Abstract
Background
Since the introduction of direct-acting antivirals, thousands of chronic hepatitis C patients have been successfully treated. However, vulnerable populations have a higher prevalence of hepatitis C virus (HCV) infection and face barriers that impede their access to antivirals. We carried out an HCV microelimination program focused on vulnerable population groups in Malaga.
Methods
People in drug addiction treatment centers and homeless shelters in Malaga who participated in the program between October 2020 and October 2021 were included. After providing participants with educational information on HCV, a dry drop test (DDT) was used to collect blood for subsequent screening for HCV infection. The participants who were diagnosed with HCV infection were scheduled for comprehensive healthcare assessments, including blood tests, ultrasonography, elastography, and the prescription of antivirals, all conducted in a single hospital visit. Sustained viral response (SVR) was analysed 12 weeks after end of treatment.
Results
Of the 417 persons invited to participate, 271 (65%) agreed to participate in the program. These participants were screened for HCV infection and 28 of them were diagnosed with HCV infection (10%). These hepatitis C-infected patients had a mean age of 53 ± 9 years; 86% were males and 93% were or had been drug users. Among 23 patients with HCV infection, HCV genotype 1a predominated (74%). Medical exams showed that 19% (4/21) had advanced fibrosis (F3–4), and 5% (1/21) had portal hypertension. Finally, 23 infected patients received treatment with glecaprevir/pibrentasvir or sofosbuvir/velpatasvir and SVR was confirmed in 22 patients (96%).
Conclusions
Drug users and homeless people have a higher prevalence of HCV infection than the general population. The microelimination program with educational activity and screening tools achieved a high participation rate, easy healthcare access, and a high rate of SVR despite the SARS-CoV-2 pandemic.
Keywords: drug users, vulnerable population, hepatitis C microelimination
Introduction
Curing hepatitis C virus (HCV) infection, a global health problem, is now achievable with current therapies. Accordingly, the World Health Organization (WHO) proposed viral hepatitis C elimination as a global target with a reduction in new HCV infections by 90% and a decrease in the mortality rate of associated complications of 65%, both by 2030 [1].
Patients with HCV infection can develop chronic hepatitis with the subsequent risk of progression to liver cirrhosis [2, 3]. Furthermore, HCV infection is one of the leading causes of chronic liver disease and hepatocellular carcinoma (HCC) in the world [4]. There are currently >70 million chronic hepatitis C patients in the world, many of whom are unaware of their infection [5]. In recent years, thanks to the commercialization of direct-acting antivirals, the treatment of HCV infection has achieved important progress with high rates of sustained virologic response (SVR), up to 95% [6, 7], and improvements in necroinflammation and liver fibrosis status.
According to the Alliance for the Elimination of Viral Hepatitis in Spain, it is estimated that 0.22% of the Spanish population is infected with HCV, 50,000 of whom are still undiagnosed, based on findings from a cross-sectional study performed between 2017 and 2018 [8]. A large proportion of HCV infections are caused by sharing drug injection utensils. Risky sexual behavior and sharing drug inhalation utensils have also been reported as possible ways of HCV transmission [9–12]. Therefore, populations with the aforementioned risk factors have a high prevalence not only of HCV infections, but also of other infections. These patients are often associated with social stigma and face difficulties in accessing health services. In addition, the lack of information on HCV infection often leads to diminished motivation to seek treatment for the disease. This situation may be resolved by new educational program strategies [13, 14].
An additional barrier to accessing HCV treatment for drug users and homeless people is complicated health systems in which patients require several medical appointments to obtain treatments. This problem may be solved through specific protocols and programs involving different specialist units: Hepatology, Infectious diseases, and Microbiology. The role of a trained nurse as a liaison between the patient and the different services has been reported to be successful [15]. In addition, problems derived from the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic have resulted in more restricted access to different health units, and have consequently created new barriers for vulnerable HCV infection populations [16].
In this particular context, we aimed to carry out a two-step HCV microelimination program focused on drug users and homeless people in Malaga during the SARS-CoV-2 pandemic.
Materials and methods
Recruitment
A prospective study was carried out to identify and treat HCV infection among drug users and homeless people in drug addiction treatment centers and homeless shelters in Malaga (Spain) during the SARS-CoV-2 pandemic. The sample population was recruited between October 2020 and October 2021.
A fully trained nurse went periodically (twice a month) to the following centers: Meeting and Reception Center–Cruz Roja, Provincial Drug Addiction Center, Homeless Refuge, “Pozos Dulces” Refuge, and “AREA” alcoholism treatment center. The nurse provided information on infection risk factors, morbidities, and mortality related to HCV infection and treatment options, and the offer of participating in the study was extended to people. The inclusion criteria were as follows: (i) individuals aged ≥18 years, (ii) individuals who attended the aforementioned centers, and (iii) individuals who provided written informed consent.
This study was performed according to the principles of the Declaration of Helsinki and was approved by the Málaga Regional Research Ethics Committee. All the participants provided written informed consent.
Dry drop test
On-site dry drop test (DDT) (TFN-Specimen Collection Card, Ahlstrom-Munksjö, Helsinki, Finland) [17] was used as a blood sample collection tool for subsequent HCV screening. The DDT consisted of pricking a finger with a lancet to draw a drop of blood that was collected and dried on the card. The cards are manufactured from filter paper made from pure absorbent linters and are used as specimen collection devices when preparing dry blood samples, and for transport and storage for in vitro diagnostic tests. To prevent damage from humidity and UV light, the cards were stored individually in zip-lock bags with desiccant and were kept in the dark and transported to the laboratory once the sample had dried. In the microbiology laboratory, HCV RNA extraction and polymerase chain reaction were done using the Cobas® 6800 System (Roche Diagnostics, Basel, Switzerland).
Management of patients
Patients with a positive HCV viral load in the DDT underwent an intravenous blood sample analysis in their centers to confirm the test. Those in whom the HCV infection was confirmed were given a multidisciplinary appointment to simplify all additional steps into a single hospital visit (HCV genotyping, hepatitis B virus [HBV], and human immunodeficiency virus [HIV] serology, biochemistry with liver profile, blood count, and coagulation). In addition, medical exams (FibroScan® and liver ultrasound) were performed, and the patient received the diagnosis of the physician and educational support about HCV as well as being provided with direct-acting antiviral treatment.
Participants with a positive viral load from DDT and a negative viral load in intravenous blood samples were not considered to have HCV infection (DDT false positive). Patients infected with HCV were treated with glecaprevir/pibrentasvir (Maviret®, AbbVie) or sofosbuvir/velpatasvir (Epclusa®, Gilead) based on the decision of their physician, taking into account their previous treatment for HCV, degree of liver fibrosis, potential risk of liver decompensation, and drug interactions. Twelve weeks after finishing treatment, SVR was analysed. Patients with an advanced fibrosis stage were offered follow-up HCC screening.
Data collection
Sociodemographic data (age, sex, comorbidities, country of origin, and attending center) and information on the previous use of drugs, type, and route of consumption were collected. The following additional variables were also collected and analysed in patients with HCV-positive viral load: HCV genotype (analysed by using the Abbott Real Time HCV genotype II assay), HBV and HIV serology, degree of fibrosis evaluated by using transient elastography (FibroScan®), ultrasound screening for HCC, and blood analysis including liver profile tests (aspartate aminotransferase [AST], alanine transaminase [ALT], gamma-glutamyl transferase, alkaline phosphatase, bilirubin, albumin, and platelets).
Statistical analysis
Mean and standard deviation or median and interquartile range (IQR) were calculated according to data sample distribution. Student’s t-test was used to analyse normally distributed parameters. Differences in qualitative variables were analysed using the chi-square test or Fisher’s exact test, as appropriate. All results were considered statistically significant when P < 0.05. All analyzes were performed using STATA (version 17, College Station, TX: StataCorp LLC).
Results
Participants and centers
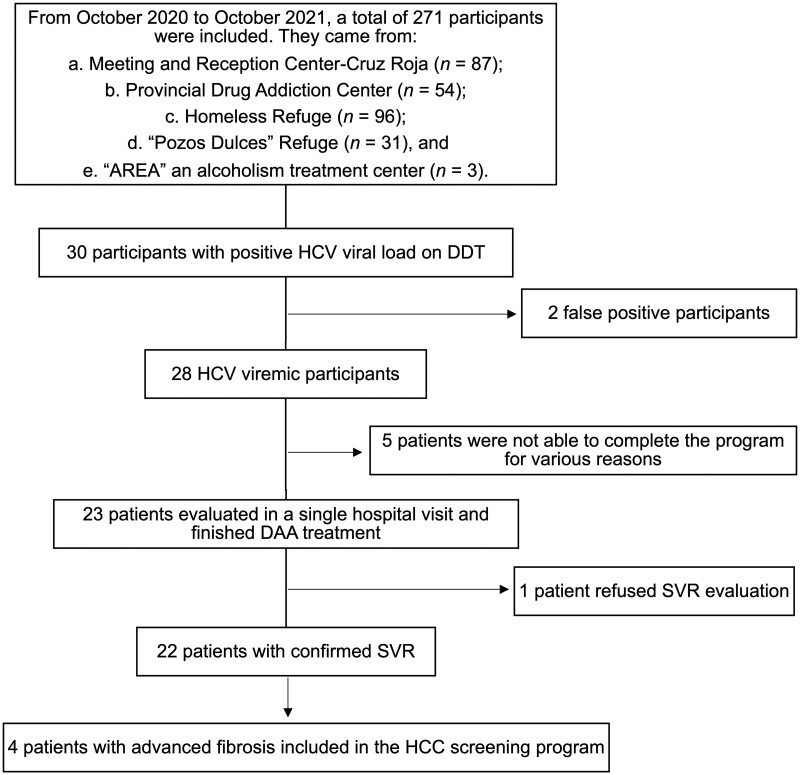
Of the 417 persons who received the offer to participate, 271 (65%) accepted and were included in the program and screened for HCV infection (Figure 1). The 271 participants were recruited from five different centers in the city of Malaga: (i) Meeting and Reception Center—Cruz Roja (87 participants), (ii) Provincial Drug Addiction Center (54 participants), (iii) Homeless Refuge (96 participants), (iv) “Pozos Dulces” Refuge (31 participants), and (v) “AREA”, an alcoholism treatment center (3 participants). Thirty participants were found to be positive for HCV by means of DDT-mediated blood testing. Of them, 28 (10% of the total population screened) were confirmed in intravenous blood samples and included in the microelimination program. Considering only participants from the Provincial Drug Addiction Center, 20% of the screened population were positive for HCV, demonstrating a higher HCV infection rate among drug users.
Figure 1.
Flow chart of participants included in the study and screened for hepatitis C virus through a dry drop test. n = number of participants, HCV = hepatitis C virus, DDT = dry drop test, DAA = direct action antiviral, SVR = sustained viral response, HCC = hepatocellular carcinoma.
Clinical characteristics
Males predominated among both the positive and negative HCV viral load participants, with a higher proportion in the positive group (86% vs 73%, P = 0.170) and similarly a slightly higher mean age in the positive group (53 vs 49 years, P = 0.089), demonstrating predominance of men among vulnerable populations.
HCV-infected and non-infected participants differed with regard to previous/current drug use (93% vs 60%, P = 0.001). Heroin was the most frequently used illicit drug among the HCV-infected participants, 79% of whom used heroin compared with 30% of non-infected participants (P < 0.001). Cocaine was the second most frequent drug, used by 75% of HCV-infected participants compared with 42% in non-infected participants (P = 0.006). Detailed information on drug use is shown in Table 1. In the HCV-infected group, 11 patients (48%) were on methadone treatment, 7 patients (30%) were on antipsychotic treatments, and 11 patients (48%) were on anxiolytic treatment (Table 2). One patient had HIV–HCV co-infection.
Table 1.
Clinical and epidemiological characteristics of vulnerable participants screened with positive HCV viral load compared with negative HCV viral load
| Characteristic | Participants with positive HCV viral load (n = 28) | Participants with negative HCV viral load (n = 243) | P-value |
|---|---|---|---|
| Age, years, mean ± SD | 53 ± 9 | 49 ± 12 | 0.089 |
| Males, n (%) | 24 (86) | 178 (73) | 0.170 |
| Country of origin, n (%) | |||
| Spain | 25 (89) | 190 (78) | 0.202 |
| Others | 3 (11) | 53 (22) | 0.651 |
| Recruitment center, n (%) | |||
| Cruz Roja | 12 (43) | 75 (31) | 0.411 |
| PDAC-M | 11 (39) | 43 (18) | 0.135 |
| HR-M | 4 (14) | 92 (38) | 0.330 |
| Pozos Dulces | 1 (4) | 30 (12) | 0.807 |
| AREA | 0 (0) | 3 (1) | – |
| Comorbidity, n (%) | |||
| Hypertension | 6 (21) | 42 (17) | 0.809 |
| Diabetes | 2 (7) | 31 (13) | 0.808 |
| Dyslipidemia | 2 (7) | 37 (15) | 0.758 |
| Previous or current drug use, way of consumption, n (%) | 26 (93) | 145 (60) | 0.001 |
| Cocaine | 21 (75) | 103 (42) | 0.006 |
| Inhaled | 10 (36) | 68 (28) | 0.603 |
| Intranasal | 6 (21) | 31 (13) | 0.608 |
| Intravenous | 1 (4) | 1 (0.4) | – |
| Othersa | 4 (14) | 3 (1) | 0.548 |
| Heroin | 22 (79) | 73 (30) | <0.001 |
| Inhaled | 9 (32) | 48 (20) | 0.424 |
| Intranasal | 1 (4) | 2 (1) | 0.875 |
| Intravenous | 3 (11) | 6 (3) | 0.625 |
| Othersa | 9 (32) | 17 (7) | 0.095 |
| Cannabis | 12 (43) | 77 (32) | 0.453 |
| Inhaled | 11 (39) | 75 (31) | 0.595 |
| Othersa | 1 (4) | 2 (0.8) | 0.884 |
| Other previous or current risky habit | |||
| Alcohol, n (%) | 19 (68) | 116 (48) | 0.106 |
| Beverages/day, median (IQR) | 10 (4–20) | 10 (5–20) | 0.825 |
| Tobacco, n (%) | 25 (89) | 174 (72) | 0.070 |
Others: tablets, infusion. HCV = hepatitis C virus, SD = standard deviation, PDAC-M = Provincial Drug Addiction Center Málaga, HR-M = Homeless Refuge Málaga, AREA = alcoholism treatment center, IQR = interquartile range.
Table 2.
Information about HCV patients who underwent treatment
| Complementary test and administered treatment | HCV patients (n = 23) |
|---|---|
| Analytical parameter, median (IQR) | |
| AST, U/L | 49 (35–92) |
| ALT, U/L | 61 (36–94) |
| GGT, U/L | 43 (27–90) |
| Alkaline phosphatase, U/L | 87 (66–102) |
| Albumin, g/dL | 4 (3.8–4.3) |
| Platelets, 103 U/µL | 172 (147–244) |
| Bilirubin, mg/dL, mean ± SD | 0.55 ± 0.22 |
| INR, mean ± SD | 1.04 ± 0.1 |
| Viral load HCV, IU/mL, median (IQR) | 3,040,000 (1,355,000–5,200,000) |
| Genotype, n (%) | |
| 1a | 17 (74) |
| 1b | 1 (4) |
| 2 | 1 (4) |
| 3 | 2 (9) |
| 4 | 2 (9) |
| Co-infections, n (%) | |
| HIV | 1 (4) |
| HBV | 0 |
| Fibrosis grade (FibroScan®), n (%) | |
| F0–2 | 17 (81) |
| F3–4 | 4 (19) |
| Liver ultrasound findings, n (%) | |
| Signs of liver steatosis | 10 (48) |
| Smooth liver margins | 19 (90) |
| Lobulated liver margins | 2 (10) |
| Signs of portal hypertension | 1 (5) |
| Ascites | 0 |
| HCC | 0 |
| Opioid substitution therapy, n (%) | 11 (48) |
| Concomitant psychiatric medication, n (%) | |
| Antipsychotics | 7 (30) |
| Anxiolytics | 11 (48) |
| HCV treatment, n (%) | |
| Glecaprevir/pibrentasvir | 9 (39) |
| Sofosbuvir/velpatasvir | 14 (61) |
| Confirmed SVR, n (%) | 22 (96) |
HCV = hepatitis C virus, IQR = interquartile range, AST = aspartate aminotransferase, ALT = alanine aminotransferase, GGT = gamma-glutamyl transferase, SD = standard deviation, INR = international normalized ratio, HIV = human immunodeficiency virus, HBV = hepatitis B virus, HCC = hepatocellular carcinoma, SVR = sustained virological response.
HCV genotype distribution was as follows: 1a (17 patients, 74%), 1b (1 patient, 4%), 2 (1 patient, 4%), 3 (2 patients, 9%), and 4 (2 patients, 9%). FibroScan® and liver ultrasound were performed in 21 patients (2 patients rejected undergoing evaluation): 17 patients (81%) had no fibrosis or mild fibrosis (F0–2), while four patients (19%) had advanced fibrosis (F3–4). Ten patients had signs of liver steatosis detected by using ultrasound (48%), 19 patients (90%) had smooth liver margins, and 2 patients (10%) had lobulated liver margins; 1 patient (5%) had signs of portal hypertension detected by using ultrasound, while none of the patients had ascites or HCC. Regarding analytical parameters, only median values of AST (49 U/L, IQR 35–92 U/L) and ALT (61 U/L, IQR 36–94 U/L) were outside the normal ranges. The clinical data of HCV-infected patients are shown in Table 2.
Treatment administration
Nine patients (39%) were treated with glecaprevir/pibrentasvir and 14 patients (61%) were treated with sofosbuvir/velpatasvir. Twelve weeks after the end of treatment, patient viral response was evaluated. Of the 23 patients included in the program, 22 were successfully treated with confirmed SVR (detectable viral load < 15 IU/mL), while 1 patient refused to undergo SVR analysis after finishing treatment. The median (IQR) time from DDT blood collection to SVR confirmation was 202 days (184–258 days).
All patients with an advanced degree of fibrosis (F3–4) were included in the hepatocellular cancer screening program (Figure 1).
Discussion
HCV microelimination programs are strategic keys for achieving the objectives of the WHO regarding viral hepatitis C elimination by 2030 [18]. The main target of these strategies involves populations that are characterized by some form of vulnerability, such as poverty, homelessness, or drug use [19–22]. The prevalence of HCV-infected participants in our study conducted in the South of Spain was 10%, which increased to 20% if only participants from drug addiction treatment centers are considered. These prevalences are higher than what is estimated in the general Spanish population (0.22%) [8] or other drug addiction treatment centers in Southern Spain (6%) [23], but lower than reported in a recent study on people who inject drugs in Northern Spain (56%) [24, 25]. Despite differences in infection rates, these data confirm that drug users and homeless people are vulnerable to HCV infection and should be considered crucial targets for specific screening and HCV microelimination programs.
Several barriers that hinder access to treatments in these populations have been described [20, 26, 27]. Social stigma, complex lifestyles, HCV not being a priority, unawareness of available effective antiviral treatments, and the need for multiple appointments and analytical tests to access HCV treatment can make the usual healthcare process difficult. In fact, five of the viremic patients in our study could not be treated, despite the facilities provided in our eradication program. These patients were unable to be localized by phone or post or were informed about the infection based on intravenous blood sample but did not attend their hospital appointments for further evaluation and treatment. In order to solve this problem, new strategies for in situ evaluation and antiviral treatment dispensation should be implemented.
In Spain, some strategies, such as “test and treat at the point-of-care”, were reported and compared with “standard of care” (hospital referral in cases with confirmed HCV infection) in harm reduction centers and addiction centers. A recent study in Northern Spain has highlighted the advantage of a “test and treat at the point-of-care” strategy in HCV elimination. Focusing on harm reduction centers, the new strategy achieved higher treatment rates (63% vs 6%), higher SVR rates (64% vs 23%), and reduced reinfections (21% vs 24%) compared with “standard of care.” Similarly, when focusing on addiction centers, the “test and treat at the point-of-care” strategy demonstrated higher treatment rates (53% vs 34%), higher SVR rates (84% vs 65%), and reduced reinfections (0% vs 6%) compared with “standard of care.” Hence, microelimination programs can be useful and should be applied not only to persons on drug rehabilitation treatments, but also to active drug users. In fact, active drug users had worse results with “standard of care” treatment and higher benefits of HCV microelimination programs in “test and treat at the point-of-care” [25]. Other strategies in vulnerable populations involving an education program and a liaising nurse facilitating HCV evaluation and treatment in a single consultation have achieved a high rate of treatment (93.3%) and SVR (78.8%) [23]. In addition to the measures taken in the previous study, in the present study, we used a simplified blood collection tool, the DDT, which could potentially be more acceptable to many persons than a vein blood extraction; this needs to be confirmed in further studies.
In our study, males predominated among the screened vulnerable population, especially among viremic patients, similarly to what has been seen in other Spanish studies [23, 25]. This is probably related to more risk-associated habits among men, such as illicit drug use. In our study, the percentage of drug use among the HCV-infected patients reached 93%, predominantly heroin use. Other risk-associated habits, such as consumption of alcohol and tobacco use, were also frequent in the screened population, especially in HCV patients. Higher prevalence of alcohol consumption has been described in epidemiological studies in HCV-infected patients [23, 28]. This highlights the need for healthcare professionals to target populations with risk-associated habits due to the increased risk of comorbidities, such as infections (HCV, HBV, HIV) or psychiatric disorders, among others [10, 11, 29, 30]. In fact, one patient with HIV–HCV co-infection was found among our patients (4%)—a small but important percentage, although lower than in other studies (20%) [24]. Similarly to other studies, an important percentage of our patients were under psychiatric treatment. Despite concomitant drug use, comorbidities, and adherence doubts, 100% of the patients treated who were retested 12 weeks after finishing the treatment obtained SVR. This highlights the importance of microelimination programs for HCV to circumvent complications that hinder access and adherence to healthcare. Such programs are not currently considered in usual protocols and health programs of the Spanish National Health System [12, 31].
The viral genotype distribution in the patients of our study was similar to that previously reported in Spain [32], with a predominance of genotype 1 followed by genotype 3. Four patients had advanced fibrosis. Such patients need to be included in HCC screening programs. This highlights the need for early HCV diagnosis and treatment in order to reduce morbidity and mortality [33].
A study of HCV patients in the USA undergoing methadone maintenance treatment found that educational level, information about antiviral therapies, and good interaction with physicians were the main issues for patients when deciding whether to start treatment [34]. It highlights the importance of programs in which this vulnerable population should not only be screened, but also informed about the risks of infection associated with particular behavior, the risk of morbidity in HCV-infected patients, and the availability of easy, safe, and effective treatments.
In our study, the SARS-CoV-2 pandemic created a significant limitation with regard to performing the study due to restrictions of mobility and access to the different centers both for drug addiction treatment center and homeless shelter users and sanitary workers. These limitations are reasons for the limited number of recruitments in our study. In fact, the expansion of the SARS-CoV-2 pandemic has been a major barrier worldwide in achieving the WHO goal of eliminating viral hepatitis as a health problem by 2030 [35]. However, despite the limitations, we were able to inform people at risk in addiction and homeless centers about HCV through a liaising nurse and collect blood samples for HCV screening using DDT, which resulted in successful HCV treatments and enhanced HCV awareness among the study population. DDT is a rapid and minimally invasive blood collection tool that is generally accepted by this population. Its use is not limited to drug treatment centers and shelters for homeless people, but could also be useful in primary care and hospitals, and is applicable to persons at risk of having HCV infection during other medical consultations. However, a limitation of DDT is the need to perform subsequent analysis to detect the viral load. A test that had the ability to diagnose with HCV infection in situ would further improve microelimination programs.
We concluded that HCV microelimination programs are effective in achieving HCV elimination in vulnerable populations. In addition, a trained nurse is an effective liaison between different hospitals and can provide recruitment services in HCV microelimination programs. Finally, these elimination programs should be promoted and organized by National Health Systems instead of being limited to isolated research efforts. This would enable HCV treatment for vulnerable populations on a larger scale.
Authors’ Contributions
Study concept and design: M.R.D., M.G.C. Case recruitments: J.A., J.J.R.R., M.M.H., V.V.L., R.M.A., J.B.J. Case diagnosis: J.M.P.B., A.M.G.G., R.A., A.O.A., M.D.G.E., F.F.G., J.P.T.O., M.G.C., R.J.A., I.V., E.C., E.C.H. Case treatment: J.M.P.B., A.M.G.G., R.A., A.O.A., M.D.G.E., F.F.G., J.P.T.O., M.G.C., R.J.A., E.S.Y. Data acquisition: J.M.P.B., J.A., M.R.D., M.G.C. Statistical analyses: J.A., I.A.A., M.R.D. Analysis and interpretation of data: J.M.P.B., M.R.D., M.G.C. Drafting of the manuscript: J.M.P.B., J.A., M.R.D. Critical revision of the manuscript: R.J.A., M.G.C. All authors read and approved the final manuscript.
Acknowledgements
We acknowledge the collaboration with the Gilead Sciences Scholarship Program.
Contributor Information
José María Pinazo-Bandera, Gastroenterology Department, Málaga Biomedicine Research Institute-IBIMA BIONAND Platform, Virgen de la Victoria University Hospital, University of Málaga, Málaga, Spain; Biomedic Research Network in Hepatic and Digestive Diseases (CIBERehd), Madrid, Spain.
Jesús Aranda, Gastroenterology Department, Málaga Biomedicine Research Institute-IBIMA BIONAND Platform, Virgen de la Victoria University Hospital, University of Málaga, Málaga, Spain.
Alberto Manuel García-García, Gastroenterology Department, Málaga Biomedicine Research Institute-IBIMA BIONAND Platform, Virgen de la Victoria University Hospital, University of Málaga, Málaga, Spain.
Ramiro Alcántara, Gastroenterology Department, Málaga Biomedicine Research Institute-IBIMA BIONAND Platform, Virgen de la Victoria University Hospital, University of Málaga, Málaga, Spain.
Aida Ortega-Alonso, Gastroenterology Department, Málaga Biomedicine Research Institute-IBIMA BIONAND Platform, Virgen de la Victoria University Hospital, University of Málaga, Málaga, Spain; Biomedic Research Network in Hepatic and Digestive Diseases (CIBERehd), Madrid, Spain.
Enrique Del Campo-Herrera, Gastroenterology Department, Málaga Biomedicine Research Institute-IBIMA BIONAND Platform, Virgen de la Victoria University Hospital, University of Málaga, Málaga, Spain.
Encarnación Clavijo, Microbiology Department, Málaga Biomedicine Research Institute-IBIMA BIONAND Platform, Virgen de la Victoria University Hospital, University of Málaga, Málaga, Spain.
M Dolores García-Escaño, Gastroenterology Department, Málaga Biomedicine Research Institute-IBIMA BIONAND Platform, Virgen de la Victoria University Hospital, University of Málaga, Málaga, Spain.
Juan Jesús Ruiz Ruiz, Provincial Center for Drug Addiction, Provincial Council of Málaga, Málaga, Spain.
Mónica Morales-Herrera, Meeting and Reception Center, (CEA-Cruz Roja), Málaga, Spain.
Vanesa Valle-López, Meeting and Reception Center, (CEA-Cruz Roja), Málaga, Spain.
Rosa Martín-Alarcón, Municipal Reception Center, Málaga, Spain.
Isabel Viciana, Microbiology Department, Málaga Biomedicine Research Institute-IBIMA BIONAND Platform, Virgen de la Victoria University Hospital, University of Málaga, Málaga, Spain.
Juan Bautista Jiménez, Provincial Center for Drug Addiction, Provincial Council of Málaga, Málaga, Spain.
Felix Fernández-García, Gastroenterology Department, Málaga Biomedicine Research Institute-IBIMA BIONAND Platform, Virgen de la Victoria University Hospital, University of Málaga, Málaga, Spain.
Juan Pedro Toro-Ortiz, Gastroenterology Department, Málaga Biomedicine Research Institute-IBIMA BIONAND Platform, Virgen de la Victoria University Hospital, University of Málaga, Málaga, Spain.
Elena Sánchez-Yáñez, Farmacy Department, Virgen de la Victoria University Hospital, Málaga, Spain.
Ismael Álvarez-Álvarez, Gastroenterology Department, Málaga Biomedicine Research Institute-IBIMA BIONAND Platform, Virgen de la Victoria University Hospital, University of Málaga, Málaga, Spain; Biomedic Research Network in Hepatic and Digestive Diseases (CIBERehd), Madrid, Spain.
Raúl J Andrade, Gastroenterology Department, Málaga Biomedicine Research Institute-IBIMA BIONAND Platform, Virgen de la Victoria University Hospital, University of Málaga, Málaga, Spain; Biomedic Research Network in Hepatic and Digestive Diseases (CIBERehd), Madrid, Spain.
Mercedes Robles-Díaz, Gastroenterology Department, Málaga Biomedicine Research Institute-IBIMA BIONAND Platform, Virgen de la Victoria University Hospital, University of Málaga, Málaga, Spain; Biomedic Research Network in Hepatic and Digestive Diseases (CIBERehd), Madrid, Spain.
Miren García-Cortés, Gastroenterology Department, Málaga Biomedicine Research Institute-IBIMA BIONAND Platform, Virgen de la Victoria University Hospital, University of Málaga, Málaga, Spain; Biomedic Research Network in Hepatic and Digestive Diseases (CIBERehd), Madrid, Spain.
Funding
This work was supported by a grant from GILEAD for hepatitis C microelimination programs and the Instituto de Salud Carlos III [Río Hortega CM21/00074 to J.M.P.B.]. The funding sources had no involvement in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the manuscript for publication.
Conflict of Interest
None declared.
References
- 1. World Health Organization. Global Health Sector Strategy on Viral Hepatitis 2016–2021. Towards Ending Viral Hepatitis.2016. https://apps.who.int/iris/handle/10665/246177 (15 January 2023, date last accessed).
- 2. Westbrook RH, Dusheiko G.. Natural history of hepatitis C. J Hepatol 2014;61:S58–68. [DOI] [PubMed] [Google Scholar]
- 3. Leone N, Rizzetto M.. Natural history of hepatitis C virus infection: from chronic hepatitis to cirrhosis, to hepatocellular carcinoma. Minerva Gastroenterol Dietol 2005;51:31–46. [PubMed] [Google Scholar]
- 4. Tenen DG, Chai L, Tan JL.. Metabolic alterations and vulnerabilities in hepatocellular carcinoma. Gastroenterol Rep (Oxf) 2020;9:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. World Health Organization. Progress Report on Access to Hepatitis C Treatment: Focus on Overcoming Barriers in Low- And Middle-Income Countries.2018. Available in: https://apps.who.int/iris/bitstream/handle/10665/260445/WHO-CDS-HIV-18.4-eng.pdf;jsessionid=56D03CC062A7C51DF284D1B4B4FEF7AF?sequence=1 (15 January 2023, date last accessed).
- 6. Janjua NZ, Wong S, Abdia Y. et al. Impact of direct-acting antivirals for HCV on mortality in a large population-based cohort study. J Hepatol 2021;75:1049–57. [DOI] [PubMed] [Google Scholar]
- 7. Janjua NZ, Darvishian M, Wong S. et al. ; British Columbia Hepatitis Testers Cohort Team. Effectiveness of ledipasvir/sofosbuvir and sofosbuvir/velpatasvir in people who inject drugs and/or those in opioid agonist therapy. Hepatol Commun 2019;3:478–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Grupo de trabajo del estudio de prevalencia de la infección por hepatitis C en población general en España. Ministerio de Sanidad. Resultados del 2° Estudio de Seroprevalencia en España. 2020. https://www.sanidad.gob.es/profesionales/saludPublica/prevPromocion/vacunaciones/comoTrabajamos/docs/EstudioSeroprevalencia_EnfermedadesInmunoprevenibles.pdf (15 January 2023, date last accessed).
- 9. Clarke A, Kulasegaram R.. Hepatitis C transmission—where are we now? Int J STD AIDS 2006;17:74–80. [DOI] [PubMed] [Google Scholar]
- 10. Macías J, Palacios RB, Claro E. et al. High prevalence of hepatitis C virus infection among noninjecting drug users: association with sharing the inhalation implements of crack. Liver Int 2008;28:781–6. [DOI] [PubMed] [Google Scholar]
- 11. Vallejo F, Barrio G, Brugal MT. et al. ; Itinere Project Group. High hepatitis C virus prevalence and incidence in a community cohort of young heroin injectors in a context of extensive harm reduction programmes. J Epidemiol Community Health 2015;69:599–603. [DOI] [PubMed] [Google Scholar]
- 12. Reyes-Urueña J, Brugal MT, Majo X. et al. Cross sectional study of factors associated to self-reported blood-borne infections among drug users. BMC Public Health 2015;15:1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Roncero C, Vega P, Martinez-Raga J. et al. Chronic Hepatitis C and people with a history of injecting drugs in Spain: population assessment, challenges for effective treatment. Adicciones 2017;29:71–3. [DOI] [PubMed] [Google Scholar]
- 14. Fortier E, Alavi M, Micallef M. et al. ; ETHOS Study Group. The effect of social functioning and living arrangement on treatment intent, specialist assessment and treatment uptake for hepatitis C virus infection among people with a history of injecting drug use: The ETHOS study. Int J Drug Policy 2015;26:1094–102. [DOI] [PubMed] [Google Scholar]
- 15. Pozza R, McCoy-Hill C, Hall K. et al. Eradicating hepatitis C virus: The APRN's role. Nurse Pract 2019;44:16–27. [DOI] [PubMed] [Google Scholar]
- 16. Joo JY, Liu MF.. Nurses' barriers to caring for patients with COVID-19: a qualitative systematic review. Int Nurs Rev 2021;68:202–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Instructions for use. Ahllstrom Munksjö TFN-Specimen Collection Card. Available in: https://gentegra.com/wp-content/uploads/2021/06/31701_TFNCard_En_R08.pdf (15 January 2023, date last accessed).
- 18. European Union HCV Collaborators. Hepatitis C virus prevalence and level of intervention required to achieve the WHO targets for elimination in the European Union by 2030: a modelling study. Lancet Gastroenterol Hepatol 2017;2:325–36. [DOI] [PubMed] [Google Scholar]
- 19. Mateu-Gelabert P, Guarino H, Quinn K. et al. Young drug users: a vulnerable population and an underutilized resource in HIV/HCV Prevention. Curr HIV/AIDS Rep 2018;15:324–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Glaspy S, Avramovic G, McHugh T. et al. Exploring and understanding HCV patient journeys- HEPCARE Europe project. BMC Infect Dis 2021;21:239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Felsher M, Tobin KE, Sulkowski M. et al. HCV communication within ego-centric networks of men and women who inject drugs. Drug Alcohol Depend 2021;229:109157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Moreno GA, Wang A, Sánchez González Y. et al. Value of comprehensive HCV treatment among vulnerable, high-risk populations. Value Health 2017;20:736–44. [DOI] [PubMed] [Google Scholar]
- 23. Corona-Mata D, Rivero-Juárez A, Camacho Á. et al. Efficacy of a comprehensive strategy for the detection and treatment of hepatitis C infection in a population attending addiction centers. Front Public Health 2023;11:1092960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lens S, Miralpeix A, Gálvez M. et al. HCV microelimination in harm reduction centres has benefits beyond HCV cure but is hampered by high reinfection rates. JHEP Rep 2022;4:100580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Forns X, Colom J, García-Retortillo M. et al. Point-of-care hepatitis C testing and treatment strategy for people attending harm reduction and addiction centres for hepatitis C elimination. J Viral Hepat 2022;29:227–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pinazo Bandera J, García García AM, Cobos Rodríguez J. et al. Pilot study of microelimination in hepatitis C: direct derivation between drug centers and the hepatoogy unit in a university hospital. RAPDOnline 2020;43:181–5. [Google Scholar]
- 27. Dhiman RK, Grover GS, Premkumar M. et al. ; MMPHCRF Investigators. Outcomes of real-world integrated HCV microelimination for people who inject drugs: an expansion of the Punjab model. E Clinical Medicine 2021;41:101148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Taylor AL, Denniston MM, Klevens RM. et al. Association of hepatitis C virus with alcohol use among U.S. Adults: NHANES 2003-2010. Am J Prev Med 2016;51:206–15. [DOI] [PubMed] [Google Scholar]
- 29. Xu HQ, Wang CG, Zhou Q. et al. Effects of alcohol consumption on viral hepatitis B and C. World J Clin Cases 2021;9:10052–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Silverstein PS, Kumar S, Kumar A.. HIV-1, HCV and alcohol in the CNS: potential interactions and effects on neuroinflammation. Curr HIV Res 2014;12:282–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cuadrado A, Cabezas J, Llerena S. et al. Prevalence of hepatitis C in patients with non-affective psychotic disorders. Rev Esp Enferm Dig 2020;112:550–4. [DOI] [PubMed] [Google Scholar]
- 32. Petruzziello A, Marigliano S, Loquercio G. et al. Hepatitis C virus (HCV) genotypes distribution: an epidemiological up-date in Europe. Infect Agent Cancer 2016;11:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cepeda JA, Thomas DL, Astemborski J. et al. Impact of hepatitis C treatment uptake on cirrhosis and mortality in persons who inject drugs: a longitudinal, community-based cohort study. Ann Intern Med 2022;175:1083–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jessop AB, Bass SB, Gutierrez M. et al. Perceptions of barriers and benefits of HCV treatment and correlates to treatment intention in methadone patients. J Health Care Poor Underserved 2019;30:1433–54. [DOI] [PubMed] [Google Scholar]
- 35. Blach S, Kondili LA, Aghemo A. et al. Impact of COVID-19 on global HCV elimination efforts. J Hepatol 2021;74:31–6. [DOI] [PMC free article] [PubMed] [Google Scholar]