Abstract
Prophylactic donor lymphocyte infusions (DLI) are used to augment post-transplant immune recovery to reduce both infectious complications and disease recurrence. Preclinical studies implicate the naive T-cell subset as the primary driver of graft versus host disease (GvHD). In this phase I dose escalation study, we assessed the safety of a DLI that was depleted of CD45RA+ naïve T-cells. Sixteen adult patients received a prophylactic DLI at a median of 113 days (range 76-280 days) following an HLA-identical, non-myeloablative allogeneic hematopoietic stem cell transplantation. Three patients each received the naive T-cell depleted DLI with a CD3+ dose of 1x105/kg, 1x106/kg, and 5x106/kg. The maximum dose of 1x107/kg was expanded to 7 patients. No dose limiting grade III/IV acute GvHD or adverse events attributable to the DLI were observed at any dose level. One patient developed grade 2 acute GvHD of skin and upper intestines, and another developed moderate chronic GvHD of the lungs following the DLI. With a median follow-up of 2.8 years, 2-year progression-free and overall survival is 50.0% and 68.8%, respectively. In conclusion, these data suggest that a DLI that has been depleted of CD45RA+ naïve T-cells is feasible and carries a low risk of acute or chronic GvHD.
Introduction
Allogeneic stem cell transplantation (HSCT) offers the potential for cure of a variety of high-risk hematologic malignancies. Unfortunately, relapse of the underlying malignancy is the primary cause of death following HSCT. The risk of relapse is particularly high when the patient is conditioned with a reduced intensity or non-myeloablative conditioning regimen [1]. Donor T-cells play a critical role in the success or failure of the allogeneic HSCT. A subset of donor T-cells mediates graft-versus-host disease (GvHD) while others provide the foundation for immune recovery as well as the graft versus tumor response. For many years, post-transplant donor lymphocyte infusions (DLI) have been used to augment the immune response to both pathogens and malignancies. However, DLI carries the risk of inducing severe acute GvHD [2, 3]. Therefore, a major challenge in allogeneic stem cell transplantation is determining how to maximally exploit the beneficial effect of T-cells without causing significant GvHD. This challenge could be overcome by selectively depleting the population of donor T-cells responsible for eliciting the GvHD response.
Naïve T-cells are T-cells that have not encountered antigens specific for their T-cell receptor. Memory T-cells have previously been exposed to their corresponding cognate antigens. Our pre-clinical murine studies have implicated the naive T-cell, defined as CD62L+CD45RB+CD44low cells, as the primary driver of alloreactivity [4]. We and others have established that memory T-cells are less capable of inducing GvHD in several different animal models [5–8]. Based on these studies, a DLI consisting of a selected population of CD8+ memory cells has been studied as a means for addressing post-transplant relapse [9]. We hypothesized that a DLI consisting of memory T-cells is well suited to prevent post-transplant relapse and aid in immunologic reconstitution. We therefore conducted a phase 1 dose escalation study to determine the safety of prophylactic naïve T-cell depleted DLI after a matched donor allogeneic HSCT.
Methods
Study Population
Eligible patients were those that had received an alemtuzumab or thymoglobulin-containing conditioning regimen followed by allogeneic transplant from an HLA-identical family donor or an 8/8 HLA-matched unrelated donor. To allow for wash-out of the serotherapy, the investigational DLI was given at least 60 days following transplantation. Other inclusion criteria include Karnofsky performance status of 50-100%, no active acute GvHD ≥ grade II, no extensive chronic GvHD and age ≥ 18 years. Donor myeloid engraftment was required to be at least 40%. Restrictions on concomitant immunosuppressive medications were as follows; prednisone (or equivalent corticosteroid) dose ≤ 20mg daily, mycophenolate mofetil dose ≤ 2000mg/day and cyclosporine/tacrolimus at ≤ therapeutic blood trough levels. Immunosuppressive medication dosing/levels were required to remain stable in the 2 weeks preceding the naïve-T cell depleted DLI and 60 days following the DLI. Planned or ongoing therapies for relapse prevention were considered an exclusion criteria.
The protocol was reviewed and approved by the Institutional Review Board of Duke University School of Medicine and was registered on Clinicaltrials.gov (NCT01627275). All subjects signed an approved consent form for participation in the study.
Naïve T-cell depletion
A single donor leukapheresis procedure processing up to 24 liters was performed at steady state (not during G-CSF therapy). The leukapheresis product was depleted of naïve T-cells using CliniMACS® Miltenyi system (murine anti-human CD45RA monoclonal antibody conjugated to superparamagnetic iron dextran particles) under Investigational New Drug agreement (IND 15088) with the FDA. The protocol defined release criteria of the investigational DLI was a viability of > 70%, purity of < 10% residual CD3+ CD45RA+ cells, endotoxin < 5EU/kg/hr and a negative gram stain. The product was infused directly after processing, without cryopreservation.
Study design
The protocol schema is illustrated in Figure 1. A standard phase I, 3+3 dose escalation schema was employed. The planned DLI dose escalation schema was as follows: 1x106 CD3/kg, 5x106/kg, 1x107 CD3+/kg, 5x107 CD3+/kg, 1x108 CD3+/kg. Once the maximum tolerated dose was determined, a 4 patient dose expansion cohort was planned. The dose-limiting toxicity of the DLI was defined as the development of grade III/IV acute GvHD within 90 days of the DLI.
Figure 1:
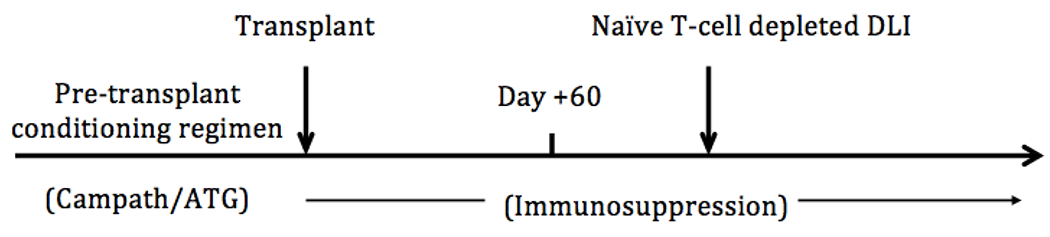
Protocol Schema
A single donor naïve T-cell depleted DLI was administered through a peripheral or central venous catheter at least 60 days following transplantation. Patients were evaluated by a healthcare professional a minimum of every other week by phone or by physical exam for evidence of therapy-related toxicity including GvHD until 12 weeks following DLI and then as clinically indicated thereafter. Blood samples for immune reconstitution assessments were collected immediately prior to the DLI and then approximately 3, 6, 12 months following the transplantation. Patients were monitored for a minimum of 1 hour following the DLI for assessment of infusion-related toxicity. Acute GvHD was assessed as per standard Glucksberg Criteria [10]. Chronic GvHD was assess as per NIH consensus criteria [11].
The primary objective was to determine the maximum tolerated dose of a naïve T-cell depleted DLI. The primary safety endpoint for the study is the occurrence of grade III/IV acute GvHD. The primary efficacy endpoint for the study is event-free and overall survival.
Statistical Analysis
Overall survival and progression-free survival since the investigational DLI were estimated using the Kaplan-Meier method. Cumulative incidences of acute and chronic GvHD since DLI were similarly estimated. Lymphocyte count was modeled as a function of days-since-DLI in a simple linear regression, and scatter plots were produced visualizing the linear relationship.
Results
Patient Characteristics
The patient characteristics are described in Table 1. Of the 28 adult patients who were evaluated for this trial, 16 received the investigational DLI. Donor ineligibilities and GvHD above the minimum specified severity were the two main reasons for exclusion (Figure 2). Nine patients had intermediate risk, 5 high risk and 2 low risk according to the Disease Risk Index for allogeneic transplantation [12]. At the time of the investigational DLI, 13 patients were receiving immunosuppressive medications for ongoing GvHD prophylaxis or treatment as follows: Mycophenolate Mofetil alone (n=7), prednisone alone (n=2), calcineurin inhibitor alone (n=1) and a combination of two agents (n=3).
Table 1:
Patient Characteristics
| (n=16) | |
|---|---|
| Age | |
| median age (range) | 54 (30-68) |
| Sex, n (%) | |
| Female | 4 (25) |
| Male | 12 (75) |
| Diagnosis, n (%) | |
| Acute myeloid leukemia | 6 (38) |
| Non Hodgkin Lymphoma | 4 (25) |
| Multiple Myeloma | 2 (13) |
| Hodgkin lymphoma | 1 (6) |
| Chronic Lymphocytic Leukemia | 1 (6) |
| Myelofibrosis | 1 (6) |
| MDS/MPN (CMML vs CML) | 1 (6) |
| Allo-Transplant Overall Disease Risk Index, n (%) [12] | |
| Low | 2 (13) |
| Intermediate | 9 (56) |
| High | 5 (31) |
| Very High | 0 (0) |
| CMV status, n (%) | |
| Recipient or donor positive | 12 (75) |
| Recipient and donor negative | 4 (25) |
| Conditioning Regimens, n (%) | |
| Fludarabine melphalan alemtuzumab | 12 (75) |
| Busulfan, fludarabine, antithymocyte globulin | 3 (19) |
| Busulfan, cyclophosphamide, antithymocyte globulin | 1 (6) |
| GvHD prophylaxis at the time of study DLI infusion, n (%) | |
| Mycophenolate Mofetil alone | 7 (44) |
| Prednisone alone | 2 (12) |
| Tacrolimus alone | 1 (6) |
| Two of Mycophenolate Mofetil, prednisone and tacrolimus | 3 (19) |
| None | 3 (19) |
Figure 2:
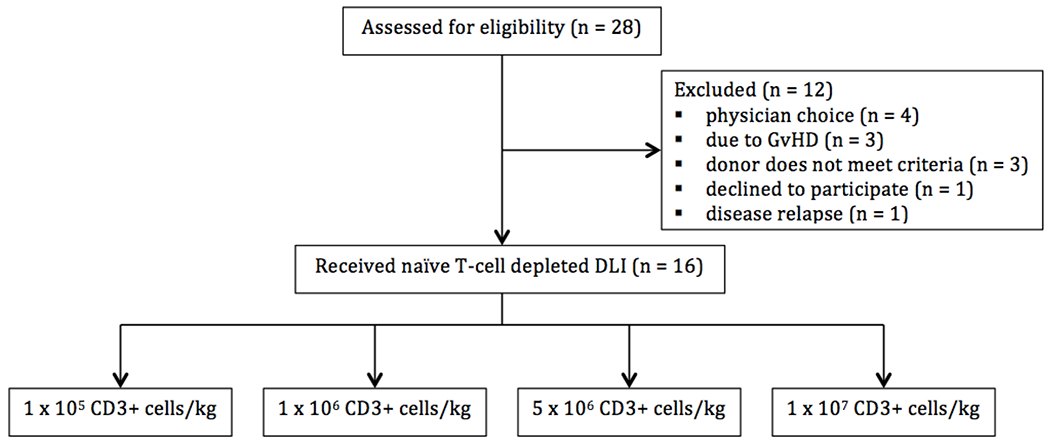
Patient Flow Chart
Graft and donor characteristics
A median of 12.4x109 CD3+ cells were collected from either matched unrelated or matched sibling donors. All donors were matched at HLA A, B, C, DRB1 and HLADQ. Matched related donors were also matched at HLA DP, however DP matching information was not available from matched unrelated donors. The donor leukapheresis product contained a median 74.4% (range 56.9% - 90.9%) CD3+ CD45RA+ cells. After depletion of CD45RA+ cells, the remaining product contained a median 2.9x109 CD3+ cells (range 1.2 - 10.1x109 CD3+ cells), which represents a median 23.1% (range 5.2% - 42.4%) of the starting CD3+ graft content. The final product retained a median of 1.1x108 (range 0.0x108 - 18.1x108) CD45RA+ cells representing a median 95% (range 70.35%-99.95%) depletion of the target cells. Cellular content of the DLI prior to and after processing is reported in Table 2. The products of four patients (patient number 1, 2,3 and 7) were out of specification due to presence of residual CD45RA+ cells. The products were infused as planned as it was felt to be in the best interest of the patient. The investigational DLI was given at a median 113 days (range 76-280 days) following transplantation.
Table 2:
Donor Lymphocyte Product Characteristics
| Variable | Value (n=16) |
|---|---|
| Source, n (%) | |
| Matched Unrelated Donor | 8 (50) |
| Matched Sibling | 8 (50) |
| Apheresis Product | |
| Total viable CD3+ cells, collected | |
| Median (109 cells) | 12.4 |
| Range (109 cells) | 6.7-23.8 |
| Total viable CD3+, CD45RA+, CD45RO− cells, collected | |
| Median (109 cells) | 3.8 |
| Range (109 cells) | 1.9-13.2 |
| Total viable CD3+, CD45RA− cells, collected | |
| Median (109 cells) | 2.8 |
| Range (109 cells) | 1.4-9.8 |
| Post-Selection Product | |
| Total viable CD3+ cells | |
| Median (109 cells) | 2.9 |
| Range (109 cells) | 1.2-10.1 |
| Total viable CD45RA+CD3+ cells (CD45RA+ T-cell contamination) | |
| Median (109 cells) | 0.1 |
| Range (109 cells) | 0.00-1.8 |
| Total viable CD45RA−CD3+ cells | |
| Median (109 cells) | 2.4 |
| Range (109 cells) | 1.1-9.3 |
| DLI Dose in CD3/kg, n (%) | |
| 1x105 | 3 (19) |
| 1x106 | 3 (19) |
| 5x106 | 3 (19) |
| 1x107 | 7 (43) |
| Days between transplant and study DLI, days | |
| Median | 112.5 |
| Range | 76-280 |
Dose-limiting toxicity and overall mortality
Due to cell dose limitations obtained from the donor leukapheresis and cell loss following depletion of CD45RA+ cells, we were unable to escalate the dose higher than 1x107 CD3+ cells/kg. With a median follow-up of 2.8 years, no dose limiting toxicity or adverse events attributable to the investigational DLI were observed at any dose level.
Relapse and non-relapse mortality
Seven subjects experienced disease relapse (43.7%). Of these, two received the lowest CD3+ DLI dose of 1x105/kg, one received the 1x106/kg dose and the remaining 4 received the highest dose of 1x107/kg. Relapse was the cause of death in 3 patients (one each with Hodgkin’s disease, multiple myeloma, and acute myeloid leukemia), resulting in a relapse-related mortality of 18.8%. Two additional patients died from non-relapse causes, one from acute pancreatitis and sepsis and the other from acute GvHD after two conventional DLIs were administered (after investigational DLI) for persistent mixed chimerism. The 2-year progression-free and overall survival was 50% and 68.8%, respectively (Figure 3).
Figure 3:
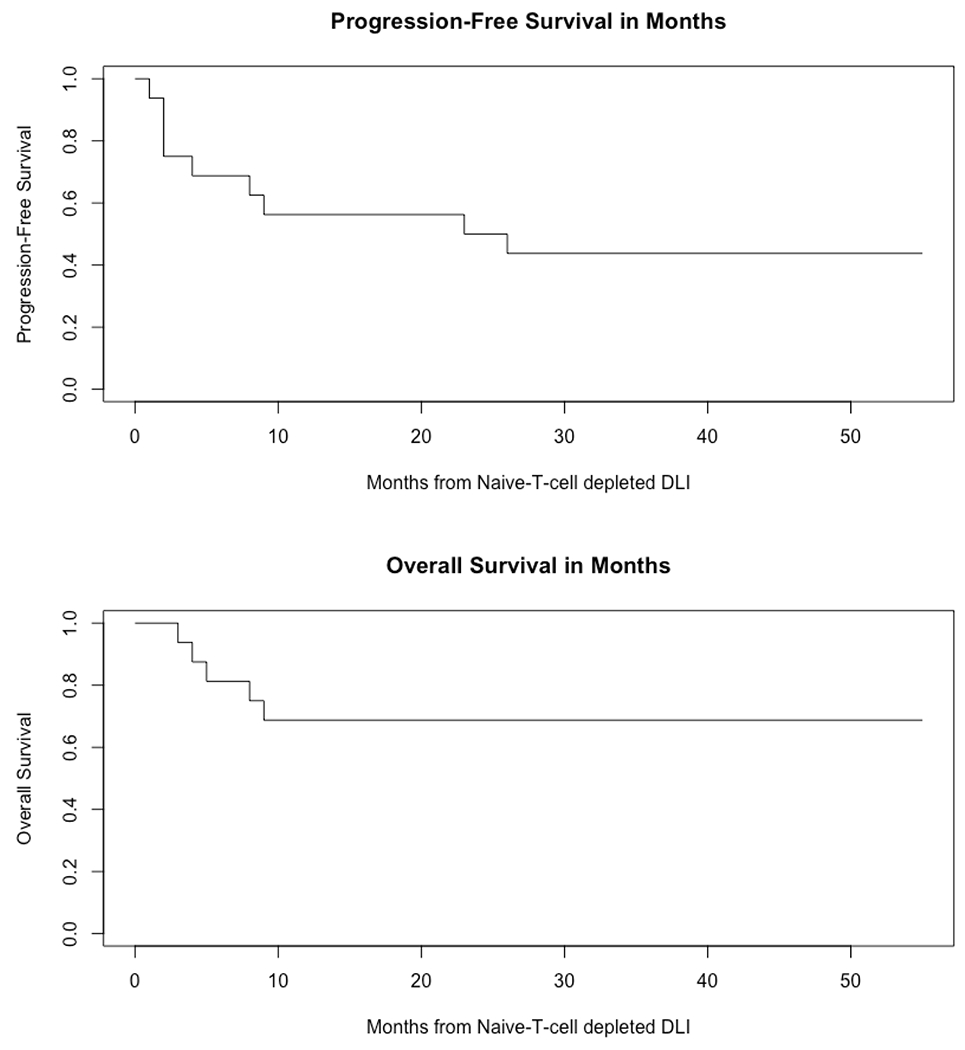
Progression free survival (50%) and overall survival (68.8%) at 24 months (n = 16)
Graft vs. Host Disease (GvHD)
While none of the study patients experience the protocol defined DLT of Grade III/IV acute GVHD, one patient developed grade II acute skin and upper intestinal tract GvHD 13 days after infusion of the study DLI at a dose of 5x106 CD3+/kg. Of note, this DLI had a high content of residual CD3+CD45RA+ cells at 20.6%. Another patient developed biopsy-proven moderate chronic GvHD involving the lungs 3.7 months following the study DLI dose of 5x106 CD3+/kg with 2.2% residual CD3+CD45RA+ cells. The cumulative incidence of acute and chronic GvHD attributable to the DLI was 6.2% (Figure 4).
Figure 4.
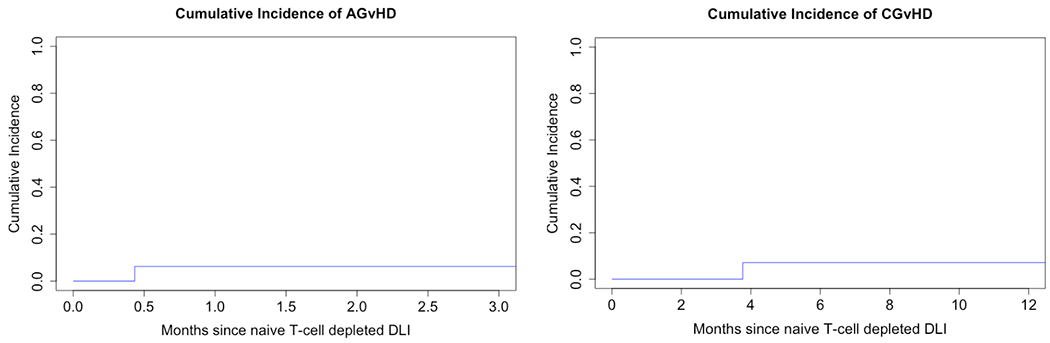
Acute grade II-IV (n = 1) and chronic (n = 1) GvHD attributable to Naïve T-cell depleted DLI
Immune reconstitution and Opportunistic Infections
Prior to administration of the investigational DLI, the patients had a median of 152 circulating CD3+ cells/μL (range 2 – 1426 cells/μL). Immune reconstitution of lymphocyte subsets is shown in Figure 5. The NK cells, CD3+, CD4+ and CD8+ lymphocytes recovered with positive correlations of 0.24, 0.40, 0.42 and 0.38, respectively over the one-year period following the study DLI.
Figure 5:
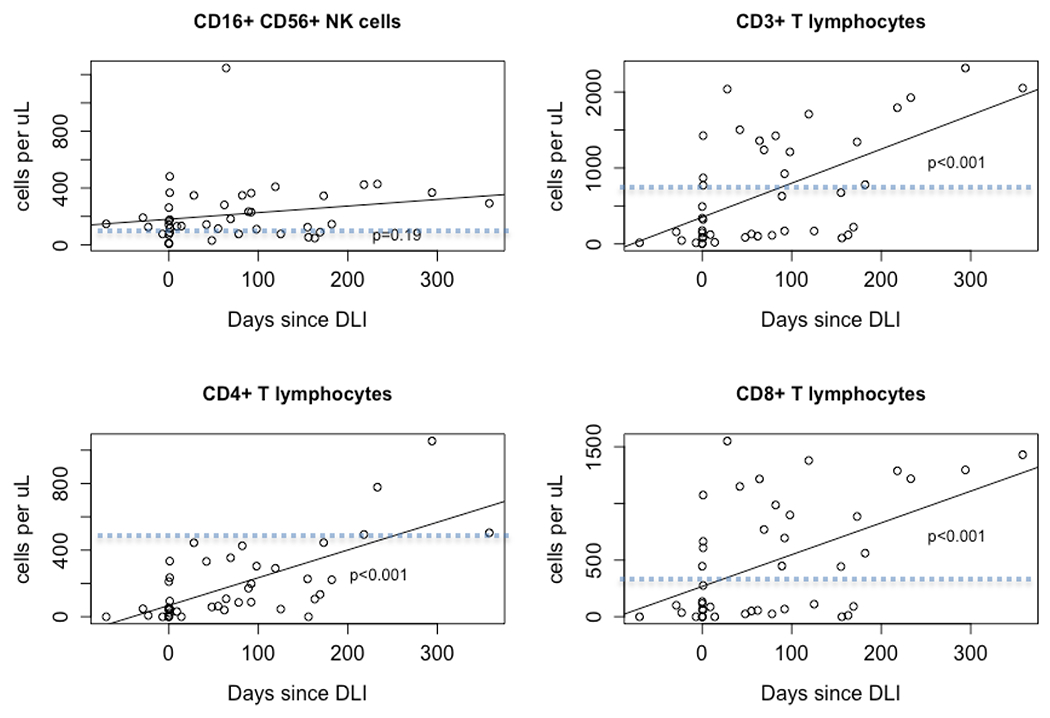
Immune Reconstitution Profiles of NK cells, CD3+, CD4+, CD8+ T Cells after Naive T-cell Depleted DLI. Dotted line indicates lower limit of the normal range.
After transplant and before the study DLI, engraftment studies showed that 8 patients had donor CD3+ fraction <90%. Engraftment studies were available in 14 patients after study DLI and 4 patients had donor CD3+ fraction <90%.
Two patients had low titer asymptomatic CMV viremia at the time of study DLI that resolved after the DLI. One additional patient developed HHV6 viremia after study DLI.
Discussion
Augmenting post-transplant immune recovery through the use of DLI has been performed for many years, but the incumbent risk of inducing potentially lethal acute graft versus host disease has limited its use. Based on our pre-clinical work suggesting that GvHD is driven by naïve T-cells [4, 5], we performed this phase I study of a naïve T-cell depleted DLI product. We found that a prophylactic naïve-T cell depleted DLI is safe. At 1x107 naïve depleted CD3+ cells/kg, the highest administered dose in this dose escalation study, there was no grade III/IV acute GvHD. The overall incidence of grade II-IV acute GvHD attributable to study DLI was also low at 6.2%.
This is the first study of a naïve T-cell depleted product used as a delayed, prophylactic DLI meant to improve post-transplant immune recovery. Our study is distinct from that of Muffly et. al. who recently reported the safety of a naïve T-cell depleted DLI that had an additional selection for CD8+ cells. This DLI was given as part of treatment for post-transplant relapse, and showed a similarly low incidence of acute GVHD, even after a relatively high dose of T-cells. Bleakley et. al. conducted a 35-patient single center trial of patients transplanted with a naïve-T cell depleted graft (median 10x106 CD3+/kg; range 1.6 – 10x106). In this study, the mobilized peripheral blood stem cell product from HLA-identical related donors was first selected for CD34+ cells. The CD34 negative, T-cell containing fraction was then depleted of CD45RA+ naïve T-cells and co-infused with the CD34+ fraction on day 0 of the transplant. The overall rate of grade II-IV acute GvHD was 66%, but only 3 patients developed grade III and 0 cases of grade IV acute GvHD. More strikingly, the incidence of chronic GvHD was only 9% [13]. These promising findings have resulted in an ongoing phase II multi-center trial for pediatric and young adult patients aged 6 months to 22 years comparing naïve T-cell depleted PBSC graft against unmanipulated T-cell replete BM graft (ClinicalTrials.gov Identifier: NCT03779854). A phase II multi-center trial is also currently recruiting to compare naïve T-cell depleted peripheral blood stem cell graft against three other PBSC grafts with the goal to reduce GvHD (ClinicalTrials.gov Identifier: NCT03970096). Use of a naïve T-cell depleted stem cell graft has also been applied to pediatric recipients of haploidentical transplantation [14] [15]. In a retrospective comparison to recipients of a pan T-cell depleted graft, the naïve T-cell depleted graft provided a more prompt immune reconstitution, which translated in a reduced incidence of viral infections. The incidence of grade III-IV acute GVHD was 23.1% in this study, which was comparable to those who received a pan T-cell depleted haploidentical graft [15].
The risk of GvHD induction following DLI decreases with time as host T-cell recovery and endogenous tolerogenic mechanisms evolve. Barrett and colleagues found that an unmanipulated DLI dose containing 1x107 CD3+ cells/kg delivered on day 30 following a T-cell depleted HLA-identical allogeneic transplantation resulted in an unacceptably high rate of acute GvHD (grade II-IV, 100%) [2]. Our historical institutional experience with delivering an unmanipulated DLI in the first 3 months following an alemtuzumab-based non-myeloablative HLA-matched donor HSCT resulted in 21 of 52 (40%) of patients developing acute GVHD (grade I, n=5, grade II, n=7, grade II, n=6 and grade IV, n=3) All but one of the GvHD events occurred after a CD3+ DLI dose that was similar to what was delivered in this study (1 x 106/kg, n=14 or 1 x 107/kg, n=6) [16]. Three Grade IV GvHD cases were from cohorts receiving a CD3+ cell dose of 1x106/kg or smaller. Bar and colleagues performed a retrospective analysis of the acute GvHD risk following a late DLI administered for treatment of disease relapse. They found that acute GvHD occurred in 21% of patients following an unmanipulated DLI of <1 x 107/kg, and 45% for those receiving a dose of between 1 x 107/kg and 10 x 107/kg [3]. As expected following an alemtuzumab or thymoglobulin based conditioning regimen, the patients in our study received their DLI early in the course of T-cell recovery. Despite median time to DLI administration of 113 days, the median CD3 count was only 152 cells/μl. This is attributable to the prolonged in vivo lymphodepletion from a serotherapy-based conditioning regimen. While the primary objective of this study was safety and feasibility, the exceedingly low rate of GvHD following a naïve T-cell depleted DLI in these highly lymphodepleted hosts suggests a reduced GvHD inducing potential of memory T-cells.
In an attempt to maximize safety and minimize discomfort, we requested a single, steady state, large volume peripheral blood apheresis session from the patient’s stem cell donor. This was particularly important since half of the donors were unrelated. This yielded 1.2x109 - 10.1x109 CD3+ cells after naïve-T cell depletion resulting in a maximum DLI dose of 1x107 CD3+/kg. Higher doses may have been possible if the donors were subjected to additional apheresis sessions.
This phase I study demonstrated that the maximum administered cell dose of 1x107 CD3+ cells/kg DLI depleted of naïve T-cells did not result in clinically significant acute GvHD. However, the sample size was too small to draw conclusions regarding its efficacy with respect to the graft versus tumor potential or functional immunologic recovery. Larger studies in a more homogeneous patient population will be needed to confirm the results. Another limitation of the study design is that the delayed DLI approach selects for patients who may be less likely to develop GvHD following a DLI. However it should also be noted that 6 subjects (37.5%) did in fact have grade I-IV acute GvHD pre DLI which resolved or improved before enrollment on the study.
Our study suggests that a prophylactic naïve T-cell depleted DLI after an in vivo T-cell depleted allogeneic stem cell transplant is safe, feasible and is unlikely to trigger severe acute GvHD. While the pre-clinical models suggest that memory cells have potent anti-tumor potential, this finding will require further investigation to confirm. Also requiring further investigation is a safety and dose finding study of a naïve T-cell depleted haploidentical cell product. Such a product would be a highly desirable adjunct to the rapidly emerging haploidentical HSCT approach using post-transplant cyclophosphamide.
Table 3;
Outcome Summary
| Patient # | sex | age at study DLI | Disease | Overall Disease Risk Index | Status at Transplant | Conditioning regimen | MUD/MRD | Acute GvHD before study DLI, grade and site (Day of transplant) | Chimerism at the time of study DLI, CD3+; CD15+ | Chimerism after study DLI CD3+; CD15+ (Days following DLI) | Days between transplant and study DLI | Reason(s) the study DLI was delayed | Study DLI CD3+/kg (106) | Residual CD45RA+ as % of CD3+ | Residual CD3+ CD45RA+ T cells/kg (106) | Immunosuppression at the time of study DLI | Absolute lymphocyte count at study DLI (x10^6) | Acute GvHD after study DLI, grade and site (days post DLI) | Chronic GvHD after study DLI, severity and site | Viral infections after study DLI | Disease Recurrence, days after study DLI | Unmanipulated DLI (Days post study DLI) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 68 | AML | High | CR | Flu/Mel/Cam | MUD | 3; skin (30) | >98%; >98% | N/A; N/A | 119 | Logistics and GvHD | 0.1 | 26.9 | 0.03 | Pred 10mg qd + MMF 1000mg bid | 1000 | No | N/A | None | 89 | No |
| 2 | F | 54 | MM | Inter | VGPR | Flu/Mel/Cam | MRD | None | 80%; >98% | 76%; >98% (120) | 76 | 0.1 | 12.4 | 0.01 | MMF 1000mg bid | 500 | No | N/A | None | No | Yes (69) | |
| 3 | F | 50 | MM | High | PR | Flu/Mel/Cam | MRD | None | 61%; >98% | 80%; >98% (112) | 105 | Logistics (patient consideration) | 0.1 | 29.6 | 0.03 | MMF 500mg bid | 1100 | No | N/A | CMV viremia | 266 | Yes (119) |
| 4 | M | 30 | HL | High | PR | Flu/Mel/Cam | MRD | None | 76%; >98% | N/A; N/A | 92 | 1 | 8.6 | 0.09 | None | 900 | None | N/A | HHV6 | 72 | No | |
| 5 | M | 65 | NHL | Low | PD | Flu/Mel/Cam | MUD | 3; GI (48) | >98%; >98% | >98%; >98% (95) | 90 | 1 | 0.3 | 0.003 | Pred 10mg qd + MMF 720mg bid | 200 | No | N/A | None | No | No | |
| 6 | M | 55 | NHL | Inter | PR | Flu/Mel/Cam | MRD | 1; skin (47) | >98%; >98% | >98%; >98% (114) | 92 | 1 | 1.2 | 0.01 | MMF 1000mg bid | 200 | No | N/A | None | No | No | |
| 7 | M | 65 | CLL/SLL | Low | CR | Flu/Mel/Cam | MUD | None | 92%; 95% | 94%; >98% (118) | 144 | Technical/logistic | 5 | 20.5 | 1.0 | MMF 500mg bid | 300 | 2, skin + GI (13) | N/A | None | No | No |
| 8 | M | 65 | NHL | Inter | PD | Flu/Mel/Cam | MRD | None | 65%; >98% | 92%; >98% (91) | 97 | Technical/logistic | 5 | 0.5 | 0.02 | MMF 1000mg bid | 100 | No | N/A | None | No | No |
| 9 | F | 41 | NHL | Inter | PD | Flu/Mel/Cam | MUD | 2; skin (68) | >98%; >98% | >98%; >98% (95) | 140 | Pre-DLI GvHD | 5 | 2.2 | 0.1 | None | 600 | No | Mod, lung | None | No | No |
| 10 | M | 61 | AML | Inter | PD | Flu/Mel/Cam | MRD | 2; skin (96) | 98%; >98% | N/A; N/A | 229 | CMV viremia and GvHD | 10 | 2.4 | 0.2 | Pred 20mg qd | 300 | No | N/A | None | No | No |
| 11 | M | 67 | MDS/MPN | Inter | SD | Flu/Mel/Cam | MRD | None | 34%; >98% | 85%; 98% (94) | 113 | Technical/logistic | 10 | 5.0 | 0.5 | MMF 1000mg bid | 200 | No | N/A | None | No | No |
| 12 | F | 44 | AML | Inter | CR | Flu/Mel/Cam | MUD | None | 89%; 88% | 98%; 98% (240) | 78 | 10 | 4.7 | 0.5 | MMF 360mg bid | 840 | No | N/A | None | 720 | Yes (779) | |
| 13 | M | 52 | AML | High | CR | Bu/Cy/ATG | MUD | 2; GI (83) | 94%; >98% | >98%; >98% (87) | 112 | Pre-DLI GvHD | 10 | 0.6 | 0.07 | Pred 10mg qd + Tac 1.5mg qd | 1590 | No | N/A | None | No | No |
| 14 | M | 53 | AML | High | CR | Bu/Cy/ATG | MUD | None | >98%; >98% | >98%; >98% (110) | 119 | Logistics (patient consideration) | 10 | 0.05 | 0.005 | Tac 0.5mg qd | 500 | No | N/A | None | 81 | Yes (109) |
| 15 | M | 47 | MF | Inter | SD | Bu/Cy/ATG | MRD | None | 91%; 98% | 92%; 98% (124) | 280 | bowel obstruction, abscess and complications | 10 | 7.5 | 0.75 | Pred 10mg qd | 2100 | No | N/A | None | 818 | Yes (905) |
| 16 | M | 56 | AML | Inter | CR | Bu/Cy/ATG | MUD | None | 60%; >98% | 88%; >98% (39) | 252 | CMV after transplant; logistics | 10 | 6.5 | 0.65 | None | 1500 | No | N/A | CMV viremia | 44 | Yes (97) |
AML; acute myeloid leukemia, MM; multiple myeloma, HL; Hodgkin lymphoma, NHL; non-hodgkin lymphoma, CLL/SLL; chronic lymphocytic leukemia/small lymphocytic leukemia, MDS/MPN; myelodysplastic syndrome/myeloproliferative disorder, MF; myelofibrosis, CR; complete remission, VGPR; very good partial remission, PR; partial remission, PD; progressive disease, Flu/Mel/Cam; fludarabine/melphalan/campath, Bu/Cy/ATG; busulfan/cyclophosphamide/anti-thymocyte globulin, MUD; matched unrelated donor, MRD, Matched related donor, N/A; not available, Pred; prednisone, MMF; mycophenolate mofetil, Tac; tacrolimus, CMV; cytomegalovirus viremia, HHV6; human herpesvirus 6 viremia
Acknowledgement
Ko Maung is supported by NIH T32HL007057 grant.
Footnotes
Conflict of Interest: None of the authors have any conflicts of interest or financial ties to disclose.
References:
- 1.Scott BL, Pasquini MC, Logan BR, Wu J, Devine SM, Porter DL, et al. Myeloablative Versus Reduced-Intensity Hematopoietic Cell Transplantation for Acute Myeloid Leukemia and Myelodysplastic Syndromes. 2017;35(11):1154–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barrett AJ, Mavroudis D, Tisdale JR, Molldrem J, Clave Emmanuel, Dunbar CE, et al. T cell-depleted bone marrow transplantation and delayed T cell add-back to control acute GVHD and conserve a graft-versus-leukemia effect. Bone Marrow Transplantation. 1998;21(6):543–51. [DOI] [PubMed] [Google Scholar]
- 3.Bar M, Sandmaier BM, Inamoto Y, Bruno B, Hari P, Chauncey T, et al. Donor Lymphocyte Infusion for Relapsed Hematological Malignancies after Allogeneic Hematopoietic Cell Transplantation: Prognostic Relevance of the Initial CD3+ T Cell Dose. Biology of Blood and Marrow Transplantation. 2013;19(6):949–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen BJ, Deoliveira D, Cui X, Le NT, Son J, Whitesides JF, et al. Inability of memory T cells to induce graft-versus-host disease is a result of an abortive alloresponse. Blood. 2007;109(7):3115–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen BJ, Cui X, Sempowski GD, Liu C, Chao NJ. Transfer of allogeneic CD62L- memory T cells without graft-versus-host disease. Blood. 2004;103(4):1534–41. [DOI] [PubMed] [Google Scholar]
- 6.Anderson BE, McNiff J, Yan J, Doyle H, Mamula M, Shlomchik MJ, et al. Memory CD4+ T cells do not induce graft-versus-host disease. Journal of Clinical Investigation. 2003;112(1):101–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xystrakis E, Bernard I, Dejean AS, Alsaati T, Druet P, Saoudi A et al. Alloreactive CD4 T lymphocytes responsible for acute and chronic graft-versus-host disease are contained within the CD45RChigh but not the CD45RClow subset. European Journal of Immunology. 2004;34(2):408–17. [DOI] [PubMed] [Google Scholar]
- 8.Dutt S, Tseng D, Ermann J, George TI, Liu YP, Davis CR, et al. Naive and Memory T Cells Induce Different Types of Graft-versus-Host Disease. The Journal of Immunology. 2007. November 15;179(10):6547–54. [DOI] [PubMed] [Google Scholar]
- 9.Muffly L, Sheehan K, Armstrong R, Jensen K, Tate K, Rezvani AR, et al. Infusion of donor-derived CD8+ memory T cells for relapse following allogeneic hematopoietic cell transplantation. Blood Advances. 2018. March 27;2(6):681–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glucksberg H, Storb R, Fefer A, Buckner CD, Neiman PE, Clift RA, et al. Thomas ED Clinical manifestations of graft-versus-host disease in human recipients of marrow from HLA-matched sibling donors. Transplantation. 1974;18:295–304. [DOI] [PubMed] [Google Scholar]
- 11.Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group report. Biology of Blood and Marrow Transplantation. 2015;21(3):389–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Armand P, Gibson CJ, Cutler C, Ho VT, Koreth J, Alyea EP, et al. A disease risk index for patients undergoing allogeneic stem cell transplantation. Blood. 2012. July 26;120(4):905–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bleakley M, Heimfeld S, Loeb KR, Jones LA, Chaney C, Seropian S, et al. Outcomes of acute leukemia patients transplanted with naive T cell–depleted stem cell grafts. J Clin Invest. 2015;125(7):2677–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shook DR, Triplett BM, Eldridge PW, Kang G, Srinivasan A, Leung W. Haploidentical stem cell transplantation augmented by CD45RA negative lymphocytes provides rapid engraftment and excellent tolerability. Pediatric Blood & Cancer. 2015;62(4):666–73. [DOI] [PubMed] [Google Scholar]
- 15.Triplett BM, Muller B, Kang G, Li Y, Cross SJ, Moen J, et al. Selective T-cell depletion targeting CD45RA reduces viremia and enhances early T-cell recovery compared with CD3-targeted T-cell depletion. Transplant Infectious Disease. 2018;20(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rizzieri DA, Dev P, Long GD, Gasparetto C, Sullivan KM, Horwitz M, et al. Response and toxicity of donor lymphocyte infusions following T cell depleted non-myeloablative allogeneic hematopoietic stem cell transplantation from 3–6/6 HLA matched donors. Bone Marrow Transplantation. 2009;43(4):327–33. [DOI] [PMC free article] [PubMed] [Google Scholar]


