Abstract
The Phoenix Epidemiology and Clinical Research Branch of the National Institute of Diabetes and Digestive and Kidney Diseases has conducted prospective studies of diabetes and its complications in the Pima Indians living in Arizona, USA for over 50 years. In this review we highlight areas in which these studies provided vital insights into the criteria used to diagnose type 2 diabetes, the pathophysiologic changes that accompany the development of type 2 diabetes, and the course and determinants of diabetes complications—focusing specifically on diabetic kidney disease. We include data from our longitudinal population-based study of diabetes and its complications, studies on the role of insulin resistance and insulin secretion in the pathophysiology of type 2 diabetes, and in-depth studies of diabetic kidney disease that include measures of glomerular function and research kidney biopsies. We also focus on the emerging health threat posed by youth-onset type 2 diabetes, which was first seen in the Pima Indians in the 1960s and is becoming an increasing issue worldwide.
Keywords: type 2 diabetes, pathophysiology, complications, American Indians
1. Introduction
Pima Indians are members of an American Indian population that mostly resides in Arizona, USA and Northern Mexico. The Arizona Pima Indians have a high prevalence of type 2 diabetes, with more than half of all adults over the age of 35 years affected 1. Diabetes diagnosed in the Pima Indians is primarily type 2 diabetes, with no cases of type 1 diabetes identified even when onset occurs in childhood 2–4. However, we recently identified a case of diabetes that may be monogenic in origin 5. From 1965 until 2007 the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) conducted a longitudinal population-based study to examine diabetes and its complications in the Pima Indians. As part of the study, community members aged 5 years and older, regardless of health status, were invited to attend a research examination every 2 years. These research examinations included a 75g oral glucose tolerance test, biospecimen collections, and a medical examination 6.
Adult participants in this longitudinal study were also invited to take part in other more in-depth studies conducted by the NIDDK which focused on specific aspects of diabetes and its complications. These included inpatient studies that investigated the pathophysiology of diabetes and obesity using hyperinsulinemic-euglycemic clamps and intravenous glucose tolerances tests (IVGTT) 7–10. Outpatient studies were also conducted that focused on kidney disease and included measures of glomerular hemodynamic function 11, kidney biopsies 12, and a clinical trial of treatment with losartan in early diabetic kidney disease (NCT00340678) 13. Pregnant women were also invited to undergo third trimester oral glucose tolerance tests and their offspring were then followed-up as part of the longitudinal study 14–16.
In this review, we highlight some of the key findings from these studies and how they fundamentally changed our understanding of type 2 diabetes and its complications, focusing specifically on diabetic kidney disease. While this review focuses on studies conducted solely in the Pima Indians it is important to note that Pima Indians also participated in three major multicenter clinical trials including the Diabetes Prevention Program (NCT00004992) 17, Action for Health in Diabetes (LookAHEAD) (NCT00017953)18 and A Comparative Effectiveness Study of Major Glycemia-Lowering Medications for Treatment of Type 2 Diabetes (GRADE) (NCT01794143) 19,20. They also participated in a multicenter observational study, The Family Investigation of Nephropathy and Diabetes Study (FIND) (NCT00301249) 21. All the studies discussed in this review were approved by the Institutional Review Board (IRB) of the National Institute of Diabetes and Digestive and Kidney Diseases or equivalent for studies that pre-dated the establishment of the IRB process.
2. Establishing the Diagnosis of Type 2 Diabetes
Diabetes and its clinical effects have been recognized for centuries. However, standardized diagnostic criteria are a relatively recent development. When the Pima Indian longitudinal study was launched in 1965, blood glucose concentration cut points considered diagnostic for diabetes varied across studies making comparison of findings difficult. NIDDK investigators initially selected an arbitrary 2-hour plasma glucose concentration cut-point of 8.9 mmol/l (160 mg/dl) to diagnose diabetes in the Pima Indians using blood samples collected after a 75-gram oral carbohydrate load (Glucola, Ames, Elkhart, IN or Dexcola, Custom Laboratories, Baltimore, MD). When they plotted the distribution of 2-hour post-load plasma glucose concentrations in the population, they noted bimodal distributions never reported previously, which were best explained by two overlapping normal distributions 22 [FIG 1a]. These bimodal distributions were apparent for all those aged 35 years and over and indicated that the population could be divided into two groups—those without diabetes who were members of the lower distribution and those with type 2 diabetes who were members of the upper distribution. At the time, a widely held view was that diabetes in older people was the result of an increase in blood glucose concentration associated with aging, whereas the findings in the Pima Indians indicated a distinct transition from no diabetes to diabetes. This finding is also informative about the way people transition from normal glucose tolerance to diabetes. If there was a gradual shift from a blood glucose concentration in the normal range to a blood glucose concentration in the diabetic range then the bimodal distribution would be less obvious, whereas the lack of many people with ‘high normal’ values suggests that people transition relatively rapidly either to diabetes or back to normal glucose tolerance 23.
FIGURE 1:
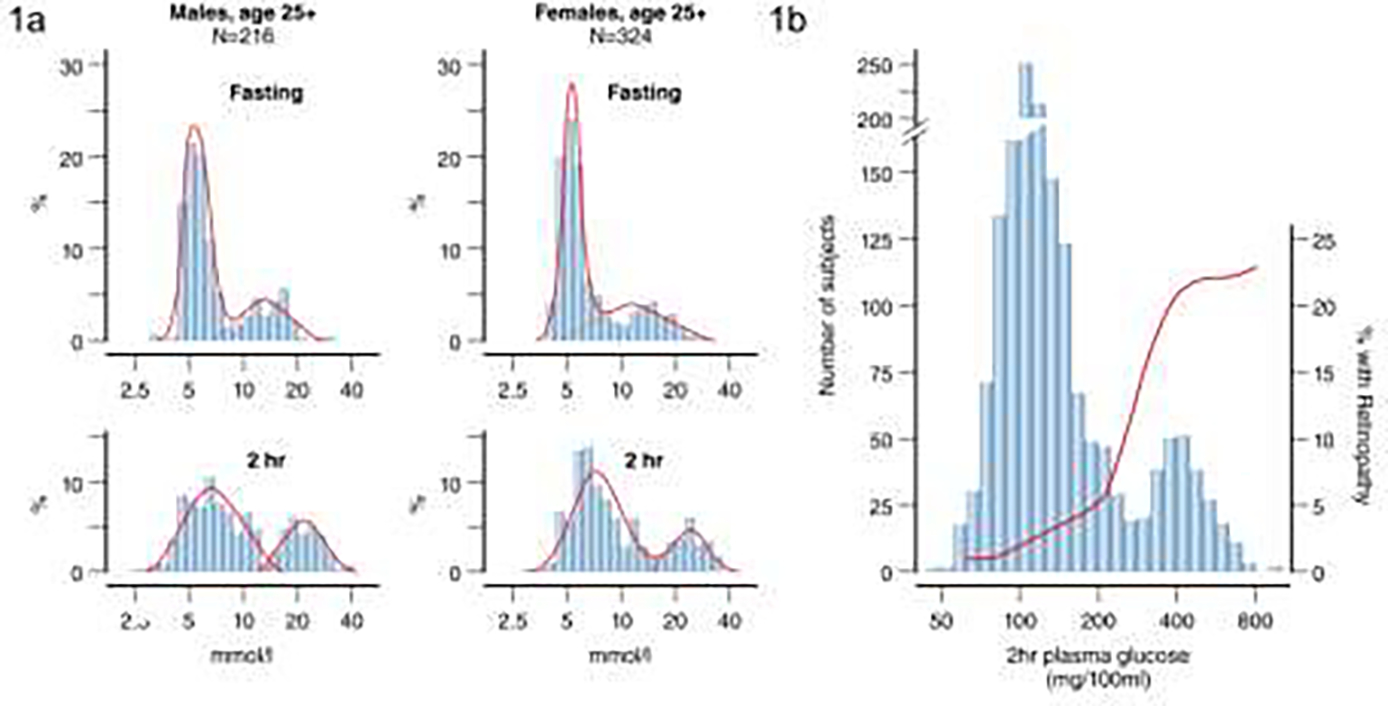
a) Distribution of fasting and 2-hour glucose in Pima Indians for men and women aged over 25 years at time of examination– data shows bimodal distribution (Adapted from Rushforth et al, 1979 95); b) Bimodal glucose distribution and prevalence of retinopathy as assessed by fundoscopy in 1640 adult Pima Indians aged 15–64 years for men and 15–74 years for women demonstrating rise in prevalence of retinopathy around a cut-point of 200 mg/dl (11.1 mmol/l) (Adapted from Dorf et al, 197624). NOTE: Figure 1b uses non-SI units.
The finding of a bimodal distribution in the Pima Indians permitted investigators to establish a diagnostic cut-point for diabetes that was based on empirical data. The 2-hour post-load plasma glucose concentration that best separated the two normal distributions in the Pima Indians was between 11.1 and 13.9 mmol/l (200–250 mg/dl), leading to a proposal to raise the cut-point for diagnosing diabetes from 8.9 mmol/l (160 mg/dl) that was originally used in the population to 11.1 mmol/l (200 mg/dl) 6. Additional support for the 11.1 mmol/l cut-point came from examining the prevalence of diabetic retinopathy, assessed by direct fundoscopy, in the Pima Indians as a function of the 2-hour post-load plasma glucose concentration. A sharp increase in prevalence of diabetic retinopathy was noted among people who had a 2-hour post-load plasma glucose concentration of 11.1 mmol/l or above, with higher prevalence associated with higher blood glucose concentrations [FIG 1b]24. Based on this work, the cut-point of 11.1 mmol/l for a 2-hour post-load plasma glucose was adopted as the diagnostic criterion for diabetes by the National Diabetes Data Group (NDDG) and World Health Organization when they proposed standardized definitions for type 2 diabetes 25,26 and remains part of the diagnostic criteria used by the American Diabetes Association 27 and the World Health Organization today 28. Since these early studies, there have been adjustments to the fasting blood glucose cut-point and more recently the addition of an HbA1c cut-point but the original observations in the Pima Indians were central to establishing a unified diagnostic criterion for diabetes.
3. Pathophysiology of the Transition to Type 2 Diabetes
3.1. Physiologic Determinants of Type 2 Diabetes
The longitudinal population study in the Pima Indians provides valuable clinical data on the transition from normal glucose tolerance (NGT) to diabetes. Obesity is a major risk factor for type 2 diabetes and in the Pima Indians body weight increases prior to the onset of diabetes, with maximal body weight in adults typically occurring around the time of diagnosis of diabetes. A steady decline in weight then follows the onset of diabetes. These changes are most marked in individuals who develop diabetes before the age of 45 years [FIG 2a]29. Changes in blood glucose concentration before the onset of type 2 diabetes are more complex. An analysis of glucose trajectories in nondiabetic Pima Indians who later developed diabetes found that the glucose trajectories are best described by an initial gradual linear increase (mean increase of 0.04 mmol/l/year (0.75 mg/dl/year)) followed by an exponential rise before the diagnosis of diabetes reflecting a marked acceleration in the rate of glucose increase so that by 2 years before diagnosis the rate of change in fasting plasma glucose concentration is about 0.83 mmol/l/year (15 mg/dl/year) [FIG 2b]30.
FIGURE 2:
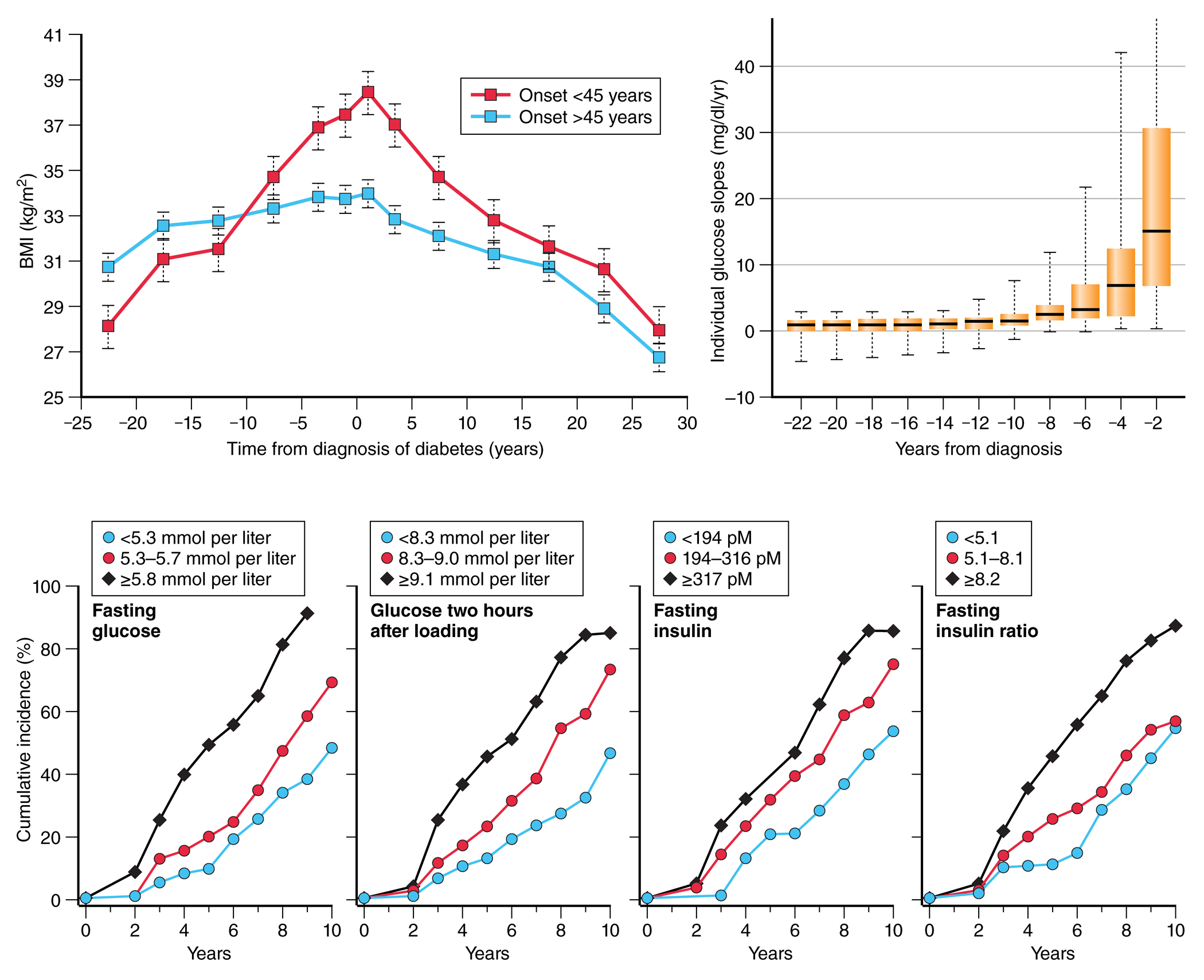
a) Change in body mass index in Pima Indian adults before and after diagnosis of diabetes. Each point represents the mean +/− standard error for each 5-year duration band, divided by age of onset of diabetes (Adapted from Looker et al, 2001 29); b) Distribution of instantaneous glucose slopes (rates of change of glucose in individuals) in 55 Pima Indians by time from diabetes diagnosis. Data are medians with 25th and 75th percentiles with ranges shown by error bars (Adapted from Mason et al, 2007 30); c) cumulative incidence of diabetes based on tertiles of fasting glucose, 2-hour glucose, fasting insulin, and 2-hour insulin showing highest cumulative incidence of diabetes in the highest tertile of each measure (Adapted from Saad et al 1988 31). NOTE: Figure 2b uses non-SI units.
In Pima Indians with impaired glucose tolerance (IGT; fasting glucose <7.8 mmol/l and 2-hour blood glucose of ≥7.8–11.1 mmol/l), 25% progress to diabetes within 5 years and 61% by 10 years. Notably, the risk of transition from IGT to diabetes increases with age until Pima Indians reach 45 years old after which risk decreases with age – suggesting depletion of the susceptible population 31. Plasma glucose and insulin concentrations (fasting or 2-hour) are all significant risk factors for progression to diabetes [FIG 2c].
3.1.1. Insulin action and insulin secretion:
Important insights into the transition from normal glucose regulation to diabetes were gained by using gold standard reference methods for measuring insulin action and secretion in the Pima Indians. These methods included the hyperinsulinemic-euglycemic clamp 32 for measuring insulin action and the IVGTT for measuring the acute insulin response (AIR) 33. In cross-sectional analyses, Pima Indians with fasting plasma glucose concentrations in the diabetic range have lower total glucose disposal rates during both maximal and submaximal insulin stimulation than those in the non-diabetic range 34. Moreover, the AIR is absent in individuals with diabetes 35. In prospective studies, both lower insulin action and secretion at baseline are risk factors for developing diabetes [FIG 3a]9.
FIGURE 3:
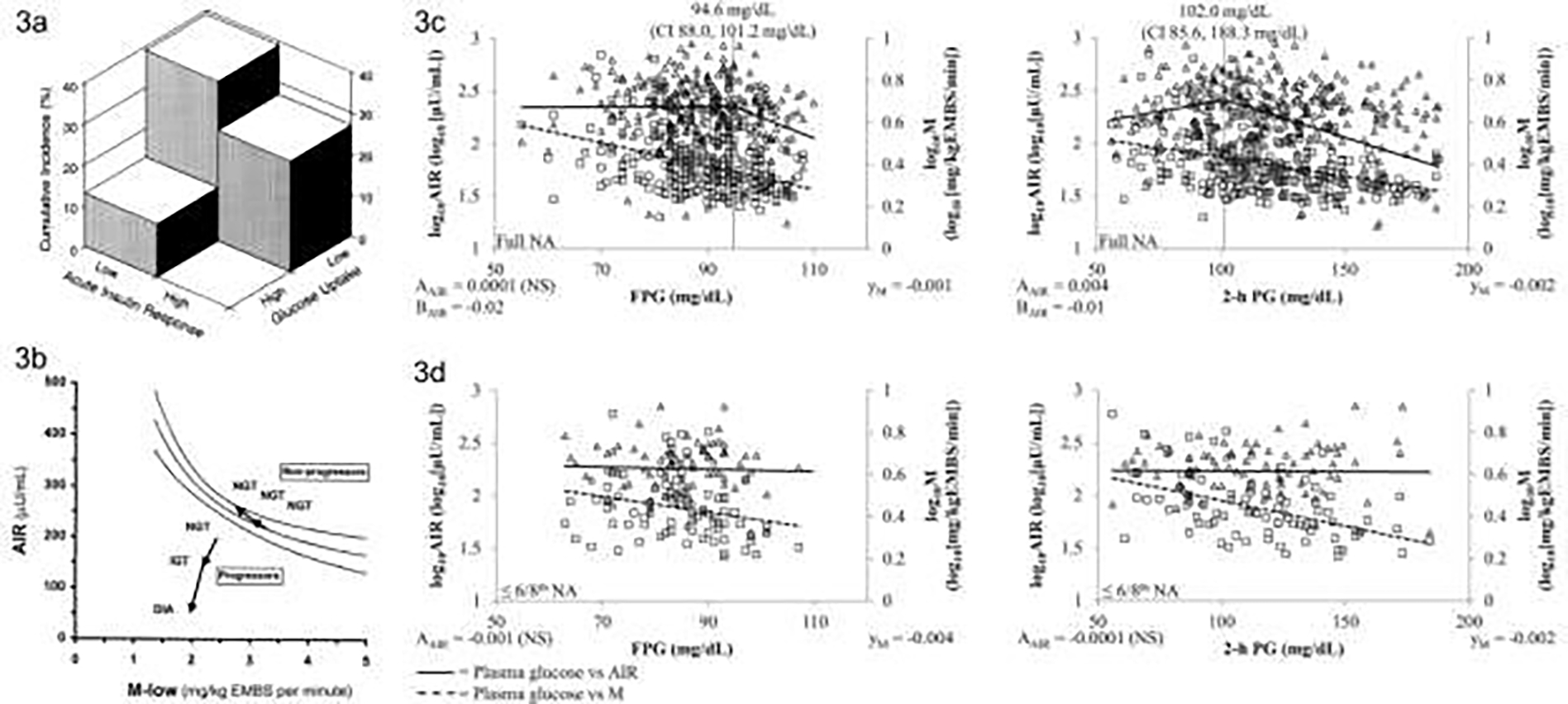
a) Cumulative incidence of diabetes according to whether measures of acute insulin response and glucose uptake fall below or above the median – the highest cumulative incidence is seen in the participants with below median values of both measures (From Lillioja et al, 19939); b) Disposition index for participants with NGT compared to participants who progressed from NGT to IGT and then diabetes – shows the relationship in the progressors does not fall on the normal hyperbolic curve even when still NGT and continues to move away from the curve as glycemia worsens (From Weyer et al, 199910); c) Acute insulin response (AIR) of full heritage Pima Indians and; d) those with ≤3/4th Pima heritage relative to increasing fasting (FPG) and 2-h plasma glucose (2-h PG) concentrations. Triangles mark the relationship of AIR and plasma glucose concentrations, whereas squares stand for the relationship between the rate of insulin-dependent glucose disposal (M) and plasma glucose concentrations. For plasma glucose concentration vs. AIR, AAIR gives the slope up to the plasma glucose concentration of the inflexion point. BAIR indicates the slope for this relationship beyond that point. The inflexion point is marked by a vertical line and its respective plasma glucose concentration with 95% confidence interval (CI) is reported. Where no inflexion point was found, AAIR gives the overall slope. In each panel, development of AIR relative to plasma glucose concentrations is indicated by a line. The trendline for plasma glucose concentrations vs. M is indicated by a dashed line, and its slope is reported (yM). Slopes and trendlines for plasma glucose concentrations vs. AIR and M, respectively, were tested for statistical significance (NS, not significant). Values for AIR and M were log10-transformed to meet linear distribution. Abbreviations: AIR, acute insulin response; DIA, type 2 diabetes; EMBS, estimated metabolic body size; IGT, impaired glucose tolerance; NA, Native Americans; NGT, normal glucose tolerance. (From Heinitz et al, 2018 36) NOTE: Figures 3c and 3d use non-SI units.
The temporal sequence with which abnormalities in insulin action and secretion occur as individuals progress from NGT to diabetes was further elucidated by repeated hyperinsulinemic-euglycemic clamps and IVGTT measurements in the same individuals during progression to diabetes 10. The transition from NGT to IGT is associated with a decline in insulin sensitivity and secretion that is accompanied by an increase in weight [Fig 3b]. Further progression from IGT to diabetes is characterized by additional weight gain and further deterioration in both insulin action and AIR. Importantly, repeated measurements of glucose disposal and AIR in those who remain NGT demonstrate increased AIR despite weight gain and worsening insulin sensitivity 10.
In cross-sectional analysis of data from Pima Indians with either NGT or impaired glucose regulation, a continuous decline in glucose disposal is associated with increasing fasting and 2-hour plasma glucose concentrations [FIG 3c]36. However, the association between insulin secretion and plasma glucose concentration is not as clear-cut. Though a decline in insulin secretion is important in the pathogenesis of diabetes, the association between glucose and AIR is not always linear, with variation observed according to the extent of Indian admixture. Among full-heritage Pima Indians the relationship between AIR and plasma glucose is non-linear, with a stable AIR at low glucose concentrations and a steep decline in AIR at higher glucose concentrations. The inflexion point for the fasting plasma glucose concentration is at 5.3 mmol/l (95 mg/dL) and for 2-hour plasma glucose concentration is at 5.7 mmol/l (102 mg/dL). Notably, both inflexion points are below the diagnostic criteria for impaired glucose regulation. These inflexion points are also influenced by the presence of obesity. By contrast, in Pima Indians who are less than full heritage (≤3/4 Pima) the AIR remains stable across the range of glucose concentrations studied with no inflexion points observed [FIG 3d]. These findings are consistent with our observation that AIR is highly heritable in this population 37. Given that impaired AIR occurs well below the cut-points for IGT suggests that interventions to prevent the onset of diabetes may be valuable when people are still NGT.
3.1.2. Endogenous glucose production:
Use of a glucose tracer prior to and during the insulin infusion phases of the hyperinsulinemic-euglycemic clamp has allowed us to examine the role of increased endogenous glucose production in the development of type 2 diabetes 38. In Pima Indians without diabetes, the rate of endogenous glucose production in the fasting state is not associated with later development of diabetes in models adjusted for glucose disposal and AIR39. However, the percent suppression of endogenous glucose production by insulin during a hyperinsulinemic euglycemic clamp is a predictor of diabetes but not after adjustment for obesity 9. Moreover, endogenous glucose production does not change in Pima Indians who progress from NGT to IGT but does increase during progression from IGT to diabetes 10. Taken together, prospective studies in Pima Indians indicate that endogenous glucose production may be a secondary factor in the development of diabetes and that increased endogenous glucose production occurs relatively late in the transition from NGT to diabetes.
3.1.3. Insulin clearance:
When considering insulin concentrations in the blood we mostly focus on insulin secretion but the rate of insulin clearance, primarily by the liver, may also play a role in the development of type 2 diabetes. Steady-state plasma insulin concentration during the insulin infusion phase of the clamp provides a measurement of whole-body insulin clearance 32. In Pima Indians without diabetes, lower whole-body insulin clearance is associated with an unfavorable metabolic phenotype characterized by higher body fat (%), higher fasting plasma insulin concentration, and a diminished ability to suppress endogenous glucose production 40. Furthermore, lower insulin clearance is a risk factor for type 2 diabetes, even after adjusting for insulin action and secretion, indicating that impairment of insulin clearance may play an additional role in the pathogenesis of diabetes independent of these factors 40.
3.2. Genetic Determinants of Type 2 Diabetes
The global prevalence of type 2 diabetes is highly influenced by the obesogenic lifestyle. However, this disease also has a significant heritable component, as discussed elsewhere in this issue (Froguel P). Studies in Pima Indians have documented that even among individuals who are equally obese (e.g., body mass index >40 kg/m2) the prevalence of type 2 diabetes differs by parental diabetes status, supporting a key role for genetic determinants of type 2 diabetes independent of obesity 41. Longitudinal studies in the Pima Indians have further documented that parental onset of type 2 diabetes before the age of 35 years is a greater risk factor for type 2 diabetes in the offspring than parental diabetes onset at a later age, suggesting that type 2 diabetes onset at younger ages is more genetically driven while at older ages is more environmentally influenced 42. Importantly, a single private missense/nonsense variant does not account for the high prevalence of type 2 diabetes in the Pima Indians. Whole exome sequencing of nearly 7,000 community members found only a few rare, apparently highly penetrant variants 43, indicating that type 2 diabetes in Pima Indians is primarily polygenic.
3.2.1. Role of established variants for type 2 diabetes:
Large, multi-ethnic genome-wide association meta-analyses have identified hundreds of non-coding variants that contribute to type 2 diabetes 44,45. Replication of these established variants in Pima Indians typically show modest directionally consistent associations with type 2 diabetes when analyzed individually. The association becomes more robust when analyzed together as a polygenic risk score, indicating that Pima Indians share many of the same risk alleles found in other ethnic groups 46. On the other hand, although TCF7L2 is the best replicated gene for type 2 diabetes among all ethnic groups 47, variation in this gene has little to no effect on type 2 diabetes in Pima Indians despite a statistically significant heterogeneity in effect 46,48. By contrast, the effect of KCNQ1 is much greater in Pima Indians than in other ethnic groups 46. Multigenerational studies in the Pima Indians demonstrated that maternal, but not paternal, inheritance of the risk allele significantly increases risk for type 2 diabetes. This maternally inherited allele is associated with a 28% decrease in insulin secretion in normal glucose tolerant subjects, providing a physiological mechanism for the increased risk of diabetes 49.
The physiologic mechanism affected by the KCNQ1 locus has been modeled using induced pluripotent stem cells (iPSCs) from Pima Indians isogenic for intronic variants in KCNQ1. These studies demonstrated that imprinting of KCNQ1 and the adjacent gene CDKN1C during pancreatic islet-like cell generation from iPSCs is consistent with known imprinting patterns in fetal pancreas and adult islets making iPSCs an ideal model system to study this locus50. Comparison of an isogenic cell line with a hemizygous deletion to the parental cell line identified CDKN1C and H19 as differentially expressed specifically during the endocrine progenitor stage of pancreatic-islet development.
3.2.2. Monogenic diabetes and obesity genes:
Several uncommon mutations in known monogenic disease genes that increase diabetes severity particularly during childhood were identified in the Pima Indians5, 51. Population-based sequencing of the MC4R gene, the most common cause of monogenic obesity, found that 2.4% of Pima Indians are heterozygous for one of six different functional MC4R coding mutations 51. Greater change of BMI over time in carriers than in noncarriers was observed during childhood but not adulthood, indicating that the effects of these mutations are more apparent during active growth51. Moreover, an increased risk for developing type 2 diabetes was found in individuals with a defective MC4R43, 51, but this risk was only independent of BMI in childhood51.
Both neonatal diabetes and hyperinsulinemic hypoglycemia of infancy are caused by mutations in ABCC8 and KCNJ11 genes. Direct sequencing of these genes in the Pima Indians identified a private Arg1420His mutation in the ABCC8 gene carried by 3.3% of the Pima Indian community 5. Heterozygous community members had a doubled risk of type 2 diabetes across all age groups [FIG 4a] with an age of onset of the disease 7 years earlier [FIG 4b] and at a leaner BMI. The loss-of- function mutation also resulted in higher mean birth weights, consistent with fetal hyperinsulinemia. One individual was homozygous for the mutation (His1420His) and had been diagnosed with severe hyperinsulinemia during infancy and type 2 diabetes at age 3.5 years.
FIGURE 4:
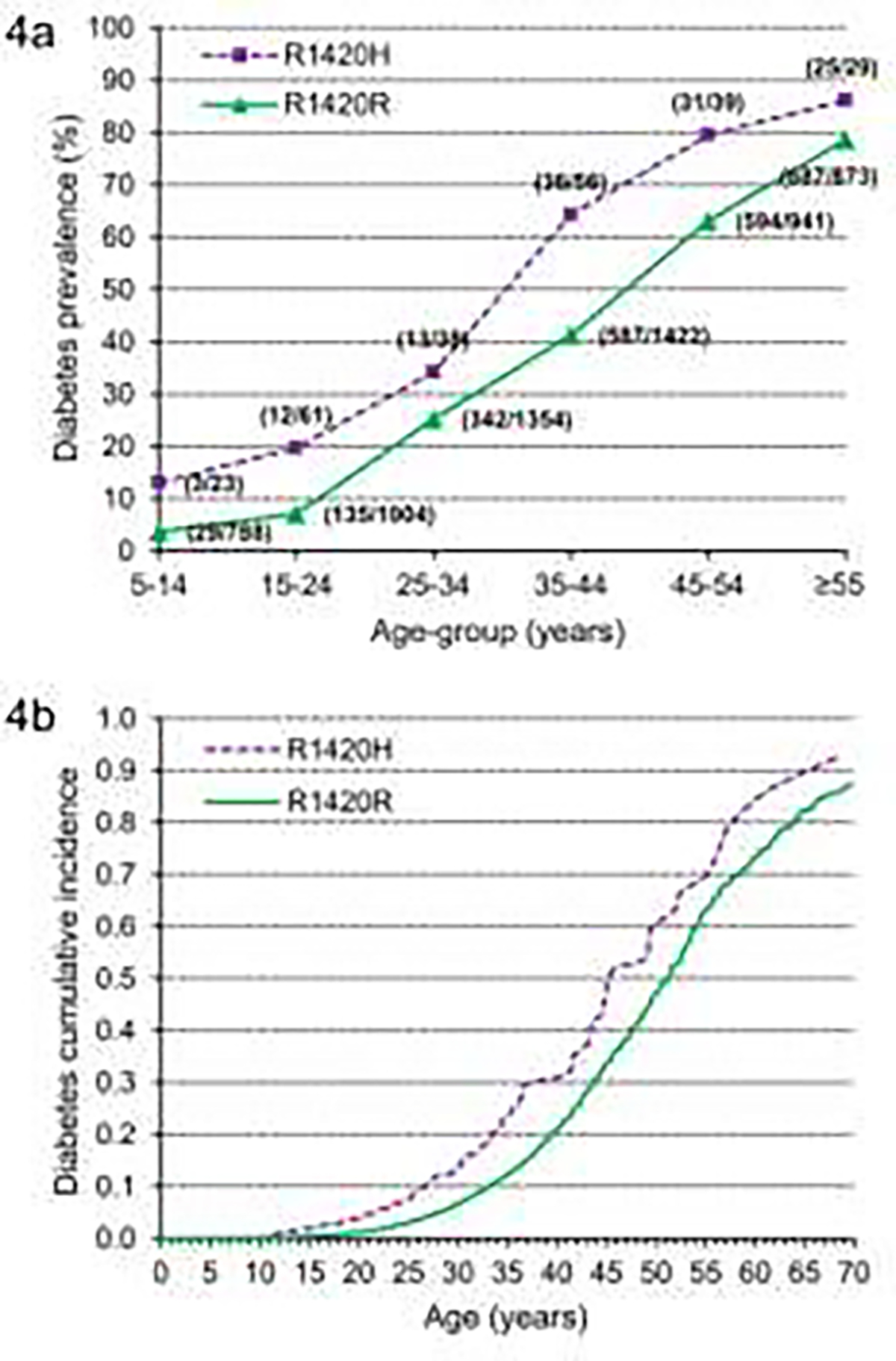
a) Prevalence of type 2 diabetes for Arg1420His carriers and noncarriers shown in individuals grouped by their age at last exam. Numbers in parentheses indicate the number of subjects with diabetes (numerator) and total number of subjects (denominator) for each age-group; (From Baier et al, 2015 5) b) Type 2 diabetes cumulative incidence adjusted for sex, birth year, and ancestry (American Indian/European admixture based on genetic markers and self-reported fraction Pima Indian heritage). Mean onset age was calculated from the parameters of the Weibull model for an individual born in 1940, and the mean age for all other covariates was 45.0 years for Arg1420His and 52.1 years for Arg1420Arg. (From Baier et al, 2015 5)
3.3. Environmental and Epigenetic Determinants of Type 2 Diabetes
By performing oral glucose tolerance tests in pregnant women and following the health outcomes of their offspring over decades, we were able to explore the role of intrauterine diabetes exposure as a risk factor for diabetes in the offspring. We found that offspring exposed to diabetes in utero had a higher risk of childhood obesity 14 and diabetes 52 than those who were not exposed to diabetes in utero. Importantly, within families in which some offspring were exposed to intrauterine diabetes and others were not, the offspring exposed to diabetes in utero were heavier and had a 3.7-fold greater risk of diabetes than those who were not exposed 53. This observation demonstrated that the higher risk of diabetes in the offspring was not attributable to variations in maternal genetic risk but to the intrauterine diabetes exposure 53, which appears to alter epigenetic regulation of gene expression at multiple genomic sites in the offspring 54. Some of these methylation sites also associate with impaired insulin secretion, increased body weight, and increased risk of type 2 diabetes.
Of concern, exposure to diabetes in utero is becoming more common in the Pima Indians – affecting around 2% of children aged 5–19 years in the period 1967–76 and affecting almost 8% of children for the period 1987–96 16. This increased frequency of exposure was accompanied by a rise in the prevalence of childhood-onset type 2 diabetes, with intrauterine diabetes exposure accounting for ~40% of childhood cases in the period 1987–1996 which is approximately double the attributable risk from the period 1967–76 [FIG 5a]. The interplay between intrauterine exposure to diabetes and the risk of the offspring developing diabetes at a young age has created a ‘vicious cycle’ 42 whereby a larger proportion of female offspring in each successive generation develop diabetes during their childbearing years which leads to an ever increasing prevalence of type 2 diabetes in youth attributable to this exposure [FIG 5b]. The increase in the incidence of childhood diabetes in the Pima Indians is strongly associated with increases of in utero exposure to diabetes as described above 55. The AIR (measured during an IVGTT) is lower in participants exposed to diabetes in utero compared to measures in participants whose mothers’ developed diabetes after the participant was born 56 which may be a mechanistic link between the exposure and the risk of early onset diabetes. The impact of youth-onset type 2 diabetes in the Pima Indians is discussed more fully later in this review.
FIGURE 5:
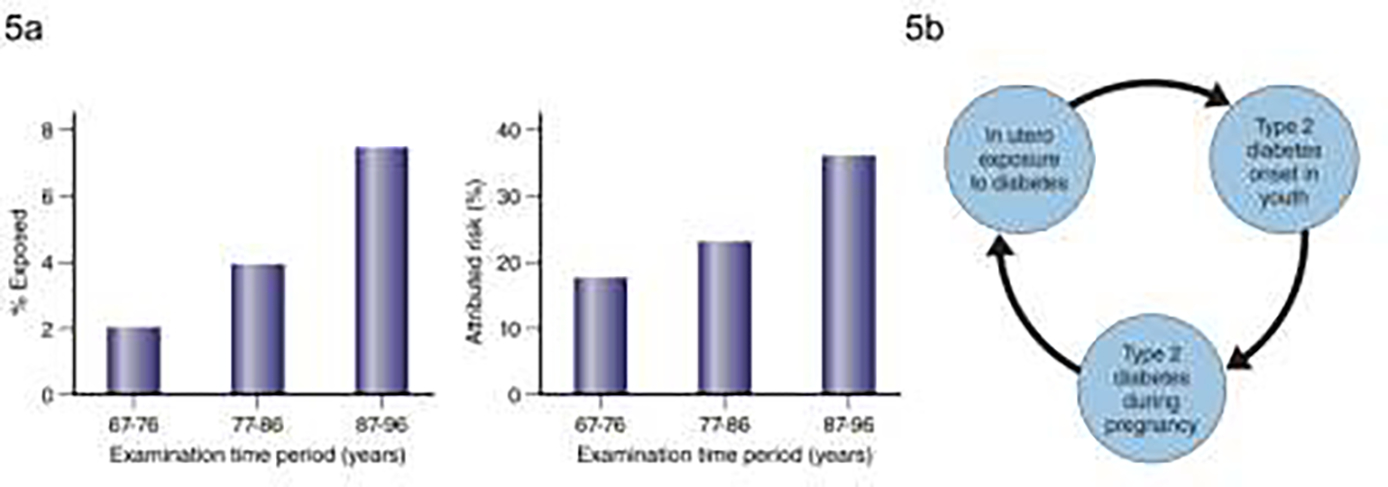
a) Prevalence of exposure to diabetes in utero in children aged 5–19 years by examination period in the left-hand panel and attributable risk for diabetes in children aged 5–19 years in the in right-hand panel (Adapted from Dabelea et al, 200016); b) the “vicious cycle” of in utero exposure to diabetes leading to young onset of diabetes in the offspring leading to more of the offspring’s children exposed to diabetes in utero (Adapted from Knowler et al, 199042).
The contribution of environmental factors in the development of type 2 diabetes was further explored by comparing diabetes among Pima Indians living in Arizona and those living in Northern Mexico. The two populations share considerable genetic similarity but have very different lifestyles – with the Pima Indians in Mexico having far higher levels of physical activity than those in Arizona. In keeping with their more active lifestyle, the Mexican Pima Indians have significantly lower rates of obesity and diabetes compared to the Arizona Pima Indians 57. Studies in the Pima Indians clearly demonstrate the importance of environmental and lifestyle determinants of obesity and diabetes and show that even in populations with high genetic risk, the development of diabetes is determined largely by environmental factors. It is noteworthy that there are substantial genetic differences between Arizona and Mexican Pimas at some loci, e.g. at HLA-DR/DQ 58, but the known differences do not appear to explain much of the difference in diabetes prevalence.
An obesogenic environment can also influence the effect of some diabetes-associated variants. For example, a haplotype across the SLC16A11 gene, with a frequency of 50% in samples from the Americas but rare in Europeans and Africans, is associated with increased diabetes risk in Mexican and other Latin American populations 59. Studies in Pima Indians in Arizona demonstrated that this haplotype strongly interacted with BMI such that the diabetes risk was only among lean individuals; among obese individuals this haplotype associated with protection against type 2 diabetes 60.
4. Pathophysiology of Diabetes Complications
Prior to the mid-1980s, type 2 diabetes was generally considered to be a relatively benign form of diabetes, compared to type 1 diabetes, and was not believed to be a major source of complications 61,62. Findings in the Pima Indians challenged this narrative and contributed to the current recognition that the complications of type 2 diabetes are a major source of morbidity and mortality worldwide. As a leading cause of death among diabetic Pima Indians, diabetic kidney disease (DKD) is a major focus of research in this population and several key findings have come from this work which will be reviewed here 12.
4.1. Diabetic Kidney Disease
The population-based studies of kidney disease in the Pima Indians initially relied on measurements of proteinuria to identify the early clinical stages of DKD. The cumulative incidence of proteinuria in these studies was higher than seen in a Caucasian cohort with type 2 diabetes 63 and nearly all the excess mortality associated with diabetes in the Pima Indians was observed in those with proteinuria 64. Kidney disease was also the leading cause of death among those with diabetes, but as kidney replacement therapy became more accessible to the population, deaths from kidney disease declined and cardiovascular disease emerged as the leading cause of death 65. The incidence of kidney failure among those with type 2 diabetes was equivalent to that in persons with type 1 diabetes 66.
A simple nephelometric immunoassay was established in the early 1980s to detect low concentrations of albumin in the population 67. Studies establishing the validity of the urine albumin-to-creatinine ratio (ACR) as a measure of urine albumin excretion in this and other populations contributed to the acceptance of this measure as a standard screening tool for DKD 68,69.
The prevalence of albuminuria in the Pima Indians, based on the urine albumin-to-creatinine ratio, increases with worsening glycemia – with higher albuminuria in participants with IGT or diabetes compared to participants with NGT. After the onset of diabetes, the prevalence of albuminuria also increases with increasing diabetes duration. By 20 years of diabetes, severe albuminuria (ACR ≥300 mg/g) was present in over 60% of the population, with moderate albuminuria (ACR 30–299 mg/g) detected in another 20% [FIG 6a] 70. The importance of severe albuminuria as a predictor of progressive DKD is highlighted by the finding that almost 50% of participants who developed severe albuminuria progressed to kidney failure within 10 years 63. The prevalence of albuminuria has declined over time in the population, reflecting the more effective management of DKD and the greater use of antihypertensive medicines including those that block the renin-angiotensin system 71.
FIGURE 6:
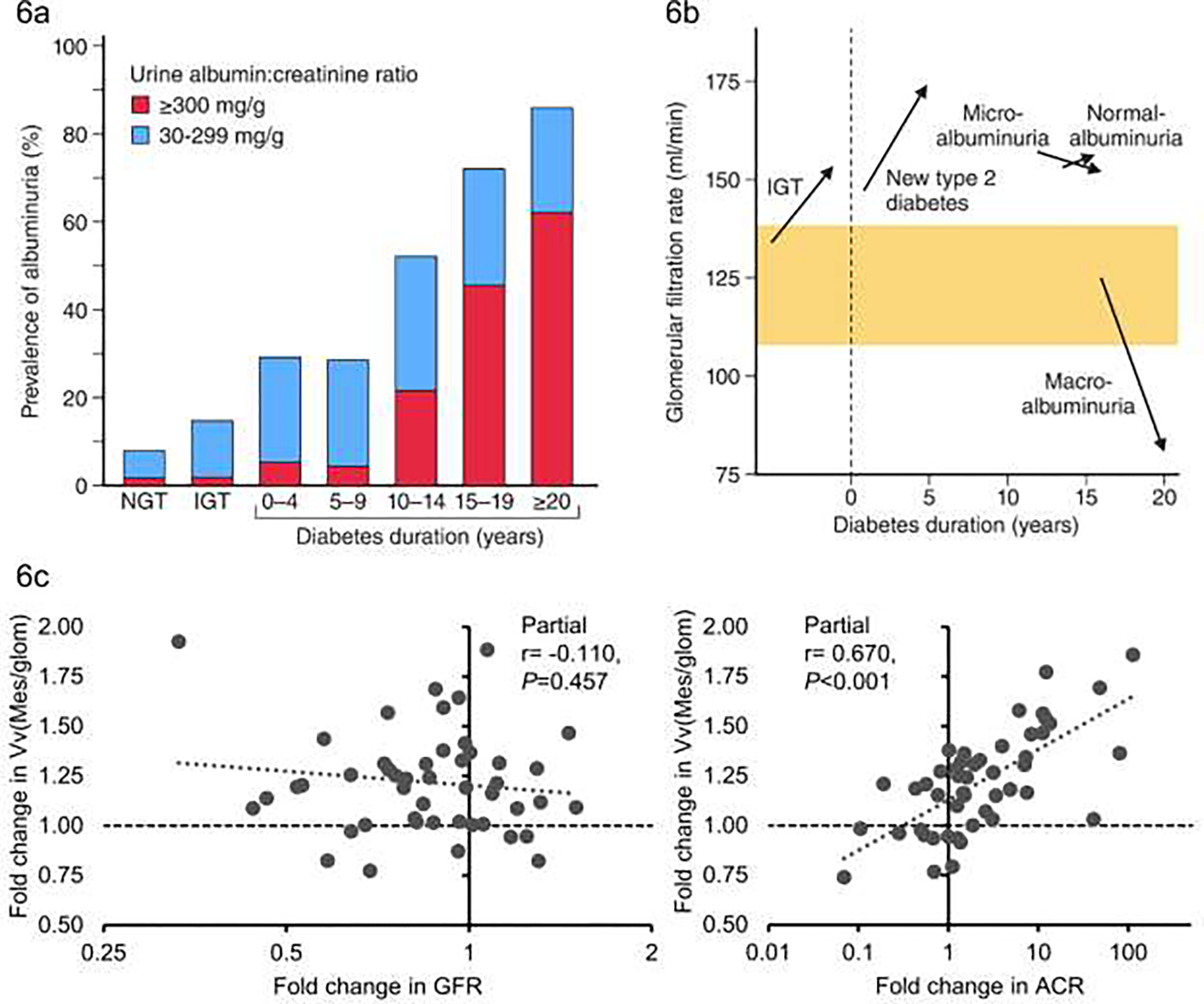
a) Prevalence of albuminuria by glycemia and diabetes duration (blue bars for ACR 30–299 mg/dl), red bars for ACR >= 300 mg/dl) NGT = normal glucose tolerance, IGT = impaired glucose tolerance (Adapted from Nelson et al 198996); b) Changes in mean glomerular filtration rate by glycemia stage, diabetes duration and degree of albuminuria - normoalbuminuria = ACR <30 mg/dl micro-albuminuria = ACR 30–299 mg/dl, also known as moderate albuminuria; macroalbuminuria = ACR >= 300 mg/dl) also known as severe albuminuria (Adapted from Nelson et al, 199611); c) Association of fold change in fractional mesangial volume with fold change in ACR (right panel) and fold change in GFR (left panel) partial correlation coefficients are adjusted for age, sex and baseline values of ACR and GFR respectively (Adapted from Looker et al, 201975).
4.2. Glomerular Hemodynamics in DKD
Following decades of research into DKD in the Pima Indians using the epidemiologic data collected in the population study, in-depth studies focusing on DKD in participants recruited from the population study were launched in 1988. These kidney studies included a more detailed assessment of kidney function including measurements of the glomerular filtration rate (GFR) by the urinary clearance of iothalamate and renal plasma flow rate by the urinary clearance of p-aminohippurate. Research kidney biopsies were performed in informative subsets of those involved in the kidney studies which provided kidney tissue for morphometric and gene expression studies, including single cell expression data from the most recent kidney biopsies.
Measurements before and after the development of diabetes demonstrated that the GFR was elevated in Pima Indians with IGT compared to those with NGT, and that GFR increased further after the onset of diabetes, remaining elevated in those with moderate albuminuria before declining as albumin excretion became more severe [FIG 6b] 11. As the kidney studies included measurement of both GFR and renal plasma flow it was possible to investigate the role of intraglomerular hemodynamics in the progression of DKD to kidney failure 72. This work is discussed elsewhere in this issue (Saulnier PJ et al).
4.3. Kidney Structure in DKD
As DKD is primarily a clinical diagnosis, kidney biopsies are not a routine part of management and biopsies are often used clinically to establish the cause of kidney disease in patients with atypical presentations. Accordingly, clinically indicated kidney biopsies among those with diabetes are biased towards nondiabetic kidney diseases. As a result, research kidney biopsies are a highly valuable source of structural and gene expression data in DKD as they are not influenced by an atypical clinical presentation. Research kidney biopsies were an integral part of the Pima Indian kidney studies 73–75. Kidney biopsy tissue was examined by light and electron microscopy and standard morphometric techniques were applied to quantify key attributes of DKD including glomerular basement membrane thickness, mesangial fractional volume, surface density of the peripheral glomerular basement membrane, glomerular volume and cortical interstitial fractional volume 13,73–76. These measurements confirmed that the structural lesions found in Pima Indians with diabetes reflect classical changes of DKD 73. Due to the younger age of onset of diabetes and lower prevalence of comorbidities, such as cardiovascular disease and hypertension, the chronic kidney disease in Pima Indians is almost exclusively caused by diabetes 73 which is unusual in other populations with type 2 diabetes and more in keeping with kidney disease seen in type 1 diabetes.
Studies of the research kidney biopsies demonstrated that a lower number of podocytes per glomerulus was strongly associated with a higher level of albuminuria 73 and predicted increasing albuminuria during follow-up 77. In addition, among Pima Indians with moderate albuminuria at the baseline kidney biopsy, we demonstrated a 35% decline, on average, in the number of podocytes per glomerulus in biopsies performed 4 years later, indicating that early loss of podocytes in type 2 diabetes is associated with progression of DKD 78. We also demonstrated that structural lesions often predate clinical evidence of DKD 73 and early structural injury, including mesangial expansion, higher percentage of global glomerulosclerosis, greater podocyte foot process width, higher mean glomerular volume, reduced endothelial fenestrations, and lower glomerular filtration surface density are associated with subsequent loss of kidney function 74. In a study of 44 Pima Indians with two kidney biopsies separated by a median interval of 9.3 years we observed progression in most morphometric measures associated with DKD. These changes in kidney structure were strongly associated with increasing albuminuria but not with declining GFR [FIG 6c] 75. Most participants had normal or elevated GFR at their first biopsy and it is possible that in more advanced DKD an association with GFR would be seen, but at least in early disease changes in kidney structure are more closely related to changes in albuminuria.
Changes in structure are some of the earliest changes seen in DKD and we explored the role of biomarkers for detecting these early structural lesions and for better understanding the underlying pathophysiology. We examined the relationships between biomarkers in blood and urine and early structural lesions in a recent review 79 and our findings from these studies are summarized and updated in the TABLE 1.
TABLE 1:
Biomarkers associated with kidney structure in type 2 diabetes
| Biomarker(s) | Sample type | Number in study | Positive associations | Negative associations | Reference |
|---|---|---|---|---|---|
|
| |||||
| Advanced Glycation End-products: | Beisswenger et al, 2005 88 | ||||
| Methylglyoxal | Serum | 45 | GBM width | Podo | |
| 3DG | GBM width | Saulnier et al, 2016 89 | |||
| CML | Serum | 95 | GS, VvMes, VvInt, FPW | %ECF, TFS | |
| CEL & MGHI | GS, VvMes, VvInt | TFS, VG | |||
| GHI | GS, VvMes, VvInt, FPW | Sv, TFS | |||
| MDGHI | GS, VvMes, VvInt | ||||
|
| |||||
| Tumor Necrosis | Serum | 83 | VvMes | %ECF | Pavkov et al 2016 90 |
| Factor Receptor 1 | Serum | 105 | VvMes, GBM width, VvInt | %ECF, TFS | Kobayashi et al, 202291 |
|
| |||||
| Tumor Necrosis Factor Receptor 2 | Serum | 83 | VvMes | %ECF | Pavkov et al 201690 |
|
| |||||
| Cardiovascular autonomic neuropathy | 63 | Wheelock et al, 201692 | |||
| E/I ratio | GBM width | ||||
| SdNN | Sv | GS, GBM width | |||
| LF | Sv | GS, GBM width | |||
| HF | Sv | GBM width, VvInt | |||
|
| |||||
| White blood cell fractions | Blood | 108 | Wheelock et al, 201793 | ||
| Neutrophils | - | %ECF | |||
| Eosinophils | GBM width | Sv | |||
| Lymphocytes | %ECF | GBM width | |||
| NLR | - | %ECF | |||
|
| |||||
| Polyubiquitinated PTEN K80 | Serum | 77 | VvMes, GBM width | Podocyte fractional volume, number density of podocytes | Looker et al 201994 |
|
| |||||
| Axon Guidance Proteins: | Serum | 105 | Satake et al 2021 72 | ||
| EFNA4& EFNA5 | VvMes, GBM width | Sv | |||
| EPHA2 | VvMes, GBM width, VvInt, FPW, PD | Sv, Podo, %ECF | |||
| EPHB2 | VvMes | Sv | |||
| EPHB6 | VvMes, GBM width | Sv, Podo | |||
| UNC5C | VvMes, GBM width | Sv, Podo, %ECF | |||
|
| |||||
| NLB1 | Serum | 105 | VvMes, GBM width, VvInt | Podo, podocyte fractional volume, %ECF, TFS | Kobayashi et al, 2022 91 |
Summary of associations between biomarkers and kidney structure in cross sectional studies among the Pima Indians. (Adapted from "Role of Kidney Biopsies for Biomarker Discovery in Diabetic Kidney Disease" Looker et al, 2018 79).
Kidney structural abbreviations: GBM = glomerular basement membrane; VvMes = mesangial fractional volume, VvInt = cortical fractional interstitial volume; Sv = surface density of the peripheral glomerular basement membrane per glomerulus; TFS = total filtration surface per glomerulus; %ECF = percentage of endothelial cell fenestrations; FPW = foot process width; Podo = number of podocytes per glomerulus; GS = percentage of sclerosed glomeruli, VG = mean glomerular volume.
Biomarker abbreviations: CEL = carboxyethyl lysine; CML = carboxymethyl lysine, GHI = glyoxal hydroimidazolone; MGHI = methylglyoxal hydroimidazolone; 3DGHI = 3 deoxyglucasone hydroimidazolone; E/I ratio = expiration:inspiration ratio; sdNN = standard deviation of the normal R-R interval; LF= low frequency signal power; HF= high frequency signal power; NLR = neutrophil:lymphocyte ratio.
4.4. Gene Expression in Kidney Tissue
Perturbations of gene expression in kidney biopsy tissue have been examined in Pima Indians with type 2 diabetes and early DKD. After microdissection of the biopsy tissue, gene expression was measured in the glomerular and tubulo-interstitial compartments. In a study including expression data from Pima Indians with early DKD, from whites with more advanced DKD, and from nondiabetic controls, we found that glomerular JAK2 expression was higher in early DKD compared to controls, whereas in advanced DKD JAK2 expression was lower in the glomerular compartment but higher in the tubulointerstitial compartment [FIG 7] 80. JAK2 is associated with fibrosis and these observations demonstrated how expression patterns evolved as DKD progresses. These observations were critical to designing a randomized clinical trial of baricitinib, a JAK1/JAK2 inhibitor (Clinical Trial NCT01683409). This study, which was not conducted in Pima Indians, showed that 6-month treatment with baricitinib reduced albuminuria in comparison with placebo and that reduction of blood and urine biomarkers of JAK-STAT activation predicted from the gene expression profiling preceded the reduction in albuminuria 81. The potential value of tissue-level molecular analysis to identify therapeutic targets is illustrated by this work.
FIGURE 7:
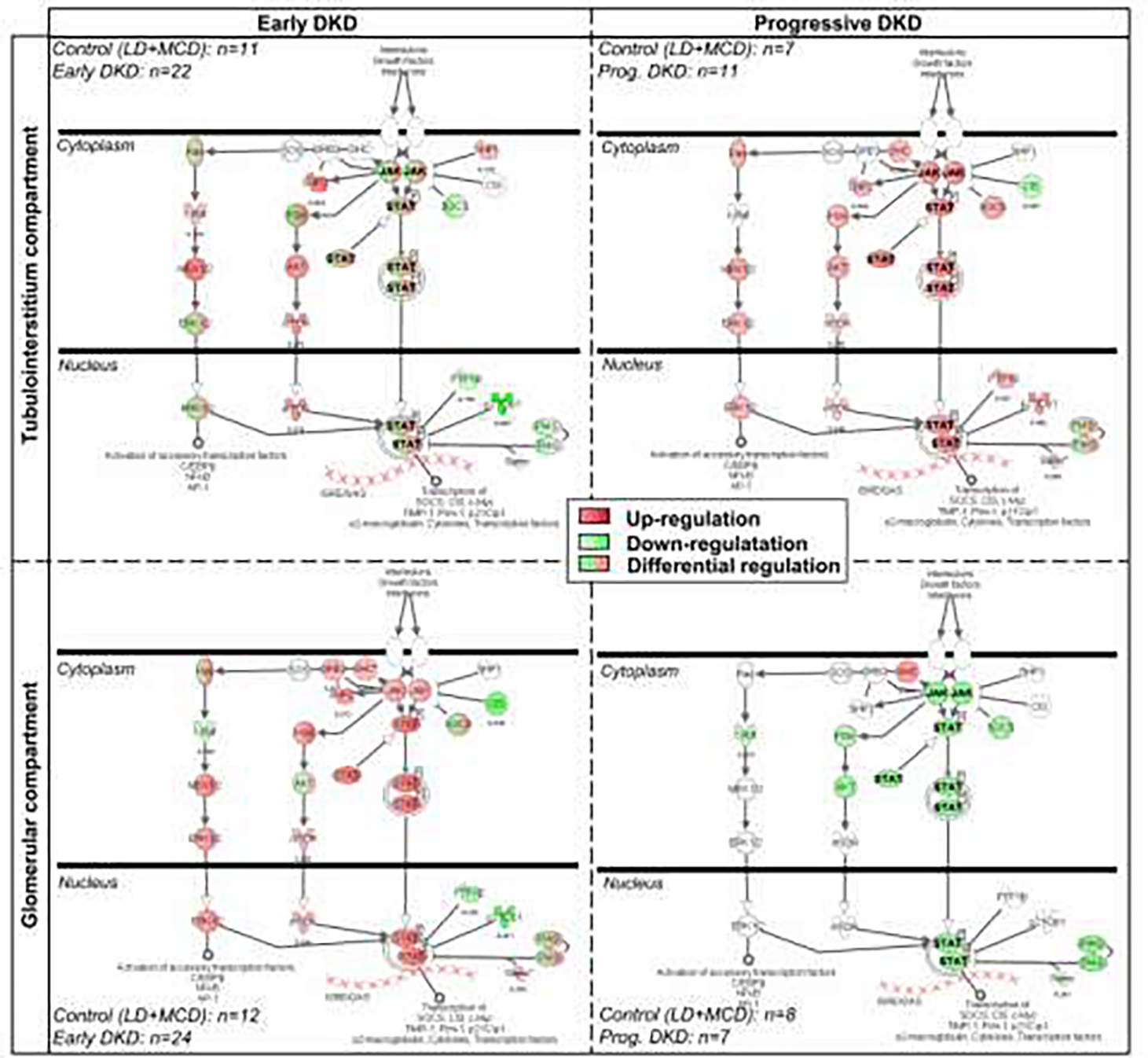
JAK/Stat canonical pathway in diabetic kidney disease as assessed by Ingenuity Pathway Analysis software. The expression patterns in the Pima Indian samples (marked ‘Early DKD’) and in the European samples (marked ‘Progressive DKD’). Red indicates increased expression, green reduced expression and green with red for differential expression (Adapted from Berthier et al, 2009 80).
Gene expression data were also used to link kidney structural measurements in the Pima Indians to kidney function. The structural parameter most strongly associated with transcriptional regulation was cortical interstitial fractional volume, a marker of tubulointerstitial damage. Transcripts associated with cortical interstitial fractional volume were enriched for inflammatory pathways, many of which are also associated with DKD progression in more advanced DKD in other populations demonstrating potential pathways which can link the changes we observe in structure with the changes in clinical progression of DKD [FIG 8] 82.
FIGURE 8:
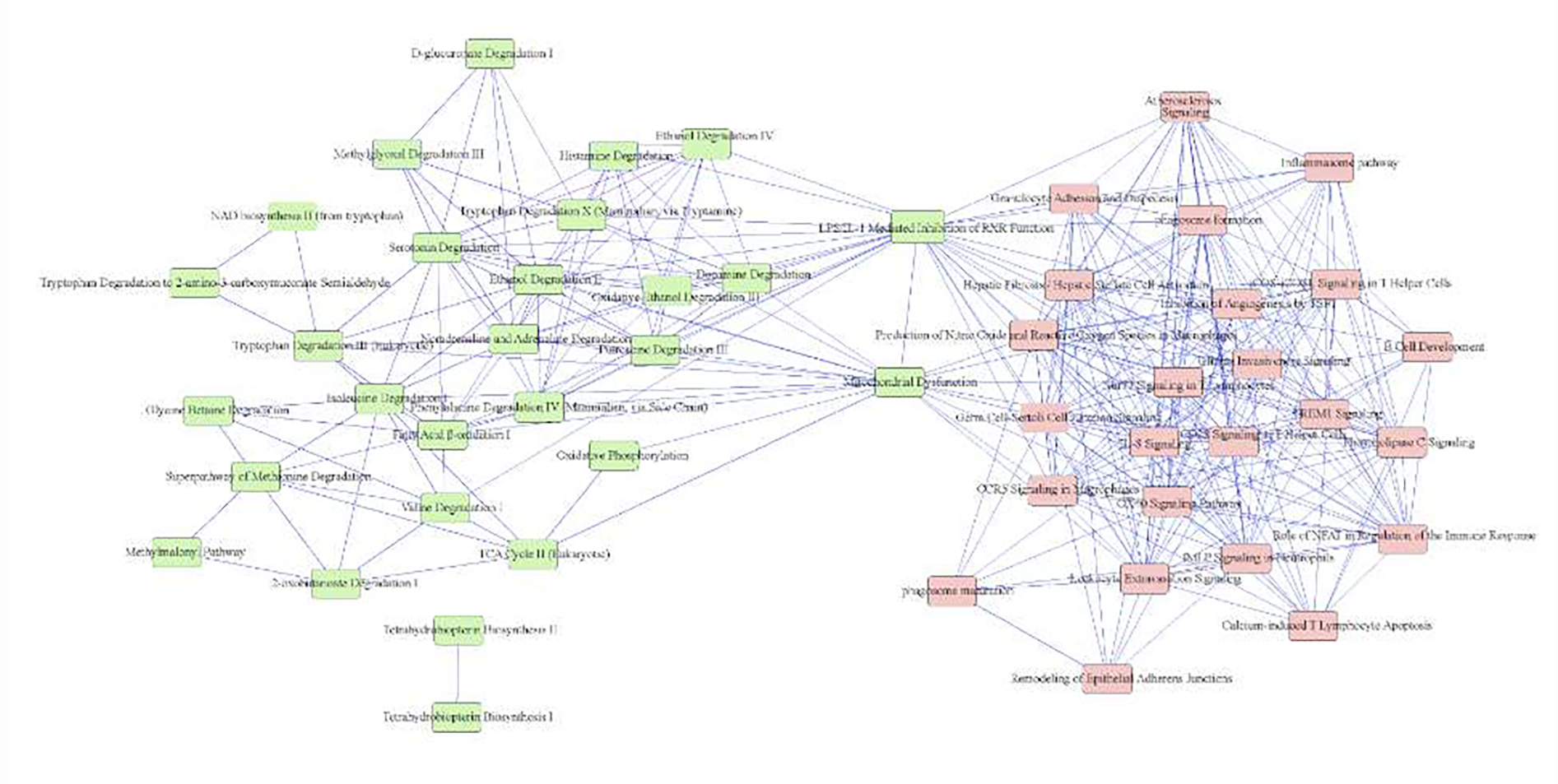
Ingenuity Pathway (Ingenuity Systems, QIAGEN, Redwood City, CA) network showing significant pathways (P ≤ 0.05) enriched by cortical interstitial fractional volume (VvInt)-correlated transcripts. Pathways are connected by 1 or more shared genes. The network displays 2 principal domains driven by negatively correlated (left) and positively correlated (right) transcripts. The nodes (pathways) are connected by (edges) genes shared among them. (From Nair et al, 2018 82).
As we described above, hyperfiltration is one of the earliest changes in DKD and may itself lead to kidney damage and progressive DKD. By examining long-term GFR trajectories and integrating them with morphometric and molecular analyses of research kidney biopsies, we identified a set of genes in the glomerular compartment in Pima Indians that were differentially expressed between participants who had their peak GFR recorded within +/− 2 years of biopsy, the hyperfiltration group, and participants who reached their peak GFR >2 years after the kidney biopsy, the pre-hyperfiltration group [FIG 9a] 83. The hyperfiltration group also exhibited structural lesions characteristic of hyperfiltration. The transcriptional signature for hyperfiltration was enriched for endothelial stress response signaling genes, with most of the transcripts mapped to endothelial and inflammatory cell clusters in kidney single cell transcriptional data collected recently in a subset of Pima Indians included in the in-depth kidney studies. We also used these single cell transcriptomic data to develop a schematic for the interplay between kidney endothelial and mesangial cells during hyperfiltration [FIG 9b]. These pathways may provide therapeutic targets for hyperfiltration in DKD.
FIGURE 9:
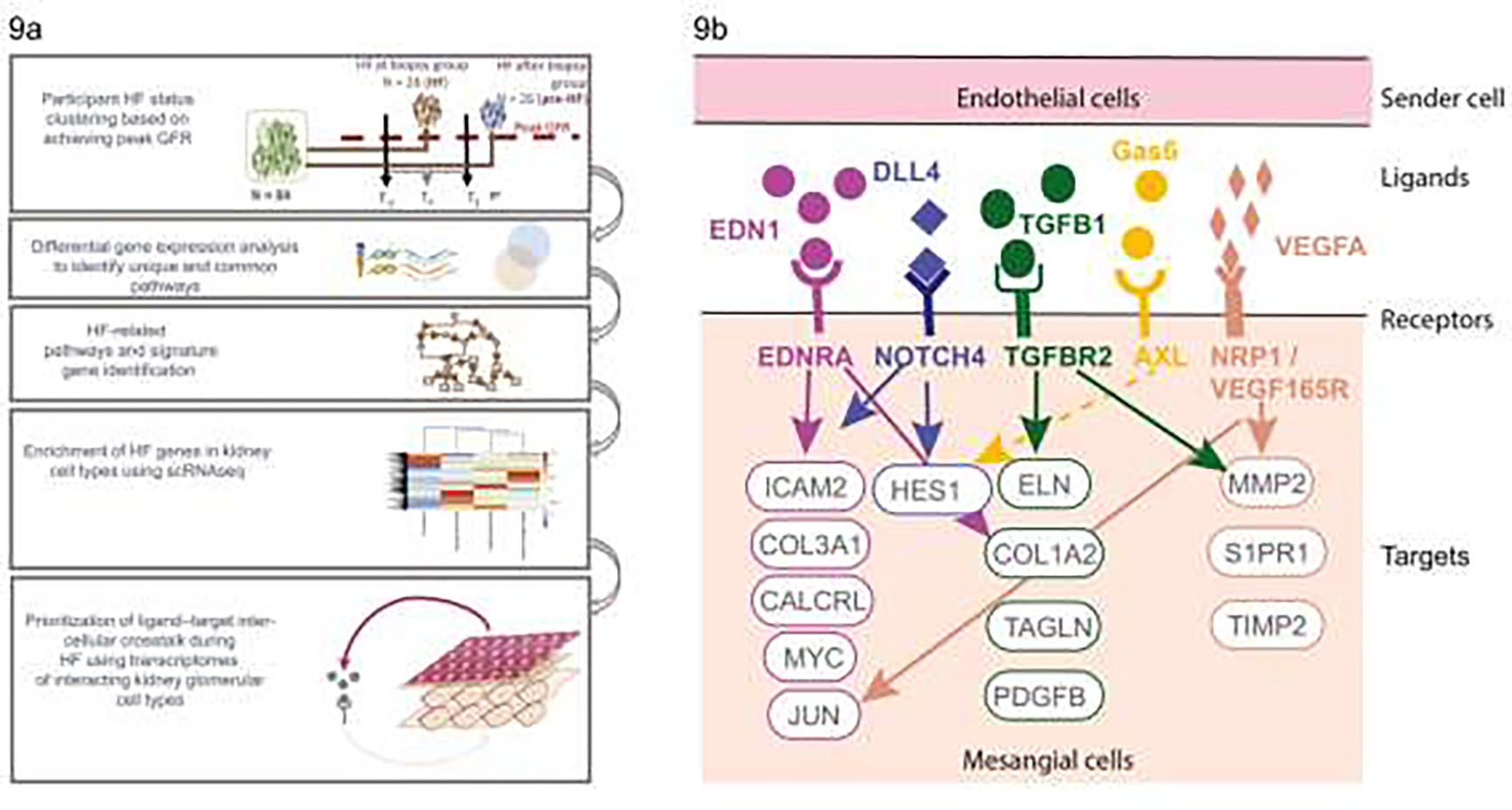
a) Diagram outlining the hyperfiltration study design showing the groups defined based on timing of peak GFR in relation to kidney biopsy, and interrogation of gene expression data to identify genes associated with hyperfiltration followed by examination of single cell data to identify cell types associated with the enriched hyperfiltration genes leading to development of a model for cross-talk between cell types in hyperfiltration (From Steffansson et al, 2022)83; b) molecular insights into hyperfiltration associated with early DKD. CALCRL, calcitonin receptor like receptor; COL1A2, collagen type I alpha 2 chain; COL3A1, collagen type III alpha 1 chain; DLL4, delta-like canonical notch ligand 4; EDN1, endothelin-1; EDNRA, endothelin receptor A; ELN, elastin; Gas6, growth arrest–specific gene 6; HES1, hes family bHLH transcription factor 1; ICAM, intercellular adhesion molecule; JUN, Jun proto-oncogene; MMP2, matrix metallopeptidase 2; MYC, MYC proto-oncogene; NOTCH4, notch receptor 4; NRP1, neuropilin 1; PDGFB, platelet derived growth factor subunit B; S1PR1, sphingosine-1-phosphate receptor 1; TAGLN, transgelin; TGFB1, transforming growth factor-β1; TGFBR2, transforming growth factor-β receptor 2; TIMP2, TIMP metallopeptidase inhibitor 2; VEGF165R, vascular endothelial growth factor 165 receptor; VEGFA, vascular endothelial growth factor A (From Steffansson et al, 202283).
5. Youth-Onset Type 2 Diabetes
Of the many insights gained from studies in the Pima Indians, one that stands out is their contribution to our understanding of youth-onset type 2 diabetes. The incidence and prevalence of youth-onset type 2 diabetes is increasing worldwide 84, but its emergence in many populations has been relatively recent. By contrast, youth-onset type 2 diabetes was first identified in the Pima Indians in the mid 1960’s at the initiation of the longitudinal diabetes study, and the decades of follow-up of this population has permitted us to characterize its course more fully than is possible in other populations.
Studies of youth-onset diabetes (age <20 years) compared with adult-onset diabetes in the Pima Indians revealed no difference between the two groups in the incidence of proteinuria by duration of diabetes [FIG 10a], indicating that those who developed type 2 diabetes in youth were far more likely to develop DKD at an earlier age than those who developed diabetes later in life 85. Accordingly, when kidney failure was considered as the end-point, youth-onset diabetes was associated with a five-fold higher incidence of kidney failure between the ages of 25 and 64 years than those who developed diabetes in adulthood [FIG 10b] 86. Moreover, in a study in which we assessed the impact of age of diabetes onset on kidney structure we showed an inverse association between age of onset and key early structural lesions including GBM width, mesangial fractional volume, and surface density of the peripheral glomerular basement membrane and podocyte number density [FIG 10c] 87. These findings are intriguing when coupled with the clinical data addressed above, as clinically we find progression of DKD similar in youth-onset and adult-onset participants for a given diabetes duration while the biopsy data suggest that structural lesions are more severe for a given duration when type 2 diabetes develops at a younger age. It is possible that the clinical impact of the more severe structural lesions is in part mitigated by the younger age with fewer age-related comorbidities in those with youth-onset diabetes, resulting in similar rates of clinical progression. The severity of the structural lesions however suggests that people with youth-onset type 2 diabetes would benefit from more aggressive reno-protective management to improve later DKD progression 87.
FIGURE 10:
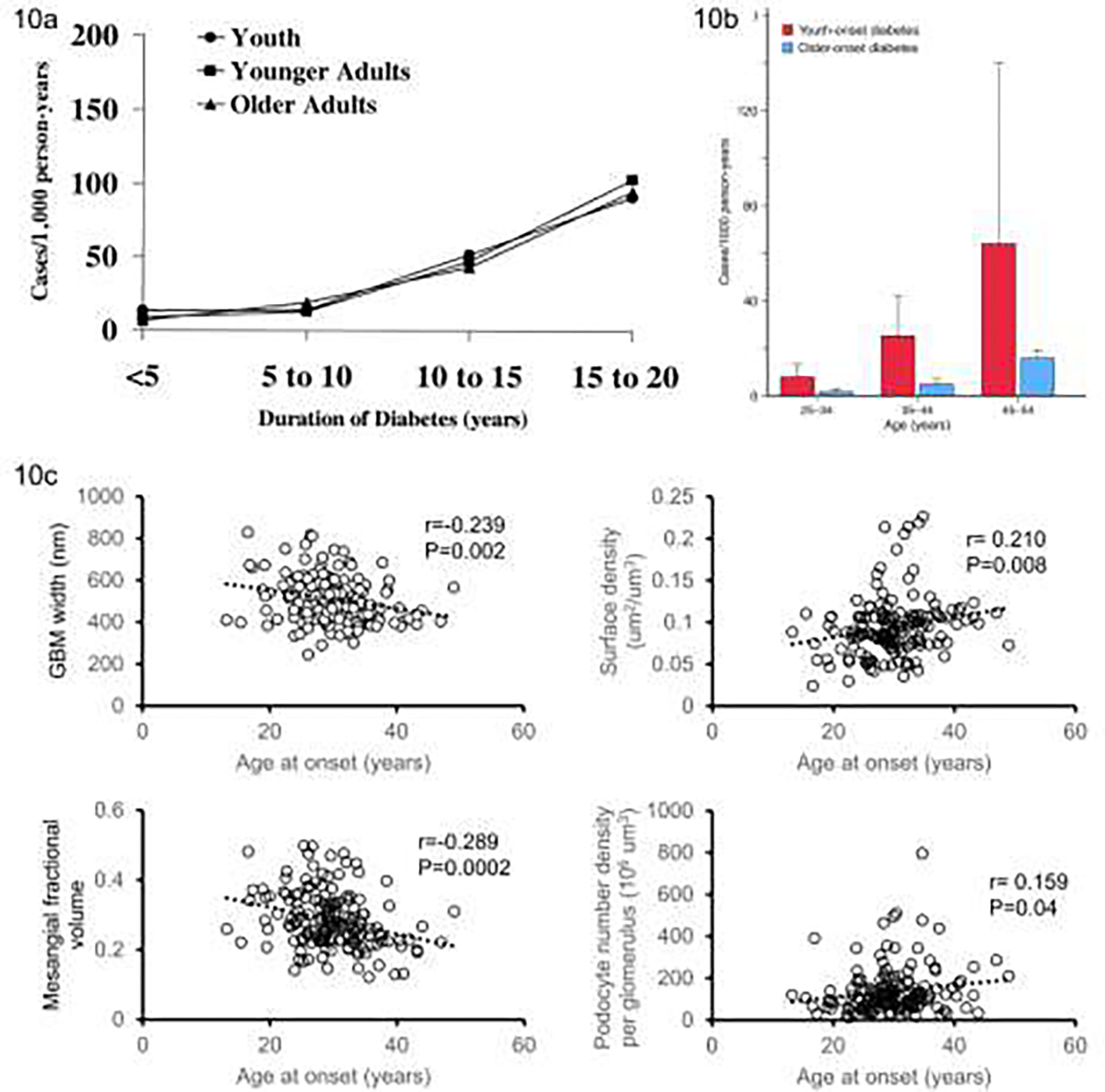
a) Incidence of proteinuria (urine protein-to-creatinine ratio ≥0.5 g/g) by age of onset of diabetes black circles – diabetes diagnosed age <20 years, black squares – diabetes diagnosed age 20–39 years, black triangles – diabetes diagnosed age 40–59 years. Test for trend, P = 0.77, calculated using Mantel-Haenzel test to compare incidence rates between age groups, controlling for diabetes duration (From Krakoff et al, 2003 85); b) Sex adjusted incidence rates of kidney failure by age of onset for Pima Indians aged between 25 and 54 years – red bars for youth-onset type 2 diabetes (age of onset under 20 years) and blue bars older-onset type 2 diabetes (age of onset over 20 years) (Adapted from Pavkov et al 86); c) Association of selected kidney structure measures with age at onset of diabetes. Plots show correlation of residuals for each structural measure and age at onset of diabetes adjusted for attained age, sex, HbA1c, BMI, and GFR (From Looker et al, 202287).
6. Conclusions
The contribution of the Pima Indians to our understanding of type 2 diabetes cannot be overstated [BOX]. Studies in the Pima Indians established the unified definition of diabetes and the dominant role of insulin resistance in the development of type 2 diabetes. The adverse impact of a diabetic intrauterine environment on the early development of diabetes in the offspring was characterized for the first time in the Pima Indians, along with the vicious cycle of increasing diabetes prevalence in successive generations that this exposure created. Yout-honset type 2 diabetes was not only identified in the 1960’s, but it was followed from its onset to the appearance of end-stage complications and death, providing the first detailed characterization of its clinical course and long-term impact. Comprehensive studies of DKD illustrated the important role that early hemodynamic changes and loss of podocytes within the kidneys played in the development and progression of kidney disease and provided new information on the mechanisms underlying this progression. Findings in the Pima Indians have foreshadowed similar trends in other populations worldwide, providing clinicians and investigators with important opportunities to develop and test new management tools to address these adverse trends before they become entrenched 12.
BOX. Selected Clinical Contributions to Type 2 Diabetes of the Pima Indian Studies.
Identified the bi-modal glucose distribution and a cut-point for diabetes diagnosis from a 2 hour plasma glucose concentration in a 75g glucose tolerance test of 11.1 mmol/l (200 mg/dl)
Demonstrated the changes in insulin sensitivity and insulin secretion in individuals followed from normal glucose tolerance through the onset of type 2 diabetes
Demonstrated the increased risk of youth-onset diabetes in offspring exposed to diabetes in utero
Highlighted the role of environmental risk factors for diabetes in studies of US and Mexican Pima Indians
Identified that 3.3% of Pima Indians are heterozygous for a private mutation in the ABCC8 gene that doubles the risk for type 2 diabetes; homozygous carriers can develop hyperinsulinemic-hypoglycemia of infancy
Demonstrated that type 2 diabetes is highly polygenic even in a population isolate
Demonstrated that extreme phenotypes commonly observed in early childhood are driven by monogenic mutations within a polygenic background
Established type 2 diabetes as a major cause of kidney failure
Developed a simple nephelometric immunoassay for measuring urine albumin concentration
Demonstrated the validity of the urine albumin-to-creatinine ratio as a measure of urine albumin excretion and contributed to the acceptance of this ratio as a standard screening and diagnostic tool for DKD
Identified numerous modifiable risk factors for DKD including
Hyperglycemia
High blood pressure
Hyperlipidemia
Obesity
Intrauterine diabetes exposure
Intrauterine growth retardation
Periodontal disease
Environmental pollutants
Characterized the risk of DKD associated with youth-onset type 2 diabetes, foreshadowing similar trends in other populations worldwide
Revealed that glomerular hemodynamic changes and structural kidney injury precede the development and predict the progression of clinical DKD in type 2 diabetes
Demonstrated the crucial role of podocyte injury in the development and progression of DKD in type 2 diabetes
Documented the risks associated with youth-onset type 2 diabetes for diabetic complications including faster development of kidney structural lesions
Many findings first reported in the Pima Indians have been confirmed in other populations, and likewise those initially identified in other populations have been corroborated in the Pima Indians, illustrating the considerable scientific value and the generalizability of this work. The studies conducted in the Pima Indians were possible because of a successful partnership with the Community that spanned five decades, and the benefits of this collaboration continue to be felt both in the Community and worldwide.
Acknowledgements
This work was supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases. The authors acknowledge the significant contributions of Peter H. Bennett, William C. Knowler, Clifton Bogardus, David J. Pettitt, Jonathan Krakoff, Lois I. Jones, Enrique Diaz, Jillian Loebel, Roselene Lovelace, Bernadine Waseta, and Camille Waseta. The authors also acknowledge the many Pima Indians who participated in the studies referenced in this paper and without whom none of this would have been possible.
Footnotes
The authors declare that they have no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Knowler WC, Bennett PH, Hamman RF, Miller M. Diabetes incidence and prevalence in Pima Indians: a 19-fold greater incidence than in Rochester, Minnesota. American journal of epidemiology 1978;108:497–505. [DOI] [PubMed] [Google Scholar]
- 2.Knowler WC, Bennett PH, Bottazzo GF, Doniach D. Islet cell antibodies and diabetes mellitus in Pima Indians. Diabetologia 1979;17:161–4. [DOI] [PubMed] [Google Scholar]
- 3.Savage PJ, Bennett PH, Senter RG, Miller M. High prevalence of diabetes in young Pima Indians: evidence of phenotypic variation in a genetically isolated population. Diabetes 1979;28:937–42. [DOI] [PubMed] [Google Scholar]
- 4.Dabelea D, Palmer JP, Bennett PH, Pettitt DJ, Knowler WC. Absence of glutamic acid decarboxylase antibodies in Pima Indian children with diabetes mellitus. Diabetologia 1999;42:1265–6. [DOI] [PubMed] [Google Scholar]
- 5.Baier LJ, Muller YL, Remedi MS, et al. ABCC8 R1420H Loss-of-Function Variant in a Southwest American Indian Community: Association With Increased Birth Weight and Doubled Risk of Type 2 Diabetes. Diabetes 2015;64:4322–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bennett PH, Burch TA, Miller M. Diabetes mellitus in American (Pima) Indians. Lancet (London, England) 1971;2:125–8. [DOI] [PubMed] [Google Scholar]
- 7.Bogardus C, Lillioja S, Howard BV, Reaven G, Mott D. Relationships between insulin secretion, insulin action, and fasting plasma glucose concentration in nondiabetic and noninsulin-dependent diabetic subjects. The Journal of clinical investigation 1984;74:1238–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lillioja S, Mott DM, Howard BV, et al. Impaired glucose tolerance as a disorder of insulin action. Longitudinal and cross-sectional studies in Pima Indians. The New England journal of medicine 1988;318:1217–25. [DOI] [PubMed] [Google Scholar]
- 9.Lillioja S, Mott DM, Spraul M, et al. Insulin resistance and insulin secretory dysfunction as precursors of non-insulin-dependent diabetes mellitus. Prospective studies of Pima Indians. The New England journal of medicine 1993;329:1988–92. [DOI] [PubMed] [Google Scholar]
- 10.Weyer C, Bogardus C, Mott DM, Pratley RE. The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. The Journal of clinical investigation 1999;104:787–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nelson RG, Bennett PH, Beck GJ, et al. Development and progression of renal disease in Pima Indians with non-insulin-dependent diabetes mellitus. Diabetic Renal Disease Study Group. The New England journal of medicine 1996;335:1636–42. [DOI] [PubMed] [Google Scholar]
- 12.Nelson RG, Knowler WC, Kretzler M, et al. Pima Indian Contributions to Our Understanding of Diabetic Kidney Disease. Diabetes 2021;70:1603–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weil EJ, Fufaa G, Jones LI, et al. Effect of losartan on prevention and progression of early diabetic nephropathy in American Indians with type 2 diabetes. Diabetes 2013;62:3224–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pettitt DJ, Baird HR, Aleck KA, Bennett PH, Knowler WC. Excessive obesity in offspring of Pima Indian women with diabetes during pregnancy. The New England journal of medicine 1983;308:242–5. [DOI] [PubMed] [Google Scholar]
- 15.Pettitt DJ, Bennett PH, Saad MF, Charles MA, Nelson RG, Knowler WC. Abnormal glucose tolerance during pregnancy in Pima Indian women. Long-term effects on offspring. Diabetes 1991;40 Suppl 2:126–30. [DOI] [PubMed] [Google Scholar]
- 16.Dabelea D, Knowler WC, Pettitt DJ. Effect of diabetes in pregnancy on offspring: follow-up research in the Pima Indians. The Journal of maternal-fetal medicine 2000;9:83–8. [DOI] [PubMed] [Google Scholar]
- 17.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. The New England journal of medicine 2002;346:393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wing RR, Bolin P, Brancati FL, et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. The New England journal of medicine 2013;369:145–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nathan DM, Lachin JM, Balasubramanyam A, et al. Glycemia Reduction in Type 2 Diabetes - Glycemic Outcomes. The New England journal of medicine 2022;387:1063–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nathan DM, Lachin JM, Bebu I, et al. Glycemia Reduction in Type 2 Diabetes - Microvascular and Cardiovascular Outcomes. The New England journal of medicine 2022;387:1075–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knowler WC, Coresh J, Elston RC, et al. The Family Investigation of Nephropathy and Diabetes (FIND): design and methods. Journal of diabetes and its complications 2005;19:1–9. [DOI] [PubMed] [Google Scholar]
- 22.Rushforth NB, Bennett PH, Steinberg AG, Burch TA, Miller M. Diabetes in the Pima Indians. Evidence of bimodality in glucose tolerance distributions. Diabetes 1971;20:756–65. [DOI] [PubMed] [Google Scholar]
- 23.Stern MP, Rosenthal M, Haffner SM. A new concept of impaired glucose tolerance. Relation to cardiovascular risk. Arteriosclerosis (Dallas, Tex) 1985;5:311–4. [DOI] [PubMed] [Google Scholar]
- 24.Dorf A, Ballintine EJ, Bennett PH, Miller M. Retinopathy in Pima Indians. Relationships to glucose level, duration of diabetes, age at diagnosis of diabetes, and age at examination in a population with a high prevalence of diabetes mellitus. Diabetes 1976;25:554–60. [DOI] [PubMed] [Google Scholar]
- 25.Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. National Diabetes Data Group. Diabetes 1979;28:1039–57. [DOI] [PubMed] [Google Scholar]
- 26.WHO Expert Committee on Diabetes Mellitus: second report. World Health Organization technical report series 1980;646:1–80. [PubMed] [Google Scholar]
- 27.Diagnosis and classification of diabetes mellitus. Diabetes care 2014;37 Suppl 1:S81–90. [DOI] [PubMed] [Google Scholar]
- 28.Organization WH. Definition and DIagnosis of Diabetes Mellitus and Intermediate Hyperglycaemia. Geneva 2006.
- 29.Looker HC, Knowler WC, Hanson RL. Changes in BMI and weight before and after the development of type 2 diabetes. Diabetes care 2001;24:1917–22. [DOI] [PubMed] [Google Scholar]
- 30.Mason CC, Hanson RL, Knowler WC. Progression to type 2 diabetes characterized by moderate then rapid glucose increases. Diabetes 2007;56:2054–61. [DOI] [PubMed] [Google Scholar]
- 31.Saad MF, Knowler WC, Pettitt DJ, Nelson RG, Mott DM, Bennett PH. The natural history of impaired glucose tolerance in the Pima Indians. The New England journal of medicine 1988;319:1500–6. [DOI] [PubMed] [Google Scholar]
- 32.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. The American journal of physiology 1979;237:E214–23. [DOI] [PubMed] [Google Scholar]
- 33.Schwartz MW, Boyko EJ, Kahn SE, Ravussin E, Bogardus C. Reduced insulin secretion: an independent predictor of body weight gain. The Journal of clinical endocrinology and metabolism 1995;80:1571–6. [DOI] [PubMed] [Google Scholar]
- 34.Bogardus C Insulin resistance in the pathogenesis of NIDDM in Pima Indians. Diabetes care 1993;16:228–31. [DOI] [PubMed] [Google Scholar]
- 35.Pratley R, Weyer C, Bogardus C. Metabolic abnormalities in the development of type 2 diabetes mellitus. In: LeRoith D, Taylor S, Olefsky J, eds. Diabetes Mellitus: A Fundamental and Clinical Text. 2nd ed. Philadelphia: Lippincott, Williams & Wilkins; 1999. [Google Scholar]
- 36.Heinitz S, Piaggi P, Bogardus C, Krakoff J. Decline in the acute insulin response in relationship to plasma glucose concentrations. Diabetes/metabolism research and reviews 2018;34. [DOI] [PubMed] [Google Scholar]
- 37.Sakul H, Pratley R, Cardon L, Ravussin E, Mott D, Bogardus C. Familiality of physical and metabolic characteristics that predict the development of non-insulin-dependent diabetes mellitus in Pima Indians. American journal of human genetics 1997;60:651–6. [PMC free article] [PubMed] [Google Scholar]
- 38.DeFronzo RA. Lilly lecture 1987. The triumvirate: beta-cell, muscle, liver. A collusion responsible for NIDDM. Diabetes 1988;37:667–87. [DOI] [PubMed] [Google Scholar]
- 39.Bunt JC, Krakoff J, Ortega E, Knowler WC, Bogardus C. Acute insulin response is an independent predictor of type 2 diabetes mellitus in individuals with both normal fasting and 2-h plasma glucose concentrations. Diabetes/metabolism research and reviews 2007;23:304–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shah MH, Piaggi P, Looker HC, Paddock E, Krakoff J, Chang DC. Lower insulin clearance is associated with increased risk of type 2 diabetes in Native Americans. Diabetologia 2021;64:914–22. [DOI] [PubMed] [Google Scholar]
- 41.Knowler WC, Pettitt DJ, Savage PJ, Bennett PH. Diabetes incidence in Pima indians: contributions of obesity and parental diabetes. American journal of epidemiology 1981;113:144–56. [DOI] [PubMed] [Google Scholar]
- 42.Knowler WC, Pettitt DJ, Saad MF, Bennett PH. Diabetes mellitus in the Pima Indians: incidence, risk factors and pathogenesis. Diabetes/metabolism reviews 1990;6:1–27. [DOI] [PubMed] [Google Scholar]
- 43.Kim HI, Ye B, Gosalia N, et al. Characterization of Exome Variants and Their Metabolic Impact in 6,716 American Indians from the Southwest US. American journal of human genetics 2020;107:251–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mahajan A, Spracklen CN, Zhang W, et al. Multi-ancestry genetic study of type 2 diabetes highlights the power of diverse populations for discovery and translation. Nature genetics 2022;54:560–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vujkovic M, Keaton JM, Lynch JA, et al. Discovery of 318 new risk loci for type 2 diabetes and related vascular outcomes among 1.4 million participants in a multi-ancestry meta-analysis. Nature genetics 2020;52:680–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hanson RL, Rong R, Kobes S, et al. Role of Established Type 2 Diabetes-Susceptibility Genetic Variants in a High Prevalence American Indian Population. Diabetes 2015;64:2646–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Del Bosque-Plata L, Martinez-Martinez E, Espinoza-Camacho MA, Gragnoli C. The Role of TCF7L2 in Type 2 Diabetes. Diabetes 2021;70:1220–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guo T, Hanson RL, Traurig M, et al. TCF7L2 is not a major susceptibility gene for type 2 diabetes in Pima Indians: analysis of 3,501 individuals. Diabetes 2007;56:3082–8. [DOI] [PubMed] [Google Scholar]
- 49.Hanson RL, Guo T, Muller YL, et al. Strong parent-of-origin effects in the association of KCNQ1 variants with type 2 diabetes in American Indians. Diabetes 2013;62:2984–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nair AK, Traurig M, Sutherland JR, et al. Generation of Isogenic hiPSCs with Targeted Edits at Multiple Intronic SNPs to Study the Effects of the Type 2 Diabetes Associated KCNQ1 Locus in American Indians. Cells 2022;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thearle MS, Muller YL, Hanson RL, et al. Greater impact of melanocortin-4 receptor deficiency on rates of growth and risk of type 2 diabetes during childhood compared with adulthood in Pima Indians. Diabetes 2012;61:250–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pettitt DJ, Aleck KA, Baird HR, Carraher MJ, Bennett PH, Knowler WC. Congenital susceptibility to NIDDM. Role of intrauterine environment. Diabetes 1988;37:622–8. [DOI] [PubMed] [Google Scholar]
- 53.Dabelea D, Hanson RL, Lindsay RS, et al. Intrauterine exposure to diabetes conveys risks for type 2 diabetes and obesity: a study of discordant sibships. Diabetes 2000;49:2208–11. [DOI] [PubMed] [Google Scholar]
- 54.Chen P, Piaggi P, Traurig M, et al. Differential methylation of genes in individuals exposed to maternal diabetes in utero. Diabetologia 2017;60:645–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dabelea D, Hanson RL, Bennett PH, Roumain J, Knowler WC, Pettitt DJ. Increasing prevalence of Type II diabetes in American Indian children. Diabetologia 1998;41:904–10. [DOI] [PubMed] [Google Scholar]
- 56.Gautier JF, Wilson C, Weyer C, et al. Low acute insulin secretory responses in adult offspring of people with early onset type 2 diabetes. Diabetes 2001;50:1828–33. [DOI] [PubMed] [Google Scholar]
- 57.Schulz LO, Bennett PH, Ravussin E, et al. Effects of traditional and western environments on prevalence of type 2 diabetes in Pima Indians in Mexico and the U.S. Diabetes care 2006;29:1866–71. [DOI] [PubMed] [Google Scholar]
- 58.Hsueh WC, Bennett PH, Esparza-Romero J, et al. Analysis of type 2 diabetes and obesity genetic variants in Mexican Pima Indians: Marked allelic differentiation among Amerindians at HLA. Ann Hum Genet 2018;82:287–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Consortium STD, Williams AL, Jacobs SB, et al. Sequence variants in SLC16A11 are a common risk factor for type 2 diabetes in Mexico. Nature 2014;506:97–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Traurig M, Hanson RL, Marinelarena A, et al. Analysis of SLC16A11 Variants in 12,811 American Indians: Genotype-Obesity Interaction for Type 2 Diabetes and an Association With RNASEK Expression. Diabetes 2016;65:510–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fabre J, Balant LP, Dayer PG, Fox HM, Vernet AT. The kidney in maturity onset diabetes mellitus: a clinical study of 510 patients. Kidney international 1982;21:730–8. [DOI] [PubMed] [Google Scholar]
- 62.Joslin EP. Joslin’s diabetes mellitus. Philadelphia: Lea & Febiger; 1985. [Google Scholar]
- 63.Nelson RG, Knowler WC, McCance DR, et al. Determinants of end-stage renal disease in Pima Indians with type 2 (non-insulin-dependent) diabetes mellitus and proteinuria. Diabetologia 1993;36:1087–93. [DOI] [PubMed] [Google Scholar]
- 64.Nelson RG, Pettitt DJ, Carraher MJ, Baird HR, Knowler WC. Effect of proteinuria on mortality in NIDDM. Diabetes 1988;37:1499–504. [DOI] [PubMed] [Google Scholar]
- 65.Pavkov ME, Sievers ML, Knowler WC, Bennett PH, Nelson RG. An explanation for the increase in heart disease mortality rates in diabetic Pima Indians: effect of renal replacement therapy. Diabetes care 2004;27:1132–6. [DOI] [PubMed] [Google Scholar]
- 66.Nelson RG, Newman JM, Knowler WC, et al. Incidence of end-stage renal disease in type 2 (non-insulin-dependent) diabetes mellitus in Pima Indians. Diabetologia 1988;31:730–6. [DOI] [PubMed] [Google Scholar]
- 67.Vasquez B, Flock EV, Savage PJ, et al. Sustained reduction of proteinuria in type 2 (non-insulin-dependent) diabetes following diet-induced reduction of hyperglycaemia. Diabetologia 1984;26:127–33. [DOI] [PubMed] [Google Scholar]
- 68.Nelson RG, Knowler WC, Pettitt DJ, Saad MF, Charles MA, Bennett PH. Assessment of risk of overt nephropathy in diabetic patients from albumin excretion in untimed urine specimens. Archives of internal medicine 1991;151:1761–5. [PubMed] [Google Scholar]
- 69.Bennett PH, Haffner S, Kasiske BL, et al. Screening and management of microalbuminuria in patients with diabetes mellitus: recommendations to the Scientific Advisory Board of the National Kidney Foundation from an ad hoc committee of the Council on Diabetes Mellitus of the National Kidney Foundation. American journal of kidney diseases : the official journal of the National Kidney Foundation 1995;25:107–12. [DOI] [PubMed] [Google Scholar]
- 70.Nelson RG, Bennett PH. Diabetic renal disease in Pima Indians. Transplantation proceedings 1989;21:3913–5. [PubMed] [Google Scholar]
- 71.Pavkov ME, Mason CC, Bennett PH, Curtis JM, Knowler WC, Nelson RG. Change in the distribution of albuminuria according to estimated glomerular filtration rate in Pima Indians with type 2 diabetes. Diabetes care 2009;32:1845–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Satake E, Saulnier P-J, Kobayashi H, et al. Comprehensive Search for Novel Circulating miRNAs and Axon Guidance Pathway Proteins Associated with Risk of End Stage Kidney Disease in Diabetes. Journal of the American Society of Nephrology 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pagtalunan ME, Miller PL, Jumping-Eagle S, et al. Podocyte loss and progressive glomerular injury in type II diabetes. The Journal of clinical investigation 1997;99:342–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fufaa GD, Weil EJ, Lemley KV, et al. Structural Predictors of Loss of Renal Function in American Indians with Type 2 Diabetes. Clinical journal of the American Society of Nephrology : CJASN 2016;11:254–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Looker HC, Mauer M, Saulnier P-J, et al. Changes in albuminuria but not GFR are associated with early changes in kidney structure in type 2 diabetes. Journal of the American Society of Nephrology 2019;30:1049–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lemley KV, Abdullah I, Myers BD, et al. Evolution of incipient nephropathy in type 2 diabetes mellitus. Kidney international 2000;58:1228–37. [DOI] [PubMed] [Google Scholar]
- 77.Meyer TW, Bennett PH, Nelson RG. Podocyte number predicts long-term urinary albumin excretion in Pima Indians with Type II diabetes and microalbuminuria. Diabetologia 1999;42:1341–4. [DOI] [PubMed] [Google Scholar]
- 78.Lemley KV, Abdullah I, Myers BD, et al. Evolution of incipient nephropathy in type 2 diabetes mellitus. Kidney International 2000;58:1228–37. [DOI] [PubMed] [Google Scholar]
- 79.Looker HC, Mauer M, Nelson RG. Role of Kidney Biopsies for Biomarker Discovery in Diabetic Kidney Disease. Advances in chronic kidney disease 2018;25:192–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Berthier CC, Zhang H, Schin M, et al. Enhanced expression of Janus kinase-signal transducer and activator of transcription pathway members in human diabetic nephropathy. Diabetes 2009;58:469–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tuttle KR, Brosius FC 3rd, Adler SG, et al. JAK1/JAK2 inhibition by baricitinib in diabetic kidney disease: results from a Phase 2 randomized controlled clinical trial. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association 2018;33:1950–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nair V, Komorowsky CV, Weil EJ, et al. A molecular morphometric approach to diabetic kidney disease can link structure to function and outcome. Kidney international 2018;93:439–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Stefansson VTN, Nair V, Melsom T, et al. Molecular programs associated with glomerular hyperfiltration in early diabetic kidney disease. Kidney international 2022;102:1345–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bjornstad P, Chao LC, Cree-Green M, et al. Youth-onset type 2 diabetes mellitus: an urgent challenge. Nature reviews Nephrology 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Krakoff J, Lindsay RS, Looker HC, Nelson RG, Hanson RL, Knowler WC. Incidence of retinopathy and nephropathy in youth-onset compared with adult-onset type 2 diabetes. Diabetes care 2003;26:76–81. [DOI] [PubMed] [Google Scholar]
- 86.Pavkov ME, Bennett PH, Knowler WC, Krakoff J, Sievers ML, Nelson RG. Effect of youth-onset type 2 diabetes mellitus on incidence of end-stage renal disease and mortality in young and middle-aged Pima Indians. Jama 2006;296:421–6. [DOI] [PubMed] [Google Scholar]
- 87.Looker HC, Pyle L, Vigers T, et al. Structural Lesions on Kidney Biopsy in Youth-Onset and Adult-Onset Type 2 Diabetes. Diabetes care 2022;45:436–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Beisswenger PJ, Drummond KS, Nelson RG, Howell SK, Szwergold BS, Mauer M. Susceptibility to diabetic nephropathy is related to dicarbonyl and oxidative stress. Diabetes 2005;54:3274–81. [DOI] [PubMed] [Google Scholar]
- 89.Saulnier PJ, Wheelock KM, Howell S, et al. Advanced Glycation End Products Predict Loss of Renal Function and Correlate With Lesions of Diabetic Kidney Disease in American Indians With Type 2 Diabetes. Diabetes 2016;65:3744–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pavkov ME, Weil EJ, Fufaa GD, et al. Tumor necrosis factor receptors 1 and 2 are associated with early glomerular lesions in type 2 diabetes. Kidney international 2016;89:226–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kobayashi H, Looker HC, Satake E, et al. Neuroblastoma suppressor of tumorigenicity 1 is a circulating protein associated with progression to end-stage kidney disease in diabetes. Science translational medicine 2022;14:eabj2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wheelock KM, Jaiswal M, Martin CL, et al. Cardiovascular autonomic neuropathy associates with nephropathy lesions in American Indians with type 2 diabetes. Journal of diabetes and its complications 2016;30:873–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wheelock KM, Saulnier PJ, Tanamas SK, et al. White blood cell fractions correlate with lesions of diabetic kidney disease and predict loss of kidney function in Type 2 diabetes. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association 2017. [DOI] [PubMed] [Google Scholar]
- 94.Looker HC, Lin C, Nair V, et al. Serum Level of Polyubiquitinated PTEN and Loss of Kidney Function in American Indians With Type 2 Diabetes. American Journal of Kidney Diseases 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rushforth NB, Miller M, Bennett PH. Fasting and two-hour post-load glucose levels for the diagnosis of diabetes. The relationship between glucose levels and complications of diabetes in the Pima Indians. Diabetologia 1979;16:373–9. [DOI] [PubMed] [Google Scholar]
- 96.Nelson RG, Kunzelman CL, Pettitt DJ, Saad MF, Bennett PH, Knowler WC. Albuminuria in type 2 (non-insulin-dependent) diabetes mellitus and impaired glucose tolerance in Pima Indians. Diabetologia 1989;32:870–6. [DOI] [PubMed] [Google Scholar]


