Summary
The aberrantly expressed microRNAs (miRNAs) including miR-29c-3p have been reported in the brains of Alzheimer’s disease (AD) patients in recent researches. Nevertheless, the functional role and underlying molecular mechanism of miR-29c-3p in AD pathogenesis are still not well elucidated. The purpose of this study was to examine whether miR-29c-3p regulated β-Ameyloid (Aβ)-induced neurotoxicity by targeting β-site amyloid precursor protein-cleaving enzyme 1 (BACE1). The expressions of miR-29c-3p and BACE1 mRNA and protein levels in Aβ-treated PC12 cellular AD model were examined by qRT-PCR and western blot analyses. Luciferase reporter assay verified the potential target of miR-29c-3p. Cell viability, apoptosis, and caspase-3 activity in PC12 cells were detected by the MTT assay, flow cytometry, and caspase-3 activity assay, respectively. Our results indicated that miR-29c-3p downregulation and BACE1 upregulation existed in the cellular AD model of PC12 cells. Moreover, miR-29c-3p directly inhibited BACE1 expression. miR-29c-3p overexpression and BACE1 knockdown strengthened Aβ-induced cell apoptosis, and caspase-3 activity in PC12 cells, which was partially eliminated by over-expression of BACE1. Conversely, BACE1 knockdown reversed the miR-29c-3p inhibition- mediated inhibitory effect on Aβ-induced cell toxicity, apoptosis, and caspase-3 activity in PC12 cells. Considering, miR-29c-3p attenuated Aβ-induced neurotoxicity through targeting BACE1 in an cellular AD model of PC12, providing a potential therapeutic target for AD treatment.
Keywords: Alzheimer’s disease, β-amyloid, miR-29c-3p, BACE1
Introduction
Alzheimer’s disease (AD), as one of the most common neural degenerative diseases, was characterized by progressively debilitating cognitive and behavioral abilities [1]. Currently, various pathological characteristics of AD have been described, such as cerebral cortical nerve cell loss, extracellular amyloid plaques, and intraneuronal neuro-fibrillary tangles [2]. The most widely accepted hypothesis is Aβ deposition, which is generated by the cleavage of the amyloid precursor protein (APP) by BACE1/β-secretase and γ-secretase [3]. However, until now, no effective treatments have been available to prevent development of AD or to delay progression of the disease, largely because of our limited understanding of mechanisms underlying the pathogenesis of AD.
MicroRNAs (miRNAs) are endogenous, small noncoding RNAs(~20–23nt), acting as post-transcriptional regulators of gene expression by binding to the 3′-UTR of the targets [4]. Previous study has been reported that multiple types miRNAs are aberrantly expressed in patients with AD and participate in biological and cellular processes [5–8]. In addition, the miRNA gene expression was affected by various stimulus, such as toxicant, hypoxia, superoxide, and so on [9–11]. miR-29a and miR-29b-1 expression were significantly down-regulated in the brain of sporadic AD patients, and regulated BACE1 activity and Aβ generation by binding to 3′-UTR of BACE1 mRNA [12]. Downregulation of miR-29c-3p in the serum of AD patients, indicating that miR-29c-3p may be involved in the pathologic development of AD [13]. Nevertheless, the role and molecular mechanism of miR-29c-3p underlying the pathogenesis of AD remain elusive.
In this study, our results indicated that miR-29c-3p downregulation and BACE1 upregulation in the cellular AD model of PC12. Functionally, miR-29c-3p attenuated Aβ-induced neurotoxicity by decreasing Aβ-mediated cell viability suppression and apoptosis induction in PC12 cells. Mechanically, miR-29c-3p exerted its neuro- protective effect through targeting BACE1 in a cellular AD model of PC12.
Materials and methods
Cell lines and culture
PC12 cells purchased from ATCC (Manassas, Virginia, USA) were cultured in DMEM medium (Gibco, Langley, Oklahoma, USA) supplemented with 10 % fetal bovine serum (FBS; Invitrogen, Carlsbad, California, USA) and 1 % penicillin/streptomycin (Sigma-Aldrich, St Louis, Missouri, USA) at 37 °C in a humidified incubator with 5 % CO2.
Cellular AD model construction and cell transfection
Aβ-Peptide (1–42) monomer was purchased from Abcam (Cambridge, UK). Aβ Peptide was dissolved in 1 % NH4OH/Water and stored in aliquots in tightly sealed vials at −20 °C. The solution was equilibrated to room temperature for at least 1 hour before use. PC12 cells were exposed to 10 μM Aβ42. miR-29c-3p mimic (miR-29c-3p), miR-29c-3p negative control (miR-NC), miR-29c-3p inhibitor, and inhibitor control were synthesized from GenePharma (Shanghai, China). siRNA especially targeting BACE1 (si-BACE1), scrambled siRNA control (si-con), the over-expression vector pcDNA-BACE1 (BACE1) of BACE1, and the empty pcDNA3.1 vector (Vector; GenePharma, Shanghai, China) were also obtained from GenePharma (Shanghai, China). Cell transfection was performed using Lipofectamine 2000 (Invitrogen) in accordance with the manufacturer’s protocol. The miRNA sequence was presented as follows:
-
miR-29c-3p mimic (miR-29c-3p):
sense: 5′-UAGCACCA UUUGAAAUCGGUUA-3′,
anti-sense: 5′-UAACCGAUUUCAAAUGGUGCUA-3′;
-
miR-29c-3p negative control (miR-NC): sense: 5′-UCACAACCUCCUAGAAAGAG UAGA-3′,
anti-sense: 5′-UCUACUCUUUCUAGGAGGUUGUGA-3′;
-
miR-29c-3p inhibitor: 5′-UAACCGAUUUCAAAUGGUGCUA-3′;
inhibitor control: 5′-UCUAC UCUUUCUAGGAGGUUGUGA-3′.
Quantitative real-time PCR (qRT-PCR)
Total RNA from tissues and cells was isolated using the Trizol reagent (Invitrogen, Carlsbad, CA, USA). cDNA production for miR-29c-3p and BACE1 was synthesized through the reverse transcription reaction. qRT-PCR was carried out using the SYBR green QPCR master mix (TaKaRa, Dalian, China) on an GFX96 Real-Time PCR System. Relative gene expression levels were analyzed using the 2−ΔΔCt method. The nuclear RNA U6 was used to normalize the miRNA qRT-PCR data. Sequences of primers (TakaRa, Dalian, China) were as follows:
-
miR-29c-3p forward, 5′-ACACTC CAGCTGGTAGCACCATTGAAAT-3′;
reverse, 5′-TGGTGTCGTGGAGTCG-3′;
-
BACE1 forward, 5′-CCAAGACGACTGTTACAA-3′;
reverse, 5′-GAAGCCCTCCAT GATAAC-3′;
-
U6 forward, 5′-CTCGCTTCGGCAGCACA-3′;
reverse, 5′-AACGCTT CACGAATTTGCGT-3′;
GAPDH forward, 5′-GGAGCGAGATCCCTCCAAAAT-3′; 5′-GGCTGTTGTCATACTTCTCATGG-3′.
Western blot
Western blot analysis was carried out as described previously [14]. Proteins were separated by 10 % SDS-PAGE gels and transferred to polyvinylidene fluoride membranes (PVDF; GE HealthCare, Little Chalfont, UK). The membranes were blocked in 5 % non-fat milk at room temperature for 1h and then incubated the corresponding primary antibody at 4 °C overnight. The primary antibody was presented as follows: anti-BACE1 antibody (1:1200; ab10716, Abcam, Cambridge, MA, USA) and anti-GAPDH (1:3000; ab9485, Abcam, Cambridge, MA, USA). Then, the membranes were incubated with HRP-conjugated secondary anti-rabbit antibodies (1:2000; ab6721, Abcam, Cambridge, MA, USA) for 1h at room temperature after washing with TBST buffer. Finally, membranes were visualized by a commercial enhanced chemiluminescent substrate (Bio-Rad, USA), and Image J was used to quantitate the expression of proteins.
MTT assay
Briefly, PC12 cells were seeded in a 96-well plate. After incubation with 10 μM Aβ for 24 h, cells were treated with 5 mg/ml MTT reagent (Sigma-Aldrich) for another 4h. Then, the absorbance at 570 nm was assessed using a microplate reader (Becton Dickinson, Mountain View, CA, USA).
Flow cytometry assay
PC12 cells were seeded into 6-well plates and exposed to 10 μM Aβ for 24 h. Cell apoptosis was assessed using the Annexin V-FITC Apoptosis Detection Kit (Invitrogen) and analyzed using flow cytometry (BD Biosciences, Franklin Lakes, New Jersey, USA).
Caspase-3 activity assay
Transfected PC12 cells were treated with 10 μM Aβ for 24 h. The absorbance was measured at 405nm using a microplate reader (Becton Dickinson, Mountain View, CA, USA) using the caspase-3 assay Kit (Solarbio, Beijing, China).
Luciferase reporter assay
The wild type or mutant-type 3′-UTR of BACE1 sequences containing putative miR-29c-3p-binding sites were synthesized and cloned into pGL3 luciferase vectors (Promega, Madison, Wisconsin, USA), creating pGL3-BACE1-Wt (Wt) or pGL3- BACE1-Mut (Mut) reporter vectors. To determine the putative target BACE1, Wt or Mut was transfected into PC12 cells with miR-con or miR-29c-3p. After transfection for 48 h, the Dual Luciferase Reporter Assay Kit (Promega) was used to analyze the luciferase activity of PC12 cells.
Statistical analysis
All data were presented as mean ± standard deviation (SD). The comparisons between two groups were performed using Student’s t-test and the difference among more than two groups was assessed by one-way analysis of variance. A P-value less than 0.05 was considered to be a significant difference.
Results
MiR-29c-3p downregulation and BACE1 upregulation in the cellular model
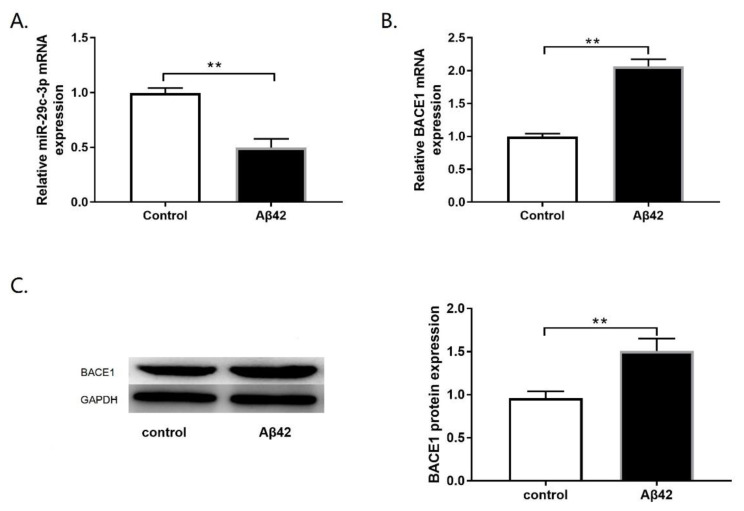
To explore the functional role of miR-29c-3p and BACE1 in the pathogenesis development of AD. For cellular AD model construction, PC12 cells were exposed to 10 μM Aβ42. Then, the mRNA and protein expression of miR-29c-3p and BACE1 were detected in the cellular AD model. Similarly, miR-29c-3p downregulation (Fig. 1A; p<0.01) and upregulation of BACE1 mRNA(Fig. 1B; p<0.01) and protein levels (Fig. 1C; p<0.01) were also found in the cellular AD model of PC12 cells. Overall, our results revealed that dysregulated miR-29c-3p and BACE1 expression may be associated with the pathogenesis development of AD.
Fig. 1.
Downregulation of miR-29c-3p expression and upregulation of BACE1 expression in the β-amyloid (Aβ42)-induced cellular AD model. (A) miR-29c-3p expression was assessed by qRT-PCR in rat neuronal differentiated PC12 cells treated with 10μM Aβ42. (B, C) BACE1 mRNA (B) and protein levels (C) were detected in rat neuronal differentiated PC12 cells treated with 10μM Aβ42 by qRT-PCR and western blot assay, respectively. **p<0.01.
MiR-29c-3p directly repressed BACE1 expression
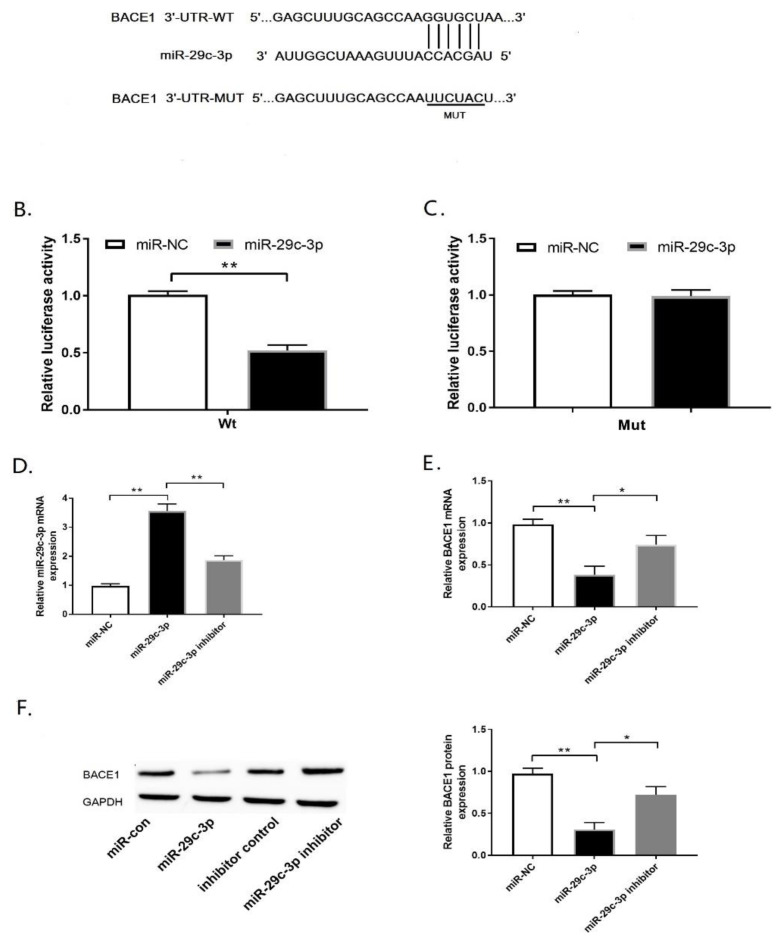
According to the inverse expression of miR-29c-3p and BACE1, we carried out predict the interaction between them through miRNA target prediction software TargetScan (http://www.targetscan.org). As expected, the 3′-UTR of BACE1 had a potential binding site of miR-29c-3p (Fig. 2A). To further verify the interaction between miR-29c-3p and BACE1, the luciferase reporter assay was performed in PC12 cells co-transfected with reporter vectors (Wt or Mut) containing the wild type or mutant BACE1 3′-UTR with miR-29c-3p or miR-NC. Our findings indicated that miR-29c-3p over-expression remarkably decreased the luciferase activity of the Wt reporter (Fig. 2B; p<0.01), but not that of the Mut reporter in PC12 cells (Fig. 2C; p>0.05). To further explore the regulatory role of miR-29c-3p on BACE1, qRT-PCR and western blot assays were carried out in PC12 cells transfected with miR-NC or miR-29c-3p or miR-29c-3p inhibitor. MiR-29c-3p transfection increased miR-29c-3p expression, whereas miR-29c-3p inhibitor repressed miR-29c-3p expression in PC12 cells (Fig. 2D; p<0.01). Moreover, miR-29c-3p overexpression inhibited BACE1 mRNA(Fig. 2E; p<0.01) and protein (Fig. 2F; p<0.01) levels in PC12 cells. Conversely, miR-29c-3p inhibitor elevated BACE1 mRNA(Fig. 2E; p<0.05) and protein (Fig. 2F; p<0.05) levels in PC12 cells. Considering, miR-29c-3p directly inhibited BACE1 expression.
Fig. 2.
BACE1 is a functional target of miR-29c-3p. (A) The 3′-UTR of BACE1 containing the putative miR-29c-3p-binding sites was presented. (B, C) The luciferase activity of PC12 cells co-transfected with the wild type (B) or the mutated (C) BACE1 reporter vector (Wt or Mut) and miR-29c-3p or miR-NC was assessed by luciferase reporter assay. (D) miR-29c-3p expression was analyzed by qRT-PCR assay. (E, F) BACE1 mRNA (E) and protein (F) levels were observed by qRT-PCR and western blot assay. *p<0.05, **p<0.01.
MiR-29c-3p attenuated β-amyloid-induced cell viability inhibition and apoptosis induction in a cellular Alzheimer’s disease model
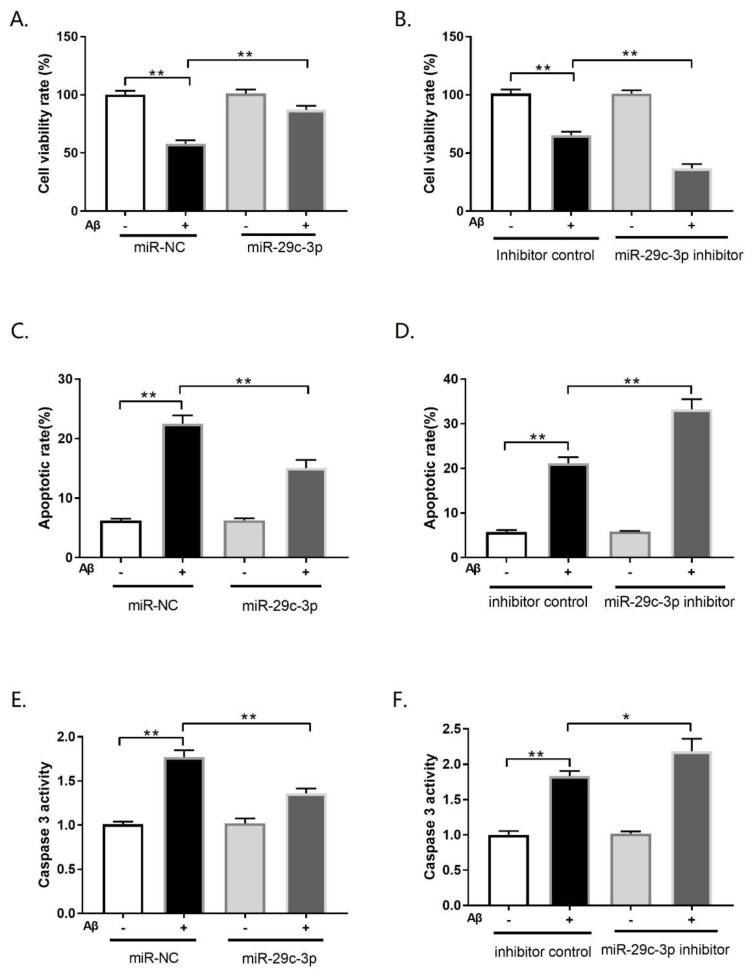
To further explore the functional role of miR-29c-3p in an Aβ insult cellular AD model of PC12, gain-of-function or loss-of-function experiments were carried out in PC12 cells by the introduction of miR-29c-3p or miR-29c-3p inhibitor. After transfection, PC12 cells were exposed to 10 μM Aβ or PBS for 24 h. MTT assay indicated that miR-29c-3p overexpression remarkably attenuated Aβ-mediated cell viability inhibition in PC12 cells (Fig. 3A; p<0.01), whereas miR-29c-3p inhibition aggravated the inhibitory effect of Aβ42 on cell viability in PC12 cells (Fig. 3B; p<0.01). Meanwhile, we also investigated the effect of miR-29c-3p on Aβ-induced cell apoptosis by flow cytometry analysis and caspase-3 activity. Our results revealed that miR-29c-3p upregualtion significantly decreased Aβ-induced apoptosis in PC12 cells (Fig. 3C; p<0.01). But, miR-29c-3p downregulation intensified the inductive effect of Aβ on apoptosis in PC12 cells (Fig. 3D; p<0.01). Moreover, forced miR-29c-3p expression decreased the increase in caspase-3 activity caused by Aβ treatment in PC12 cells (Fig. 3E; p<0.01). Conversely, miR-29c-3p inhibition exaggerated the promotive effect of Aβ on caspase-3 activity in PC12 cells (Fig. 3F; p<0.05). Overall, miR-29c-3p attenuated Aβ-induced cell toxicity and apoptosis in PC12 cells.
Fig. 3.
MiR-29c-3p attenuated β-amyloid (Aβ)-induced cell viability suppression and apoptosis induction in the cellular Alzheimer’s disease (AD) model. PC12 cells transfected with (miR-29c-3p or miR-NC) or (miR-29c-3p inhibitor or inhibitor control) were treated with 10μM Aβ42. (A, B) The viability of PC12 cells was evaluated through the MTT assay. (C, D) The apoptosis of PC12 cells was analyzed by the flow cytometry. (E, F) Caspase-3 activity was detected by the caspase-3 activity assay in PC12 cells. *p<0.05; **p<0.01.
MiR-29c-3p exerted its neuroprotective effect through targeting BACE1 in the cellular Alzheimer’s disease model
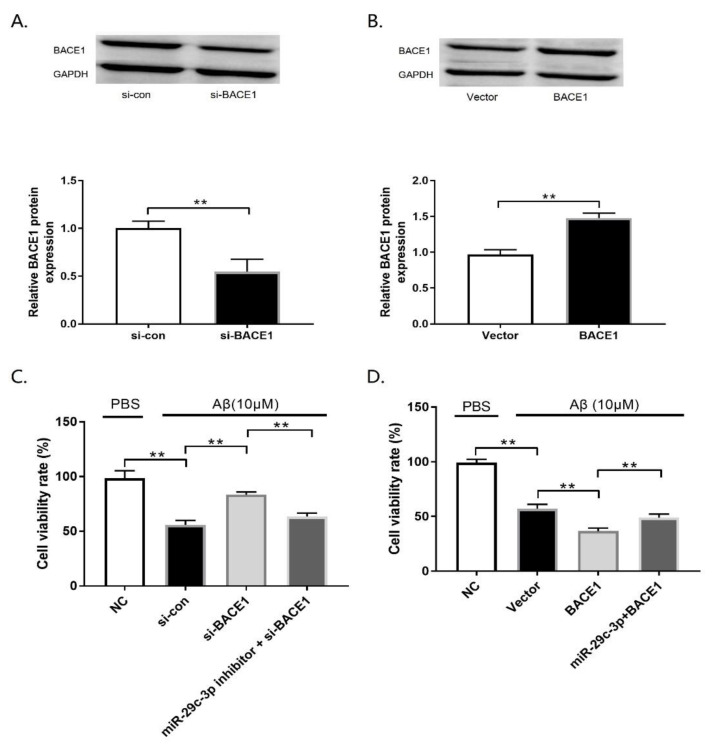
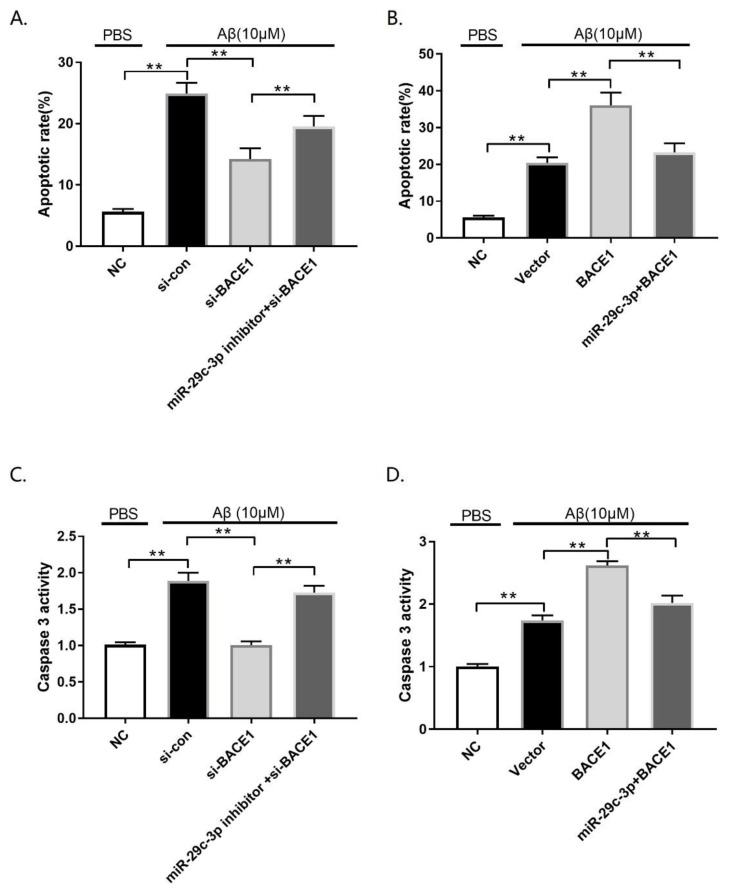
To verify whether the neuroprotective effect of miR-29c-3p in the Aβ42 insult cellular AD model of PC12 cells was mediated by BACE1, PC12 cells were transfected with si-con, si-BACE1, or miR-29c-3p + si-BACE1 or vector, BACE1, or a miR-29c-3p inhibitor + BACE1 and then treated with 10 μM Aβ42 for 24 h. Firstly, the transfection efficiency of si-BACE1 or BACE1 was analyzed by western blot assay. We found that si-BACE1 contributed toward a significant reduction in the BACE1 protein level (Fig. 4A; p<0.01), whereas BACE1 transfection results in a significant elevation in BACE1 protein expression (Fig. 4B; p<0.01). MTT assay indicated that BACE1 knockdown rescued Aβ-mediated cell viability inhibition, however, the inductive effect of si-BACE1 on cell viability was partially over-turned by miR-29c-3p inhibition (Fig. 4C; p<0.01). In contrast, BACE1 overexpression remarkably elevated the inhibited cell viability triggered by Aβ treatment, which was eliminated by miR-29c-3p overexpression (Fig. 4D; p<0.01). Furthermore, BACE1 silencing remarkably reduced Aβ-induced apoptosis in PC12 cells; however, miR-29c-3p inhibition significantly eliminated these effects (Fig. 5A; p<0.01). In contrast, elevated BACE1 expression increased Aβ-induced apoptosis in PC12 cells, whereas forced miR-29c-3p expression decreased the enhancing effect of BACE1 on Aβ-induced apoptosis in PC12 cells (Fig. 5B; p<0.01). Similarly, si-BACE1 weakened the increased caspase-3 activity caused by Aβ42 treatment (Fig. 5C; p<0.01); however, the Aβ-induced increase in caspase-3 activity was heightened by BACE1 upregulation in PC12 cells (Fig. 5C; p<0.01). Furthermore, anti-miR-29c-3p reversed the inhibitory effect of si-BACE1 on caspase-3 activity (Fig. 5D; p<0.01), whereas enforced miR-29c-3p overexpression eliminated BACE1-mediated enhancement of caspase-3 activity (Fig. 5D; p<0.01). Overall, these findings indicated that miR-29c-3p contributed toward neuroprotection by targeting BACE1 in the cellular AD model of PC12.
Fig. 4.
MiR-29c-3p exerts its neuroprotective effect by targeting BACE1 in a cellular Alzheimer’s disease (AD) model. (A) BACE1 protein level in PC12 cells transfected with si-BACE1 or si-con was measured by western blot. (B) BACE1 protein level in PC12 cells transfected with BACE1 or vector was measured by western blot. (C) Cell viability in PC12 cells was analyzed by MTT assay, (D) Cell viability in PC12 cells was analyzed by MTT assay after transfected with BACE1 or miR-29c-3p+BACE1. **p<0.01
Fig. 5.
MiR-29c-3p exerts its neuroprotective effect by targeting BACE1 in a cellular Alzheimer’s disease (AD) model. (A) PC12 cells transfected with si-con, si-BACE1, or miR-29c-3p inhibitor + si-BACE1 was exposed to 10 μM β-amyloid (Aβ) and the untransfected cells (NC) were treated with PBS. (B) PC12 cells transfected with vector, BACE1 or miR-29c-3p + BACE1 was exposed to 10 μM β-amyloid (Aβ) and the untransfected cells (NC) were treated with PBS. (C) The caspase-3 activity of PC12 cells transfected with si-con, si-BACE1, or miR-29c-3p inhibitor + si-BACE1 was detected by the caspase-3 activity assay. (D) The caspase-3 activity of PC12 cells transfected with vector, BACE1 or miR-29c-3p + BACE1 was detected by the caspase-3 activity assay. **p<0.01.
Discussion
Aβ overproduction is considered as the leading cause of synaptic and neuronal loss, which led to the development of AD [15]. The therapeutic interventions on the processing and metabolism of Aβ peptides in AD patients have failed up to the present day. The etiological factors of AD are very complicated and the underlying molecular pathological mechanism is not understood well. Several miRNAs have been reported to play crucial roles in AD and may be developed as potential therapeutic targets for AD [16]. In our study, an in vitro neuronal cell injury model was established, which provided evidences on the inhibitory effect of miR-29c-3p on Aβ-induced neurotoxicity in PC12 cells.
Aβ was generated from APP through sequential cleavage by a rate-limiting enzyme BACE1, which causes neurotoxicity, such as neuronal apoptosis [17]. Therefore, molecules acting as APP and BACE1 inhibitors are of interest as potential therapeutic targets for both AD and AD mouse models. In our study, miR-29c-3p downregulation and BACE1 upregulation in Aβ-induced PC12 cell model of AD were found. A previous study reported that miR-29c-3p was decreased in AD [13]. In addition, BACE1 was identified as a direct functional target of miR-29c-3p. Hence, we indicated that miR-29c-3p decreased Aβ-induced cell toxicity and apoptosis through directly inhibiting BACE1.
Previous study reported that abnormal expression of miR-29c-3p was related to pathogenesis and progression in human neurodegenerative disorders [18]. miR-29c contributed to APP protein accumulation in an AD PC12 cellular model and an SAMP8 mice model [19]. Meanwhile, our results elucidated that miR-29c-3p expression was decreased in Aβ-induced PC12 cells. Gain-of-function experiments found that miR-29c-3p overexpression reduced Aβ-induced cell toxicity and apoptosis in PC12 cells. Conversely, loss-of-function experiments found that miR-29c-3p inhibition aggravated Aβ-induced neurotoxicity in PC12 cells. Overall, these findings revealed that miR-29c-3p exerts neuroprotective effects in a cellular AD model of PC12, which may be a potential therapeutic target for AD patients.
BACE1, a rate-limiting enzyme in the generation of Aβ from APP, was led to the majority of Aβ production in AD pathology [20]. Previous study has been reported that BACE1 is abundantly expressed in the brain and neurons, which are upregulated in AD patients [21, 22]. In addition, the physiological role BACE1 has been reported in AD [23]. BACE1 expression was elevated in AD patients and the cellular AD model and inhibition of BACE1 by miR-15b enhanced Aβ accumulation [24, 25]. Similarly, our study also found that BACE1 expression were increased in the Aβ-treated PC12 cellular AD model.
Moreover, BACE1 knockdown attenuated Aβ-induced neurotoxicity in PC12 cells. BACE1 overexpression aggravated Aβ-induced neurotoxicity in PC12 cells. Overall, BACE1 could enrage Aβ-induced neurotoxicity in PC12 cells, for which the explanation may be that elevated BACE1 strengthened the endogenous Aβ production in cells that added to the exogenous Aβ treatment of the cells, then, elevated BACE1 may simply increase the Aβ production in the cells. Our study found that miR-29c-3p could reverse the functional role of BACE1 in an Aβ-induced PC12 cell model of AD, indicating that miR-29c-3p attenuated Aβ-induced neurotoxicity by targeting BACE1. In consistent with our study, miRNAs downregulation, such as miR-38 [26], miR-124 [27], and miR-15b [25], could contribute toward AD pathology by modulating their target gene BACE1.
In conclusion, these results indicated that miR-29c-3p exerted its neuroprotective effect through targeting BACE1 in PC12 cells, elaborating the functional mechanism of miR-29c-3p in an Aβ-induced AD cell model, which may become a promising therapeutic target for AD treatment.
Footnotes
Conflict of Interest: There is no conflict of interest.
References
- 1.François M, Fenech MF, Thomas P, Hor M, Rembach A, Martins RN, et al. High Content, Multi-Parameter Analyses in Buccal Cells to Identify Alzheimer’s Disease. Curr Alzheimer Res. 2016;13:787–799. doi: 10.2174/1567205013666160315112151. [DOI] [PubMed] [Google Scholar]
- 2.Selkoe DJ. Alzheimer’s disease is a synaptic failure. Science. 2002;298:789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- 3.Chinchalongporn V, Shukla M. Melatonin ameliorates Aβ-induced alteration of βAPP-processing secretases via the melatonin receptor through the Pin1/GSK3β/NF-κB pathway in SH-SY5Y cells. J Pineal Res. 2018;64:e12470. doi: 10.1111/jpi.12470. [DOI] [PubMed] [Google Scholar]
- 4.Shukla GC, Singh J, Barik S. MicroRNAs: processing, maturation, target recognition and regulatory functions. Mol Cell Pharmacol. 2011;3:83–92. [PMC free article] [PubMed] [Google Scholar]
- 5.Im HI, Kenny PJ. MicroRNAs in neuronal function and dysfunction. Trends Neurosci. 2012;35:325–334. doi: 10.1016/j.tins.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang M, Qin L, Tang B. MicroRNAs in Alzheimer’s disease. Front Genet. 2019;10:153. doi: 10.3389/fgene.2019.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu H, Wang L, Wang M, Song B. MicroRNA-195 downregulates Alzheimer’s disease amyloid-β production by targeting BACE1. Brain Res Bull. 2012;88:596. doi: 10.1016/j.brainresbull.2012.05.018. [DOI] [PubMed] [Google Scholar]
- 8.Dehghani R, Rahmani F, Rezaei N. MicroRNA in Alzheimer’s disease revisited: implications for major neuropathological mechanisms. Rev Neurosci. 2017;11:80. doi: 10.1515/revneuro-2017-0042. [DOI] [PubMed] [Google Scholar]
- 9.Chen Z, Li Y, Zhang H, Huang P, Luthra R. Hypoxia-regulated microRNA-210 modulates mitochondrial function and decreases ISCU and COX10 expression. Oncogene. 2010;29:4362–4368. doi: 10.1038/onc.2010.193. [DOI] [PubMed] [Google Scholar]
- 10.Lema C, Cunningham MJ. MicroRNAs and their implications in toxicological research. Toxicol Lett. 2010;198:100–105. doi: 10.1016/j.toxlet.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 11.Liu X, Yu J, Jiang L, Wang A, Shi F, Ye H, Zhou X. MicroRNA-222 regulates cell invasion by targeting matrix metalloproteinase 1 (MMP1) and manganese superoxide dismutase 2 (SOD2) in tongue squamous cell carcinoma cell lines. Cancer Genomics Proteomics. 2009;6:131–139. [PMC free article] [PubMed] [Google Scholar]
- 12.Hébert SS, Horré K, Nicolaï L, Papadopoulou AS, Mandemakers W, Silahtaroglu AN, et al. Loss of microRNA cluster miR-29a/b-1 in sporadic Alzheimer’s disease correlates with increased BACE1/beta-secretase expression. Proc Natl Acad Sci USA. 2008;105:6415–6420. doi: 10.1073/pnas.0710263105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu Y, Xu J, Xu J, Cheng J, Jiao D, Zhou C, et al. Lower Serum Levels of miR-29c-3p and miR-19b-3p as Biomarkers for Alzheimer’s Disease. Tohoku J Exp Med. 2010;242:129–136. doi: 10.1620/tjem.242.129. [DOI] [PubMed] [Google Scholar]
- 14.Bai WD, Ye XM, Zhang MY, Zhu HY, Xi WJ, Huang X, et al. MiR-200c suppresses TGF-β signaling and counteracts trastuzumab resistance and metastasis by targeting ZNF217 and ZEB1 in breast cancer. Int J Cancer. 2014;135:1356–1368. doi: 10.1002/ijc.28782. [DOI] [PubMed] [Google Scholar]
- 15.Benilova I, Karran E, De Strooper B. The toxic Aβ oligomer and Alzheimer’s disease: an emperor in need of clothes. Nat Neurosci. 2012;15:349–357. doi: 10.1038/nn.3028. [DOI] [PubMed] [Google Scholar]
- 16.Lukiw WJ. Micro-RNA speciation in fetal, adult and Alzheimer’s disease hippocampus. Neuroreport. 2007;18:297–300. doi: 10.1097/WNR.0b013e3280148e8b. [DOI] [PubMed] [Google Scholar]
- 17.Fedotova J, Soultanov V, Nikitina T, Roschin V, Ordyan N, Hritcu L. Cognitive-enhancing activities of the polyprenol preparation Ropren® in gonadectomized β-amyloid (25–35) rat model of Alzheimer’s disease. Physiol Behav. 2016;157:55–62. doi: 10.1016/j.physbeh.2016.01.035. [DOI] [PubMed] [Google Scholar]
- 18.Patel AA, Ganepola GAP, Rutledge JR, Chang DH. The potential role of dysregulated miRNAs in Alzheimer’s disease pathogenesis and progression. J Alzheimers Dis. 2019;67:1123–1145. doi: 10.3233/JAD-181078. [DOI] [PubMed] [Google Scholar]
- 19.Yang G, Song Y, Zhou X, Deng Y, Liu T, Weng G, et al. MicroRNA-29c targets β-site amyloid precursor protein-cleaving enzyme 1 and has a neuroprotective role in vitro and in vivo. Mol Med Rep. 2015;12:3081–3088. doi: 10.3892/mmr.2015.3728. [DOI] [PubMed] [Google Scholar]
- 20.Crunkhorn S. Alzheimer disease: BACE1 inhibitor reduces β-amyloid production in humans. Nat Rev Drug Discov. 2016;16:18. doi: 10.1038/nrd.2016.272. [DOI] [PubMed] [Google Scholar]
- 21.Zhao J, Fu Y, Yasvoina M, Shao P, Hitt B, O’Connor T, et al. Beta-site amyloid precursor protein cleaving enzyme 1 levels become elevated in neurons around amyloid plaques: implications for Alzheimer’s disease pathogenesis. J Neurosci. 2007;27:3639–3649. doi: 10.1523/JNEUROSCI.4396-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hampel H, Shen Y. Beta-site amyloid precursor protein cleaving enzyme 1 (BACE1) as a biological candidate marker of Alzheimer’s disease. Scand J Clin Lab Invest. 2009;69:8–12. doi: 10.1080/00365510701864610. [DOI] [PubMed] [Google Scholar]
- 23.Barão S, Moechars D, Lichtenthaler SF, De SB. BACE1 physiological functions may limit its use as therapeutic target for Alzheimer’s disease. Trends Neurosci. 2016;39:158–169. doi: 10.1016/j.tins.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 24.Gong G, An F, Wang Y, Bian M, Yu LJ, Wei C. miR-15b represses BACE1 expression in sporadic Alzheimer’s disease. Oncotarget. 2017;8:91551–9155. doi: 10.18632/oncotarget.21177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li J, Wang H. miR-15b reduces amyloid-β accumulation in SH-SY5Y cell line through targeting NF-κB signaling and BACE1. Biosci Rep. 2018:38. doi: 10.1042/BSR20180051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu CG, Wang JL, Li L, Wang PC. MicroRNA-384 regulates both amyloid precursor protein and β-secretase expression and is a potential biomarker for Alzheimer’s disease. Int J Mol Med. 2014;34:160–166. doi: 10.3892/ijmm.2014.1780. [DOI] [PubMed] [Google Scholar]
- 27.Du X, Huo X, Yang Y, Hu Z, Botchway BOA, Jiang Y, et al. MiR-124 downregulates BACE 1 and alters autophagy in APP/PS1 transgenic mice. Toxicol Lett. 2017;280:195–205. doi: 10.1016/j.toxlet.2017.08.082. [DOI] [PubMed] [Google Scholar]