Abstract
Biological diversity can be divided into: alpha (α, local), beta (β, difference in assemblage composition among locals), and gamma (γ, total diversity). We assessed the partitioning of taxonomic diversity of Ephemeroptera, Plecoptera and Trichoptera (EPT) and of functional feeding groups (FFG) in neotropical savanna (southeastern Brazilian cerrado) streams. To do so, we considered three diversity components: stream site (α), among stream sites (β1), and among hydrologic units (β2). We also evaluated the association of EPT genera composition with heterogeneity in land use, instream physical habitat structure, and instream water quality variables. The percentage of EPT taxonomic α diversity (20.7%) was smaller than the β1 and β2 diversity percentages (53.1% and 26.2%, respectively). The percentage of EPT FFG collector-gatherer α diversity (26.5%) was smaller than that of β1 diversity (55.8%) and higher than the β2 (17.7%) diversity. The collector-gatherer FFG was predominant and had the greatest β diversity percentage among stream sites (β1, 55.8%). Our findings support the need for implementing regional scale conservation strategies in the cerrado biome, which has been degraded by anthropogenic activities.
Keywords: Beta diversity, EPT assemblages, Functional feeding groups, Brazilian cerrado
1. Introduction
Whittaker (1960) first proposed the concepts of alpha (α), beta (β), and gamma (γ) diversities. In general terms, alpha diversity corresponds to local diversity, beta diversity corresponds to difference in assemblage composition among locals, and gamma diversity corresponds to total regional diversity. In the additive partitioning of species diversity, α diversity is typically expressed as the mean number of taxa observed at any given scale (Lande, 1996; Veech et al., 2002; Anderson et al., 2011). β diversity is the difference in the α diversities between two scales in the spatial hierarchy, and γ diversity represents the sum of the α diversity and all involved β diversities within a given region (Jost et al., 2010). Knowing how the taxa of a regional pool are distributed among multiple scales is an important issue in ecology (Jankowski et al., 2009; Heino et al., 2015a). Therefore, evaluating the pattern of diversity distribution through additive partitioning is important when determining scales of major interest for conserving and rehabilitating aquatic ecosystems (Diniz-Filho et al., 2009; Molozzi et al., 2013). Determining the scale where most biological variability occurs helps managers and conservationists focus their efforts and resources where they are most likely to have the greatest effect.
Recent studies focusing on the additive partitioning of species diversity have sought to understand the distribution patterns of assemblages at several spatial scales (Frissell et al. 1986; Rietkerk et al., 2002; Jost et al., 2010; Ligeiro et al., 2010; Ávila et al., 2011). Such studies are needed because ecological processes and distribution patterns vary with the scale of spatial observation, which can range from centimeters to kilometers (Allan and Castillo, 2007). In addition, enhancing the understanding of spatial differences in organism interactions with their habitats and the processing of food resources facilitates the refinement of biomonitoring programs. For example, Boyero (2003) showed that assemblages of Ephemeroptera, Plecoptera and Trichoptera (EPT) varied substantially among habitats, streams and basins. So, biotic indices and ranges of measures must be analyzed and calibrated at different scales to adjust for such geographic differences if one wishes to increase the sensitivity of those indices (Stoddard et al., 2008a). Similarly, several authors have shown that habitat heterogeneity, which supports biological diversity in aquatic ecosystems, is organized in a spatial hierarchy (Cortes et al., 2010; Ligeiro et al., 2010; Ávila et al., 2011; Hepp et al., 2012). Determining how biological traits and ecological processes vary with spatial scale aids managers and conservationists in refining biological metrics and indices and in separating natural variability from anthropogenic disturbances.
In lotic ecosystems, local scales show heterogeneity in assemblage structure and abiotic conditions (Ligeiro et al., 2010; Macedo et al., 2014; Heino et al., 2015a). Taxonomic surveys, the evaluation of functional feeding groups (FFGs), and studies of the heterogeneity and distribution of assemblage characteristics among discrete sites are all important for elucidating how taxonomic diversity and biological traits are distributed in ecosystems (Boyero, 2003). In particular, including functional aspects of biological assemblages is important for making more comprehensive assessments of ecological condition than are possible with taxonomic assessments alone. Although FFGs are essential for understanding many processes in aquatic ecosystems, studies of the distribution of the taxonomic composition of functional feeding groups (e.g., predators, shredders, collectors, and scrapers) across spatial scales are lacking for tropical regions (Boyero, 2005). Each of these groups relies on specific food resources, which are in turn influenced by different habitat characteristics. For instance, collector-gatherers feed on fine particulate organic matter (FPOM), and are more common in fine substrates and still waters, whereas collector-filterers position themselves on substrates exposed to flowing water, from which they sieve FPOM that is suspended in the water (Cummins et al., 2005; Merritt et al., 2008). Thus, differences in resources and habitat characteristics (Boyero, 2003) are likely to produce differences in the proportions of various functional feeding groups. Studies dealing only with assemblage taxa richness miss important variations in the functional composition of those assemblages (Marzin et al., 2012; Leitão et al., 2016).
The EPT are generally sensitive to changes in aquatic environments (Bonada et al., 2006; Stoddard et al., 2008a). Because of their sensitivity to anthropogenic disturbances, the EPT are among the most commonly used ecological indicators in large-scale (regional and national) biological assessments (e.g. Stoddard et al., 2008a; Moya et al., 2011; Chen et al., 2014). Therefore, it is useful to evaluate how geographic scale and functional feeding groups (FFGs) affect EPT diversity.
In this study we evaluated how the taxonomic composition of whole EPT assemblages and of individual FFG are distributed among spatial scales, using a hierarchical series of three diversity components in neotropical savanna streams: stream site (α), among stream sites (β1), and among hydrologic units (β2). We evaluated two hypotheses: (i) β diversity is not evenly distributed among spatial scales. Environmental variables at the local scale (e.g., substrate type, current velocity, width and water depth) greatly influence biological communities (Ligeiro et al., 2010; Hepp et al., 2012) and for this reason we expected that taxonomic composition and FFG would show greater variability among sites than among hydrologic units. (ii) Taxonomic diversity distribution follows distinct patterns among the different FFG. Considering that food resources and habitat availability vary at different scales (Boyero 2003), we expected that individual FFG would display distinct α and β diversities and that the partition pattern of whole EPT assemblages would be defined by the partition of the most abundant FFG.
2. Material and Methods
2.1. Study area and site selection
We sampled 160 wadeable stream sites (stream orders ranging from 1–3 on 1:100,000 scale maps) (Strahler, 1957) belonging to the Araguari, São Francisco, Rio Grande, and Paranaíba River Basins in the states of Minas Gerais, São Paulo, and Goiás, southeastern Brazil (Figure 1). The hydrologic units (Seaber et al., 1987) were defined as the contributing drainage areas within 35 km upstream of each of four major hydropower reservoirs (Nova Ponte, Três Marias, Volta Grande, São Simão). The sites are all located in the neotropical savanna, which has a humid tropical and seasonal climate with approximately 1,600 mm mean annual rainfall (Brasil, 1992). Regional climate is characterized by a dry season from May-September, with monthly precipitation between 10–55 mm, and a rainy season between October-April, with monthly precipitation between 100–300 mm. The neotropical savanna, which is one of the most threatened biomes worldwide, is a priority hotspot for biodiversity conservation (Myers et al., 2000). Since the 1950s, agriculture and pasture have progressively replaced natural areas (Diniz-Filho et al., 2009), resulting in clearing of more than half of the original ~2 million km2 forested area (Klink and Machado, 2005; Wantzen et al., 2006). We selected sampling sites by using a randomized, spatially balanced, systematic sample design adapted from one the U.S. Environmental Protection Agency developed for its National Rivers and Streams Assessment (Olsen and Peck, 2008). Each year (2009–2012) during the dry season, we sampled 40 wadeable stream sites in one of the four regions for a total of 160 sites.
Figure 1.
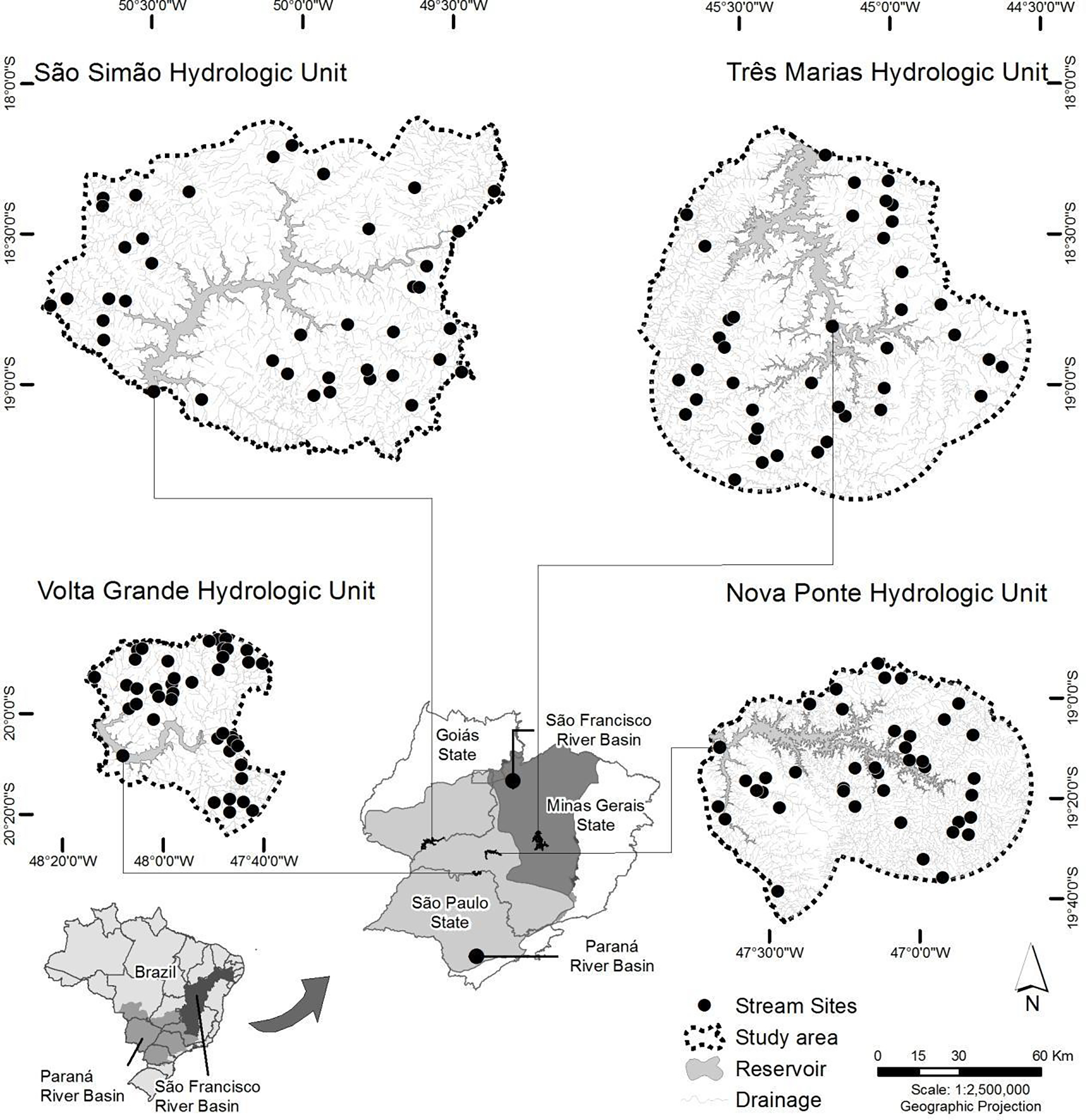
Locations of wadeable stream sites (n=160) in four hydrologic units in the neotropical savanna, Minas Gerais, southeastern Brazil.
2.2. Catchment land use and land cover
We classified land use and cover within the catchment upstream of each site by interpreting a combination of high-resolution satellite images (0.6–5.0 m spatial resolution, Google Earth data: Google 2010) and Landsat multispectral satellite images (R4G3B2 false color band combination). This method is very accurate because the high-resolution satellite images better distinguish the shape of units, while multispectral images better distinguish vegetation leaf structure (e.g., more- or less-dense canopy and biomass concentration) (Macedo et al., 2014). We identified four natural vegetation cover types (woodland savanna, grassy-woody savanna, parkland savanna, and wetland palm swamps) and four land uses (pasture, agriculture, Eucalyptus forest, and urban areas) in the 160 catchments.
2.3. Site physical habitat structure and water quality
We characterized physical habitat structure and water quality at each sampling site with standardized field methods (Kaufmann et al., 1999; Peck et al., 2006); this included multiple metrics of channel morphology, riparian structure, flow type, substrate type, and instream habitat cover (Kaufmann et al., 1999, 2008). Metrics were selected from a master list (see Table 1) by removing redundant metrics through use of correlation analysis and principal component analysis (PCA; Ferreira et al., 2014). To assess water quality, we measured temperature (°C), electrical conductivity (μS.cm−1), pH, turbidity (NTU), and total dissolved solids (mg.L−1) in situ with a multi-probe (YSI, 650 MDS, model 6920). Total nitrogen (mg.L−1) and dissolved oxygen concentrations (mg.L−1) were determined from preserved water samples in the lab following Standard Methods (APHA, 2005).
Table 1.
Candidate physical habitat structure and water quality variables (from Ferreira et al., 2014)
| Variables group and name | Variable code |
|---|---|
|
| |
| Channel morphology | |
| Mean depth of cross-section (cm) | xdepth_s |
| Mean wetted width (m) | xwidth |
| Mean bankfull width (m) | xbkf_w |
| Mean residual depth (cm) | rp100 |
| Channel water surface slope - reach mean (%) | xslope |
| Channel sinuosity (m.m−1) | Sinu |
| Bed substrate | |
| Mean embeddedness of channel and margin (%) | xembed |
| Standard deviation of embeddedness in channel + margin (%) | vembed |
| Log10 (Relative bed stability) | lrbs* |
| Substrate - log10(geometric mean diameter mm) | lsub_dmm* |
| Substrate % cobbles (diameter 64 – 250 mm) | pct_cb |
| Riparian | |
| Mean mid-channel canopy density (%) | xcdenmid |
| Standard deviation - mid-channel canopy density (%) | vcdenmid |
| Riparian vegetation canopy+mid+ground cover (%) | xcmg |
| Flow type | |
| Glide (% of reach) | pct_gl |
| Pools - all types (% of reach) | pct_pool |
| Slow water habitat (% glide + pool) | pct_slow |
| Shelter | |
| Coarse litter (%) | pct_bf |
| Large wood debris in bankfull channel (number/m2 - all size classes) | c1w_msq |
| Brush and small debris (areal proportion) | xfc_brs |
| Undercut banks (areal proportion) | xfc_ucb |
| Anthropogenic fish cover (areal proportion) | xfc_ant |
| Water quality | |
| Dissolved oxygen (mg.L−1) | DO |
| Negative log hydrogen ion concentration | pH |
| Turbidity (NTU) | Turbidity |
| Total nitrogen (mg.L−1) | N-total |
| Electrical conductivity (μS.cm−1) | Cond. |
| Total dissolved solids (mg.L−1) | TDS |
| Temperature of water | T 0C |
lrbs and lsub_dmm (Kaufmann et al., 2008).
2.4. Benthic macroinvertebrate sampling and functional feeding group classification
We sampled aquatic insects at all 160 sites with a D-framed kick net (30 cm aperture, 500 μm mesh, and 0.09 m2 area) for a total of 1 m2 per site. Sampling was performed at 11 equidistant transects via a systematic zigzag pattern throughout each site (minimum of 150 m), as described in Hughes and Peck (2008). Insect samples were fixed in 4% formalin then returned to the Benthos Ecology Laboratory of the Federal University of Minas Gerais, Brazil. In the laboratory, samples were rinsed on a 500-μm mesh sieve, then sorted EPT specimens were identified to genus-level with a stereo microscope (80x) using published keys (Wiggins, 1996; Pes et al., 2005; Salles, 2006; Merritt et al., 2008; Mugnai et al., 2010). The EPT FFG classifications were assigned to each taxa using published descriptions (Merritt et al., 2008; Oliveira and Nessimian, 2010; Shimano et al., 2012; Brasil et al., 2014). Scrapers feed on periphyton; shredders feed on leaves; collector-gatherers feed on fine particulate organic matter from stream bottoms; filterers feed on fine particulate organic matter in the water column; and predators eat live invertebrates.
2.5. Data analysis
We used additive partitioning to assess EPT genera and FFG diversities (Lande, 1996; Crist et al., 2003; Jost et al., 2010; Hepp and Melo, 2013). All data were assessed within a hierarchical scheme defined by each stream site (α), among stream sites (β1), among hydrologic units (β2), and total study area, where γ = α + β1 + β2 (Figure 2). We compared the observed diversity of each component with the expected diversity, as defined with the random individual-based (Type I) model of Crist et al. (2003). The additive partitioning randomizes macroinvertebrate individuals among the smallest sampling units, thereby removing any taxonomic or FFG aggregation that may exist among individuals on the analyzed scales (Crist et al., 2003; Ligeiro et al., 2010). We assessed the significance of our results by comparing the percents of diversity components from the observed values with the percents obtained from 999 randomizations. A high proportion of the data generated at random with values higher than the observed values (Propexp>obs > 0.975) indicates that the observed values were significantly less than those expected at random. In contrast, low proportions (Propexp>obs < 0.025) indicates that the observed values were significantly greater than those expected at random. Randomizations were performed with Partition v 3.0 software (Veech and Crist 2009).
Figure 2:
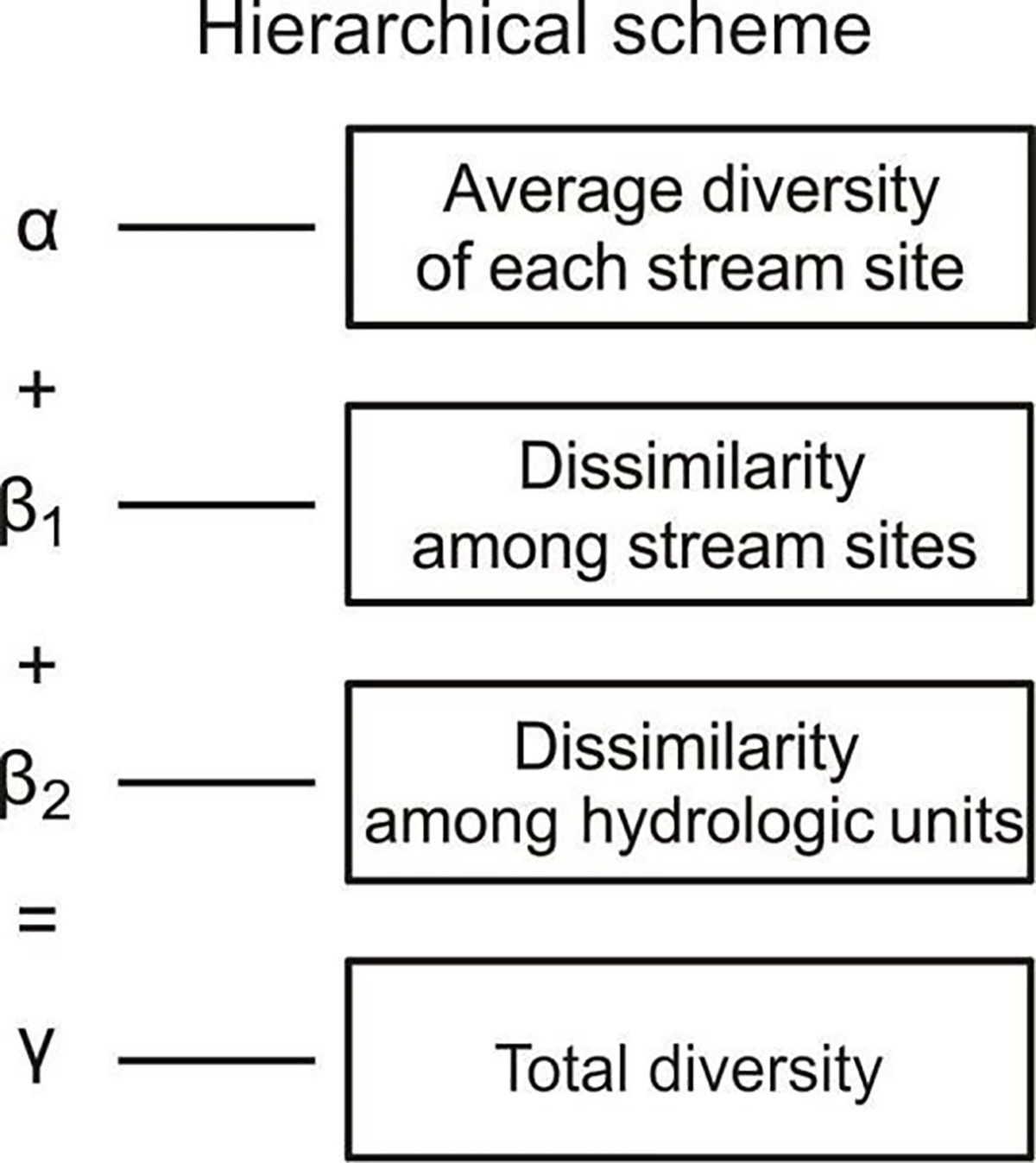
Schematic of diversity partitioning at different hierarchical levels.
The stream site and catchment environmental metrics (Table 1) were subjected to PCA to select those that had the highest correlation with PCA principal component 1. We selected metrics within each metric group (see Table 2) that contributed most to the dispersion of the data in the multivariate space of a principal component analysis (PCA). The 1st axis of each PCA (PCA 1) represents the clearest univariate gradient formed by the habitat metrics in each group (Ferreira et al., 2014). For example, PCA 1 for the channel-morphology metric group in the hydrologic units represents mean cross-section depth (cm) and mean residual depth (cm) (Table 2). We compared the selected metrics among the four hydrologic units via one-way ANOVA. Then, we subjected the selected metrics to a nested ANOVA by using the stream sites and the hydrologic units as random factors. We transformed the data (log x+1) to fulfill the variance homogeneity assumption for the nested ANOVA.
Table 2.
Physical habitat structure and water quality variables (mean, standard deviation) selected by PCA plus land use metrics that explain EPT distributions in the Neotropical Savanna.
| Variable groups and names | Variable code | Nova Ponte | Comparison of hydrologic units |
nested ANOVA (Random factors) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Três Marias | Volta Grande | São - Simao | ANOVA | Stream sites | Hydrologic units | ||||||
|
|
|
||||||||||
| F-value | p-value | F-value | p-value | F-value | p-value | ||||||
|
| |||||||||||
| Site | |||||||||||
| Channel morphology | |||||||||||
| Mean depth of cross-section (cm) | xdepth_s | 20.6 (10.4) | 27.8 (11.68) | 26.8 (11.37) | 25.2 (11.86) | 3.76 | 0.01* | 1.07 | 0.38 | 3.82 | 0.01* |
| Mean residual depth (cm) | rp100 | 10.3 (5.6) | 23.7 (12.8) | 17.5 (6.1) | 17.0 (8.6) | 14.16 | < 0.001* | 0.82 | 0.75 | 13.53 | <0.001* |
| Bed substrate | |||||||||||
| Substrate % cobbles (diameter 64 – 250 mm) | pct_cb | 7.9 (10.5) | 8.8 (12.9) | 10.3 (12.5) | 8.1 (9.9) | 0.26 | 0.85 | 1.35 | 0.11 | 0.27 | 0.84 |
| Riparian | |||||||||||
| Standard deviation: | |||||||||||
| mid-channel canopy density (%) | vcdenmid | 13.4 (10.50) | 14.2 (8.59) | 13.9 (9.1) | 13.6 (9.4) | 2.88 | 0.03* | 1.08 | 0.35 | 2.95 | 0.03* |
| Flow type | |||||||||||
| Pools - all types (% of reach) | pct_pool | 14.6 (18.3) | 31.1 (30.8) | 5.0 (12.4) | 4.4 (12.9) | 19.38 | < 0.001* | 0.98 | 0.50 | 19.32 | <0.001* |
| Shelter | |||||||||||
| Anthropogenic fish cover (%) | xfc_ant | 3.3 (9.0) | 6.7 (11.8) | 1.7 (4.8) | 7.9 (19.8) | 3.62 | 0.01* | 1.07 | 0.37 | 3.68 | 0.01* |
| Water quality | |||||||||||
| Dissolved oxygen (mg.L−1) | DO | 7.5 (1.16) | 7.7 (2.9) | 8.4 (3.2) | 7.6 (1.7) | 0.41 | 0.74 | 0.93 | 0.59 | 0.39 | 0.75 |
| Total dissolved solids (mg.L−1) | TDS | 15.2 (11.8) | 41.1 (33.5) | 19.8 (23.9) | 38.6 (31.5) | 12.20 | < 0.001* | 0.75 | 0.84 | 11.46 | <0.001* |
| Catchment | |||||||||||
| Woodland savanna (%) | wood_sav | 15.3 (8.0) | 16.0 (13.0) | 12.1 (5.8) | 13.6 (6.5) | 1.28 | 0.19 | 0.82 | 0.75 | 0.90 | 0.44 |
| Park land savanna (%) | park_sav | 9.6 (23.7) | 17.7 (18.9) | 0.00 (0.00) | 0.8 (2.3) | 24.99 | < 0.001* | 1.43 | 0.07 | 50.51 | <0.001* |
| Grassy-woody savanna (%) | gras_sav | 11.0 (14.8) | 10.3 (9.0) | 1.36 (1.47) | 1.5 (2.9) | 19.78 | < 0.001* | 1.05 | 0.4 | 23.30 | <0.001* |
| Wetland palm swamps (%) | palm_sav | 0.6 (1.3) | 1.4 (3.2) | 0.4 (1.1) | 2.4 (2.6) | 12.34 | < 0.001* | 0.91 | 0.61 | 12.18 | <0.001* |
| Pasture (%) | pasture | 16.6 (18.3) | 40.7 (18.2) | 11.6 (10.7) | 10.6 (14.6) | 29.25 | < 0.001* | 0.92 | 0.59 | 20.68 | <0.001* |
| Agriculture (%) | agriculture | 46.6 (29.7) | 3.0 (4.1) | 70.4 (15.8) | 68.1 (20.4) | 105.28 | < 0.001* | 1.19 | 0.22 | 116.99 | <0.001* |
| Eucalyptus forest (%) | eucalyptus | 0.00 (0.00) | 8.9 (11.1) | 0.2 (1.3) | 0.7 (1.4) | 30.83 | < 0.001* | 1.10 | 0.34 | 32.03 | <0.001* |
| Urban (%) | urban | 0.5 (2.6) | 2.1 (11.0) | 3.9 (12.1) | 2.3 (13.4) | 2.23 | 0.55 | 2.84 | <0.001* | 6.33 | <0.001* |
significant difference
3. Results
3.1. EPT assemblage composition and functional feeding groups
We collected 45,481 EPT specimens distributed among 80 genera in the 160 stream sites sampled (Figure 3a). The greatest abundance was found in Três Marias (14,943 individuals), and the lowest was found in Nova Ponte (5,463 individuals). The most abundant FFG was collector-gatherers (47%) and the lowest was predators (3.3%).
Figure 3.
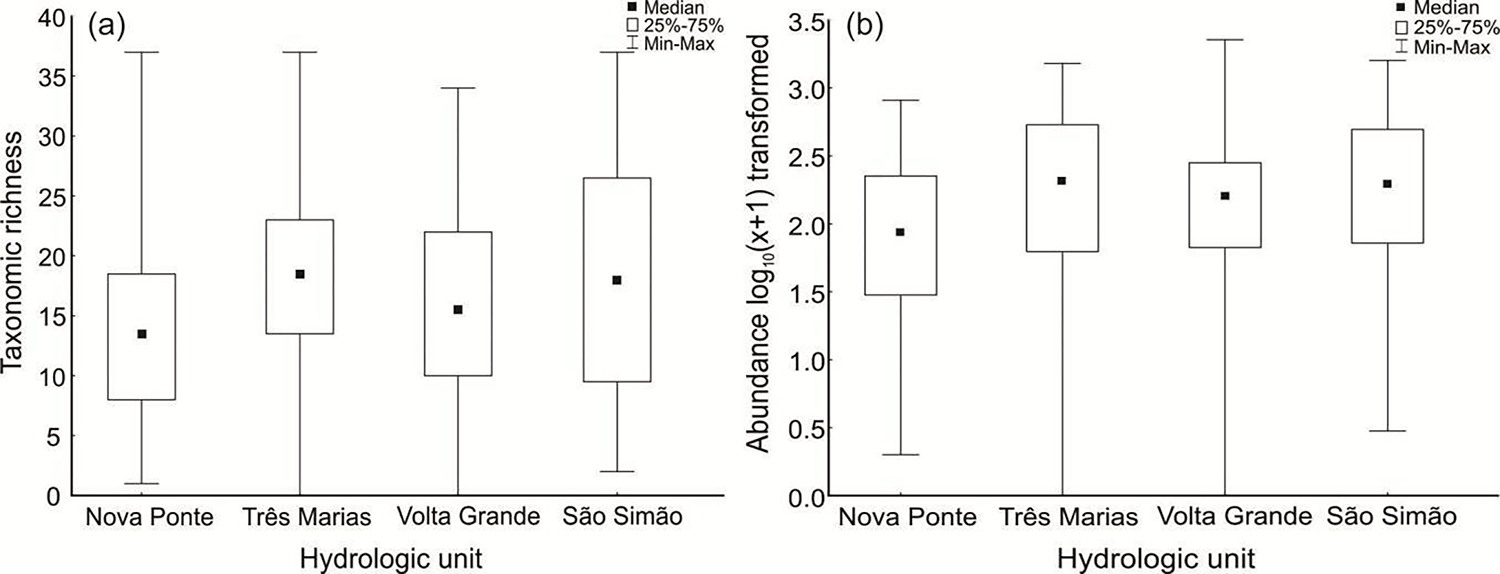
Distribution of (a) richness and (b) abundance of EPT genera in hydrologic units in the neotropical savanna, Minas Gerais, southeastern Brazil.
3.2. Physical habitat, land use, and cover
Site-specific channel morphology, riparian cover, flow types, instream habitat cover, and water quality were all significantly different among hydrologic units (one-way ANOVA: p < 0.05, Table 2), but dissolved oxygen and percent cobble were not. Significant differences were observed among the four hydrologic units for all land use and land cover types except percent woodland savanna (F(3, 156) = 1.3, p > 0.05) and percent urban area (F(3, 156) = 1.3, p > 0.05). Três Marias had the greatest proportion of pasture (40.7%), whereas Nova Ponte, Volta Grande and São Simão had large proportions of agriculture (46.6%, 70.4% and 68.1%, respectively, Table 2). The nested ANOVA indicated that variation in environmental metrics was almost always greater among hydrologic units than among sites (Table 2).
The sites showed good water quality values by Brazilian water quality standards (Brasil, 2005; Table 2, 3), but the hydrologic units differed in electrical conductivity (F(3, 156) = 18.5, p < 0.001) and total dissolved solids (F(3, 156) = 12.2, p < 0.001). The highest mean conductivity value (76.1 ± 92.3 μS.cm−1) was observed in Três Marias, and the lowest (43.6 ± 29.3 μS.cm−1) in Volta Grande. The highest mean value of total dissolved solids (41.1 ± 33.5 mg.L−1) also occurred in Três Marias, and the lowest (15.2 ± 11.8 mg.L−1) occurred in Nova Ponte (Table 2).
Table 3.
Mean and standard deviation of water quality variables and one-way ANOVA of the differences among the four hydrologic units.
| Hydrologic unit | Water quality variables |
||||
|---|---|---|---|---|---|
| pH | Turbidity (NTU) | Electrical conductivity (μS.cm−1) | Temperature (°C) | Total Nitrogen (mg.L−1) | |
|
| |||||
| Nova Ponte | 6.89 (0.46) | 7.6 (10.5) | 23.3 (17.7) | 20.3 (1.8) | 0.05 (0.01) |
| Três Marias | 7.7 (0.5) | 8.2 (14.5) | 76.1 (92.3) | 17.3 (1.8) | 0.24 (0.98) |
| Volta Grande | 7.1 (1.8) | 5.8 (4.7) | 43.6 (29.3) | 20.6 (1.4) | 0.11 (0.03) |
| São Simão | 6.8 (0.5) | 6.9 (4.2) | 68.7 (51.2) | 19.7 (3.6) | 0.10 (0.03) |
| ANOVA, F(3, 156) - value | 2.09 | 0.82 | 18.52 | 3.88 | 1.72 |
| p - value | 0.10 | 0.48 | < 0.001 | 0.01 | 0.16 |
3.3. Additive partitioning of taxonomic composition of EPT assemblages and individual FFG categories
The percentage of observed mean stream site (α) diversity (20.7%) for EPT taxonomic composition was lower than that observed among stream sites (β1) and among hydrologic units (β2) (53.1 and 26.2%, respectively). The expected α taxonomic diversity percentage (42.7%) was slightly less than that for expected β1 diversity (47.8%) and greater than that for expected β2 (9.4%) diversity. The observed β1 taxonomic diversity percentage (53.1%) was slightly greater than that expected at random (47.8%) (Figure 4). In contrast, the observed β2 taxonomic diversity percentage was nearly three times greater than the expected value (9.4%).
Figure 4.
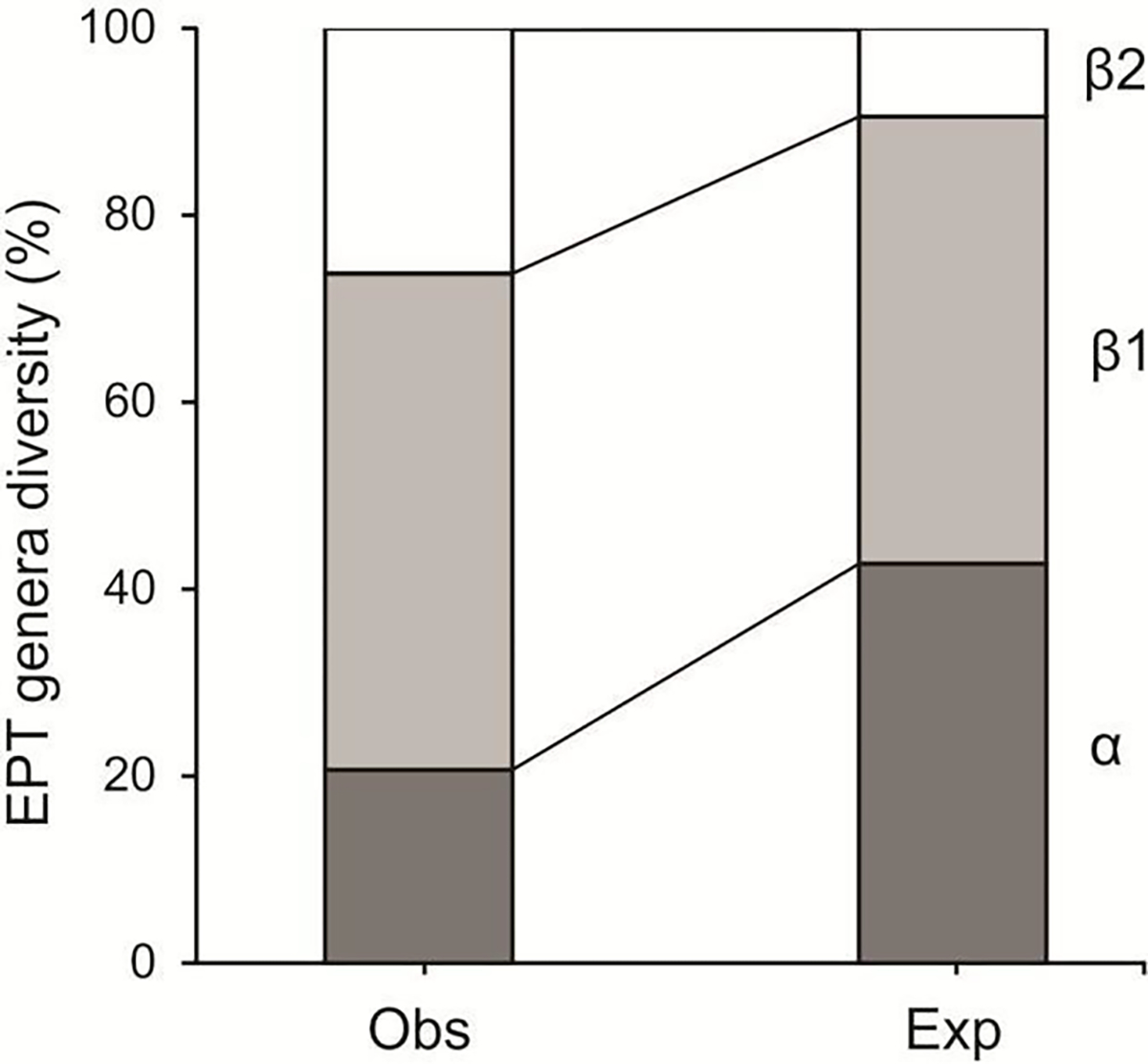
Additive partitioning of EPT observed and expected taxonomic composition in wadeable stream sites (α; Propexp>obs: > 0.999), among stream sites (β1; Propexp<obs: < 0.001), and among hydrologic units (β2; Propexp<obs: < 0.001) in the neotropical savanna, Minas Gerais, southeastern Brazil.
Separate functional analysis of the α and β diversity was possible only for the collector-gatherer FFG. Abundances of all other FFG were too low. Therefore, individuals from the remaining FFG were pooled to facilitate further diversity partitioning. The observed α diversity percentage for the collector-gatherer FFG (26.5%) was less than the observed β1 diversity (55.8%) and greater than the observed β2 diversity (17.7%), whereas the expected percentage of α diversity (53.25%) was greater than the expected percentage for β1 and β2 diversities (41.96% and 4.83%, respectively). The observed percentage of β1 diversity for the collector-gatherer FFG was greater (55.8%) than that expected at random and greater than that for the observed β2 collector-gatherer diversity (20.7%) (Figure 5). The same pattern was observed for the combined FFG (predators + shredders + filterers + scrapers) in which the observed percentage of β1 diversity was slightly greater (55.6%) than that expected at random (50.5%) and greater than for the observed β2 diversity (17.7%) (Figure 6).
Figure 5.
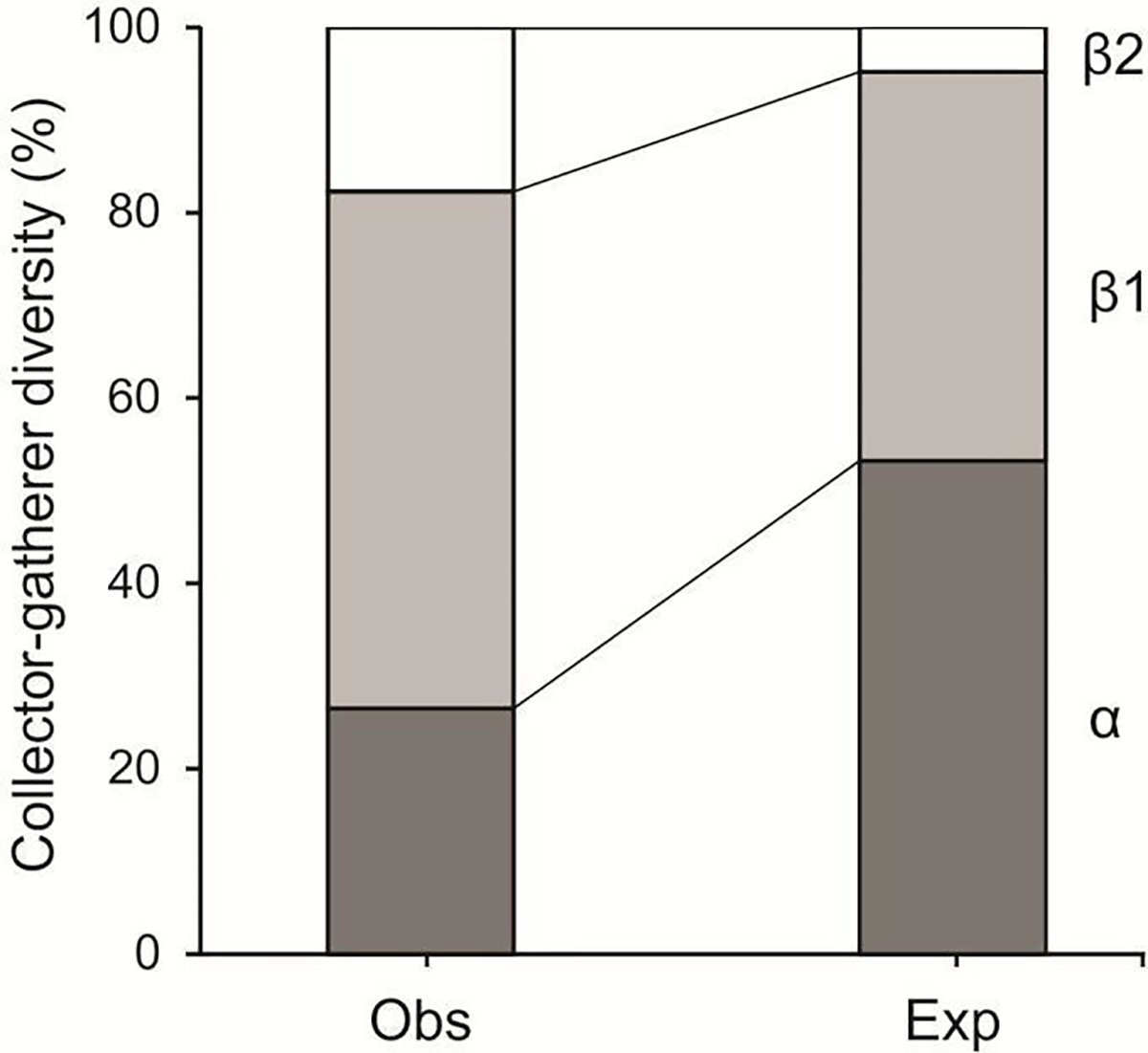
Additive partitioning of observed and expected EPT functional feeding group composition for collector-gatherers in wadeable stream sites (α; Propexp>obs: > 0.999), among stream sites (β1; Propexp<obs: < 0.001), and among hydrologic units (β2; Propexp<obs: < 0.001) in the neotropical savanna, Minas Gerais, southeastern Brazil.
Figure 6.
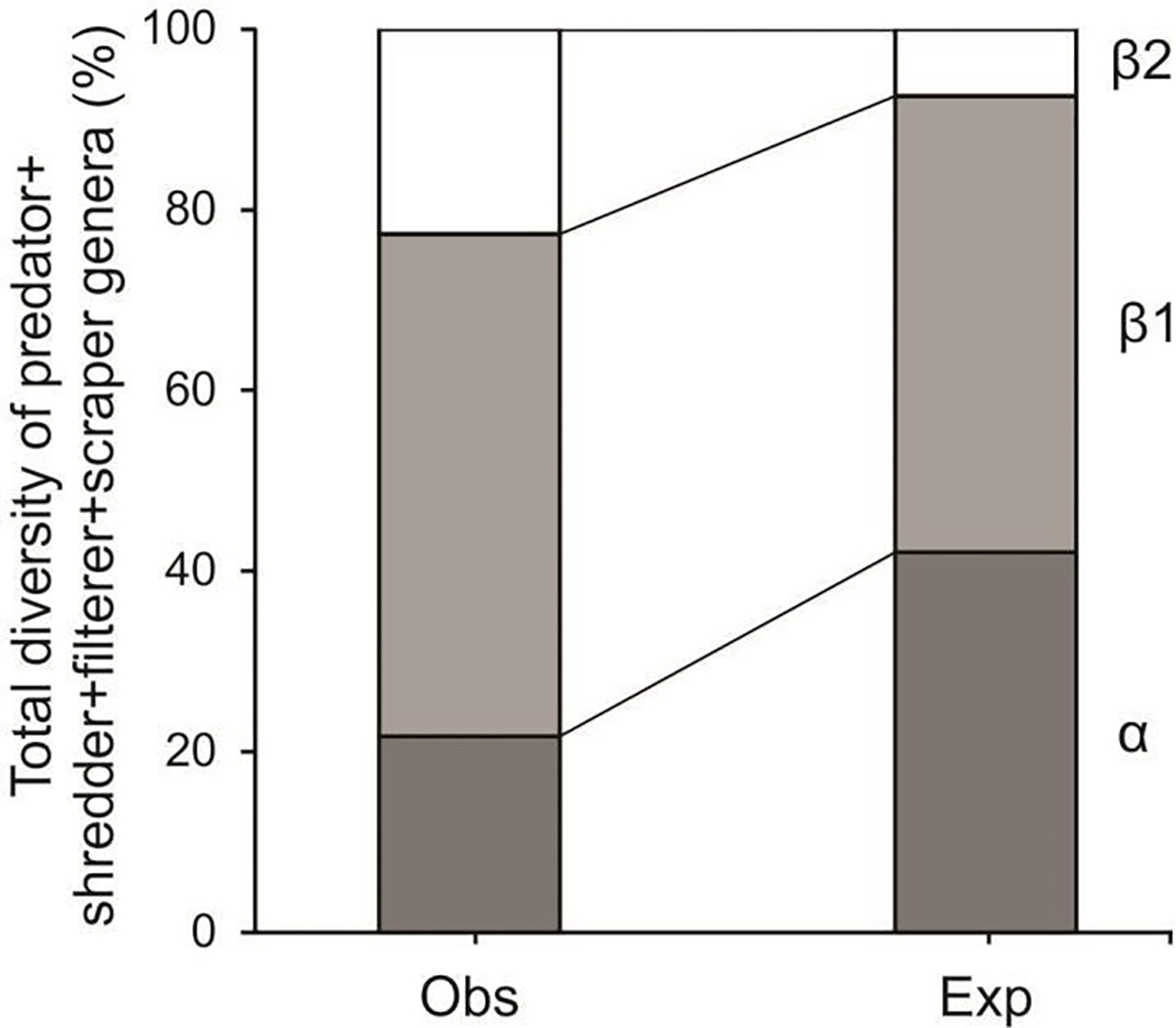
Additive partitioning of observed and expected of EPT functional feeding group composition for predators+shredders+filterers+scrapers in stream sites (α; Propexp>obs: > 0.999), among stream sites (β1; Propexp<obs: > 0.001), and among hydrologic units (β2; Propexp<obs: < 0.001) in the neotropical savanna, Minas Gerais, southeastern Brazil.
4. Discussion
In our investigation of the distribution patterns of the α and β diversities of EPT taxonomic composition, the largest β diversity was observed among stream sites (β1) and not among hydrological units (β2) or within sites (α). Both β diversities were much higher than the α diversity, indicating that each stream site represented only a small part of the total (γ) diversity. In addition, the environmental metrics usually varied more among hydrologic units than among sites (see nested ANOVA, Table 2). Thus, the β diversity was not evenly distributed throughout the spatial scales, which supported our first hypothesis.
As observed in previous studies (Ferreira et al., 2014; Macedo et al., 2014), our results indicate that the taxonomic and functional composition of EPT assemblages in neotropical savanna streams is determined by local (stream physical habitat structure and water quality) and catchment (land use, land cover) factors. Heino et al. (2015a) studied the relationship between β diversity and habitat heterogeneity and proposed two models. i) Assemblage compositions are scale-dependent, and β diversity varies positively with habitat heterogeneity based on the observation that organisms disperse at all spatial scales. ii) The relationship between β diversity and habitat heterogeneity may be affected by the dispersal ability of the organisms. According to those authors, evaluations of β diversity at multiple spatial scales are required for clear observations of the relationship between β diversity and habitat scale. We did not evaluate the dispersal ability of organisms, but EPT individuals have relatively low dispersion capacity along stream channels (Greenwood et al., 2001; Petersen et al., 2004; Yaegashi et al., 2014). However, we observed that β diversity was more strongly associated with habitat heterogeneity among sites than among hydrologic units. Additionally, our study corroborates the statements of Heino et al. (2015a) regarding habitat heterogeneity, because we observed significant differences in physical habitat among the hydrologic units and in land use and cover of site catchments (Table 2). Hepp and Melo (2013) also highlighted the importance of stream site heterogeneity. According to them, when there is only a spatial effect, the trend in assemblage composition is similar in different stream sites of the same river because of insect dispersion characteristics (especially for EPT). In an earlier study conducted in the Nova Ponte and Três Marias hydrologic units, Ferreira et al. (2014) observed the importance of site-scale physical habitat on the distribution of EPT richness and concluded that channel morphology (width and depth), riparian structure, substrate composition, and water quality were important for structuring macroinvertebrate richness in neotropical savanna streams. This study corroborates those results because we also showed that the among-site scale (β1) was most important for the distribution of EPT genera and for biological variation. Similarly, Ligeiro et al. (2010) investigated the distribution patterns of diversity in neotropical savanna streams and emphasized the importance of the stream site scale for β diversity, as did Hepp et al. (2012).
We observed similar EPT genera richness among hydrologic units; however, slightly fewer sites in the Três Marias hydrologic unit had low richness of EPT genera compared with the other hydrologic units. This probably occurred because the land use in most Três Marias catchments is pasture and small farms versus row-crop agriculture (Ferreira et al., 2014). On the other hand, land use in most Nova Ponte, Volta Grande and São Simão catchments is primarily row-crop agriculture (soy, coffee, corn, and sugar cane), which generally disturbs streams to a greater degree than pasture (Ligeiro et al., 2013). Agricultural practices tend to increase the introduction of sediments, nutrients and biocides, which tend to reduce instream insect richness, especially EPT (Hepp and Santos, 2009), which are sensitive to anthropogenic disturbance (e.g., Stoddard et al., 2008b). The low EPT abundances observed in the Nova Ponte hydrologic unit also may have resulted from higher mean annual precipitation in 2009, compared with 2010 in Três Marias, 2011 in Volta Grande, and 2012 in São Simão (1939 mm vs 958 mm, 968 mm and 1155 mm, respectively; ANA, 2014). The Nova Ponte hydrologic unit also had greater long term annual precipitation than the others (1657 mm vs 1449 mm, 1591 mm, 1589 mm for Três Marias, Volta Grande and São Simão, respectively; Hijmans et al., 2005). That greater precipitation may have produced greater natural environmental disturbance (flushing flows in headwaters) and greater runoff of agricultural pollutants, affecting the abundance and richness of EPT assemblages. Macedo et al. (2014) showed that decreased benthos richness was associated with increased rainfall in the Nova Ponte and Três Marias hydrologic units. Lastly, the similarity in EPT genera richness among hydrologic units is likely due to the fact that all four units occurred in the neotropical savanna, an ecoregion with broadly similar climate, potential natural vegetation, and land surface form that has persisted for a very long time (Myers et al., 2000; Wantzen et al., 2006; Brasil et al., 2014). Such a region tends to support generally similar aquatic biota (Omernik, 1987; Whittier et al., 1988; Stoddard et al., 2008a; Pinto et al., 2009).
Our data revealed that the collector-gatherers drove the patterns of diversity partitioning of the EPT assemblage. The collector-gatherer FFG exhibited greater EPT richness and genera abundance compared with the other FFG, which supported our second hypothesis. Our study revealed low shredder abundance in the neotropical savanna, which supports reports of shredder scarcity in tropical regions (Wantzen and Wagner, 2006; Boyero et al., 2011; Rezende et al., 2016). However, the abundance and importance of shredders may differ among biomes because of differing availabilities of leaf litter as food and for providing physical habitat structure (Ferreira et al., 2015).
Another important factor regarding the FFG that we did not consider is the food plasticity of macroinvertebrates, which could contribute to a greater proportion of collector-gatherers in our sites. It is usually accepted that macroinvertebrates exhibit plasticity in their feeding habits (Tomanova et al., 2006; Carvalho and Graça, 2007). Our specimens were classified according to the literature (Merritt et al., 2008; Oliveira and Nessimian, 2010; Shimano et al., 2012; Brasil et al., 2014). However, a rigorous assessment of their gut contents would be required for a more precise EPT FFG classification. Ferreira et al. (2015) evaluated the gut contents of Phylloicus larvae, which are typically considered shredders, in the Nova Ponte and Três Marias hydrologic units. They noted that the larvae exhibited collector-gatherer behavior in Nova Ponte based on the predominance of fine particulate organic matter (particles <50 μm) in their digestive tracts. Thus, Ferreira et al. (2015) concluded that Phylloicus larvae could change their feeding strategies depending on food availability and habitat type, which are affected by land use, human population density, and the geomorphic characteristics within each hydrologic unit.
The four hydrologic units differed in several environmental variables (Table 2). For example, Três Marias has experienced replacement of its natural areas with pasture, whereas agriculture has replaced natural vegetation in the other hydrologic units, with greater intensities in the São Simão and Volta Grande hydrologic units. Because of such anthropogenic activities, the neotropical savanna has reduced habitat heterogeneity, which has affected the composition and distribution of benthic macroinvertebrate assemblages (Ferreira et al., 2014; Macedo et al., 2014, 2016). Such changes help explain the differences in EPT taxonomic composition among the stream sites as well as their habitat heterogeneity, resulting in greatest β diversity among stream sites. Hepp and Santos, (2009) observed decreased EPT richness in Araucaria rain forest sites over an urban and agriculture gradient. Sensolo et al. (2012) studied Atlantic rain forest streams and observed that substantial changes in the composition of Chironomidae (Diptera) genera were influenced by changes in catchment land use and cover. Ligeiro et al. (2013) also studied neotropical savanna streams and observed that increased human pressures resulted in decreased EPT richness and abundance.
We observed the greatest variability in β diversity among stream sites, although there were also substantial differences among hydrologic units. This suggests that the regional effects on the biota were indirect and that the invertebrate taxa were directly affected by local physical habitat structure and water quality. In our study, only the percentage of urban area showed significant variability at both the stream site and hydrologic unit scales, indicating the direct effects on sites of urban point sources of pollution and land use. As an example of these local and regional influences, Macedo et al. (2014) evaluated the influence of the geophysical landscape, land use and cover, and site physical habitat structure on macroinvertebrate richness. They concluded that site physical habitat structure determined benthic assemblage composition either independently or in combination with a gradient of anthropogenic pressures. Moreno et al. (2010) studied streams in another hydrologic unit in the São Francisco River Basin and evaluated the factors determining the structure and distribution of benthic invertebrate assemblages. They determined that the main structuring factors were degree of habitat conservation around the sites, nutrient concentration, and substrate size. Heino et al. (2015b) investigated the relationship between ecological factors and the β diversity of aquatic insect meta-communities within and among catchments. They reported that environmental metrics explained insect β diversity better than spatial metrics in aquatic ecosystems, although they might be weak predictors.
Conclusions and recommendations
We identified the scales of major importance for biodiversity in neotropical savanna streams through use of additive partitioning to analyze EPT genera diversity and FFG. Our findings should help focus sampling efforts for future biodiversity surveys and local and regional bioassessments. Our results also can facilitate decision-making aimed at the recovery and conservation of watersheds by underlining the primary factors and spatial scales for mitigating disturbance and maintaining aquatic life in neotropical savanna streams. Taxonomic and functional biodiversity is inextricably linked to the preservation of the essential functions of ecosystems and preservation of biodiversity is currently an urgent concern of environmental policy (Di Battista et al., 2016). We highlight the need for further studies evaluating the relationship among β diversity at multiple spatial scales and the incorporation of meta-community studies to assess the dispersal ability of benthic organisms and the relationships between organism traits and their habitats. Additional studies of FFG partitions also are necessary because individual partitioning of groups other than collector-gatherers was not possible because of low numbers and because ecological function is a critically important component of ecological condition. Finally, we recommend further gut content evaluations of macroinvertebrates to obtain more accurate FFG classification. Such studies are needed because classifications based only on the literature and mouthparts may incorporate substantial errors when regional differences in available food and habitats are considered.
We stress the importance of studying river basin condition at multiple scales. Such approaches help us understand ecological patterns and processes with greater certainty, which can lead to more scientifically rigorous and rational conservation and management of water resources. Our results indicate that multiple scale approaches would greatly benefit effective design and evaluation of measures aimed at conserving biodiversity and the habitats that foster it. Such measures might include: (1) Identifying small streams to include preserves, conservation areas, and protected areas because they support high levels of biological variability (beta diversity) and represent over 80% of the stream length in drainage basins. (2) Maintaining and restoring natural riparian zone vegetation cover and complexity to buffer streams from land use disturbances and provide alternative food and energy sources to streams. (3) Focusing financial investments into riparian zone recovery programs. (4) Developing public policies and biomonitoring programs focused on conservation and assessment of water resources such as those resulting from the U.S. Clean Water Act and National Rivers and Streams Assessment, the European Union Water Framework Directive, and the Australian Sustainable Rivers Audit.
Acknowledgements
We thank the Fundação de Amparo à Pesquisa do Estado de Minas Gerais/FAPEMIG/CAPES/Programa Mineiro de Pós-Doutorado – PMPD II/Processo CRA-BPD-00494-13, Peixe-Vivo Program of Companhia Energética de Minas Gerais, Pesquisa & Desenvolvimento/Agência Nacional de Energia Elétrica/Companhia Energética de Minas Gerais-P&D ANEEL/CEMIG (GT-487), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), and Fulbright-Brasil for financial support; Carlos Bernardo Mascarenhas Alves for logistical support; and colleagues from the Federal University of Minas Gerais Benthic Ecology Laboratory, Federal Center of Technological Education of Minas Gerais, Federal University of Lavras, and Pontifical Catholic University of Minas Gerais for help with field collections. MC was awarded research productivity CNPq (no. 303380/2015-2), research project CNPq (no. 446155/2014-4), and Minas Gerais research grant FAPEMIG PPM-IX - 00525-15. LUH received financial support from CNPq (no. 471572/2012-8). We thank Dan McGarvey for his review of an earlier draft of this manuscript, which was also reviewed by the U.S.EPA National Health and Environmental Effects Research Laboratory’s Western Ecology Division and approved for publication. Approval does not signify that the contents reflect the views of the USEPA, nor does mention of trade names or commercial products constitute endorsement or recommendation for use.
References
- Allan JD, Castillo MM, 2007. Stream Ecology: Structure and Function of Running Waters, 2nd ed. Springer. [Google Scholar]
- ANA, 2014. Hydroweb: Sestema de informações hidrológicas [WWW Document]. URL http://hidroweb.ana.go.br (accessed 1.16.16).
- Anderson MJ, Crist TO, Chase JM, Vellend M, Inouye BD, Freestone AL, Sanders NJ, Cornell HV, Comita LS, Davies KF, Harrison SP, Kraft NJB, Stegen JC, Swenson NG, 2011. Navigating the multiple meanings of β diversity: a roadmap for the practicing ecologist. Ecol. Lett. 14, 19–28. doi: 10.1111/j.1461-0248.2010.01552.x [DOI] [PubMed] [Google Scholar]
- APHA, 2005. Standard Methods for the Examination of Water and Wastewater, 21st ed. American Public Health Association, Washington, DC. [Google Scholar]
- Ávila AC, Stenert C, Maltchik L, 2011. Partitioning macroinvertebrate diversity across different spatial scales in southern Brazil coastal wetlands. Wetlands 31, 459–469. doi: 10.1007/s13157-011-0178-3 [DOI] [Google Scholar]
- Bonada N, Prat N, Resh VH, Statzner B, 2006. Developments in aquatic insect biomonitoring: a comparative analysis of recent approaches. Annu. Rev. Entomol. 51, 495–523. doi: 10.1146/annurev.ento.51.110104.151124 [DOI] [PubMed] [Google Scholar]
- Boyero L, 2003. Multiscale patterns of spatial variation in stream macroinvertebrate communities. Ecol. Res. 18, 365–379. doi: 10.1046/j.1440-1703.2003.00562.x [DOI] [Google Scholar]
- Boyero L, 2005. Multiscale variation in the functional composition of stream macroinvertebrate communities in low-order mountain streams. Limnetica 24, 245–250. [Google Scholar]
- Boyero L, Pearson RG, Dudgeon D, Graça MAS, Gessner MO, Albariño RJ, Ferreira V, Yule CM, Boulton AJ, Arunachalam M, Callisto M, Chauvet E, Ramírez A, Chará J, Moretti MS, Gonçalves JF, Helson JE, Chará-Serna AM, Encalada AC, Davies JN, Lamothe S, Cornejo A, Li AOY, Buria LM, Villanueva VD, Zúñiga MC, Pringle CM, 2011. Global distribution of a key trophic guild contrasts with common latitudinal diversity patterns. Ecology 92, 1839–1848. [DOI] [PubMed] [Google Scholar]
- Brasil, 1992. Normais Climatológicas (1960–1990). Ministério da Agricultura e Reforma Agrária, Secretaria Nacional de Irrigação, Departamento Nacional de Meteorologia, Brasília. [Google Scholar]
- Brasil. Conselho Nacional de Meio-Ambiente, 2005. Resolution 357 of National Council for Environmental, March 17th 2005 (Resolução n. 357 do Conselho Nacional de Meio-Ambiente, de 17 de março de 2005). [Google Scholar]
- Brasil LS, Juen L, Batista JD, Pavan MG, Cabette HSR, 2014. Longitudinal distribution of the functional feeding groups of aquatic insects in streams of the Brazilian cerrado savanna. Neotrop. Entomol. 43, 421–428. doi: 10.1007/s13744-014-0234-9 [DOI] [PubMed] [Google Scholar]
- Carvalho EM, Graça MAS, 2007. A laboratory study on feeding plasticity of the shredder Sericostoma vittatum Rambur (Sericostomatidae). Hydrobiologia 575, 353–359. doi: 10.1007/s10750-006-0383-x [DOI] [Google Scholar]
- Chen K, Hughes RM, Xu S, Zhang J, Cai D, Wang B, 2014. Evaluating performance of macroinvertebrate-based adjusted and unadjusted multi-metric indices (MMI) using multi-season and multi-year samples. Ecol. Indic. 36, 142–151. doi: 10.1016/j.ecolind.2013.07.006 [DOI] [Google Scholar]
- Cortes RUI, Varandas S, Hughes S, Magalhães M, Teixeira A, 2010. Environmental drivers of benthic communities: the importance of landscape metrics, in: Azevedo JC, Feliciano M, Castro J, Pinto MA (Eds.), IUFRO Landscape Ecology International Conference. Instituto Politécnico de Bragança, Bragança, Portugal, p. 6. [Google Scholar]
- Crist TO, Veech JA, Gering JC, Summerville KS, 2003. Partitioning species diversity across landscapes and regions: a hierarchical analysis of α, β, and γ diversity. Am. Nat. 162, 734–743. doi: 10.1086/378901 [DOI] [PubMed] [Google Scholar]
- Cummins KW, Merritt RW, Andrade PC, 2005. The use of invertebrate functional groups to characterize ecosystem attributes in selected streams and rivers in south Brazil. Stud. Neotrop. Fauna Environ. 40, 69–89. doi: 10.1080/01650520400025720 [DOI] [Google Scholar]
- Di Battista T, Fortuna F, Maturo F, 2016. Environmental monitoring through functional biodiversity tools. Ecol. Indic. 60, 237–247. doi: 10.1016/j.ecolind.2015.05.056 [DOI] [Google Scholar]
- Diniz-Filho JAF, Terribile LC, De-Oliveira G, Rangel TF, 2009. Padrões e processos ecológicos e evolutivos em escala regional. Megadiversidade 5, 5–16. [Google Scholar]
- Ferreira WR, Ligeiro R, Macedo DR, Hughes RM, Kaufmann PR, Oliveira LG, Callisto M, 2015. Is the diet of a typical shredder related to the physical habitat of headwater streams in the Brazilian Cerrado? Ann. Limnol. - Int. J. Limnol. 51, 115–127. doi: 10.1051/limn/2015004 [DOI] [Google Scholar]
- Ferreira WR, Ligeiro R, Macedo DR, Hughes RM, Kaufmann PR, Oliveira LG, Callisto M, 2014. Importance of environmental factors for the richness and distribution of benthic macroinvertebrates in tropical headwater streams. Freshw. Sci. 33, 860–871. doi: 10.1086/676951 [DOI] [Google Scholar]
- Frissell CA, Liss WJ, Warren CE, Hurley MD 1986. A hierarchical framework for stream habitat classification: viewing streams in a watershed contest. Environ. Mgmt. 10(2):199–214. [Google Scholar]
- Greenwood MT, Bickerton MA, Petts GE, 2001. Assessing adult Trichoptera communities of small streams: a case study from Charnwood Forest, Leicestershire, UK. Aquat. Conserv. Mar. Freshw. Ecosyst. 11, 93–107. doi: 10.1002/aqc.435 [DOI] [Google Scholar]
- Heino J, Melo AS, Bini LM, 2015a. Reconceptualising the beta diversity-environmental heterogeneity relationship in running water systems. Freshw. Biol. 60, 223–235. doi: 10.1111/fwb.12502 [DOI] [Google Scholar]
- Heino J, Melo AS, Bini LM, Altermatt F, Al-Shami SA, Angeler DG, Bonada N, Brand C, Callisto M, Cottenie K, Dangles O, Dudgeon D, Encalada A, Göthe E, Grönroos M, Hamada N, Jacobsen D, Landeiro VL, Ligeiro R, Martins RT, Miserendino ML, Rawi CSM, Rodrigues ME, Roque FO, Sandin L, Schmera D, Sgarbi LF, Simaika JP, Siqueira T, Thompson RM, Townsend CR, 2015b. A comparative analysis reveals weak relationships between ecological factors and beta diversity of stream insect metacommunities at two spatial levels. Ecol. Evol. 5, 1235–48. doi: 10.1002/ece3.1439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepp LU, Melo AS, 2013. Dissimilarity of stream insect assemblages: effects of multiple scales and spatial distances. Hydrobiologia 703, 239–246. doi: 10.1007/s10750-012-1367-7 [DOI] [Google Scholar]
- Hepp LU, Santos S, 2009. Benthic communities of streams related to different land uses in a hydrographic basin in southern Brazil. Environ. Monit. Assess. 157, 305–318. doi: 10.1007/s10661-008-0536-7 [DOI] [PubMed] [Google Scholar]
- Hepp LU, Landeiro VL, Melo AS, 2012. Experimental assessment of the effects of environmental factors and longitudinal position on alpha and beta diversities of aquatic insects in a neotropical stream. Int. Rev. Hydrobiol. 97, 157–167. doi: 10.1002/iroh.201111405 [DOI] [Google Scholar]
- Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A, 2005. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 25, 1965–1978. doi: 10.1002/joc.1276 [DOI] [Google Scholar]
- Hughes RM, Peck DV, 2008. Acquiring data for large aquatic resource surveys: the art of compromise among science, logistics, and reality. J. N. Amer. Benth. Soc 27,837–859. [Google Scholar]
- Jankowski JE, Ciecka AL, Meyer NY, Rabenold KN, 2009. Beta diversity along environmental gradients: Implications of habitat specialization in tropical montane landscapes. J. Anim. Ecol. 78, 315–327. doi: 10.1111/j.1365-2656.2008.01487.x [DOI] [PubMed] [Google Scholar]
- Jost L, DeVries P, Walla T, Greeney H, Chao A, Ricotta C, 2010. Partitioning diversity for conservation analyses. Divers. Distrib. 16, 65–76. doi: 10.1111/j.1472-4642.2009.00626.x [DOI] [Google Scholar]
- Kaufmann PR, Faustini JM, Larsen DP, Shirazi MA, 2008. A roughness-corrected index of relative bed stability for regional stream surveys. Geomorphology 99, 150–170. doi: 10.1016/j.geomorph.2007.10.007 [DOI] [Google Scholar]
- Kaufmann PR, Levine P, Robison EG, Seeliger C, Peck DV, 1999. Quantifying Physical Habitat in Wadeable Streams. EPA/620/R-99/003. U.S. Environmental Protection Agency, Washington, DC. [Google Scholar]
- Klink CA, Machado RB, 2005. Conservation of the Brazilian cerrado. Conserv. Biol. 19, 707–713. [Google Scholar]
- Lande R, 1996. Statistics and partitioning among multiple communities. Oikos 76, 5–13. [Google Scholar]
- Leitão RP, Zuanon J, Villéger S, Williams SE, Baraloto C, Fortunel C, Mendonça FP, Mouillot D, 2016. Rare species contribute disproportionately to the functional structure of species assemblages. Proc. R. Soc. B Biol. Sci. 283, 20160084. doi: 10.1098/rspb.2016.0084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligeiro R, Hughes RM, Kaufmann PR, Macedo DR, Firmiano KR, Ferreira WR, Oliveira D, Melo AS, Callisto M, 2013. Defining quantitative stream disturbance gradients and the additive role of habitat variation to explain macroinvertebrate taxa richness. Ecol. Indic. 25, 45–57. doi: 10.1016/j.ecolind.2012.09.004 [DOI] [Google Scholar]
- Ligeiro R, Melo AS, Callisto M, 2010. Spatial scale and the diversity of macroinvertebrates in a Neotropical catchment. Freshw. Biol. 55, 424–435. doi: 10.1111/j.1365-2427.2009.02291.x [DOI] [Google Scholar]
- Macedo DR, Hughes RM, Ferreira WR, Firmiano KR, Silva DRO, Ligeiro R, Kaufmann PR, Callisto M, 2016. Development of a benthic macroinvertebrate multimetric index (MMI) for neotropical savanna headwater streams. Ecol. Indic 64, 132–141. doi: 10.1016/j.ecolind.2015.12.019 [DOI] [Google Scholar]
- Macedo DR, Hughes RM, Ligeiro R, Ferreira WR, Castro MA, Junqueira NT, Oliveira DR, Firmiano KR, Kaufmann PR, Pompeu PS, Callisto M, 2014. The relative influence of catchment and site variables on fish and macroinvertebrate richness in cerrado biome streams. Landsc. Ecol. 29, 1001–1016. doi: 10.1007/s10980-014-0036-9 [DOI] [Google Scholar]
- Marzin A, Archaimbault V, Belliard J, Chauvin C, Delmas F, Pont D, 2012. Ecological assessment of running waters: do macrophytes, macroinvertebrates, diatoms and fish show similar responses to human pressures? Ecol. Ind. 23, 56–65. [Google Scholar]
- Merritt RW, Cummins KW, Berg MB, 2008. An Introduction to the Aquatic Insects of North America, 3rd ed. Kendall Hunt Publishing Company, Dubuque, IA. [Google Scholar]
- Molozzi J, Hepp LU, Callisto M, 2013. The additive partitioning of macroinvertebrate diversity in tropical reservoirs. Mar. Freshw. Res. 64, 609–617. doi: 10.1071/MF12354 [DOI] [Google Scholar]
- Moreno P, França J, Ferreira W, Paz A, Monteiro I, Callisto M, 2010. Factors determining the structure and distribution of benthic invertebrate assemblages in a tropical basin. Neotrop. Biol. Conserv. 5, 135–145. doi: 10.4013/nbc.2010.53.01 [DOI] [Google Scholar]
- Moya N, Hughes RM, Domínguez E, Goitia E, 2011. Macroinvertebrate-based multimetric predictive models for evaluating the human impact on biotic condition of Bolivian streams. Ecol. Indic. 11, 840–847. doi: 10.1016/j.ecolind.2010.10.012 [DOI] [Google Scholar]
- Mugnai R, Nessimian JL, Baptista DF, 2010. Manual de Identificação de Macroinvertebrados Aquáticos do Estado do Rio de Janeiro. Techical Books Editora, Rio de Janeiro. [Google Scholar]
- Myers N, Mittermeier RA, Mittermeier CG, da Fonseca GAB, Kent J, 2000. Biodiversity hotspots for conservation priorities. Nature 403, 853–858. doi: 10.1038/35002501 [DOI] [PubMed] [Google Scholar]
- Oliveira ALH, Nessimian JL, 2010. Spatial distribution and functional feeding groups of aquatic insect communities in Serra da Bocaina streams, southeastern Brazil. Acta Limnol. Bras. 22, 424–441. doi: 10.4322/actalb.2011.007 [DOI] [Google Scholar]
- Olsen AR, Peck DV, 2008. Survey design and extent estimates for the Wadeable Streams Assessment. J. North Am. Benthol. Soc. 27, 822–836. doi: 10.1899/08-050.1 [DOI] [Google Scholar]
- Omernik JM, 1987. Ecoregions of the conterminous United States. Ann. Assoc. Amer. Geogr. 77, 118–125. [Google Scholar]
- Peck DV, Herlihy AT, Hill BH, Hughes RM, Kaufmann PR, Klemm DJ, Lazorchak JM, McCormick FH, Peterson SA, Ringold PL, Magee T, Cappaert MR, 2006. Environmental Monitoring and Assessment Program – Surface Waters Western Pilot Study: Field Operations Manual for Wadeable Streams. EPA Report EPA 600/R-05/xxx, U.S. Environmental Protection Agency, Office of Research and Development, Washington, DC. [Google Scholar]
- Pes AMO, Hamada N, Nessimian JL, 2005. Chaves de Identificação de Larvas para Famílias e Gêneros de Trichoptera 181 (Insecta) da Amazônia Central, Brasil. Rev. Bras. Entomol. 49, 181–204. [Google Scholar]
- Petersen I, Masters Z, Hildrew AG, Ormerod SJ, 2004. Dispersal of adult aquatic insects in catchments of differing land use. J. Appl. Ecol. 41, 934–950. [Google Scholar]
- Pinto BCT, Araujo FG, Rodriguez VD, Hughes RM, 2009. Local and ecoregion effects on fish assemblage structure in tributaries of the Rio Paraíba do Sul, Brazil. Freshw, Biol 54,2600–2615. [Google Scholar]
- Rezende RS, Graça MAS, Santos AM, Medeiros AO, Santos PF, Nunes YR, Gonçalves JFJ, 2016. Organic matter dynamics in a tropical gallery forest in a grassland landscape. Biotropica. doi: 10.1111/btp.12308 [DOI] [Google Scholar]
- Rietkerk M, van de Koppel J, Kumar L, van Langevelde F, Prins HHT, 2002. The ecology of scale. Ecol. Modell. 149, 1–4. [Google Scholar]
- Salles FF, 2006. A Ordem Ephemeroptera no Brasil (insecta): Taxonomia e Diversidade. Universidade Federal de Viçosa, Viçosa. [Google Scholar]
- Seaber PR, Kapinos FP, Knapp GL, 1987. Hydrologic Unit Maps [WWW Document]. U. S. Geol. Surv. Water-Supply Pap. URL http://pubs.usgs.gov/wsp/wsp2294/pdf/wsp_2294.pdf (accessed 1.5.16).
- Sensolo D, Hepp LU, Decian V, Restello RM, 2012. Influence of landscape on assemblages of Chironomidae in neotropical streams. Ann. Limnol. - Int. J. Limnol 48, 391–400. doi: 10.1051/limn/2012031 [DOI] [Google Scholar]
- Shimano Y, Salles FF, Faria LRR, Cabette HSR, Nogueira DS, 2012. Distribuição espacial das guildas tróficas e estruturação da comunidade de Ephemeroptera (Insecta) em córregos do Cerrado de Mato Grosso, Brasil. Iheringia. Série Zool. 102, 187–196. doi: 10.1590/S0073-47212012000200011 [DOI] [Google Scholar]
- Stoddard JL, Herlihy AT, Peck DV, Hughes RM, Whittier TR, Tarquinio E, 2008a. A process for creating multi-metric indices for large-scale aquatic surveys. J. N. Amer. Benth. Soc. 27, 878–891. [Google Scholar]
- Stoddard MT, Huffman DW, Alcoze TM, Fule PZ, 2008b. Effects of slash on herbaceous communities in pinyon–juniper woodlands of northern Arizona. Rangel. Ecol Manag. 61, 485–495. [Google Scholar]
- Strahler AN, 1957. Quantitative analysis of watershed geomorphology. Trans. Am. Geophys. Union 38, 913–920. [Google Scholar]
- Tomanova S, Goitia E, Helešic J, 2006. Trophic levels and functional feeding groups of macroinvertebrates in neotropical streams. Hydrobiologia 556, 251–264. doi: 10.1007/s10750-005-1255-5 [DOI] [Google Scholar]
- Veech JA, Summerville KS, Crist TO, Gering JC, 2002. The additive partitioning of species diversity: recent revival of an old idea. Oikos 1, 3–9. [Google Scholar]
- Veech J, Crist T 2009. PARTITION: software for hierarquical partitioning of species diversity, version 3.0. Available at http://www.user.muohio.edu/cristto/partition.htm [Accessed 12 February 2015].
- Wantzen KM, Wagner R, 2006. Detritus processing by invertebrate shredders: a neotropical–temperate comparison. J. North Am. Benthol. Soc. 25, 216–232. doi: 10.1899/0887-3593(2006)25[216:DPBISA]2.0.CO;2 [DOI] [Google Scholar]
- Wantzen KM, Siqueira A, Cunha CN, Sá MFP, 2006. Stream-valley systems of the Brazilian cerrado: impact assessment and conservation scheme. Aquat. Conserv. Mar. Freshw. Ecosyst. 16, 713–732. doi: 10.1002/aqc.807 [DOI] [Google Scholar]
- Whittaker RH, 1960. Vegetation of the Siskiyou Mountains, Oregon and California. Ecol. Monogr. 30, 279–338. [Google Scholar]
- Whittier TR, Hughes RM, Larsen DP, 1988. The correspondence between ecoregions and spatial patterns in stream ecosystems in Oregon. Can. J. Fish. Aquat. Sci. 45, 1264–1278. [Google Scholar]
- Wiggins GB, 1996. Larvae of the North American Caddisfly Genera (Trichoptera), 2nd ed. University of Toronto Press, Toronto. [Google Scholar]
- Yaegashi S, Watanabe K, Monaghan MT, Omura T, 2014. Fine-scale dispersal in a stream caddisfly inferred from spatial autocorrelation of microsatellite markers. Freshw. Sci. 33, 172–180. doi: 10.1086/675076 [DOI] [Google Scholar]


