Abstract
Antipsychotic-induced weight gain is a well-established but poorly understood clinical phenomenon. New mechanistic insights into how antipsychotics modulate adipose physiology are sorely needed, in hopes of either devising a therapeutic intervention to ameliorate weight gain or contributing to improved design of future agents. In this study, we have hypothesized that the weight gain-associated tricyclic antipsychotics clozapine and chlorpromazine directly impact adipose tissue by potentiating adipogenic differentiation of preadipocytes. Utilizing a well-established in vitro model system (3T3-L1 preadipocyte cell line), we demonstrate that, when applied specifically during induction of adipogenic differentiation, both clozapine and chlorpromazine significantly potentiate in vitro adipogenesis, observed as morphological changes and increased intracellular lipid accumulation. These persistent effects, observed at endpoints well after the end of antipsychotic exposure, are accompanied by increased transcript- and protein-level expression of the mature adipocyte marker perilipin-1, as indicated by RT-qPCR and Western blotting, but not by further upregulation of pro-adipogenic transcription factors versus positive controls. Our findings point to a possible physiological mechanism of antipsychotic-induced hyperplasia, with potentiated expression of mature adipocyte markers enhancing the differentiation and maturation of preadipocytes.
Keywords: 3T3-L1 cell, adipogenesis, antipsychotic, perilipin, preadipocyte
Introduction
Antipsychotics are the primary therapeutic tool for management of psychosis, a debilitating condition associated with schizophrenia and other psychiatric disorders [1]. Although efficacious and widely used, many antipsychotics are notorious for severe metabolic side effects, including average weight gain of up to 2 lbs/month [2], dyslipidemia, and even Type 2 diabetes mellitus [1-3]. These effects are most strongly associated with the atypical antipsychotics clozapine (Clz) and olanzapine, but similar outcomes have been reported for the typical agent chlorpromazine (Cpz), which shares a common tricyclic chemical structure [1].
Antipsychotics may exert their metabolic side effects related to weight gain via central mechanisms (e.g., effects on appetite and satiety), or via peripheral mechanisms, whereby the compounds act directly on adipose tissue. Both possibilities are under intensive study. However, a cogent unifying molecular mechanism remains elusive, although a peripheral one is attractive; while a central mechanism would likely be difficult to counteract, a peripheral mechanism allows for the possibility of ameliorating weight gain with an intervention that spares centrally-mediated therapeutic effects.
In the present study, we have hypothesized that tricyclic antipsychotics potentiate adipogenic differentiation of preadipocyte cells into mature adipocytes via enhancement of the adipogenic gene expression program. Such an effect would roughly correspond to an in vivo mechanism of weight gain by hyperplasia, wherein new adipocytes are added by differentiation from precursor cell pools, rather than hypertrophy of existing adipocytes. Previous work shows that olanzapine and Clz may directly impact lipid accumulation in in vitro differentiated adipocytes [4-8] or enhance adipogenic differentiation from stem cells (e.g., mesenchymal stem cells and adipose-derived stem cells) and the well-established [9,10] 3T3-L1 mouse preadipocyte cell line [11-13]. However, these studies have been largely inconclusive regarding drug concentrations that more closely approximate physiologically relevant levels, often operating at concentrations as high as 50 μM to achieve significant effects. Our study complements the existing literature by presenting a careful series of experiments on the precise timing of Clz application, at or below 10 μM (ranging from slightly above to well within reported therapeutic concentrations [14]), during differentiation and maturation of preadipocytes (the 3T3-L1 mouse preadipocyte line, selected as a model system to represent mammalian physiology more broadly); this is an important distinction given that adipogenesis in vivo can involve commitment of stem cells into preadipocytes and/or final terminal differentiation of preadipocytes into mature adipocytes [15,16]. We show that Clz, applied precisely during induction of adipogenic differentiation of preadipocytes, potentiates apparent adipogenesis, by a mechanism involving specific modulation of adipogenic genes, and we show that Cpz also potentiates adipogenesis.
Methods
3T3-L1 cell culture
Undifferentiated (UD) mouse 3T3-L1 cells (ATCC CL-173) were maintained routinely below 70% confluency in Dulbecco’s modified Eagle’s medium (DMEM, Thermo Fisher) with 10% calf serum (ATCC 30-2030) and 100 units/mL penicillin/streptomycin (Thermo Fisher) in a humidified 5% CO2 incubator at 37°C. To induce adipogenic differentiation, cells were initially seeded onto either 96- or 24-well tissue culture plates (USA Scientific) at sub-confluency, allowed to proliferate to full confluency over a 48-hour period, then incubated for another 48 hours.
Adipogenic induction protocols
For induction of adipogenic differentiation, we opted for a commercially-available complete adipogenic induction supplement (StemXVivo adipogenic supplement, R&D Systems), to streamline workflow and improve lot-to-lot consistency. Optimization revealed that 2x StemXVivo supplement applied for a total of 4 days (following the initial 4-day proliferation/incubation period) was a sufficient threshold-level stimulus for significant adipogenic differentiation in 3T3-L1 cells, while 1x StemXVivo was a sub-threshold stimulus. At induction of adipogenic differentiation (4 days post-seeding), cultures were exposed to one of three adipogenic induction stimuli: antipsychotic alone, sub-threshold (1x StemXVivo, alone or with antipsychotic), or threshold-level (2x StemXVivo, alone or with antipsychotic). After the 4-day induction, induced cells continued in maintenance medium (DMEM with 10% fetal bovine serum and 1 μg/mL insulin, Sigma Aldrich) until termination. On each culture plate, a negative control condition was also maintained; these continued in standard UD 3T3-L1 growth medium throughout. All cultures were fed with appropriate medium, replenishing approximately half of the well volume every 2-3 days, until termination. Clz and Cpz were obtained from Sigma-Aldrich; stock solutions were prepared and diluted to final concentrations for use as a standalone induction stimulus, treatment in combination with a sub-threshold or threshold-level stimulus, or treatment of induced cultures several days post-induction (PI).
Visual and ORO-based assessment of adipogenesis
Cells were seeded onto 96-well plates, each 8-well row considered as a set of technical replicates averaged into a single biological replicate. At endpoints of 10, 14, or 17 days PI (i.e., from the initial start of induction), cultures were visually inspected, using an Olympus inverted scope and attached camera to obtain brightfield photomicrographs at 100x magnification, for morphological assessment of adipogenic differentiation (i.e., appearance of characteristic golden spherical cytoplasmic lipid droplets). Cultures were then subjected to ORO staining, which stains accumulated intracellular lipid a characteristic red color: fixation in 4% formaldehyde, washing with 60% isopropanol, incubation for 10 minutes in 0.3% ORO in isopropanol (Sigma-Aldrich). Stained cells were again imaged, with effort made to document the same regions of the same wells as before. ORO was eluted in 100% isopropanol prior to absorbance measurements (500 nm) in a microplate reader (Biotek). Data are reported as either raw absorbance values, for direct comparison of induced cultures with corresponding negative controls, or as fold changes in absorbance for antipsychotic-treated cultures versus corresponding positive controls, with negative controls treated as background (internal negative control absorbance values for each biological replicate were subtracted from all other group values).
Reverse transcription quantitative PCR (RT-qPCR)
Cells were seeded onto 24-well plates, with 2 wells per condition combined into a single sample to ensure sufficient material. At endpoints of 18-24 (data pooled into a single “24 hour” group due to lack of resources), 48, or 72 hours PI (i.e., from initial application of induction stimulus), cultures were used for total RNA isolation (RNA/Protein Purification Kit, Norgen Biotek). RNA isolates were assayed by NanoDrop and electrophoresis (2% E-Gel Ex, Thermo Fisher) and subjected to DNase treatment (Turbo DNA-free kit, ibid). Reverse transcription was carried out (High Capacity RNA-to-cDNA kit, ibid), generating first-strand cDNA. All of the above procedures were done according to the manufacturer’s instructions. cDNA was used as template for quantitative (real-time) PCR (qPCR) with TaqMan gene expression assays (ibid) for the genes CEBPA (encoding CEBPα), PPARG (encoding PPARγ), or PLIN1 (encoding perilipin-1), plus 18S rRNA always analyzed as an endogenous control gene for all samples. Reactions were prepared in 2-3 technical replicates per biological replicate, in 10 μL total consisting of: 1 μL cDNA, 5 μL TaqMan Gene Expression master mix (ibid), 0.5 μL TaqMan assay primer/probe mix, 3.5 μL PCR-grade water. qPCR was performed in an ABI 2500 Fast RT Thermocycler, using the pre-programmed TaqMan conditions. Resulting CT data (technical replicate average) for the gene of interest (i.e., CEBPA, PPARG, or PLIN1) were normalized to endogenous control (ΔCT), then analyzed by: the ΔΔCT method, for fold expression changes in the gene of interest in induced/positive control samples versus non-induced/negative control samples; the 2^-ΔΔCT method, for fold expression changes in the gene of interest in antipsychotic-treated samples versus matched positive control samples; or the 2^-ΔCT method, for showing absolute amounts of expression across unmatched conditions [17].
Western blotting
Cells were initially prepared as described above for RT-qPCR experiments, but lysed in RIPA buffer at 14 or 17 days PI and. Whole-cell homogenate was mixed 1:1 with 2x Laemmli buffer (BioRad) containing 5% β-mercaptoethanol (Sigma Aldrich) and heated at 95°C for 5 minutes. Western blotting was carried out as previously described [18,19], except the iBlot 2 dry electroblotting system (Thermo Fisher, 8.5 minutes at 15 V) was used for PVDF membrane transfer. Primary antibodies against perilipin-1 (Plpn-1, rabbit mAb, Cell Signaling) and β-actin (mouse mAb, Thermo Fisher), were diluted 1:5,000 in 5% BSA/TBST; anti-rabbit and -mouse HRP-linked secondary antibodies (Cell Signaling) were diluted 1:20,000 and 1:10,000 in 5% nonfat milk/TBST. Chemiluminescent images of comparable exposure from each biological replicate set were analyzed densitometrically using ImageJ. Resulting optical density measurements for Plpn-1 and corresponding loading control (β-actin) bands were inverted, background adjusted, and used to calculate a Plpn-1:β-actin ratio.
Data analysis
Final analysis was carried out and graphs prepared using Microsoft Excel with the Analyse-it add-in. Student’s t-test was used for simple direct statistical comparisons between only two groups. For datasets involving more than two groups (e.g., non-induced/negative control and induced/positive control conditions versus multiple antipsychotic-treated conditions), a one-way ANOVA plus post-hoc multiple comparisons test was performed. Dunnett’s post-test was used for comparisons between a single common control and multiple experimental conditions, while Tukey’s post-test was used when a single common control was not present. For all statistical tests, p values of less than 0.05 considered statistically significant.
Results
Evaluating Clz as a lipogenic and/or adipogenic stimulus
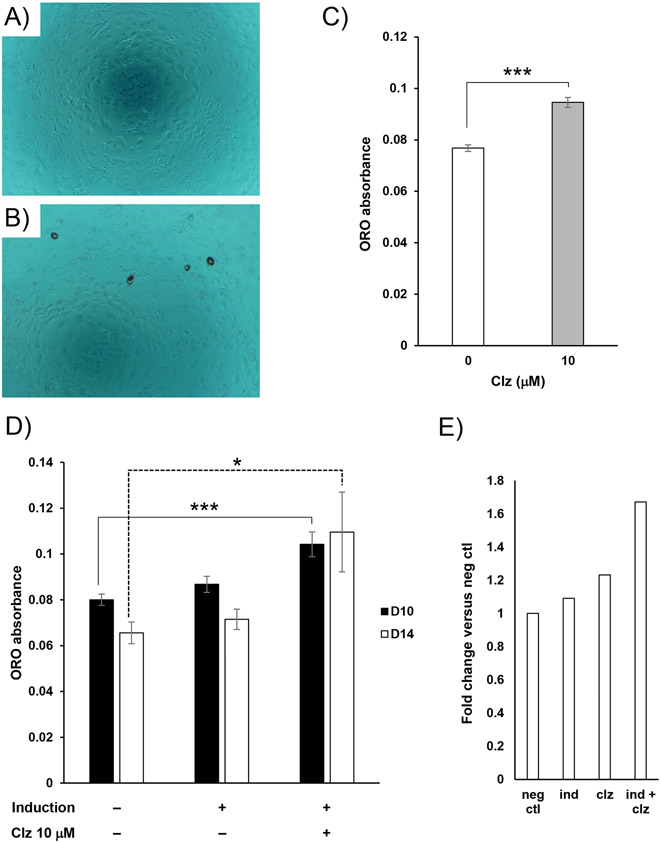
We initially evaluated Clz as a lipogenic stimulus, acting on existing adipocytes to drive hypertrophy. From 8 days PI, cultures already differentiated into adipocytes without antipsychotic were exposed to Clz for 6 days, prior to termination at 14 days PI; there was no impact of Clz on lipid accumulation under these conditions (Figure S1), indicating that Clz alone is not a sufficient lipogenic stimulus in this model system. We next investigated whether Clz alone could induce adipogenic differentiation of preadipocytes. For these experiments, UD 3T3-L1 cells were prepared for adipogenic induction, then exposed to 10 μM Clz alone during the 4-day induction. After maintenance without Clz to the culture endpoint (14 days PI), cells were analyzed visually for morphological changes and by ORO staining, revealing that Clz alone did drive a small but significant degree of apparent adipogenic differentiation (Figure 1A-C). Because the effect persisted for 10 days after Clz exposure ended, this strongly suggests that Clz impacts the adipogenic gene expression program directly. Furthermore, when we applied a sub-threshold induction stimulus to preadipocyte cultures, which was insufficient to drive significant adipogenesis alone, we found that addition of 10 μM Clz along with that stimulus, again during the 4-day induction only, drove a significant degree of apparent adipogenic differentiation (Figure 1D). In addition, a greater fold change in lipid accumulation was observed for Clz combined with the sub-threshold stimulus (Figure 1E). These findings indicate that Clz drives persistent pro-adipogenic alterations in preadipocytes.
Figure 1.
Clz functions as a mild adipogenic stimulus. A-C. UD 3T3-L1 cells were exposed to 10 μM Clz as a standalone adipogenic induction stimulus (as described in the methods section). At 14 days PI, cultures were terminated and ORO-stained, with representative (n = 4) photomicrographs obtained at 100x from negative control and 10 μM Clz cultures shown in panels A and B, and quantitative ORO absorbance data shown in panel C. Data are mean ± SEM (n = 4) of raw absorbance values; ***p<0.001 by Student’s t-test. D. UD 3T3-L1 cells were exposed to a sub-threshold adipogenic induction stimulus, alone or in combination with 10 μM Clz. At 10 and 14 days PI, cultures were terminated and ORO-stained. Data are mean ± SEM (n = 7) of raw absorbance values; *p<0.05, ***p<0.001 by Dunnett’s post-test. E. The same ORO absorbance data as in preceding panels, collected at D14, but expressed as fold change versus negative control (set as 1.0), showing the sub-threshold induction and Clz exposure alone (“ind” and “clz”) and in combination (“ind + clz”).
Clz potentiates adipogenesis
In a physiological setting, the antipsychotic would be but one component of a complex chemical milieu regulating adipose hyperplasia. To more closely represent an in vivo environment, which may already be driving the adipogenic differentiation of preadipocytes, we assessed Clz effects by combining it with a threshold-level stimulus (which drives significant adipogenesis alone). To reiterate, in our experimental design, each culture plate (single biological replicate) includes a non-induced/negative control condition, induced/positive control condition (induction stimulus alone, without antipsychotic), and various antipsychotic-treated induced groups, all processed at the same time, in parallel. For experiments involving the threshold-level stimulus, we also tested multiple concentrations of Clz (10, 5, and 1 μM) in combination with the stimulus. As shown in Figure 2A, combining the threshold-level stimulus with 10 μM Clz, during the 4-day induction only, caused clear potentiation versus matched positive control in adipocyte-like morphological changes and lipid accumulation. In our estimation, 5 μM Clz also resulted in obvious potentiation, with a greater number of lipid-accumulating adipocyte-like cells versus positive controls; 1 μM Clz did not display any clear trends.
Figure 2.
Clz potentiates adipogenic differentiation, as indicated by intracellular lipid accumulation, in response to a threshold-level stimulus. UD 3T3-L1 cells were exposed to a threshold-level adipogenic stimulus alone (“Positive control” or “0 μM Clz”) or in combination with the various Clz concentrations indicated (as described in the methods section). A. At 14 days PI, photomicrographs were obtained of cultures at 100x (left-hand panels, intracellular lipid as characteristic golden spheres). Cultures were terminated and ORO-stained; after staining, photomicrographs of cultures were again obtained at 100x (right-hand panels). Photomicrographs are from a single biological replicate, but are broadly representative in trend of n = 6 total biological replicates. B. Quantitative data for ORO staining of Clz-treated cultures, obtained at 14 and 17 days PI. Data (mean ± SEM) are expressed as fold change versus internal positive control (0 μM Clz), with internal negative control as background, for each biological replicate (n = 6 for D14, n = 3 for D17); *p<0.05 by Dunnett’s post-test.
Quantitative measurement of lipid accumulation after ORO staining demonstrated significant potentiation for 10 μM Clz versus matched positive controls at 14 days PI (Figure 2B); trends toward significant potentiation were also apparent for both 10 and 5 μM Clz at 17 days PI (Figure 2B), but results were just short of statistical significance (p ≈ 0.07-0.09). At 17 days, all individual biological replicates showed greater lipid accumulation for the 10 and 5 μM Clz conditions versus their matched positive controls (fold changes of ≈ 1.4-3.7). However, there was great variability in the precise change, a reasonable outcome given the long culture time involved enabling greater experiment-to-experiment divergence by the endpoint. Overall, these findings further support the ability of Clz to drive persistent pro-adipogenic changes, here in the context of an existing sufficient adipogenic induction stimulus.
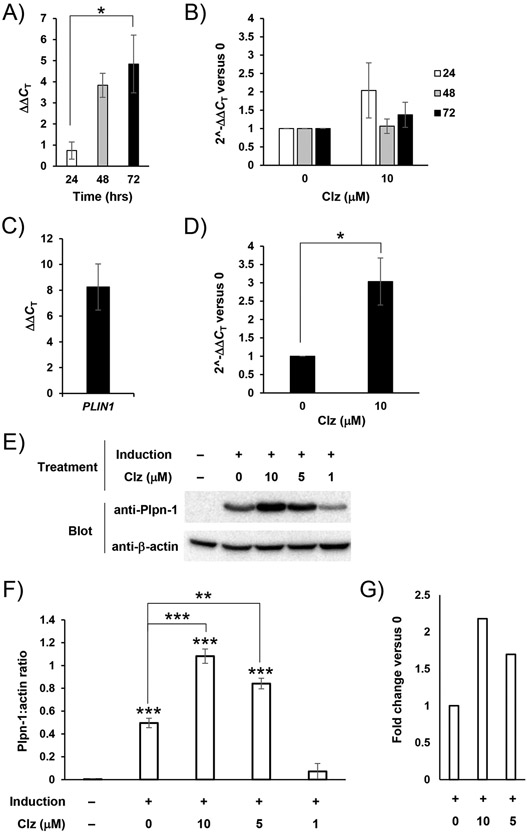
Clz impacts the adipogenic gene expression program
The timing of Clz exposure (coming during the critical adipogenic induction period), and the persistent nature of the Clz effects observed to this point (specifically persisting for many days after the end of exposure) point to effects on the adipogenic gene expression program. To address such effects, we employed RT-qPCR for analysis of relevant transcript levels in differentiating cells. We began by specifically analyzing cells for expression of PPARG, a gene encoding the PPARγ transcription factor which acts as an early response master regulator of adipogenic differentiation [16,20]. We found clear evidence of PPARG upregulation in cells subjected to a threshold-level induction stimulus versus matched non-induced controls, particularly by 72 hours PI (Figure 3A). In comparing cells induced with 10 μM Clz, we did not observe any significant changes or clear trends toward antipsychotic-enhanced PPARG upregulation (Figure 3B). We also analyzed expression of another pro-adipogenic transcription factor, CEBPα, however we observed no effects of antipsychotic exposure on the CEBPA gene (Figure S2).
Figure 3.
Clz specifically increases expression of mature adipocyte markers but not master regulators of adipogenesis. A. UD 3T3-L1 cells were exposed to a threshold-level adipogenic stimulus for the indicated lengths of time, then analyzed by RT-qPCR (as described in the methods section) for PPARG. Resulting data were analyzed according to the ΔΔCT method, showing PPARG upregulation in induced relative to non-induced cells. Data are mean ± SEM (n = 3-4 biological replicates); *p<0.05 by Tukey’s post-test. B. As in A, however this panel includes both the positive control (0 μM Clz) and a Clz treatment condition (threshold-level stimulus plus 10 μM Clz). Resulting data were analyzed according to the 2^-ΔΔCT method, showing fold change in PPARG expression versus positive control (0 μM Clz) cells. Data are mean ± SEM (n = 3-5). C. As in A, but for 72 hours total only and analyzing for PLIN1. Resulting data were analyzed according to the ΔΔCT method, now showing PLIN1 upregulation in induced relative to non-induced cells. Data are mean ± SEM (n = 3). D. As in C, however this panel includes both the positive control (0 μM Clz) and a Clz treatment condition (threshold-level stimulus plus 10 μM Clz). Resulting data were analyzed according to the 2^-ΔΔCT method, showing fold change in PLIN1 expression versus positive control (0 μM Clz) cells. Data are mean ± SEM (n = 3); *p<0.05 by Student’s t-test. E. Cells were prepared and induced to differentiate as in previous panels. At culture termination (now 14 or 17 days PI), whole-cell homogenate was obtained and analyzed by Western blotting (as described in the methods section). Chemiluminescent images shown feature samples from a single biological replicate, representative of n = 3 total biological replicates. F. Chemiluminescent images from Western blotting described in E were analyzed by densitometry. Data are presented as a ratio of Plpn-1 bands to their corresponding loading control (β-actin) bands, and are mean ± SEM (n = 3); **p<0.01 versus positive control (0 μM Clz, indicated by lines), ***p<0.001 versus negative control, or versus positive control (indicated by lines), by Tukey’s post-test. G. The same data as in F, but expressed as fold change versus the internal positive control (0 μM Clz, set as 1.0).
Although we did not see any clear impacts of Clz exposure on upregulation of adipogenic transcription factors, we were able to observe a greater upregulation of the gene for Plpn-1 (PLIN1), an essential protein in adipocyte function, contributing to the amphipathic coating necessary around cytoplasmic lipid droplets [21]. At 72 hours PI, we observed not only a clear induction of this delayed response gene in induced versus non-induced controls (Figure 3C), but also an even greater upregulation of PLIN1 in Clz-treated cells (Figure 3D). This early transcript-level upregulation was further accompanied by a persistent potentiation of Plpn-1 protein levels, shown by Western blotting analysis of cultures terminated at 14 or 17 days PI (Figure 3E-G).
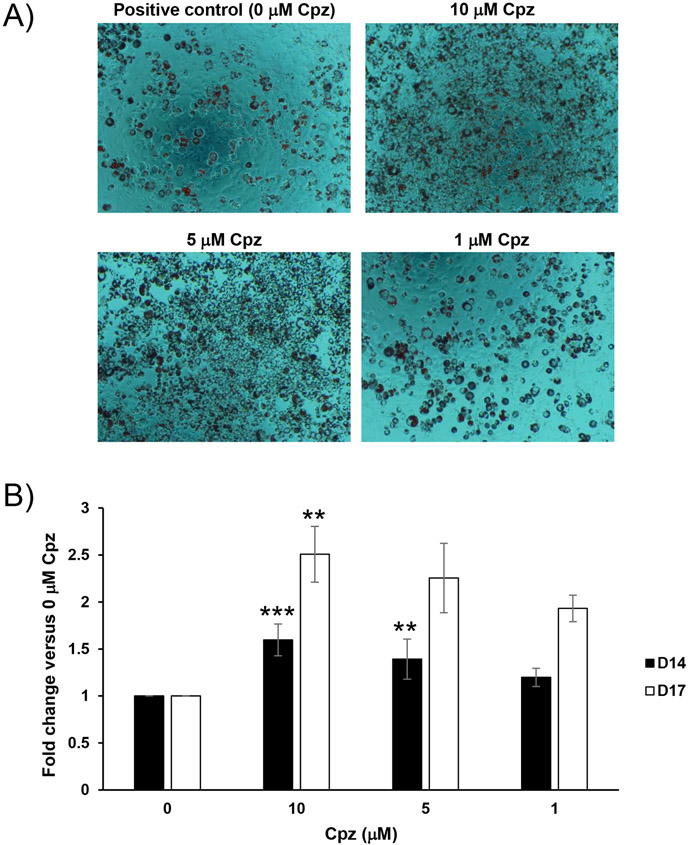
Cpz also potentiates adipogenesis
As a final addition to our study, we assessed whether the chemically and clinically related tricyclic antipsychotic chlorpromazine (Cpz) could drive a similar potentiation of adipogenic differentiation in our model system; we repeated the experiments already described for Figure 2, substituting Cpz for Clz. Results clearly demonstrated that Cpz, when applied together with a threshold-level adipogenic stimulus during the 4-day induction only, does indeed significantly potentiate apparent adipogenic differentiation (Figure 4; see also Figure S3 for additional representative photomicrographs). Results were broadly similar to those obtained under the same overall conditions with Clz (Figure 2), but with more Cpz-treated conditions displaying statistically significant quantitative potentiation of adipogenesis.
Figure 4.
Cpz potentiates adipogenic differentiation in response to a threshold-level stimulus. UD 3T3-L1 cells were exposed to a threshold-level adipogenic stimulus alone (“Positive control” or “0 μM Cpz”) or in combination with various Cpz concentrations indicated (as described in the methods section). A. At 14-days PI, cultures were terminated and ORO-stained; after staining, photomicrographs of cultures were again obtained at 100x. Photomicrographs are from a single biological replicate, but are broadly representative in trend of n = 6 total biological replicates. B. Quantitative data for ORO staining of Cpz-treated cultures, obtained at 14 and 17 days PI. Data (mean ± SEM) are expressed as fold change versus internal positive control (0 μM Cpz), with internal negative control as background, for each biological replicate (n = 6 for D14, n = 5 for D17); **p<0.01, ***p<0.001, versus corresponding 0 μM Cpz condition, by Dunnett’s post-test.
Discussion
Our hypothesis that tricyclic antipsychotics potentiate adipogenesis via enhancement of the underlying adipogenic gene expression program is strongly supported by our findings. Clz acts alone (Figure 1) or in combination with an independent threshold-level stimulus (Figure 2) to induce adipogenic differentiation of preadipocytes. Clear potentiation of morphological changes and lipid accumulation are accompanied by greater upregulation in expression of the mature adipocyte marker Plpn-1 (Figure 3C-G). We did not see Clz-dependent effects on expression of the major pro-adipogenic transcription factors PPARγ or CEBPα (Figure 3A-B, Figure S2), which are upstream of mature markers like Plpn-1 in the adipogenic gene expression program; these results are broadly consistent with a previous report regarding high-dose olanzapine in stem cell differentiation [13]. These results clearly demonstrate that Clz acts during the adipogenic induction period to directly and persistently potentiate the expression of a key adipocyte marker. Our findings regarding Cpz (Figure 4, Figure S3) further raise the possibility that Plpn-1 upregulation-based potentiation of adipogenesis may be a general property shared across the weight gain-inducing tricyclic antipsychotic class.
Like all antipsychotics, Clz binds multiple cell-surface targets [1], including to the adrenergic receptors which normally regulate lipolysis and lipogenesis in mature adipocytes [21,22], primarily acting as an antagonist with respect to canonical heterotrimeric G protein signaling, but also with other possible functional outcomes including recruitment of arrestins (key multifunctional intracellular effectors [23,24]) to the receptor [14]. However, our results indicate that Clz alone is an insufficient stimulus for lipogenesis in differentiated adipocytes (Figure S1), suggesting much more than an acute effect on signal transduction; this is consistent with a similar previous report [6] and further supports a mechanism wherein Clz affects adipose by driving hyperplasia, driving differentiation and addition of new adipocytes to the tissue, rather than hypertrophy, driving increases in size of existing adipocytes.
It remains unclear how Clz modulates Plpn-1 expression. Our results indicate that Clz does not impact the induction of the classic master adipogenic transcriptional regulators PPARγ and CEBPα [16,20]. Regarding CEBPα, this is not entirely unexpected; CEBPα is generally thought to be involved more critically later, in maintenance rather than induction of adipogenesis [20], and is generally upregulated later anyway, consistent with previous similar studies [12,13]. As to PPARγ, given the early timing of our analysis, within the initial 4-day induction period (i.e., precisely when this immediate early gene is being induced), our finding of no additional antipsychotic-dependent upregulation is somewhat more unexpected. However, the antipsychotic may still act on some other related adipose-related transcription factor, e.g. SREBP1c [12]. Alternatively, it may act directly on some other molecular target entirely, or indirectly via a signal transduction pathway, for example through the aforementioned modulation of arrestin mediator recruitment to adrenergic receptors [14]. Given the diverse array of known cell-surface targets for these drugs, and their lipophilicity, which raises the possibility of plasma membrane permeance and interaction with intracellular targets [1], determining the precise molecular players involved in our Clz effects represents a daunting task that will require significant future efforts. It should also be noted, once again, that our study relies on a mouse-derived cell line; although it is common practice to use mouse model systems as a general physiological stand-in for human ones in studies of such clinically relevant problems, the precise translatability of any specific finding cannot be guaranteed.
Despite its limitations, our study nevertheless advances mechanistic knowledge in the field. In contrast to previous work on this subject, we have used a range of lower Clz concentrations that more closely approximate physiologically-relevant values and evaluated the precise timing of antipsychotic exposure during preadipocyte differentiation and maturation under a consistent general set of experimental conditions, while our finding of Cpz effects (again, over a range of concentrations that approximate physiologically-relevant values) is an entirely novel contribution. Overall, we have highlighted a sequence of events in our mouse preadipocyte model system potentially relevant to antipsychotic-induced weight gain, wherein a tricyclic antipsychotic enhances upregulation of Plpn-1 in preadipocytes experiencing adipogenic stimuli, contributing to adipose hyperplasia.
Supplementary Material
Acknowledgments
The authors thank Kurt Gibbs (Morehead State University) for advice regarding RT-qPCR assays, Amber Hall (formerly of Morehead State University) for contributions to RT-qPCR data collection, and Qin Wang (University of Alabama at Birmingham) for providing frozen 3T3-L1 stock.
Funding
This work was supported by the National Institutes of Health [grant number P20GM103436].
Abbreviations
- Clz
clozapine
- Cpz
chlorpromazine
- CEBP
CCAAT-enhancer-binding protein
- ORO
Oil Red O
- PPAR
peroxisome proliferator-activated receptor
- Plpn-1
perilipin-1
- PI
post-induction
- RT-qPCR
reverse-transcription quantitative (real-time) PCR
- UD
undifferentiated
Footnotes
Compliance with ethical standards
The authors declare that they have no conflict of interest. Other ethics approvals are not applicable to this study.
References
- [1].Meyer JM, Pharmacotherapy of psychosis and mania, in: Goodman & Gilman’s The Pharmacological Basis of Therapeutics, 13th ed., McGraw-Hill Education, 2018: pp. 279–302. [Google Scholar]
- [2].Lieberman JA, Rosenheck RA, Davis SM, Hsiao JK, Effectiveness of Antipsychotic Drugs in Patients with Chronic Schizophrenia, The New England Journal of Medicine. (2005) 15. [DOI] [PubMed] [Google Scholar]
- [3].Bak M, Fransen A, Janssen J, van Os J, Drukker M, Almost All Antipsychotics Result in Weight Gain: A Meta-Analysis, PLOS ONE. 9 (2014) e94112. 10.1371/journal.pone.0094112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Li Y, Zhao X, Feng X, Liu X, Deng C, Hu C-H, Berberine Alleviates Olanzapine-Induced Adipogenesis via the AMPKα–SREBP Pathway in 3T3-L1 Cells, Int J Mol Sci. 17 (2016). 10.3390/ijms17111865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Yang Z, Yin J-Y, Gong Z-C, Huang Q, Chen H, Zhang W, Zhou H-H, Liu Z-Q, Evidence for an effect of clozapine on the regulation of fat-cell derived factors, Clinica Chimica Acta. 408 (2009) 98–104. 10.1016/j.cca.2009.07.021. [DOI] [PubMed] [Google Scholar]
- [6].Tsubai T, Yoshimi A, Hamada Y, Nakao M, Arima H, Oiso Y, Noda Y, Effects of clozapine on adipokine secretions/productions and lipid droplets in 3T3-L1 adipocytes, Journal of Pharmacological Sciences. 133 (2017) 79–87. 10.1016/j.jphs.2017.01.004. [DOI] [PubMed] [Google Scholar]
- [7].Chen C-C, Hsu L-W, Huang K-T, Goto S, Chen C-L, Nakano T, Overexpression of Insig-2 inhibits atypical antipsychotic-induced adipogenic differentiation and lipid biosynthesis in adipose-derived stem cells, Sci Rep. 7 (2017) 1–10. 10.1038/s41598-017-11323-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Sertié AL, Suzuki AM, Sertié RAL, Andreotti S, Lima FB, Passos-Bueno MR, Gattaz WF, Effects of antipsychotics with different weight gain liabilities on human in vitro models of adipose tissue differentiation and metabolism, Progress in Neuro-Psychopharmacology and Biological Psychiatry. 35 (2011) 1884–1890. 10.1016/j.pnpbp.2011.07.017. [DOI] [PubMed] [Google Scholar]
- [9].Green H, Kehinde O, An established preadipose cell line and its differentiation in culture II. Factors affecting the adipose conversion, Cell. 5 (1975) 19–27. 10.1016/0092-8674(75)90087-2. [DOI] [PubMed] [Google Scholar]
- [10].Green H, Meuth M, An established pre-adipose cell line and its differentiation in culture, Cell. 3 (1974) 127–133. 10.1016/0092-8674(74)90116-0. [DOI] [PubMed] [Google Scholar]
- [11].Hemmrich K, Gummersbach C, Pallua N, Luckhaus C, Fehsel K, Clozapine enhances differentiation of adipocyte progenitor cells, Mol Psychiatry. 11 (2006) 980–981. 10.1038/sj.mp.4001892. [DOI] [PubMed] [Google Scholar]
- [12].Yang L-H, Chen T-M, Yu S-T, Chen Y-H, Olanzapine induces SREBP-1-related adipogenesis in 3T3-L1 cells, Pharmacological Research. 56 (2007) 202–208. 10.1016/j.phrs.2007.05.007. [DOI] [PubMed] [Google Scholar]
- [13].Nimura S, Yamaguchi T, Ueda K, Kadokura K, Aiuchi T, Kato R, Obama T, Itabe H, Olanzapine promotes the accumulation of lipid droplets and the expression of multiple perilipins in human adipocytes, Biochemical and Biophysical Research Communications. 467 (2015) 906–912. 10.1016/j.bbrc.2015.10.045. [DOI] [PubMed] [Google Scholar]
- [14].Cottingham C, Che P, Zhang W, Wang H, Wang RX, Percival S, Birky T, Zhou L, Jiao K, Wang Q, Diverse arrestin-recruiting and endocytic profiles of tricyclic antipsychotics acting as direct α 2A adrenergic receptor ligands, Neuropharmacology. 116 (2017) 38–49. 10.1016/j.neuropharm.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Rosen ED, MacDougald OA, Adipocyte differentiation from the inside out, Nature Reviews Molecular Cell Biology. 7 (2006) 885–896. 10.1038/nrm2066. [DOI] [PubMed] [Google Scholar]
- [16].Cristancho AG, Lazar MA, Forming functional fat: a growing understanding of adipocyte differentiation, Nature Reviews Molecular Cell Biology. 12 (2011) 722–734. 10.1038/nrm3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Schmittgen TD, Livak KJ, Analyzing real-time PCR data by the comparative CT method, Nat Protoc. 3 (2008) 1101–1108. 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- [18].Cottingham C, Chen Y, Jiao K, Wang Q, The Antidepressant Desipramine Is an Arrestin-biased Ligand at the α 2A -Adrenergic Receptor Driving Receptor Down-regulation in Vitro and in Vivo, Journal of Biological Chemistry. 286 (2011) 36063–36075. 10.1074/jbc.M111.261578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Cottingham C, Jones A, Wang Q, Desipramine selectively potentiates norepinephrine-elicited ERK1/2 activation through the α2A adrenergic receptor, Biochem Biophys Res Commun. 420 (2012) 161–165. 10.1016/j.bbrc.2012.02.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Tang QQ, Lane MD, Adipogenesis: From Stem Cell to Adipocyte, Annual Review of Biochemistry. 81 (2012) 715–736. 10.1146/annurev-biochem-052110-115718. [DOI] [PubMed] [Google Scholar]
- [21].Brasaemle DL, Thematic review series: Adipocyte Biology . The perilipin family of structural lipid droplet proteins: stabilization of lipid droplets and control of lipolysis, Journal of Lipid Research. 48 (2007) 2547–2559. 10.1194/jlr.R700014-JLR200. [DOI] [PubMed] [Google Scholar]
- [22].Lafontan M, Berlan M, Fat Cell α2-Adrenoceptors: The Regulation of Fat CellFunction and Lipolysis*, Endocrine Reviews. 16 (1995) 716–738. 10.1210/edrv-16-6-716. [DOI] [PubMed] [Google Scholar]
- [23].Shenoy SK, Lefkowitz RJ, Multifaceted roles of beta-arrestins in the regulation of seven-membrane-spanning receptor trafficking and signalling, Biochem. J 375 (2003) 503–515. 10.1042/BJ20031076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Rajagopal S, Rajagopal K, Lefkowitz RJ, Teaching old receptors new tricks: biasing seven-transmembrane receptors, Nat Rev Drug Discov. 9 (2010) 373–386. 10.1038/nrd3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.