Abstract
Heat-labile enterotoxin subunit B (LTB) is a noncatalytic protein derived from Escherichia coli that binds to ganglioside GM1, a glycosphingolipid on the surface of mammalian cells. In this study, the effects of recombinant LTB (rLTB) on murine lymphocytes were examined in vitro. T and B cells readily bound fluorescein isothiocyanate-labeled rLTB. CD8+ T cells bound twice as much as CD4+ T cells and B cells. Exposure of T-cell subsets and B cells to rLTB abrogated mitogen-driven proliferation. CD8+ T cells were more susceptible to rLTB than either CD4+ T cells or B cells. There were differences in the sensitivity of lymphocytes from various strains of mice to rLTB. This was attributed to qualitative and quantitative differences in the CD4+ T cells. rLTB induced apoptosis in both T-cell subsets, but the level was significantly higher in CD8+ T cells. Apoptosis peaked at around 8 h after exposure to rLTB and incubation at 37°C. Binding to ganglioside GM1 was essential for suppression, since rLTB/G33D, a mutant which does not bind GM1, failed to inhibit proliferation or induce apoptosis. Naive T cells, which were acutely sensitive to rLTB, became more resistant after activation. Conversely, activated T cells regained their sensitivity to rLTB when they reverted back to a resting state. A 1-h pulse with rLTB was sufficient to inhibit T-cell proliferation and cytotoxic-T-lymphocyte generation in primary mixed lymphocyte reaction cultures. CD8+ T cells were preferentially depleted in these cultures. rLTB also induced functional modifications in T cells as indicated by inhibition of gamma interferon secretion after polyclonal activation. Thus, rLTB may have immunomodulatory properties independent of its ability to induce apoptosis.
Novel agents that bind to and modulate the function of immune cells are of interest for transplantation immunology, autoimmune diseases, vaccine development, and other related fields. ADP-ribosylating bacterial enterotoxins are a novel class of agents that bind to gangliosides in the membranes of mammalian cells and perturb cellular function. Gangliosides are glycosphingolipids which contain exposed carbohydrate and sialic acid moieties with a lipophilic ceramide tail that is inserted into the membrane bilayer (13). Membrane sphingolipids have been implicated in pathways of signal transduction involving ceramide as a second messenger. The physiological roles of membrane glycosphingolipids and sphingolipids are still being elucidated, but they are widely recognized as active participants in the regulation of proliferation, differentiation, transformation, and death of mammalian cells (16). Bacterial enterotoxins which bind to membrane glycosphingolipids on lymphocytes may be useful as immunomodulatory agents to prevent or modulate T-cell-mediated disorders. This report describes the results of in vitro studies examining the immunomodulatory effects of a recombinant, noncatalytic bacterial enterotoxin on murine T- and B-cell function.
Escherichia coli heat-labile enterotoxin (LT) is a bacterial ADP-ribosylating exotoxin composed of six noncovalently linked polypeptides, including a single A subunit (27 kDa) with ADP-ribosyltransferase activity and five monomeric noncatalytic subunits (11.6 kDa) which form a pentamer (B subunit or LTB). LT is a potent immunosuppressive and immunomodulatory protein which has ∼80% primary amino acid homology and shares many structure-function properties with the more extensively characterized cholera toxin (CT) (5, 18, 19). Until recently, the immunosuppressive properties of LT, like those of CT, were thought to be mediated by delivery of the toxic A subunit to intracellular GS proteins through formation of endocytotic vesicles after binding to target cells expressing the receptor for the B subunit (21). Work by others using the B subunit of CT (CTB) purified from the holotoxin has suggested that the B subunit has some immunomodulatory effects in vitro and immunoregulatory effects in vivo that are independent of the A subunit (8, 34–37). However, the possible presence of residual holotoxin in these preparations complicates the assignment of a role to the B subunit in eliciting these effects. By using recombinant B subunits, which lack any enzymatic activity, it can be shown conclusively that the B subunit has immunomodulatory properties independent of the holotoxin (reference 25 and this report). If fully understood, the mechanisms involved in the immunomodulatory effects of the noncatalytic B subunits might be exploited for therapeutic benefit in T-cell-mediated diseases.
The primary goal of this study was to gain insight into the mechanism(s) responsible for the regulatory effects of recombinant LTB (rLTB) on T cells in order to guide the rational development of strategies for its use as a T-cell immunomodulator in graft-versus-host disease (GVHD) or other T-cell-mediated diseases. Previously published data suggests that rLTB affects CD8+ T cells but not CD4+ T cells (25); however, the influence of T-cell activation on relative sensitivity to rLTB has not been examined. Although native B subunits (CTB) have been tested for their effect on naive T cells responding to allogeneic histocompatibility antigens (36), recombinant B subunits, and rLTB in particular, have not been evaluated. Therefore, we compared the effects of rLTB on naive, mitogen-activated, and alloantigen-specific CD4+ and CD8+ T cells in vitro. Finally, because LTB, like CTB, preferentially binds and cross-links ganglioside GM1 (5, 18, 19) and does so only weakly with several other gangliosides (GM2 and asialo-GM1) (31), we examined the role of binding to ganglioside GM1 by using a non-GM1-binding mutant (rLTB/G33D) (25).
We report that rLTB preferentially inhibited T-cell responses to T-cell receptor (TCR) cross-linking with monoclonal antibody (MAb) and to allogeneic histocompatibility antigens in a dose-dependent manner. GM1 binding was essential for the effects. The primary mechanism of T-cell suppression appeared to be rapid induction of apoptosis. There was significant variation in the susceptibilities of T cells from different strains of mice to rLTB, which was attributed to differential sensitivity within T subsets. CD8+ T cells were acutely sensitive to the effects of rLTB, and naive T cells were more sensitive to rLTB than activated T cells. CD4 and CD8 T cells that survived exposure to suboptimal doses of rLTB proliferated in response to TCR cross-linking, but they failed to secrete gamma interferon (IFN-γ), indicating that functional modulation had occurred.
MATERIALS AND METHODS
Expression and purification of rLTB and rLTB/G33D.
T7 vectors were used to express rLTB (pT7-rLTB) and rLTB/G33D (pT7-rLTB/G33D) in E. coli. Briefly, the genes encoding rLTB (porcine isolate; accession number M15363) (a gift of Rino Rappuoli, Chiron Vaccines, Siena, Italy) or rLTB/G33D were engineered with NdeI and BamHI restriction sites at the 5′ and 3′ ends of the respective open reading frames by PCR amplification (Ultima polymerase; Perkin-Elmer Corp.). DNA was amplified by PCR and sequenced to assure that secondary-site mutations were not introduced into the open reading frames.
rLTB or rLTB/G33D was expressed as a nonfusion, secreted protein in E. coli BL21(DE3). Overnight cultures were diluted 1/50 in L broth (1.6 liters) and incubated with rotation at 250 rpm for 2 h, when 0.75 mM IPTG (isopropyl-β-d-thiogalactopyranoside) was added. Cultures were harvested after 4 h (rLTB) or 2 h (rLTB/G33D), and periplasms were prepared as previously described (1).
For rLTB, the periplasm was subjected to gel filtration chromatography (Sephacryl S-200HR; Sigma) (450-ml column equilibrated in 0.05 M Tris [pH 8.0] containing 1 mM EDTA and 0.2 M NaCl). Fractions enriched for rLTB were identified by sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis followed by staining with Coomassie blue and then subjected to affinity chromatography on Bio-Gel A0.5 M resin (Bio-Rad) (20 ml of resin equilibrated in 10 mM Tris [pH 7.6] containing 20 mM NaCl). The resin was washed with 30 ml of equilibation buffer, and rLTB was eluted with 10 mM Tris (pH 7.6) containing 20 mM NaCl and 400 mM galactose.
For rLTB/G33D, the periplasm was equilibrated to 30% saturation with ammonium sulfate, kept on ice for 1 h, and then centrifuged for 20 min at 12,000 × g at 4°C. The ammonium sulfate-soluble material was subjected to hydrophobic chromatography (phenyl-Sepharose CL-4B; Sigma) (2 ml of resin equilibrated in 25 mM Tris-HCl [pH 7.6] containing 1 M ammonium sulfate). The resin was washed with equilibration buffer and rLTB/G33D was eluted by sequential washes with 25 mM Tris-HCl (pH 7.6) containing 0.7, 0.6, and 0.5 M ammonium sulfate. Fractions enriched for rLTB/G33D were pooled and subjected to gel filtration on Sephacryl-200HR (Sigma) (450 ml of resin equilibrated in 10 mM Tris-HCl [pH 7.6] containing 20 mM NaCl). Fractions enriched for rLTB/G33D were pooled. Purified rLTB and rLTB/G33D were concentrated on a YM10 membrane (Amicon), and the retentate was dialyzed into 20 mM NaPO4 (pH 7.4) containing 100 mM NaCl (for rLTB) or 25 mM Tris-HCl (pH 7.6) (for rLTB/G33D). Purified rLTB and rLTB/G33D were stored in aliquots at −80°C.
The gene for LTB did not encode ADP-ribosyltransferase activity. Nevertheless, we confirmed the absence of catalytic activity by using microgram quantities of rLTB in biological assays with murine lymphocytes and Chinese hamster ovary (CHO) cells. rLTB failed to stimulate cyclic AMP accumulation in lymphocytes and did not induce elongation of CHO cells, indicating complete absence of ADP-ribosyltransferase activity.
Conjugation of FITC to rLTB or rLTB/G33D.
rLTB and rLTB/G33D were conjugated with fluorescein isothiocyanate (FITC) by published protocols (Sigma Immunochemicals Flurotag FITC Conjugation Kit, no. FITC-1). By using the extinction coefficient of 7,800 at 480 nm for FITC, the ratio of moles of FITC bound per mole of rLTB was determined to be approximately 4:1.
Animals and preparation of cells.
AKR (H-2k), B10.BR (H-2k), BALB/c (H-2d), C57BL/6 (H-2b), DBA/2 (H-2d), and SJL (H-2s) mice were purchased from The Jackson Laboratory (Bar Harbor, Maine). Spleens and lymph nodes were aseptically collected from the mice and pressed through sterile wire mesh screens with the plunger from a 3-ml syringe into tissue culture medium to produce single-cell suspensions. After pelleting by centrifugation, erythrocytes were removed from spleen cell suspensions by hypotonic lysis with sterile distilled water. After washing, the number of viable cells was determined with a hemocytometer and trypan blue dye exclusion (usually >90% were viable).
The MACS Cell Separator System (Miltenyi Biotec, Auburn, Calif.) was used to positively or negatively select for B cells, T cells, and T-cell subsets. Immunomagnetic microbeads conjugated to MAbs specific for B220 or CD4 and CD8 (Miltenyi) were used to positively select for B cells or T-cell subsets. T-cell-enriched suspensions were prepared either by negative selection with the MACS Cell Separator and anti-B220 microbeads (B-cell depleted and T-cell enriched) or by positive selection with anti-Thy1.2 microbeads. One cycle of negative selection generally resulted in enrichment to >80%. Positively selected Thy1.2+ T cells, as well as CD4 and CD8 T-cell subsets, were generally greater than 90% pure with less than 2% contamination from the nonselected CD4 or CD8 population. Purity was assessed by flow cytometry with the following T- and B-cell-specific MAbs: FITC–anti-Thy-1.2 (CD90; Becton Dickinson, Mountain View, Calif.), phycoerythrin (PE)–anti-CD8 (CalTag Laboratories, Burlingame, Calif.), PE–anti-CD4 (PharMingen, San Diego, Calif.), and FITC–anti-Ly5 (CD45R/B220, CalTag). The cells were analyzed on a FACScan flow cytometer with Consort 32 computer support by using Lysis II software (Becton Dickinson).
SCDA assays.
The standard cell dilution analysis (SCDA) assay of Pechhold et al. (27) was used to determine the absolute number of lymphocyte subsets in heterogenous cell cultures. Briefly, duplicate samples of the cell cultures were stained with either PE–anti-CD4 or PE–anti-CD8 MAb, washed with phosphate-buffered saline (PBS) plus 1% bovine serum albumin and 0.1% NaN3 (PBS-BSA-azide), and combined with 50,000 paraformaldehyde-fixed standard cells (SC) in the presence of propidium iodide (PI) (0.2 μg/ml) to discriminate between live and dead cells (total volume = 200 μl). SC were murine thymocytes stained with FITC–anti-Thy1.2 MAb and fixed in PBS containing 4% paraformaldehyde. The cell suspensions were analyzed immediately after addition of SC and PI. Gating of the cells for analysis was done as described in detail elsewhere (27). The absolute number of CD4 or CD8 T cells was determined by multiplying the T cell subset ratio (relative proportion of PI-negative, PE-positive T-subset/relative proportion of PI-positive, FITC-positive SC) by the number of SC added to the sample. See reference 27 for a detailed description of the SCDA assay and gating procedures.
Polyclonal T- and B-cell activation.
T cells were activated by binding the ɛ chain of the TCR with immobilized anti-CD3 MAb. Round-bottom 96-microwell plates and flat-bottom 24-macrowell plates were coated with anti-CD3 MAb (10 μg/ml) for 3 h at 37°C and then washed thoroughly with PBS. Purified anti-CD3 (clone 145 2C11) MAb was purchased from PharMingen. B cells were activated by the addition of E. coli lipopolysaccharide (LPS) (0111:B4, 2 μg/ml; Calbiochem, San Diego, Calif.) to the culture wells. Cells were suspended in Dulbecco’s modified Eagles’ medium (Gibco) containing 10% fetal bovine serum and supplemented as described elsewhere (32). The cultures were incubated at 37°C in humidified air plus 10% CO2 for the lengths of time indicated for individual experiments. Stimulation of cell proliferation was measured in SCDA assays as described above and/or by incorporation of [3H]thymidine ([3H]TdR) as an indicator of DNA synthesis. For the latter, 1 μCi of [3H]TdR was added to triplicate microwells for the final 18 to 24 h of cell culture. The cells were collected onto filter paper disks. The disks were washed to remove unincorporated radioisotope and dried, and radioactivity was counted in a liquid scintillation counter. Negative controls consisted of triplicate uncoated microwells containing responder cells only.
Clonal T cell activation and establishment of antigen-specific T-cell lines.
Primary mixed lymphocyte reaction (MLR) cultures were used for clonal activation of T cells. Responder spleen cells from C57BL/6 (H-2b) mice were cocultured with mitomycin C-treated stimulator cells from major histocompatibility complex (MHC)-mismatched B10.BR (H-2k) mice in a 24-well plate (2 × 106 viable cells per 2 ml per well). The ratio between these responder and stimulator cells was 1:3. At the end of 5 days, the cells were gently triturated and triplicate 100-μl samples of each cell suspension were transferred to microwells for labeling with 1 μCi of [3H]TdR for 6 h. Cell-mediated lympholysis (CML) assays were done as described elsewhere (7) with effector cells generated in the MLR cultures. Mitogen-activated B10.BR lymphoblasts were labeled with 51Cr (as sodium chromate) and used as antigen-specific target cells in 3.5 h chromium release CML assays. Lytic activity in the cultures was calculated as lytic units (number of cells necessary to lyse 30% of the target cells [LU30]) per million effector cells as described elsewhere (7). The remaining cells in the MLR cultures were analyzed by flow cytometry in SCDA assays.
Long-term CD4 and CD8 T-cell lines were established from MLR cultures under limiting-dilution conditions. The T-cell lines were maintained and expanded over a 6-month period by antigen-specific stimulation with irradiated H-2k-spleen cells, followed 3 to 4 days later by feeding of the cells with exogenous recombinant interleukin-2 (rIL-2) (10 U/ml). The stimulation-feeding cycle was repeated every 7 to 14 days. T-cell lines were >99% CD4+ or CD8+ and H-2-k specific (19a).
Assays for cell death.
Multiple flow cytometric assays were used to identify apoptotic and exclude necrotic cell death of T cells exposed to rLTB (6).
(i) DNA cleavage (hypodiploid state).
The nuclear DNA contents of T-cell suspensions were measured with a Becton Dickinson Cycle TEST PLUS DNA Reagent Kit. Briefly, cell membrane lipids were dissolved with nonionic detergent, cellular proteins and RNA were eliminated by enzymatic digestion, and nuclear chromatin was stabilized by addition of spermine. The isolated nuclei were then stained with PI according to the kit instructions and analyzed by flow cytometry on a FACScan equipped with a doublet discriminator. Diploid and hypodiploid nuclei were identified by using CellFIT software (Becton Dickinson).
(ii) Binding of FITC-Annexin V.
FITC-conjugated Annexin V was purchased from R&D Systems (Minneapolis, Minn.). Cells were stained with PE-conjugated CD4- or CD8-specific MAbs and then exposed to FITC-Annexin V in the presence of PI (2 μg/ml) according to the manufacturer’s instructions. Three-color analysis was done on a FACScan. In some experiments, 7-amino-actinomycin D (10 μg/ml; Calbiochem) was substituted for PI.
(iii) Retention of rhodamine 123.
Rhodamine 123 (R123) was purchased from Sigma. Cells were washed in PBS-BSA-azide and then incubated with R123 (10 μg/ml) for 30 min at 37°C. After being stained, the cells were kept on ice and analyzed immediately to determine membrane integrity as assessed by retention of the dye. After analysis on the FACScan, the remaining labeled cells were heated to 56°C for 30 min to induce necrotic cell death and reanalyzed to confirm loss of membrane integrity, i.e., leakage of the dye.
Cytokine assays.
IFN-γ secreted into the culture supernatant by T cells was measured by enzyme-linked immunosorbent assay (ELISA) 24 h after activation on immobilized anti-CD3 MAb by using capture and detection antibodies purchased from PharMingen.
RESULTS
rLTB inhibits mitogen-driven proliferation of T and B cells.
Treatment of spleen cells with rLTB significantly inhibited (P < 0.0001) the response of T and B cells to polyclonal activation with anti-CD3 MAb or LPS, respectively (Table 1). This effect was entirely dependent on binding of the recombinant B subunit to ganglioside GM1, since rLTB/G33D, a mutant which does not bind GM1, failed to inhibit proliferation of either T or B cells. Failure to bind rLTB/G33D was confirmed by flow cytometry with FITC-labeled rLTB/G33D (data not shown).
TABLE 1.
Response of T cells and B cells to mitogens is suppressed by rLTB but not by rLTB/G33D, a non-GM1-binding mutanta
| Treatment | Mean cpm ± SD (% of control)b for:
|
||
|---|---|---|---|
| CD8+ T cells | CD4+ T cells | Ly5+ B cells | |
| Medium | 34,589 ± 760 | 106,678 ± 4,960 | 40,728 ± 1,160 |
| rLTB (10 μg/ml) | 422 ± 123* (0.2) | 29,994 ± 5,322* (28.1) | 8,249 ± 1,585* (20.3) |
| rLTB/G33D (10μg/ml) | 37,241 ± 330 (108) | 132,215 ± 1,115 (124) | 38,591 ± 1,586 (93.7) |
CD8+ (94%), CD4+ (91%), and Ly5+ (96%) cells were positively selected from the spleens of B10.BR mice and activated with immobilized anti-CD3 MAb (T cells) or LPS (B cells) (see Materials and Methods). rLTB and rLTB/G33D were present throughout the 72-h incubation period.
Data are for triplicate microwells labeled with [3H]TdR for the last 20 h of culture. *, P < 0.0001 versus medium control and rLTB/G33D.
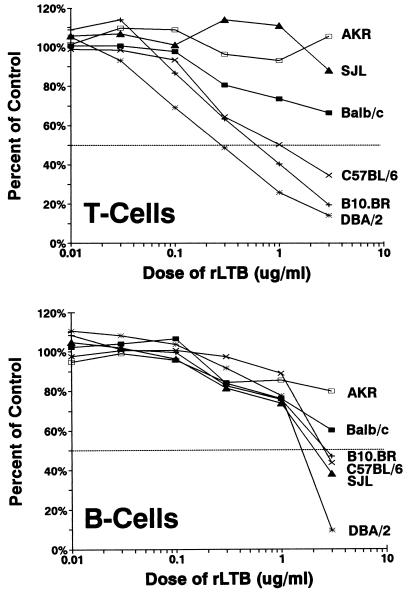
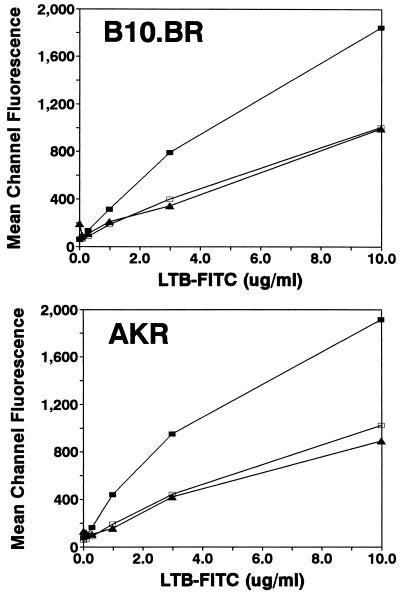
In contrast to previously published reports by others (25), we found that polyclonal activation of CD4+ T cells was inhibited by rLTB but that CD8+ T cells were significantly more sensitive to rLTB than CD4+ cells (P < 0.01). When T and B cells from different strains of mice were compared, significant variation in relative sensitivities to rLTB-mediated immune suppression was observed (Fig. 1). To determine whether the difference between strains was related to the ability to bind rLTB, we stained T-cell subsets and B cells with FITC-labeled rLTB (Fig. 2). CD8+ T cells bound approximately twice as much rLTB as either CD4+ T cells or Ly5+ B cells. These results were confirmed with 125I-labeled rLTB and in competition assays with unlabeled LTB (data not shown). There was no difference in the binding of LTB to cells from a strain with high (B10.BR) versus low (AKR) sensitivity to rLTB (Fig. 2). Thus, the different sensitivities of the strains cannot be attributed solely to binding differences.
FIG. 1.
T and B cells from different strains of mice vary in their susceptibility to immunosuppression by rLTB. Spleen cells (2 × 105/200 μl) were added to microwells coated with anti-CD3 MAb (10 μg/ml) or containing LPS (2 μg/ml) in the presence of rLTB at the concentrations indicated. The cultures were incubated for 72 h at 37°C and pulse-labeled with [3H]TdR for the final 20 h. Data are expressed as percentages of the control response (no LTB added), which ranged from 31,710 (±485) to 70,938 (±3,275) cpm. The background for unstimulated cultures (no rLTB, MAb, or LPS) ranged from 240 to 3,665 cpm. Results from one of two experiments giving similar results are shown. For clarity, the standard deviation bars for each data point are not shown. Generally, they were <10% of the mean counts per minute.
FIG. 2.
Strain differences in susceptibility to rLTB were not due to differences in ability of T and B cells to bind rLTB. Unseparated spleen cells from B10.BR, a high-susceptibility strain, and AKR, a low-susceptibility strain, were stained with FITC-LTB and PE-CD4 (open squares), PE-CD8 (closed squares), or PE-Ly5 (triangles). The mean fluorescence intensity for each subset in relation to the concentration of FITC-LTB is shown. The twofold-greater intensity of staining for CD8+ compared to CD4+ T cells was consistently observed in multiple experiments and was confirmed with binding of 125I-LTB to purified subsets.
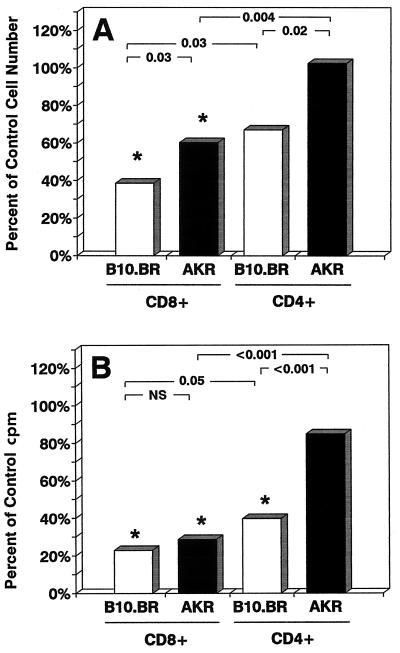
To directly compare the susceptibilities to rLTB of T-cell subsets from high- and low-sensitivity strains, we isolated CD4+ and CD8+ cells from B10.BR and AKR mice and compared the effects of rLTB on their survival (SCDA assay) and proliferative capacity ([3H]TdR uptake) in response to anti-CD3 MAb in parallel but independent assays (Fig. 3). CD8+ cells from both B10.BR and AKR mice were significantly more susceptible to rLTB-induced cell death (Fig. 3A) and inhibition of proliferation (Fig. 3B) than were CD4+ T cells (P ≤ 0.05). In both assays, CD4+ T cells from AKR mice were significantly more resistant than CD4+ cells from the B10.BR strain (P ≤ 0.02). Since the spleens of AKR mice had a higher proportion of CD4+ T cells than those of B10.BR mice (data not shown), the splenic T-cell populations used in these experiments were inherently skewed toward a more resistant population. Collectively, these results indicate that the variation in strain sensitivity to rLTB as shown in Fig. 1 (with unseparated spleen cells) was due to both qualitative (CD4 T cells being less sensitive to rLTB) and quantitative (relative CD4 T-cell content) factors rather than to differences in rLTB binding between the strains.
FIG. 3.
There are qualitative and quantitative differences in the effects of rLTB on CD4 and CD8 T-cell subsets from high- and low-sensitivity strains. (A) T-cell-enriched cell suspensions (B220 negative) from B10.BR and AKR spleens were treated with 10 μg of rLTB per ml for 2 h on ice, washed, and then incubated at 37°C for 24 h. The numbers of viable CD4 and CD8 T cells at 24 h were measured in SCDA assays. Data are normalized to the numbers of cells present in untreated control wells (AKR CD4, 53,171 ± 3,778; AKR CD8, 23,363 ± 1,750; B10.BR CD4, 31,271 ± 4,299; B10.BR CD8, 31,245 ± 408). ∗, P < 0.05 versus untreated control cells. P values between LTB-treated groups are shown at the top. (B) Positively selected CD4 and CD8 T cells isolated from the spleens of B10.BR and AKR mice were treated with 10 μg of rLTB per ml for 2 h on ice, washed, and then plated on anti-CD3-coated microwells for 72 h. Cell proliferation was measured by [3H]TdR uptake during the last 20 h of culture in triplicate microwells. Data are expressed as percentages of counts per minute in untreated control wells (AKR CD4, 50,578 ± 9,882; AKR CD8, 27,537 ± 8,452; B10.BR CD4, 91,918 ± 13,167; B10.BR CD8, 29,430 ± 5,384). Statistical comparisons are as described for panel A. NS, not significant.
rLTB induces apoptosis in both CD4+ and CD8+ T cells.
To determine whether rLTB induced apoptosis in T-cell subsets, CD4+ and CD8+ T cells were enriched by positive or negative selection by using immunomagnetic cell separation and cultured for 24 h in various doses of rLTB or the non-GM1-binding mutant rLTB/G33D in the absence of mitogen. Apoptosis was measured by flow cytometry with four techniques: analysis of DNA content (6), binding of FITC-Annexin V and exclusion of PI (33), changes in light scatter properties (23), and retention of R123 (6). Multiple assays were employed to ensure detection of apoptotic cells and exclusion of necrotic cells.
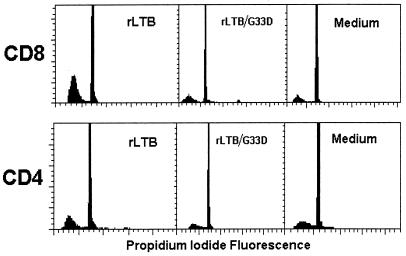
As a consequence of DNA cleavage by endonucleases, apoptotic cells exhibit a DNA content that is less than that of diploid cells in G0/G1 phase (i.e., sub-G0 or hypodiploid cells). Selected DNA histograms from one experiment are shown in Fig. 4. Average results from two to four experiments are presented in Table 2. rLTB stimulated a dose-dependent apoptotic response in CD8+ T cells. Within 24 h, an average of 50% of the CD8+ cells were hypodiploid (apoptotic) (P < 0.0001), compared to 12% of CD4+ cells incubated with 10 μg of rLTB per ml. With CD4+ cells, however, the apoptotic response did not increase proportionally to the dose of rLTB. The optimum effect was observed with 1 μg of rLTB per ml in three of four experiments (average, 20%; median, 23%) (Table 2). This suggests that the response to rLTB within the CD4+ cells may be more complex than that within the CD8+ population. Such differences may relate to the relative activation state of CD4+ T cells within heterogeneous populations, as described below.
FIG. 4.
Induction of apoptosis in CD8+ and CD4+ T cells by rLTB, but not by the non-GM1-binding mutant rLTB/G33D, as detected by DNA ploidy analysis. Representative DNA histograms from CD8-enriched (73% CD8+, 6% CD4+, and 20% Ly5+) (top row) and CD4-enriched (83% CD4+, 3% CD8+, and 12% Ly5+) (bottom row) spleen cells after treatment with 10 μg of rLTB or rLTB/G33D per ml for 24 h are shown. DNA ploidy analysis was done as described in Materials and Methods. The percentages of hypodiploid cells (left to right) were 51, 14, and 10% for CD8 T cells and 28, 11, and 12% for CD4 T cells. See Table 2 for average results from four experiments.
TABLE 2.
Preferential induction of apoptosis in CD8+ T cells by rLTB and failure of the non-GM1-binding mutant rLTB/G33D to induce apoptosisa
| Cells | Treatment | Concn (μg/ml) | % Hypodiploid nuclei (mean ± SEM) | No. of expts |
|---|---|---|---|---|
| CD8+ | rLTB | 10 | 49.6 ± 2.6* | 4 |
| 1 | 27.9 ± 9.9 | 4 | ||
| 0.1 | 14.5 ± 3.1 | 3 | ||
| rLTB/G33D | 10 | 14.8 ± 1.0 | 3 | |
| 1 | 11.8 | 1 | ||
| Medium | 12.2 ± 2.3 | 4 | ||
| CD4+ | rLTB | 10 | 11.7 ± 6.4 | 4 |
| 1 | 20.4 ± 5.8 | 4 | ||
| 0.1 | 5.4 ± 0.07 | 3 | ||
| rLTB/G33D | 10 | 11.9 ± 0.8 | 3 | |
| 1 | 9.2 ± 0.8 | 2 | ||
| Medium | 8.8 ± 2.2 | 4 |
DNA analysis was done as described in Materials and Methods. Data are the percentages of hypodiploid nuclei (sub-G0) detected after treatment for 24 h with rLTB, rLTB/G33D, or medium alone. Three of four experiments used positively selected T-cell subsets; one experiment used negatively selected subsets with equivalent results. *, P < 0.0001 versus medium, rLTB/G33D, and CD4+ cells; for all other comparisons, P > 0.05.
Background levels of hypodiploid cells incubated in medium alone were 9 and 12% for CD4+ and CD8+ cells, respectively. The percentage of hypodiploid cells after culture with the non-GM1-binding mutant rLTB/G33D was not significantly different from that for control cells. These results demonstrate that there is preferential, but not exclusive, induction of apoptosis in CD8+ T cells and that binding to ganglioside GM1 is required for induction of apoptosis. We were unable to detect apoptosis in enriched populations of Ly5+ B cells (>90% pure) by DNA ploidy analysis and gel electrophoresis (data not shown), confirming that B cells are relatively resistant to rLTB. Higher doses and longer exposure times, however, appear to induce death in B cells (Fig. 1 and unpublished data).
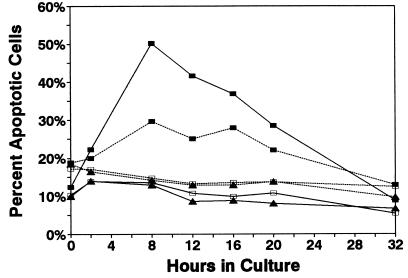
The kinetics of apoptosis induction in T cells was determined by monitoring the binding of FITC-Annexin V and exclusion of PI as a function of time after exposure to rLTB. Annexin V, an anticoagulant protein, binds to exposed phosphatidylserine on the plasma membrane during the early stages of apoptosis (33). Early apoptotic cells can be distinguished from necrotic cells, which also bind Annexin V, by their ability to exclude nonvital dyes such as PI. Lymph node cells, containing both CD8+ and CD4+ T cells (as well as B cells), were used in order to detect any preferential effect of rLTB on one or the other T-cell subset when tested within the same cell suspension. Three-color flow cytometry analysis was used to identify apoptotic CD8 and CD4 subsets within the mixture (i.e., Annexin positive and PI negative). Combined results from two overlapping experiments (0 to 20 h and 8 to 32 h) are shown in Fig. 5. The percentage of apoptotic cells peaked after 8 h in both CD8+ and CD4+ T cells and then declined as the cells lost membrane integrity and moved into a state of advanced apoptosis (Annexin bright and PI positive). These experiments demonstrated that CD8+ T cells were more sensitive to rLTB than CD4+ cells but that CD4+ T cells were susceptible to rLTB-induced apoptosis, in contrast to the reports by others (25). The non-GM1-binding mutant rLTB/G33D failed to induce apoptosis, confirming the essential role of GM1 binding. Concurrent T-cell stimulation with anti-CD3 MAb did not alter the kinetics of LTB-induced apoptosis (data not shown). Similar results were obtained by using uptake of 7-amino-actinomycin D and forward light scatter to monitor the kinetics of apoptosis (data not shown).
FIG. 5.
Kinetics of apoptosis induction by rLTB in CD8 and CD4 T cells. Lymph node cells (2 × 105 in 200 μl per well) from B10.BR mice were cultured in the presence of rLTB (5 μg/ml) (closed squares), rLTB/G33D (5 μg/ml) (open squares), or medium alone (triangles) for up to 32 h. At various times, cells were stained with PE–anti-CD4 (dashed lines) or PE–anti-CD8 (solid lines) along with FITC-Annexin V in the presence of PI and analyzed by three-color flow cytometry. The data represent the percentages of early apoptotic CD4 or CD8 T cells in mixed cultures as defined by increased binding of Annexin V and ability to exclude PI (i.e., Annexin positive and PI negative).
A significant decrease in forward light scatter of cells has been reported for other apoptosis systems (6, 23). We observed a progressive shift in light-scattering properties of rLTB-treated cells over time. This shift correlated with the gradual decline in Annexin-positive, PI-positive cells observed after 8 h (Fig. 5) and loss of membrane integrity. We interpreted these data to indicate progression of cells from an early (Annexin-low, PI-negative) to an advanced (Annexin-bright, PI-positive) stage of apoptosis. Because R123 is retained by early apoptotic cells but not by necrotic cells (6), we used retention of R123 to confirm membrane integrity and further exclude necrotic cell death. R123 was retained by CD8+ T cells cultured for 4 to 8 h in the absence (viable cells) and presence (apoptotic cells) of rLTB at 10 μg/ml (84 versus 80% R123 positive, respectively). However, when the cells were heated to 56°C to induce necrotic cell death, the percentage of R123+ cells declined to as low as 1% as membrane integrity was lost. Collectively, the results described in this section indicate that rLTB, but not the non-GM1-binding mutant, rapidly (i.e., within 8 h) induced apoptosis in both CD4+ and CD8+ T cells. The heightened susceptibility of CD8+ T cells to rLTB-induced apoptosis compared to CD4+ T cells correlated with increased binding of FITC-LTB (Fig. 2), suggesting that the concentration of GM1 available for cross-linking by rLTB may contribute to the relative sensitivities of T cell subsets.
Activated T cells are more resistant to LTB-mediated inhibition.
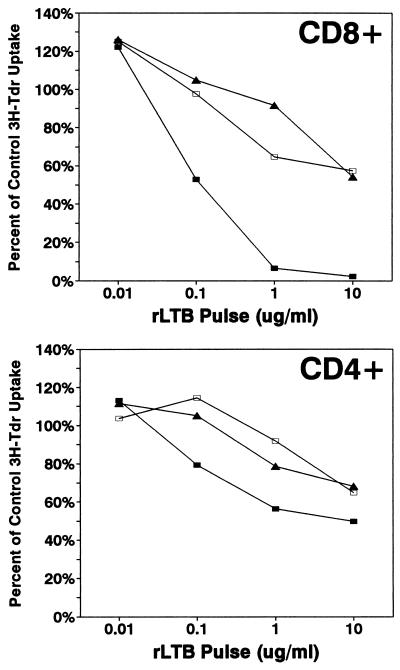
In the next set of experiments, we varied the time at which B10.BR T cells were exposed to rLTB in relation to TCR ligation in order to determine whether their state of activation altered the outcome. Naive CD4+ and CD8+ T cells were pulsed for 2 h with rLTB immediately before or 24 and 48 h after activation with immobilized anti-CD3 MAb. DNA synthesis (as measured by [3H]TdR uptake) and secretion of cytokine growth factors (e.g., IL-2) peak approximately 48 h after activation of T cells with anti-CD3 MAb. The results from a typical cell proliferation experiment are shown in Fig. 6. As noted earlier, naive CD8+ T cells were significantly more sensitive to inhibition than naive CD4+ T cells (P < 0.0001). At the higher concentrations of rLTB, essentially all of the naive CD8+ T cells were inhibited, whereas a proportion of naive CD4+ T cells remained functionally responsive to polyclonal activation. These data suggest the presence of a subpopulation of CD4+ T cells which were insensitive to the inhibitory effects of rLTB. Activated T cells of both subsets were significantly more resistant to inhibition by rLTB than naive cells (P < 0.01). The increased resistance to inhibition of proliferation was not due to decreased binding of rLTB, since activated T-cell subsets bound FITC-LTB at levels comparable to or slightly higher than those of naive cells (23a).
FIG. 6.
Activated T cells are less susceptible than naive cells to suppression by rLTB. Positively selected CD4+ (94% pure) and CD8+ (92% pure) B10.BR T cells were divided into three aliquots and treated as follows. The first aliquot (naive cells) (closed squares) was treated with 0, 0.01, 0.1, 1, or 10 μg of rLTB per ml (106/ml) for 2 h at 37°C, washed, exposed to immobilized anti-CD3 MAb for 24 h, and then transferred to uncoated microwells and incubated for an additional 48 h. Cells in the second aliquot (24-h activated) (open squares) were activated for 24 h on immobilized anti-CD3 MAb, collected, counted, treated with 0 to 10 μg of rLTB per ml for 2 h, and washed, and then a constant number of cells were transferred to uncoated wells and incubated for an additional 48 h. Cells in the third aliquot (48-h activated) (triangles) were exposed to immobilized anti-CD3 MAb for the first 24 h of culture, transferred to uncoated wells, incubated for 24 h, recollected, counted, treated with 0 to 10 μg of rLTB per ml for 2 h, and washed, and then a constant number were distributed to uncoated wells and incubated for an additional 24 h. This design kept the length of time that cells were exposed to anti-CD3 MAb (24 h) as well as rLTB (2 h) and the total time in culture (72 h) constant; only the timing of when the cells were exposed to rLTB changed. All cultures were set up in quadruplicate and labeled with [3H]TdR for the last 22 h of culture. Cultures without rLTB served as an internal control within each set, and the data are normalized to percentages of the untreated control response. Key statistical comparisons: for naive CD8 cells, P < 0.01 versus 24- and 48-h-activated cells at 10, 1, and 0.1 μg of rLTB per ml; for naive CD4 cells, P < 0.01 versus 24-h-activated cells at 10, 1, and 0.1 μg of rLTB per ml; for naive CD8 and CD4 T cells, P < 0.01 versus medium control at 10, 1, and 0.1 μg of rLTB per ml; and for activated CD8 and CD4 T cells, P < 0.05 versus medium control only at 10 μg of rLTB per ml.
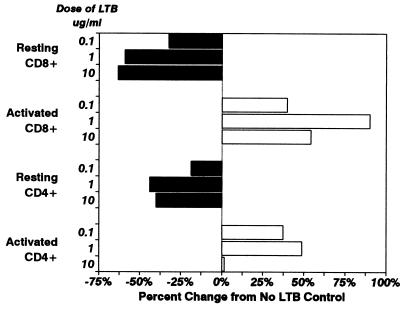
We next compared the effects of rLTB on proliferation of alloantigen-specific CD4+ and CD8+ T-cell lines established from long-term MLR cultures. The T-cell lines were allowed to rest for 10 days after allostimulation and then washed free of exogenous rIL-2 and held overnight (resting T cells). The quiescent T-cell lines were pulse-treated with rLTB, and the number of viable cells was determined 24 h later by quantitative flow cytometry with the SCDA assay (Fig. 7). Parallel cultures of the same T-cell lines were exposed to specific allostimulator cells for 48 h in the presence of rIL-2 (activated T cells) and then pulsed with rLTB for 2 h, and the number of viable cells remaining after 24 h was measured by SCDA (Fig. 7). The results show that both CD8+ and CD4+ alloantigen-specific T cells were susceptible to rLTB-induced cell death when they were in a resting state but that they were relatively resistant to rLTB when in an activated state. Resting CD8+ T cells were more sensitive to rLTB than resting CD4+ cells. The two T-cell lines bound FITC-LTB at comparable levels whether in a quiescent or stimulated state (data not shown).
FIG. 7.
Activated T cells become susceptible to suppression by rLTB when they return to a resting state. Long-term MHC antigen-specific CD4 and CD8 T-cell lines were established from MLR cultures as described in Materials and Methods. The T-cell lines were allowed to rest for 10 days and then washed free of rIL-2 and exposed to rLTB (solid bars), or they were reactivated with specific allostimulator cells 48 h prior to treatment with rLTB (open bars). The cells were exposed to rLTB (0.1, 1, and 10 μg/ml) for 2 h, washed, and reincubated. The number of viable cells remaining after 48 h was determined by quantitative flow cytometry with the SCDA assay. Data are presented as the percent change from values for untreated (no-rLTB) control cultures: viable cells in resting CD4 cells, 18,037; in resting CD8 cells, 14,047; in activated CD4 cells, 58,592; in activated CD8 cells, 50,858. Negative values indicate a decrease in cells; positive values indicate an increase from control levels.
rLTB inhibits proliferation of CD8+ T cells and generation of CTL in MLR cultures.
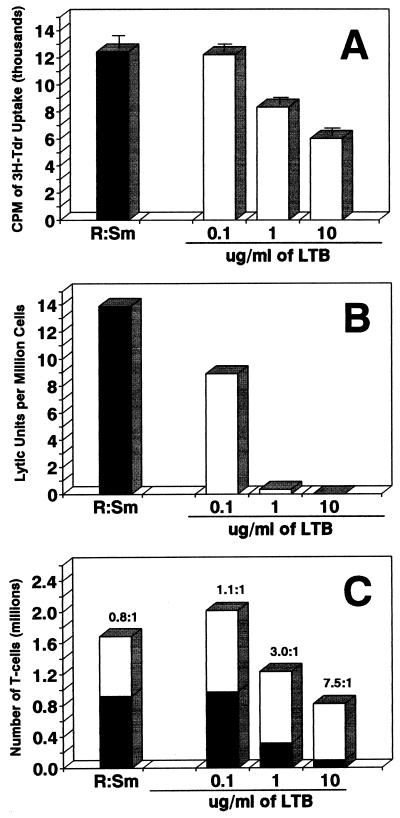
MLR and CML assays were used to examine the effects of rLTB on antigen-specific T-cell responses, i.e., clonal responses to allogeneic histocompatibility antigens. Spleen cells from C57BL/6 (H-2b) mice were stimulated with MHC-mismatched, mitomycin C-treated spleen cells from B10.BR (H-2k) mice in a 5-day MLR assay. Proliferation was measured by [3H]TdR uptake. As shown in Fig. 8A, pretreatment of C57BL/6 responder cells with rLTB for 1 h was sufficient to inhibit alloantigen-driven T-cell proliferation in a dose-dependent manner. In addition, generation of cytotoxic T lymphocytes (CTL) within the MLR cultures was inhibited in an LTB-dose-dependent manner (Fig. 8B). The numbers of LU30 per million cells in the 5-day MLR cultures (pulsed for 1 h on day 0) with 0.1, 1, and 10 μg of rLTB per ml were 64, 2, and 0% of control levels, respectively.
FIG. 8.
Pretreatment with rLTB preferentially depletes CD8+ responder T cells from MLR/CML cultures. (A) Responder spleen cells (R) from C57BL/6 (H-2b) mice were pretreated (107/ml) with 0.1, 1.0, or 10 μg of rLTB per ml for 1 h at 37°C (open bars) or with no rLTB (solid bar), washed, recounted, checked for viability, and then stimulated with mitomycin C-treated MHC-mismatched B10.BR (H-2k) spleen cells (Sm) in MLR cultures for 5 days (see Materials and Methods). Cell proliferation was measured by pulsing with [3H]TdR for the final 6 h of culture. Mean counts per minute and standard deviations for triplicate samples are shown. (B) Effector CTL generated in the MLR culture were mixed with 51Cr-labeled target cells at various effector/target cell ratios in a 3.5-h CML assay (see Materials and Methods). The number of LU30 per million effector cells was calculated for each culture. (C) Responding CD4 and CD8 T cells in the MLR cultures were quantified by SCDA assays as described in Materials and Methods. The data shown are average numbers of CD4+ (open bars) and CD8+ (solid bars) T cells per well on day 5 (means for duplicate samples). The value above each bar shows the ratio of CD4 to CD8 T cells.
The T cells responding to alloantigen in the 5-day MLR cultures were phenotyped and quantitated by flow cytometry with the SCDA assay (Fig. 8C). As expected, both CD4+ and CD8+ T cells proliferated in untreated cultures (Fig. 8C). The ratio of CD4 to CD8 cells was 0.8:1. A 1-h pretreatment with rLTB had a profound and preferential effect on CD8+ T cells. While there was a loss of CD8+ T cells, the absolute number of CD4+ T cells did not change significantly in comparison to that in the control culture. With increasing amounts of rLTB, the CD4/CD8 ratio was skewed toward CD4 cells (1:1, 3:1, and 8:1 with 0.1, 1, and 10 μg/ml, respectively). The loss of CD8+ T cells correlated with an LTB dose-dependent decrease in CTL (cf. Fig. 8B and C). The number of CD4+ T cells in MLR cultures decreased significantly only when rLTB was present continuously during the 5-day culture and at higher concentrations (e.g., 10 μg/ml) (data not shown).
Altered cytokine secretion in LTB-treated CD8+ and CD4+ T cells.
The immunosuppressive properties of rLTB in vitro were attributed in large part to a loss of CD8+ and CD4+ T cells through induction of apoptosis. However, a significant number of CD4+ T cells and some CD8+ T cells survived exposure to rLTB (see, e.g., Fig. 6 and 8C). To determine whether functional activity was altered in surviving cells, CD4+ and CD8+ T cells were pulse-treated with rLTB for 1 h and then stimulated with immobilized anti-CD3 MAb to induce cytokine secretion and proliferation. Secretion of IFN-γ and IL-2 into the culture supernatant was monitored after 24 h. Others have reported decreased IFN-γ production after in vivo treatment with rLTB (25).
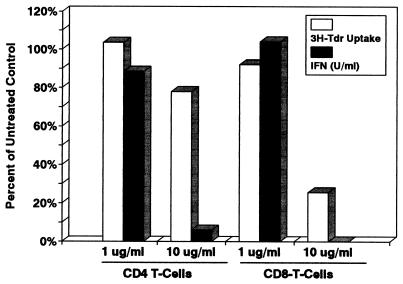
CD4+ T cells pulsed with 10 μg of rLTB per ml proliferated in response to TCR signaling (75% of control [3H]TdR uptake) but failed to secrete significant amounts of IFN-γ (6% of control levels) (Fig. 9). The effect of rLTB treatment on proliferation and cytokine secretion was LTB dose dependent. As expected, proliferation of CD8+ T cells, which are more sensitive to rLTB, was affected to a greater extent than CD4+ cells, but IFN-γ secretion by the residual CD8+ T cells was not detected. Similar results were obtained when IL-2 secretion was measured (data not shown). These results suggest that rLTB has immunomodulatory properties independent of its ability to induce apoptosis.
FIG. 9.
rLTB inhibits secretion of IFN-γ by CD4+ and CD8+ T cells in response to activation with immobilized anti-CD3 MAb. Positively selected CD4+ and CD8+ T cells were pretreated for 1 h with rLTB at 1.0 and 10 μg/ml, washed, and then activated by being cultured in microwells coated with anti-CD3 MAb. The culture supernatant was collected 24 h later and frozen until tested by ELISA for the presence of IFN-γ (solid bars). The remaining cells were resuspended in fresh medium and labeled with 1 μCi of [3H]TdR overnight to assess cell proliferation (open bars). Data are normalized as percentages of control values for untreated activated T cells. Control values were 21 and 98 U of IFN-γ per ml and 80,653 and 51,582 cpm of [3H]TdR uptake for untreated CD4+ and CD8+ T cells, respectively.
DISCUSSION
The data presented here substantiate that recombinant LTB is a potent immunosuppressive and immunomodulatory agent. We present data for (i) preferential suppression of T cells versus B cells in vitro, (ii) suppression of T-cell proliferation to antibody-mediated TCR cross-linking and allostimulation, (iii) induction of apoptosis in T cells, especially CD8+ cells, after exposure to rLTB, (iv) a stronger effect of rLTB on naive cells than on activated T cells, and (v) functional alteration in secretion of IFN-γ by CD8+ and CD4+ T cells that survived exposure to rLTB. These properties are dependent on binding of rLTB to ganglioside GM1, based on the inactivity of the mutant rLTB/G33D, which does not bind to GM1.
The x-ray crystallographic structure of LT has provided considerable insight into the toxin’s biological and biophysical properties (29, 30). LT holotoxin is composed of five GM1 binding sites. Each GM1 binding site is formed by contiguous regions of adjacent B-subunit monomers (30). The GM1 binding sites are exposed to solvent and do not appear to undergo conformational change upon binding to ganglioside on the cell membrane (29). Structural analysis has also resolved the critical role of glycine 33 (G33) within the B-subunit monomers. G33 appears to interact with solvent and contribute to high-affinity GM1 binding (29). CT binds exclusively to GM1, while LT is more tolerant, binding primarily to GM1 but also weakly to several other gangliosides, including GM2 and asialo-GM1 (31). Thus, the biological range of LT may extend beyond GM1 receptor-mediated mechanisms. However, we were unable to detect binding of FITC-labeled rLTB/G33D to murine lymphocytes (data not shown), suggesting that ganglioside GM1 is the dominant receptor in this system.
Use of a GM1 receptor-binding mutant to elucidate the importance of receptor binding in the activity of LTB was first described by Nashar and colleagues (25). These investigator found that rLTB, but not rLTB/G33D, caused complete depletion of murine CD8+ T cells, an increase in the activation of CD4+ T cells, an increase in IL-2 and decrease in IFN-γ secretion, and activation of CD25+ (IL2R+) B cells. However, their initial studies used cells from mice primed with rLTB or rLTB/G33D in vivo. More recently, the same group has reported that rLTB has a differential effect on CD4+ and CD8+ lymph node cells from ovalbumin-primed mice that were restimulated with antigen in vitro (26). Exposure of responder cells to rLTB for 5 days in ovalbumin-stimulated cultures led to depletion of CD8+ T cells and enhanced expression of CD25 (IL2Rα) on CD4+ T cells (26). The investigators did not establish, however, whether antigen (ovalbumin) priming in vivo or restimulation in vitro affected the response to rLTB.
In contrast to Nashar and colleagues (25, 26), who concluded that CD4+ T cells were resistant to rLTB, we found that both CD4+ and CD8+ T cells were susceptible to induction of apoptosis but differed in dose response (Fig. 5 and Table 2). This is similar to the effects of CT as reported by Yankelevich et al. (37), who found that treatment with the B subunit of native CT stimulated apoptosis in both CD4 and CD8 T cells, although apoptosis was preferentially induced in CD8+ T cells. Thus, CD4+ T cells do not appear to be inherently resistant to immunosuppression by either LTB or CTB. The immunosuppressive effect may be dose dependent. Nashar and colleagues (26) used higher doses of rLTB than we employed, and they did not describe dose-response studies using T-cell subsets. We observed that at high doses of rLTB, some CD4+ T cells were spared in comparison to CD8+ T cells (Fig. 6 and Table 2); i.e., complete suppression of the CD4+ T-cell response to polyclonal activation was seldom achieved. There may be a subpopulation of CD4+ T cells that are relatively resistant to rLTB. Constitutive activation of cells within the CD4+ population in vivo might affect the extent of inhibition or cell death observed in vitro.
We did reproduce the observation of Nashar and colleagues (26), who used ovalbumin-specific cell cultures to show preferential depletion of CD8+ T cells by rLTB, by using primary MLR cultures. In the present study, we found that CD8+ T cells were preferentially depleted from the MLR culture, while CD4+ T cells were spared (Fig. 8C). However, we extended the observations of Nashar et al. by showing in two different ways that the activation state of the T cells significantly altered susceptibility to rLTB-mediated suppression. First, naive CD4+ and CD8+ T cells, which were sensitive to the effects of rLTB, acquired a more resistant phenotype when activated (Fig. 6). Second, T cells in an activated, rLTB-resistant state reverted to a more LTB-sensitive phenotype when they were allowed to rest, i.e., to return to a nondividing state (Fig. 7). Both polyclonal activation with anti-CD3 MAb (Fig. 6) and clonal activation with alloantigen (Fig. 7) decreased the relative sensitivity of T cells to rLTB. In the studies reported by Nashar et al. (26), the predominant responding cells were ovalbumin-specific CD4+ T cells that had been primed in vivo and then reactivated in vitro. Our data suggest that such cells may be relatively resistant to LTB-induced apoptosis, whereas nonactivated CD8+ T cells present in the same culture remain susceptible to rLTB.
Yankelevich and colleagues (37) did not detect differential binding of CTB to CD8+ or CD4+ T cells by using FITC-labeled CTB, but others have reported preferential binding to CD8+ T cells (8). We observed that CD8+ T cells bind almost twice as much rLTB as CD4+ cells in three different assays (Fig. 2 and unpublished data). Although the difference in binding of rLTB did not explain either strain variation in response to rLTB or the effects on naive versus activated T cells, it did correlate with the increased sensitivity of CD8+ T cells to LTB-induced apoptosis. Binding to GM1 was essential for the induction of apoptosis in T cells, as indicated by the lack of effect with the LTB/G33D mutant. The relative level of GM1 expression and availability for cross-linking may be important factors in the dose of rLTB required to suppress T cells.
The mechanism by which rLTB induces apoptosis is not known. Membrane sphingolipids constitute a unique signaling pathway that links certain cell surface receptors to the nucleus with ceramide and other sphingomyelin breakdown products serving as second messengers (15, 28). Although associated with the induction of apoptosis, the sphingomyelin pathway, and ceramide in particular, have also been linked to T-cell activation via the CD28 cosignaling pathway (2). Current models for glycosphingolipid-triggered apoptosis generally favor an endocytotic vesicle pathway with ceramide release from the lysosomal compartment (14). Thus, LTB-mediated cross-linking and endocytosis of glycoshpingolipids may be responsible for LTB-induced apoptosis. Support for an endocytotic pathway comes from our observation that rLTB failed to induce apoptosis when T cells were kept at 4°C to inhibit endocytosis but induced cell death when they were transferred to 37°C (31a). A number of extracellular agents have been shown to activate the sphingomyelin pathway, including tumor necrosis factor alpha, IFN-γ, IL-1, and dexamethasone (15). Fas-mediated apoptosis may also involve activation of the sphingomyelin pathway and ceramide release (4, 12). However, Fas does not appear to be involved in LTB-induced apoptosis, since cells from Fas-deficient C3H.MRL (lpr/lpr) mice were as sensitive to rLTB as cells from normal C3H/He mice (31a).
There is emerging evidence for cross-talk between phospholipid and sphingolipid signal transduction pathways (17, 20, 22). Ligation of the TCR activates phospholipases, leading to calcium mobilization and signal transduction, with diacylglyerol and inositol-1,4,5-triphosphate, among others, acting as secondary messengers (3). This pathway culminates in mitogenic T-cell activation. In opposition to this system, sphingomyelin breakdown products, such as ceramide and sphingosine, act as antiproliferative agents and regulators of apoptosis (16, 20, 28). These observations and the data reported here showing decreased sensitivity of activated T cells to rLTB encourage us to propose the following hypothesis. In naive T cells, cross-linking of ganglioside GM1 by rLTB leads to endocytosis and release of ceramide from the lysosomal compartment, activating the sphingomyelin pathway and inducing apoptosis. In activated cells, however, the apoptosis signal induced by rLTB binding is countered by cross-talk from the phospholipid pathway triggered by TCR ligation. We further speculate that functional alterations occur when both pathways are activated and the apoptotic signal is blocked. Under this scenario, T-cell activation with alloantigen or anti-CD3 MAb would be predicted to decrease the susceptibility of CD8+ and CD4+ T cells to LTB-induced apoptosis but not to prevent immunomodulation (such as decreased cytokine secretion). This is consistent with the cellular data reported here, but further experimentation is necessary to confirm involvement of the biochemical pathways cited.
Elson and colleagues have extensively characterized the effects of CTB on murine lymphocytes in vitro (8, 34, 35). These investigators found that native CTB is a strong inhibitor of mitogen- and antigen-driven T-cell activation (34), that direct binding of CTB to T cells is required (34), that a short pulse with CTB is sufficient for suppression (8), and that both CD4 and CD8 T cells are inhibited by CTB, although CD8+ T cells bind more CTB than CD4+ cells (8). The results that we obtained with recombinant LTB were comparable to those of Elson and colleagues (8, 34, 35), indicating that the B subunits of CT and LT act in similar, if not identical, manners to inhibit T cells. In another study, Elson and associates (35) found that T-cell inhibition by CTB was not mediated through interference with the phospholipid signal transduction pathway. Although induction of apoptosis by CTB was not examined, Elson et al. (8) found that CTB induced a reduction in CD8+ intraepithelial lymphocytes shortly after mucosal exposure by gavage. Yankelevich et al. (37) have reported that native CTB induces apoptosis in murine T cells.
In the present study, we found that recombinant LTB suppressed secretion of IFN-γ and IL-2 measured 24 h after stimulation with immobilized anti-CD3 MAb while having a variable effect on the proliferation of both CD4+ and CD8+ T-cell subsets in vitro (Fig. 9 and unpublished data). Levels of IL-4 and IL-10 at 24 h were negligible (not shown). Similar observations have been reported previously for native CTB (8, 35). This raises the possibility that at least some of the immunomodulatory properties associated with B subunits of bacterial enterotoxins may be mediated not by apoptosis but by functional alterations through signal transduction as a result of binding to glycosphingolipids on the cell membrane. Yankelvich et al. (36) report that a 1-h pretreatment of immunocompetent donor spleen cells with the B subunit of native CT is sufficient to prevent acute GVHD following allogeneic bone marrow transplantation in mice. Whether induction of apoptosis or immune deviation or both contribute to decreased GVHD is unclear at present. Acute GVHD is mediated by TH1-type cytokines, which include IFN-γ and IL-2 (10, 11), and CD8+ T cells typically exhibit a TH1-like cytokine profile (9). CT has been reported to have a differential effect on TH1 and TH2 CD4+ T cells (24), and like LT, it binds to ganglioside GM1 via the B subunit. Thus, glycosphingolipids may provide unique target molecules for immunosuppression or immunomodulation of T-cell activity with genetically engineered enterotoxins.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grants CA73738 (to R.L.T. and J.T.B.) from the National Cancer Institute and AI30162 (to J.T.B.) from the National Institute of Allergy and Infectious Diseases, by a grant from the Cancer Center of the Medical College of Wisconsin, and by the Midwest Athletes Against Childhood Cancer Fund (Milwaukee, Wis.).
We are indebted to Bryon D. Johnson for providing the T-cell lines used in these studies and to Rino Rappuoli for providing the gene encoding LTB.
REFERENCES
- 1.Barbieri J T, Moloney B, Mende-Mueller L. Expression and secretion of the S-1 subunit of pertussis toxin in Escherichia coli. J Bacteriol. 1989;171:4362–4369. doi: 10.1128/jb.171.8.4362-4369.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boucher L-M, Wiegmann K, Fütterer A, Pfeffer K, Machleidt T, Schütze S, Mak T, Krönke M. CD28 signals through acidic sphingomyelinase. J Exp Med. 1995;181:2059–2068. doi: 10.1084/jem.181.6.2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cantrell D. T cell antigen receptor signal transduction pathways. Annu Rev Immunol. 1996;14:259–274. doi: 10.1146/annurev.immunol.14.1.259. [DOI] [PubMed] [Google Scholar]
- 4.Cifone M G, De Maria R, Roncaioli P, Rippo M R, Azuma M, Lanier L L, Santoni A, Testi R. Apoptotic signaling through CD95 (Fas/Apo-1) activates an acidic sphingomyelinase. J Exp Med. 1993;177:1547–1552. doi: 10.1084/jem.180.4.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Craig S W, Cuatrecasas P. Mobility of cholera toxin receptors on rat lymphocyte membranes. Proc Natl Acad Sci USA. 1975;72:3844–3848. doi: 10.1073/pnas.72.10.3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Darzynkiewicz Z, Bruno S, Del Bino G, Gorczyca W, Hotz M A, Lassota P, Traganos F. Features of apoptotic cells measured by flow cytometry. Cytometry. 1992;13:795–808. doi: 10.1002/cyto.990130802. [DOI] [PubMed] [Google Scholar]
- 7.Drobyski W R, Piaskowski V, Ash R C, Casper J T, Truitt R L. Preservation of lymphokine-activated killer activity following T cell depletion of human bone marrow. Transplantation. 1990;50:625–632. doi: 10.1097/00007890-199010000-00020. [DOI] [PubMed] [Google Scholar]
- 8.Elson C O, Holland S P, Dertzbaugh M T, Cuff C F, Anderson A O. Morphologic and functional alterations of mucosal T cells by cholera toxin and its B subunit. J Immunol. 1995;154:1032–1040. [PubMed] [Google Scholar]
- 9.Erard F, Wild M T, Garcia-Sanz J A, LeGros G. Switch of CD8 T cells to noncytolytic CD8−CD4− cells that make TH2 cytokines and help B cells. Science. 1993;260:1802–1805. doi: 10.1126/science.8511588. [DOI] [PubMed] [Google Scholar]
- 10.Ferrara J L M. Paradigm shift for graft-versus-host disease. Bone Marrow Transplant. 1994;14:183–184. [PubMed] [Google Scholar]
- 11.Fowler D H, Kurasawa K, Husebekk A, Cohen P A, Gress R E. Cells of Th2 cytokine phenotype prevent LPS-induced lethality during murine GVH reactions. J Immunol. 1994;152:1004–1013. [PubMed] [Google Scholar]
- 12.Gulbins E, Bissonnette R, Mahboubi A, Martin S, et al. Fas-induced apoptosis is mediated via a ceramide-initiated ras signalling pathway. Immunity. 1995;2:341–351. doi: 10.1016/1074-7613(95)90142-6. [DOI] [PubMed] [Google Scholar]
- 13.Hakomori S I. Bifunctional role of glychosphingolipids: modulators for transmembrane signaling and mediators for cellular interactions. J Biol Chem. 1990;265:18713–18716. [PubMed] [Google Scholar]
- 14.Hakomori S I, Igarashi Y. Glycosphingolipids and spingolipids closely associated with or causing apoptosis. Acta Histochem Cytochem. 1995;28:77–82. [Google Scholar]
- 15.Hannun Y A. The sphingomyelin cycle and the second messenger function of ceramide. J Biol Chem. 1994;269:3125–3128. [PubMed] [Google Scholar]
- 16.Hannun Y A, Linardic C M. Sphingolipid breakdown products: anti-proliferative and tumor-suppressor lipids. Biochim Biophys Acta. 1993;1154:223–236. doi: 10.1016/0304-4157(93)90001-5. [DOI] [PubMed] [Google Scholar]
- 17.Hannun Y A, Obeid L M. Ceramide: an intracellular signal for apoptosis. Trends Biochem Sci. 1995;20:73–77. doi: 10.1016/s0968-0004(00)88961-6. [DOI] [PubMed] [Google Scholar]
- 18.Hirst T R. Biogenesis of cholera toxin and related oligomeric enterotoxins. Handb Nat Toxins. 1995;8:123–184. [Google Scholar]
- 19.Holmgren J. Actions of cholera toxin and the prevention and treatment of cholera. Nature. 1981;292:413–417. doi: 10.1038/292413a0. [DOI] [PubMed] [Google Scholar]
- 19a.Johnson, B. Unpublished data.
- 20.Kolesnick R, Fuks Z. Ceramide: a signal for apoptosis or mitogenesis? J Exp Med. 1995;181:1949–1952. doi: 10.1084/jem.181.6.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lencer W I, Delp C, Neutra M, Madare J L. Mechanism of cholera toxin action on a polarized human intestinal epithelial cell line: role of vesicular traffic. J Cell Biol. 1992;117:1197–1209. doi: 10.1083/jcb.117.6.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liscovitch M. Crosstalk among multiple signal-activated phospholipases. Trends Biochem Sci. 1992;17:393–399. doi: 10.1016/0968-0004(92)90007-v. [DOI] [PubMed] [Google Scholar]
- 23.Lyons A B, Samuel K, Sanderson A, Maddy A H. Simultaneous analysis of immunophenotype and apoptosis of murine thymocytes by single laser flow cytometry. Cytometry. 1992;13:809–821. doi: 10.1002/cyto.990130803. [DOI] [PubMed] [Google Scholar]
- 23a.Mueller, R. Unpublished data.
- 24.Munoz E, Zubiaga A M, Merrow M, Sauter N P, Huber B T. Cholera toxin discriminates between T helper 1 and 2 cells in T cell receptor-mediated activation: role of cAMP in T cell proliferation. J Exp Med. 1990;172:95–103. doi: 10.1084/jem.172.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nashar T O, Webb H M, Eaglestone S, Williams N A, Hirst T R. Potent immunogenicity of the B subunit of Escherichia coli heat-labile enterotoxin: receptor binding is essential and induces differential modulation of lymphocyte subsets. Proc Natl Acad Sci USA. 1996;93:226–230. doi: 10.1073/pnas.93.1.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nashar T O, Williams N A, Hirst T R. Cross-linking of cell surface ganglioside GM1 induces the selective apoptosis of mature CD8+ T lymphocytes. Int Immunol. 1996;8:731–736. doi: 10.1093/intimm/8.5.731. [DOI] [PubMed] [Google Scholar]
- 27.Pechhold K, Pohl T, Kabelitz D. Rapid quantification of lymphocyte subsets in heterogeneous cell populations by flow cytometry. Cytometry. 1994;16:152–159. doi: 10.1002/cyto.990160209. [DOI] [PubMed] [Google Scholar]
- 28.Pushkareva M, Obeid L M, Hannun Y A. Ceramide: an endogenous regulator of apoptosis and growth suppression. Immunol Today. 1995;16:294–297. doi: 10.1016/0167-5699(95)80184-7. [DOI] [PubMed] [Google Scholar]
- 29.Sixma T K, Kalk K H, van Zanten B A, Dauter Z, Kingma J, Witholt B, Hol W G. Refined structure of Escherichia coli heat-labile enterotoxin, a close relative of cholera toxin. J Mol Biol. 1993;230:890–918. doi: 10.1006/jmbi.1993.1209. [DOI] [PubMed] [Google Scholar]
- 30.Sixma T K, Stein P E, Hol W G, Read R J. Comparison of the B-pentamers of heat-labile enterotoxin and verotoxin-1: two structures with remarkable similarity and dissimilarity. Biochemistry. 1993;32:191–198. doi: 10.1021/bi00052a025. [DOI] [PubMed] [Google Scholar]
- 31.Teneberg S, Hirst T R, Angstrom J, Karlsson K A. Comparison of the glycolipid-binding specificities of cholera toxin and porcine Escherichia coli heat-labile enterotoxin: identification of a receptor-active non-ganglioside glycolipid for the heat-labile toxin in infant rabbit small intestine. Glycoconjugate J. 1994;11:533–540. doi: 10.1007/BF00731304. [DOI] [PubMed] [Google Scholar]
- 31a.Truitt, R. Unpublished data.
- 32.Truitt R L, Shih C-Y, LeFever A V, Tempelis L D, Andreani M, Bortin M M. Characterization of alloimmunization-induced T lymphocytes reactive against AKR leukemia in vitro and correlation with graft-vs-leukemia activity in vivo. J Immunol. 1983;131:2050–2058. [PubMed] [Google Scholar]
- 33.Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C. A novel assay for apoptosis: flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled annexin V. J Immunol Methods. 1995;184:39–51. doi: 10.1016/0022-1759(95)00072-i. [DOI] [PubMed] [Google Scholar]
- 34.Woogen S D, Ealding W, Elson C O. Inhibition of murine lymphocyte proliferation by the B subunit of cholera toxin. J Immunol. 1987;139:3764–3770. [PubMed] [Google Scholar]
- 35.Woogen S D, Turo K, Dieleman L A, Beagley K W, Elson C O. Inhibition of murine T cell activation by cholera toxin B subunit is not mediated through the phosphatidylinositol second messenger system. J Immunol. 1993;150:3274–3283. [PubMed] [Google Scholar]
- 36.Yankelevich B, Brown E, Mazumder A. Prevention of acute graft-versus-host disease by treatment with a novel immunosuppressant—cholera toxin B subunit. J Immunol. 1995;154:3611–3617. [PubMed] [Google Scholar]
- 37.Yankelevich B, Soldatenkov V A, Hodgson J, Polotsky A J, Creswell K, Mazumder A. Differential induction of programmed cell death in CD8+ and CD4+ T cells by the B subunit of cholera toxin. Cell Immunol. 1996;168:229–234. doi: 10.1006/cimm.1996.0070. [DOI] [PubMed] [Google Scholar]