Abstract
Although promising therapeutics are in the pipeline, bariatric surgery (also known as metabolic surgery) remains our most effective strategy for the treatment of obesity and type 2 diabetes mellitus (T2DM). Of the many available options, Roux-en-Y gastric bypass (RYGB) and vertical sleeve gastrectomy (VSG) are currently the most widely used procedures. RYGB and VSG have very different anatomical restructuring but both surgeries are effective, to varying degrees, at inducing weight loss and T2DM remission. Both weight loss-dependent and weight loss-independent alterations in multiple tissues (such as the intestine, liver, pancreas, adipose tissue and skeletal muscle) yield net improvements in insulin resistance, insulin secretion and insulin-independent glucose metabolism. In a subset of patients, post-bariatric hypoglycaemia can develop months to years after surgery, potentially reflecting the extreme effects of potent glucose reduction after surgery. This Review addresses the effects of bariatric surgery on glucose regulation and the potential mechanisms responsible for both the resolution of T2DM and the induction of hypoglycaemia.
Introduction
Despite promising pharmacotherapies in the pipeline, bariatric surgery (also known as metabolic surgery) remains the most effective strategy for the treatment of obesity and type 2 diabetes mellitus (T2DM)1-3. Pioneering work by Pories et al.4 first proposed that bariatric surgery could treat T2DM given its robust effects in reducing body weight as well as blood levels of glucose, fasting insulin and HbA1c. Indeed, multiple subsequent studies demonstrated the efficacy of bariatric surgery in improving glucose homeostasis, reducing the need for glucose-lowering medications, and reducing both microvascular and macrovascular complications of T2DM3. Some patients even have remission of T2DM, defined as a normal HbA1c without glucose-lowering medications for at least 3 months5. Moreover, patients who have bariatric surgery are less likely to be diagnosed with T2DM 15 years postoperatively than those who don't have surgery6.
The precise mechanisms responsible for improved glucose control after surgery remain uncertain. Some suggest that the profound weight loss induced by surgery is the critical factor necessary for T2DM resolution7. Thus, those with greater weight loss have a greater propensity for T2DM control and remission after surgery than those with less weight loss; however, additional factors exist, including duration of disease, age and the level of glycaemic control, thereby suggesting weight loss-independent mechanisms8,9. Given that these factors are associated with β-cell functional capacity, T2DM might be more easily reversed in patients with shorter duration of disease, younger age and better glycaemic control. However, even some patients with T2DM who were taking insulin prior to bariatric surgery have reported cessation of insulin therapy10.
This Review examines the clinical and preclinical literature to identify the physiological and cellular responses to bariatric surgery that might be mechanistically linked to changes in glucose homeostasis. Although weight loss-associated mechanisms are not within the main scope of this Review, we highlight potential roles for both weight loss-dependent and weight loss-independent responses as mediators of glycaemic improvement.
Current bariatric procedures
The most widely utilized bariatric surgeries at present are vertical sleeve gastrectomy (VSG) and Roux-en-Y gastric bypass (RYGB). VSG involves the removal of ~80% of the stomach along the greater curvature. By contrast, RYGB involves gastric size restriction with the creation of a small gastric pouch and re-routing of the intestinal tract, such that ingested nutrients empty directly into the jejunum, thereby bypassing 95% of the stomach, the duodenum and proximal jejunum. Other types of bariatric surgeries, including gastric banding and biliopancreatic diversion (BPD), are less commonly employed than RYGB and VSG due to decreased efficacy (gastric banding) for weight loss and T2DM resolution, or the increased magnitude of micronutrient and macronutrient malabsorption (BPD).
Although RYGB has long been considered the gold standard bariatric procedure, utilization of VSG has steadily increased. VSG is now the most widely performed bariatric surgery worldwide, in large part because it is a more straightforward procedure that requires a shorter operative time than RYGB. RYGB and VSG have generally comparable efficacy for weight loss, which is potentially related to similar physiological responses, including rapid nutrient entry into the intestine, increased postprandial secretion of gut peptides and increased plasma levels of bile acids. Comparisons of RYGB and VSG indicate equal potency for T2DM control in some studies11-13, whereas others demonstrate a somewhat greater efficacy of RYGB1,14. In those studies, reported differences in the degree of T2DM remission generally correlate with the degree of weight loss. For example, the STAMPEDE trial in patients with obesity and uncontrolled T2DM found that weight loss was greater with RYGB than VSG and, while RYGB was similar to VSG for glucose control, patients who underwent RYGB required less medication to achieve that control1. Where applicable in this Review, we discuss potential differences between the surgeries. However, given the similar physiological responses between RYGB and VSG, we speculate that the dominant mechanisms for improvements in glucose metabolism are also similar.
Effect of weight loss on glucose control
The degree of weight loss achieved with bariatric surgery is generally associated with the degree of resolution of T2DM9,15,16, highlighting the important role of weight loss-dependent mechanisms. However, most studies that directly compare surgical versus medical weight loss are small, with heterogeneity of disease duration or severity limiting the statistical power. Thus, randomized clinical trials that compare surgical versus medical management of T2DM are especially important. Such trials have demonstrated the marked superiority of RYGB, BPD and VSG for both durable weight loss and improved glycaemic control compared with medical management1-3. Likewise, gastric banding, which does not alter nutrient flow, typically induces more modest weight loss and improvements in glycaemia than the other forms of surgery17.
Weight loss yields reductions in total, visceral and pancreatic adipose tissue, reductions in intrahepatic levels of lipids, and improved insulin sensitivity, all of which are expected to improve systemic glucose metabolism18. However, dissecting the weight loss-dependent and independent mechanisms of bariatric surgery in humans is highly challenging as long-term control of food intake is not possible. The situation is further complicated by the fact that medical management of obesity is less likely to achieve equivalent weight loss and takes longer to do so than surgery. One carefully performed study analysed glucose metabolism and insulin sensitivity in 22 participants who achieved a matched 18% weight loss after surgery or medical therapy7. Interestingly, the authors observed similar increases in liver, adipose tissue, and muscle insulin sensitivity and increases in β-cell function when weight loss was matched. Thus, the authors suggested that mechanisms mediating improved glucose metabolism after surgery are primarily related to the magnitude of weight loss. However, additional mechanisms beyond insulin action were not evaluated.
Several lines of evidence suggest that additional mechanisms might contribute to both early and sustained metabolic improvements after bariatric surgery. First, glycaemic control improves rapidly after surgery, enabling discontinuation of insulin and other glucose-lowering medications, even before substantial weight loss has occurred. In one study, patients who had undergone BPD had greater reductions in glucose tolerance and basal and glucose-stimulated blood levels of insulin compared with non-surgical control individuals matched for weight loss17. Additionally, patients with T2DM who underwent adjustable gastric banding10 or dietary-induced weight loss19 have lower rates of insulin cessation compared with those who underwent surgery, even when controlling for weight loss. Together, these data suggest that there are additional weight loss-independent mechanisms for T2DM resolution after bariatric surgery (Fig. 1).
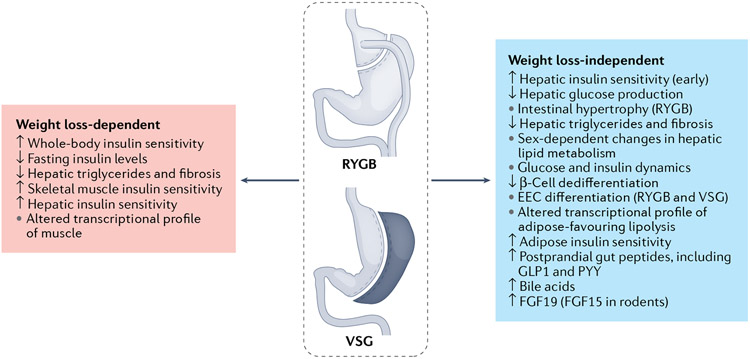
Fig. 1 ∣. Weight loss-dependent and weight loss-independent mechanisms of bariatric surgery on glucose metabolism.
Bariatric surgery has important weight loss-dependent and weight loss-independent mechanisms for driving improvements in glucose homeostasis. In itself, weight loss improves whole-body and specifically muscle and adipose insulin sensitivity. By contrast, the liver seems to be more susceptible to weight loss-independent effects on improving hepatic insulin sensitivity. Weight loss-independent effects also include changes in postprandial glucose and insulin dynamics, with high peaks and rapid returns towards baseline. Bariatric surgery leads to increases in postprandial levels of multiple gut peptides, bile acids and fibroblast growth factor 19 (FGF19; FGF15 in rodents). Changes in the nutrient profile and increases in bile acid signalling contribute to increases in intestinal cell proliferation (Roux-en-Y gastric bypass (RYGB)) and differentiation towards the enteroendocrine cell (EEC) lineages (both RYGB and vertical sleeve gastrectomy (VSG)). There are probably combined weight loss-dependent and weight loss-independent effects on hepatic levels of triglycerides. GLP1, glucagon-like peptide 1; PYY, peptide YY.
Systemic glycaemic effects of surgery
In this section, we review the available preclinical and clinical data that highlight potential target organs mediating improvements in glucose metabolism after bariatric surgery (Fig. 2).
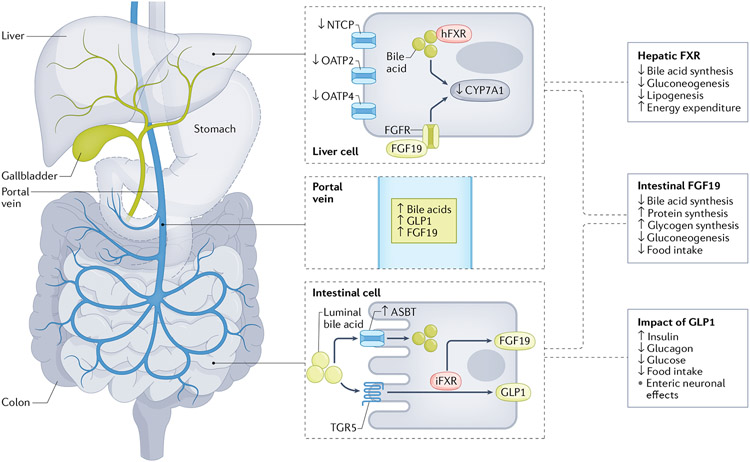
Fig. 2 ∣. Bariatric surgery-induced changes in bile acid dynamics induce alterations in glucose metabolism.
Plasma levels of bile acids increase after bariatric surgery via alterations in the enterohepatic circulation. Increased expression of the intestinal bile acid transporter ASBT increases the resorption of bile acids from the intestinal lumen into the portal vein. Decreases in hepatic bile acid transporters (NTCP, OATP2 and OATP4) reduce the reuptake of bile acids into the liver, which leaves increased levels of bile acids in the circulation. Within the intestine and adipose tissue, bile acids can activate Takeda G protein receptor 5 (TGR5); although the data are conflicting with VSG, this might be one mechanism by which luminal bile acids stimulate glucagon-like peptide 1 (GLP1) and consequently impact glucose metabolism and food intake. Also within the intestine, bile acids activate farnesoid X receptor (FXR), which increases expression and plasma levels of fibroblast growth factor 19 (FGF19; FGF15 in rodents). In the liver, FXR is also activated and the combination of these signalling pathways leads to reductions in bile acid synthesis and decreased gluconeogenesis and lipogenesis, and could also impact aspects of energy homeostasis. Dashed outline around the stomach indicates that it has undergone bariatric surgery. iFXR, intestinal FXR; hFXR, hepatic FXR.
Nutrient absorption and processing
After either RYGB or VSG, nutrients enter the gastrointestinal tract very rapidly20. With RYGB, there is no pylorus or antrum to slow nutrient entry into the intestine and therefore undigested nutrients rapidly enter the Roux limb. With VSG, postprandial increases in gastric pressure result in increased gastric emptying20. This change in nutrient exposure probably affects intestinal structure and function in both surgeries. Both human and rodent studies demonstrate intestinal hypertrophy after RYGB21, with shifts in glucose metabolism towards pathways that support tissue growth22. Tissue growth is energetically expensive and might contribute to changes in total energy expenditure and/or glucose disposal. Indeed, surgical procedures that modulate gastric anatomy or function, including gastrectomy, RYGB, VSG or Billroth II (partial gastrectomy and intestinal rearrangement), increase 18F-fluorodeoxyglucose uptake in multiple intestinal segments in humans23,24 and rats22 in correlation with reduced fasting blood levels of glucose.
The expression of genes regulating glycolysis (potentially mediated by hypoxia-inducible factor 1α) and of genes involved in fatty acid, triglyceride and cholesterol synthesis is increased in the jejunum after RYGB in rats25. These changes parallel increases in markers of intestinal stem cells and proliferation. Similar changes are observed in human jejunal biopsy samples taken before and 1 month after RYGB. Although VSG does not induce intestinal hypertrophy like RYGB21, intestinal stem cell differentiation is shifted away from absorptive cells and towards secretory cell lineages in mice26. Collectively, these data suggest that surgery-induced intestinal rearrangement modulates intestinal gene expression and cell lineage, potentially via the effect of altered luminal nutrients and metabolites.
Insulin sensitivity
Hepatic insulin sensitivity.
In rats fed a high-fat diet (HFD) before and after bariatric surgery, either RYGB or VSG improved hepatic insulin sensitivity (measured by insulin-induced suppression of hepatic glucose production) within 2 weeks of surgery and in a weight loss-independent manner27. This finding is linked to a reduction in gluconeogenesis after RYGB as suggested by the RYGB-induced downregulation of genes that regulate gluconeogenesis in a rat model of T2DM28. In lean rats without T2DM, plasma levels of gluconeogenic amino acids were increased in a weight loss-independent manner after bariatric surgery, suggesting that substrate availability is not the limiting factor for reduced gluconeogenesis29. By contrast, hepatic glycogen content does not differ after VSG in mice30. Together, these data suggest that reduced hepatic glucose production after bariatric surgery is mediated via suppression of gluconeogenesis rather than glycogen breakdown.
Increased lipid accumulation in the liver is associated with impaired hepatic insulin sensitivity. Bariatric surgery is highly effective at lowering the levels of hepatic lipids and is an effective treatment for non-alcoholic fatty liver disease31. In humans, reductions in hepatic levels of triglycerides and fibrosis occur after either VSG or RYGB31-33. Although this effect could be weight loss-dependent34, the reduction in hepatic levels of triglyceride after VSG in mice35,36 and after RYGB in rats37 is greater than in weight-matched sham-surgery control animals, which indicates additional weight loss-independent mechanisms.
Previous work in humans and rodents has demonstrated widespread transcriptomic changes early after RYGB during active weight loss and during subsequent weight-stable periods25, which could explain changes in the metabolic phenotype of the liver. In the early postoperative phase in rodents, increases are observed in the hepatic expression of lipid oxidative genes as well as in the downregulation of lipogenic genes (such as Cd36 and Scd1) after RYGB and VSG35. These changes are a probable contributor to the greater lipid utilization and rapid reduction in hepatic levels of triglycerides that occurs in RYGB versus sham-surgery rodents. At later time points, gene expression of hepatic target genes of Ppara is repressed. In male and female VSG-treated rats, these transcriptomic changes are accompanied by shifts in systemic lipid trafficking, including reductions in postprandial chylomicron production; however, differences are observed in hepatic VLDL production in female (but not male) rats after VSG38. Similar patterns in intestinal chylomicron and VLDL production are observed after RYGB in humans39. Changes in hepatic lipid metabolism were greater in individuals who underwent bariatric surgery than in weight loss-matched control individuals, again suggesting weight loss-independent mechanisms.
Hepatic metabolism is also influenced by changes in metabolites that are absorbed or produced by the intestine. One study examined the portal vein metabolome after RYGB in lean rats to gain an understanding of the intestinally derived signals reaching the liver and nerves that innervate the portal vein29. RYGB-treated rats demonstrated alterations in intestinal lipid metabolism and reduced levels of the endocannabinoid N-oleoylethanolamide (OEA) in the portal vein. These patterns could also be linked to post-RYGB reductions in hepatic expression of PPARα (a downstream target of OEA), which were observed after RYGB in mice25. Of note, these data, obtained from a lean mouse RYGB model, differ from data in a HFD-fed rat RYGB model40 and a HFD-fed mouse VSG model41, in which intestinal OEA content was increased after surgery. However, in HFD-fed mice, neither OEA signalling within the intestine (via G-coupled protein receptor 119) nor within the liver (via PPARα or CD36) was necessary for the effect of VSG on weight loss and/or glucose homeostasis41.
A total of 80% of patients who undergo bariatric surgery are women, yet most preclinical studies favour male animals. Both human and animal data demonstrate a clear biological effect of sex on lipid metabolism42. Female rats and mice fed a HFD are more resistant than males to surgery-induced reductions in hepatic levels of triglycerides despite similar weight loss38,43. In addition, female rodents generally have lower HFD-induced increases in hepatic levels of triglycerides compared with males, suggesting a potential floor effect. However, when female mice are switched to a chow diet after VSG, they then have a substantial reduction in hepatic levels of triglycerides43. Thus, females are more sensitive than males to the effect of dietary modifications, as compared with that of surgery, on hepatic triglycerides. The mechanism for these sex differences is unknown, but male versus female mice, regardless of diet, have notable differences in the hepatic expression of key genes and microRNAs (for example, miR-103, miR-107, miR-802 and let-7e, all well-established markers of obesity) as well as in hepatic lipid accumulation and energy homeostasis38,43-47. In male mice fed a HFD, these microRNAs were ‘rescued’ by VSG (that is, their levels became similar to those in chow-fed animals) but neither diet nor surgery had any effect on the levels of these microRNAs in female mice43. Altogether, the data indicate a profound weight loss-independent effect of bariatric surgery on hepatic glucose and lipid metabolism in rodents, but also suggest important sex differences in how the liver responds to surgery.
Adipose and skeletal muscle insulin sensitivity.
Consistent with the anticipated effect of major weight loss to improve adipose tissue mass and function, analysis of subcutaneous adipose tissue gene expression in RYGB-treated mice revealed upregulation of genes of the tricarboxylic acid cycle, β-oxidation, electron transport chain and fatty acid synthesis as well as of Ucp1 and other brown and beige adipose tissue markers25. These changes were not observed in weight loss-matched control mice, providing further evidence for weight-independent effects even in adipose tissue. In contrast, this study also found that RYGB did not induce long-term transcriptional changes in skeletal muscle25. Another study that examined temporal changes in the metabolic function of adipose and skeletal muscle in humans after RYGB or VSG found no improvements in skeletal muscle insulin sensitivity and mitochondrial function 2 weeks postoperatively despite weight loss48. The authors hypothesized that persistent adipose tissue lipolysis and consequently increased plasma levels of free fatty acids prevented improvements in skeletal muscle insulin sensitivity.
In humans, both short-term and long-term studies find improvements in whole-body insulin sensitivity that are not correlated with a patient’s weight loss49,50. However, when compared to control groups matched for weight loss, bariatric surgery induces no additional improvements in muscle insulin sensitivity in either rats27 or humans7,51. The discrepancy as to whether skeletal muscle insulin sensitivity occurs in direct relation to weight loss or not is worthy of further study; however, this question is challenging to study given coexisting adipose tissue lipolysis during rapid weight loss as well as inflammation that occurs early in the postoperative period. Statistical integration of metabolomic and genomic data from muscle and adipose tissues is a promising avenue to further dissect the metabolic effects of bariatric surgery.
Insulin secretion
Higher circulating levels of glucagon are observed in the postprandial state in humans after bariatric surgery compared with before52-54, suggesting that pancreatic α-cell function is altered by bariatric surgery. However, the effects of bariatric surgery on β-cells have been more widely studied. Generally, fasting insulin and the total insulin output in response to intravenous glucose are reduced after bariatric surgery55, a pattern consistent with enhanced insulin sensitivity occurring in response to postoperative weight loss. Conversely, the dynamic response of insulin to nutrient ingestion considerably differs after surgery and is distinct from that observed after non-surgical weight loss. After both RYGB and VSG, rapid increases in postprandial blood levels of glucose are paralleled by rapid surges in insulin secretion that quickly return to baseline27,55. These patterns are attributed to the increased rate of nutrient entry and absorption by the intestine.
Besides its effects on nutrient absorption, bariatric surgery directly impacts β-cell function. Islets isolated from obese mice after VSG showed improved glucose-stimulated insulin secretion, enhanced calcium responses and improved intra-islet connectivity compared with sham-operated control mice, meaning that individual β-cells are better coordinated to release insulin after surgery56. Single-cell molecular profiling of mouse islets 2 weeks after VSG in non-diabetic obese mice demonstrated distinct changes in β-cell redifferentiation and proliferation, an effect that was independent of weight loss57. These latter data are translationally important as loss of functional β-cells with T2DM has been linked to dedifferentiation and reduced insulin secretion58. Even in diabetic animal models, RYGB and/or VSG reduced β-cell dedifferentiation (RYGB, diabetic Goto–Kakizaki rats)59, improved β-cell identity markers (VSG, young db/db mice60,61) and increased islet insulin content (RYGB, Zucker diabetic fatty rats62). However, RYGB did not reverse hyperglycaemia or increase β-cell activity in older diabetic mice or mice with chemical ablation of β-cells63. This finding echoes clinical data that demonstrates improved rates of T2DM remission in patients with shorter versus longer duration of preoperative disease8.
Another major contributor to increased postprandial plasma levels of insulin after surgery is the marked increase in plasma levels of incretin hormones, that is, gut peptides which stimulate insulin secretion (see ref.64 for a review). The >10-fold increase in the incretin hormone glucagon-like peptide 1 (GLP1) after bariatric surgery has received the greatest attention. In fact, a whole cocktail of gut peptides is increased postprandially; this effect could result from rapid nutrient entry into the intestine and/or changes in intestinal morphology or cell differentiation (discussed later). In sum, rapid nutrient entry has both direct and indirect effects on β-cell mass and function that are critical for surgery-induced improvements in T2DM.
Organ crosstalk in bariatric surgery
Beyond effects on insulin sensitivity and secretion, bariatric surgery leads to physiological changes across multiple organ systems that could also contribute to improvements in whole-body glucose metabolism. For example, the increases in plasma levels of bile acids and the changes in neural communication between the gut and central nervous system (CNS) are striking. We predict that the combination of these responses contributes to a more sustained and potent effect on glucose and lipid metabolism than caloric restriction alone. In this section, we review the crosstalk between organ systems after bariatric surgery, including both clinical and preclinical data.
Incretins
The secretion of a whole host of gut peptides is enhanced by bariatric surgery, with some surgery-specific differences between RYGB and VSG. After RYGB, ghrelin (produced in the stomach) as well as duodenally produced gastric inhibitory peptide (GIP) and cholecystokinin are not consistently changed in the plasma in humans and rodent models. Conversely, after VSG in humans, plasma levels of ghrelin11,65 are notably reduced and plasma levels of cholecystokinin and GIP are substantially increased66. Gut peptides traditionally recognized to be secreted by L cells in the distal gut, such as GLP1 and peptide YY (PYY), have greater postprandial increases in both rodents and humans after VSG and RYGB compared with controls12,51,67-69. Of these, GIP and GLP1 are critical glucoregulatory peptides; a 10-fold increase in prandial GLP1 secretion after surgery is widely believed to have a role in mediating the improvements in glucose homeostasis that occur very early in the postoperative period even before weight loss has occurred. However, as discussed later, data exist that do not support this hypothesis. With RYGB, oral nutrient delivery potently stimulates increases in plasma levels of GLP1 but this effect is not observed after direct nutrient delivery to the bypassed stomach70. However, work from our group found that rapid nutrient delivery to the distal gut, in itself, does not drive the increase in plasma levels of GLP1 after VSG in rats20.
Bile acids also contribute to postprandial increases in GLP1 occurring after bariatric surgery via several potential mechanisms. Luminal bile acids are known to acutely increase GLP1 secretion via a Takeda G protein receptor 5 (TGR5)-dependent mechanism71. Interestingly, the surgery-induced increase in GLP1 observed in wild-type mice was blunted in Tgr5-null mice in one72 but not in another study73. However, bile acids might also mediate surgical (RYGB and VSG) increases in GLP1 by increasing enteroendocrine cell numbers through intestinal tissue hypertrophy in rodents21 and/or increasing enteroendocrine cell differentiation in rodents21,26,74,75. Moreover, bile acid receptor expression (including farnesoid X receptor (Fxr) but not Tgr5) was increased in stem cells from VSG-treated mice. Furthermore, bile acids increased the number of GLP1-positive cells in a mouse intestinal organoid system, an effect that was blocked with an FXR antagonist26. Thus, we hypothesize that bile acids increase GLP1 by inducing increased numbers of L cells via an FXR-dependent pathway.
Whether increases in postprandial GLP1 secretion are necessary for improved glucose control after bariatric surgery remains debatable. Chronic impairment of GLP1 signalling using multiple genetic strategies in mice76-78 has no effect on surgical results. In fact, VSG was effective in normalizing glucose tolerance in Glp1r-knockout mice76. Thus, the 10-fold increase in plasma levels of GLP1 occurring after surgery might be viewed as a response to rapid nutrient entry into the intestine; however, given the mechanistic mouse data, this increase in GLP1 might not be critical for T2DM resolution.
Bile acids
Bile acids are a particularly interesting target for the success of bariatric surgery. Synthesized in the liver and secreted into the intestine, plasma levels of bile acids increase markedly in the postprandial state79 in both humans and rodents. Bile acids are recognized as key metabolic regulators of glucose and lipid metabolism, feeding behaviour, and neuronal activation80, in part via actions in the enterohepatic circulation. After bariatric surgery, plasma levels of total and specific species of bile acids are increased in humans81 and rodents73,82,83. In rats and mice, VSG has been shown to alter the enterohepatic circulation such that reabsorption from the intestine is increased. By contrast, hepatic bile acid transporters and genes that regulate hepatic bile acid synthesis are downregulated, with a net elevation in plasma levels of bile acids83. This increase enables bile acids to serve as signalling molecules at multiple target organs via FXR (highly expressed in the intestine, liver, adipose tissue, pancreas and adrenal gland)84 and TGR5 (expressed in the gall bladder, ileum, colon, adipose tissue, liver, skeletal muscle85 and immune cells)86.
Critical mouse work has supported the role of bile acid signalling as a mechanism for the success of bariatric surgery. For example, whole-body Fxr-knockout mice regain body mass and do not improve glucose tolerance after VSG82. Although this work established an important role for bile acids in surgical outcomes, it did not parse out whether FXR signalling in a specific tissue or downstream pathway was dominant. Activation of hepatic FXR leads to downstream activation of short heterodimer partner (SHP). In diet-induced obese mice after VSG, viral knockdown of SHP was necessary to improve hepatic inflammation; however, SHP was not necessary for changes in body weight, glucose homeostasis or hepatic levels of triglycerides35.
FXR activation in the intestine drives expression and secretion of fibroblast growth factor 19 (FGF19, FGF15 in rodents) into the circulation87, which activates FGF receptors in the liver, brain and adipose tissue to regulate metabolism. Plasma levels of FGF19 are increased in humans after VSG and RYGB88-90 and ileal expression of Fgf15 is increased in mice after VSG30. Whole-body Fgf15-knockout mice after VSG had increased liver accumulation of cholesterol, phosphatidylinositol, diacylglycerol and phosphatidylglycerol, and increased expression of genes reflecting exaggerated endoplasmic reticulum stress and inflammation compared with wild-type mice; however, Fgf15-knockout mice still lost weight after VSG83. Intestine-specific knockout of Fgf15 in mice led to a similar hepatic phenotype after VSG, with higher free cholesterol and no reduction in hepatic levels of triglycerides despite even greater weight loss compared with wild-type mice30. VSG also further increased plasma levels of bile acids in intestine-specific Fgf15-knockout mice versus wild-type control mice but glucose tolerance did not improve. Collectively, these data suggest that integration of both hepatic and intestinal FXR signalling pathways is necessary for the full benefits of VSG on hepatic lipid metabolism and potentially on body weight and glucose homeostasis.
Bile acids also activate TGR5, but the role of TGR5 activation in the success of surgery remains uncertain. One study found that whole-body Tgr5-knockout mice had reduced weight loss, no change in hepatic levels of triglycerides, and no improvements in glucose tolerance or hepatic insulin sensitivity after VSG but still had substantial elevations of plasma levels of bile acids72. By contrast, two other studies showed that TGR5 was not necessary for the benefits of either VSG73 or RYGB91. One possibility is that bile acids act through both FXR and TGR5 to mediate the metabolic success of surgery but that differences between studies reflect variation in the ability of the FXR system to compensate for the loss of TGR5.
In humans, fasting and postprandial plasma levels of both bile acids and FGF19 are increased progressively over time after RYGB or VSG92-94. One study identified an increase in a primary bile acid species, cholic acid-7-sulfate (CA7S), in the caecal content of both humans and mice who had undergone VSG94. Strikingly, CA7S activated TGR5 signalling in human HEK293T cultured cells, increased GLP1 secretion in vivo in mice and reduced plasma levels of glucose after gavage. Taken together, both mouse and human studies indicate profound and potentially mechanistic alterations in the bile acid signalling axis after bariatric surgery (Fig. 3).
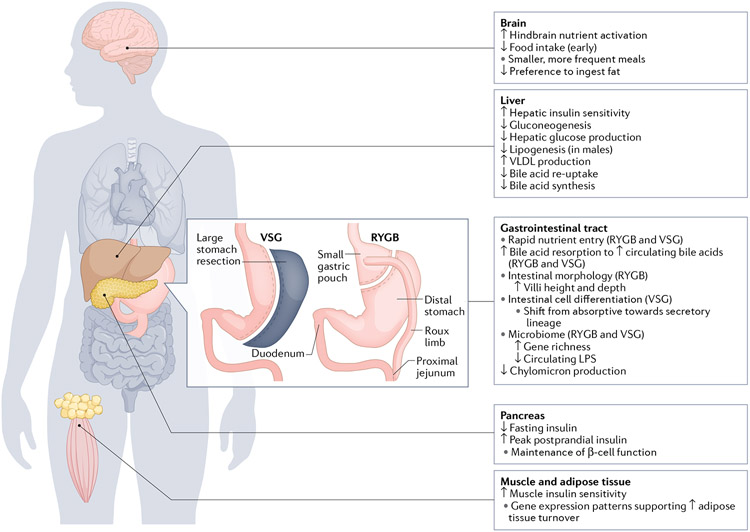
Fig. 3 ∣. Target organ responses to RYGB and VSG.
In the gastrointestinal tract, changes in nutrient entry and intestinal adaptations occur after bariatric surgery. Key changes in other target organs, including the brain, liver, pancreas, skeletal and adipose tissue, are summarized. LPS, lipopolysaccharide; RYGB, Roux-en-Y gastric bypass; VSG, vertical sleeve gastrectomy.
Other potential mediators of crosstalk
Amino acids.
Beyond bile acids, RYGB profoundly alters the circulating levels of other metabolites with the potential to serve as signalling molecules that regulate glucose homeostasis. For example, circulating branched-chain amino acids (that is, leucine, isoleucine and valine) are positively related to obesity and T2DM in both human and rodent studies (see ref.95 for a review). After RYGB in patients with T2DM, fasting plasma levels of both total amino acids and branched-chain amino acids and their metabolites are decreased; however, this same decrease is not observed in patients with matched weight loss96 or with medical management of T2DM92. Interestingly, these patterns in levels of amino acids correlated with changes in both insulin secretion and insulin sensitivity. Net absorption of ingested protein does not differ in humans after RYGB, but the postprandial absorption of amino acids is accelerated after RYGB similar to the postprandial time course for glucose54. Thus, the decrease in circulating amino acids is not due to increases in protein absorption from the diet. In rodents after RYGB, the expression of amino acid catabolic enzymes is increased when compared to weight loss-matched control rodents25. This finding suggests that decreased circulating amino acids might be due to increased catabolism rather than to decreased absorption. Given that weight loss-matched individuals do not see the same changes in circulating amino acids as do individuals who undergo surgery, perhaps surgery-induced changes in amino acids direct alterations in glucose homeostasis rather than in weight loss.
The microbiome.
Alterations in the gut microbiome are implicated in a spectrum of metabolic complications, including obesity and control of food intake, hyperglycaemia, and hypercholesterolaemia. The microbiome has metabolic, immunological and neural functions in the body and is also necessary for the conversion of primary to secondary bile acids97. Thus, it is not surprising that the profound changes occurring after bariatric surgery in intestinal anatomy and function, and the resultant shifts in dietary intake, weight loss, L cell secretory function, levels of bile acids and alterations in the gut–brain axis, occur in concert with surgery-induced changes in the composition and diversity of the intestinal microbiome in humans and rodents97,98. A shift from ‘bad’ to ‘good’ bacteria in the gut is suggested to contribute to weight loss, to changes in bile acid metabolism over time99 and to long-term rates of T2DM control100. In essence, surgery is thought to correct the dysbiosis associated with metabolic disease.
One study showed that gut microbial changes that occurred 1 year after RYGB in humans persisted for 9 years97. Although these studies are correlative, faecal microbial transplantation from patients who underwent bariatric surgery to obese mice lead to reductions in weight gain and improved metabolic phenotypes in recipient mice97,98,100. These types of studies are interesting as they support a mechanistic link between changes in the microbiome and surgical success. We are still at the cusp of trying to understand the role of these gut organisms in health, disease and surgical success and, more importantly, whether this information can be leveraged for the treatment of metabolic disease.
Growth hormone signalling.
To identify additional mechanisms for improved glucose homeostasis after RYGB, we analysed the plasma proteome and metabolome in fasted individuals with T2DM who had been randomized to RYGB or medical therapy92,101. High-throughput mediation analysis demonstrated that the top-ranking mediator of improved glycaemia after RYGB was the growth hormone receptor (GHR); postoperatively, reductions in plasma signals that represent GHR signalling at 3 months mediated the improvement in HbA1c at 1 year. Strikingly, the relationship between GHR signalling and improvements in HbA1c was stronger than the relationship between reductions in BMI and HbA1c. Given that GH signalling is diabetogenic in adults, these data suggest that RYGB induced the development of GH resistance.
Human data cannot ascertain the source of GHR regulation after RYGB, but similar reductions in GHR expression were observed in rodents from as early as 9 days after RYGB in multiple tissues, including the liver and intestine25. Moreover, experimental reduction of GHR in mouse primary hepatocytes reduced glucose production. Whether these effects are directly related to reductions in GHR signalling or to reductions in insulin-like growth factor 1 (IGF1)-independent GH signalling and the subsequent increases in plasma levels of IGF-binding protein 1 (IGFBP1) and IGFBP2 remain unclear. Interestingly, a 2021 study demonstrated that IGFBP2 is required for the full effect of RYGB on weight loss and early improvements in insulin action in mice102. While future studies are required to expand on these findings, GH signalling represents an underappreciated pathway in metabolic changes occurring after bariatric surgery.
The gut–brain axis
The gut–brain axis refers to the tight coupling between neural, endocrine and/or nutrient signals that originate from the gut and signal the CNS to modify feeding behaviour and metabolism103. Given the profound effect of bariatric surgery on body mass and metabolism in both humans and rodents, it is logical to hypothesize the gut–brain axis as a major driver but the precise signals are unknown. The gut–brain axis probably contributes primarily to body weight regulation after bariatric surgery as reviewed previously104,105. Whether this axis also regulates glucose homeostasis is understudied and worthy of future work.
Interestingly, all of the factors discussed in this section (incretins, bile acids, amino acids, the microbiome and GH signalling) could act via both peripheral and CNS signalling. For example, GLP1 is produced in the gut but also in a discrete set of neurons within the hindbrain; GLP1 action in the brain has demonstrated effects on reducing food intake and consequently decreasing body weight in rats, mice and humans (see ref.106 for a review). Bile acids, certain amino acids, like leucine, and GHR have all been found to have receptors or signalling mechanisms in the CNS and to regulate either feeding behaviour or glucose control. Likewise, gut–brain–pancreas neural connections have also been mapped107 and could have a role in mediating the pancreatic changes that occur in response to nutrient ingestion after surgery.
Vagotomy studies have been used to determine the necessity of vagal innervation in bariatric surgery outcomes. These studies have found that portal vein vagal innervation is not necessary for weight loss in rats108. Furthermore, individuals with obesity that underwent RYGB with vagotomy have similar weight loss as those without vagotomy109. Although subdiaphragmatic vagotomy in rodents did not prevent weight loss after RYGB, it did prevent surgery-induced shifts in macronutrient preference40. Specific ligation of the coeliac vagal branches, which innervate the intestine, blunts RYBG-induced weight loss and hypophagia in rodents110. These latter two studies suggest that bariatric surgery acts to induce weight loss via targeting specific subpopulations of vagal neurons. The application of new genetic tools to study subpopulations of neurons within the vagus might help to decipher the contribution of the vagus to the metabolic success of bariatric surgery.
Hypoglycaemia after bariatric surgery
Unfortunately, the benefits of bariatric surgery on glucose metabolism come with an increased risk for hypoglycaemia, termed post-bariatric hypoglycaemia (PBH) (reviewed in ref.111). PBH occurs in a subset of individuals and represents extreme glucose reduction after surgery. Although recognized most commonly after RYGB, hypoglycaemia also occurs after VSG112-114, duodenal switch115, gastrectomy or oesophagectomy116, and Nissen fundoplication117 but only rarely after gastric banding118 or BPD119.
Characteristics and epidemiology
Symptoms of PBH reflect sympathetic and parasympathetic activation in response to hypoglycaemia such as palpitations, lightheadedness and sweating; these symptoms overlap with those of post-surgical dumping syndrome or other disorders, sometimes making diagnosis challenging. Severe hypoglycaemia is associated with neuroglycopenia, with decreased cognition, confusion, somnolence, falls, seizures and loss of consciousness. More broadly, severe hypoglycaemia notably impacts quality of life, threatens safety and can be disabling. With recurrent hypoglycaemia, counterregulatory responses are reduced, resulting in hypoglycaemia unawareness and neuroglycopenia as the first manifestation.
The prevalence of hypoglycaemia in individuals who have had bariatric surgery varies by methods of ascertainment and definition but ranges from <1% for severe hypoglycaemia requiring hospitalization (rare)120 to 10–30% with self-reported symptoms121,122. Without documentation of low blood levels of glucose or neuroglycopenia, determining whether symptoms are indeed related to hypoglycaemia, dumping syndrome or other disorders with overlapping symptoms is difficult. Continuous glucose monitoring (CGM) can overestimate the frequency of low blood levels of glucose; however, one study showed that 75% of asymptomatic patients post-RYGB (~7 years post-surgery) had sensor glucose below 55 mg/dl, whereas non-surgical control individuals remained above that threshold123.
Two distinct features of PBH offer clues to its pathophysiology. First, hypoglycaemia typically occurs 1–3 h after meals, which suggests an important role in postprandial nutrient transit and hormonal responses. In patients who underwent RYGB, the postprandial blood concentration of glucose spikes to high levels within 30 min, with spikes of lesser magnitude in patients who underwent VSG. These high blood levels of glucose, together with even higher levels of GLP1, stimulate excessive insulin secretion with subsequent rapid drops in glucose by 1–3 h after meals124,125. Additional contributors to high postprandial levels of insulin levels include reduced insulin clearance and increased β-cell sensitivity to glucose112,124,126. Excessive glycaemic variability (both high and low glucose) is observed during CGM127 (Fig. 4). Some patients also develop hypoglycaemia during physical activity, suggesting additional insulin-independent mechanisms.
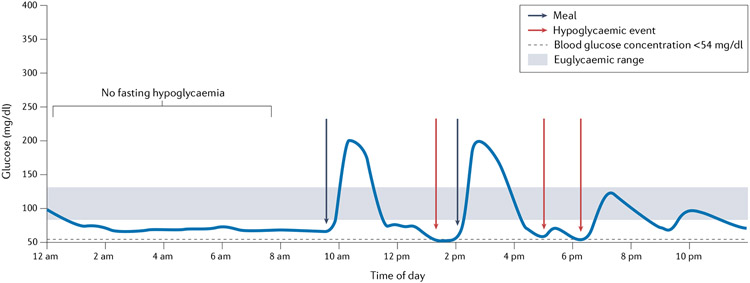
Fig. 4 ∣. An example of a daily pattern of glucose in a patient with post-bariatric hypoglycaemia.
This figure represents a typical pattern of plasma levels of glucose throughout the day in a patient with post-bariatric hypoglycaemia. Nocturnal hypoglycaemia is rarely observed. After a meal, sharp increases occur in plasma levels of glucose, followed by rapid drops that often lead to hypoglycaemia.
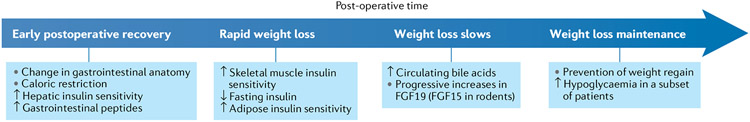
Second, PBH typically manifests clinically >1 year postoperatively, which suggests that remodelling of intestinal structure and function and changes in hormonal secretion after surgery are required to yield the full syndrome. Potential factors that parallel this pattern include the progressive increases in plasma levels of FGF19 seen over time in patients after bariatric surgery. These changes are exaggerated in patients with PBH, who have threefold higher plasma levels of FGF19 (ref.90) and increased postprandial bile acids93 as compared with patients after RYGB without hypoglycaemia. Thus, changes in intestinal lumen contents and intestinal adaptations postoperatively might be exaggerated in individuals with PBH and contribute to excessive secretion of both GLP1 and FGF19 (Fig. 5).
Fig. 5 ∣. Timeline of physiological changes occurring after bariatric surgery.
Shown are key changes occurring during the early postoperative recovery, during the phase of rapid weight loss, during the phase where weight loss slows and during the weight loss maintenance phase. FGF19, fibroblast growth factor 19.
Risk factors for PBH include female sex, absence of preoperative diabetes mellitus, large postoperative weight loss, use of selective serotonin reuptake inhibitor or serotonin and noradrenaline reuptake inhibitor antidepressants, RYGB (compared with VSG), prior cholecystectomy128, and a history of symptoms potentially related to hypoglycaemia prior to surgery122,129,130. We do not fully understand the mechanisms responsible for these relationships. However, these associations again point to postprandial metabolic shifts and the potential contribution of genetic variation to risk for PBH.
Identifying and targeting mechanisms
Like humans131, a high degree of glucose variability is observed in rodents after bariatric surgery132, and hypoglycaemia has been noted after both RYGB133 and VSG132. Other parallels between rodents and humans are the increased rate of nutrient entry and high peak postprandial plasma levels of glucose, GLP1 and insulin, all of which are factors that have been implicated in PBH. These parallels offer an opportunity to explore the mechanisms of PBH and to identify new potential therapeutic targets.
GLP1 and insulin secretion-dependent mechanisms.
Given that postprandial peaks in insulin are thought to be a dominant mechanism contributing to PBH, reducing postprandial insulin secretion has been a key therapeutic target. Initial strategies focused on reducing peak postprandial glucose and subsequent insulin secretion by reducing the intake of rapidly absorbed carbohydrates. Controlled portions of complex carbohydrates as well as adequate protein and healthy fat intake are also recommended. If this strategy is not sufficient to control PBH, α-glucosidase inhibitors (for example, acarbose) to slow down or sodium–glucose co-transporter 1 (SGLT1) or SGLT2 inhibitors to reduce glucose absorption and glucose-induced inflammatory responses134 have been used. Diazoxide or somatostatin analogues might additionally be required to reduce insulin secretion. Initial efforts to reduce β-cell mass via partial pancreatectomy resulted in PBH recurrence in a substantial fraction of patients135, suggesting that islet function rather than mass drives surgery-induced insulin secretion136.
Therapies under development have focused on reducing GLP1 signalling to reduce postprandial insulin. Multiple studies demonstrate that the GLP1 receptor antagonist exendin 9–39 reduces peak postprandial circulating levels of insulin and raises the glucose nadir126,137. We have seen similar effects of GLP1 receptor antagonists in VSG-treated rats, with increases in the glucose nadir132. However, exendin 9–39 also increases the glucose nadir in sham-surgery control rats. Thus, whether GLP1 signalling is an actual mechanism that underlies postprandial hypoglycaemia is unclear as is whether it is a therapeutic target that improves but does not directly address the primary cause of PBH.
Insulin secretion-independent mechanisms.
Several insulin secretion-independent mechanisms might also contribute to PBH. The robust weight loss occurring after both RYGB and VSG increases insulin sensitivity, as predicted, and might be expected to increase hypoglycaemia. Indeed, in one study, increased weight loss at 1 year was associated with an increased incidence of hypoglycaemia112; however, insulin sensitivity was not an independent risk factor in the stepwise regression analysis112. By contrast, one cross-sectional study using intravenous glucose tolerance testing demonstrated that glucose effectiveness (that is, insulin-independent glucose uptake), and not systemic insulin sensitivity, distinguished individuals with PBH from those who were unaffected post-surgery138. Additional studies will be required to address these possibilities.
Bariatric surgery also reduces counterregulatory hormonal responses to hypoglycaemia in both humans and animals. In rodents, administration of 2-deoxyglucose (a glucoprivic agent that inhibits the first step in glycolysis and prevents the CNS from utilizing glucose) leads to increases in feeding and circulating levels of counterregulatory hormones. The feeding response to 2-deoxyglucose estimates counterregulatory function and is blunted in VSG compared with sham-surgery control rats132. Although this finding will have to be validated using a direct assessment of counterregulatory hormones, these data suggest that VSG animals have a blunted autonomic response to glucoprivation132.
Likewise, bariatric surgery in humans is associated with reduced secretion of glucagon, cortisol and catecholamines during hyperinsulinaemic–hypoglycaemic clamps as early as 6 months postoperatively115. Furthermore, similar reductions in counterregulatory hormones occur after meals in patients with PBH139. Thus, altered counterregulatory hormone levels and/or action, especially in the postprandial period53, are possible central and early contributors to PBH.
The mechanisms responsible for altered counterregulation are unknown. Reduced counterregulation could be linked to recurrent but unrecognized hypoglycaemia, changes in glucose sensing, or hormonal and metabolite signalling in the CNS. Further studies in animal models with experimental ablation of candidate pathways will be required to dissect these possibilities. When hypoglycaemia is predicted in patients with severe PBH, the therapeutic closed-loop delivery of glucagon is effective to prevent severe hypoglycaemia140. Likewise, the use of CGM to predict or detect hypoglycaemia, especially in patients with hypoglycaemia unawareness, permits treatment before hypoglycaemia becomes life-threatening. These approaches are viewed as ‘supplementary’ approaches to augment defective counterregulation in patients with severe PBH.
Several other factors should be considered when evaluating PBH, including potential undernutrition, hepatic, renal or adrenal disease, the use of medications associated with hypoglycaemia (such as selective serotonin reuptake inhibitors, serotonin and noradrenaline reuptake inhibitors, and tramadol)122,141, and the presence of an insulin-producing tumour142.
Given that surgery-induced intestinal rearrangement and altered nutrient delivery are ultimately responsible for hypoglycaemia, some patients with severe PBH require more extreme approaches to therapy, including a feeding tube143 or even reversal of surgery (when feasible)144. Unfortunately, even these approaches are not uniformly successful in preventing hypoglycaemia, implicating a role for critical vascular, neuronal or intestinally mediated adaptations that are not fully blocked by surgery reversal (Fig. 6).
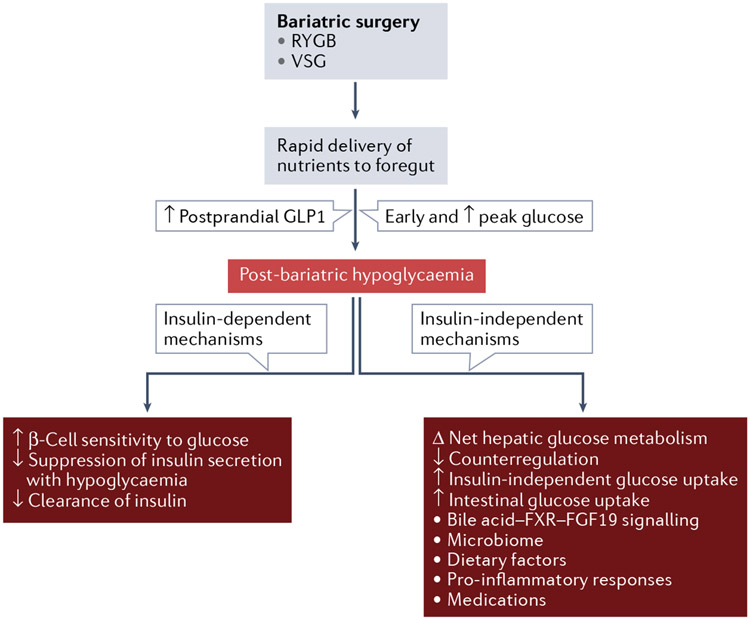
Fig. 6 ∣. Changes in glucose regulation after bariatric surgery that hwave been linked to post-bariatric hypoglycaemia.
After bariatric surgery (Roux-en-Y gastric bypass (RYGB) or vertical sleeve gastrectomy (VSG)), nutrients are delivered rapidly to the foregut after a meal, which leads to an early and increased peak in blood levels of glucose and increased postprandial levels of glucagon-like peptide 1 (GLP1). Post-bariatric hypoglycaemia is thought to be caused by insulin-dependent mechanisms (increased insulin levels) or insulin-independent mechanisms (decreased counterregulatory hormones and changes in glucose production and uptake). FGF19, fibroblast growth factor 19; FXR, farnesoid X receptor.
Conclusions
In this Review, we discuss the multiple target organ systems that respond to surgery and the potential mechanisms for improvements or changes in glucose control after bariatric surgery. A full understanding of the diverse mechanisms mediating these effects could uncover key biological pathways and identify novel non-surgical approaches in the treatment of T2DM. From a clinical perspective, many key unanswered questions remain. For example, which patients are most likely to reap durable benefits of bariatric surgery as compared with medical therapy? Can we identify those predisposed to severe hypoglycaemia prior to consideration of bariatric surgery? Finally, can we identify novel approaches to reduce severe hypoglycaemia in PBH?
Key points.
Roux-en-Y gastric bypass and vertical sleeve gastrectomy are the two most widely used forms of bariatric surgery; both induce considerable weight loss and can induce remission of type 2 diabetes mellitus (T2DM) in some patients.
Bariatric surgery has important weight loss-dependent and weight loss-independent mechanisms for the induction of resolution of T2DM.
Bariatric surgery affects the glucoregulatory function of multiple target organs, including the intestine, liver, pancreas, adipose tissue and skeletal muscle.
In a subset of patients, post-bariatric hypoglycaemia can develop months to years after surgery, thereby representing a potential extreme example of altered glucose metabolism.
Footnotes
Competing interests
M.E.P. reports personal consulting fees from Astra Zeneca, Fractyl, Hanmi Pharmaceutical, MBX Biosciences, Recordati, Poxel, Eiger Pharmaceuticals, and Xeris and grants from Chan-Zuckerberg Initiative, Dexcom and Helmsley Trust, outside the submitted work. D.A.S. reports consulting fees from Metis Therapeutics, outside the submitted work.
References
- 1. Schauer PR et al. Bariatric surgery versus intensive medical therapy for diabetes — 5-year outcomes. N. Engl. J. Med 376, 641–651 (2017). This 5-year follow-up to the STAMPEDE clinical trial randomized patients with T2DM to RYGB, VSG or medical management, and showed that RYGB and VSG were superior to medical therapy in terms of weight loss, glycaemic control and reduction in medication use.
- 2.Kirwan JP et al. Diabetes remission in the alliance of randomized trials of medicine versus metabolic surgery in type 2 diabetes (ARMMS-T2D). Diabetes Care 45, 1574–1583 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mingrone G. et al. Metabolic surgery versus conventional medical therapy in patients with type 2 diabetes: 10-year follow-up of an open-label, single-centre, randomised controlled trial. Lancet 397, 293–304 (2021). [DOI] [PubMed] [Google Scholar]
- 4.Pories WJ et al. Is type II diabetes mellitus (NIDDM) a surgical disease? Ann. Surg 215, 633–642 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Riddle MC et al. Consensus report: definition and interpretation of remission in type 2 diabetes. J. Clin. Endocrinol. Metab 44, 2438–2444 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carlsson LM et al. Bariatric surgery and prevention of type 2 diabetes in Swedish obese subjects. N. Engl. J. Med 367, 695–704 (2012). [DOI] [PubMed] [Google Scholar]
- 7. Yoshino M. et al. Effects of diet versus gastric bypass on metabolic function in diabetes. N. Engl. J. Med 383, 721–753 (2020). This study examined key metabolic phenotypes related to glucose metabolism and insulin sensitivity in participants undergoing matched weight loss via surgery or dietary restriction, showing similar metabolic and physiological responses.
- 8.Dang JT et al. Predictive factors for diabetes remission after bariatric surgery. Can. J. Surg 62, 315–319 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sjöholm K, Sjöström E, Carlsson LMS & Peltonen M Weight change-adjusted effects of gastric bypass surgery on glucose metabolism: two- and 10-year results from the Swedish obese subjects (SOS) study. Diabetes Care 39, 625–631 (2016). [DOI] [PubMed] [Google Scholar]
- 10.Mcglone E. et al. Bariatric surgery for patients with type 2 diabetes mellitus requiring insulin: clinical outcome and cost-effectiveness analyses. PLoS Med. 17, 5 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nosso G. et al. Comparative effects of Roux-en-Y gastric bypass and sleeve gastrectomy on glucose homeostasis and incretin hormones in obese type 2 diabetic patients: a one-year prospective study. Horm. Metab. Res 48, 312–317 (2016). [DOI] [PubMed] [Google Scholar]
- 12.Nannipieri M. et al. Roux-en-Y gastric bypass and sleeve gastrectomy: mechanisms of diabetes remission and role of gut hormones. J. Clin. Endocrinol. Metab 98, 4391–4399 (2013). [DOI] [PubMed] [Google Scholar]
- 13.Keidar A. et al. Roux-en-Y gastric bypass vs sleeve gastrectomy for obese patients with type 2 diabetes: a randomised trial. Diabetologia 56, 1914–1918 (2013). [DOI] [PubMed] [Google Scholar]
- 14.Lee W-J et al. Gastric bypass vs sleeve gastrectomy for type 2 diabetes mellitus: a randomized controlled trial. Arch. Surg 146, 143–148 (2011). [DOI] [PubMed] [Google Scholar]
- 15.Blackstone R, Bunt J, Celaya Cortes M & Sugerman H Type 2 diabetes after gastric bypass: remission in five models using HbA1c, fasting blood glucose, and medication status. Surg. Obes. Relat. Dis 8, 548–555 (2012). [DOI] [PubMed] [Google Scholar]
- 16.Zechner JF et al. Weight-independent effects of Roux-en-Y gastric bypass on glucose homeostasis via melanocortin-4 receptors in mice and humans. Gastroenterology 144, 580–590.e7 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pontiroli AE, Gniuli D & Mingrone G Early effects of gastric banding (LGB) and of biliopancreatic diversion (BPD) on insulin sensitivity and on glucose and insulin response after OGTT. Obes. Surg 20, 474–479 (2010). [DOI] [PubMed] [Google Scholar]
- 18.Petrov MS & Taylor R Intra-pancreatic fat deposition: bringing hidden fat to the fore. Nat. Rev. Gastroenterol. Hepatol 19, 153–168 (2022). [DOI] [PubMed] [Google Scholar]
- 19.Steven S. et al. Very low-calorie diet and 6 months of weight stability in type 2 diabetes: pathophysiological changes in responders and nonresponders. Diabetes Care 39, 808–815 (2016). [DOI] [PubMed] [Google Scholar]
- 20.Chambers AP et al. Regulation of gastric emptying rate and its role in nutrient-induced GLP-1 secretion in rats after vertical sleeve gastrectomy. AJP Endocrinol. Metab 306, E424–E432 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cavin JB et al. Differences in alimentary glucose absorption and intestinal disposal of blood glucose after Roux-en-Y gastric bypass vs sleeve gastrectomy. Gastroenterology 150, 454–464.e9 (2016). [DOI] [PubMed] [Google Scholar]
- 22.Saeidi N. et al. Reprogramming of intestinal glucose metabolism and glycemic control in rats after gastric bypass. Science 341, 406–410 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ku CR et al. Intestinal glycolysis visualized by FDG PET/CT correlates with glucose decrement after gastrectomy. Diabetes 66, 385–391 (2017). [DOI] [PubMed] [Google Scholar]
- 24.Franquet E. et al. PET-CT reveals increased intestinal glucose uptake after gastric surgery. Surg. Obes. Relat. Dis 15, 643–649 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ben-Zvi D. et al. Time-dependent molecular responses differ between gastric bypass and dieting but are conserved across species. Cell Metab. 28, 310–323.e6 (2018). This study examined the molecular changes in multiple tissues after RYGB in mice and humans, identifying key molecular responses.
- 26. Kim K-S et al. Vertical sleeve gastrectomy induces enteroendocrine cell differentiation of intestinal stem cells through bile acid signaling. JCI Insight 1, e154302 (2022). This paper finds bile acid-driven increases in enteroendocrine cell differentiation in a mouse model of VSG.
- 27.Chambers AP et al. Weight-independent changes in blood glucose homeostasis after gastric bypass or vertical sleeve gastrectomy in rats. Gastroenterology 141, 950–958 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yan Y. et al. Roux-en-Y gastric bypass surgery suppresses hepatic gluconeogenesis and increases intestinal gluconeogenesis in a T2DM rat model. Obes. Surg 26, 2683–2690 (2016). [DOI] [PubMed] [Google Scholar]
- 29. Stefater MA et al. Portal venous metabolite profiling after RYGB in male rats highlights changes in gut-liver axis. J. Endocrinol 4, bvaa003 (2020). This paper compared the metabolomic profile of metabolites in the portal vein after RYGB in rats to sham surgery control rats.
- 30.Bozadjieva-Kramer N. et al. Intestinal-derived FGF15 protects against deleterious effects of vertical sleeve gastrectomy in mice. Nat. Commun 12, 4768 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rabl C & Campos GM The impact of bariatric surgery on nonalcoholic steatohepatitis. Semin. Liver Dis 32, 80–91 (2012). [DOI] [PubMed] [Google Scholar]
- 32.Whang E. et al. Vertical sleeve gastrectomy attenuates the progression of non-alcoholic steatohepatitis in mice on a high-fat high-cholesterol diet. Obes. Surg 29, 2420–2429 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Verbeek J. et al. Roux-en-y gastric bypass attenuates hepatic mitochondrial dysfunction in mice with non-alcoholic steatohepatitis. Gut 64, 673–683 (2015). [DOI] [PubMed] [Google Scholar]
- 34.Romero-Gómez M, Zelber-Sagi S & Trenell M Treatment of NAFLD with diet, physical activity and exercise. J. Hepatol 67, 829–846 (2017). [DOI] [PubMed] [Google Scholar]
- 35.Myronovych A. et al. The role of small heterodimer partner in nonalcoholic fatty liver disease improvement after sleeve gastrectomy in mice. Obesity 22, 2301–2311 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ben-Haroush Schyr R. et al. Sleeve gastrectomy suppresses hepatic glucose production and increases hepatic insulin clearance independent of weight loss. Diabetes 70, 2289–2298 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mazzini GS et al. Gastric bypass increases circulating bile acids and activates hepatic farnesoid X receptor (FXR) but requires intact peroxisome proliferator activator receptor alpha (PPARα) signaling to significantly reduce liver fat content. J. Gastrointest. Surg 25, 871–879 (2021). [DOI] [PubMed] [Google Scholar]
- 38.Grayson BE et al. Bariatric surgery emphasizes biological sex differences in rodent hepatic lipid handling. Biol. Sex Differ 8, 4 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Klein S. et al. Gastric bypass surgery improves metabolic and hepatic abnormalities associated with nonalcoholic fatty liver disease. Gastroenterology 130, 1564–1572 (2006). [DOI] [PubMed] [Google Scholar]
- 40.Hankir MK et al. Gastric bypass surgery recruits a gut PPAR-α-striatal D1R pathway to reduce fat appetite in obese rats. Cell Metab. 25, 335–344 (2017). [DOI] [PubMed] [Google Scholar]
- 41.Hutch CR et al. Oea signaling pathways and the metabolic benefits of vertical sleeve gastrectomy. Ann. Surg 271, 509–518 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Karthickeyan CK, Mehrabian M & Lusis AJ Sex differences in metabolism and cardiometabolic disorders. Curr. Opin. Lipidol 29, 404–410 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hutch CR et al. Diet-dependent sex differences in the response to vertical sleeve gastrectomy. Am. J. Physiol. Endocrinol. Metab 321, E11–E23 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bhatia H, Pattnaik BR & Datta M Inhibition of mitochondrial β-oxidation by miR-107 promotes hepatic lipid accumulation and impairs glucose tolerance in vivo. Int. J. Obes 40, 861–869 (2016). [DOI] [PubMed] [Google Scholar]
- 45.Bhatia H, Verma G & Datta M MiR-107 orchestrates ER stress induction and lipid accumulation by post-transcriptional regulation of fatty acid synthase in hepatocytes. Biochim. Biophys. Acta Gene Regul. Mech 1839, 334–343 (2014). [DOI] [PubMed] [Google Scholar]
- 46.Kornfeld JW et al. Obesity-induced overexpression of miR-802 impairs glucose metabolism through silencing of Hnf1b. Nature 494, 111–115 (2013). [DOI] [PubMed] [Google Scholar]
- 47.Trajkovski M. et al. MicroRNAs 103 and 107 regulate insulin sensitivity. Nature 474, 649–653 (2011). [DOI] [PubMed] [Google Scholar]
- 48.Gancheva S. et al. Dynamic changes of muscle insulin sensitivity after metabolic surgery. Nat. Commun 10, 4179 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Angelini G. et al. Small intestinal metabolism is central to whole-body insulin resistance. Gut 70, 1098–1109 (1098). [DOI] [PubMed] [Google Scholar]
- 50.Salinari S. et al. Insulin sensitivity and secretion changes after gastric bypass in normotolerant and diabetic obese subjects. Ann. Surg 257, 462–468 (2013). [DOI] [PubMed] [Google Scholar]
- 51.Dirksen C. et al. Exaggerated release and preserved insulinotropic action of glucagon-like peptide-1 underlie insulin hypersecretion in glucose-tolerant individuals after Roux-en-Y gastric bypass. Diabetologia 56, 2679–2687 (2013). [DOI] [PubMed] [Google Scholar]
- 52.Garibay D. et al. β-Cell glucagon-like peptide-1 receptor contributes to improved glucose tolerance after vertical sleeve gastrectomy. Endocrinology 157, 3405–3409 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Salehi M, Gastaldelli A & Defronzo R Prandial hepatic glucose production during hypoglycemia is altered after gastric bypass surgery and sleeve gastrectomy. Metabolism 131, 155199 (2022). [DOI] [PubMed] [Google Scholar]
- 54.Svane MS et al. Postprandial nutrient handling and gastrointestinal hormone secretion after Roux-en-Y Gastric bypass vs sleeve gastrectomy. Gastroenterology 156, 1627–1641.e1 (2019). [DOI] [PubMed] [Google Scholar]
- 55.Ferrannini E & Mingrone G Impact of different bariatric surgical procedures on insulin action and β-cell function in type 2 diabetes. Diabetes Care 32, 514–520 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Akalestou E. et al. Intravital imaging of islet Ca2+ dynamics reveals enhanced β cell connectivity after bariatric surgery in mice. Nat. Commun 12, 5165 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Oppenländer L. et al. Vertical sleeve gastrectomy triggers fast β-cell recovery upon overt diabetes. Mol. Metab 54, 101330 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Talchai C, Xuan S, Lin HV, Sussel L & Accili D Pancreatic β cell dedifferentiation as a mechanism of diabetic β cell failure. Cell 150, 1223–1234 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Qian B. et al. Reduction of pancreatic β-cell dedifferentiation after gastric bypass surgery in diabetic rats. J. Mol. Cell Biol 6, 531–534 (2014). [DOI] [PubMed] [Google Scholar]
- 60.Abu-Gazala S. et al. Sleeve gastrectomy improves glycemia independent of weight loss by restoring hepatic insulin sensitivity. Diabetes 67, 1079–1085 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li F. et al. Preventative sleeve gastrectomy contributes to maintaining β cell function in db/db diabetic mouse. Obes. Surg 26, 2402–2410 (2016). [DOI] [PubMed] [Google Scholar]
- 62.Mosinski JD et al. Roux-en-Y gastric bypass restores islet function and morphology independent of body weight in ZDF rats. Am. J. Physiol. Endocrinol. Metab 320, E392–E398 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhu C, Xu R, Li Y, Andrade M & Yin DP Gastric bypass prevents diabetes in genetically modified mice and chemically induced diabetic mice. PLoS One 16, e0258942 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim K-S & Sandoval DA Endocrine function after bariatric surgery. Compr. Physiol 7, 783–798 (2017). [DOI] [PubMed] [Google Scholar]
- 65.Casajoana A. et al. Predictive value of gut peptides in T2D remission: randomized controlled trial comparing metabolic gastric bypass, sleeve gastrectomy and greater curvature plication. Obes. Surg 27, 2235–2245 (2017). [DOI] [PubMed] [Google Scholar]
- 66.Jacobsen SH et al. Changes in gastrointestinal hormone responses, insulin sensitivity, and beta-cell function within 2 weeks after gastric bypass in non-diabetic subjects. Obes. Surg 22, 1084–1096 (2012). [DOI] [PubMed] [Google Scholar]
- 67.Korner J. et al. Differential effects of gastric bypass and banding on circulating gut hormone and leptin levels. Obesity 14, 1553–1561 (2006). [DOI] [PubMed] [Google Scholar]
- 68.Shin AC, Zheng H, Townsend RL, Sigalet DL & Berthoud H-RR Meal-induced hormone responses in a rat model of Roux-en-Y gastric bypass surgery. Endocrinology 151, 1588–1597 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yousseif A. et al. Differential effects of laparoscopic sleeve gastrectomy and laparoscopic gastric bypass on appetite, circulating acyl-ghrelin, peptide YY3-36 and active GLP-1 levels in non-diabetic humans. Obes. Surg 24, 241–252 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McLaughlin T, Peck M, Holst J & Deacon C Reversible hyperinsulinemic hypoglycemia after gastric bypass: a consequence of altered nutrient delivery. J. Clin. Endocrinol. Metab 95, 1851–1855 (2010). [DOI] [PubMed] [Google Scholar]
- 71.Thomas C et al. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 10, 167–177 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ding L. et al. Vertical sleeve gastrectomy activates GPBAR-1/TGR5 to sustain weight loss, improve fatty liver, and remit insulin resistance in mice. Hepatology 64, 760–773 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McGavigan AK et al. TGR5 contributes to glucoregulatory improvements after vertical sleeve gastrectomy in mice. Gut 66, 226–234 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nausheen S, Shah IH, Pezeshki A, Sigalet DL & Chelikani PK Effects of sleeve gastrectomy and ileal transposition, alone and in combination, on food intake, body weight, gut hormones, and glucose metabolism in rats. AJP Endocrinol. Metab 305, E507–E518 (2013). [DOI] [PubMed] [Google Scholar]
- 75.Li F, Peng Y, Zhang M, Yang P & Qu S Sleeve gastrectomy activates the GLP-1 pathway in pancreatic β cells and promotes GLP-1-expressing cells differentiation in the intestinal tract. Mol. Cell. Endocrinol 436, 33–40 (2016). [DOI] [PubMed] [Google Scholar]
- 76.Wilson-Pérez HE et al. Vertical sleeve gastrectomy is effective in two genetic mouse models of glucagon-like peptide-1 receptor deficiency. Diabetes 62, 2380–2385 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mokadem M, Zechner JF, Margolskee RF, Drucker DJ & Aguirre V Effects of Roux-en-Y gastric bypass on energy and glucose homeostasis are preserved in two mouse models of functional glucagon-like peptide-1 deficiency. Mol. Metab 3, 191–201 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kim K-S et al. Glycemic effect of pancreatic preproglucagon in mouse sleeve gastrectomy. JCI Insight 4, e129452 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shaham O. et al. Metabolic profiling of the human response to a glucose challenge reveals distinct axes of insulin sensitivity. Mol. Syst. Biol 4, 214 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Evers SS, Sandoval DA & Seeley RJ The physiology and molecular underpinnings of the effects of bariatric surgery on obesity and diabetes. Annu. Rev. Physiol 79, 313–334 (2017). [DOI] [PubMed] [Google Scholar]
- 81.Patti M-EE et al. Serum bile acids are higher in humans with prior gastric bypass: potential contribution to improved glucose and lipid metabolism. Obesity 17, 1671–1677 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ryan KK et al. FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature 509, 183–188 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Myronovych A. et al. Assessment of the role of FGF15 in mediating the metabolic outcomes of murine vertical sleeve gastrectomy (VSG). Am. J. Physiol. Gastrointest. Liver Physiol 319, G669–G684 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lefebvre P, Cariou B, Lien F, Kuipers F & Staels B Role of bile acids and bile acid receptors in metabolic regulation. Physiol. Rev 89, 147–191 (2009). [DOI] [PubMed] [Google Scholar]
- 85.Watanabe M. et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature 439, 484–489 (2006). [DOI] [PubMed] [Google Scholar]
- 86.Thomas C, Auwerx J & Schoonjans K Bile acids and the membrane bile acid receptor TGR5-connecting nutrition and metabolism. Thyroid 18, 167–174 (2008). [DOI] [PubMed] [Google Scholar]
- 87.Cicione C, Degirolamo C & Moschetta A Emerging role of fibroblast growth factors 15/19 and 21 as metabolic integrators in the liver. Hepatology 56, 2404–2411 (2012). [DOI] [PubMed] [Google Scholar]
- 88.Haluzíková D. et al. Laparoscopic sleeve gastrectomy differentially affects serum concentrations of FGF-19 and FGF-21 in morbidly obese subjects. Obesity 21, 1335–1342 (2013). [DOI] [PubMed] [Google Scholar]
- 89.Sachdev S. et al. FGF 19 and bile acids increase following Roux-en-Y gastric bypass but not after medical management in patients with type 2 diabetes. Obes. Surg 26, 957–965 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mulla CM et al. Plasma FGF-19 levels are increased in patients with post-bariatric hypoglycemia. Obes. Surg 29, 2092–2099 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hao Z. et al. Roux-en-Y gastric bypass surgery-induced weight loss and metabolic improvements are similar in TGR5-deficient and wildtype mice. Obes. Surg 28, 3227–3236 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Dreyfuss JM et al. High-throughput mediation analysis of human proteome and metabolome identifies mediators of post-bariatric surgical diabetes control. Nat. Commun 12, 6951 (2021). This study utilized fasting plasma samples obtained longitudinally from patients with T2DM randomized to RYGB or medical management, showing RYGB-associated progressive increases in multiple bile acid species and FGF19, and reductions in branched-chain amino acid-related metabolites over time.
- 93.van den Broek M. et al. Altered bile acid kinetics contribute to postprandial hypoglycaemia after Roux-en-Y gastric bypass surgery. Int. J. Obes 45, 619–630 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chaudhari SN et al. Bariatric surgery reveals a gut-restricted TGR5 agonist with anti-diabetic effects. Nat. Chem. Biol 17, 20–29 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.White PJ & Newgard CB Branched-chain amino acids in disease. Science 363, 582–583 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Laferrère B. et al. Differential metabolic impact of gastric bypass surgery versus dietary intervention in obese diabetic subjects despite identical weight loss. Sci. Transl. Med 3, 80re2 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tremaroli V. et al. Roux-en-Y gastric bypass and vertical banded gastroplasty induce long-term changes on the human gut microbiome contributing to fat mass regulation. Cell Metab. 22, 228–238 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fouladi F. et al. The role of the gut microbiota in sustained weight loss following Roux-en-Y gastric bypass surgery. Obes. Surg 29, 1259–1267 (2019). [DOI] [PubMed] [Google Scholar]
- 99.Dang JT et al. Ileal microbial shifts after Roux-en-Y gastric bypass orchestrate changes in glucose metabolism through modulation of bile acids and L-cell adaptation. Sci. Rep 11, 23813 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Debédat J. et al. The human gut microbiota contributes to type-2 diabetes non-resolution 5-years after Roux-en-Y gastric bypass. Gut Microbes 14, 2050635 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Simonson DC, Halperin F, Foster K, Vernon A & Goldfine AB Clinical and patient-centered outcomes in obese patients with type 2 diabetes 3 years after randomization to Roux-en-Y gastric bypass surgery versus intensive lifestyle management: the SLIMM-T2D study. Diabetes Care 41, 670–679 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Faramia J. et al. IGFBP-2 partly mediates the early metabolic improvements caused by bariatric surgery. Cell Rep. Med 2, 100248 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kim K-S, Seeley RJ & Sandoval DA Signalling from the periphery to the brain that regulates energy homeostasis. Nat. Rev. Neurosci 19, 185–196 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Martinou E, Stefanova I, Iosif E & Angelidi AM Neurohormonal changes in the gut-brain axis and underlying neuroendocrine mechanisms following bariatric surgery. Int. J. Mol. Sci 23, 1–40 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bethea M & Sandoval DA Gut factors mediating the physiological impact of bariatric surgery. Curr. Diab. Rep 22, 371–383 (2022). [DOI] [PubMed] [Google Scholar]
- 106.Müller TD et al. Glucagon-like peptide 1 (GLP-1). Mol. Metab 30, 72–130 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rosario W. et al. The brain-to-pancreatic islet neuronal map reveals differential glucose regulation from distinct hypothalamic regions. Diabetes 65, 2711–2723 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shin AC, Zheng H & Berthoud H-R Vagal innervation of the hepatic portal vein and liver is not necessary for Roux-en-Y gastric bypass surgery-induced hypophagia, weight loss, and hypermetabolism. Ann. Surg 255, 294–301 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Okafor P. et al. Effect of vagotomy during Roux-en-Y gastric bypass surgery on weight loss outcomes. Obes. Res. Clin. Pract 9, 274–280 (2015). [DOI] [PubMed] [Google Scholar]
- 110.Hao Z. et al. Vagal innervation of intestine contributes to weight loss after Roux-en-Y gastric bypass surgery in rats. Obes. Surg 24, 2145–2151 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Salehi M, Vella A, Mclaughlin T & Patti M, Society, E. Hypoglycemia after gastric bypass surgery: current concepts and controversies. J. Clin. Endocrinol. Metab 103, 2815–2826 (2018). This review summarizes the pathophysiology, diagnosis and treatment of PBH.
- 112.Capristo E. et al. Incidence of hypoglycemia after gastric bypass versus sleeve gastrectomy: a randomized trial. J. Clin. Endocrinol. Metab 103, 2136–2146 (2018). [DOI] [PubMed] [Google Scholar]
- 113.Lazar LO et al. Symptomatic and asymptomatic hypoglycemia post three different bariatric procedures: a common and severe complication. Endocr. Pract 10.4158/EP-2019-0185 (2019). [DOI] [PubMed] [Google Scholar]
- 114.Lee CJ et al. Comparison of hormonal response to a mixed meal challenge in hypoglycemia after sleeve gastrectomy versus gastric bypass. J. Clin. Endocrinol. Metab 107, e4159–e4166 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Abrahamsson N, Engströ BE, Sundbom M & Karlsson FA Hypoglycemia in everyday life after gastric bypass and duodenal switch. Eur. J. Endocrinol 173, 91–100 (2015). [DOI] [PubMed] [Google Scholar]
- 116.Kubota T. et al. Utility of continuous glucose monitoring following gastrectomy. Gastric Cancer 23, 699–706 (2020). [DOI] [PubMed] [Google Scholar]
- 117.Zaloga GP & Chernow B Postprandial hypoglycemia after Nissen fundoplication for reflux esophagitis. Gastroenterology 84, 840–842 (1983). [PubMed] [Google Scholar]
- 118.Bairdain S. et al. Laparoscopic adjustable gastric banding and hypoglycemia. Case Rep. Endocrinol. 2013, 671848 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sessa L. et al. Effect of single anastomosis duodenal-ileal bypass with sleeve gastrectomy on glucose tolerance test: comparison with other bariatric procedures. Surg. Obes. Relat. Dis 15, 1091–1097 (2019). [DOI] [PubMed] [Google Scholar]
- 120.Marsk R, Jonas E, Rasmussen F & Näslund E Nationwide cohort study of post-gastric bypass hypoglycaemia including 5,040 patients undergoing surgery for obesity in 1986–2006 in Sweden. Diabetologia 53, 2307–2311 (2010). [DOI] [PubMed] [Google Scholar]
- 121.Lee CJ, Brown TT, Schweitzer M, Magnuson T & Clark JM The incidence and risk factors associated with developing symptoms of hypoglycemia after bariatric surgery. Surg. Obes. Relat. Dis 14, 797–802 (2018). [DOI] [PubMed] [Google Scholar]
- 122. Fischer LE et al. Postbariatric hypoglycemia: symptom patterns and associated risk factors in the Longitudinal Assessment of Bariatric Surgery study. Surg. Obes. Relat. Dis 17, 1787–1798 (2021). This longitudinal cohort study examined hypoglycaemia symptoms following RYGB and laparoscopic adjustable gastric banding to identify risk factors for PBH.
- 123. Kefurt R. et al. Hypoglycemia after Roux-En-Y gastric bypass: detection rates of continuous glucose monitoring (CGM) versus mixed meal test. Surg. Obes. Relat. Dis 11, 564–569 (2015). This study utilized CGM to evaluate hypoglycaemia in 40 individuals post-RYGB, showing asymptomatic low sensor glucose levels (<55 mg/dl) in 75% of the patients and nocturnal hypoglycaemia in 38%.
- 124.Salehi M, Gastaldelli A & D’Alessio DA Altered islet function and insulin clearance cause hyperinsulinemia in gastric bypass patients with symptoms of postprandial hypoglycemia. J. Clin. Endocrinol. Metab 99, 2008–2017 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Goldfine AB et al. Patients with Neuroglycopenia after gastric bypass surgery have exaggerated incretin and insulin secretory responses to a mixed meal. J. Clin. Endocrinol. Metab 92, 4678–4685 (2007). [DOI] [PubMed] [Google Scholar]
- 126.Salehi M, Gastaldelli A & D’Alessio DA Blockade of glucagon-like peptide 1 receptor corrects postprandial hypoglycemia after gastric bypass. Gastroenterology 146, 669–680.e2 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lee D. et al. Glycemic patterns are distinct in post-bariatric hypoglycemia after gastric bypass (PBH-RYGB). J. Clin. Endocrinol. Metab 106, 2291–2303 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.van Furth AM et al. Cholecystectomy increases the risk of dumping syndrome and postbariatric hypoglycemia after bariatric surgery. Surg. Obes. Relat. Dis 16, 1939–1947 (2020). [DOI] [PubMed] [Google Scholar]
- 129.Lee CJ et al. Risk of post-gastric bypass surgery hypoglycemia in nondiabetic individuals: a single center experience. Obesity 24, 1342–1348 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Rebelos E. et al. Impact of postprandial hypoglycemia on weight loss after bariatric surgery. Obes. Surg 30, 2266–2273 (2020). [DOI] [PubMed] [Google Scholar]
- 131.Tharakan G. et al. Roles of increased glycaemic variability, GLP-1 and glucagon in hypoglycaemia after Roux-en-Y gastric bypass. Eur. J. Endocrinol 177, 455–464 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Evers SS et al. Continuous glucose monitoring reveals glycemic variability and hypoglycemia after vertical sleeve gastrectomy in rats. Mol. Metab 32, 148–159 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Abegg K. et al. Effect of bariatric surgery combined with medical therapy versus intensive medical therapy or calorie restriction and weight loss on glycemic control in Zucker diabetic fatty rats. Am. J. Physiol. Regul. Integr. Comp. Physiol 308, 321–329 (2015). [DOI] [PubMed] [Google Scholar]
- 134.Hepprich M. et al. Postprandial hypoglycemia in patients after gastric bypass surgery is mediated by glucose-induced IL-1β. Cell Metab. 31, 699–709 (2020). [DOI] [PubMed] [Google Scholar]
- 135.Vanderveen KA et al. Outcomes and quality of life after partial pancreatectomy for noninsulinoma pancreatogenous hypoglycemia from diffuse islet cell disease. Surgery 148, 1237–1245 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Meier JJ, Butler AE, Galasso R & Butler PC Hyperinsulinemic hypoglycemia after gastric bypass surgery is not accompanied by islet hyperplasia or increased -cell turnover. Diabetes Care 29, 1554–1559 (2006). [DOI] [PubMed] [Google Scholar]
- 137.Craig CM, Liu L-F, Deacon CF, Holst JJ & McLaughlin TL Critical role for GLP-1 in symptomatic post-bariatric hypoglycaemia. Diabetologia 60, 531–540 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Patti ME, Li P & Goldfine AB Insulin response to oral stimuli and glucose effectiveness increased in neuroglycopenia following gastric bypass. Obesity 23, 798–807 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Øhrstrøm CC et al. Counterregulatory responses to postprandial hypoglycemia after Roux-en-Y gastric bypass. Surg. Obes. Relat. Dis 17, 55–63 (2021). This study evaluated counterregulatory responses during postprandial hypoglycaemia in individuals with PBH who underwent RYGB.
- 140.Mulla CM et al. A randomized, placebo-controlled double-blind trial of a closed-loop glucagon system for postbariatric hypoglycemia. J. Clin. Endocrinol. Metab 105, 1260–1271 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Nakhaee S. et al. The effect of tramadol on blood glucose concentrations: a systematic review. Expert Rev. Clin. Pharmacol 13, 531–543 (2020). [DOI] [PubMed] [Google Scholar]
- 142.Mulla CM et al. Insulinoma after bariatric surgery: diagnostic dilemma and therapeutic approaches. Obes. Surg 26, 874–881 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zanley E. et al. Guidelines for gastrostomy tube placement and enteral nutrition in patients with severe, refractory hypoglycemia after gastric bypass. Surg. Obes. Relat. Dis 17, 456–465 (2021). [DOI] [PubMed] [Google Scholar]
- 144.Davis DB et al. Roux en Y gastric bypass hypoglycemia resolves with gastric feeding or reversal: confirming a non-pancreatic etiology. Mol. Metab 9, 15–27 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]