolated from the duodenum or ileum were assessed by using MAb G10/4 for immunofluorescence assay as described elsewhere (6). For detection of the immunoreactivity of the antibody with the surface antigen, a goat anti-mouse IgG (IgG Fab′ specific; Sigma, St. Louis, Mo.) conjugated to TRITC was used at a dilution of 1:50. For double labeling to simultaneously detect IgA-bearing trophozoites originating from intestinal sections (isolated from three animals per experimental group), a goat anti-mouse IgA (α chain specific; Sigma) conjugated to fluorescein isothiocyanate (FITC) was used at the same dilution. Isotype specificity of the two antibody conjugates had previously been confirmed in control experiments by proving the absence of reactivities upon incubation of (i) IgA-bearing trophozoites (isolated from offspring of nonpreinfected mothers at days 8 and 9 p.i., see Results and Fig. 1) with anti-mouse IgG-TRITC conjugate and (ii) MAb G10/4-labeled and non-IgA-bearing trophozoites (isolated from offspring of nonpreinfected mothers at day 5 p.i.; see Results and Fig. 1) with anti-mouse IgA-FITC conjugate. The numbers of VSP H7-type (TRITC-labeled) and/or IgA-bearing (FITC-labeled) trophozoites were determined by inspection of four randomly chosen microscopical fields (at a 100-fold magnification).
FIG. 1.
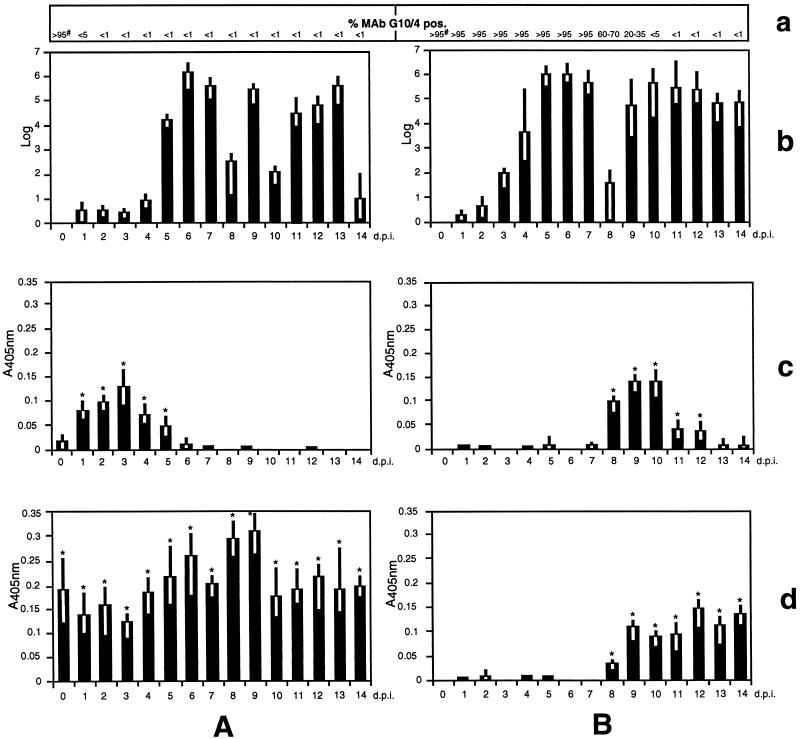
Follow-up analysis of G. lamblia (clone GS/M-83-H7)-infected offspring from experimentally preinfected (A) and nonpreinfected (B) mothers in terms of the intestinal parasite burden (given as log numbers of parasites detectable in a 1-cm section from the duodenum) (b) and incorporation of maternal milk IgA against purified recombinant MBP fused to the 314-aa N-terminal (c) and 171-aa C-terminal (d) parts of VSP H7. The parasite burden and milk anti-VSP H7 IgA from six mice per time point (given in days p.i. of offspring) were analyzed. The mean values of absorbance at 405 nm (A405) for the VSP H7-specific reactions as well as the ranges are shown. Mean values of <0.01 (including negative values) are indicated by a blank position. Significant differences between the values were determined by using Student’s t test (∗, P <0.05). (a) The numbers indicate percentages of intestinal parasites with an MAb G10/4 (VSP H7)-positive variant antigen type. #, numbers represent percentages of MAb G10/4-positive parasites in the original inoculum.
Cytotoxicity assay.
Cytotoxicity assays were performed in triplicates as outlined previously (1, 15), using 96-well microtiter plates for incubation of 4 × 104 Giardia trophozoites per 100 μl of culture medium in the presence or absence of 1-μl aliquots of milk supernatants (see above). During incubation, trophozoites were maintained and expanded in TYI-S-33 medium (8) under anaerobic conditions. Detachment and aggregation of trophozoites were investigated immediately after addition of the reagent, and after 5 min, 15 min, 1 h, 6 h, and 24 h. Cytotoxicity was determined after 24 h by estimating the number of adherent viable parasites through microscopical examination. Test cultures containing a predetermined number of adherent cells less than about 10% of the number of adherent cells visible in a parallel control culture were scored positive with respect to cytotoxicity. Test cultures containing a number of adherent cells visually indistinguishable from the number in a parallel control culture were scored negative. All cytotoxicity assays were reproduced in two separate experiments. In each experiment, all milk supernatants were analyzed in parallel using trophozoites from only one in vitro culture for the individual tests.
Bacterial expression and purification of recombinant VSP H7.
The gene segment coding for the 314-aa N-terminal or the 171-aa C-terminal region of VSP H7 were cloned into the Escherichia coli expression vector pMalc2 (New England Biolabs, Beverly, Mass.), and respective recombinant proteins (produced as C-terminal fusions to an N-terminal portion of the E. coli maltose-binding protein [MBP]) were purified as described elsewhere (9).
ELISA and Western blot.
For analysis of milk IgA antibodies against the different VSP H7 peptide fragments, the following IgA-capture enzyme-linked immunosorbent assay (ELISA) was performed. Goat anti-mouse-IgA antibody (α chain specific; Sigma) was used at a 1:100 dilution (in 0.1 M NaHCO3, pH 8.2) for coating of microELISA plates (Maxisorp F 96 Immunoplates; Nunc, Roskilde, Denmark). Anti-IgA-coated wells were incubated for 4 h at room temperature with 100-μl aliquots of milk supernatant (see above) diluted 1:5 (milk supernatants from stomach content) or 1:50 (milk supernatants from mammary gland) in blocking solution (PBS containing 0.3% Tween 20, 10% TYI-S-33 medium, and 0.5% skim milk). Subsequently, wells were incubated for 2 h at room temperature with 100 μl of antigen solution (1 μg of antigen per ml of blocking solution) containing purified MBP′-VSP H7 (314-aa N-terminal region), MBP′-VSP H7 (171-aa C-terminal region), or MBP′ control antigen, respectively. Antigen-antibody complexes were detected by a consecutive incubation (2 h at room temperature) with a rabbit anti-MBP IgG antibody (New England Biolabs) and a goat anti-rabbit antibody (Fcγ specific; Promega, Madison, Wis.) conjugated to alkaline phoshatase, diluted 1:10,000 and 1:500, respectively. Test results were validated only when both variability within triplicate determinations was less than 15% and A405 (absorbance at 405 nm) values obtained for all MBP-specific control reactions were below 0.05. The individual VSP H7-specific values were calculated by subtracting the mean value (plus 2 standard deviations) for a triplicate determination of MBP control reactions from the mean value for the respective MBP′-VSP H7-specific reactions.
Western blot analysis of soluble G. lamblia clone GS/M-83-H7 antigens was done as described by Gottstein and coworkers (6). Milk supernatants (milk supernatants from mammary gland; see above), MAb G10/4, and alkaline phosphatase-conjugated goat anti-mouse IgA (α chain specific; Promega) were diluted 1:50, 1:200, and 1:500, respectively.
Statistical methods.
The significance of the differences among the control and experimental groups was determined by Student’s t test using the Microsoft Excel program. P values of <0.05 were considered statistically significant.
RESULTS
Course of G. lamblia infection and antigenic variation of the parasite.
In order to study the immunological and infection parameters related to the process of antigenic variation, gravid ZU.ICR mice were either kept uninfected (nonpreinfected mothers) or were perorally infected three times with G. lamblia trophozoites (preinfected mothers). Three-day-postpartum offspring from these animals were infected by intragastric injection with the same type of trophozoites. At different time points during the lactation phase, six offspring from different mothers were sacrificed by CO2 euthanasia. Microscopical determination of the number of intestinal parasites (isolated from the duodenum) in the suckling offspring revealed that the course of the infection was associated with a fluctuation of the parasite burden (Fig. 1b). In offspring from preinfected mothers, three waves of high-degree infection were apparent at days 5 to 7 (first wave), at day 9 (second wave), and at days 11 to 13 (third wave) postinfection (p.i.). The course of infection in offspring from nonpreinfected mothers exhibited a first wave between days 3 and 7 and a second wave emerging at day 9 and extending beyond the lactation phase. In the infection experiments, transmission of the parasite infection from mothers to the offspring could be ruled out by showing that no parasites were detectable in intestinal biopsy material from experimentally noninfected offspring derived from preinfected mothers (data not shown). All data described in this paragraph were successfully reproduced in a second experiment which included an equivalent follow-up study of the parasite burden in offspring from nonpreinfected and preinfected mothers (data not shown).
Immunofluorescence analysis of the parasite’s antigenic variation in offspring from preinfected mothers revealed that trophozoites from the first wave had already undergone diversification from MAb G10/4 positive to negative types (Fig. 1a). In contrast, antigenic diversification of parasites in offspring from nonpreinfected mothers essentially occurred between days 8 and 10 p.i. concurrent with the second wave of high-degree infection.
Taken together, these observations indicated that preinfection of mothers with G. lamblia clone GS/M-83-H7 had an influence on both the course of the infection in offspring and on the antigenic variation of the intestinal parasite population in these animals.
Milk anti-VSP H7 IgA production.
Using recombinant peptide fragments from both the variant-specific 314-aa N-terminal and the 171-aa C-terminal part of VSP H7 as antigenic reagents, the maternal milk IgA response against the parasite infection was investigated by ELISA (Fig. 1c and d). Milk was sampled from the stomach of suckling mice at different times. The ELISAs revealed that at the time of inoculation of the offspring (day 0), preinfected mothers preferentially synthesized milk IgA against the C-terminal part of VSP H7 (Fig. 1Ad). During early-phase infection of the offspring (days 1 to 5 p.i.), milk IgA from preinfected mothers was directed against both the N-terminal (Fig. 1Ac) and C-terminal (Fig. 1Ad) regions of VSP H7. From day 6 p.i., antibodies against the N-terminal part had disappeared, whereas antibodies against the C-terminal part were present throughout the whole lactation period. In the nonpreinfected mothers, milk IgA production against both parts of VSP H7 (between days 8 and 12 p.i.) (Fig. 1Bc and d) was coincident with the initial appearance of antigenic variants among the intestinal parasite population in the offspring. This time correlation suggested a selective function of these antibody specificities within the process of antigenic variation of the parasite. A selective function of anti-VSP H7 IgM and IgG antibodies within this process was ruled out by demonstrating the absence of such antibodies in any of the milk samples of either experimental animal group investigated (data not shown).
The ELISA for which results are shown in Fig. 1 further revealed that both the experimentally preinfected and the nonpreinfected mothers produced milk anti-VSP H7 IgA antibodies only a relatively short period after infection of the offspring (day 1 p.i., preinfected mothers; day 8 p.i., nonpreinfected mothers). This indicated that the mothers from both experimental groups had received an immunological stimulus by ingestion of parasite contaminations on the offspring (most likely resulting from leakage of trophozoites during intragastric injection of the inoculum). The occurrence of such an experimentally uncontrolled infection was confirmed by demonstrating the presence of trophozoites in the intestine from experimentally nonpreinfected mother animals (data not shown). On the other hand, infection of mothers by parasite cysts fecally excreted by the offspring was excluded due to prepatency and was also ruled out by coprological examinations.
Milk antibody cytotoxicity test and characterization of parasiticidal antibody specificities.
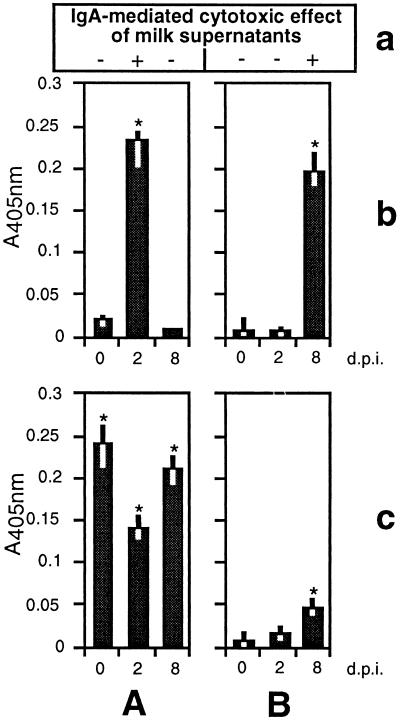
Previous investigations revealed that serum antibodies against the 314-aa N-terminal region of VSP H7 displayed a direct cytotoxic and agglutinating effect on in vitro-cultivated trophozoites of G. lamblia clone GS/M-83-H7 (15). In order to test the potential of milk IgA antibodies to mediate cytotoxicity, in vitro-cultivated trophozoites from clone GS/M-83-H7 were incubated in the presence of milk supernatants from the mammary glands of preinfected and nonpreinfected mothers sampled at 0, 2, and 8 days p.i. (Fig. 2a). This analysis revealed that milk supernatants with detectable antibodies against the 314-aa N-terminal part of VSP H7 (day 2 p.i., preinfected mothers; day 8 p.i., nonpreinfected mothers) (Fig. 2b) were cytotoxic and had a strong and immediate agglutinating effect on trophozoites, visible after about 5 min. In contrast, milk supernatants containing only IgA against the 171-aa C-terminal part of VSP H7 (days 0 and 8 p.i., preinfected mothers) (Fig. 2c) were not cytotoxic and caused only a weak and transient agglutination of trophozoites, visible after about 1 h and disappearing during a further 24-h incubation in culture medium. Milk supernatants containing no anti-VSP H7 IgA antibodies at all (days 0 and 2 p.i., nonpreinfected mothers) (Fig. 2b and c) did not exhibit any of these cytological effects, even after an extended incubation period of about 24 h. Furthermore, analogous testing of the entire set of milk supernatants with non-VSP H7-type trophozoites, isolated at day 12 p.i. from offspring of nonpreinfected mothers, did not show any significant effect (data not shown). All of the cytotoxicity tests described above were performed under conditions in which supernatants had been preincubated for 40 min at 56°C to inactivate complement components. The outcome of these experiments was essentially the same as that described for the assays with untreated milk supernatants.
FIG. 2.
ELISA-based determination of milk IgA against purified recombinant MBP fused to the 314-aa N-terminal (b) and 171-aa C-terminal (c) parts of VSP H7. Milk was taken at different time points (given in days p.i. of offspring) from the mammary gland of G. lamblia (clone GS/M-83-H7)-preinfected (A) and nonpreinfected (B) mothers. The mean values of absorbance at 405 nm (A405) for the VSP H7-specific reactions as well as the ranges are indicated. Significant differences between the values were determined by using Student’s t test (∗, P <0.05). (a) Milk supernatants with (+) and without (−) a cytotoxic effect towards G. lamblia clone GS/M-83-H7 trophozoites are indicated.
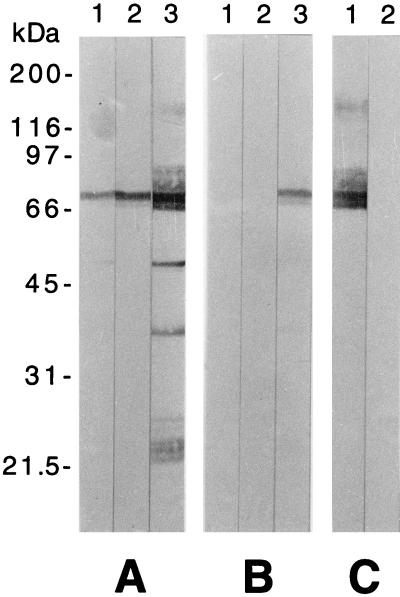
In order to further characterize the specificities of the anti-Giardia IgA antibodies, a Western blot experiment was performed using crude trophozoite extracts as antigenic reagents and the same milk supernatants as used above (Fig. 3A and B). This experiment revealed that IgA from the cytotoxic milk supernatants (day 2 p.i., preinfected mothers; day 8 p.i., nonpreinfected mothers) was essentially directed against VSP H7. On the other hand, the noncytotoxic VSP H7-reactive milk supernatants obtained from preinfected mothers at day 8 p.i. exhibited additional IgA specificities to non-VSP H7 antigens of the parasite. In this Western blot analysis, the VSP H7 band was identified by its immunoreactivity with MAb G10/4 (Fig. 3C).
FIG. 3.
Western blot analysis of parasite-specific milk IgA production in G. lamblia (clone GS/M-83-H7)-preinfected (A) and nonpreinfected (B) mothers. Sodium dodecyl sulfate-10% polyacrylamide gel-fractionated and blotted total protein from G. lamblia clone GS/M-83-H7 was incubated with milk supernatants sampled from mothers at days 0 (lanes 1), 2 (lanes 2), and 8 (lanes 3) after infection of offspring. (C) Reference blots incubated with VSP H7-reactive MAb G10/4 (lane 1) and a negative control, MAb 6E7 (lane 2), are shown. On the left, sizes of the protein markers are given in kilodaltons.
Determination of relative amounts of VSP H7-type trophozoites from different intestinal sites.
As demonstrated by immunofluorescence assay (Fig. 1), offspring from nonpreinfected mothers at day 8 p.i. harbored a population of trophozoites which were heterogeneous in variant-antigen-type composition. The parasite population in the duodenum of these animals had undergone partial antigenic diversification and consisted of only 60 to 70% MAb G10/4-positive trophozoites. In a further experiment, additional staining of duodenal trophozoites with an anti-murine IgA-specific second antibody revealed that, in contrast to the MAb G10/4-negative cells, the large majority (>90%) of positive cells were covered with intestinal IgA antibodies (Table 1). An analogous investigation of the trophozoites isolated from the ileum of the same animals showed that the relative amount of MAb G10/4-positive cells was much higher (>95%) and these parasites were essentially also covered with IgA (>95%). As assessed by short-term (2-day) in vitro cultivation and subsequent immunofluorescence staining, the proliferative (viable) group of duodenal trophozoites consisted of MAb G10/4-negative cells. In contrast, the MAb G10/4-positive trophozoites from the ileum (>95% of the parasites) partially appeared as aggregates of cells and could not be subsequently grown in vitro.
TABLE 1.
Determination of MAb G10/4-positive and IgA-bearing parasites in the duodenum and ileum of G. lamblia clone GS/M-83-H7-infected micea
| Parasites from: | Duodenum
|
Ileum
|
||
|---|---|---|---|---|
| MAb G10/4 positive (%) | MAb G10/4 positive, IgA bearing (%) | MAb G10/4 positive (%) | MAb G10/4 positive, IgA bearing (%) | |
| Day 5 p.i. | >95b | <1 | —c | — |
| Day 8 p.i. | 60–70d | >90 | >95e | >95 |
In all examinations, the relative amount of MAb G10/4-negative, IgA-bearing parasites was <1%.
In vitro-proliferative (viable) parasites were MAb G10/4 positive.
No parasites detectable by direct microscopical examination and after in vitro cultivation.
In vitro-proliferative (viable) parasites were MAb G10/4 negative.
Parasites partially appeared as aggregates and did not proliferate in vitro.
As a control, intestinal parasite populations taken from the offspring of nonpreinfected mothers were investigated at day 5 p.i., at which time the initial antigenic diversification had not yet occurred. In this case, more than 95% of trophozoites were MAb G10/4 positive and did not bear detectable amounts of IgA. In separate examinations, adherent and proliferative parasites were demonstrated in the duodenum whereas no parasites were detectable in the ileum either under the microscope or in long-term (6-day) in vitro cultures. After short-term in vitro cultivation of the parasites from the duodenum, the majority (>95%) of the cells maintained the MAb G10/4-positive variant antigen type.
The findings described above were obtained in three parallel experiments representing investigations of duodenal and ileal trophozoites from three animals per experimental group. Taken together, these examinations demonstrated that the population of viable G. lamblia trophozoites was essentially located in the duodenum. Considering the immunofluorescence assays and the in vitro cultivation tests of the diverse intestinal parasite isolates, our data further suggested that those trophozoites which were opsonized with IgA represented a population of nonviable parasites predominantly located in the ileum (see also Discussion).
DISCUSSION
We performed a series of experimental G. lamblia (clone GS/M-83-H7) infections of neonatal mice and their mothers to study the function of local anti-VSP H7 IgA antibodies in the process of antigenic variation of the parasite. Our study included an analysis of milk IgA from both primarily noninfected mothers and mothers that had previously received a strong immunological stimulus through repetitive peroral infection with the parasite. By using the combined mother-offspring model, we were able to circumvent two problems which hamper pathogenic and immunological investigations of G. lamblia infections in the conventional murine system. First, inoculation of G. lamblia trophozoites into adult mice has been shown to result in a strong anti-Giardia immune response but in a relatively low-degree infection (2), which complicates analysis of the respective parasite populations in terms of their variant-antigen-type composition. Secondly, neonatal mice generally develop a high-degree infection (7) but possess an immature immune system which does not significantly respond during the early-phase infection. Therefore, we developed the present strategy to test the influence of milk anti-VSP H7 IgA antibodies from infected mothers on the G. lamblia populations in infected offspring. In this context, the elaboration of a highly sensitive IgA capture ELISA (see Materials and Methods) was an important prerequisite for reliable and reproducible monitoring of milk anti-VSP H7 IgA production in infected animals. A conventional ELISA (data not shown) had previously exhibited both a higher level of nonspecific background reactions and greater variability within the individual triplicate A405 values (see also Materials and Methods).
Our investigations revealed that IgA antibodies against the relatively conserved epitopes of the 171-aa C-terminal VSP H7 region displayed weak trophozoite-agglutinating effects which apparently did not significantly interfere with the in vitro growth of the parasite. In contrast, maternal milk IgA antibodies against the variant-specific 314-aa N-terminal part of VSP H7 caused immediate aggregation of, and direct cytotoxicity to, in vitro-cultivated VSP H7-type trophozoites. The in vivo relevance of these antibody-mediated parasiticidal effects was evidenced by an investigation which determined the relative amount of VSP H7-type trophozoites in both the duodenum and the ileum of G. lamblia-infected offspring. When the variant antigen types in these particular intestinal sites were specified during the initial phase of antigenic variation, the proportion of VSP H7-type trophozoites versus non-VSP H7-type trophozoites was revealed to be much higher in the ileum than in the duodenum of the offspring. Further analyses indicated that MAb G10/4-positive (VSP H7-type) trophozoites were specifically covered with IgA antibodies and also represented collections of nonviable cells which did not proliferate in vitro. In concordance with results from our previous studies (9, 14, 15), the findings described above suggested that an antibody-mediated and growth-selective mechanism is involved in the process of antigenic diversification within a G. lamblia (clone GS/M-83-H7) population in suckling mice. The following mechanism of action is proposed. Ingested milk IgA antibodies against the 314-aa N-terminal part of VSP H7 specifically react with, and cause direct parasiticidal effects to, VSP H7-type trophozoites in the duodenum. While non-VSP H7-type trophozoites remain unaffected and reside as adherent, viable cells in the duodenum, nonviable and nonadherent VSP H7-type trophozoites are entrapped by the mucus and are subsequently transported via intestinal peristalsis to the ileum. In this distal section of the murine intestine, such trophozoites appear as nonadherent cell aggregates exclusively consisting of nonviable, VSP H7-type parasites.
The course of the G. lamblia infection in suckling mice was associated with a fluctuation of the parasite burden. Interestingly, proliferation of the trophozoites during the progressive phase of the second and third wave of high-intensity infection resulted in growth rates that were at least three times higher than those observed in vitro. The quasielimination of the VSP H7-type trophozoites which constituted the first wave of high-intensity infection in offspring from nonpreinfected mothers coincided with both an antigenic diversification from an essentially MAb G10/4-positive to a -negative parasite population and a production of parasite-agglutinating and cytotoxic milk antibodies directed against the variant-specific 314-aa N-terminal part of VSP H7. These findings again provided strong evidence that in vivo growth of such individual populations of variant antigen types was controlled by a selective pressure through the parasiticidal activity of variant-specific IgA antibodies.
Further investigations exploring the G. lamblia GS/M-83-H7-mouse model system will address the following questions. (i) Do the individual waves within the fluctuating infection represent distinct and consecutively emerging populations of variant antigen types? (ii) Does an individual wave consist of one or several variant antigen types? (iii) Does a consecutive and transient production of cytotoxic IgA antibodies against the VSPs of successively emerging variant antigen types account for the fluctuating course of infection? (iv) Does antigenic variation in vivo occur randomly or in a “programmed” manner which leads to the same sequence of G. lamblia variant antigen types in different host individuals? From these studies, we expect to obtain further insights into both the strategy of antigenic variation, adopted by the parasite to deal with the immune system, and the immunological effector mechanisms generated by the murine host to eliminate the different variant antigen types of the parasite from their intestinal habitat.
ACKNOWLEDGMENTS
We acknowledge V. Zimmermann for excellent technical assistance, A. Hemphill for fruitful discussion, B. Connolly for carefully reading the manuscript, and T. E. Nash (NIH, Bethesda, Md.) for his gift of MAbs, the vspH7 cDNA fragment, and G. lamblia clone GS/M-83-H7.
This work was supported by grants obtained from the Swiss National Science Foundation (no. 31-37607.93 and 31-49439.96).
REFERENCES
- 1.Aggarwal A, Merritt J W, Nash T E. Cysteine-rich variant surface proteins of Giardia lamblia. Mol Biochem Parasitol. 1989;32:39–48. doi: 10.1016/0166-6851(89)90127-8. [DOI] [PubMed] [Google Scholar]
- 2.Byrd L G, Conrad J T, Nash T E. Giardia lamblia infections in adult mice. Infect Immun. 1994;62:3583–3585. doi: 10.1128/iai.62.8.3583-3585.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farthing M J G. Giardia lamblia. In: Blaser M J, Smith P D, Radvin J I, Greenberg H G, Guerrant R L, editors. Infections of the gastrointestinal tract. New York, New York: Raven Press; 1995. pp. 1081–1105. [Google Scholar]
- 4.Faubert G M. The immune response to Giardia. Parasitol Today. 1996;12:140–145. doi: 10.1016/0169-4758(96)10004-1. [DOI] [PubMed] [Google Scholar]
- 5.Gottstein B, Deplazes P, Tanner I. In vitro synthesized immunoglobulin A from nu/+ and reconstituted nu/nu mice against a dominant surface antigen of Giardia lamblia. Parasitol Res. 1993;79:644–648. doi: 10.1007/BF00932506. [DOI] [PubMed] [Google Scholar]
- 6.Gottstein B, Harriman G R, Conrad J T, Nash T E. Antigenic variation in Giardia lamblia: cellular and humoral immune response in a mouse model. Parasite Immunol. 1990;12:659–673. doi: 10.1111/j.1365-3024.1990.tb00995.x. [DOI] [PubMed] [Google Scholar]
- 7.Gottstein B, Nash T E. Antigenic variation in Giardia lamblia: infection of congenitally athymic nude and scid mice. Parasite Immunol. 1991;13:649–659. doi: 10.1111/j.1365-3024.1991.tb00560.x. [DOI] [PubMed] [Google Scholar]
- 8.Keister D B. Axenic culture of Giardia lamblia in TYI-S-33 medium supplemented with bile. Trans R Soc Trop Med Hyg. 1983;77:487–488. doi: 10.1016/0035-9203(83)90120-7. [DOI] [PubMed] [Google Scholar]
- 9.Müller N, Stäger S, Gottstein B. Serological analysis of the antigenic heterogeneity of Giardia lamblia variant surface proteins. Infect Immun. 1996;64:1385–1390. doi: 10.1128/iai.64.4.1385-1390.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nash T E. Surface antigen variability and variation in Giardia lamblia. Parasitol Today. 1992;8:229–234. doi: 10.1016/0169-4758(92)90119-m. [DOI] [PubMed] [Google Scholar]
- 11.Nash T E, Herrington D A, Levine M M, Conrad J H, Merritt J W. Antigenic variation of Giardia lamblia in experimental human infections. J Immunol. 1990;144:4362–4369. [PubMed] [Google Scholar]
- 12.Nash T E, Merritt J W, Conrad J T. Isolate and epitope variability in susceptibility of Giardia lamblia to intestinal proteases. Infect Immun. 1991;59:1334–1340. doi: 10.1128/iai.59.4.1334-1340.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parr E L, Bozzola J J, Parr M B. Purification and measurement of secretory IgA in mouse milk. J Immunol Methods. 1995;180:147–157. doi: 10.1016/0022-1759(94)00310-s. [DOI] [PubMed] [Google Scholar]
- 14.Stäger S, Müller N. Giardia lamblia infections in B-cell-deficient transgenic mice. Infect Immun. 1997;65:3944–3946. doi: 10.1128/iai.65.9.3944-3946.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stäger S, Felleisen R, Gottstein B, Müller N. Giardia lamblia variant surface protein (VSP) H7 stimulates a heterogeneous repertoire of antibodies displaying differential cytological effects on the parasite. Mol Biol Parasitol. 1997;85:113–124. doi: 10.1016/s0166-6851(96)02818-6. [DOI] [PubMed] [Google Scholar]